Abstract
Purpose
To review our experience in the management of malignant transformation of teratoma (MTT).
Materials and methods
Nine patients with MTT were identified from January 1980 to August 2005, with all pathological specimens re-reviewed by a single genitourinary pathologist.
Results
Two patients presented with clinical stage I disease in which malignant transformation occurred within the primary testis tumor (rhabdomyosarcoma in 1 and adenocarcinoma in 1). These patients underwent a primary retroperitoneal lymph node dissection (RPLND). No viable tumor was identified in the specimen, and both patients were alive without disease at 16 months follow-up. Of the remaining 7 patients, the clinical stages were IIA (N = 1), IIB (N = 3), and III (N = 3), and all were treated with chemotherapy followed by RPLND. The MTT histology of these RPLND specimens consisted of adenocarcinoma (N = 3), rhabdomyosarcoma (N = 2), angiosarcoma (N = 1), and astrocytoma (N = 1). Following preoperative chemotherapy, a significant radiologic response (defined as more than a 25% reduction in maximum tumor circumferential diameter) was demonstrated in 1 patient, and normalization of serum tumor markers was demonstrated in 6. At a mean follow-up of 5 years, 3 of these 7 patients were alive with no evidence of disease, 1 had persistent disease, and 3 had died of disease, and their median disease-specific survival duration was 4.6 years.
Conclusions
In our experience, MTT is significantly resistant to current chemotherapeutic regimens, as demonstrated by its poor radiologic response to treatment. Alternative therapeutic strategies targeted to MTT are thus needed.
Keywords: Malignant transformation, Teratoma, Postchemotherapy Retroperitoneal lymph node dissection, Testis cancer
1. Introduction
Malignant transformation of teratoma (MTT) is defined as the transformation of a somatic teratomatous component of a germ cell tumor (GCT) to a non-germ cell tumor malignant phenotype, with the most frequent histologic sub-types consisting of rhabdomyosarcoma, adenocarcinoma, and primitive neuroectodermal tumors [1]. MTT is a relatively rare clinical entity, with one study estimating that it constitutes only approximately 3% to 6% of metastatic GCT cases [2,3].
In contrast to conventional GCTs, which have an excellent response to platinum-based chemotherapy with overall cure rates of over 90% [1,4,5], MTT is a highly aggressive tumor with a propensity for systemic progression. In a study of 21 patients with MTT, Comiter et al. reported an 81% recurrence rate following aggressive platinum-based chemotherapy and surgical resection, with a median time to recurrence of 6 months and an overall disease-specific mortality rate of 24% at a median follow-up of 50 months [3]. Other studies have reported similar treatment-related outcomes for patients with MTT, and they implicate chemoresistance to conventional platinum-based chemotherapy as a possible explanation for its poor prognosis [1,3,6–8].
The purpose of this study was to review our experience in the management of MTT and determine its response to conventional systemic chemotherapy followed by retroperitoneal lymph node dissection (RPLND).
2. Materials and methods
2.1. Study design
Prior to conducting this study, our retrospective chart review protocol was approved by the Institutional Review Board. From our patient database, we were able to identify 9 patients diagnosed as having MTT from January 1980 to August 2005. Complete medical records for all 9 patients were reviewed, and pertinent clinical information (i.e., clinical presentation, treatment, and disease-specific outcomes) was entered into a database. Clinical staging was assessed using the 1997 TNM staging system. All patients had a complete metastatic evaluation, including assessment of serum tumor markers [α fetoprotein (AFP), β-human chorionic gonadotropin (HCG), lactate dehydrogenase (LDH)], and radiologic evaluation including chest and abdomen/ pelvis computed tomography (CT) scans.
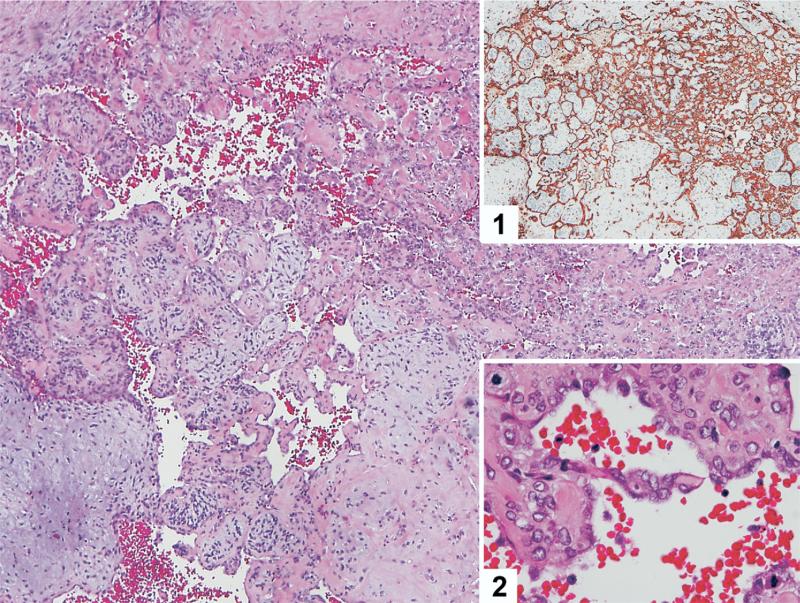
The pathologic specimens from the testis and subsequent resection(s) were re-reviewed by a single genitourinary pathologist (J.A.G.) who confirmed the diagnoses of MTT, with the malignant component of these tumors being of epithelial, mesenchymal, or neural tissue origin (Fig. 1). The diagnosis of MTT was determined on the basis of the presence of non-germ cell malignant cancer in the presence of predominantly mature teratoma, with the tumor suspected to have resulted from malignant transformation of the teratomatous elements. If viable GCT were present within the specimen, it constituted only a small focus.
Fig. 1.
Low-power magnification of angiosarcoma arising within a background of teratoma. The tumor cells are positive for CD31 (inset 1) and have prominent cytologic atypia (inset 2). (Color version of figure is available online.)
Two patients had clinical stage I disease, and both had MTT within the primary testis tumor (1 rhabdomyosarcoma transformation and 1 adenocarcinoma transformation). These patients had normal serum tumor marker levels at diagnosis and were treated by a modified-template RPLND. Of the remaining 7 patients, 1 had stage IIA disease, 3 had stage IIB, and 3 had stage III. To subjectively quantify the treatment response of these tumors to preoperative systemic chemotherapy, we established criteria to define a good serum tumor marker response and a good radiologic response to preoperative chemotherapy after its completion. A good serum tumor marker response was defined as a normalization of serum tumor marker levels in patients previously having elevated serum tumor marker levels with the reference ranges for normal serum tumor markers being an AFP < 5 ng/ml, HCG < 1 mIU/ml, and LDH < 618 IU/l. A good radiologic response was defined as a 25% or greater decrease in the maximum circumferential diameter of the tumor from the start to the completion of systemic chemo-therapy as measured on preoperative CT imaging. Following RPLND, patients with MTT were either offered adjuvant chemotherapy or were followed at regular surveillance intervals (every 3 to 6 months) with serum tumor markers and imaging of the chest and abdomen/pelvis. Patients with local recurrence or systemic metastasis were offered salvage therapy (surgery, chemotherapy, or both) if they had a good performance status (Eastern Cooperative Oncology Group Score of 0–1) and if they were deemed good salvage therapy candidates.
2.2. Statistical analysis
Descriptive statistics were collected, including the median and range of values for age at diagnosis, serum tumor marker levels prior to orchiectomy and RPLND, and largest diameter of retroperitoneal mass on CT. The methods of Kaplan and Meier [9] were used to estimate the median disease-specific survival (DSS) and recurrence-free survival (RFS).
3. Results
3.1. Patient characteristics
A summary of the patients’ demographic and clinical characteristics is shown in Table 1. The median age of patients at diagnosis was 32.1 years (range 17–44 years). All 9 patients presented with local symptoms, 6 with a painless testicular mass, and 3 with a painful testicular mass (one of whom had abdominal/back pain). The median serum tumor marker levels prior to orchiectomy were AFP 25.8 ng/ml (1.8–122.0 ng/ml); HCG 1.7 mIU/ml (1.0–491.0 mIU/ml); and LDH 515.0 IU/L (451.0–579.0 IU/l). The clinical stages of the primary testis tumors were T1 in 6 patients, T2 in 2, and T3 in 1. The primary tumor histologies were mixed nonseminomatous GCT in 6 patients and pure teratoma in 3. The clinical stages of testis cancer were I in 2 patients, IIA in 1, IIB in 3, and III in 3.
Table 1.
Patient characteristics (N = 9)
| Variable | Mean | Median | Range |
|---|---|---|---|
| Age at diagnosis (in years) | 31.1 | 32.1 | 17–44 |
| Serum tumor markers prior to orchiectomy | |||
| AFP (ng/ml) | 44.2 | 25.8 | 1.8–122.0 |
| HCG (mIU/ml) | 83.4 | 1.7 | 1–491.0 |
| LDH (IU/l) | 515.0 | 515.0 | 451.0–579.0 |
| Serum tumor markers prior to RPLND | |||
| AFP (ng/ml) | 2.7 | 2.6 | 1.0–6.5 |
| HCG (mIU/ml) | 1.0 | 1.0 | 1.0–1.0 |
| LDH (IU/l) | 1,023.0 | 794.5 | 489.0–2,014.0 |
| Largest preoperative diameter of retroperitoneal mass on CT (in cm) | 7.1 | 3.8 | 1.0–17.0 |
| Variable | Number (%) |
|---|---|
| Clinical stage of primary tumor | |
| T1 | 6 (66.7%) |
| T2 | 2 (22.2%) |
| T3 | 1 (11.1%) |
| Vascular invasion | |
| No | 8 (88.9%) |
| Yes | 1 (11.1%) |
| Histology of testis primary tumor | |
| Mixed NSGCT | 6 (66.7%) |
| Teratoma | 3 (33.3%) |
| Clinical stage of disease | |
| I | 2 (22.2%) |
| IIA | 1 (11.1%) |
| IIB | 3 (33.3%) |
| III | 3 (33.3%) |
| Metastatic sites of disease | |
| Confined to testis | 2 (22.2%) |
| Retroperitoneum | 4 (44.4%) |
| Retroperitoneum and liver | 1 (11.1%) |
| Retroperitoneum and lung | 2 (22.2%) |
Both patients with clinical stage I disease underwent a primary RPLND (modified template), with no viable tumor found in the surgical specimens (1 patient had normal lymphatic tissue and 1 had teratoma alone). Neither of these patients developed any subsequent recurrences; both were alive without disease at a mean follow-up duration of 16 months (15.2, 16.6 months). Patients with clinical stage II and III testis cancer received a median of 4 cycles of chemotherapy prior to RPLND. On the basis of our definitions, the majority of patients (6 of 7) had a good serum tumor marker response; however, only 1 had a good radiologic response. Pathologic review of the RPLND specimens revealed adenocarcinoma in 3 patients (mucinous adenocarcinoma in 1 case), rhabdomyosarcoma in 2, angiosarcoma in 1, and astrocytoma in 1. Systemic metastases developed in 4 patients with the lung (N = 2), abdomen (N = 2), and bone (N = 1) being the sites of disease progression. Three patients with MTT (2 with clinical stage IIB disease and 1 with stage IIA disease) did not receive adjuvant or salvage chemotherapy, yet they are still alive with no evidence of disease. Salvage therapy consisting of systemic chemotherapy (doxorubicin, paclitaxel, and cisplatin for 3 cycles) followed by consolidative surgery (repeat abdominal resection and lung resection) was administered to 1 patient but he nevertheless died of disease. At last follow-up, 1 patient is still alive with persistent disease however he has recent evidence of bony metastases. This patient is being considered for salvage chemotherapy. Two patients with disease progression following RPLND were not offered salvage therapy because of their poor performance status, and received palliative care. At a mean follow-up duration of 5.0 years, 3 patients had died of disease, 3 were alive with no evidence of disease, and 1 was alive with persistent disease.
4. Discussion
The present study summarizes our experience in the management of 9 testis cancer patients with MTT treated at our center over the past 25 years. Our study is limited by the rarity of this condition combined with the diverse treatment strategies and chemotherapeutic regimens used. As such, we are clearly limited in our ability to make definitive conclusions on what may be the optimal treatment of this condition. Nevertheless, there are several important points that can be drawn from our study and are useful to the urologic oncologist managing these patients. Using specific criteria to define good radiologic and good serum tumor marker responses to treatment, we noted that MTT tumors have a poor radiologic response to chemotherapy despite frequent normalization of serum tumor marker levels. Although previous studies have commented on the chemoresistance of these tumors, this is one of the first studies to specifically quantify their response rates to conventional testis cancer therapies [1,3,6]. In addition, our study demonstrates a good outcome associated with MTT within the primary testis tumor for patients with stage I disease. In our study, both of the patients with clinical stage I testis cancer were surgically managed by primary RPLND, with none of the patients having any viable tumor within the RPLND specimen and both still alive with no evidence of disease at last follow-up. Similarly, in a previous study, all 5 patients with MTT confined to the testis were alive with no evidence of disease with follow-up durations ranging from 20 to 120 months [2]. Nevertheless, we feel that primary RPLND is a key diagnostic and therapeutic modality in patients with MTT within the testis as 1 of 2 patients in our series had teratoma within the RPLND specimen. We hope future studies will provide additional insight into the role of primary RPLND in this patient population.
In the present study, patients with stage II or III testis cancer all underwent preoperative chemotherapy (median 4 cycles); however, only 1 patient (14%) exhibited a good radiologic response. The normalization of serum tumor marker levels without noticeable radiologic response in many of these patients may be explained by the effects of chemotherapy on the remaining germ cell elements within these tumors. This finding indicates that tumor marker response following systemic chemotherapy in these patients may not be the best surrogate determinant of treatment response. The poor radiologic response of many of these tumors to chemotherapy further supports their chemorefractory behavior.
Our incidence of MTT among testis cancer patients treated at our center over the past 2 decades is 2.2%, which is quite similar to the incidence rates of 2.9% and 3.5% previously reported in 2 retrospective studies [2,3]. Patients diagnosed with MTT within the RPLND specimen have often been treated with chemotherapy and surgery [2,3,6]. Three of the patients in our study received adjuvant chemo-therapy following RPLND but metastasis developed in all 3, and they died of their disease. One of these patients received salvage chemotherapy and surgery that did not appear to alter the fate of his disease although he survived beyond 7 years after RPLND. Three of our patients were alive with no evidence of disease at last follow-up, offering some hope to patients, particularly those with low clinical stage testis cancer. However, it is to be noted that 2 of our patients have been followed for less than 12 months from the time of RPLND. Overall, we report a 42% disease-specific mortality rate at a mean follow-up duration of 5 years in patients with MTT having clinical stage II and III testis cancer, which is consistent with previous studies that have reported 25% to 70% disease-specific mortality rates, with median survival durations ranging from 23 to 30.5 months [2,3,6].
Although we were unable to identify clinical or radiologic predictors of treatment response, due to the limited number of cases, we believe clinical stage may constitute an important predictor of disease-related outcome, because all patients with stage I disease were alive with no evidence of disease whereas all patients with clinical stage III disease died of disease. Although somewhat intuitive, clinical stage has never been explicitly stated in prior studies and should be taken into account when selecting a treatment strategy and counseling patients.
We recognize that there are several limitations to our study. One is its retrospective nature, which is unavoidable due to the rarity of this tumor. Another limitation is that the chemotherapeutic regimens were not uniform among patients, making it difficult to evaluate the efficacy of a specific chemotherapeutic regimen in treating MTT. Nevertheless, the present study helps better define the chemorefractory behavior of MTT using well-delineated treatment parameters. We advise urologists and oncologists treating this diverse group of patients to consider alternative therapeutic strategies (e.g., novel chemotherapeutic agents and/or gene targeted therapy) to treat MTT than are currently being used. MTT is a distinct yet rare clinical entity derived from conventional GCT and characterized by a high-risk of disease progression and death. Future studies should focus on delineating such treatment strategies (most likely as multi-center prospective studies because of the rarity of this condition), with the goal of improving the prognosis associated with these tumors.
5. Conclusions
MTT develops in a small subset (2.2%) of testis cancer patients. Patients with stage I disease have an excellent prognosis and we feel primary RPLND is an excellent diagnostic and therapeutic option in these patients. MTT in patients with clinical stage II and III testis cancer is characterized by a poor radiologic response to preoperative chemotherapy despite a favorable tumor marker response. The poor prognosis seen in these patients is due largely to the chemoresistance of MTT, underscoring the need for more effective alternative therapeutic strategies to be developed.
References
- 1.Motzer RJ, Amsterdam A, Prieto V, et al. Teratoma with malignant transformation: Diverse malignant histologies arising in men with germ cell tumors. J Urol. 1998;159:133. doi: 10.1016/s0022-5347(01)64035-7. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed T, Bosl GJ, Hajdu SI. Teratoma with malignant transformation in germ cell tumors in men. Cancer. 1985;56:860. doi: 10.1002/1097-0142(19850815)56:4<860::aid-cncr2820560426>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 3.Comiter CV, Kibel AS, Richie JP, et al. Prognostic features of teratomas with malignant transformation: A clinicopathological study of 21 cases. J Urol. 1998;159:859. [PubMed] [Google Scholar]
- 4.Stephenson AJ, Bosl GJ, Motzer RJ, et al. Retroperitoneal lymph node dissection for nonseminomatous germ cell testicular cancer: Impact of patient selection factors on outcome. J Clin Oncol. 2005;23:2781. doi: 10.1200/JCO.2005.07.132. [DOI] [PubMed] [Google Scholar]
- 5.Donohue JP, Leviovitch I, Foster RS, et al. Integration of surgery and systemic therapy: Results and principles of integration. Semin Urol Oncol. 1998;16:65. [PubMed] [Google Scholar]
- 6.Donadio AC, Motzer RJ, Bajorin DF, et al. Chemotherapy for teratoma with malignant transformation. J Clin Oncol. 2003;21:4285. doi: 10.1200/JCO.2003.01.019. [DOI] [PubMed] [Google Scholar]
- 7.Asano T, Kawakami S, Okuno T, et al. Malignant transformation in a mature testicular teratoma left untreated for more than 50 years since childhood. Scand J Urol Nephrol. 2003;37:177. doi: 10.1080/00365590310008947. [DOI] [PubMed] [Google Scholar]
- 8.Ito K, Iigaya T, Umezawa A. Case of testicular cancer with malignant transformation in the residual retroperitoneal mature teratoma eight years after the initial chemotherapy. Nippon Hinyokika Gakkai Zasshi. 1998;89:622. doi: 10.5980/jpnjurol1989.89.622. [DOI] [PubMed] [Google Scholar]
- 9.Kaplan EL, Meier EP. Nonparametric estimation from incomplete observations. J Am Stat. 1958;53:457. [Google Scholar]