Abstract
Objective
Angiogenesis is the formation of new blood vessels through endothelial cell sprouting. This process requires the mitogen activated protein kinases (MAPK), signaling molecules that are negatively regulated by the MAPK phosphatase, MKP-1. The purpose of this study was to evaluate the role of MKP-1 in neovascularization in vivo and identify associated mechanisms in endothelial cells.
Approach and Results
We used murine hindlimb ischemia as a model system to evaluate the role of MKP-1 in angiogenic growth, remodeling, and arteriogenesis in vivo. Genomic deletion of MKP-1 blunted angiogenesis in the distal hindlimb and microvascular arteriogenesis in the proximal hindlimb. In vitro, endothelial MKP-1 depletion/deletion abrogated VEGF-induced migration and tube formation, and reduced proliferation. These observations establish MKP-1 as a positive mediator of angiogenesis and contrast with the canonical function of MKP-1 as a MAPK phosphatase, implying an alternative mechanism for MKP-1-mediated angiogenesis.
Cloning and sequencing of MKP-1-bound chromatin identified localization of MKP-1 to exonic DNA of the angiogenic chemokine fractalkine, and MKP-1 depletion reduced histone H3 serine 10 dephosphorylation on this DNA locus and blocked fractalkine expression. In vivo, MKP-1 deletion abrogated ischemia-induced fractalkine expression and macrophage and T-lymphocyte infiltration in distal hindlimbs, while fractalkine delivery to ischemic hindlimbs rescued the effect of MKP-1 deletion on neovascular hindlimb recovery.
Conclusions
MKP-1 promoted angiogenic and arteriogenic neovascular growth, potentially through dephosphorylation of H3S10 on coding-region DNA to control transcription of angiogenic genes such as fractalkine. These observations reveal a novel function for MKP-1 and identify MKP-1 as a potential therapeutic target.
Keywords: angiogenesis, MKP-1, hindlimb ischemia
Introduction
Angiogenesis, the process of endothelial cell (EC) sprouting from existing vessels to form new vessels is essential for myriad physiological processes and pathological conditions including development and growth, regeneration and repair, and tumor growth and metastasis1,2. Despite intense study, the mechanisms underlying the activation, progression, and regulation of angiogenesis remain incompletely understood1. The vascular endothelial growth factor (VEGF) has been identified as one of the most potent cytokines capable of initiating and maintaining this process, and has long been a target for both pro- and anti-angiogenic therapies3,4. Despite its potency in model systems, both delivery of VEGF, through recombinant proteins or gene therapy, and inhibition of VEGF for cancer therapy have had mixed clinical success 4–6. Thus, elucidating the molecular and regulatory mechanisms underlying this process is essential for both therapeutic formation of new vessels that feed growing and regenerating tissues as well as prevention of aberrant vascular growth that drives tumor size and aggression1,6.
One of the key mechanisms that regulates the cellular response to growth factors, cytokines, and environmental stresses is the MAPK signaling pathway7. This pathway consists of several families of primary kinase effectors, extracellular-related kinase (Erk), p38, and c-Jun N-terminal kinase (JNK)8. These MAPK are required for vascular growth and development9–12 and positively mediate angiogenic processes in endothelial cells, including migration, proliferation, and tube formation13–17. They are deactivated through Thr/Tyr dephosphorylation by the nuclear phosphatase, mitogen-activated protein kinase phosphatase-1, MKP-1 (also known as DUSP1 or CL100)18.
In this study, we investigated the role of MKP-1 in angiogenic and arteriogenic neovascularization. Given the requirement of MAPK activation for vascular development and angiogenesis, we previously hypothesized that MKP-1 negatively regulates angiogenesis; surprisingly, however, we found that MKP-1 positively mediated EC migration and aortic ring sprouting in response to VEGF stimulation in vitro19, suggesting that MKP-1 may also have a non-canonical function that plays a positive role in neovascularization independently or concurrently with its action on MAPK.
Further recent observations from our laboratory suggested a potential effector of this putative non-canonical signaling: MKP-1-mediated chromatin modification. Using a “substrate trap” cysteine-to-serine (C259S) mutant of MKP-1 (CS-MKP-1), which results in stable binding of MKP-1 to its substrates, we identified MKP-1 as the only known mammalian histone H3 serine 10 (H3S10) phosphatase, which is required for VEGF-induced H3S10 dephosphorylation20. Here, we describe the effect of genomic deletion of MKP-1 on angiogenic and arteriogenic recovery from hindlimb ischemia in vivo and demonstrate a positive role for endothelial MKP-1 in angiogenic gene expression associated with MKP-1-mediated exonic histone H3 dephosphorylation on the angiogenic and inflammatory gene, fractalkine.
Materials and Methods
Materials and Methods are available in the online-only supplement available at atvb.ahajournals.org.
Results
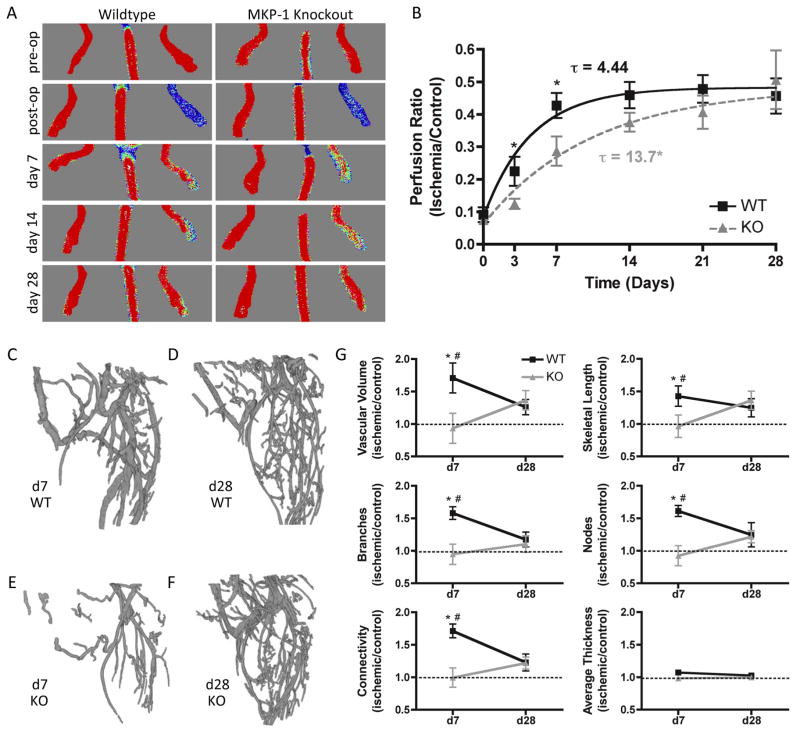
MKP-1 knockout (KO) and littermate wildtype (WT) mice (N=11–20 per group) underwent surgical induction of hindlimb ischemia21,22, and angiogenic and arteriogenic recovery was evaluated over 28 days by longitudinal laser Doppler perfusion imaging (LDPI, Figure 1A, B). Exponential curve-fit analysis demonstrated that MKP-1 KO mice featured significantly slower recovery compared to WT (τWT = 4.44 ± 0.99 days, τKO = 13.7 ± 4.08 days; p = 0.04), but recovered to the same plateau by day 28 (PWT = 0.48 ± 0.03, PKO = 0.54 ± 0.09; p = 0.58). The perfusion ratio was significantly lower in KO limbs at days 3 and 7, and KO mice recovered to WT levels by day 28.
Figure 1. Ischemic limb recovery in WT and MKP-1 KO mice.
Mice (N = 11–20 per time point per genotype) received surgical induction of ischemia in L hindlimbs, with R limbs evaluated as contralateral controls. Shown are representative images (A) and quantification (B) of laser Doppler perfusion to ischemic foot, normalized by contralateral control. Data were fit to an exponential recovery curve, y = S e−t/τ+P; MKP-1 knockout mice had a significantly longer time constant, τ, but recovered to the same plateau, P, by day 28. (C–G): MicroCT angiography generated 3D reconstructions of WT and KO vascular structures in the calf region at day 7 (A,C) and day 28 (B,D) (N = 4–9 per time point per genotype). Quantification of vascular network parameters (E) revealed significantly greater angiogenic network formation in ischemic limbs of WT mice at d7 compared to ischemic limbs of KO mice (*p ≤ 0.05 vs. KO at same time point) and contralateral controls (dotted lines, #p ≤ 0.05 vs. control at same time point). By day 28, there were no significant differences between genotypes or compared with controls. Ischemic limbs of KO mice did not differ from controls at either time point.
Based on these observations, days 7 and 28 were selected for quantitative microCT angiography analysis. Angiogenesis and arteriogenesis are linked in the hindlimb ischemia model, as sufficient collateralization of the upper hindlimb is necessary for perfusion and angiogenesis of the lower hindlimb and sufficient capillary networks are required for arteriogenesis and collateralization. Thus, angiogenesis and collateral arteriogenesis act in concert to establish a functional vascular network in both proximal and distal parts of the ischemic limb. However, while these mechanisms of new vessel formation cannot be spatially divorced, they are dominant in different regions of the limb, as has been described previously23,24. Vascular growth in the thigh occurs primarily through arteriogenesis stemming from increased wall shear stress in collateral vessels, whereas it proceeds predominantly through angiogenesis in the lower hindlimb (calf) as a result of tissue hypoxia23,24. Therefore, these two regions of interest were selected for independent analysis. In the distal hindlimb, WT mice featured a biphasic recovery characterized by increased vascularity above contralateral controls at day 7 (p < 0.05 vs. control) and remodeling to control values by day 28 (p > 0.05 vs. control). In contrast, vascular parameters of KO mice failed to exceed unoperated controls at either time point, with significantly lower vascularity compared with ischemic WT limbs at day 7 and equivalent values at day 28 (Figure 1C–G; unoperated control values indicated by dotted lines). In the proximal hindlimb, there were no differences in vascular parameters between genotypes or between control and ischemic limbs, with the exception of connectivity at day 7, in which ischemic limbs of WT mice had significantly greater connectivity than the contralateral control and ischemic limbs of KO mice (Supplemental Figure I).
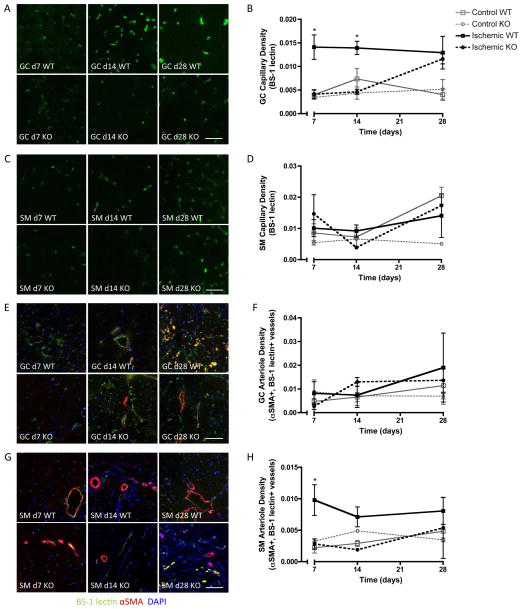
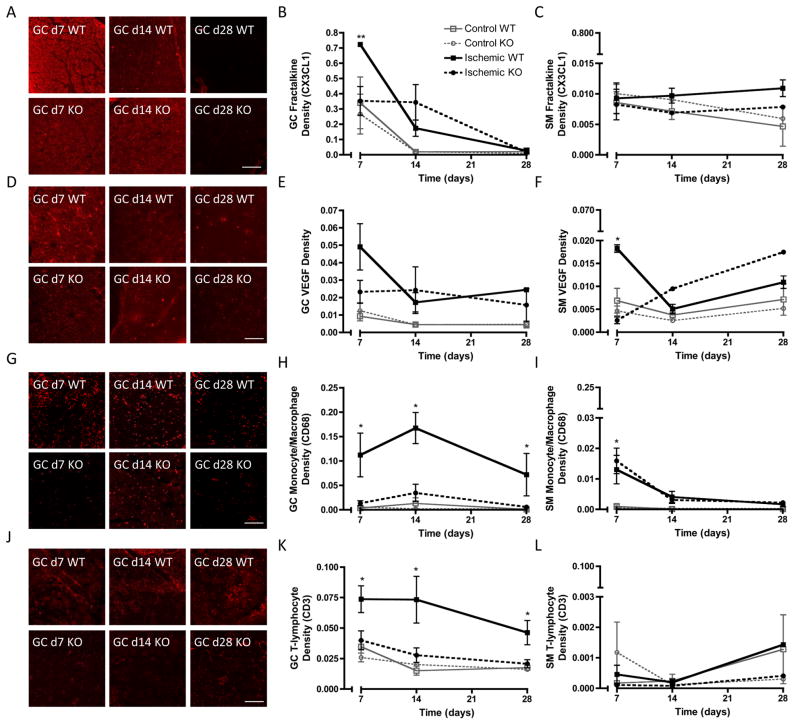
As microCT angiography is limited by resolution to assess patent vessels of ~20μm in diameter, microvascular angiogenesis and arteriogenesis were evaluated in both regions of interest by immunofluorescent staining for capillaries (EC marker BS-1 lectin) and arterioles (αSMA+, BS-1 lectin-stained vessels) at days 7, 14, and 28. In the gastrocnemius (GC) muscle of the lower hindlimb, ischemia significantly increased capillary density in WT mice compared to controls (Figure 2A, B). This increase was delayed in MKP-1 KO mice, with significantly lower values at days 7 and 14 but recovery to WT values by day 28. In contrast, capillary density was not significantly altered by MKP-1 deletion in the soleus muscle (SM) of the upper hindlimb (Figure 2C, D). Arteriole formation was not affected by MKP-1 deletion in the GC muscle (Figure 2E, F), but in the SM muscle of the upper hindlimb, arteriole density was significantly higher in WT mice compared to KO and controls at day 7 (Figure 2G, H).
Figure 2. Immunofluorescent evaluation of microvascular capillaries and arterioles.
Shown are representative micrographs and quantification of endothelial cell (BS-1 lectin, A–D), and arteriole (αSMA+, BS1-lectin+, E–H) staining in transverse sections of gastrocnemius (GC; calf) and soleus (SM; thigh) muscles of ischemic limbs of WT and MKP-1 KO mice (N = 3–8 per time point per genotype). Capillary density was significantly lower in GC muscles of KO mice at day 7 and 14, but equivalent to WT at day 28, while arteriole density was lower in SM muscles of KO mice at day 7. *p ≤ 0.05 vs. KO at same time point. Scale bar: 25 μm.
To evaluate the time course of MKP-1 expression in ischemic hindlimbs, SM and GC sections of WT mice were immunostained for MKP-1 at days 7, 14, and 28. MKP-1 was induced by hindlimb ischemia in both regions at day 7 and decreased to control levels by day 28 (Supplemental Figure II).
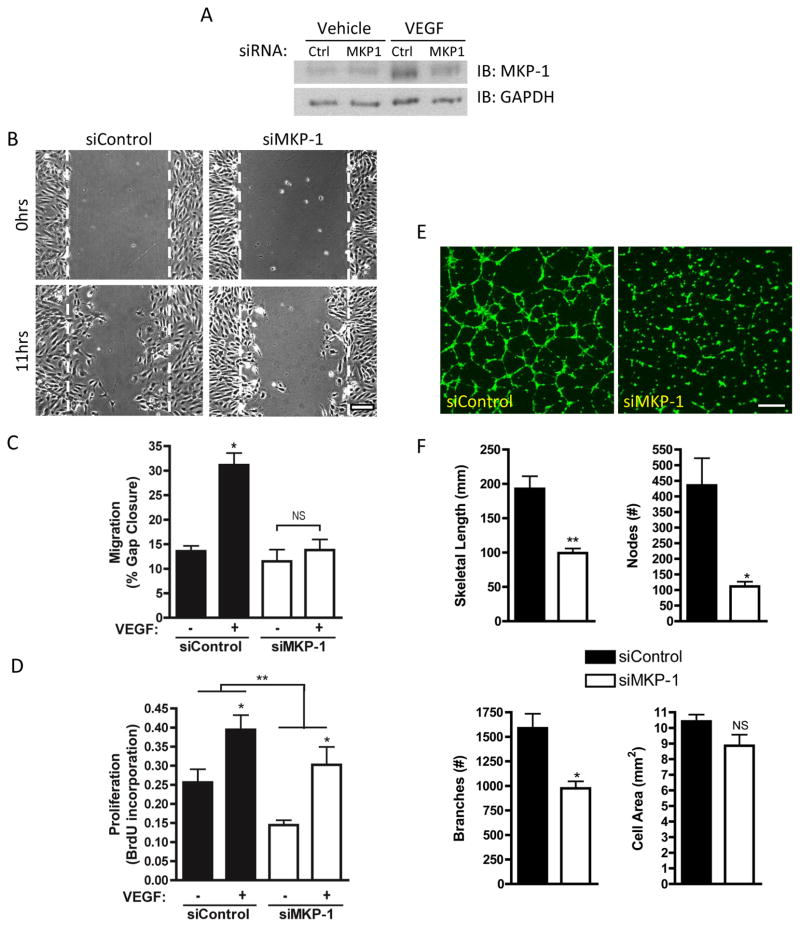
In vitro, MKP-1 depletion by siRNA (Figure 3A) completely abolished VEGF-induced human umbilical vein endothelial cell (HUVEC) migration (Figure 3B, C). Similarly, mouse aortic endothelial cells (MAEC) from KO mice exhibited the same migratory deficit (Supplemental Figure IIIa, b). MKP-1 depletion significantly reduced HUVEC proliferation overall, though cells remained responsive to VEGF stimulation (Figure 3D). MKP-1 depletion also abrogated VEGF-induced HUVEC tube formation on Matrigel with reduced length, number of nodes, and number of branches without altering cell number (Figure 3E, F).
Figure 3. Effect of MKP-1 depletion on in vitro angiogenesis.
HUVEC were depleted of MKP-1 by siRNA (A) and serum starved 2hrs prior to treatment with 50 ng/ml VEGF. VEGF-induced migration (B, C), proliferation (D) and tube formation (E, F) were evaluated using the scratch wound, BrdU, and Matrigel assays, respectively. Representative migration images in (B) show initial wound (top) and migration over 11 hrs (bottom) in VEGF-treated samples. Representative tube formation images in (E) show tube formation over 6 hrs after VEGF treatment. MKP-1 depletion abrogated VEGF-induced migration and tube formation and reduced overall proliferation but did not block the pro-proliferative effect of VEGF. *p ≤ 0.05, **p ≤ 0.01, NS = not significant. Scale bars: 100 μm.
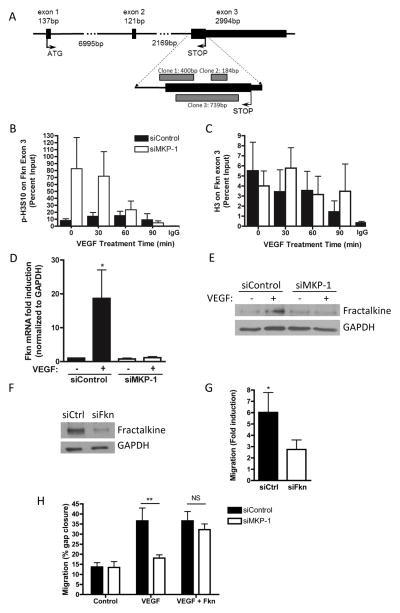
Next, to identify putative gene targets for MKP-1 regulation through chromatin interactions in HUVEC, we immunoprecipitated CS-MKP-1-bound chromatin, and cloned and sequenced associated DNA. While sequencing of vehicle-treated samples returned only pBluescript plasmid, in VEGF treated samples, three independent clones were identified; these fragments overlapped one another on the proximal end of exon 3 of the angiogenic and inflammatory gene, fractalkine, also known as cx3cl1 (Figure 4A).
Figure 4. MKP-1-chromatin interactions & fractalkine regulation.
HUVEC were transfected with substrate trap mutant CS-MKP-1 (myc-tagged), and treated 10 min with 50ng/ml VEGF or vehicle; chromatin was immunoprecipitated using an anti-myc antibody, and associated DNA were cloned and sequenced. In VEGF-treated samples, three independent clones were identified overlapping exon 3 of the angiogenic chemokine, fractalkine, as illustrated (A). Vehicle treated samples had no DNA binding. HUVEC depleted of MKP-1 by siRNA were treated with 50ng/ml VEGF over a 90 minute time course, and chromatin was immunoprecipitated using antibodies against phospho-histone H3S10 (B) or H3 (C) on this DNA locus. MKP-1 depletion enriched the DNA for p-H3S10 at 0 and 30 minutes after VEGF treatment, but had no effect on total H3 binding. Fractalkine mRNA (D) and protein (E) expression were induced under control conditions, but completely ablated when MKP-1 was depleted. HUVEC depleted of fractalkine (F) exhibited a blunted migratory response to VEGF (G), but treatment with recombinant fractalkine rescued the migratory deficit of MKP-1-depleted cells (H). *p ≤ 0.05, **p ≤ 0.01 NS = not significant.
To test whether MKP-1 specifically dephosphorylates histone H3 serine 10 on this gene locus, we immunoprecipitated phospho-H3S10-bound chromatin in control or MKP-1 depleted HUVEC and performed RT-PCR for the proximal region of fractalkine exon 3. MKP-1 depletion prevented dephosphorylation of H3S10 on this gene locus at 0 and 30 minutes after VEGF treatment, returning to control levels by 90 minutes, but remained dephosphorylated at all time points in control cells (Figure 4B). In contrast, binding of total histone H3 to this DNA locus was not affected by MKP-1 depletion (Figure 4C).
Next, we evaluated fractalkine induction by VEGF in HUVEC. In cells depleted of MKP-1, VEGF-induced fractalkine expression was completely ablated at both the message (Figure 4D) and protein (Figure 4E) levels. Similarly, MKP-1-null MAEC exhibited reduced VEGF-induced fractalkine expression (Supplemental Figure IIIc). Functionally, fractalkine-depleted HUVEC were no longer significantly responsive to VEGF-induced migration (Figure 4F, G), and treatment of HUVEC (Figure 4H) or MAEC (Supplemental Figure IIId, e) with recombinant fractalkine (100ng/ml) rescued the effect of MKP-1 deletion on EC migration.
To verify that MKP-1 mediated fractalkine gene regulation in vivo, we evaluated fractalkine protein expression in both GC and SM muscles of control and ischemic hindlimbs of WT and MKP-1 KO mice at days 7, 14, and 28 by immunofluorescence. Fractalkine staining co-localized with endothelial cells in the ischemic hindlimbs of both WT and KO mice (Supplemental Figure IV), demonstrating that ECs in the ischemic hindlimb express fractalkine, though fractalkine staining was not exclusive to ECs, consistent with its role as a soluble signaling molecule in its cleaved form. In GC muscle of the lower hindlimb, fractalkine expression was significantly induced over control levels in ischemic limbs of WT, but not KO, mice at day 7, confirming the MKP-1 dependence of fractalkine expression. Over time, expression decreased to negligible levels in both genotypes by day 28 (Figure 5A, B). Background fractalkine levels were moderately elevated in control limbs at day 7 compared with days 14 and 28, suggesting a systemic circulation of cytokines that resulted in contralateral induction, though this was insufficient to cause inflammatory cell infiltration in distal KO or control limbs (Figure 5G, H, J, K). This may suggest a threshold expression level necessary for inflammatory cell infiltration, which was not met in either control or KO limbs at any time point. In SM muscle of the upper hindlimb, fractalkine expression was not significantly induced in either ischemic or control limbs of either genotype (Figure 5C). While monocyte/macrophage infiltration was significantly induced by ischemia at day 7 in SM sections, it was less robust than in GC muscle, and there were no differences between WT and MKP-1 KO mice (Figure 5I). T-lymphocyte staining in SM sections was minimal and exhibited no differences between genotypes (Figure 5L).
Figure 5. MKP-1 regulation of fractalkine & VEGF expression and inflammation in vivo.
Shown are representative micrographs of transverse GC sections (A, D, G, J) and area density quantification of fractalkine (B,C), VEGF (E, F), monocyte/macrophage (CD68; H, I), and T-lymphocyte (CD3; K, L) staining in transverse sections of both GC and SM muscles of WT and MKP-1 KO mice (N = 3–8 per time point per genotype). Fractalkine expression in GC tissue was significantly elevated in ischemic limbs of WT, but not knockout mice, at day 7 (B). Fractalkine was not elevated in SM sections for either genotype (C). VEGF was transiently elevated at day 7 in WT GC and SM sections (E, F, respectively). In GC sections, monocyte/macrophage and T-lymphocyte infiltration was significantly elevated at all time points in WT mice over MKP-1 KO (H, K), but was not different between genotypes in SM sections (I, L). *p ≤ 0.05 vs. KO at same time point. Scale bars: 50 μm.
Next, we quantified the time course of VEGF protein expression in GC and SM muscles of control and ischemic hindlimbs of WT and MKP-1 KO mice. In the GC muscle, VEGF expression was induced by ischemia in WT mice, though expression did not differ significantly from KO levels (Figure 5D, E). There were no differences at days 14 or 28. In WT SM muscle, VEGF was significantly induced by hindlimb ischemia at day 7 and decreased through day 28, while in KO mice VEGF expression was not elevated at day 7 but increased over time (Figure 5F). VEGF expression levels were lower in SM than in GC muscles for both genotypes.
Fractalkine is a potent chemotactic agent for inflammatory leukocytes25–27, so to evaluate the role of MKP-1 in the time course of inflammation, GC and SM sections from WT and MKP-1 KO mice were probed for monocytes/macrophages (CD68, Figure 5G–I), and T-lymphocytes (CD3, Figure 5J–K) at 7, 14, and 28 days. In GC muscle, monocyte/macrophage and T-lymphocyte infiltration was minimal in control limbs and in ischemic limbs of KO mice, but was significantly elevated in ischemic limbs of WT mice at all time points evaluated (Figure 5G, H, J, K). In contrast, in SM muscle, monocyte/macrophage infiltration was elevated at day 7 in both WT and KO mice, and diminished to negligible levels by day 28. T-lymphocyte staining was minimimal in all groups at all time points in SM sections. Immunofluorescence specificity was demonstrated in positive and negative controls (Supplemental Figure V).
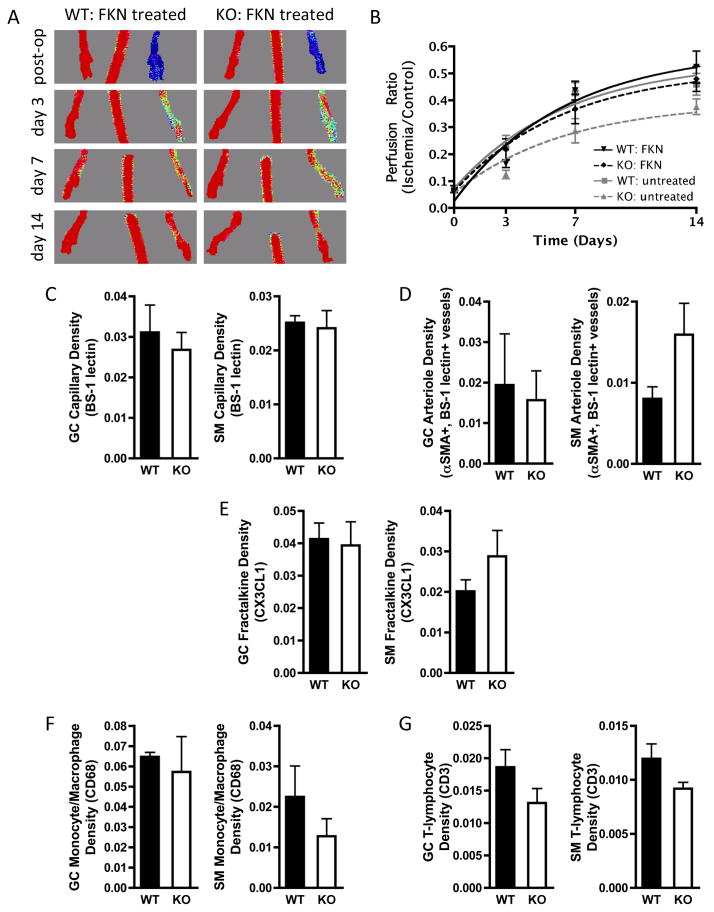
Finally, to test whether delivery of recombinant fractalkine would rescue the effect of MKP-1 deletion, WT and MKP-1 KO mice (N = 6 per genotype) received hindlimb ischemia and were then injected intramuscularly immediately after surgery with 5μg recombinant mouse fractalkine, divided evenly between GC and SM muscles, as described previously in rats28. While FKN delivery unexpectedly had no effect in WT mice, perfusion recovery of MKP-1 KO mice was restored to WT untreated levels (data reproduced from Figure 1 in gray), with no significant differences between WT untreated, WT treated, or KO treated groups at any time point (Figure 6A, B). Exponential curve-fit analysis revealed no differences in characteristic time scales between fractalkine-treated WT and fractalkine treated KO mice (τWT = 9.29 days, τKO = 8.03 days; p = 0.83). GC and SM muscles at day 7 post-ischemia (N = 3 per group) were sectioned and immunostained for capillaries (Figure 6C), arterioles (Figure 6D), fractalkine (Figure 6E), monocyte/macrophages (Figure 6F), and T-lymphocytes (Figure 6G). For each measure, there were no differences between WT or MKP-1 KO mice.
Figure 6. Rescue of MKP-1 phenotype by recombinant fractalkine delivery.
Ischemic WT and MKP-1 KO mice (N = 6 per group) were intramuscularly injected immediately post-surgery with 5 μg recombinant mouse fractalkine, divided evenly between GC and SM muscles. Recovery over 14 days was evaluated by LDPI (A, B), and GC and SM muscles at day 7 (N = 3 per group) were immunostained for capillaries (C), arterioles (D), fractalkine (E), monocytes/macrophages (F), and T-lymphocytes (G). Fractalkine delivery rescued the effect of MKP-1 deletion on perfusion recovery, and there were no differences in fractalkine treated samples between genotypes for immunostained parameters.
Discussion
In this study, we evaluated the role of the nuclear phosphatase, MKP-1, in neovascularization using hindlimb ischemia as a model system to evaluate angiogenesis, arteriogenesis, and vascular remodeling. We found that MKP-1 was transiently induced by hindlimb ischemia and positively mediated neovascular growth in vivo and in vitro, in part through regulation of the angiogenic and inflammatory chemokine, fractalkine.
Hindlimb ischemia recovery can be characterized as an under-damped feedback control system with a biphasic response profile featuring an initial phase of vessel growth peaking above contralateral controls and a later phase of vessel rarefaction and remodeling in which unnecessary and inefficient vessels are pruned away without changes in functional perfusion29. In this study, WT mice followed this under-damped biphasic profile, with vascular parameters elevated above contralateral controls at day 7, and normalized by day 28. In contrast, MKP-1 KO mice responded as an over-damped system, failing to supersede control values at any time point.
These data demonstrate a critical role for MKP-1 in the early growth phase of neovascular growth, attributable to a combination of angiogenesis and arteriogenesis. These processes can be evaluated by 3D vascular network analysis and immunostaining for both capillary and arteriole formation. MKP-1 deletion inhibited hypoxia-induced angiogenesis in the distal hindlimb, but did not affect arteriole formation in this region. In the proximal hindlimb, MKP-1 KO limbs did not show differences in capillary density, but exhibited transiently reduced arteriogenesis, as measured by arteriole staining. The MKP-1-associated defect in arteriogenesis was largely limited to the microvasculature as microCT angiography did not detect differences in vascular network parameters besides connectivity. However, of the microCT-based parameters, connectivity has been shown to correlate best with functional perfusion21, suggesting a potential functional importance of MKP-1 in arteriogenesis. Together, these data implicate MKP-1 as an important regulator of the early response to ischemia, with downstream effects mirroring the kinetics of MKP-1 expression in the ischemic limb. These data are consistent with reports that MKP-1 is induced in ischemic tumor microenvironments30 and is required for VEGF expression and maintenance of vascular density in hypoxia-exposed lung30. Importantly, the early angiogenic response is critical for prevention of irreversible myocardial necrosis in ischemic heart disease and is an indicator of functional outcome32–33, and mediates revival of tumor cells from dormancy34, highlighting the relevance of these findings beyond accelerated recovery from peripheral artery disease.
To evaluate the molecular mechanisms by which MKP-1, a negative regulator of MAPK, may serve a positive role in angiogenic function of endothelial cells, we evaluated the effect of MKP-1 depletion/deletion on HUVEC/MAEC angiogenesis in vitro. We found that endothelial MKP-1 is required for VEGF-induced migration and tube formation, and positively contributed to EC proliferation independently of VEGF. Initially, these data appear to conflict with a recent report that MKP-1 overexpression reduced endothelial cell motility and proliferation35. In those experiments, however, MKP-1 was expressed to supra-physiological levels, which may lead to non-physiological outcomes. Indeed, in that report, depletion of MKP-1 reduced VEGF-stimulated proliferation, consistent with the pro-angiogenic role described here. These data are consistent with our prior observations that MKP-1 is required for VEGF-induced EC migration and aortic ring sprouting18. Together, this study implicates MKP-1 as an important regulatory molecule in angiogenesis and suggests that tight control of MKP-1 levels is required for proper EC function.
Our prior discovery that MKP-1 is an H3S10 phosphatase19, led us to hypothesize that MKP-1 may regulate EC function through chromatin modification. Using ChIP, we identified MKP-1 association with exon 3 of the angiogenic chemokine fractalkine and found that MKP-1 dephosphorylated H3S10 on this DNA locus and was required for fractalkine induction by VEGF. This histone modification occurred in exonic DNA, immediately proximal to the stop codon, rather than in the promoter region, suggesting that MKP-1 may regulate transcriptional arrest and progression rather than initiation. These data support the hypothesis that histone tail modifications downstream of the promoter also contribute to the histone code, regulating not only transcription initiation & transcription factor recruitment, but also elongation and propagation of chromatin disruption36. Such coding region histone modification for gene regulation has been reported previously. For example, H3 and H4 acetylation within the coding region of the angiogenic VEGF-cofactor, CCN1, mediate chromatin folding and transcription37. In addition, H3S10 phosphorylation is required for the initiation of chromatin condensation during mitosis38,39 as well as priming H3 for subsequent Lys14 acetylation40, a switch between permissive and repressive chromatin41, consistent with the observations seen here. Further, H3S10 phosphorylation within the FOSL1 enhancer has been shown to trigger a cascade required for transcription elongation42. Thus, MKP-1 dephosphorylation of H3S10 may alter chromatin structure and accessibility to transcriptional machinery, allowing transcriptional progression and gene expression; however, in the absence of MKP-1, chromatin may be closed and transcription arrested. Consistent with these molecular signaling links, treatment of both HUVEC and MAEC with recombinant fractalkine rescued the migratory deficit caused by MKP-1 depletion/deletion, demonstrating the importance of this factor in MKP-1 mediated endothelial cell activation.
Given the ubiquity of histone H3, fractalkine may be representative of more general gene regulation by MKP-1. We therefore explored gene regulation of fractalkine as a prototype for MKP-1-mediated angiogenic gene expression. Fractalkine43 is a multifunctional chemokine, expressed primarily by endothelial cells, that exists in both membrane-bound and soluble forms, the latter derived by proteolytic cleavage25. It exerts its angiocrine effect through cleavage from the endothelial surface and signaling through its receptor, CX3CR1 on nearby endothelial cells10,25–28. In the context of hindlimb ischemia, activated CX3CR1 induced HIF-1a expression, VEGF production, and signaling through VEGFR228, consistent with the timecourse and MKPO-1-dependence of VEGF expression in the present study.
Fractalkine mRNA has been shown to be upregulated in ischemic hindlimbs, peaking at 1–3 days post-surgery, and, in that study, delivery of recombinant fractalkine stimulated hindlimb recovery in a rat hindlimb ischemia model28. In a separate study, fractalkine induced angiogenesis in the chick chorioallantoic membrane and mouse subcutaneous pocket models with similar strength as recombinant VEGF42. In the present study, we found that MKP-1 KO mice exhibited reduced fractalkine expression associated with deficient neovascularization, and fractalkine treatment rescued the effect of MKP-1 deletion, with no differences in functional perfusion recovery or day 7 microvascularity. However, in contrast to the rat study, exogenous fractalkine delivery failed to enhance perfusion recovery in WT mice, suggesting that in ischemic mice, endogenous fractalkine levels are sufficient to mediate neovascular recovery. Together, these data confirms the role of fractalkine in MKP-1-mediated neovascularization.
In addition to its angiogenic functions, fractalkine also mediates chemotaxis and firm adhesion of monocytes and T-lymphocytes to activated endothelium25–27, cells which play important roles in recovery from hindlimb ischemia45–48. Consistent with these observations, we found MKP-1 deletion dramatically reduced both monocyte/macrophage and T-lymphocyte invasion in the distal hindlimb. In the proximal hindlimb, however, inflammatory cell invasion was less than in the distal limb, and there were no differences between genotypes, which may partially explain the merely modest effect of MKP-1 deletion on arteriogenesis in that region of interest. Upon fractalkine delivery, there were no differences between genotypes in monocyte/macrophage or T-lymphocyte invastion in either region of interest, suggesting this effect is mediated, at least in part, by MKP-1-regulated fractalkine expression.
Together, these data implicate a dual role for MKP-1 in neovascularization: through endothelial cell migration, proliferation and tube formation, and through inflammatory cell infiltration. This is consistent with our previous findings in the ApoE−/− background that MKP-1 mediates macrophage activation21. In that study, MKP-1 null mice featured decreased atherosclerotic lesion size, decreased inflammatory cytokines (IL1α, TNFα) and increased levels of anti-inflammatory IL10 in the circulation, as well as impaired macrophage spreading, migration, and tissue infiltration21. Mechanistically, defective ERK1/2 signaling was implicated in the reduced migration of MKP−/−;ApoE−/− macrophages through pharmacologic Mek1/2 kinase inhibition21. Thus, MKP-1 may play dual roles in recovery from hindlimb ischemia, involving both its canonical function as a MAPK phosphatase in inflammatory cells and its chromatin modifying function in endothelial cells.
In addition, increasing evidence suggests that chemokine pathways such as CCL2/CCR2 and fractalkine/CX3CR1 control imflammatory cell mobilization from the bone marrow to the bloodstream49,50. For example, Conchain et al. showed that while the CCL2/CCR2 axis regulated recruitment of Ly6Chi monocytes to the circulation and contributed to hindlimb ischemia recovery, CX3CR1−/− mice exhibited a reduction in only Ly6Clo monocytes and did not present a deficit in hindlimb neovascularization50. Future studies will evaluate the role of MKP-1 in regulation of other chemokine pathways and MKP-1/chemokine-mediated monocyte mobilization and their role in neovascularization.
These data do not rule out the possibility that canonical MAPK deactivation may contribute to the functional effects observed here as numerous studies have verified the MAPK phosphatase activity of MKP-1 in endothelial cells18,51,52. Indeed, we have previously shown that VEGF induction of MKP-1 is JNK MAPK dependent, and VEGF-induced MKP-1 regulates phosphorylation of both JNK and p38, but not ERK1/2, in HUVEC18. Thus, the observations described here may result from a combination of these various MKP-1 substrates, which may explain the blunted angiogenic growth phase concurrent with decreased fractalkine expression but eventual recovery, perhaps due to prolonged MAPK activation.
Together, these data reveal a novel role for MKP-1 in angiogenic gene expression and neovascularization in vivo, identify a putative epigenetic mechanism for MKP-1-mediated gene regulation in endothelial cells, and validate MKP-1 as a potential therapeutic target that can be activated specifically through combinatorial growth factor stimulation51,53.
Supplementary Material
Significance.
Angiogenesis is critical to many physiological and pathophysiological processes, and despite intense study, the regulatory mechanisms controlling this process remain incompletely understood. MKP-1 is typically considered an off-switch for the mitogen activated protein kinases (MAPK), signaling molecules that promote angiogenesis. Here, however, we show in the hindlimb ischemia model that MKP-1 positively mediates the early growth phase of neovascularization, a process critical in diseases such as myocardial ischemia and tumor revival. We show that MKP-1 binds to DNA of the angiogenic chemokine, fractalkine, dephosphorylates histone H3 on this DNA locus, and is required for expression of this gene in vitro and in vivo. Finally, the deficit in neovascular recovery from hindlimb ischemia in MKP-1 KO mice is rescued by in vivo fractalkine delivery. Together, these observations identify MKP-1 as a potential therapeutic target in vascular regeneration and disease.
Acknowledgments
We would like to thank Lisa Dechert and Chad Braley for technical assistance, Drs. Yi Fan and Paul Fox for assistance with the hindlimb ischemia model, and Drs. Judy Drazba, Rick Rozic and Amit Vasanji for imaging assistance. HUVEC were harvested by the Clinical and Translational Science Collaborative of Cleveland (supported by grant UL1TR000439 from the National Center for Advancing Translational Sciences, a component of the NIH Roadmap for Medical Research).
Funding
This work was supported by NIH grants P01-HL29582 (to P.E.D.) and F32-HL118969-01 (to J.D.B.).
Non-standard abbreviations
- MKP-1
mitogen-activated protein kinase phosphatase
- MAPK
mitogen activated protein kinase
- H3S10
histone H3 serine 10
- LDPI
laser Doppler perfusion imaging
- CS-MKP-1
MKP-1 C259S mutant
- ERK
Extracellular related kinase
- JNK
c-Jun N-terminal kinase
- VEGF
vascular endothelial growth factor
- KO
knockout
- WT
wildtype
- EC
endothelial cell
- HUVEC
human umbilical vein endothelial cell
- MAEC
murine aortic endothelial cell
- GC
gastrocnemius muscle
- SM
soleus muscle
Footnotes
Disclosures
The authors have no conflicts of interest to disclose.
References
- 1.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011 May 19;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Folkman J. Angiogenesis. Annual review of medicine. 2006;57:1–18. doi: 10.1146/annurev.med.57.121304.131306. [DOI] [PubMed] [Google Scholar]
- 3.Loges S, Roncal C, Carmeliet P. Development of targeted angiogenic medicine. J Thromb Haemost. 2009 Jan;7:21–33. doi: 10.1111/j.1538-7836.2008.03203.x. [DOI] [PubMed] [Google Scholar]
- 4.De Bock K, Mazzone M, Carmeliet P. Antiangiogenic therapy, hypoxia, and metastasis: risky liaisons, or not? Nat Rev Clin Oncol. 2011 Jul;8:393–404. doi: 10.1038/nrclinonc.2011.83. [DOI] [PubMed] [Google Scholar]
- 5.Cao Y, Arbiser J, D’Amato RJ, D’Amore PA, Ingber DE, Kerbel R, Klagsbrun M, Lim S, Moses MA, Zetter B, Dvorak H, Langer R. Forty-year journey of angiogenesis translational research. Sci Transl Med. 2011 Dec 21;3:114rv113. doi: 10.1126/scitranslmed.3003149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jain RK, Duda DG, Clark JW, Loeffler JS. Lessons from phase III clinical trials on anti-VEGF therapy for cancer. Nat Clin Pract Oncol. 2006 Jan;3:24–40. doi: 10.1038/ncponc0403. [DOI] [PubMed] [Google Scholar]
- 7.Owens DM, Keyse SM. Differential regulation of MAP kinase signalling by dual-specificity protein phosphatases. Oncogene. 2007 May 14;26:3203–3213. doi: 10.1038/sj.onc.1210412. [DOI] [PubMed] [Google Scholar]
- 8.Marshall CJ. MAP kinase kinase kinase, MAP kinase kinase and MAP kinase. Curr Opin Genet Dev. 1994 Feb;4:82–89. doi: 10.1016/0959-437x(94)90095-7. [DOI] [PubMed] [Google Scholar]
- 9.Srinivasan R, Zabuawala T, Huang H, Zhang J, Gulati P, Fernandez S, Karlo JC, Landreth GE, Leone G, Ostrowski MC. Erk1 and Erk2 regulate endothelial cell proliferation and migration during mouse embryonic angiogenesis. PLoS One. 2009;4:e8283. doi: 10.1371/journal.pone.0008283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giroux S, Tremblay M, Bernard D, Cardin-Girard JF, Aubry S, Larouche L, Rousseau S, Huot J, Landry J, Jeannotte L, Charron J. Embryonic death of Mek1-deficient mice reveals a role for this kinase in angiogenesis in the labyrinthine region of the placenta. Current biology : CB. 1999 Apr 8;9:369–372. doi: 10.1016/s0960-9822(99)80164-x. [DOI] [PubMed] [Google Scholar]
- 11.Mudgett JS, Ding J, Guh-Siesel L, Chartrain NA, Yang L, Gopal S, Shen MM. Essential role for p38alpha mitogen-activated protein kinase in placental angiogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2000 Sep 12;97:10454–10459. doi: 10.1073/pnas.180316397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Regan CP, Li W, Boucher DM, Spatz S, Su MS, Kuida K. Erk5 null mice display multiple extraembryonic vascular and embryonic cardiovascular defects. Proceedings of the National Academy of Sciences of the United States of America. 2002 Jul 9;99:9248–9253. doi: 10.1073/pnas.142293999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eliceiri BP, Klemke R, Stromblad S, Cheresh DA. Integrin alphavbeta3 requirement for sustained mitogen-activated protein kinase activity during angiogenesis. The Journal of cell biology. 1998 Mar 9;140:1255–1263. doi: 10.1083/jcb.140.5.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Volin MV, Huynh N, Klosowska K, Reyes RD, Woods JM. Fractalkine-induced endothelial cell migration requires MAP kinase signaling. Pathobiology. 2010;77:7–16. doi: 10.1159/000272949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rousseau S, Houle F, Landry J, Huot J. p38 MAP kinase activation by vascular endothelial growth factor mediates actin reorganization and cell migration in human endothelial cells. Oncogene. 1997 Oct;15:2169–2177. doi: 10.1038/sj.onc.1201380. [DOI] [PubMed] [Google Scholar]
- 16.Mavria G, Vercoulen Y, Yeo M, Paterson H, Karasarides M, Marais R, Bird D, Marshall CJ. ERK-MAPK signaling opposes Rho-kinase to promote endothelial cell survival and sprouting during angiogenesis. Cancer Cell. 2006 Jan;9:33–44. doi: 10.1016/j.ccr.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 17.Caunt CJ, Keyse SM. Dual-specificity MAP kinase phosphatases (MKPs): shaping the outcome of MAP kinase signalling. The FEBS journal. 2013 Jan;280:489–504. doi: 10.1111/j.1742-4658.2012.08716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chandrasekharan UM, Yang L, Walters A, Howe P, DiCorleto PE. Role of CL-100, a dual specificity phosphatase, in thrombin-induced endothelial cell activation. The Journal of biological chemistry. 2004 Nov 5;279:46678–46685. doi: 10.1074/jbc.M406441200. [DOI] [PubMed] [Google Scholar]
- 19.Kinney CM, Chandrasekharan UM, Mavrakis L, DiCorleto PE. VEGF and thrombin induce MKP-1 through distinct signaling pathways: role for MKP-1 in endothelial cell migration. American journal of physiology. Cell physiology. 2008 Jan;294:C241–250. doi: 10.1152/ajpcell.00187.2007. [DOI] [PubMed] [Google Scholar]
- 20.Kinney CM, Chandrasekharan UM, Yang L, Shen J, Kinter M, McDermott MS, DiCorleto PE. Histone H3 as a novel substrate for MAP kinase phosphatase-1. American journal of physiology. Cell physiology. 2009 Feb;296:C242–249. doi: 10.1152/ajpcell.00492.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shen J, Chandrasekharan UM, Ashraf MZ, Long E, Morton RE, Liu Y, Smith JD, DiCorleto PE. Lack of mitogen-activated protein kinase phosphatase-1 protects ApoE-null mice against atherosclerosis. Circulation research. 2010 Mar 19;106:902–910. doi: 10.1161/CIRCRESAHA.109.198069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duvall CL, Taylor WR, Weiss D, Guldberg RE. Quantitative microcomputed tomography analysis of collateral vessel development after ischemic injury. American journal of physiology. Heart and circulatory physiology. 2004 Jul;287:H302–310. doi: 10.1152/ajpheart.00928.2003. [DOI] [PubMed] [Google Scholar]
- 22.Duvall CL, Weiss D, Robinson ST, Alameddine FM, Guldberg RE, Taylor WR. The role of osteopontin in recovery from hind limb ischemia. Arteriosclerosis, thrombosis, and vascular biology. 2008 Feb;28:290–295. doi: 10.1161/ATVBAHA.107.158485. [DOI] [PubMed] [Google Scholar]
- 23.Limbourg A, Korff T, Napp LC, Schaper W, Drexler H, Limbourg FP. Evaluation of postnatal arteriogenesis and angiogenesis in a mouse model of hind-limb ischemia. Nat Protoc. 2009;4:1737–1746. doi: 10.1038/nprot.2009.185. [DOI] [PubMed] [Google Scholar]
- 24.Lloyd PG, Yang HT, Terjung RL. Arteriogenesis and angiogenesis in rat ischemic hindlimb: role of nitric oxide. American journal of physiology. Heart and circulatory physiology. 2001 Dec;281:H2528–2538. doi: 10.1152/ajpheart.2001.281.6.H2528. [DOI] [PubMed] [Google Scholar]
- 25.Boerckel JD, Uhrig BA, Willett NJ, Huebsch N, Guldberg RE. Mechanical regulation of vascular growth and tissue regeneration in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2011 Sep 13;108:E674–680. doi: 10.1073/pnas.1107019108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu H, Jiang D. Fractalkine/CX3CR1 and atherosclerosis. Clinica chimica acta; international journal of clinical chemistry. 2011 Jun 11;412:1180–1186. doi: 10.1016/j.cca.2011.03.036. [DOI] [PubMed] [Google Scholar]
- 26.Ancuta P, Rao R, Moses A, Mehle A, Shaw SK, Luscinskas FW, Gabuzda D. Fractalkine preferentially mediates arrest and migration of CD16+ monocytes. The Journal of experimental medicine. 2003 Jun 16;197:1701–1707. doi: 10.1084/jem.20022156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang XP, Mattagajasingh S, Su S, Chen G, Cai Z, Fox-Talbot K, Irani K, Becker LC. Fractalkine upregulates intercellular adhesion molecule-1 in endothelial cells through CX3CR1 and the Jak Stat5 pathway. Circulation research. 2007 Nov 9;101:1001–1008. doi: 10.1161/CIRCRESAHA.107.160812. [DOI] [PubMed] [Google Scholar]
- 28.Ryu J, Lee CW, Hong KH, Shin JA, Lim SH, Park CS, Shim J, Nam KB, Choi KJ, Kim YH, Han KH. Activation of fractalkine/CX3CR1 by vascular endothelial cells induces angiogenesis through VEGF-A/KDR and reverses hindlimb ischaemia. Cardiovascular research. 2008 May 1;78:333–340. doi: 10.1093/cvr/cvm067. [DOI] [PubMed] [Google Scholar]
- 29.Landazuri N, Joseph G, Guldberg RE, Taylor WR. Growth and regression of vasculature in healthy and diabetic mice after hindlimb ischemia. American journal of physiology. Regulatory, integrative and comparative physiology. 2012 Jul 1;303:R48–56. doi: 10.1152/ajpregu.00002.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laderoute KR, Mendonca HL, Calaoagan JM, Knapp AM, Giaccia AJ, Stork PJ. Mitogen-activated protein kinase phosphatase-1 (MKP-1) expression is induced by low oxygen conditions found in solid tumor microenvironments. A candidate MKP for the inactivation of hypoxia-inducible stress-activated protein kinase/c-Jun N-terminal protein kinase activity. The Journal of biological chemistry. 1999 Apr 30;274:12890–12897. doi: 10.1074/jbc.274.18.12890. [DOI] [PubMed] [Google Scholar]
- 31.Shields KM, Panzhinskiy E, Burns N, Zawada WM, Das M. Mitogen-activated protein kinase phosphatase-1 is a key regulator of hypoxia-induced vascular endothelial growth factor expression and vessel density in lung. The American journal of pathology. 2011 Jan;178:98–109. doi: 10.1016/j.ajpath.2010.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee SH, Wolf PL, Escudero R, Deutsch R, Jamieson SW, Thistlethwaite PA. Early Expression of Angiogenesis Factors in Acute Myocardial Ischemia and Infarction. New England Journal of Medicine. 2000 Mar;342:626–633. doi: 10.1056/NEJM200003023420904. [DOI] [PubMed] [Google Scholar]
- 33.Isner JM. Tissue responses to ischemia: local and remote responses for preserving perfusion of ischemic muscle. The Journal of clinical investigation. 2000 Sep;106:615–619. doi: 10.1172/JCI10961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Naumov GN, Akslen LA, Folkman J. Role of Angiogenesis in Human Tumor Dormancy: Animal Models of the Angiogenic Switch. Cell Cycle. 2006 Aug;5:1779–1787. doi: 10.4161/cc.5.16.3018. [DOI] [PubMed] [Google Scholar]
- 35.Bellou S, Hink MA, Bagli E, Panopoulou E, Bastiaens PI, Murphy C, Fotsis T. VEGF autoregulates its proliferative and migratory ERK1/2 and p38 cascades by enhancing the expression of DUSP1 and DUSP5 phosphatases in endothelial cells. American journal of physiology. Cell physiology. 2009 Dec;297:C1477–1489. doi: 10.1152/ajpcell.00058.2009. [DOI] [PubMed] [Google Scholar]
- 36.Li B, Carey M, Workman JL. The role of chromatin during transcription. Cell. 2007 Feb 23;128:707–719. doi: 10.1016/j.cell.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 37.Hanna M, Liu H, Amir J, Sun Y, Morris SW, Siddiqui MA, Lau LF, Chaqour B. Mechanical regulation of the proangiogenic factor CCN1/CYR61 gene requires the combined activities of MRTF-A and CREB-binding protein histone acetyltransferase. The Journal of biological chemistry. 2009 Aug 21;284:23125–23136. doi: 10.1074/jbc.M109.019059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wei Y, Yu L, Bowen J, Gorovsky MA, Allis CD. Phosphorylation of histone H3 is required for proper chromosome condensation and segregation. Cell. 1999 Apr 2;97:99–109. doi: 10.1016/s0092-8674(00)80718-7. [DOI] [PubMed] [Google Scholar]
- 39.Van Hooser A, Goodrich DW, Allis CD, Brinkley BR, Mancini MA. Histone H3 phosphorylation is required for the initiation, but not maintenance, of mammalian chromosome condensation. Journal of cell science. 1998 Dec;111:3497–3506. doi: 10.1242/jcs.111.23.3497. [DOI] [PubMed] [Google Scholar]
- 40.Lo WS, Trievel RC, Rojas JR, Duggan L, Hsu JY, Allis CD, Marmorstein R, Berger SL. Phosphorylation of serine 10 in histone H3 is functionally linked in vitro and in vivo to Gcn5-mediated acetylation at lysine 14. Molecular cell. 2000 Jun;5:917–926. doi: 10.1016/s1097-2765(00)80257-9. [DOI] [PubMed] [Google Scholar]
- 41.Eberharter A, Becker PB. Histone acetylation: a switch between repressive and permissive chromatin. Second in review series on chromatin dynamics. EMBO reports. 2002 Mar;3:224–229. doi: 10.1093/embo-reports/kvf053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zippo A, Serafini R, Rocchigiani M, Pennacchini S, Krepelova A, Oliviero S. Histone crosstalk between H3S10ph and H4K16ac generates a histone code that mediates transcription elongation. Cell. 2009 Sep 18;138:1122–1136. doi: 10.1016/j.cell.2009.07.031. [DOI] [PubMed] [Google Scholar]
- 43.Bazan JF, Bacon KB, Hardiman G, Wang W, Soo K, Rossi D, Greaves DR, Zlotnik A, Schall TJ. A new class of membrane-bound chemokine with a CX3C motif. Nature. 1997 Feb 13;385:640–644. doi: 10.1038/385640a0. [DOI] [PubMed] [Google Scholar]
- 44.Lee SJ, Namkoong S, Kim YM, Kim CK, Lee H, Ha KS, Chung HT, Kwon YG. Fractalkine stimulates angiogenesis by activating the Raf-1/MEK/ERK- and PI3K/Akt/eNOS-dependent signal pathways. American journal of physiology. Heart and circulatory physiology. 2006 Dec;291:H2836–2846. doi: 10.1152/ajpheart.00113.2006. [DOI] [PubMed] [Google Scholar]
- 45.Egami K, Murohara T, Aoki M, Matsuishi T. Ischemia-induced angiogenesis: role of inflammatory response mediated by P-selectin. Journal of leukocyte biology. 2006 May;79:971–976. doi: 10.1189/jlb.0805448. [DOI] [PubMed] [Google Scholar]
- 46.Zouggari Y, Ait-Oufella H, Waeckel L, Vilar J, Loinard C, Cochain C, Recalde A, Duriez M, Levy BI, Lutgens E, Mallat Z, Silvestre JS. Regulatory T cells modulate postischemic neovascularization. Circulation. 2009 Oct 6;120:1415–1425. doi: 10.1161/CIRCULATIONAHA.109.875583. [DOI] [PubMed] [Google Scholar]
- 47.Stabile E, Kinnaird T, la Sala A, Hanson SK, Watkins C, Campia U, Shou M, Zbinden S, Fuchs S, Kornfeld H, Epstein SE, Burnett MS. CD8+ T lymphocytes regulate the arteriogenic response to ischemia by infiltrating the site of collateral vessel development and recruiting CD4+ mononuclear cells through the expression of interleukin-16. Circulation. 2006 Jan 3;113:118–124. doi: 10.1161/CIRCULATIONAHA.105.576702. [DOI] [PubMed] [Google Scholar]
- 48.Heil M, Schaper W. Insights into the pathways of arteriogenesis. Curr Pharm Biotechnol. 2007 Feb;8:35–42. doi: 10.2174/138920107779941408. [DOI] [PubMed] [Google Scholar]
- 49.Tsou CL, Peters W, Si Y, Slaymaker S, Aslanian AM, Weisberg SP, Mack M, Charo IF. Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. Journal of Clincal Investigation. 2007 Apr;117:902–909. doi: 10.1172/JCI29919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cochain C, Rodero MP, Vilar J, et al. Regulation of monocyte subset systemic levels by distinct chemokine receptors controls post-ischemic neovascularization. Cardiovascular Research. 2010 Oct;88:186–195. doi: 10.1093/cvr/cvq153. [DOI] [PubMed] [Google Scholar]
- 51.Chandrasekharan UM, Waitkus M, Kinney CM, Walters-Stewart A, DiCorleto PE. Synergistic induction of mitogen-activated protein kinase phosphatase-1 by thrombin and epidermal growth factor requires vascular endothelial growth factor receptor-2. Arteriosclerosis, thrombosis, and vascular biology. 2010 Oct;30:1983–1989. doi: 10.1161/ATVBAHA.110.212399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weber NC, Blumenthal SB, Hartung T, Vollmar AM, Kiemer AK. ANP inhibits TNF-alpha-induced endothelial MCP-1 expression--involvement of p38 MAPK and MKP-1. Journal of leukocyte biology. 2003 Nov;74:932–941. doi: 10.1189/jlb.0603254. [DOI] [PubMed] [Google Scholar]
- 53.Waitkus MS, Chandrasekharan UM, Willard B, Haque SJ, DiCorleto PE. STAT3- mediated coincidence detection regulates noncanonical immediate early gene induction. Journal of Biological Chemistry. 2013 Apr;288:11988–12003. doi: 10.1074/jbc.M112.428516. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.