Abstract
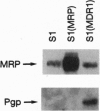
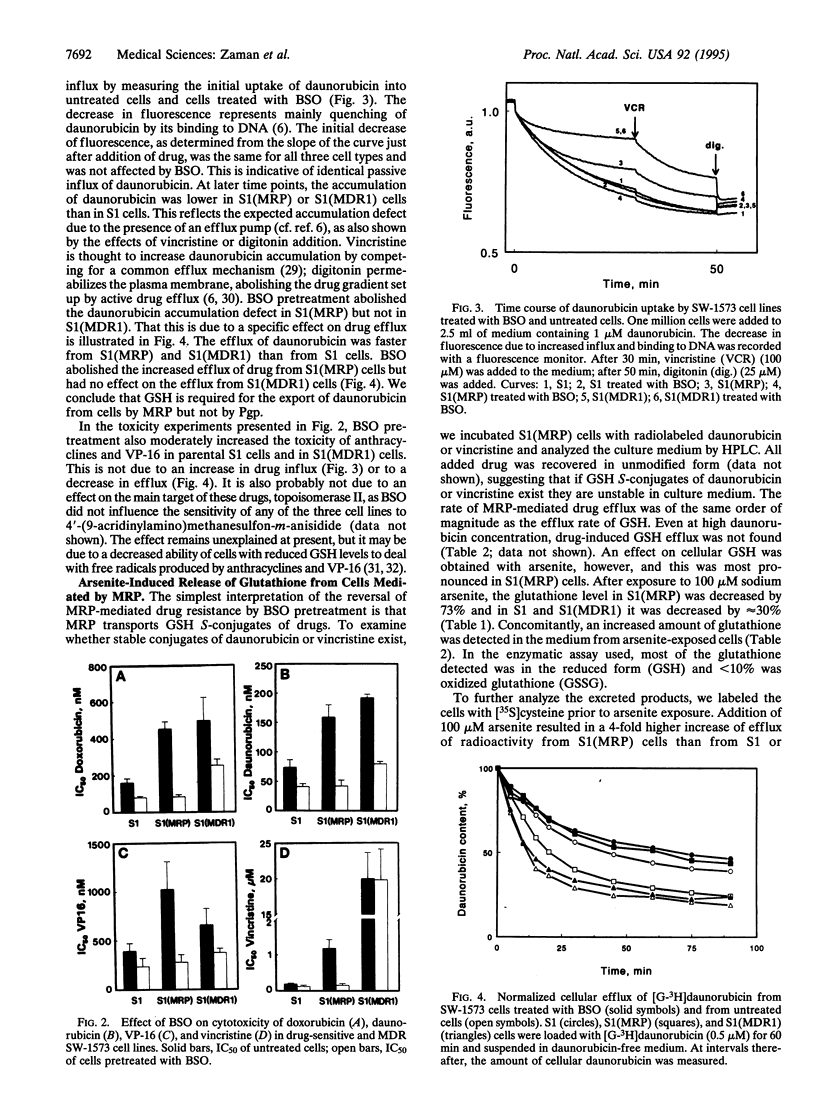
Multidrug-resistance-associated protein (MRP) is a plasma membrane glycoprotein that can confer multidrug resistance (MDR) by lowering intracellular drug concentration. Here we demonstrate that depletion of intracellular glutathione by DL-buthionine (S,R)-sulfoximine results in a complete reversal of resistance to doxorubicin, daunorubicin, vincristine, and VP-16 in lung carcinoma cells transfected with a MRP cDNA expression vector. Glutathione depletion had less effect on MDR in cells transfected with MDR1 cDNA encoding P-glycoprotein and did not increase the passive uptake of daunorubicin by cells, indicating that the decrease of MRP-mediated MDR was not due to nonspecific membrane damage. Glutathione depletion resulted in a decreased efflux of daunorubicin from MRP-transfected cells, but not from MDR1-transfected cells, suggesting that glutathione is specifically required for the export of drugs from cells by MRP. We also show that MRP increases the export of glutathione from the cell and this increased export is further elevated in the presence of arsenite. Our results support the hypothesis that MRP functions as a glutathione S-conjugate carrier.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almquist K. C., Loe D. W., Hipfner D. R., Mackie J. E., Cole S. P., Deeley R. G. Characterization of the M(r) 190,000 multidrug resistance protein (MRP) in drug-selected and transfected human tumor cell. Cancer Res. 1995 Jan 1;55(1):102–110. [PubMed] [Google Scholar]
- Cole S. P., Bhardwaj G., Gerlach J. H., Mackie J. E., Grant C. E., Almquist K. C., Stewart A. J., Kurz E. U., Duncan A. M., Deeley R. G. Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science. 1992 Dec 4;258(5088):1650–1654. doi: 10.1126/science.1360704. [DOI] [PubMed] [Google Scholar]
- Cole S. P., Sparks K. E., Fraser K., Loe D. W., Grant C. E., Wilson G. M., Deeley R. G. Pharmacological characterization of multidrug resistant MRP-transfected human tumor cells. Cancer Res. 1994 Nov 15;54(22):5902–5910. [PubMed] [Google Scholar]
- Delnomdedieu M., Basti M. M., Otvos J. D., Thomas D. J. Reduction and binding of arsenate and dimethylarsinate by glutathione: a magnetic resonance study. Chem Biol Interact. 1994 Feb;90(2):139–155. doi: 10.1016/0009-2797(94)90099-x. [DOI] [PubMed] [Google Scholar]
- Eijdems E. W., Borst P., Jongsma A. P., de Jong S., de Vries E. G., van Groenigen M., Versantvoort C. H., Nieuwint A. W., Baas F. Genetic transfer of non-P-glycoprotein-mediated multidrug resistance (MDR) in somatic cell fusion: dissection of a compound MDR phenotype. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3498–3502. doi: 10.1073/pnas.89.8.3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Checa J. C., Takikawa H., Horie T., Ookhtens M., Kaplowitz N. Canalicular transport of reduced glutathione in normal and mutant Eisai hyperbilirubinemic rats. J Biol Chem. 1992 Jan 25;267(3):1667–1673. [PubMed] [Google Scholar]
- Flens M. J., Izquierdo M. A., Scheffer G. L., Fritz J. M., Meijer C. J., Scheper R. J., Zaman G. J. Immunochemical detection of the multidrug resistance-associated protein MRP in human multidrug-resistant tumor cells by monoclonal antibodies. Cancer Res. 1994 Sep 1;54(17):4557–4563. [PubMed] [Google Scholar]
- Fujii R., Mutoh M., Sumizawa T., Chen Z. S., Yoshimura A., Akiyama S. Adenosine triphosphate-dependent transport of leukotriene C4 by membrane vesicles prepared from cisplatin-resistant human epidermoid carcinoma tumor cells. J Natl Cancer Inst. 1994 Dec 7;86(23):1781–1784. doi: 10.1093/jnci/86.23.1781. [DOI] [PubMed] [Google Scholar]
- Gottesman M. M., Pastan I. Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu Rev Biochem. 1993;62:385–427. doi: 10.1146/annurev.bi.62.070193.002125. [DOI] [PubMed] [Google Scholar]
- Gyurasics A., Varga F., Gregus Z. Glutathione-dependent biliary excretion of arsenic. Biochem Pharmacol. 1991 Jul 15;42(3):465–468. doi: 10.1016/0006-2952(91)90306-p. [DOI] [PubMed] [Google Scholar]
- Ishikawa T., Ali-Osman F. Glutathione-associated cis-diamminedichloroplatinum(II) metabolism and ATP-dependent efflux from leukemia cells. Molecular characterization of glutathione-platinum complex and its biological significance. J Biol Chem. 1993 Sep 25;268(27):20116–20125. [PubMed] [Google Scholar]
- Ishikawa T. The ATP-dependent glutathione S-conjugate export pump. Trends Biochem Sci. 1992 Nov;17(11):463–468. doi: 10.1016/0968-0004(92)90489-v. [DOI] [PubMed] [Google Scholar]
- Ishikawa T., Wright C. D., Ishizuka H. GS-X pump is functionally overexpressed in cis-diamminedichloroplatinum (II)-resistant human leukemia HL-60 cells and down-regulated by cell differentiation. J Biol Chem. 1994 Nov 18;269(46):29085–29093. [PubMed] [Google Scholar]
- Jedlitschky G., Leier I., Buchholz U., Center M., Keppler D. ATP-dependent transport of glutathione S-conjugates by the multidrug resistance-associated protein. Cancer Res. 1994 Sep 15;54(18):4833–4836. [PubMed] [Google Scholar]
- Juliano R. L., Ling V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim Biophys Acta. 1976 Nov 11;455(1):152–162. doi: 10.1016/0005-2736(76)90160-7. [DOI] [PubMed] [Google Scholar]
- Katki A. G., Kalyanaraman B., Sinha B. K. Interactions of the antitumor drug, etoposide, with reduced thiols in vitro and in vivo. Chem Biol Interact. 1987;62(3):237–247. doi: 10.1016/0009-2797(87)90025-1. [DOI] [PubMed] [Google Scholar]
- Kramer R. A., Zakher J., Kim G. Role of the glutathione redox cycle in acquired and de novo multidrug resistance. Science. 1988 Aug 5;241(4866):694–697. doi: 10.1126/science.3399900. [DOI] [PubMed] [Google Scholar]
- Kruh G. D., Chan A., Myers K., Gaughan K., Miki T., Aaronson S. A. Expression complementary DNA library transfer establishes mrp as a multidrug resistance gene. Cancer Res. 1994 Apr 1;54(7):1649–1652. [PubMed] [Google Scholar]
- Leier I., Jedlitschky G., Buchholz U., Cole S. P., Deeley R. G., Keppler D. The MRP gene encodes an ATP-dependent export pump for leukotriene C4 and structurally related conjugates. J Biol Chem. 1994 Nov 11;269(45):27807–27810. [PubMed] [Google Scholar]
- Mans D. R., Schuurhuis G. J., Treskes M., Lafleur M. V., Retèl J., Pinedo H. M., Lankelma J. Modulation by D,L-buthionine-S,R-sulphoximine of etoposide cytotoxicity on human non-small cell lung, ovarian and breast carcinoma cell lines. Eur J Cancer. 1992;28A(8-9):1447–1452. doi: 10.1016/0959-8049(92)90541-9. [DOI] [PubMed] [Google Scholar]
- Meijer C., Mulder N. H., Timmer-Bosscha H., Peters W. H., de Vries E. G. Combined in vitro modulation of adriamycin resistance. Int J Cancer. 1991 Oct 21;49(4):582–586. doi: 10.1002/ijc.2910490419. [DOI] [PubMed] [Google Scholar]
- Meister A. Glutathione deficiency produced by inhibition of its synthesis, and its reversal; applications in research and therapy. Pharmacol Ther. 1991;51(2):155–194. doi: 10.1016/0163-7258(91)90076-x. [DOI] [PubMed] [Google Scholar]
- Morgenstern J. P., Land H. A series of mammalian expression vectors and characterisation of their expression of a reporter gene in stably and transiently transfected cells. Nucleic Acids Res. 1990 Feb 25;18(4):1068–1068. doi: 10.1093/nar/18.4.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow C. S., Cowan K. H. Glutathione S-transferases and drug resistance. Cancer Cells. 1990 Jan;2(1):15–22. [PubMed] [Google Scholar]
- Mülder H. S., Lankelma J., Dekker H., Broxterman H. J., Pinedo H. M. Daunorubicin efflux against a concentration gradient in non-P-glycoprotein multidrug-resistant lung-cancer cells. Int J Cancer. 1994 Oct 15;59(2):275–281. doi: 10.1002/ijc.2910590221. [DOI] [PubMed] [Google Scholar]
- Müller M., Meijer C., Zaman G. J., Borst P., Scheper R. J., Mulder N. H., de Vries E. G., Jansen P. L. Overexpression of the gene encoding the multidrug resistance-associated protein results in increased ATP-dependent glutathione S-conjugate transport. Proc Natl Acad Sci U S A. 1994 Dec 20;91(26):13033–13037. doi: 10.1073/pnas.91.26.13033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Dwyer P. J., Hamilton T. C., Young R. C., LaCreta F. P., Carp N., Tew K. D., Padavic K., Comis R. L., Ozols R. F. Depletion of glutathione in normal and malignant human cells in vivo by buthionine sulfoximine: clinical and biochemical results. J Natl Cancer Inst. 1992 Feb 19;84(4):264–267. doi: 10.1093/jnci/84.4.264. [DOI] [PubMed] [Google Scholar]
- Sacchetta P., Di Cola D., Federici G. Alkaline hydrolysis of N-ethylmaleimide allows a rapid assay of glutathione disulfide in biological samples. Anal Biochem. 1986 Apr;154(1):205–208. doi: 10.1016/0003-2697(86)90516-6. [DOI] [PubMed] [Google Scholar]
- Scott N., Hatlelid K. M., MacKenzie N. E., Carter D. E. Reactions of arsenic(III) and arsenic(V) species with glutathione. Chem Res Toxicol. 1993 Jan-Feb;6(1):102–106. doi: 10.1021/tx00031a016. [DOI] [PubMed] [Google Scholar]
- Sinha B. K., Katki A. G., Batist G., Cowan K. H., Myers C. E. Differential formation of hydroxyl radicals by adriamycin in sensitive and resistant MCF-7 human breast tumor cells: implications for the mechanism of action. Biochemistry. 1987 Jun 30;26(13):3776–3781. doi: 10.1021/bi00387a006. [DOI] [PubMed] [Google Scholar]
- Tew K. D. Glutathione-associated enzymes in anticancer drug resistance. Cancer Res. 1994 Aug 15;54(16):4313–4320. [PubMed] [Google Scholar]
- Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969 Mar;27(3):502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- Versantvoort C. H., Broxterman H. J., Feller N., Dekker H., Kuiper C. M., Lankelma J. Probing daunorubicin accumulation defects in non-P-glycoprotein expressing multidrug-resistant cell lines using digitonin. Int J Cancer. 1992 Apr 1;50(6):906–911. doi: 10.1002/ijc.2910500615. [DOI] [PubMed] [Google Scholar]
- Wang H. F., Lee T. C. Glutathione S-transferase pi facilitates the excretion of arsenic from arsenic-resistant Chinese hamster ovary cells. Biochem Biophys Res Commun. 1993 May 14;192(3):1093–1099. doi: 10.1006/bbrc.1993.1529. [DOI] [PubMed] [Google Scholar]
- Wilce M. C., Parker M. W. Structure and function of glutathione S-transferases. Biochim Biophys Acta. 1994 Mar 16;1205(1):1–18. doi: 10.1016/0167-4838(94)90086-8. [DOI] [PubMed] [Google Scholar]
- Zaman G. J., Flens M. J., van Leusden M. R., de Haas M., Mülder H. S., Lankelma J., Pinedo H. M., Scheper R. J., Baas F., Broxterman H. J. The human multidrug resistance-associated protein MRP is a plasma membrane drug-efflux pump. Proc Natl Acad Sci U S A. 1994 Sep 13;91(19):8822–8826. doi: 10.1073/pnas.91.19.8822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaman G. J., Versantvoort C. H., Smit J. J., Eijdems E. W., de Haas M., Smith A. J., Broxterman H. J., Mulder N. H., de Vries E. G., Baas F. Analysis of the expression of MRP, the gene for a new putative transmembrane drug transporter, in human multidrug resistant lung cancer cell lines. Cancer Res. 1993 Apr 15;53(8):1747–1750. [PubMed] [Google Scholar]