Abstract
Pattern recognition receptors recognize signals originating from pathogens and comprise a large part of the arsenal in innate immune responses. The NOD-like receptors (NLRs) are one particular class of these receptors that survey the cytoplasm for signs of pathogen invasion. Upon detection, they trigger the formation of a macromolecular complex called the inflammasome that is required for elimination of the pathogen, as well as amplifying a pro-inflammatory response. Although the core machinery has been defined, recent data emphasize the complexity of how NLR inflammasomes function. Here, we highlight new discoveries that reveal how precisely fine-tuned NLR inflammasome functions are, and how that may be modulated by antagonistic effects of concomitant inflammasome activation as well as novel regulatory factors.
Introduction
In the last decade, an explosion of research has helped define the core mechanisms involved in detecting intracellular bacterial infections. As part of the first line of defense, the innate immune response employs several classes of receptors (pattern recognition receptors, PRRs) that respond to specific pathogen-associated and danger-associated molecular patterns (PAMPs and DAMPs) such as lipopolysaccharide, reactive oxygen species (ROS), and dsDNA [1]. Extracellular detection of these signals relies on Toll-like (TLRs) and C-type lectin receptors that facilitate signal transduction across cell membranes, leading to pro-inflammatory gene expression through the transcription factor, NFκB [2,3]. For a more comprehensive review of TLR signaling, see [2]. If bacteria invade the cell, cytosolic PRRs belonging to the NOD-like receptor (NLR), retinoic-acid inducible gene-I (RIG-I), and PYHIN (e.g. AIM2) families aid in amplifying pro-inflammatory responses [4]. A subset of TLRs also monitor endosomal compartments [2].
NOD-like receptors: domains and functions in inflammasomes
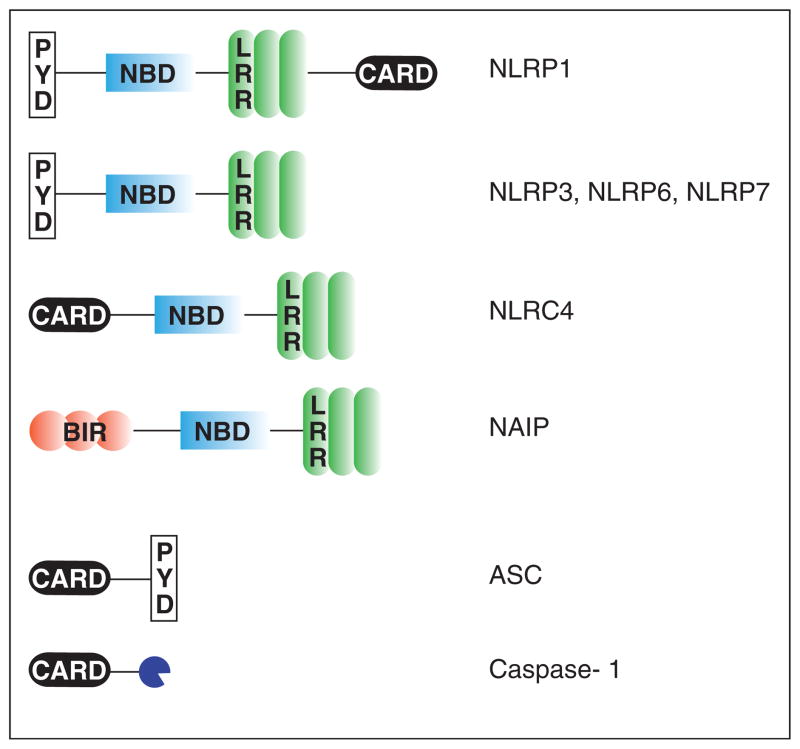
To date, there are over 20 NLRs identified in humans and mice that are characterized by a central nucleotide-binding domain (NBD/NACHT) required for oligomerization and a leucine-rich repeat domain (LRR) at the C-terminal end that is thought to mediate auto-regulation of activity and ligand-sensing. NLRs can vary in the number of LRRs as well as their N-terminal homophilic interacting domains that include caspase activation and recruitment (CARD) or PYRIN (PYD) domains (Figure 1) [5]. Upon detection of their respective ligands, NLRs recruit the apoptosis-associated speck-like protein containing a CARD (ASC). Because ASC contains both CARD and PYD domains, it oligomerizes to form the cytosolic structure, ASC speck, and also recruits CARD-containing pro-caspase-1. Together, they form a macromolecular complex called the inflammasome that is capable of initiating a specialized cell death called pyroptosis as well as cleavage and secretion of the zymogen forms of caspase-1 and the pro-inflammatory cytokines, IL-18 and IL-1β [5,6]. Based on this model, researchers have evaluated cytosolic-sensing utilizing any of the following readouts: ASC speck formation (represents oligomerization), the release of cytosolic compounds owing to pyroptosis, and processing and/or secretion of caspase-1, IL-1α/β, IL-18, and HMGB1 (danger signal).
Figure 1.
NLR domains and inflammasome components. NLRs are characterized by a common central nucleotide-binding/NACHT domain and a leucine-rich repeat domain. Different members of the NLR family are distinguished by domains residing in the variable N-terminal regions. They can include caspase-activation and recruitment (CARD), PYRIN (PYD), or BIR (baculovirus IAP repeat) domains. The ASC adaptor contains both CARD and PYD domains, while caspase-1 contains a CARD domain.
Here, we will describe recent developments that contribute to our understanding of how intracellular bacteria are sensed and controlled through different NLR inflammasomes. These studies provide further evidence that simultaneous engagement of multiple NLRs can occur upon bacterial infection. Several reports also describe new inflammasome regulatory factors that intersect with TLR and type I interferon (IFN) signaling pathways. Thus, the integration of multiple host signaling pathways appears to be essential in effectively mounting the appropriate innate immune response.
Novel implications for NLR inflammasome functions
NLRP12 teams up with NLRP3 and NLRC4 to combat Yersinia infections
Vladimer et al. recently reported pro-inflammatory responses upon NLRP12 inflammasome activation in contrast to a previous report of anti-inflammatory effects [7,8]. When the authors infected murine bone-marrow derived macrophages (BMDMs) with Y. pestis expressing hexa-acylated lipid A that is normally absent in Y. pestis grown at the host temperature, they observed strong engagement of TLR4, upregulation of Nlrp12 expression, and NLRP12-dependent pro-inflammatory cytokine secretion [7,9]. Furthermore, the authors show that NLRP12 inflammasome activation is dependent on the Type III secretion system (T3SS) of Y. pestis, similar to NLRP3 and NLRC4 [10]. Thus, all three NLRs appear to be involved in inducing pro-inflammatory cytokine secretion to successfully clear the infection in mice.
NLRP7-dependent recognition of bacterial lipoproteins stimulates cytokine processing but not cell death
Work on cytosolic detection of microbial lipopeptides, the prominent PAMP of Mycoplasma spp., has also revealed a role for NLRP7 in THP-1 cells [11••]. Interestingly, NLRP7-ASC-caspase-1 complex formation leads to processing of pro-IL-1β but not pyroptosis. A separation of inflammasome function has also been reported for NLRC4 inflammasomes [12••]. Although the regulation of this division of labor is unknown, a weak interaction between the PYD domains of NLRP7 and ASC may be important [13•]. It is possible that distinct inflammasome subcomplexes can be formed to yield alternate outputs.
NLRP6 inflammasomes have differential impacts on systemic infections
Surprisingly, studies on NLRP6 implicate cell-type specific effects [14••,15]. In mouse colonic epithelial cells, NLRP6 deficiency leads to an increased risk of severe colitis associated with reduced IL-18 levels and altered fecal microbiota [14••]. Thus, NLRP6, in cooperation with NLRP3, seems to be required for the maintenance of a functional intestinal microflora [14••,16]. By contrast, intraperitoneal infections with Listeria monocytogenes and Salmonella typhimurium lead to enhanced resistance in NLRP6 deficient mice [15]. In this model, NLRP6 appears to act as a molecular buffer to dampen undesired pro-inflammatory responses caused by NLR activation in hematopoietic cells [15].
Emerging mechanisms of inflammasome regulation
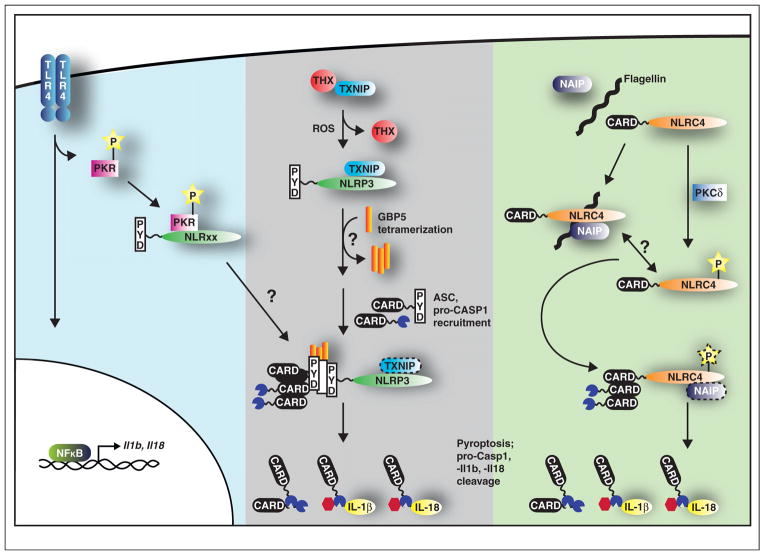
Tight regulation of caspase-1 activity is crucial because uncontrolled pro-inflammatory responses and cell death have severe negative consequences. A number of recent studies have shed light on regulatory mechanisms of NLR and caspase-1 activation, including the double-stranded RNA-dependent protein kinase, PKR. This kinase acts downstream of TLR4 signaling and was previously implicated in the response to viral infections and various PAMPs and DAMPs [17–19]. However, the molecular mechanism was unclear. Lu et al. now suggest that the broad influence of PKR is owing to its ability to associate with several inflammasomes including NLRP1, NLRP3, NLRC4, and AIM2 (Figure 2) [20]. Surprisingly, although gel filtration of LPS/ATP-stimulated BMDMs lysed under low-stringency conditions indicates a stable interaction between PKR, NLRP3, caspase-1, and ASC, it appears that the bulk of caspase-1 and ASC elute in the lower molecular weight fractions. Perhaps NLRs interact transiently and only long enough to oligomerize ASC-caspase-1 complexes.
Figure 2.
(Blue, left) TLR4 activation induces signaling pathways that upregulate transcription through NFκB. PKR is also phosphorylated upon activation of TLR4 signaling. Binding to NLRP1, NLRP3, NLRC4, and AIM2 enhances inflammasome function. (Gray, middle) The inhibitory association of thioredoxin (THX) to TXNIP is relieved in the presence of ROS. TXNIP is free to bind NLRP3 that enhances inflammasome function. GBP5 tetramerization is triggered by an as yet unknown stimulus that then promotes ASC oligomerization to amplify inflammasome activation. It is not clear if TXNIP remains bound to NLRP3 during this time. (Green, right) NLRC4 activation requires both flagellin and NAIP5/6 to induce an inflammasome response. PKCδ phosphorylation of NLRC4 may also enhance NLRC4 inflammasome activity. The kinetics of the NLRC4 phosphorylation and NAIP association during inflammasome function is not clear. Regardless of how inflammasomes are regulated, their engagement is required for pyroptosis and processing of pro-caspase-1, pro-IL-1β, and pro-IL-18.
NLRC4 regulation has also come under the spotlight. Prior work implicated NLRC4 in cytosolic-sensing of T3SS components, flagellin and PrgJ, though no direct interactions had been reported [21–23]. Recent biochemical analyses from two independent groups demonstrate that different NAIP (NLR family, apoptosis inhibitory protein) paralogues determine the specificity of NLRC4 inflammasomes [24••,25••]. NAIP2 and NAIP5/6 control ligand-dependent NLRC4 oligomerization upon association with PrgJ and flagellin, respectively (Figure 2) [24••,25••,26,27]. Interestingly, this family of proteins contains N-terminal BIR domains in addition to the basal NLR structure (Figure 1). Although the precise function of the BIR domain is unclear, it certainly contributes to NLRC4 inflammasome formation whose EM structure was recently solved [28•]. The resultant disk-like structure is believed to form the platform on which caspase-1 is activated [28•]. In addition, Qu et al. report that NLRC4 activity is modulated by PKCδ phosphorylation between the NACHT and LRR domain that licenses it towards inflammasome activation in response to S. typhimurium infection (Figure 2) [29]. In the future, it will be interesting to determine whether the phospho-specific NLRC4 antibody developed by Qu et al. recognizes NLRC4 in the absence of NAIP 2/5/6 to assess the interplay between these two regulatory pathways.
Although the ligands involved in NAIP/NLRC4 inflammasome activation have been identified, those for NLRP3 inflammasomes are less well defined. They include, but are not limited to, viral, bacterial, and fungal PAMPs in addition to DAMPs such as ROS, cathepsins, ATP, and crystalline substances [30]. Several recent reports suggest that these diverse stimuli may all converge upon mitochondrial dysregulation through ROS generation. NLRP3 was found to localize with the perinuclear endoplasmic reticulum and mitochondria in THP-1 cells while NLRP3 inflammasome activation was attenuated by downregulating voltage-dependent anion channels that affect mitochondrial metabolism [31]. The presence of ROS also has been shown to alleviate the inhibitory interaction of thioredoxin with thioredoxin-interacting protein, TXNIP, allowing TXNIP to associate with NLRP3, and activate caspase-1 (Figure 2) [32••].
Guanylate binding protein 5 (GBP5) was also recently identified as a mediator in NLRP3 inflammasome activation in response to various bacterial ligands [33]. Loss of GBP5 resulted in a reduction of IL-1β secretion and caspase-1 activity, in both THP-1 cells and murine macrophages. Shenoy et al. propose an intriguing model; tetrameric GBP5 binds NLRP3 to promote ASC oligomerization, and hence, inflammasome activation (Figure 2) [33]. Since GBP5 activity seems to be restricted to NLRP3 inflammasomes, it would be interesting to assess the potential involvement of GBPs with other inflammasomes [33]. How all these novel factors integrate together to fine-tune a concerted inflammasome response will require further examination.
The NAIPs, PKR, and GBP5 may aid in transitioning NLRs from an inactive to active conformation. Consistent with this, previous studies have found that deletion of the LRR domains of NLRP1 and NLRC4 leads to constitutive caspase-1 activation in the absence of ligand [24••,34,35]. In addition, anthrax lethal toxin-mediated cleavage of NLRP1 causes caspase-1 activation [36,37]. Because Frew et al. observe that the cleaved fragments remain associated, it is possible that the cleavage event brings the NLRP1 domains that are critical for activation into close proximity.
Caspase-11: a new player in cytosolic-sensing pathways
Cytosolic sensing through NLR inflammasomes can result in pyroptotic cell death mediated by caspase-1 or caspase-11. Using different stimuli, Kayagaki et al. demonstrate that caspase-1 mediated responses (IL-1β secretion) can be uncoupled from those of caspase-11 (cell death) [38]. Importantly, we show that caspase-11 dependent cell death induced by S. typhimurium is detrimental to the host in the absence of caspase-1 mediated innate immunity, resulting in increased susceptibility to disease [39]. Although, the sensor that activates caspase-11 during S. typhimurium infection remains a mystery, it is dependent on both TLR as well as IFN-α/β receptor (IFNAR) signaling in response to the type-I IFNs (IFN-α/β) [39–41]. Recent results from our lab indicate that the addition of exogenous IFN-β does not affect caspase-11 expression, though it does bypass the requirement for the TLR adaptor genes, Myd88/Trif, to induce caspase-11 mediated cell death in the context of S. typhimurium infection in primary BMDMs [39]. This suggests that two, as yet unidentified, signals are necessary for caspase-11-mediated cell death: (1) an IFN-β inducible gene product and (2) an infection-dependent response.
Although Broz et al. find that caspase-11 can only be activated in the context of S. typhimurium infection, Rathinam et al. report that IFN-β or IFN-γ treatment alone can increase CASP11 levels that is sufficient to induce cell death [40]. However, these contradictory results may be attributed to the use of immortalized versus primary BMDMs since the immortalization process requires the use of retroviruses that maintain their replication-proficiency post-transduction (unpublished observation). Rathinam et al. further argue that caspase-11 expression is sufficient for activation because they observe caspase-11 auto-catalytic processing upon ectopic expression in HEK293 cells, but the physiological relevance of this finding is unclear. Caspase-1, whose activation is dependent on NLR engagement in BMDMs, can also be auto-activated upon expression in HEK293 cells [42].
The downstream effects of caspase-11 activation are also controversial. Broz et al. demonstrate using confocal microscopy that inflammasome assembly upon S. typhimurium infection is disrupted in the absence of caspase-11 [39]. By contrast, Rathinam et al. conclude that ASC oligomerization does not depend on caspase-11. However, these differences are probably owing to the methods employed. Rathinam et al. assessed ASC oligomerization by analyzing BMDM lysates from two different mouse backgrounds (C57BL/6 caspase-11+/+ and 129S6 caspase-11−/−). However, the use of immunoblotting to detect ASC complexes does not discriminate between dimers and multi-oligomers in the same manner as confocal microscopy.
Conclusions and future directions
Several cytosolic receptors can nucleate inflammasome assembly. However, the recurring observation of concomitant NLR inflammasome activation in response to bacterial infections emphasizes the necessity to understand their spatiotemporal relationships upon infection. Furthermore, the precise biochemical composition of inflammasomes is still unknown, thus limiting our understanding of the underlying molecular mechanisms of assembly. This has also restricted our methods used to assess cytosolic-sensing as outlined in “NOD-like receptors: domains and functions in inflammasomes.” Because these are thought to be the most downstream events, it can skew interpretations of results. For instance, we now know that cytosolic-sensing can lead to cell death irrespective of caspase-1 processing [12••]. However, new emerging data are building upon this solid foundation. Distilling molecular pathways to their fundamental biochemistry such as with the NAIP-NLRC4-flagellin complex has led to a minimal NLRC4 inflammasome EM structure that is sure to generate new, testable hypotheses [28•]. Further investigation will also be necessary to understand how TLR, NLR, and type I IFN signaling pathways are integrated together in response to bacterial infections. The misregulation of these pathways has been implicated in numerous human diseases, including auto-inflammatory diseases [43]. Thus, an increased understanding of how immune signaling pathways are interwoven during bacterial infections and manipulated by pathogens will likely lead to the development of more directed and improved therapies to combat bacterial infections and inflammatory diseases.
Acknowledgments
We thank members of the Monack lab for critical reading of the manuscript. We apologize if any references were left out owing to space constraints. This work was supported by awards AI095396 and AI08972 from the National Institute of Allergy and Infectious Diseases to D.M., award AI007328-25 from the National Institute of Health and Dean’s Postdoctoral Fellowship from Stanford University to T.M.N., and award Ko 4584/1-1 from the German Research Foundation (DFG) to J.K.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Barton GM, Kagan JC. A cell biological view of toll-like receptor function: regulation through compartmentalization. Nat Rev Immunol. 2009;9:535–542. doi: 10.1038/nri2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Neill LA, Bowie AG. The family of five: Tir-domain-containing adaptors in toll-like receptor signalling. Nat Rev Immunol. 2007;7:353–364. doi: 10.1038/nri2079. [DOI] [PubMed] [Google Scholar]
- 3.Kingeter LM, Lin X. C-type lectin receptor-induced nf-kappab activation in innate immune and inflammatory responses. Cell Mol Immunol. 2012;9:105–112. doi: 10.1038/cmi.2011.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vance RE, Isberg RR, Portnoy DA. Patterns of pathogenesis: discrimination of pathogenic and nonpathogenic microbes by the innate immune system. Cell Host Microbe. 2009;6:10–21. doi: 10.1016/j.chom.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elinav E, Strowig T, Henao-Mejia J, Flavell RA. Regulation of the antimicrobial response by nlr proteins. Immunity. 2011;34:665–679. doi: 10.1016/j.immuni.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 6.Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol. 2009;7:99–109. doi: 10.1038/nrmicro2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vladimer GI, Weng D, Paquette SW, Vanaja SK, Rathinam VA, Aune MH, Conlon JE, Burbage JJ, Proulx MK, Liu Q, et al. The nlrp12 inflammasome recognizes yersinia pestis. Immunity. 2012;37:96–107. doi: 10.1016/j.immuni.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zambetti LP, Laudisi F, Licandro G, Ricciardi-Castagnoli P, Mortellaro A. The rhapsody of nlrps: master players of inflammation… And a lot more. Immunol Res. 2012;53:78–90. doi: 10.1007/s12026-012-8272-z. [DOI] [PubMed] [Google Scholar]
- 9.Montminy SW, Khan N, McGrath S, Walkowicz MJ, Sharp F, Conlon JE, Fukase K, Kusumoto S, Sweet C, Miyake K, et al. Virulence factors of yersinia pestis are overcome by a strong lipopolysaccharide response. Nat Immunol. 2006;7:1066–1073. doi: 10.1038/ni1386. [DOI] [PubMed] [Google Scholar]
- 10.Brodsky IE, Palm NW, Sadanand S, Ryndak MB, Sutterwala FS, Flavell RA, Bliska JB, Medzhitov R. A yersinia effector protein promotes virulence by preventing inflammasome recognition of the type iii secretion system. Cell Host Microbe. 2010;7:376–387. doi: 10.1016/j.chom.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11••.Khare S, Dorfleutner A, Bryan NB, Yun C, Radian AD, de Almeida L, Rojanasakul Y, Stehlik C. An nlrp7-containing inflammasome mediates recognition of microbial lipopeptides in human macrophages. Immunity. 2012;36:464–476. doi: 10.1016/j.immuni.2012.02.001. Using THP1 cells, the authors demonstrate that sensing of bacterial lipopeptides results in formation of an NLRP7-ASC-caspase-1 inflammasome complex that triggers interleukin release but not cell death. Taken together, this work not only presents a novel PAMP, but broadens the spectrum of possible inflammasome-mediated immune responses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12••.Broz P, von Moltke J, Jones JW, Vance RE, Monack DM. Differential requirement for caspase-1 autoproteolysis in pathogen-induced cell death and cytokine processing. Cell Host Microbe. 2010;8:471–483. doi: 10.1016/j.chom.2010.11.007. Caspase-1 mediated cell death has historically been presumed to require auto-proteolytic cleavage. Here, the authors clearly demonstrate that caspase-1 activity can be uncoupled between the initiation of cell death, which does not require autoproteolytic cleavage, and IL-1β secretion, which does. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13•.Pinheiro AS, Proell M, Eibl C, Page R, Schwarzenbacher R, Peti W. Three-dimensional structure of the nlrp7 pyrin domain: insight into pyrin-pyrin-mediated effector domain signaling in innate immunity. J Biol Chem. 2010;285:27402–27410. doi: 10.1074/jbc.M110.113191. Using NMR spectroscopy, the authors determine the structure of NLRP7 PYD and also conduct comparison analyses with the PYD domains from NLRP1 and ASC. The absence of changes in NMR spectra between NLRP7 PYD and NLRP7 PYD/ASC PYD suggests a weak and/or transient interaction that has broad implications for inflammasome assembly and structure. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14••.Elinav E, Strowig T, Kau AL, Henao-Mejia J, Thaiss CA, Booth CJ, Peaper DR, Bertin J, Eisenbarth SC, Gordon JI, Flavell RA. Nlrp6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell. 2011;145:745–757. doi: 10.1016/j.cell.2011.04.022. The authors nicely show that the NLRP6 inflammasome is needed to establish a functional gut flora that is essential to prevent colon inflammation. This is the first paper unraveling a connection between intestinal microbiota and the inflammasome and has broader implications for the importance of studying inflammasome activation on a global context in an in vivo model. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anand PK, Malireddi RK, Lukens JR, Vogel P, Bertin J, Lamkanfi M, Kanneganti TD. Nlrp6 negatively regulates innate immunity and host defence against bacterial pathogens. Nature. 2012;488:389–393. doi: 10.1038/nature11250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henao-Mejia J, Elinav E, Jin C, Hao L, Mehal WZ, Strowig T, Thaiss CA, Kau AL, Eisenbarth SC, Jurczak MJ, et al. Inflammasome-mediated dysbiosis regulates progression of nafld and obesity. Nature. 2012;482:179–185. doi: 10.1038/nature10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horng T, Barton GM, Medzhitov R. Tirap: an adapter molecule in the toll signaling pathway. Nat Immunol. 2001;2:835–841. doi: 10.1038/ni0901-835. [DOI] [PubMed] [Google Scholar]
- 18.Pfaller CK, Li Z, George CX, Samuel CE. Protein kinase pkr and rna adenosine deaminase adar1: new roles for old players as modulators of the interferon response. Curr Opin Immunol. 2011;23:573–582. doi: 10.1016/j.coi.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsu LC, Park JM, Zhang K, Luo JL, Maeda S, Kaufman RJ, Eckmann L, Guiney DG, Karin M. The protein kinase pkr is required for macrophage apoptosis after activation of toll-like receptor 4. Nature. 2004;428:341–345. doi: 10.1038/nature02405. [DOI] [PubMed] [Google Scholar]
- 20.Lu B, Nakamura T, Inouye K, Li J, Tang Y, Lundback P, Valdes-Ferrer SI, Olofsson PS, Kalb T, Roth J, et al. Novel role of pkr in inflammasome activation and hmgb1 release. Nature. 2012;488:670–674. doi: 10.1038/nature11290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miao EA, Mao DP, Yudkovsky N, Bonneau R, Lorang CG, Warren SE, Leaf IA, Aderem A. Innate immune detection of the type iii secretion apparatus through the nlrc4 inflammasome. Proc Natl Acad Sci USA. 2010;107:3076–3080. doi: 10.1073/pnas.0913087107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miao EA, Alpuche-Aranda CM, Dors M, Clark AE, Bader MW, Miller SI, Aderem A. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1beta via ipaf. Nat Immunol. 2006;7:569–575. doi: 10.1038/ni1344. [DOI] [PubMed] [Google Scholar]
- 23.Franchi L, Amer A, Body-Malapel M, Kanneganti TD, Ozoren N, Jagirdar R, Inohara N, Vandenabeele P, Bertin J, Coyle A, et al. Cytosolic flagellin requires ipaf for activation of caspase-1 and interleukin 1beta in salmonella-infected macrophages. Nat Immunol. 2006;7:576–582. doi: 10.1038/ni1346. [DOI] [PubMed] [Google Scholar]
- 24••.Kofoed EM, Vance RE. Innate immune recognition of bacterial ligands by naips determines inflammasome specificity. Nature. 2011;477:592–595. doi: 10.1038/nature10394. The authors beautifully engineer an inflammasome reconstitution system which they use to delineate the minimal components required for activation of NLRC4 inflammasomes through the murine NAIP proteins and components of the T3SS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25••.Zhao Y, Yang J, Shi J, Gong YN, Lu Q, Xu H, Liu L, Shao F. The nlrc4 inflammasome receptors for bacterial flagellin and type iii secretion apparatus. Nature. 2011;477:596–600. doi: 10.1038/nature10510. Similar to Kofoed and Vance, the authors demonstrate roles for murine NAIP proteins in NLRC4 inflammasome function. Here, they push the system one step further and demonstrate a role for human NAIP as well. [DOI] [PubMed] [Google Scholar]
- 26.Lightfield KL, Persson J, Trinidad NJ, Brubaker SW, Kofoed EM, Sauer JD, Dunipace EA, Warren SE, Miao EA, Vance RE. Differential requirements for naip5 in activation of the nlrc4 inflammasome. Infect Immun. 2011;79:1606–1614. doi: 10.1128/IAI.01187-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lightfield KL, Persson J, Brubaker SW, Witte CE, von Moltke J, Dunipace EA, Henry T, Sun YH, Cado D, Dietrich WF, et al. Critical function for naip5 in inflammasome activation by a conserved carboxy-terminal domain of flagellin. Nat Immunol. 2008;9:1171–1178. doi: 10.1038/ni.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28•.Halff EF, Diebolder CA, Versteeg M, Schouten A, Brondijk TH, Huizinga EG. Formation and structure of a naip5-nlrc4 inflammasome induced by direct interactions with conserved n- and c-terminal regions of flagellin. J Biol Chem. 2012;287:38460–38472. doi: 10.1074/jbc.M112.393512. This is the first EM image described for a minimal NLR inflammasome complete with ligand whose architecture provides broad implications for mechanisms of inflammasome function. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qu Y, Misaghi S, Izrael-Tomasevic A, Newton K, Gilmour LL, Lamkanfi M, Louie S, Kayagaki N, Liu J, Komuves L, et al. Phosphorylation of nlrc4 is critical for inflammasome activation. Nature. 2012;490:539–542. doi: 10.1038/nature11429. [DOI] [PubMed] [Google Scholar]
- 30.Franchi L, Munoz-Planillo R, Nunez G. Sensing and reacting to microbes through the inflammasomes. Nat Immunol. 2012;13:325–332. doi: 10.1038/ni.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in nlrp3 inflammasome activation. Nature. 2011;469:221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 32••.Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol. 2010;11:136–140. doi: 10.1038/ni.1831. NLRP3 is engaged by a plethora of stimuli. The authors here elucidate an elegant molecular mechanism by which reactive oxygen species induces NLRP3 inflammasome activation through disengagment of the inhibitor, thioredoxin. [DOI] [PubMed] [Google Scholar]
- 33.Shenoy AR, Wellington DA, Kumar P, Kassa H, Booth CJ, Cresswell P, MacMicking JD. Gbp5 promotes nlrp3 inflammasome assembly and immunity in mammals. Science. 2012;336:481–485. doi: 10.1126/science.1217141. [DOI] [PubMed] [Google Scholar]
- 34.Stehlik C, Reed JC. The pyrin connection: novel players in innate immunity and inflammation. J Exp Med. 2004;200:551–558. doi: 10.1084/jem.20032234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Faustin B, Lartigue L, Bruey JM, Luciano F, Sergienko E, Bailly-Maitre B, Volkmann N, Hanein D, Rouiller I, Reed JC. Reconstituted nalp1 inflammasome reveals two-step mechanism of caspase-1 activation. Mol Cell. 2007;25:713–724. doi: 10.1016/j.molcel.2007.01.032. [DOI] [PubMed] [Google Scholar]
- 36.Frew BC, Joag VR, Mogridge J. Proteolytic processing of nlrp1b is required for inflammasome activity. PLoS Pathog. 2012;8:e1002659. doi: 10.1371/journal.ppat.1002659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levinsohn JL, Newman ZL, Hellmich KA, Fattah R, Getz MA, Liu S, Sastalla I, Leppla SH, Moayeri M. Anthrax lethal factor cleavage of nlrp1 is required for activation of the inflammasome. PLoS Pathog. 2012;8:e1002638. doi: 10.1371/journal.ppat.1002638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kayagaki N, Warming S, Lamkanfi M, Vande Walle L, Louie S, Dong J, Newton K, Qu Y, Liu J, Heldens S, et al. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479:117–121. doi: 10.1038/nature10558. [DOI] [PubMed] [Google Scholar]
- 39.Broz P, Ruby T, Belhocine K, Bouley DM, Kayagaki N, Dixit VM, Monack DM. Caspase-11 increases susceptibility to salmonella infection in the absence of caspase-1. Nature. 2012;490:288–291. doi: 10.1038/nature11419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rathinam VA, Vanaja SK, Waggoner L, Sokolovska A, Becker C, Stuart LM, Leong JM, Fitzgerald KA. Trif licenses caspase-11-dependent nlrp3 inflammasome activation by gram-negative bacteria. Cell. 2012;150:606–619. doi: 10.1016/j.cell.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gurung P, Malireddi RK, Anand PK, Demon D, Walle LV, Liu Z, Vogel P, Lamkanfi M, Kanneganti TD. Trif-mediated caspase-11 production integrates tlr4- and nlrp3 inflammasome-mediated host defense against enteropathogens. J Biol Chem. 2012;287:34474–34483. doi: 10.1074/jbc.M112.401406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Imamura R, Wang Y, Kinoshita T, Suzuki M, Noda T, Sagara J, Taniguchi S, Okamoto H, Suda T. Anti-inflammatory activity of pynod and its mechanism in humans and mice. J Immunol. 2010;184:5874–5884. doi: 10.4049/jimmunol.0900779. [DOI] [PubMed] [Google Scholar]
- 43.Lamkanfi M, Dixit VM. Inflammasomes and their roles in health and disease. Annu Rev Cell Dev Biol. 2012;28:137–161. doi: 10.1146/annurev-cellbio-101011-155745. [DOI] [PubMed] [Google Scholar]