Abstract
Objective
To examine the independent contributions of objectively measured sleep duration and fragmentation on cardiometabolic risk accumulation in free-living obese adolescents.
Study design
Characteristics of metabolic syndrome (waist circumference, mean arterial pressure, fasting high-density lipoprotein cholesterol, triglycerides, glucose) were measured in obese adolescents and standardized residuals(z-scores) were summed (inverse high-density lipoprotein cholesterol) to create a continuous cardiometabolic risk score (cMetScore), adjusted for age, sex, and race. Sleep and physical activity were objectively measured in habitual, free-living conditions for 7 days (SenseWear Pro3, BodyMedia, Pittsburgh, Pennsylvania; n = 37; 54% female, ages 11–17 years). Associations between sleep duration and cMetScore were assessed via multiple linear regression.
Results
Body mass index, total sleep time, and sleep session length were each correlated with cMetScore (P < .05 all). Total sleep time was inversely and independently associated with cMetScore (r = −0.535, P = .001) and was the best independent predictor of metabolic risk.
Conclusions
Sleep duration inversely predicts cardiometabolic risk in obese adolescents, even when we controlled for various measures of physical activity, anthropometry, and adiposity. Further research should investigate the biological mechanism of this relationship and the potential treatment effect of sleep intervention in decreasing cardiometabolic risk in this population.
More than 30% of US adolescents are overweight, and more than 15% are obese,1 placing them at increased cardiometabolic risk. Compared with normal-weight peers, overweight adolescents (12–19 years) exhibit greater risk of cardiovascular disease,2 and obese youth (5–15 years) have increased glucose, blood pressure, insulin, and lipids as well as increased left ventricular mass.3 Metabolic syndrome has been diagnosed in 25%–50% of obese pediatrics.4–6 Each half-unit increase in body mass index (BMI) z-score is associated with increased metabolic syndrome risk in overweight adolescents.6 Childhood BMI tracks into adulthood,7 as do cardiovascular8–13 and cardiometabolic14 risk. Fortunately, obese adolescents who become nonobese by adulthood experience similar cardiometabolic risk as those who were never obese,15 suggesting that the treatment of obesity during youth can indeed improve lifelong health.
Due to multiple factors,16–22 most adolescents do not obtain adequate sleep.23 Short sleep duration has been associated with cardiometabolic risk and type 2 diabetes mellitus in adults.24,25 In pediatric patients, short sleep duration has been associated with greater BMI26–35 and risk for being overweight,36 although the strength of these relationships often is stronger in children than in older adolescents.37,38 Sleep duration may be associated with insulin resistance35 and increased waist circumference in youth39,40 but has not been consistently associated with metabolic risk or insulin resistance in adolescents.27,41 In a recent study, overweight youth exhibited greater cardiovascular risk than normal-weight peers, but meeting step count and sleep duration recommendations did not mitigate these differences.42 Thus, the relationship between sleep duration and cardiometabolic risk in obese adolescents remains uncertain.
An objective field measurement of sleep duration via a SenseWear armband (SWA; BodyMedia, Pittsburgh, Pennsylvania) offers a minimally invasive assessment of habitual sleep that can be easily incorporated into many settings. The purpose of this study was to determine the independent association between objectively measured habitual sleep duration and cardiometabolic risk in obese adolescents. We hypothesized that both decreased sleep duration and increased sleep fragmentation would be associated with cardiometabolic risk in this population, independent of body mass and composition.
Methods
Obese (BMI ≥95th percentile) adolescents (11–17 years) were recruited from a family-based, multidisciplinary weight-loss program (Michigan Pediatric Outpatient Weight Evaluation & Reduction program43) at the University of Michigan. Baseline assessments were included in the present study. This study was approved by the University of Michigan Institutional Review Board (HUM00028810).
Standing height (to 0.5 cm) and body mass (to 0.1 kg) were measured using a wall-mounted stadiometer (“Stow-a-weigh”; Scale-Tronix, White Plains, New York). BMI was calculated as weight (kg) divided by height (m) squared (kg•m−2). Body composition was measured via air-displacement plethysmography per the manufacturer’s directions and accounted for measured lung volume (Bod Pod; COSMED Inc, Rome, Italy). Waist circumference was measured at the natural waist.44 Seated blood pressure was measured manually. Blood was collected from the patient via an antecubital vein after an overnight (12-hour) fast. Serum was analyzed for high-density lipoprotein cholesterol (HDL-C; enzymatic colormetric assay), triglycerides (enzymatic colormetric assay), and glucose (hexokinase, nicotinamide adenine dinucleotide end point) by the Clinical Chemistry Laboratories in the Department of Pathology at the University of Michigan (accredited by the College of American Pathologists). Mean arterial pressure was calculated as diastolic blood pressure plus one-third pulse pressure.45
Each participant was fitted on the upper arm with physical activity (PA) monitor (SWA, SenseWear Pro3 or the newer model “mini”; BodyMedia) and instructed to wear it continuously for 7 days, except during activities in water. The SWA uses a tri-axial activity monitor, combined with sensors that measure galvanic skin response, near-body ambient temperature, skin temperature, and heat flux. The information collected via SWA permits the measurement of objective PA and sleep in habitual conditions, as well as assessment of activity intensity (ie, metabolic equivalents [METs]) and differentiation between inactivity and sleep. A MET is a measure of the intensity of PA expressed as a multiple of resting metabolic rate. A greater MET indicates greater energy cost. These monitors have been validated for use in pediatrics for both PA46–48 and sleep.49 A minimum of 22 hours of data capture per day were required for inclusion in analysis. This value was chosen as determined by estimates of nonwear time due to personal grooming (ie, bath, shower) and required charge-time. Two consecutive days of monitoring were required to capture overnight sleep. PA was assessed during a 24-hour period between midnight and midnight, and sleep was assessed during a 24-hour period between noon and noon. Because sleep patterns and duration generally differ between weekday and weekends, only participants with at least 3 weekdays and 2 weekend days meeting inclusion criteria were included in analyses. Five days of data were required to match current recommendations for free-living sleep assessments in pediatrics.50
Time in PA was calculated as the sum of all time spent in moderate or vigorous PA (≥3 METs). Both total duration (time) and average intensity (METs) were included in the regression model.
Total sleep time and the sessions that composed sleep were assessed. SWA returned minute-by-minute data for the entire time it was worn (ie, 1440 lines of data/day when worn for 24 hours). Sleep session was defined as at least 15 continuous minutes of sleep and sleep fragmentation as at least 15 minutes of wakefulness. This value was chosen as determined by expected typical wakefulness after sleep onset.51 If a participant awoke for less than 15 minutes, this was not considered a break in sleep, and the sleep cycle was deemed continuous. Total sleep time within a 24-hour period was measured and could include short periods (<15 minutes) of being “awake” as noted by SWA. Weekly sums were created: 5×weekday average + 2× weekend average. Weekly average = weekly sum/7.
Metabolic characteristics (waist circumference, mean arterial pressure, fasting HDL-C, triglycerides, glucose)52 were individually regressed onto age, sex, and race to create a standardized residual (z-score) for each characteristic. These residuals were then summed (inverse HDL-C) to create a continuous cardiometabolic risk score (cMetScore).53 Because a high HDL-C is indicative of decreased risk, the inverse of HDL-C was included in this summation.
Statistical Analyses
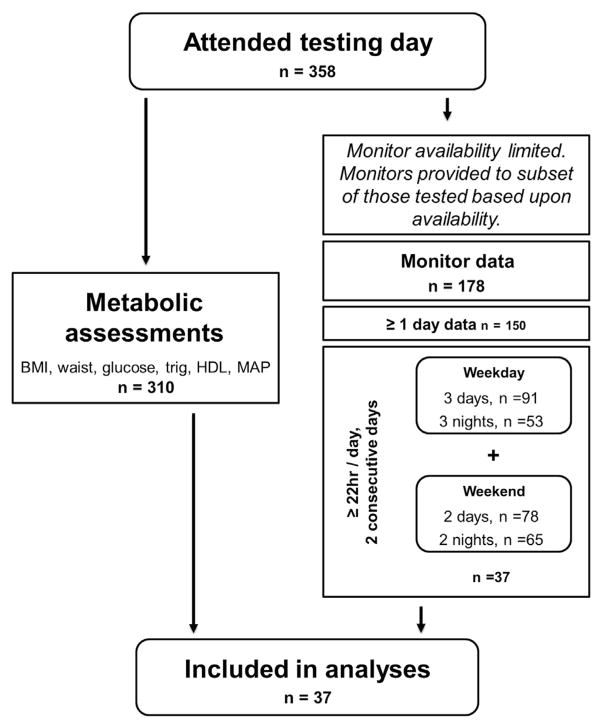
Thirty-seven participants were included in analysis (Figure 1; available at www.jpeds.com). Baseline characteristics were assessed via ANOVA. Sex-specific differences were tested via t test. Pearson bivariate correlation coefficient was used to assess the relationships of cMetScore and primary outcomes. Independent associations between sleep duration and cMetScore were assessed with multiple linear regression. In each model, cMetScore was entered as the continuous dependent variable, and the adolescents’ sleep, PA duration, and intensities (METs) were entered as independent predictors, along with potential covariates (ie, BMI). Multicollinearity of explanatory factors was assessed via variance inflation factor. Statistics were completed via SPSS versions 18.0 and 20.0 (IBM, Armonk, New York). Statistical significance was defined as P < .05.
Figure 1.
Flow chart for subject inclusion procedure. HDL, high density lipoprotein; MAP, mean arterial pressure.
Results
Participants (17 male, 20 female) were 11–17 years of age, were from varying racial backgrounds, and were obese per age- and sex-specific standards (≥95th BMI percentile; Figure 1 and Table I). Other than BMI percentile, subject characteristics did not differ by sex (Table I). One-third of participants met the minimum recommendation of being physically active at least 60 minutes/day.54 Participants slept approximately 7 hours per night, separated into 2 sleep sessions (Table I), which is less than the National Sleep Foundation recommendation for 10–17 year olds (8.5–9.25 hours/night).55 Five participants (14%) met the minimum guideline and slept at least 8.5 hours per night (≥8.5 and ≤9 hours/night, n = 3; > 9 hours/night, n = 2). The length and number of sleep sessions were inversely correlated (r = −0.804, P < .001), and the length of sleep sessions and total weekly sleep were positively correlated (r = 0.722, P < .001), revealing that less sleep fragmentation was indicative of greater volume of sleep over time.
Table I.
Subject characteristics
| Males (n = 17) | Females (n = 20) | |
|---|---|---|
| Age, y | 14 ± 0 (11–17) | 14 ± 0 (12–17) |
| Race | ||
| White, n (%) | 12 (71) | 9 (45) |
| Black, n (%) | 5 (29) | 9 (45) |
| Other, n (%) | 0 (0) | 2 (10) |
| Medicaid, n (%) | 8 (47) | 10 (50) |
| Waist circumference and body composition | ||
| Adiposity (%) | 48 ± 1 (38–61) | 45 ± 2 (26–56) |
| BMI (kg·m−2) | 41.3 ± 2.2 (31.0–66.0) | 40.7 ± 1.6 (32.0–56.0) |
| BMI z-score | 2.70 ± 0.08 (2.24–3.47) | 2.47 ± 0.05 (2.16–2.88)* |
| Waist circumference, cm | 120 ± 5 (87–174) | 112 ± 4 (85–147) |
| Blood pressure, mmHg | ||
| Systolic | 108 ± 3 (94–138) | 109 ± 2 (88–130) |
| Diastolic | 65 ± 2 (52–90) | 65 ± 2 (58–78) |
| Mean arterial pressure | 80 ± 2 (66–106) | 80 ± 2 (71–95) |
| Fasting serum chemistries (mg/dL, [mmol/L]) | ||
| HDL-C | 42 ± 1 (33–55) [1.1 ± 0.0 (0.9–1.4)] | 40 ± 3 (27–81) [1.0 ± 0.1 (0.7–2.1)] |
| Triglycerides | 116 ± 12 (45–210) [1.3 ± 0.1 (0.5–2.4)] | 140 ± 18 (63–351) [1.6 ± 0.2 (0.7–4.0)] |
| Glucose | 93 ± 1 (84–102) [5.1 ± 0.1 (4.6–5.6)] | 92 ± 2 (82–119) [5.1 ± 0.1 (4.5–6.6)] |
| Free-living sleep and PA, daily | ||
| Sleep sessions, no. | 2 ± 0 (1–4) | 2 ± 0 (1–4) |
| Sleep session length, h | 3.9 ± 0.4 (1.4–7.1) | 4.1 ± 0.3 (1.7–7.8) |
| Total sleep, h | 7.1 ± 0.3 (3.6–9.3) | 7.3 ± 0.3 (4.4–10.6) |
| METs | 1.4 ± 0.0 (1.1–1.6) | 1.2 ± 0.0 (1.0–1.6) |
| PA duration, h | 1.4 ± 0.1 (0.7–2.8) | 1.3 ± 0.2 (0.4–3.0) |
n = 31 for adiposity, n = 37 for all others. Unless otherwise noted, data presented as mean ± SEM (range).
P < .05 vs males.
cMetScore was inversely correlated with total sleep time (r = −0.535, P < .001) and sleep session length (r = −0.365, P = .026) but not PA (Table II). Total weekly sleep remained a predictor of cardiometabolic risk, even after controlling for PA time and intensity (METs), and body composition (Table III and Figure 2). Among sleep variables, total sleep time demonstrated the greatest correlation with cMetScore and was included in the model, as were PA duration and METs (Tables II and III). Model 1 includes adiposity as measured via air-displacement plethysmography. Because this assessment is generally not available clinically, an alternative model using BMI z-score was also tested. PA duration and METs demonstrated moderate multicollinearity in both versions of model 1 (variance inflation factor 3.6–3.7). Therefore, the least significant of these factors, PA duration, was removed for model 2.
Table II.
Correlation with cMetScore
| r | P | |
|---|---|---|
| Adiposity (%) | 0.171 | .358 |
| BMI, kg·m−2 | 0.540 | <.001* |
| BMI z-score | 0.523 | <.001* |
| Sleep sessions, no. | 0.182 | .282 |
| Sleep session length, h | −0.365 | .026† |
| Total sleep, h | −0.535 | <.001* |
| METs | −0.221 | .189 |
| PA duration, h | −0.159 | .348 |
n = 31 for adiposity, n = 37 for all others. r = Pearson correlation coefficient.
One MET is the amount of energy used at rest; a MET value of 3 would indicate that the energy cost is 3 times that at rest.
P < .001.
P < .05.
Table III.
Average weekly sleep duration predicts cardiometabolic risk even when controlling for various measures of PA, anthropometry, and body composition
| Factor
|
Model
|
||||||
|---|---|---|---|---|---|---|---|
| β | t | P | R | R2 | Adjusted R2 | P | |
| Model 1 | |||||||
| Total sleep | −0.507 | −3.110 | .005* | 0.583 | 0.339 | 0.238 | .025† |
| PA duration | 0.082 | .272 | .788 | ||||
| METs | −0.299 | −.983 | .335 | ||||
| Adiposity (%) | 0.025 | .152 | .880 | ||||
| Model 1: alternative | |||||||
| Total sleep | −0.410 | −2.813 | .008* | 0.656 | 0.431 | 0.359 | .001* |
| PA duration | 0.086 | .339 | .737 | ||||
| METs | −0.216 | −.837 | .409 | ||||
| BMI z-score | 0.339 | 2.272 | .030† | ||||
| Model 2 | |||||||
| Total sleep | −0.402 | −2.835 | .008* | 0.655 | 0.429 | 0.377 | <.001‡ |
| METs | −0.141 | −1.055 | .299 | ||||
| BMI z-score | 0.349 | 2.419 | .021† | ||||
n = 31 in model 1, n = 37 alternative model 1 and model 2.
P < .01.
P < .05.
P < .001.
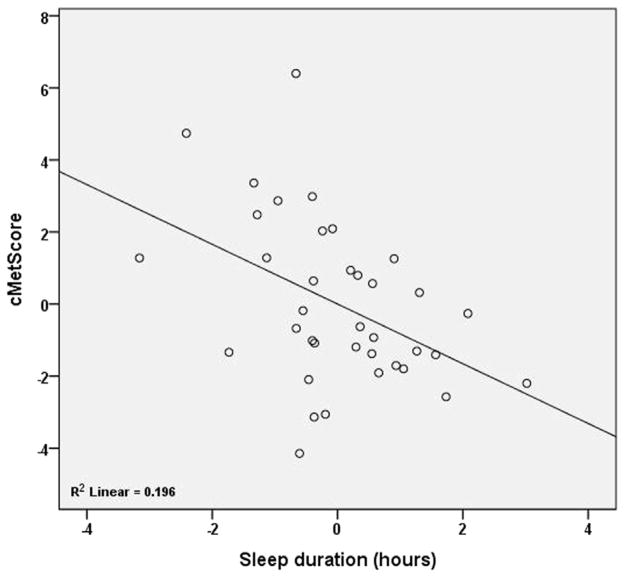
Figure 2.
Partial regression plot of sleep and cMetScore when we controlled for METs and BMI z-score (model 2). This partial correlation plot illustrates the association between cMetScore and sleep duration after adjusting for effects of BMI z-score and METs.
Discussion
Our finding of inadequate sleep in obese adolescents aligns with previous reports, such as the National Sleep Foundation 2006 Sleep in America Poll of 1602 adolescents and their caregivers, which found that the average adolescent sleeps only 7.6 hours on weeknights, with only 20% of adolescents getting the optimal amount of sleep (>9 hours).23 Potential contributing factors are many,16–18 including but not limited to decreased time for sleep due to increased academic and extracurricular expectations,23 misalignment of academic19 and biologically ideal20 schedules,21 and increased sedentary behavior22 as well as family conflict and diminished parental mental health.21
In the present study, 30% of participants met the current PA guidelines of 60 minutes/day, which is consistent with the range of PA duration identified in the Centers for Disease Control and Prevention’s 2011 US Youth Risk Behavior Survey56 in which 49.5% of respondents self-reported being physically active at least 60 minutes/day for 5 days and 28.7% reported being physically active at least 60 minutes/day on all 7 days before the survey.56
Sleep session length was inversely associated with cMetScore, whereas the number of sleep sessions (indicative of awakenings) was not associated with cMetScore. Our definition of sleep fragmentation was at least 15 minutes of wakefulness as detected by orientation, motion, temperature, and skin conductivity versus typically measured arousals or awakening using an electroencephalogram along with an electromyogram and may have limited our ability to detect an association between awakenings and cMetScore. The low variation in number of sleep sessions (range 1–4 sessions/night) may have further limited this ability. The importance of sleep fragmentation in cardiometabolic health recently has been demonstrated in a mouse model, as instrumentally fragmented sleep elicited a metabolic response similar to that of sleep deprivation.57 Within only 14 days, sleep fragmentation caused increased food intake, an inability to appropriately decrease brain temperature during sleep, glucose intolerance, and slightly increased circadian peak glucocorticoids, suggesting that sleep fragmentation may lead to the development of metabolic disturbances.57
Previous research has inconsistently documented a relationship between sleep duration and the individual risk factors of insulin resistance and waist circumference in adolescents.39,40 Most research has not focused solely on obese adolescents but has instead included normal-weight subjects or those with a range of weight. Because we included only obese adolescents, the objective measurement of habitual sleep and a continuous cMetScore likely enhanced our ability to detect an inverse relationship between sleep and cardiometabolic risk in this population.
Among 183 adolescents (88 girls, ages 13–17 years, BMI 21.6 ± 3.5 kg•m−2), self-reported sleep duration was inversely associated with C-reactive protein, but only after controlled for sex, age, and pubertal status.58 This association was not affected by additional correction for objectively measured moderate-to-vigorous PA (GT1M uniaxial accelerometer; ActiGraph, Pensacola, Florida),58 similar to our finding that the inclusion of PA duration and intensity within the model did not alter the ability of sleep duration to predict cMetScore. Among 414 children and adolescents (50% female, ages 6.1–19.9 years, BMI range 16.9–22.0 kg•m−2), insulin, homeostasis model assessment-estimated insulin resistance, and leptin were negatively associated with parental/self-reported sleep duration in girls but not boys.40 It is possible that a larger sample size in our study would have permitted the detection of sex-specific differences in the association of sleep duration and cardiometabolic risk. In a different study, total sleep duration, when adjusted for age, race, sex, BMI, and waist circumference, was inversely associated with homeostasis model assessment-estimated insulin resistance among 245 high school students (n = 116 males, age 15.7 ± 1.3 years, ~50% overweight or obese).41 Although the models, subject weight status and age, manner of sleep and activity collection (objective vs subjective), and specific variables differ, findings from these and the present study suggest that sleep duration is associated with cardiometabolic risk in adolescents.
Other studies have not observed a relationship between sleep and cardiometabolic risk in adolescents. In a cross-sectional study of 133 obese adolescents, neither objective (actigraph, Mini-Motionlogger; Ambulatory Monitoring, Inc, Ardsley, New York) nor subjective (parent and self-report) sleep duration were associated with individual metabolic outcomes nor did any measures of sleep duration differ in the presence versus absence of metabolic syndrome.59 The authors noted the unexpected finding that short sleep duration was associated with lower triglyceride and increased HDL-C levels. Our use of a continuous cMetScore rather than evaluation of its individual components may, in part, explain the differences in findings.
A study of minority adolescents found that self-reported sleep was associated with cardiometabolic outcomes,60 further supporting our findings. The model suggested that reduced sleep duration, as well as the combination of poor sleep quality and fatigue, are associated with decreased fitness, and therefore increased cardiometabolic risk.60 Our ability to detect an association between sleep duration and cardiometabolic risk within a much smaller, racially diverse and obese population was likely aided by the objective measurement of sleep duration. Although the relationship between sleep duration and cardiometabolic risk may not be directly causal, our findings indicate that sleep duration is a robust predictor in this population.
However, as with all cross-sectional investigations, a limitation of this study is the inability to disentangle the cause-effect relationship between predictors and outcomes. Indeed, whether lack of sleep “causes” cardiometabolic declines, or obesity itself is a cause of disturbances in sleep (ie, reverse causality), is an interesting and complex topic. It is also possible that inclusion of additional behavioral, demographic, and parental factors may provide further understanding to our results. For example, parental income, neighborhood stress, pubertal status, and depression have previously been noted as associated with sleep duration and metabolic health in adolescents.31 However, the strong association between sleep duration and cMetScore independent of the effects of body composition and PA suggest a potential influence of sleep duration on cardiometabolic health in obese adolescents. SWA was recently found to agree with polysomnography for sleep duration assessment, but because of individual variation the authors did not recommend it for an individual clinical evaluation of sleep.49 Nonetheless, our ability to identify a relationship between habitual sleep duration and cardiometabolic risk despite the potential variability of sleep duration assessment via SWA indicates that this measure may be acceptable. Furthermore, because we used stringent inclusion criteria, we were limited to a small sample size, a limitation that may have limited our ability to detect an association between PA and cardiometabolic risk in this cohort. Given the relatively small sample size in the present versus other studies that included a continuous metabolic risk score, future research including more obese subjects should be completed. Future research should also include adolescents with a range of weights (ie, underweight, normal, and overweight as well) to determine whether this relationship holds within a more heterogeneous population.
Habitual measures of sleep duration and PA provide evidence of a significant association between sleep duration and cardiometabolic risk accumulation in obese adolescents. The amalgamation of rigorous sleep assessment and a continuous metabolic risk score strengthen our findings, perhaps explaining why we found a clear inverse association between total sleep duration and cardiometabolic risk in obese adolescents whereas others have not. We have demonstrated that objective sleep assessment may be a useful screening tool to identify “at risk” obese adolescents. This noninvasive screening tool would enable physicians to efficiently reach and treat more patients, ultimately benefiting public health through enhanced clinical practice. Future research should explore the mechanism of the relationship between sleep and cardiometabolic health in obese adolescents as well as the efficacy of altering cardiometabolic risk in this population by targeting sleep enhancement interventions.
Acknowledgments
We acknowledge Caroline F. Tang for her assistance in SWA data preparation (Undergraduate Research Opportunity Program, University of Michigan; received financial aid as part of this program).
Supported by the National Institutes of Health (DK089503 to the University of Michigan).
Glossary
- BMI
Body mass index
- cMetScore
Cardiometabolic risk score
- HDL-C
High-density lipoprotein cholesterol
- MET
Metabolic equivalent
- PA
Physical activity
- SWA
SenseWear armband
Footnotes
The authors declare no conflicts of interest.
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. Jama. 2012;307:483–90. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.May AL, Kuklina EV, Yoon PW. Prevalence of cardiovascular disease risk factors among US adolescents, 1999–2008. Pediatrics. 2012;129:1035–41. doi: 10.1542/peds.2011-1082. [DOI] [PubMed] [Google Scholar]
- 3.Friedemann C, Heneghan C, Mahtani K, Thompson M, Perera R, Ward AM. Cardiovascular disease risk in healthy children and its association with body mass index: systematic review and meta-analysis. BMJ. 2012;345:e4759. doi: 10.1136/bmj.e4759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sabin MA, Ford AL, Holly JM, Hunt LP, Crowne EC, Shield JP. Characterisation of morbidity in a UK, hospital based, obesity clinic. Arch Dis Child. 2006;91:126–30. doi: 10.1136/adc.2005.083485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duncan GE, Li SM, Zhou XH. Prevalence and trends of a metabolic syndrome phenotype among U.S. adolescents, 1999–2000. Diabetes Care. 2004;27:2438–43. doi: 10.2337/diacare.27.10.2438. [DOI] [PubMed] [Google Scholar]
- 6.Weiss R, Dziura J, Burgert TS, Tamborlane WV, Taksali SE, Yeckel CW, et al. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med. 2004;350:2362–74. doi: 10.1056/NEJMoa031049. [DOI] [PubMed] [Google Scholar]
- 7.Singh AS, Mulder C, Twisk JW, van Mechelen W, Chinapaw MJ. Tracking of childhood overweight into adulthood: a systematic review of the literature. Obes Rev. 2008;9:474–88. doi: 10.1111/j.1467-789X.2008.00475.x. [DOI] [PubMed] [Google Scholar]
- 8.Juhola J, Magnussen CG, Viikari JS, Kahonen M, Hutri-Kahonen N, Jula A, et al. Tracking of serum lipid levels, blood pressure, and body mass index from childhood to adulthood: the Cardiovascular Risk in Young Finns Study. J Pediatr. 2011;159:584–90. doi: 10.1016/j.jpeds.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 9.Chen X, Wang Y. Tracking of blood pressure from childhood to adulthood: a systematic review and meta-regression analysis. Circulation. 2008;117:3171–80. doi: 10.1161/CIRCULATIONAHA.107.730366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freedman DS, Patel DA, Srinivasan SR, Chen W, Tang R, Bond MG, et al. The contribution of childhood obesity to adult carotid intima-media thickness: the Bogalusa Heart Study. Int J Obes. 2008;32:749–56. doi: 10.1038/sj.ijo.0803798. [DOI] [PubMed] [Google Scholar]
- 11.Baker JL, Olsen LW, Sorensen TI. Childhood body-mass index and the risk of coronary heart disease in adulthood. N Engl J Med. 2007;357:2329–37. doi: 10.1056/NEJMoa072515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morrison JA, Glueck CJ, Wang P. Childhood risk factors predict cardiovascular disease, impaired fasting glucose plus type 2 diabetes mellitus, and high blood pressure 26 years later at a mean age of 38 years: the Princeton-lipid research clinics follow-up study. Metabolism. 2012;61:531–41. doi: 10.1016/j.metabol.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gunnell DJ, Frankel SJ, Nanchahal K, Peters TJ, Davey Smith G. Childhood obesity and adult cardiovascular mortality: a 57-y follow-up study based on the Boyd Orr cohort. Am J Clin Nutr. 1998;67:1111–8. doi: 10.1093/ajcn/67.6.1111. [DOI] [PubMed] [Google Scholar]
- 14.Camhi SM, Katzmarzyk PT. Tracking of cardiometabolic risk factor clustering from childhood to adulthood. Int J Pediatr Obes. 2010;5:122–9. doi: 10.3109/17477160903111763. [DOI] [PubMed] [Google Scholar]
- 15.Juonala M, Magnussen CG, Berenson GS, Venn A, Burns TL, Sabin MA, et al. Childhood adiposity, adult adiposity, and cardiovascular risk factors. N Engl J Med. 2011;365:1876–85. doi: 10.1056/NEJMoa1010112. [DOI] [PubMed] [Google Scholar]
- 16.Olds T, Blunden S, Petkov J, Forchino F. The relationships between sex, age, geography and time in bed in adolescents: a meta-analysis of data from 23 countries. Sleep Med Rev. 2010;14:371–8. doi: 10.1016/j.smrv.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 17.Gradisar M, Gardner G, Dohnt H. Recent worldwide sleep patterns and problems during adolescence: a review and meta-analysis of age, region, and sleep. Sleep Med. 2011;12:110–8. doi: 10.1016/j.sleep.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 18.Gozal D, Kheirandish-Gozal L. Childhood obesity and sleep: relatives, partners, or both? A critical perspective on the evidence. Ann N Y Acad Sci. 2012;1264:135–41. doi: 10.1111/j.1749-6632.2012.06723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lamberg L. Pediatric sleep medicine comes of age. JAMA. 2005;293:2327–9. doi: 10.1001/jama.293.19.2327. [DOI] [PubMed] [Google Scholar]
- 20.Owens JA, Witmans M. Sleep problems. Curr Probl Pediatr Adolesc Health Care. 2004;34:154–79. doi: 10.1016/j.cppeds.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 21.Smaldone A, Honig JC, Byrne MW. Sleepless in America: inadequate sleep and relationships to health and well-being of our nation’s children. Pediatrics. 2007;119(Suppl 1):S29–37. doi: 10.1542/peds.2006-2089F. [DOI] [PubMed] [Google Scholar]
- 22.Must A, Parisi SM. Sedentary behavior and sleep: paradoxical effects in association with childhood obesity. Int J Obes. 2009;33(Suppl 1):S82–6. doi: 10.1038/ijo.2009.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Sleep Foundation. [Accessed November 25, 2012];Sleep in America Poll. 2006 http://www.sleepfoundation.org/sites/default/files/2006_summary_of_findings.pdf.
- 24.Van Cauter E, Spiegel K, Tasali E, Leproult R. Metabolic consequences of sleep and sleep loss. Sleep Med. 2008;9(Suppl 1):S23–8. doi: 10.1016/S1389-9457(08)70013-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knutson KL. Sleep duration and cardiometabolic risk: a review of the epidemiologic evidence. Best Pract Res Clin Endocrinol Metab. 2010;24:731–43. doi: 10.1016/j.beem.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cappuccio FP, Taggart FM, Kandala NB, Currie A, Peile E, Stranges S, et al. Meta-analysis of short sleep duration and obesity in children and adults. Sleep. 2008;31:619–26. doi: 10.1093/sleep/31.5.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen X, Beydoun MA, Wang Y. Is sleep duration associated with childhood obesity? A systematic review and meta-analysis. Obesity. 2008;16:265–74. doi: 10.1038/oby.2007.63. [DOI] [PubMed] [Google Scholar]
- 28.Patel SR, Hu FB. Short sleep duration and weight gain: a systematic review. Obesity. 2008;16:643–53. doi: 10.1038/oby.2007.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marshall NS, Glozier N, Grunstein RR. Is sleep duration related to obesity? A critical review of the epidemiological evidence. Sleep Med Rev. 2008;12:289–98. doi: 10.1016/j.smrv.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 30.Hart CN, Jelalian E. Shortened sleep duration is associated with pediatric overweight. Behav Sleep Med. 2008;6:251–67. doi: 10.1080/15402000802371379. [DOI] [PubMed] [Google Scholar]
- 31.Moore M, Kirchner HL, Drotar D, Johnson N, Rosen C, Redline S. Correlates of adolescent sleep time and variability in sleep time: the role of individual and health related characteristics. Sleep Med. 2011;12:239–45. doi: 10.1016/j.sleep.2010.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spruyt K, Gozal D. The underlying interactome of childhood obesity: the potential role of sleep. Childhood Obes. 2012;8:38–42. doi: 10.1089/chi.2011.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nielsen LS, Danielsen KV, Sorensen TI. Short sleep duration as a possible cause of obesity: critical analysis of the epidemiological evidence. Obes Rev. 2011;12:78–92. doi: 10.1111/j.1467-789X.2010.00724.x. [DOI] [PubMed] [Google Scholar]
- 34.Garaulet M, Ortega FB, Ruiz JR, Rey-Lopez JP, Beghin L, Manios Y, et al. Short sleep duration is associated with increased obesity markers in European adolescents: effect of physical activity and dietary habits. The HELENA study. Int J Obes. 2011;35:1308–17. doi: 10.1038/ijo.2011.149. [DOI] [PubMed] [Google Scholar]
- 35.Bornhorst C, Hense S, Ahrens W, Hebestreit A, Reisch L, Barba G, et al. From sleep duration to childhood obesity—what are the pathways? Eur J Pediatr. 2012;171:1029–38. doi: 10.1007/s00431-011-1670-8. [DOI] [PubMed] [Google Scholar]
- 36.Lumeng JC, Somashekar D, Appugliese D, Kaciroti N, Corwyn RF, Bradley RH. Shorter sleep duration is associated with increased risk for being overweight at ages 9 to 12 years. Pediatrics. 2007;120:1020–9. doi: 10.1542/peds.2006-3295. [DOI] [PubMed] [Google Scholar]
- 37.Lytle LA, Pasch KE, Farbakhsh K. The relationship between sleep and weight in a sample of adolescents. Obesity. 2011;19:324–31. doi: 10.1038/oby.2010.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Calamaro CJ, Park S, Mason TB, Marcus CL, Weaver TE, Pack A, et al. Shortened sleep duration does not predict obesity in adolescents. J Sleep Res. 2010;19:559–66. doi: 10.1111/j.1365-2869.2010.00840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Verhulst SL, Schrauwen N, Haentjens D, Rooman RP, Van Gaal L, De Backer WA, et al. Sleep duration and metabolic dysregulation in overweight children and adolescents. Arch Dis Child. 2008;93:89–90. doi: 10.1136/adc.2007.124768. [DOI] [PubMed] [Google Scholar]
- 40.Hitze B, Bosy-Westphal A, Bielfeldt F, Settler U, Plachta-Danielzik S, Pfeuffer M, et al. Determinants and impact of sleep duration in children and adolescents: data of the Kiel Obesity Prevention Study. Eur J Clin Nutr. 2009;63:739–46. doi: 10.1038/ejcn.2008.41. [DOI] [PubMed] [Google Scholar]
- 41.Matthews KA, Dahl RE, Owens JF, Lee L, Hall M. Sleep duration and insulin resistance in healthy black and white adolescents. Sleep. 2012;35:1353–8. doi: 10.5665/sleep.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maximova K, Kuhle S, Davidson Z, Fung C, Veugelers PJ. Cardiovascular risk factor profiles of normal and overweight children and adolescents: insights from the Canadian Health Measures Survey. Can J Cardiol. 2013;29:976–82. doi: 10.1016/j.cjca.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 43.Woolford SJ, Sallinen BJ, Clark SJ, Freed GL. Results from a clinical multi-disciplinary weight management program. Clin Pediatr. 2011;50:187–91. doi: 10.1177/0009922810384845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.American College of Sports Medicine. ACSM’s guidelines for exercise testing and prescription. 8. Philadelphia (PA): Wolter Kluwer Health, Lippincott Williams & Wilkins; 2010. [Google Scholar]
- 45.Rocchini AP, Katch V, Schork A, Kelch RP. Insulin and blood pressure during weight loss in obese adolescents. Hypertension. 1987;10:267–73. doi: 10.1161/01.hyp.10.3.267. [DOI] [PubMed] [Google Scholar]
- 46.Calabro MA, Welk GJ, Eisenmann JC. Validation of the SenseWear Pro Armband algorithms in children. Med Sci Sports Exerc. 2009;41:1714–20. doi: 10.1249/MSS.0b013e3181a071cf. [DOI] [PubMed] [Google Scholar]
- 47.Catharina B, Gunnevi S, Christel L. Validity of armband measuring energy expenditure in overweight and obese children. Med Sci Sports Exerc. 2010;42:1154–61. doi: 10.1249/MSS.0b013e3181c84091. [DOI] [PubMed] [Google Scholar]
- 48.Calabro MA, Stewart JM, Welk GJ. Validation of pattern-recognition monitors in children using doubly labeled water. Med Sci Sports Exerc. 2013;45:1313–22. doi: 10.1249/MSS.0b013e31828579c3. [DOI] [PubMed] [Google Scholar]
- 49.Soric M, Turkalj M, Kucic D, Marusic I, Plavec D, Misigoj-Durakovic M. Validation of a multi-sensor activity monitor for assessing sleep in children and adolescents. Sleep Med. 2013;14:201–5. doi: 10.1016/j.sleep.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 50.Acebo C, Sadeh A, Seifer R, Tzischinsky O, Wolfson AR, Hafer A, et al. Estimating sleep patterns with activity monitoring in children and adolescents: how many nights are necessary for reliable measures? Sleep. 1999;22:95–103. doi: 10.1093/sleep/22.1.95. [DOI] [PubMed] [Google Scholar]
- 51.Scholle S, Beyer U, Bernhard M, Eichholz S, Erler T, Graness P, et al. Normative values of polysomnographic parameters in childhood and adolescence: quantitative sleep parameters. Sleep Med. 2011;12:542–9. doi: 10.1016/j.sleep.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 52.Cook S, Auinger P, Li C, Ford ES. Metabolic syndrome rates in United States adolescents, from the National Health and Nutrition Examination Survey, 1999–2002. J Pediatr. 2008;152:165–70. doi: 10.1016/j.jpeds.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 53.Eisenmann JC. On the use of a continuous metabolic syndrome score in pediatric research. Cardiovasc Diabetol. 2008;7:17. doi: 10.1186/1475-2840-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Physical Activity Guidelines Advisory Committee. Physical Activity Guidelines Advisory Committee Report, 2008. Washington (DC): U.S. Department of Health and Human Services; 2008. [DOI] [PubMed] [Google Scholar]
- 55.National Sleep Foundation. [Accessed December 2, 2012];How much sleep do we really need? 2012 http://www.sleepfoundation.org/article/how-sleep-works/how-much-sleep-do-we-really-need.
- 56.Eaton DK, Kann L, Kinchen S, Shanklin S, Flint KH, Hawkins J, et al. Youth risk behavior surveillance—United States, 2011. MMWR Surveill Summ. 2012;61:1–162. [PubMed] [Google Scholar]
- 57.Baud MO, Magistretti PJ, Petit JM. Sustained sleep fragmentation affects brain temperature, food intake and glucose tolerance in mice. J Sleep Res. 2013;22:3–12. doi: 10.1111/j.1365-2869.2012.01029.x. [DOI] [PubMed] [Google Scholar]
- 58.Martinez-Gomez D, Eisenmann JC, Gomez-Martinez S, Hill EE, Zapatera B, Veiga OL, et al. Sleep duration and emerging cardiometabolic risk markers in adolescents. The AFINOS study. Sleep Med. 2011;12:997–1002. doi: 10.1016/j.sleep.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 59.Sung V, Beebe DW, Vandyke R, Fenchel MC, Crimmins NA, Kirk S, et al. Does sleep duration predict metabolic risk in obese adolescents attending tertiary services? A cross-sectional study. Sleep. 2011;34:891–8. doi: 10.5665/SLEEP.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Countryman AJ, Saab PG, Llabre MM, Penedo FJ, McCalla JR, Schneiderman N. Cardiometabolic risk in adolescents: associations with physical activity, fitness, and sleep. Ann Behav Med. 2013;45:121–31. doi: 10.1007/s12160-012-9428-8. [DOI] [PubMed] [Google Scholar]