Abstract
Acute and chronic ethanol (EtOH) administration is known to affect function, surface expression, and subunit composition of γ-aminobutyric acid (A) receptors (GABAARs) in different parts of the brain, which is believed to play a major role in alcohol dependence and withdrawal symptoms. The basolateral amygdala (BLA) participates in anxiety-like behaviors including those induced by alcohol withdrawal. In the present study we assessed the changes in cell surface levels of select GABAAR subunits in the BLA of a rat model of alcohol dependence induced by chronic intermittent EtOH (CIE) treatment and long-term (>40 days) withdrawal and investigated the time-course of such changes after a single dose of EtOH (5 g/kg, gavage). We found an early decrease in surface expression of α4 and γ subunits at 1 h following single dose EtOH treatment. At 48 h post-EtOH and after CIE treatment there was an increase in α4 and γ2, while α1, α2, and γ surface expression were decreased. To relate functional changes in GABAARs to changes in their subunit composition we analyzed miniature inhibitory postsynaptic currents (mIPSCs) and the picrotoxin-sensitive tonic current (Itonic) 48 h after EtOH intoxication. The Itonic magnitude and most of the mIPSC kinetic parameters (except faster mIPSC decay) were unchanged at 48 h post-EtOH. At the same time, Itonic potentiation by acute EtOH was greatly reduced, whereas mIPSCs became significantly more sensitive to potentiation by acute EtOH. These results suggest that EtOH intoxication-induced GABAAR plasticity in the BLA might contribute to the diminished sedative/hypnotic and maintained anxiolytic effectiveness of EtOH.
Keywords: Alcohol, Amygdala, Dependence, Synaptic, Tonic, Subunits, GABA, Plasticity, CIE
Introduction
Elevated anxiety and low tolerance to stress are major factors in the development of alcohol dependence. The basolateral nucleus of the amygdala (BLA) mediates emotions such as anxiety and is involved in drug craving and drug-related relapse [1, 2]. γ-Aminobutyric acid type A receptors (GABAARs) have been shown to be important contributors to the regulation of BLA-related anxiety [3–5]. GABAARs are ligand-gated heteropentameric chloride-channels composed of several classes of subunits (α1-6, β1-3, γ1-3, δ, ε, θ, π, and ρ1-3) with usually two α- and β-subunits and one γ or δ subunit associated in one pentamer [6]. The channel kinetics, physiological function and pharmacological sensitivity of GABAARs are largely dictated by their subunit composition [6] and subject to some fine tuning by phosphorylation/dephosphorylation states, e.g., [7]. The most abundant subunits in the BLA are α1, α2, β1-3 and γ2. GABAAR subunits α3, α4, α5, γ3 and δ are also expressed [8]. The major synaptic subunits mediating phasic inhibition in the BLA are α1 and α2 [9], while α3 and α4/δ are extrasynaptic and mediate the tonic GABA current [10, 11].
Acutely, alcohol (ethanol, EtOH) at pharmacologically relevant concentrations (<60 mM) is known to potentiate GABAARs, particularly those composed of the extrasynaptic subunit combinations containing α4 and δ [12, 13], whereas chronic EtOH administration results in decreased GABAAR activity with concomitant changes in their subunit composition in various brain regions such as the cerebral cortex [14–16], hippocampus [16–18], and amygdala [19–21], reviewed in [22].
In the rat BLA, withdrawal from chronic intermittent ethanol treatment (CIE) has previously been reported to affect GABAAR subunit peptide levels including decreases in the α1 and increases in α4 subunit surface proteins and γ2 total protein [19]. These changes are similar to those found in rat hippocampus after CIE treatment [17, 18] and at 2 days after a single intoxicating dose of EtOH (5 g/kg, gavage) [18]. Examining the time course after one-dose ethanol intoxication in rat hippocampal slices and neuronal cultures, there was an early decrease in the surface levels of the α4 and the δ subunit after 1 h. After 2 days the δ subunit was still decreased and also α1 was decreased, while α4 and γ2 membrane expression was increased compared to vehicle-treated controls. All these changes went back to normal after 2 weeks [18, 23].
Different brain regions can exhibit different EtOH-induced plastic changes [16, 24]. Therefore, we studied the surface expression of GABAAR subunits α1, α2 α4, γ2 and δ in rat BLA slices 40 days after CIE treatment. Further-more, we looked at the time-course of these plastic changes 1, 48 h and 2 weeks after one single intoxicating dose of EtOH (5 g/kg, gavage). Since the CIE-induced plasticity was similar to that observed at 48 h after single dose EtOH we obtained whole-cell patch clamp recordings in BLA neurons in slices from rats at 48 h after single dose EtOH or vehicle. We analyzed the picrotoxin-sensitive miniature inhibitory postsynaptic currents (mIPSCs) and tonic current (Itonic) to relate functional changes in GABAARs to GABAAR subunit composition.
Materials and Methods
Animals
All protocols approved by the University of California Institutional Animal Care and Use Committee following NIH guidelines. Sprague–Dawley rats (male, Harlan), 200–350 g, were housed in the vivarium under a light/dark cycle (12 h) and had free access to food and water.
EtOH Administration
Chronic intermittent EtOH (CIE) rats were produced as follows: rats received 5 g/kg, 25 % (w/v) EtOH solution in drinking water (Pharmco Products, Brookfield, CT) for the first five times once every other day and then, for the following 55 doses, 6 g/kg EtOH 30 % (w/v) once every day. This EtOH regimen led rats to experience multiple cycles of intoxication and withdrawal. The control group, chronic intermittent vehicle (CIV) rats, was treated with water (20 ml/kg) in parallel. To study acute EtOH effects rats were administered a single dose of EtOH (5 g/kg) by gavage. Control rats received drinking water (20 ml/kg of body weight; gavage). Rats were euthanized and brain tissue collected at >40 days after CIE/CIV treatment or at 1 h, 48 h, or 2 weeks after acute EtOH.
Tissue Collection
Transverse brain slices (400 μm thick) containing the BLA were obtained using standard techniques. Briefly, rats were decapitated under isoflurane anesthesia, brains were quickly removed, trimmed with a razor blade and glued to the base of a cutting chamber (VT1200S, LeicaMicrosystems Inc., Buffalo Grove, IL) filled with cold (∼4 °C) artificial cerebrospinal fluid (ACSF) composed of 125 mM NaCl, 2.5 mM KCl, 2 mM CaCl2, 2 mM MgCl2, 26 mM NaHCO3, and 10 mM d-glucose (Sigma, USA). The ACSF was continuously bubbled with a 95/5 % mixture of O2/CO2 to ensure adequate oxygenation of slices and a pH of 7.4. Slices containing the BLA were then used for the biochemical determination of surface proteins or for electrophysiological recordings.
Measurement of Surface Expressed Receptor Subunits by Cross-Linking
To measure surface protein expression in slices from in vivo treated rats, cell surface cross-linking experiments followed by Western blot analysis were performed. The BLA was microdissected in ice-cold ACSF from individual slices with the aid of a surgical microscope (Carl Zeiss Surgical GmbH, Oberkochen, Germany) guided by anatomical landmarks as illustrated in the rat brain atlas [25]. The microdissected slices were incubated at 4 °C either in ACSF alone, or ACSF containing 1 mg/ml bis(sulfosuccinimidyl)suberate (BS3) (Pierce) for 45 min. The cell-impermeable BS3 cross-links all surface proteins leading to high molecular weight aggregates which do not enter the gel. Therefore, only internal proteins are detectable in the BS3 sample. The cross-link reaction was quenched washing with 20 mM Tris wash buffer, pH 7.6, and the slices were homogenized in a buffer containing 10 mM Tris, pH 8.0, 1 mM EDTA and 1 % SDS. Protein concentrations were determined using the BCA Protein Assay Kit (Pierce).
SDS-PAGE and Western Blot Analysis
Proteins were separated on SDS-polyacrylamide gels using the Mini-Protean 3 cell system (Bio-Rad), transferred on a polyvinylidene difluoride (PDVF) membrane (Bio-Rad) and blocked with 4 % non-fat dry milk in TTBS. Blots were incubated overnight at 4 °C with the following primary antibodies: anti-α1 (aa328-382), anti-α2 (aa322-357), anti-α4 (aa379-421), anti-γ2 (aa319-366), anti-δ (aa1-44) (all at 1 mg/ml, gift of W. Sieghart), anti-β-actin (Sigma, 1:1,000), or βIII-tubulin (Sigma, 1:1,000), followed by HRP-conjugated secondary antibodies (1:5,000, Rockland). The GABAAR subunit antibodies have been previously extensively verified by various methods including immunoprecipitation, Western blotting and immunocytochemistry [26–28]. Bands from different samples corresponding to the appropriate subunit were analyzed and absorbance values compared by densitometry using ImageQuant 5.2 (Molecular Dynamics) analysis systems. The surface amount of the protein of interest is calculated by the difference between the signal of internal and total protein and normalized to β-actin or βIII-tubulin.
Electrophysiological Recordings
For recordings, individual slices containing the BLA were transferred to a custom-built Plexiglass recording chamber. Patch electrode filling solutions contained: 135 mM cesium gluconate, 2 mM MgCl2, 1 mM CaCl2, 11 mM ethylene glycol-bis (β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA), 10 mM HEPES, 2 mM ATP-K2, 0.2 mM GTP-Na2; pH adjusted to 7.25 with CsOH. Patch electrode and probe assembly targeting the region of interest were advanced using a 4-axis motorized manipulator (MX7600, Siskiyou Corporation, Grants Pass, OR) and controller (MC1000e, Siskiyou Corp.) with the aid of a dissecting microscope (7–45×, SZ61, Olympus). Whole-cell patch clamp recordings were obtained from cells of the BLA region at 34 ± 0.5 °C during perfusion with oxygenated ACSF. Cells were voltage-clamped at 0 mV. Pharmacological separation of GABAAR-mediated currents was done by application of TTX (0.5 μM), CNQX (10 μM), APV (40 μM) and CGP54626 (1 μM) in the ACSF. EtOH (10–60 mM) was applied after appropriate dilution in the ACSF.
Detection and Analysis of Miniature Inhibitory Postsynaptic Currents (mIPSCs) and Tonic Currents
The recordings were low-pass filtered off-line (Clampfit software, Molecular Devices, Sunnyvale, CA) at 2 kHz. The mIPSCs were detected (Mini Analysis Program) with threshold criteria of: 5 pA, amplitude and 20 fC, charge transfer. Frequency of mIPSCs was determined from all automatically detected events in a given 100 s recording period. For kinetic analysis, only single event mIPSCs with a stable baseline, sharp rising phase and exponential decay were chosen during visual inspection of the recording trace. Double and multiple peak mIPSCs were excluded. The mIPSC kinetics were obtained from analysis of the averaged chosen single events (>120 events/100 s recording period) aligned with half rise-time in each cell. Decay time constants were obtained by fitting a double exponential equation to the falling phase of the averaged mIPSCs of the form I(t) = If* exp(−t/τf) +Is* exp(−t/τs), where If and Is are the amplitudes of the fast and slow decay components, and τf and τs are their respective decay time constants used to fit the data. To compare decay times, we used a weighted mean decay time constant: τw = [If/(If + Is)] *tf + [Is/(If + Is)] *ts. The tonic current magnitudes were obtained from the mean baseline current of a given recording period.
Statistical Analysis
The investigators who performed and analyzed the patch clamp recordings and Western blots were blind to the treatment (vehicle, CIE, or single dose EtOH) that the rats received. All values are presented as mean ± SEM. Group differences were evaluated by unpaired Student's t test or ANOVA with post hoc comparisons where appropriate. P < 0.05 was considered statistically significant.
Results
CIE-Induced Changes in GABAAR Subunit Surface Expression in the BLA
To measure the long-lasting changes in the levels of GABAAR subunits in the plasma membrane we compared Western blots of microdissected BLA slices from CIE and CIV rats incubated with or without the membrane-impermeable cross-linking reagent BS3 (see “Materials and Methods” section). We found that the α1 GABAAR subunit surface expression was significantly decreased after CIE treatment and >40 days of withdrawal (concomitantly with total levels, CIV: 100.0 ± 10.4, CIE: 60.3 ± 12.5, n = 5, p = 0.035 unpaired t test). The surface expression of the α4 (without significant changes in total levels, CIV: 100.0 ± 19.3, CIE: 128.8 ± 27.1, n = 7, p = 0.572 unpaired t test) and γ2 (without significant changes in total protein levels; CIV: 100.0 ± 37.9, CIE: 118.4 ± 40.7.1, n = 5, p = 0.749 unpaired t test) subunits was significantly increased (Fig. 1, p < 0.05, unpaired t test). GABAAR δ and α2 subunits were also significantly decreased compared to CIV controls (Fig. 1, p < 0.05 unpaired t test). Their total protein expression was not significantly changed (δ CIV: 100.0 ± 9.9, CIE: 84.9 ± 11.9, n = 4, p = 0.368 unpaired t test; α2 CIV: 100.0 ± 16.0, CIE: 83.0 ± 20.1, n = 5, p = 0.526 unpaired t test).
Fig. 1.
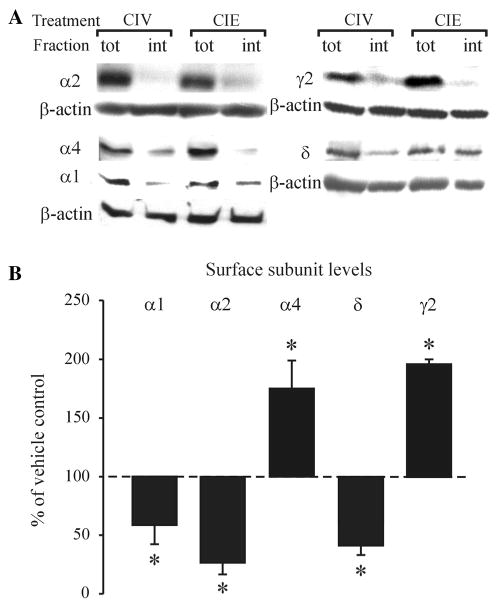
Long-lasting changes in the surface expression of the α1, α2, α4, δ and γ2 GABAAR subunits in the BLA after CIE treatment. a Representative Western blots for α1, α2, α4, &delta, γ2 GABAAR subunits and β-actin after cell surface cross-linking; tot total amount of protein/sample, int intracellular protein/sample. b Bar graph summarizing the subunit alterations after CIE treatment and >40 - days of withdrawal compared to CIV-treated controls, set as 100 % (dashed line). *p < 0.05 (unpaired t test) compared to CIV-treated controls, n = 5–6 rats/group
Time-Dependent Changes in BLA GABAAR Subunit Surface Expression After a Single Intoxicating Dose of EtOH
Previous studies in the rat hippocampus demonstrated that the CIE-induced GABAAR plasticity could be reproduced, albeit transiently, by a single high dose of EtOH [18]. Since function and pharmacological sensitivity of GABAARs are largely dictated by their subunit composition [6], this allowed some insight into the mechanisms of EtOH-induced GABAAR alterations. Applying similar principles to the BLA, we studied the time-course of GABAAR subunit changes at 1 h, 48 h and 2 weeks after administering a single dose of EtOH (5 g/kg; gavage). At 1 h after EtOH dosing the intracellular levels of the a4 subunit significantly increased (Fig. 2a, p < 0.05, unpaired t test), without significant changes in total levels (vehicle: 100.0 ± 30.1, EtOH: 106.6 ± 39.0, n = 5, p = 0.871 unpaired t test), suggesting rapid internalization of GABAARs containing this subunit. However, by 48 h after EtOH α4 subunit surface levels (but not total levels, vehicle: 100.0 ± 22.5, EtOH: 122.6 ± 24.3, n = 5, p = 0.508 unpaired t test) were increased compared to vehicle-treated controls, with return to control levels at 2 weeks following EtOH treatment (Fig. 2). By contrast, there was no detectable change in the α1 subunit surface levels at 1 h after EtOH, but they were significantly decreased after 48 h (without significant decreases in total protein level; vehicle: 100.0 ± 47.1, EtOH: 80.5 ± 48.5, n = 5, p = 0.782 unpaired t test) with return to control levels at 2 weeks following EtOH treatment (Fig. 2). Although we did not examine all three time points, at 48 h post-EtOH the α2 subunit surface levels were also significantly decreased compared to vehicle-treated controls (Fig. 2). No changes in total protein amounts of α2 were detectable (vehicle: 100.0 ± 14.9, EtOH: 113.9 ± 12.8, n = 5, p = 0.498 unpaired t test).
Fig. 2.
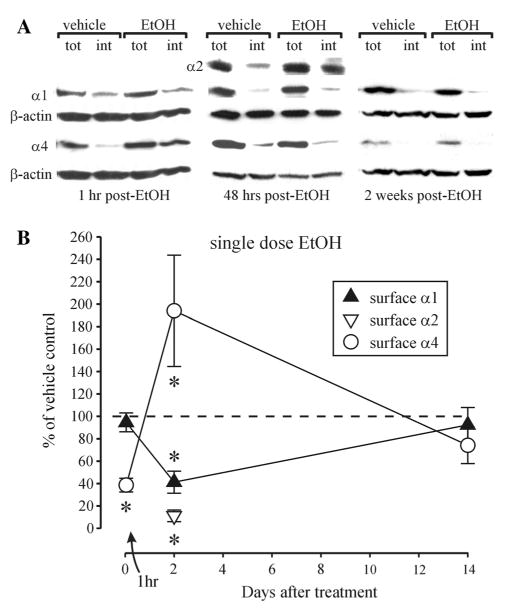
Reversible changes in α1 and α4 GABAAR subunits in the BLA after single-dose EtOH intoxication. a Representative Western blots for α1, α4 and β-actin after cell surface cross-linking at 1 h, 48 h and 2 weeks after single-dose EtOH intoxication. Cell-surface content was also measured for the α2 subunit 48 h after EtOH treatment; tot total amount of protein/sample, int intracellular protein/sample. b Summary graph showing the alterations in cell surface expression of the subunits compared to vehicle-treated controls, set as 100 % (dashed line). *p < 0.05 (unpaired t test) compared to vehicle-treated controls, n = 4–7 rats/group
Estimates of the δ subunit surface levels revealed their significant decreases at 1 h post-EtOH (Fig. 3). Similar to the α4 subunit changes at this time point, the decreases in δ subunit surface levels were associated with an increase in their intracellular fraction, but not in the total levels (vehicle: 100.0 ± 20.0; EtOH: 112.8 ± 36.1, n = 5, p = 0.761 unpaired t test). The δ subunit surface levels remained decreased at 48 h after EtOH, but recovered to control levels at 2 weeks after EtOH. The γ2 subunit surface levels were unchanged 1 h after EtOH, but there was a significant increase at the 48 h time point (without significant changes in the total levels, vehicle: 100.0 ± 16.4, EtOH: 117.9 ± 22.3, n = 6, p = 0.525 unpaired t test), with return to control levels at two weeks following EtOH treatment (Fig. 3).
Fig. 3.
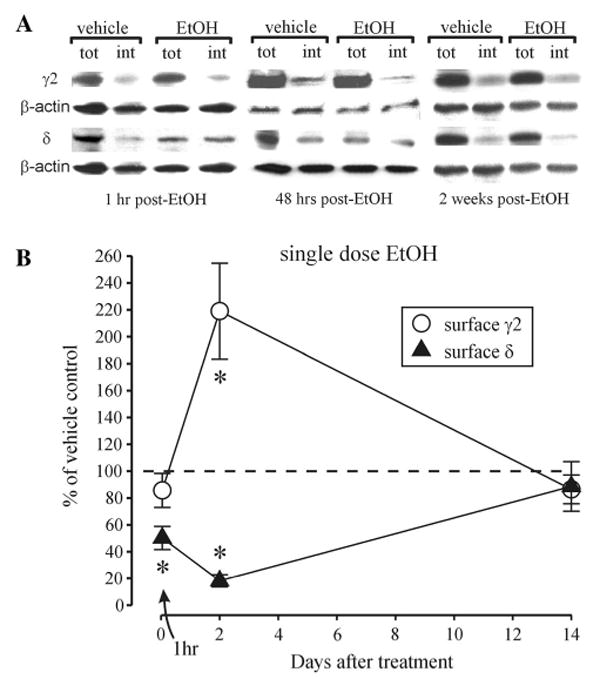
Reversible changes in δ and γ2 in the BLA following single-dose EtOH treatment. a Representative Western blots for δ, γ2 and β-actin GABAAR subunits after cell surface cross-linking at 1 h, 48 h and 2 weeks after single-dose EtOH intoxication; tot total amount of protein/sample, int intracellular protein/sample. b Summary graph showing the alterations in cell surface expression of δ and γ2 subunits at 1 h, 48 h and 2 weeks after single-dose intoxication with EtOH compared to vehicle-treated controls, set as 100 % (dashed line). *p < 0.05 (unpaired t test) compared to vehicle-treated controls, n = 6–7 rats/group
Changes in BLA GABAAR Function After a Single Intoxicating Dose of EtOH
GABAAR currents were recorded during pharmacological blockade of ionotropic glutamate receptors, GABABRs and voltage-gated sodium channels. Under these recording conditions GABAAR currents could be separated into 2 components: a non-desensitizing tonic current (Itonic) and phasic mIPSCs which are known to be mediated by extrasynaptic and synaptic GABAAR activation, respectively [29]. The bulk of mIPSCs detected in recordings from BLA principal neurons were previously shown to arise from local circuit GABAergic interneurons [30]. Since the surface GABAAR subunit changes after CIE treatment were remarkably similar to those observed at 48 h after a single EtOH dose, we concentrated our recordings on this latter time point. At 48 h after single dose EtOH, the picrotoxin-sensitive Itonic magnitude was not significantly altered compared to vehicle-treated rats (Table 1). However there was a trend to decreased mIPSC charge transfer, primarily via speeding up of the mIPSC rise time and significantly faster weighted decay time constant (τw) of mIPSCs (Table 1). Acute application of EtOH (10–60 mM) in BLA recordings from vehicle-treated rats preferentially potentiated the picrotoxin-sensitive Itonic (Fig. 4a). The phasic mIPSCs were not significantly affected by acute EtOH (10–60 mM) in these recordings (Fig. 4a). Recordings at 48 h after EtOH treatment from EtOH-treated rats showed that the mIPSCs were now significantly potentiated by 60 mM EtOH (Fig. 4c), whereas the Itonic was no longer potentiated by EtOH (10-60 mM) (Fig. 4b). The acute EtOH-induced increases in mIPSC charge transfer resulted primarily from the increases in τw and the rise time without changes in mIPSC amplitude (Fig. 4d–g). The frequency of mIPSCs was unaffected by acute EtOH application in either vehicle or EtOH-treated rats (Fig. 4h).
Table 1.
Kinetic properties of mIPSCs in BLA neurons from water-and EtOH (5 g/kg, gavage)-exposed rats. All data are expressed as mean ± SEM
| Post-vehicle (48 h) | Post-EtOH (48 h) | |
|---|---|---|
| Itonic (pA) | 15.8 ± 2.2 | 19.7 ± 5.5 |
| mIPSC area (fC) | 293.2 ± 38.4 | 224 ± 37.1 |
| mIPSC amplitude (pA) | 13.4 ± 2.0 | 14.4 ±2.1 |
| mIPSC weighted decay τ (ms) | 22.8 ± 4.8 | 13.7 ± 1.6* |
| mIPSC rise time (ms) | 2.3 ± 0.4 | 1.6 ± 0.1 |
| mIPSC frequency (Hz) | 3.4 ± 0.6 | 3.2 ± 0.9 |
| n (cells/rats) | 13/10 | 7/4 |
p < 0.05 from post-vehicle (unpaired t test)
Fig. 4.
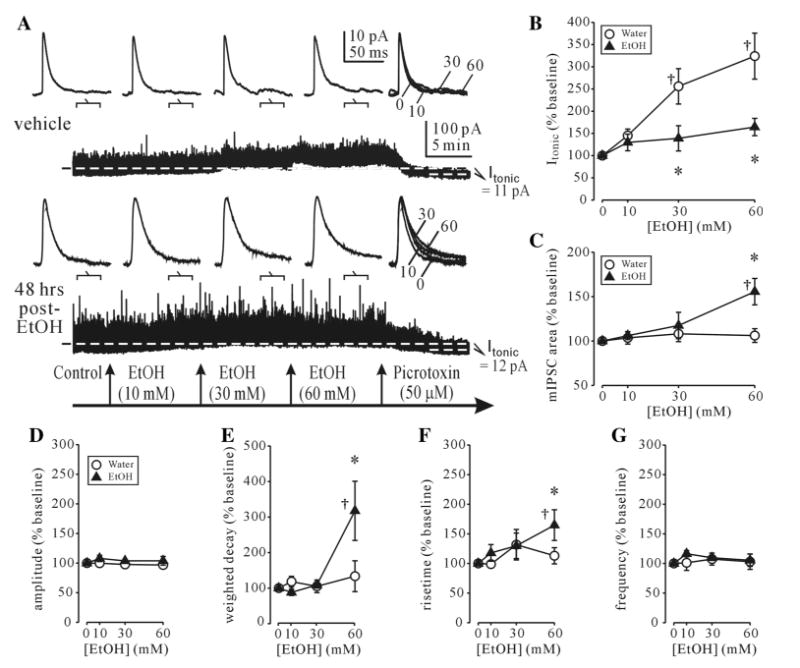
Altered EtOH sensitivity of synaptic and tonic GABAAR currents after single-dose EtOH intoxication. a Examples of individual BLA neuron recordings from vehicle-treated (upper traces) and single dose EtOH-treated rats (lower traces). The holding current (Ihold) needed to clamp the voltage at 0 mV before EtOH application is indicated by a dashed line. In a control recording, the kinetics of mIPSCs (top traces) averaged over the indicated 100 s periods during continuous recordings (lower trace) are unaffected, while Ihold is visibly potentiated by EtOH (10–60 mM). Subsequent application of picrotoxin (50 μM) reveals the GABAAR-mediated tonic current component (Itonic). At 48 h post-EtOH, Itonic (bottom trace) potentiation is reduced and mIPSC potentiation by acute EtOH is increased. b Itonic is significantly potentiated by acute application of EtOH (10–60 mM) from vehicle- but not EtOH-treated rats. c mIPSC area in EtOH-treated but not vehicle-treated rats is significantly potentiated by EtOH (60 mM) application. d-g summary graphs of acute EtOH effects on other kinetic parameters of mIPSCs from vehicle- and single dose EtOH (5 g/kg, gavage)-treated rats. For all panels, each point represents a mean of % baseline ± SEM value from 4-13 neurons (4–9 rats/group). †p < 0.05 from pre-drug; *p < 0.05 from vehicle-treated group (two-way ANOVA with post hoc Bonferroni multiple comparisons)
Discussion
Subunit Changes and Comparison to Other Brain Regions
With the cross-linking assay and Western blot analysis in microdissected BLA slices, we observed decreases in the surface expression of the GABAAR subunits α1 and δ at ≥40 days after CIE treatment. In contrast, α4 and γ2 subunit surface expression was increased. These changes are similar to what has been shown previously after ≥40 days withdrawal from CIE treatment for hippocampal CA1 and dentate gyrus regions [18]. The increase in surface α4- and the decrease in surface α1-containing GABAARs we observed in the BLA at 40 days after CIE is in agreement with what has been previously reported for BLA at 24 h after CIE treatment using vapor chambers [19].
Studying the time-course after a single intoxicating dose of EtOH in the BLA there were early (1 h) decreases in cell-surface α4 and δ subunits. The α4 and δ subunits often co-assemble in one pentamer at extrasynaptic GABAARs which mediate the tonic current and have been suggested to display high EtOH sensitivity [12, 13]. Under control conditions we found that the GABAergic Itonic is enhanced by EtOH in BLA principal neurons. EtOH has been shown to promote binding of the clathrin adapter protein AP2-μ2 to δ-subunits [31], suggesting agonist-induced endocytosis after EtOH intoxication. The δ subunit is still down-regulated 48 h after EtOH. At this point we found that the Itonic is no longer enhanced by EtOH. Like in the hippocampus, the α1 subunit surface expression is decreased 48 h after single-dose EtOH and CIE, and the α4 and γ2 subunits are both up-regulated suggesting less partnering of γ2 with α1, but forming γ2/α4-containing receptors, which have been shown to localize at synaptic sites [32]. In cultured cortical neurons, EtOH-induced increases in α4 and decreases in α1 surface subunits were shown to be dependent on an intricate time-dependent interplay between activities of protein kinase A and different isoforms of protein kinase C at GABAARs [33–36]. The BLA subunit alterations likely account for the faster rise and decay kinetics and the trend towards decreased charge transfer of mIPSCs observed in the present study (Table 1), and similar to the changes observed after CIE and single dose EtOH treatment in hippocampal neurons [18]. Another parallel to the hippocampus is the reversibility of all the plastic changes in GABAAR subunits after a single dose of EtOH, returning to control conditions in the BLA as well, while still present ≥40 d after CIE.
The bulk of the GABAergic postsynaptic currents in the BLA are mediated through α1- and α2-containing GABAARs [9]. We measured α1 and α2 surface levels ≥40 d after CIE and 48 h after single-dose EtOH and observed decreases in both subunits at both time-points. Decreases in BLA α2, α3, and a trend to decreased α1 mRNA expression have been reported in non-human primates after long-term EtOH self-administration [37], consistent with the decreases in benzodiazepine (flunitrazepam) sensitivity in BLA recordings from such animals [38]. In the rat cerebral cortex decreases in α1, α2, and α5 subunit mRNA have been reported after chronic EtOH [39]. In cultured rat hippocampal neurons [40] and cultured cerebellar granule cells [41] increases in α2 mRNA have been demonstrated. This suggests, that not all brain regions have the exact same plasticity, which is also dependent on the animal species, cell-type, neuromodulators, G-proteins, other neurotransmitter systems or co-transmitters or the subunits expressed.
Interestingly, no changes in α2 total protein levels were found in rats after CIE treatment [19]. We also detected no differences in the amount of total α2 protein, but its surface level was significantly decreased. This suggests that even in the absence of changes in expression and production of total α2 protein, EtOH treatment may result in the preferential trafficking of selective subunit combinations of GABAARs to the membrane surface.
Compared to other brain regions the α3 subunit has a notably strong expression in the BLA [42], participating to a major amount in the tonic current [10]. However, this subunit, most likely associated with γ2 is also found at synaptic sites and has been reported to interact with the postsynaptic protein gephyrin at inhibitory synapses [43]. After EtOH treatment there might be compensation not only by α4/γ2 but also by α3/γ2, explaining an increase in surface γ2 after CIE and 48 h after single dose EtOH even though surface α1 and α2 are down-regulated. Since total α3 protein levels were reported unchanged in the BLA after CIE treatment [19], this hypothesized compensation would involve selective increases in surface α3 subunits. Compensatory increases in the surface levels of α4/γ2 and α3/γ2 might also account for the lack of changes in the magnitude of Itonic observed at 48 h post-EtOH in this study.
Possible Behavioral Consequences
GABAARs play a crucial role in the BLA. Blocking GABAARs induces anxious behaviors [4], while enhancing GABAARs attenuates fear and anxiety-like behaviors [3, 44]. The down-regulation of certain GABAARs subtypes we observed in the BLA after EtOH could be a possible cellular substrate of withdrawal-anxiety. Both the α1 and the α2 subunits are down-regulated. Different groups of interneurons signal through different GABAAR subtypes, so that they participate in distinct neurocircuits [9]. For example, the α1 subunit is primarily located at cholecystokinin/cannabinoid 1 receptor-positive synapses, while other GABAergic synapses in the BLA exhibit greater expression of α2 subunit-containing GABAARs [9]. Parvalbumin-expressing interneurons can coordinate and synchronize firing of the principal neurons and may underlie network oscillations in the BLA related to fear [45, 46].
Mice expressing α2 point-mutations in the benzodiazepine binding pocket are resistant to anxiolytic-like effects of benzodiazepines and therefore have been thought to be involved in anxiolysis [47]. A decrease in conditioned fear has been suggested to be correlated with an up-regulation of the α2 subunit in the amygdala [48]. Thus, decreases in the surface α2 subunit in the BLA might contribute to the increased anxiety profile of rats during withdrawal from alcohol intoxication [32] and possibly to the emergence of a heightened response to stress in rats during protracted withdrawal from CIE treatment [49]. Studies with global α1 point-mutated knock-in (diazepam-insensitive) mice have shown that the sedative-like but not anxiolytic actions of benzodiazepines are mediated by the α1 subunit [50] and studies using amygdala-specific knockdown techniques confirmed that the α1 subunit mediates sedative and anticonvulsant, but not anxiolytic properties of benzodiazepines [51]. Thus, decreases in the α1 subunit in the BLA might contribute to the hyperexcitability, decreased seizure threshold [52], and disruption of sleep patterns [53] during withdrawal from alcohol.
Despite clear changes in the relative levels of individual GABAAR subunits, we did not detect any significant changes in the magnitude of Itonic or the kinetics of mIPSCs at 48 h after single EtOH dosing. Others have also reported small increases in mIPSC total charge transfer after withdrawal from CIE treatment [19]. The bulk of mIPSCs detected in recordings from BLA principal neurons arise from input of local circuit GABAergic interneuron rather than the paracapsular interneurons [30]. Taken together, these observations suggest that the increases in anxiety observed during withdrawal from single dose EtOH or CIE treatment might not be mediated by a functional decrement in postsynaptic GABAAR function within the BLA. However, previous studies have also demonstrated that glutamatergic neurotransmission is enhanced in the BLA after withdrawal from CIE treatment [54, 55]. Since the GABA and glutamate systems work in concert to drive BLA-mediated behaviors it is likely that the CIE-induced increases in anxiety behaviors are mediated by the enhanced glutamatergic function [54].
Despite the lack of change in the magnitude of Itonic and in mIPSC kinetics, their sensitivity to acute EtOH application was clearly altered at 48 h after single EtOH dosing. The “switch” in acute EtOH sensitivity from extrasynaptic to synaptic GABAARs was previously demonstrated in hippocampal CA1 pyramidal and dentate granule cells after single dose EtOH and CIE treatment [18, 32]. This sensitivity switch appears to account for the development of tolerance to the sedative/anesthetic effects of EtOH and its maintained anxiolytic effectiveness after CIE treatment [32, 56]. Analogous maintenance of EtOH's anxiolytic effectiveness of may account for the high rates of stress-induced relapse to drinking in alcohol-dependent individuals.
Acknowledgments
We thank Werner Sieghart (Medical University of Vienna, Austria) for the generous gift of GABAAR subunit antibodies. We also thank Erin Wenzel for help with some Western blots.
Contributor Information
A. Kerstin Lindemeyer, Department of Molecular and Medical Pharmacology, David Geffen School of Medicine, University of California, Los Angeles, CA, USA.
Jing Liang, Department of Molecular and Medical Pharmacology, David Geffen School of Medicine, University of California, Los Angeles, CA, USA; Division of Oral Biology and Medicine, School of Dentistry, University of California, 10833 Le Conte Avenue, 63-078 CHS, Los Angeles, CA 90095-1668, USA.
Vincent N. Marty, Division of Oral Biology and Medicine, School of Dentistry, University of California, 10833 Le Conte Avenue, 63-078 CHS, Los Angeles, CA 90095-1668, USA
Edward M. Meyer, Division of Oral Biology and Medicine, School of Dentistry, University of California, 10833 Le Conte Avenue, 63-078 CHS, Los Angeles, CA 90095-1668, USA
Asha Suryanarayanan, Department of Molecular and Medical Pharmacology, David Geffen School of Medicine, University of California, Los Angeles, CA, USA; Division of Oral Biology and Medicine, School of Dentistry, University of California, 10833 Le Conte Avenue, 63-078 CHS, Los Angeles, CA 90095-1668, USA.
Richard W. Olsen, Department of Molecular and Medical Pharmacology, David Geffen School of Medicine, University of California, Los Angeles, CA, USA
Igor Spigelman, Email: igor@ucla.edu, Division of Oral Biology and Medicine, School of Dentistry, University of California, 10833 Le Conte Avenue, 63-078 CHS, Los Angeles, CA 90095-1668, USA.
References
- 1.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.See RE, Fuchs RA, Ledford CC, McLaughlin J. Drug addiction, relapse, and the amygdala. Ann N Y Acad Sci. 2003;985:294–307. doi: 10.1111/j.1749-6632.2003.tb07089.x. [DOI] [PubMed] [Google Scholar]
- 3.Davis M, Rainnie D, Cassell M. Neurotransmission in the rat amygdala related to fear and anxiety. Trends Neurosci. 1994;17:208–214. doi: 10.1016/0166-2236(94)90106-6. [DOI] [PubMed] [Google Scholar]
- 4.Quirk GJ, Gehlert DR. Inhibition of the amygdala: key to pathological states? Ann N Y Acad Sci. 2003;985:263–272. doi: 10.1111/j.1749-6632.2003.tb07087.x. [DOI] [PubMed] [Google Scholar]
- 5.Sanders SK, Shekhar A. Regulation of anxiety by GABAA receptors in the rat amygdala. Pharmacol Biochem Behav. 1995;52:701–706. doi: 10.1016/0091-3057(95)00153-n. [DOI] [PubMed] [Google Scholar]
- 6.Olsen RW, Sieghart W. International Union of Pharmacology. LXX. Subtypes of γ-aminobutyric acidA receptors: classification on the basis of subunit composition, pharmacology, and function. Update. Pharmacol Rev. 2008;60:243–260. doi: 10.1124/pr.108.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Houston CM, He Q, Smart TG. CaMKII phosphorylation of the GABAA receptor: receptor subtype- and synapse-specific modulation. J Physiol. 2009;587:2115–2125. doi: 10.1113/jphysiol.2009.171603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABAA receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101:815–850. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- 9.Marowsky A, Fritschy JM, Vogt KE. Functional mapping of GABA A receptor subtypes in the amygdala. Eur J Neurosci. 2004;20:1281–1289. doi: 10.1111/j.1460-9568.2004.03574.x. [DOI] [PubMed] [Google Scholar]
- 10.Marowsky A, Rudolph U, Fritschy JM, Arand M. Tonic inhibition in principal cells of the amygdala: a central role for alpha3 subunit-containing GABAA receptors. J Neurosci. 2012;32:8611–8619. doi: 10.1523/JNEUROSCI.4404-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Semyanov A, Walker MC, Kullmann DM, Silver RA. Tonically active GABAA receptors: modulating gain and maintaining the tone. Trends Neurosci. 2004;27:262–269. doi: 10.1016/j.tins.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 12.Sundstrom-Poromaa I, Smith DH, Gong QH, Sabado TN, Li X, Light A, Wiedmann M, Williams K, Smith SS. Hormonally regulated α4β2δ GABAA receptors are a target for alcohol. Nat Neurosci. 2002;5:721–722. doi: 10.1038/nn888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wallner M, Hanchar HJ, Olsen RW. Ethanol enhances α4β3δ and α6β3δ γ-aminobutyric acid type A receptors at low concentrations known to affect humans. Proc Natl Acad Sci U S A. 2003;100:15218–15223. doi: 10.1073/pnas.2435171100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Devaud LL, Fritschy JM, Sieghart W, Morrow AL. Bidirectional alterations of GABAA receptor subunit peptide levels in rat cortex during chronic ethanol consumption and withdrawal. J Neurochem. 1997;69:126–130. doi: 10.1046/j.1471-4159.1997.69010126.x. [DOI] [PubMed] [Google Scholar]
- 15.Mhatre MC, Pena G, Sieghart W, Ticku MK. Antibodies specific for GABAA receptor α subunits reveal that chronic alcohol treatment down-regulates α-subunit expression in rat brain regions. J Neurochem. 1993;61:1620–1625. doi: 10.1111/j.1471-4159.1993.tb09795.x. [DOI] [PubMed] [Google Scholar]
- 16.Matthews DB, Devaud LL, Fritschy JM, Sieghart W, Morrow AL. Differential regulation of GABAA receptor gene expression by ethanol in the rat hippocampus versus cerebral cortex. J Neurochem. 1998;70:1160–1166. doi: 10.1046/j.1471-4159.1998.70031160.x. [DOI] [PubMed] [Google Scholar]
- 17.Cagetti E, Liang J, Spigelman I, Olsen RW. Withdrawal from chronic intermittent ethanol treatment changes subunit composition, reduces synaptic function, and decreases behavioral responses to positive allosteric modulators of GABAA receptors. Mol Pharmacol. 2003;63:53–64. doi: 10.1124/mol.63.1.53. [DOI] [PubMed] [Google Scholar]
- 18.Liang J, Suryanarayanan A, Abriam A, Snyder B, Olsen RW, Spigelman I. Mechanisms of reversible GABAA receptor plasticity after ethanol intoxication. J Neurosci. 2007;27:12367–12377. doi: 10.1523/JNEUROSCI.2786-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diaz MR, Christian DT, Anderson NJ, McCool BA. Chronic ethanol and withdrawal differentially modulate lateral/basolateral amygdala paracapsular and local GABAergic synapses. J Pharmacol Exp Ther. 2011;337:162–170. doi: 10.1124/jpet.110.177121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCool BA, Frye GD, Pulido MD, Botting SK. Effects of chronic ethanol consumption on rat GABAA and strychnine-sensitive glycine receptors expressed by lateral/basolateral amygdala neurons. Brain Res. 2003;963:165–177. doi: 10.1016/s0006-8993(02)03966-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Papadeas S, Grobin AC, Morrow AL. Chronic ethanol consumption differentially alters GABAA receptor α1 and α4 subunit peptide expression and GABAA receptor-mediated 36 Cl− uptake in mesocorticolimbic regions of rat brain. Alcohol Clin Exp Res. 2001;25:1270–1275. [PubMed] [Google Scholar]
- 22.Kumar S, Porcu P, Werner DF, Matthews DB, Diaz-Granados JL, Helfand RS, Morrow AL. The role of GABAA receptors in the acute and chronic effects of ethanol: a decade of progress. Psychopharmacology. 2009;205:529–564. doi: 10.1007/s00213-009-1562-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shen Y, Lindemeyer AK, Spigelman I, Sieghart W, Olsen RW, Liang J. Plasticity of GABAA receptors following ethanol pre-exposure in cultured hippocampal neurons. Mol Pharmacol. 2011;79:432–442. doi: 10.1124/mol.110.068650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grobin AC, Fritschy JM, Morrow AL. Chronic ethanol administration alters immunoreactivity for GABAA receptor subunits in rat cortex in a region-specific manner. Alcohol Clin Exp Res. 2000;24:1137–1144. [PubMed] [Google Scholar]
- 25.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; San Diego: 1998. [DOI] [PubMed] [Google Scholar]
- 26.Mossier B, Togel M, Fuchs K, Sieghart W. Immunoaffinity purification of c-aminobutyric acidA (GABAA) receptors containing γ1-subunits. Evidence for the presence of a single type of γ-subunit in GABAA receptors. J Biol Chem. 1994;269:25777–25782. [PubMed] [Google Scholar]
- 27.Zezula J, Fuchs K, Sieghart W. Separation of α1, α2 and α3 subunits of the GABAA-benzodiazepine receptor complex by immunoaffinity chromatography. Brain Res. 1991;563:325–328. doi: 10.1016/0006-8993(91)91556-g. [DOI] [PubMed] [Google Scholar]
- 28.Zimprich F, Zezula J, Sieghart W, Lassmann H. Immu-nohistochemical localization of the α1, α2 and α3 subunit of the GABAA receptor in the rat brain. Neurosci Lett. 1991;127:125–128. doi: 10.1016/0304-3940(91)90910-l. [DOI] [PubMed] [Google Scholar]
- 29.Mody I, Pearce RA. Diversity of inhibitory neurotransmission through GABAA receptors. Trends Neurosci. 2004;27:569–575. doi: 10.1016/j.tins.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 30.Silberman Y, Ariwodola OJ, Weiner JL. Differential effects of GABAB autoreceptor activation on ethanol potentiation of local and lateral paracapsular GABAergic synapses in the rat basolateral amygdala. Neuropharmacol. 2009;56:886–895. doi: 10.1016/j.neuropharm.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gonzalez C, Moss SJ, Olsen RW. Ethanol promotes clathrin adaptor-mediated endocytosis via the intracellular domain of δ-containing GABAA receptors. J Neurosci. 2012;32:17874–17881. doi: 10.1523/JNEUROSCI.2535-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liang J, Zhang N, Cagetti E, Houser CR, Olsen RW, Spigelman I. Chronic intermittent ethanol-induced switch of ethanol actions from extrasynaptic to synaptic hippocampal GABAA receptors. J Neurosci. 2006;26:1749–1758. doi: 10.1523/JNEUROSCI.4702-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carlson SL, Kumar S, Werner DF, Comerford CE, Morrow AL. Ethanol activation of protein kinase A regulates GABAA α1 receptor function and trafficking in cultured cerebral cortical neurons. J Pharmacol Exp Ther. 2013;345:317–325. doi: 10.1124/jpet.112.201954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumar S, Kralic JE, O'Buckley TK, Grobin AC, Morrow AL. Chronic ethanol consumption enhances internalization of α1 subunit-containing GABAA receptors in cerebral cortex. J Neurochem. 2003;86:700–708. doi: 10.1046/j.1471-4159.2003.01894.x. [DOI] [PubMed] [Google Scholar]
- 35.Kumar S, Lane BM, Morrow AL. Differential effects of systemic ethanol administration on protein kinase cε, γ, and β isoform expression, membrane translocation, and target phosphorylation: reversal by chronic ethanol exposure. J Pharmacol Exp Ther. 2006;319:1366–1375. doi: 10.1124/jpet.106.110890. [DOI] [PubMed] [Google Scholar]
- 36.Werner DF, Kumar S, Criswell HE, Suryanarayanan A, Alex FJ, Comerford CE, Morrow AL. PKCγ is required for ethanol-induced increases in GABAA receptor α4 subunit expression in cultured cerebral cortical neurons. J Neurochem. 2011;116:554–563. doi: 10.1111/j.1471-4159.2010.07140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Floyd DW, Friedman DP, Daunais JB, Pierre PJ, Grant KA, McCool BA. Long-term ethanol self-administration by cynomolgus macaques alters the pharmacology and expression of GABAA receptors in basolateral amygdala. J Pharmacol Exp Ther. 2004;311:1071–1079. doi: 10.1124/jpet.104.072025. [DOI] [PubMed] [Google Scholar]
- 38.Anderson NJ, Daunais JB, Friedman DP, Grant KA, McCool BA. Long-term ethanol self-administration by the nonhuman primate, Macaca fascicularis, decreases the benzodiazepine sensitivity of amygdala GABA(A) receptors. Alcohol Clin Exp Res. 2007;31:1061–1070. doi: 10.1111/j.1530-0277.2007.00394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mhatre MC, Ticku MK. Chronic ethanol administration alters gamma-aminobutyric acidA receptor gene expression. Mol Pharmacol. 1992;42:415–422. [PubMed] [Google Scholar]
- 40.Sanna E, Mostallino MC, Busonero F, Talani G, Tranquilli S, Mameli M, Spiga S, Follesa P, Biggio G. Changes in GABAA receptor gene expression associated with selective alterations in receptor function and pharmacology after ethanol withdrawal. J Neurosci. 2003;23:11711–11724. doi: 10.1523/JNEUROSCI.23-37-11711.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Follesa P, Biggio F, Mancuso L, Cabras S, Caria S, Gorini G, Manca A, Orru A, Biggio G. Ethanol withdrawal-induced up-regulation of the α2 subunit of the GABAA receptor and its prevention by diazepam or gamma-hydroxybutyric acid. Brain Res Mol Brain Res. 2004;120:130–137. doi: 10.1016/j.molbrainres.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 42.Fritschy JM, Mohler H. GABAA-receptor heterogeneity in the adult rat brain: differential regional and cellular distribution of seven major subunits. J Comp Neurol. 1995;359:154–194. doi: 10.1002/cne.903590111. [DOI] [PubMed] [Google Scholar]
- 43.Tretter V, Kerschner B, Milenkovic I, Ramsden SL, Ramerstorfer J, Saiepour L, Maric HM, Moss SJ, Schindelin H, Harvey RJ, Sieghart W, Harvey K. Molecular basis of the γ-amino-butyric acid A receptor α3 subunit interaction with the clustering protein gephyrin. J Biol Chem. 2011;286:37702–37711. doi: 10.1074/jbc.M111.291336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sanders SK, Shekhar A. Anxiolytic effects of chlordiazepoxide blocked by injection of GABAA and benzodiazepine receptor antagonists in the region of the anterior basolateral amygdala of rats. Biol Psychiatry. 1995;37:473–476. doi: 10.1016/0006-3223(94)00183-4. [DOI] [PubMed] [Google Scholar]
- 45.Woodruff AR, Sah P. Networks of parvalbumin-positive interneurons in the basolateral amygdala. J Neurosci. 2007;27:553–563. doi: 10.1523/JNEUROSCI.3686-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ryan SJ, Ehrlich DE, Jasnow AM, Daftary S, Madsen TE, Rainnie DG. Spike-timing precision and neuronal synchrony are enhanced by an interaction between synaptic inhibition and membrane oscillations in the amygdala. PLoS ONE. 2012;7:e35320. doi: 10.1371/journal.pone.0035320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Low K, Crestani F, Keist R, Benke D, Brunig I, Benson JA, Fritschy JM, Rulicke T, Bluethmann H, Mohler H, Rudolph U. Molecular and neuronal substrate for the selective attenuation of anxiety. Science. 2000;290:131–134. doi: 10.1126/science.290.5489.131. [DOI] [PubMed] [Google Scholar]
- 48.Heldt SA, Ressler KJ. Training-induced changes in the expression of GABAA-associated genes in the amygdala after the acquisition and extinction of Pavlovian fear. Eur J Neurosci. 2007;26:3631–3644. doi: 10.1111/j.1460-9568.2007.05970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao Y, Weiss F, Zorrilla EP. Remission and resurgence of anxiety-like behavior across protracted withdrawal stages in ethanol-dependent rats. Alcoholism: Clin Exp Res. 2007;31:1505–1515. doi: 10.1111/j.1530-0277.2007.00456.x. [DOI] [PubMed] [Google Scholar]
- 50.Rudolph U, Crestani F, Benke D, Brunig I, Benson JA, Fritschy JM, Martin JR, Bluethmann H, Mohler H. Benzodiazepine actions mediated by specific γ-aminobutyric acidA receptor subtypes. Nature. 1999;401:796–800. doi: 10.1038/44579. [DOI] [PubMed] [Google Scholar]
- 51.Heldt SA, Ressler KJ. Amygdala-specific reduction of α1-GABAA receptors disrupts the anticonvulsant, locomotor, and sedative, but not anxiolytic, effects of benzodiazepines in mice. J Neurosci. 2010;30:7139–7151. doi: 10.1523/JNEUROSCI.0693-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kokka N, Sapp DW, Taylor AM, Olsen RW. The kindling model of alcohol dependence: similar persistent reduction in seizure threshold to pentylenetetrazol in animals receiving chronic ethanol or chronic pentylenetetrazol. Alcohol Clin Exp Res. 1993;17:525–531. doi: 10.1111/j.1530-0277.1993.tb00793.x. [DOI] [PubMed] [Google Scholar]
- 53.Ehlers CL, Slawecki CJ. Effects of chronic ethanol exposure on sleep in rats. Alcohol. 2000;20:173–179. doi: 10.1016/s0741-8329(99)00077-4. [DOI] [PubMed] [Google Scholar]
- 54.Lack AK, Diaz MR, Chappell A, DuBois DW, McCool BA. Chronic ethanol and withdrawal differentially modulate pre- and postsynaptic function at glutamatergic synapses in rat basolateral amygdala. J Neurophysiol. 2007;98:3185–3196. doi: 10.1152/jn.00189.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lack AK, Christian DT, Diaz MR, McCool BA. Chronic ethanol and withdrawal effects on kainate receptor-mediated excitatory neurotransmission in the rat basolateral amygdala. Alcohol. 2009;43:25–33. doi: 10.1016/j.alcohol.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liang J, Spigelman I, Olsen RW. Tolerance to sedative/hypnotic actions of GABAergic drugs correlates with tolerance to potentiation of extrasynaptic tonic currents of alcohol-dependent rats. J Neurophysiol. 2009;102:224–233. doi: 10.1152/jn.90484.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sommer WH, Rimondini R, Hansson AC, Hipskind PA, Gehlert DR, Barr CS, Heilig MA. Upregulation of voluntary alcohol intake, behavioral sensitivity to stress, and amygdala crhr1 expression following a history of dependence. Biol Psychiatry. 2008;63:139–145. doi: 10.1016/j.biopsych.2007.01.010. [DOI] [PubMed] [Google Scholar]


