Abstract
Scrapie in sheep is spread laterally by placental transmission of an infectious misfolded form (PrPSc) of a normal prion protein (PrPC) used as a template in PrPSc formation. We hypothesized that PrPC would be expressed in uterine and placental tissues and estradiol-17B (E2) would affect uterine PrPC expression. PrPC expression was evaluated in the uterus of long-term ovarietomized (OVX) ewes treated with an E2 implant for 2 to 24 h and in uteroplacental tissues from day 20 through 30 of pregnancy. PrPC mRNA and protein were increased in the uterus after E2 treatment of OVX ewes. In the maternal placenta, expression of PrPC mRNA and protein were unchanged, but in the fetal membranes PrPC mRNA and protein expression increased from days 20 through 28. In the nonpregnant uterus, PrPC protein was immunolocalized at apical borders of the surface epithelium, in outer smooth muscle layers of large blood vessels, and in scattered stromal cells of the deep intercaruncular areas of the uterus. In the maternal placenta, PrPC protein was immunolocalized in the cytoplasm of flattened luminal epithelial cells apposed to the fetal membranes, whereas in the fetal membranes PrPC protein was in trophoblast cells and was also in several tissues of the developing embryo during early pregnancy. These data linking estrogen stimulation to increases in PrPC expression in uteroplacental tissues suggest that PrPC has a specific function during the estrous cycle and early pregnancy. Future studies should determine whether or not estrogen influences PrPC expression in other tissues, such as the nervous system and brain.
Introduction
Scrapie is a fatal and incurable neurological disease in sheep and belongs to a family of prion diseases known as transmissible spongiform encephalophies (TSE). Other well-known members of the prion disease family include bovine spongiform encephalophy (BSE), variant Creuzfeldt-Jakob disease (vCJD; the disease associated with BSE transmission to humans), and chronic wasting disease of cervidae (deer, elk, moose, and related forms). Scrapie is believed to be transmitted laterally (from sheep to sheep) via ingestion of the infected placenta at lambing. However, the mechanisms of scrapie transmission are not fully understood nor are the cells responsible for transfer and conversion of the normal prion protein (PrPC) to the abnormal infectious protein (PrPSc) positively identified. Nevertheless, PrPC must be present as it acts as a template for the conversion to PrPSc (Brandner et al. 1996, Colby & Pruisner, 2011). PrPC has previously been found in numerous sheep reproductive tissues and shown to increase during pregnancy in caruncular endometrium (Tuo et al. 2001). PrPC has also been detected in sheep blood (Halliday et al. 2005).
The precise physiological role of PrPC is presently unknown. However, a few of the functions that have been proposed for PrPC include neuroprotection, protection against oxidative stress, promotion of cell signaling cascades, involvement in cell adhesion, a role in cancer processes involving either inhibition or promotion of apoptosis, and a role in progression of Alzheimers disease (Reviewed in Nicholas et al. 2009, Mehrpour & Codogno 2010). Additionally, Alfaidy and others (2013) have reported that PrPC affects key angiogenic processes necessary for establishment of fetomaternal circulation and subsequent growth of the placenta through modulation of copper (Cu) homeostasis in humans. Either overexpression or no expression of PrPC in genetically engineered mice was shown to cause intrauterine growth retardation (IUGR), which is a major complication of pregnancy (Alfaidy et al. 2013). Thus, it seems apparent that PrPC expression plays a role in early placental development, but quantification of PrPC expression during early pregnancy and determination of which cells express PrPC has not been reported.
Estradiol-17B (E2) is a known modulator of the cyclic patterns of uterine growth and tissue remodeling that occurs during the estrous cycle (Reynolds & Redmer 2001, Reynolds et al. 2002). The uterotropic effects of E2 during early pregnancy promote increased blood flow to support embryonic development and placentation (Magness 1998, Reynolds et al. 1998a,b, 2002, 2004, 2010). Besides promoting blood flow, E2 has profound effects on various factors that promote angiogenesis and affect uteroplacental cell proliferation, growth, and differentiation (Reynolds & Redmer 1992, 1995, 2001, Reynolds et al. 1998a,b 2010, Johnson et al. 1997, 2006). Similarly, based on the interaction of PrPC with proteins known to be active in cell signaling pathways, a role for PrPC in promoting cell survival, differentiation, and prevention of apoptosis has been proposed (Nicholas et al. 2009, Mehrpour & Codogno 2010, Rigter et al. 2010). However, whether or not E2 might promote increased expression of PrPC during growth of uterine tissues has not been evaluated.
We have developed a well-tested ovariectomized (OVX) ewe model for examining the effects of E2 on growth and vascularization of endometrial tissues (Johnson et al. 1997, 2006, Reynolds et al. 1998a,b) and we have established another model for studying placental development during early pregnancy (Reynolds & Redmer 1992 1995, Grazul-Bilska et al. 2010 2011). These models are used in this study to examine whether or not PrPC expression is influenced by E2 and is developmentally regulated during early pregnancy. Our hypothesis was that expression of PrPC mRNA and/or protein would increase in a cell specific manner in the uterus after E2 treatment in long-term OVX ewes and would increase in uteroplacental tissues and the developing embryo from day 20 through day 30 after mating during early pregnancy in sheep. Our objectives were: 1) to quantify the effects of E2 treatment on uterine PrPC mRNA and protein expression in OVX ewes, 2) to quantify levels of uteroplacental PrPC mRNA and protein expression from day 20 through 30 of early pregnancy, and 3) to localize PrPC protein in specific endometrial cells after E2 treatment and in placental and embryonic tissues during early pregnancy.
Materials and Methods
Animals and Treatments
For both experiments, all animal use and care was approved by the Institutional Animal Care and Use Committee at NDSU.
Experiment 1
To examine the effects of E2 treatment on PrPC mRNA and protein expression, we used an OVX ewe model that we have previously validated and described in several studies (Johnson et al. 1997, 2006, Reynolds et al. 1998a,b). Briefly, on days 10–12 after estrus, ewes (n=32) of mixed breed were OVX and allowed to recover for at least 30 days before steroid treatment was initiated. Two silicone elastomer implants containing 100 mg of E2 were inserted subcutaneously into each ewe, and the uterus was collected at 0 h (controls), or at 2, 4, 8, 16, or 24 h after receiving the E2 implant (n=4–6 per time point; Johnson et al. 1997, Reynolds et al. 1998a,b). After weighing the intact uterus, caruncular (CAR) and intercaruncular (ICAR) tissues were dissected from the endometrium; one portion was snap-frozen and stored at −70°C for isolation of mRNA and protein, and a second portion was fixed in formalin for immunolocalization of PrPC protein. In the sheep endometrium, the CAR contains numerous blood vessels, but no glands, whereas ICAR contains secretory glands and blood vessels. Therefore these two areas of the endometrium are not only morphologically different, but also functionally different tissues, which is why we collected them separately.
Experiment 2
The animal model for this experiment has been described in detail by Grazul-Bilska et al. (2010, 2011). Briefly, mature, nonpregnant, Western range-type ewes (n = 38) of mixed breeding (predominantly Targhee x Rambouillet) were checked twice daily for behavioral estrus by using vasectomized rams, and were bred at estrus (day 0 = day of estrus) by intact rams. Maternal placenta (CAR), fetal placenta (fetal membranes; FM [corresponding to chorioallantois]), and developing embryos (n = 1–3/ewe) were collected from ewes on days 20, 22, 24, 26, 28, and 30 of pregnancy (n=5/day), and CAR was collected from nonpregnant (NP) ewes (n=5) on day 10 of the estrous cycle (controls). Similar to Experiment 1, a portion of CAR, and FM were snap-frozen and stored at −70°C for isolation of mRNA and protein, and developing embryos (n = 1/ewe) as well as a cross-section of uterus containing placental tissue were fixed in formalin for immunolocalization of PrPC protein.
In both experiments, quantitative real-time RT-PCR (qRT-PCR) was used for analysis of PrPC mRNA expression, immunohistochemistry was used for localization of PrPC protein to specific cell/tissue compartments, and Western analysis was used for quantification of PrPC protein expression.
Quantitative Real-time RT-PCR analysis of PrPC mRNA expression
The procedures for determining the expression of mRNA for PrPC in ovine E2-treated endometrium, uteroplacenta, and embyro by qRT-PCR were reported previously (Johnson et al. 2006, Grazul-Bilska et al. 2010). Briefly, snap-frozen tissues were homogenized in Tri-Reagent (Molecular Research Inc, Cincinnati, OH) according to the manufacturer’s specifications. The quality and quantity of total RNA were determined via capillary electrophoresis using the Agilent 2100 Bioanalyzer (Agilent Technologies, Wilmington, DE). Real-time, quantitative RT-PCR reagents, Taqman probes, and primers were purchased from and used as recommended by Applied Biosystems (Foster City, CA). For each sample, 30 ng of total RNA was reverse transcribed with random hexamers in triplicate 20-μl reactions. PrPC sequence-specific Taqman probe and primer sets were designed from 100% homologous regions in the 5′ end of the sheep prion gene (Forward = 5′-TTTGTGGCCATGTGGAGTGA-3′; Reverse=5′TCCATCCTCCGCC AGGTT-3′; and FAM-labeled MGB probe = 5′-CCTCTGCAAGAAGCGA-3; Accession numbers JX187517 through J187539) using the Primer Express Software from Applied Biosystems (Johnson et al. 2006, Evoniuk et al. 2007). The sequences were validated using NCBI BLAST separately on the primers, probe and entire amplicon.
The ABI PRISM 7000 was used for detection of sequences amplified in a 12.5-μl reaction volume at 60°C for 40 cycles (Applied Biosystems) in triplicate wells. Quantification was determined from a relative standard curve of dilutions of the cDNA generated from tcRNA pooled from placentomes collected on day 130 of pregnancy. To control for variations in the amount of RNA used, individual samples were simultaneously analyzed in a multiplex reaction for concentrations of 18S RNA using the 18S PDAR kit from Applied Biosystems, as we have previously described (Grazul-Bilska et al. 2010).
Western analysis
For CAR, ICAR and FM, protein fractions from TriReagent extractions were used for Western analysis following the manufacturer’s revised protocol. Briefly, proteins were precipitated with 3 volumes of 100% isopropanol, and the pellet was washed 3 times in 0.3 M guanidine hydrochloride in 95% ethanol + 2.5% glycerol (V:V) with a final wash in ethanol + 2.5% glycerol. After briefly drying the pellet, it was solubilized in 1% SDS and quantified with Bradford reagent (Wenrich & Trumbo, 2012). To quantify PrPC expression by Western analysis, 30 μg of PrPC protein was electrophoresed in a 12% SDS-PAGE gel, and transferred to an Immobilon-P membrane (©EMD Millipore Corporation, Billerica, MA) that had been wet in methanol and rinsed 3 times in dH2O. The membrane was blocked in 5% skim milk in PBS containing 0.1% Tween-20 (PBST) for 4 h at room temperature and then incubated overnight at 4°C with primary monoclonal antibody (SAF-32; Cayman Chemical Co.; Ann Arbor, MI) at 1 μg/ml in 5% skim milk containing 0.1% PBST. After rinsing the membrane 3 times for 15 min in PBST, secondary goat anti-mouse antibody (Pierce; Rockford, IL) was added to 5% milk containing 0.1% PBST and the membrane was incubated 1 h at room temperature. The membrane was washed 3 times for 15 min each in PBST, and PrPC protein was quantified using Pierce ECL Western blotting substrate (Thermo Fisher Scientific Inc., Rockford, IL) according to the manufacturer’s instruction and densitometry using the AlphaEase FC Image and Analysis software and the FluroChem FC2 Imaging System from Alpha Innotech (ProteinSimple; Santa Clara, CA).
Immunolocalization of PrPC protein
PrPC was detected in formalin-fixed 4-μm tissue sections (n=4–6 slides/h, Exp. 1; n=5 slides/day, Exp. 2; each slide was from a different ewe) as previously described (Evoniuk et al. 2007). Two 5-min incubations in citrate buffer (10mM, pH 6.0 at 90%) at 100°C were used for antigen retrieval with a 5-min cooling period between incubations, and a final 30-min cooling period before rinsing in dH20. Endogenous peroxidase was blocked for 5 min in 3% H202 and neutralized for 5 min in water and 10 min in PBS containing 0.3% Triton X (EM Science, Darmstadt, Germany). Sections were then transferred to an automated staining system (DAKO Autostainer Universal Staining System, Cupertino, CA) for primary anti-PrPC monoclonal antibody (SAF 32 at 1:150; Cayman Chemical Company, Ann Arbor, MI) staining. Primary antibody was detected with horse anti-mouse secondary antibody conjugated with horseradish peroxidase complex reagents (ImmPress™ kit Vector Laboratories Burlingame, CA) and Silver Grey (SG, Vector Laboratories) as the substrate. Sections were then counterstained with nuclear fast red (Sigma Chemical Co, St. Louis, MO). Control sections were processed identically but with mouse IgG in place of the primary antibody. Images were taken using an Eclipse E600 Nikon microscope and digital camera (Nikon Instruments Inc., Melville, NY, USA) or Zeiss Imager M2 epifluorescence microscope equipped with Zeiss piezo automated stage and AxioCam HRm camera (Carl Zeiss International, Jena, Germany).
Data Analysis
Data were analyzed by using the General Linear Models (GLM) procedure of the Statistical Analysis System (SAS), with time after E2 or day of pregnancy as the main effect and the interaction of time (day) and tissue included in the model (SAS, 2008). Data for real-time detection of PrPC mRNA and Western analysis of PrPC protein in Experiment 1 were converted to fold-increase above controls (0 h) which were set at 1, whereas in Experiment 2 the raw data obtained directly from qRT-PCR or densitometry analyses were statistically analyzed. Differences between specific means were determined using post-hoc analysis with the Least Significance Difference test (Kirk, 1982).
Results
Experiment 1
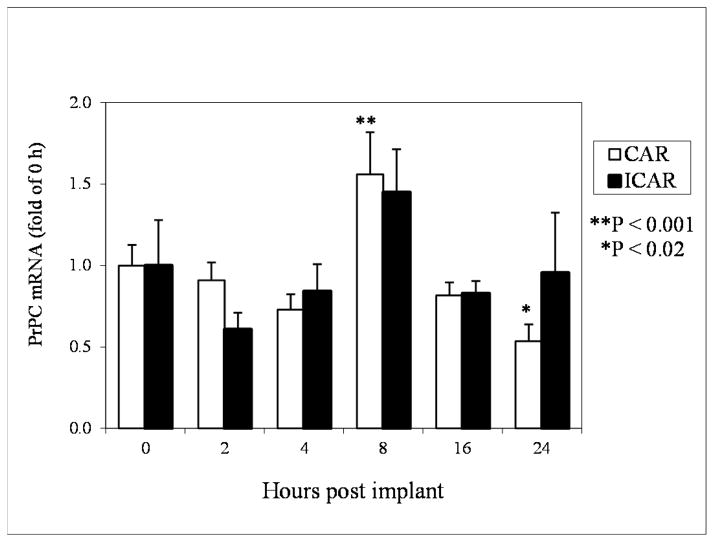
Expression of PrPC mRNA in CAR was increased (P<0.01) by approximately 1.5-fold after 8 h of E2 treatment and then decreased (P<0.02) to half that of controls at 24 h after E2 (Fig. 1). However, in ICAR, PrPC mRNA expression was statistically unchanged from 2 through 24 h after E2 treatment (Fig. 1) even though the pattern of expression was similar to CAR.
Figure 1.
Expression of PrPC mRNA in CAR and ICAR of E2-treated OVX ewes. Data were converted to fold-increase over 0 h (controls), which were set at 1.0. *P < 0.02; **P < 0.001; means ± S.E.M are from real time quantitative RT-PCR. PrPC mRNA increased by 1.5-fold at 8 h after E2 treatment and decreased to half that of 0 h by 24 h after E2 treatment in CAR, but PrPC mRNA expression was unchanged in ICAR.
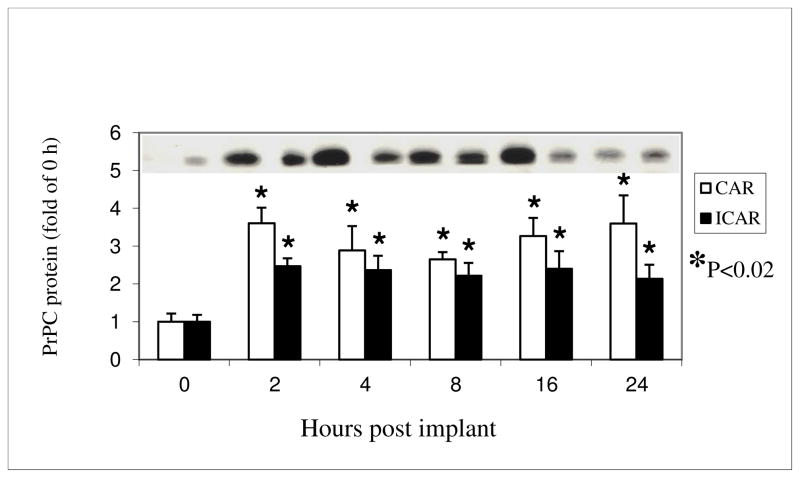
PrPC protein, with a size estimated between 30- and 40-kd was detected by Western immunoblot analysis in both CAR and ICAR tissues (shown at top of Fig. 2). PrPC protein expression was increased (P<0.02) by approximately 2- to 3-fold in both CAR and ICAR, at 2 through 24 h after E2 treatment (Fig. 2).
Figure 2.
Expression of PrPC protein in CAR and ICAR of E2-treated OVX ewes. Data were converted to fold-increase over 0 h (controls), which were set at 1.0. PrPC was present in both CAR and ICAR as a single protein band having a molecular weight between 30 and 45 kd. A representative membrane of the Western blots is shown on the top of the graph. The tissues and samples are shown in the same order as they are on the bar graph below. *P < 0.02; means ± S.E.M are from Western analysis.
Because the patterns of mRNA and protein expression were similar in CAR and ICAR, there was no significant tissue by time interaction for either of them (P>0.1).
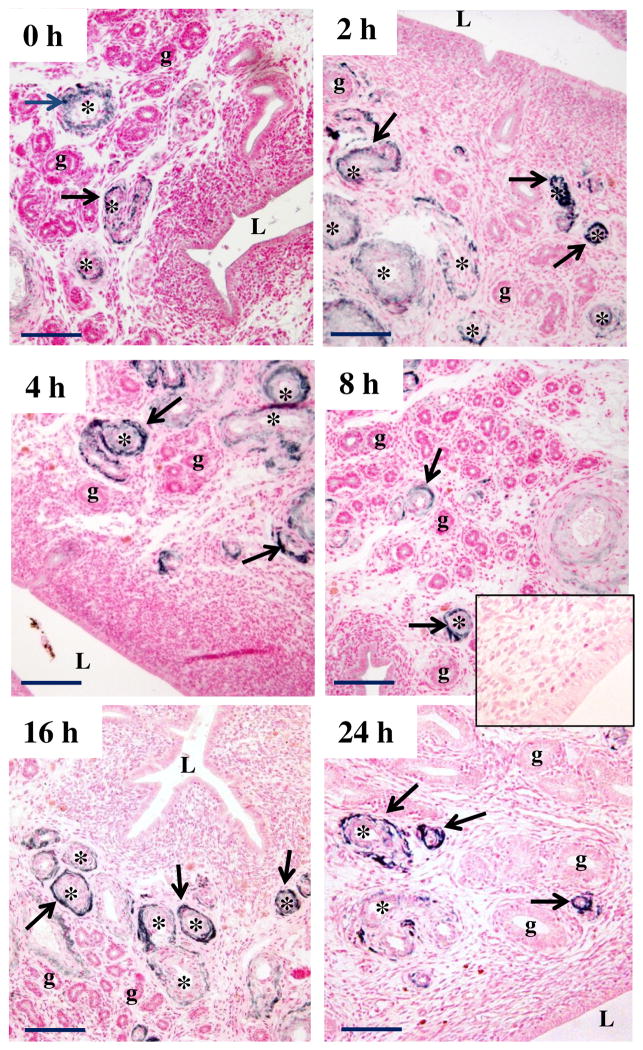
Immunolocalization of PrPC protein in the endometrium of E2-treated OVX ewes revealed that PrPC was predominantly found in arterioles and venules of both CAR and ICAR of all samples (Fig. 3). PrPC was not found in uterine glands. The intense staining for PrPC protein seen in blood vessels was present in cells that appeared to be vascular smooth muscle and adventitial cells, but was not found in the endothelial cell layer of these blood vessels. At 16 to 24 h after E2 treatment, PrPC protein was present sporadically in the outer layers of smooth muscle and adventitia of larger blood vessels, when PrPC protein had disappeared from most of the smaller blood vessels (Fig. 3). PrPC was also found in the myometrium (not shown).
Figure 3.
Representative image of immunolocalization of PrPC protein in endometrium of E2-treated OVX ewes. Arrows indicate PrPC-positive (dark) staining; counterstaining (pink) was with nuclear fast red. Note a lack of positive staining in control (inset), where primary antibody was replaced with mouse IgG. L = lumen of uterus, g = uterine gland, * = blood vessel. Note that PrPC was expressed in arterioles and venules (primarily vascular smooth muscle and adventitia) at 0 to 24 h after E2, but PrPC was not present in glands or luminal epithelium. Size bar = 50 μm.
Experiment 2
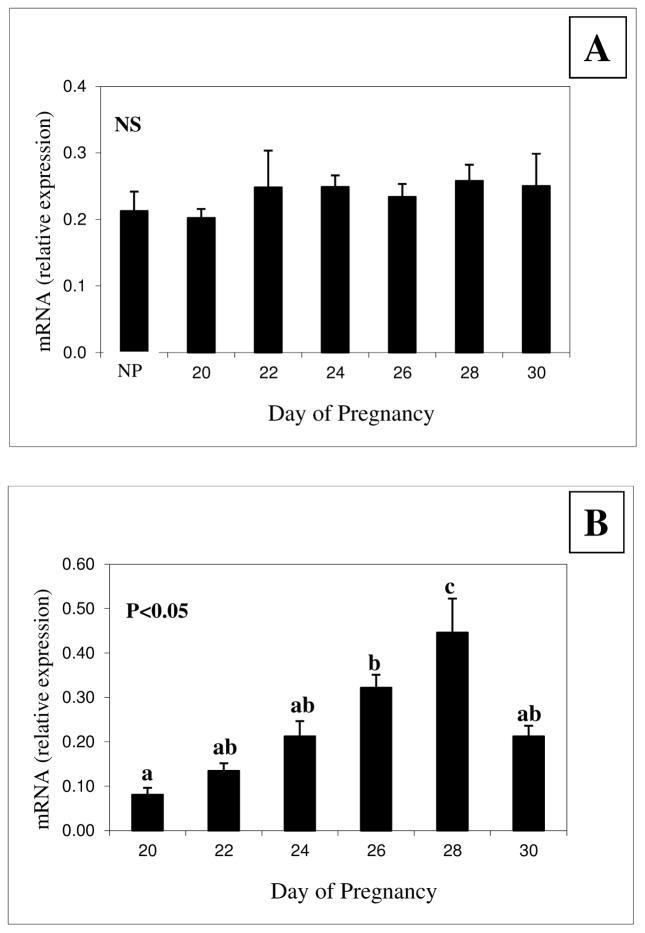
Compared with NP animals, expression of PrPC mRNA in CAR was unchanged across all days of early pregnancy (Fig. 4a). In FM, however, expression of PrPC mRNA increased (P<0.05) from days 20 to 28 of pregnancy and then decreased (P<0.05) on day 30 to the levels present on day 24 (Fig. 4b).
Figure 4.
Expression of PrPC mRNA in (A) caruncle (CAR) and (B) fetal membranes (FM) of ewes during early pregnancy. NS = not significant; a,b,cP means ± S.E.M with different superscripts differ as indicated on the graph. Note that expression of PrPC mRNA was unchanged in CAR, but was increased in FM from days 20 through 28 and on day 30 returned to levels seen on days 22–26.
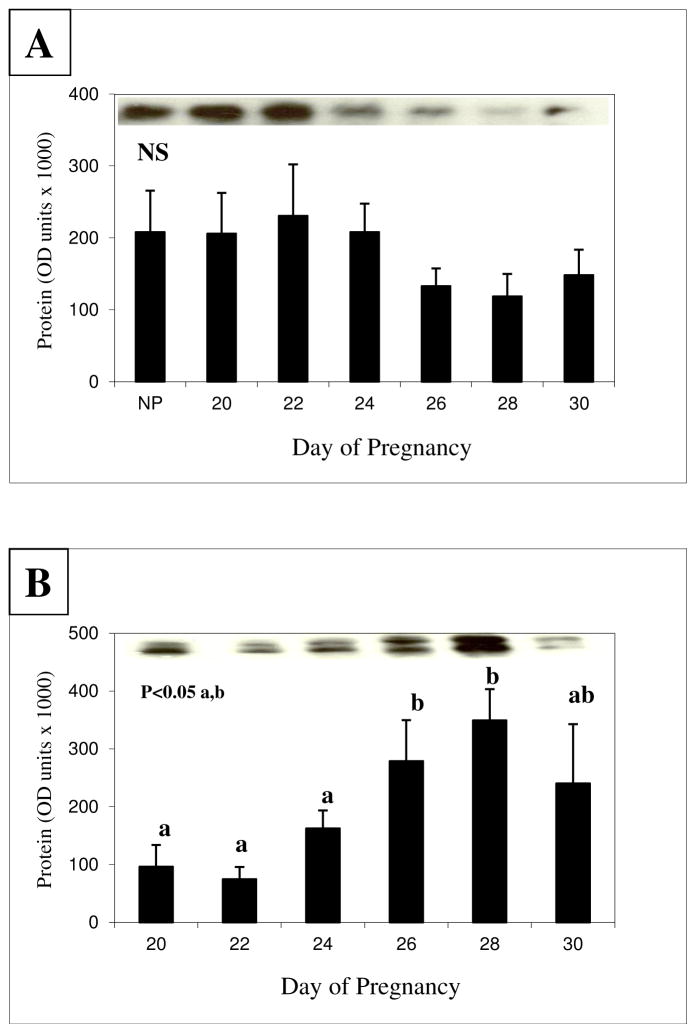
PrPC protein was detected by Western immunoblot analysis at a size estimated between 30 to 45-kd in both CAR and FM in all stages of pregnancy that were evaluated (top of Fig. 5a and 5b). However, PrPC protein was expressed as a single band in CAR and a doublet in FM. Compared with NP animals, expression of PrPC protein in CAR was unchanged across all days of early pregnancy (Fig. 5a). Expression of PrPC protein in FM (Fig. 5b), however, increased (P<0.05) from days 20 to 28 of pregnancy and then decreased (P<0.05) on day 30. Therefore, in CAR neither PrPC mRNA (Fig. 4a) nor PrPC protein (Fig. 5a) expression changed, whereas in FM the pattern of change for PrPC mRNA (Fig. 4b) and PrPC protein (Fig. 5b) expression closely paralleled each other. Thus, there was a significant (P<0.01) tissue by day interaction for PrPC mRNA and protein expression.
Figure 5.
Expression of PrPC protein in caruncle (CAR; A) and fetal membranes (FM; B) during early pregnancy in ewes. A representative membrane of the Western blots is shown on the top of the graph, where the tissues and samples are shown in the same order as they are on the bar graph below. PrPC protein had a molecular weight between 30 and 45 kd. Note that PrPC protein was a single band in CAR, but was expressed as two protein bands in FM. NS = not significant; a,bP means ± S.E.M with different superscripts differ as indicated on the graph. Values are from densitometry of the Western blots. The pattern of change in FM for PrPC mRNA (Fig. 4) and protein expression closely paralleled each other.
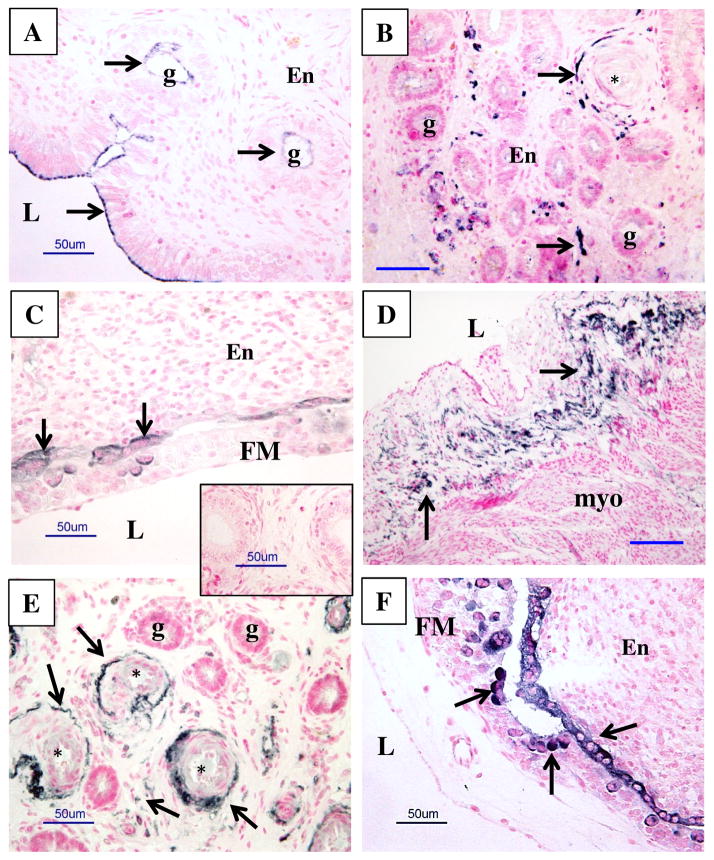
In NP ICAR, PrPC protein was present at the apical borders of the luminal epithelium and that of a few luminal glands (Fig. 6). PrPC protein was also present in the outer smooth muscle and adventitial layers of arterioles and venules and in scattered stromal cells of the deep ICAR of the uterus (Fig. 6).
Figure 6.
Representative images of PrPC protein localization (dark staining) in uterine and uteroplacental tissues of nonpregnant (NP; A and B) ewes and pregnant ewes from days 20–30 after mating (C–F). Note a lack of positive staining in control (inset in C), where primary antibody was replaced with mouse IgG. Arrows indicate some areas of PrPC-positive (dark) staining; the counterstaining (pink) was with nuclear fast red. L = lumen of uterus, g = uterine gland, En = endometrium, FM = fetal membranes (chorioallantois), myo = myometrium, * = blood vessels. Note the presence of PrPC protein in NP at the apical borders of the luminal epithelia and epithelia of luminal glands, larger blood vessels (primarily vascular smooth muscle and adventitia) and in some cells in stromal tissues of endometrium (A and B), and in tissues from pregnancy in luminal epithelia adjacent to the FM, in luminal epithelial cells of maternal caruncle (F; arrow), in bi- and multinucleated cells of the placenta (F; arrowheads), and in larger blood vessels and some cells in stromal tissues (E). Size bars = 50 μm.
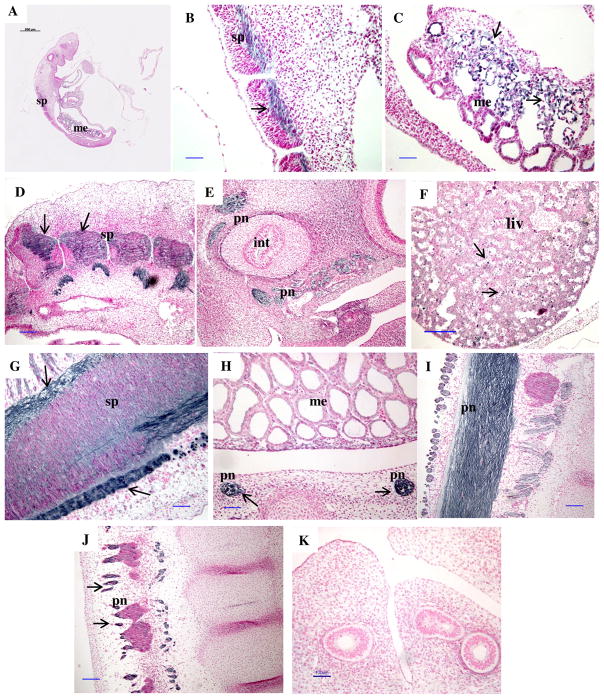
For CAR, from day 20 through 30 of pregnancy, PrPC was seen in the cytoplasm of flattened luminal epithelial cells apposed to the fetal membranes; whereas in FM, PrPC was localized to mononucleate, binucleate, and multinucleate trophoblast cells (Fig. 6). In embryos, PrPC protein was predominantly present in the mesonephros on days 20 through 26 of pregnancy but was also found in the spinal cord and midbrain (Fig. 7). By day 26 of pregnancy, PrPC protein was also found in binucleate cells of the fetal liver. By days 28 through 30, PrPC protein was expressed in fetal mesonephros and in peripheral nerves in areas surrounding the spinal cord and the intestinal tract (Fig. 7).
Figure 7.
Representative images of immunolocalization of PrPC protein in a longitudinal section of an embryo on day 20 (A), and fetal tissues from days 20 (B, C), 22 (D), 24 (E), 26 (F), 28 (G) and 30 (H–J) of early pregnancy. Note a lack of positive staining in control (K) where primary antibody was replaced with mouse IgG. Arrows indicate areas of PrPC positive (dark) staining in contrast to the background (pink) stained with nuclear fast red. sp = spinal cord, pn = peripheral nerves, liv = liver, int = intestine, me = mesonephros. Note the presence of PrPC in the spinal cord (B, D, G), peripheral nerves associated with the intestine and other peripheral nerves (E, H, J, I), liver (F), and mesonephros (C). Size bars = 500 μm on A, 100 μm on B–E and G–K, and 200 μm on F.
Discussion
To our knowledge, this research presents the first data linking levels of expression of PrPC in the sheep uterus to stimulation by the uterotropic effects of E2 and to placentation and uterine growth during early pregnancy in sheep. There are few reports of this phenomenon; however, many suggested functions for PrPC (Nicholas et al. 2009, Mehrpour & Codogno 2010, Rigter et al. 2010) are similar to those known to be associated with E2-stimulated processes, such as cell proliferation and prevention of apoptosis (Johnson et al. 1997, Reynolds et al. 1998a,b). Additionally, our data extend our knowledge of which uteroplacental cells are expressing PrPC and how PrPC expression changes during the early stages of pregnancy, a time when some of the most dramatic developmental processes such as implantation and placentation are occurring under the influence of steroids and other factors (Zheng et al. 1996, Reynolds et al. 1998a,b 2002, 2004, 2010, 2013, Johnson et al. 2006).
We found that E2 up-regulated PrPC mRNA expression in CAR, but not in ICAR, by 8 h after E2 treatment, followed by a decrease at 24 h. At 8 h following E2 treatment, we have shown that cell proliferation dramatically increases in association with maximal expression of mRNA for VEGF and FGF2 (Reynolds et al. 1998a,b). In this study, within 2 h after E2 treatment, PrPC protein expression increased more than two-fold in both the highly vascularized CAR and in the glandular ICAR, a reaction that was maintained through 24 h after E2. The significance of these changes is unclear because the function of PrPC in reproductive tissues is still unknown. Speculation that PrPC functions as a receptor for ligand uptake or transmembrane signaling is based on the fact that PrPC is found on cell surfaces and is recycled through endocytotic pathways, as are numerous other reproductive signaling molecules, including LH, FSH, TGFβ, and IGF (Harris 1999, Forde et al. 2008, Rigter et al. 2010).
Although we cannot explain the discrepancy between mRNA and protein expression in this study, other investigators have reported dynamic regulation of prion protein turnover independent of gene expression or mRNA stability (Choi et al., 2010). Thus, differential regulation of uterine prion protein and mRNA levels seems like a reasonable speculation and a worthwhile topic of future studies.
Our data demonstrate that E2 treatment not only influenced levels of PrPC protein expression in the nonpregnant uterus, but also affected which uterine cells expressed PrPC protein. PrPC was mostly present in small and large blood vessels after E2 treatment. This finding is interesting given the role of E2 in promoting vascularization and increased blood flow to the uterus in preparation for uterine growth, implantation, and placentation (Magness 1998, Reynolds et al. 1998a,b, 2002, 2004, 2010). PrPC was mainly present in the muscular and adventitial layers of the blood vessels. This may point to PrPC having a role in cell signaling cascades promoting the vascular development that is stimulated by E2. Alternatively, it has been suggested that PrPC has a protective function that occurs after injury to blood vessels (Zocche et al. 2011).
Similar to others, we found that PrPC was present in the myometrium of nonpregnant and pregnant ewes but was unaffected by E2 treatment (Tanji et al. 1995, Tuo et al. 2001; Thumdee et al. 2007). The role of PrPC in regulation of myometrial function remains to be determined.
PrPC was found in apical areas of luminal epithelial cells and the epithelia of a few luminal glands in nonpregnant intact ewes used for controls in the early pregnancy study, but was not seen in epithelial cells of the E2-treated OVX ewes. However, PrPC was found in the flattened epithelial cells of the maternal placenta during early pregnancy. These maternal epithelial cells are in close contact with the trophoblast cells of the fetal placenta, where PrPC was also present. PrPC has been found in the apical domain of human intestinal epithelial cells as well (De Keukeleire et al. 2007). Thus, we could speculate that epithelial cells and trophoblast cells might be where PrPC is converted to PrPSc in the placenta. In fact, PrPC has been shown to be overexpressed in multinucleate trophoblast cells, which are the sites where PrPSc accumulates and spreads to uninucleate trophoblast cells (Lacroux et al. 2007).
PrPC mRNA and protein expression have been shown to be greatest in the first trimester of human placenta growth (Donadio et al. 2007). In that study, expression of PrPC mRNA and protein were unchanged in the maternal placenta during early pregnancy, but increased linearly until day 28 in the fetal placenta, similar to our observations in the present study. These data suggest a need for further studies of the functions(s) of PrPC, especially during the rapid growth and differentiation of the fetal placenta and the developing embryo. For example, under the influence of E2, the capillary volume of the placenta increases dramatically after day 18 of pregnancy and becomes increasingly more branched in the fetal placenta during later pregnancy resulting in smaller, more numerous capillaries, whereas in the maternal placenta capillary numbers remain about the same but increase in size to accommodate increased blood flow (Borowicz et al. 2007; Reynolds et al. 2010). Others have reported a role for PrPC in Cu homeostasis and regulation of hypoxic stress that may lead to IUGR (Alfraidy et al. 2012). In fact, during hypoxic conditions, PrPC expression is induced by HIF1-α, which in turn is activated by Cu (Feng et al. 2009; Jeong et al. 2012). Thus, a role for placental PrPC in placental and fetal development seems likely.
It was noteworthy that PrPC protein presented as two bands in the Western analysis of FM, but only one in CAR and the OVX ewe E2-treated endometrium. These differences likely reflect the pattern of PrPC glycosylation but also could be related to the length of the PrPC transcript after its activation by proteolytic cleavage. PrPC can exist in three variable forms, as un-, mono- and di-glycosylated at either of two asparagine residues in its mature length of 208 residues (Cancellotti et al. 2013). Thus, the size of PrPC protein in Western blots has been reported as varying from 23 to 44 kd in sheep reproductive tissues (Horiuchi et al. 1995, Tuo et al. 2001). However, recent reports have suggested that there is a well conserved hydrophobic core containing a site, commonly referred to as the α-cleavage site, where PrPC is cleaved during normal proteolysis into two fragments, C1 (C-terminal) and N1 (N-terminal) (Yusa et al. 2012). These C1 and N1 fragments were detected in Western blots after removal of glycosylation (Yusa et al. 2012, Cancellotti et al. 2013). When proteolysis of PrPC is blocked, it apparently becomes neurotoxic, suggesting that the normal physiological function of PrPC depends on its cleavage into C1 and N1 (Yusa et al. 2012). Therefore, the two bands for PrPC present in the FM may suggest that PrPC has a specific function in the fetal placenta but not in the maternal placenta. The increase in PrPC expression in the fetal placenta from day 20 until day 28 of pregnancy that we report herein also supports this suggestion because it corresponds with rapid growth and vascularization of the placenta (Reynolds & Redmer 1992; Grazul-Bilska et al. 2010, 2011).
Expression of PrPC mRNA and protein in bovine and ovine fetuses and maternal tissues has been evaluated previously, and the recently reported timing and tissue-specific expression of PrPC in bovine embryos closely resembles the results of our study (Thumdee et al. 2007, Peralta et al. 2012). Early sheep pregnancy (gestation = ~145 days) from day 20 through day 30 closely correlates with the intervals reported for bovine early pregnancy (gestation = ~278 days) from day 27 through 39 (Peralta et al. 2012). In this study, PrPC was predominantly present in ovine neural tissues of the embryo in early pregnancy, but was also present in liver and mesonephros, as early as day 20 of pregnancy and was expressed in the same cell-specific manner as in cows. On day 26, PrPC expression in the liver was found in scattered multi-nucleated cells, and these likely were macrophages, similar to the findings in cows (Peralta et al. 2012). The expression of PrPC in neural tissues of the developing embryo suggests that PrPC has a role in neural differentiation (Peralta et al. 2011, 2012). We found PrPC in the spinal cord in day 20 sheep embryos, and PrPC expression in peripheral nerves by days 26 to 30, which also was similar to PrPC expression in cows (Peralta et al. 2012).
Because our research has demonstrated that E2 increased PrPC expression in the uterus, research to evaluate E2 effects on PrPC expression in the brain should be encouraged. In fact, E2 has been recently found to prevent PrPSc infection of healthy cells and to enable PrPC maintenance as a treatment for TSE infection (Molloy & McMahon, 2014). Interesting comparisons are also being made between onset of Alzheimers disease (AD) and actions of PrPC in the brain. The research demonstrates that PrPC may be a receptor for soluble amyloid B (AB) peptide fragments or may serve as an inhibitor of the neurotoxic effects of insoluble AB fragments (Laurén et al. 2009, Nieznanski et al. 2012). Additionally, E2 has been shown to have neuroprotective effects in postmenopausal women including delay of the onset of AD (Tang et al. 1996, Dubal & Wise 2002, Wise et al. 2009, Barron & Pike 2012). Thus, if PrPC is an important factor in neuroprotection against AD, future studies should continue evaluating how E2 affects prion expression in the brain and possibly blocks AD.
In summary, we have presented novel data linking E2 with increases in expression of PrPC in the uterus and have shown that PrPC expression increases in a time-specific manner in the fetal placenta during early pregnancy. Moveover, we have demonstrated localization of PrPC to blood vessels after E2-treatment, suggesting a role for PrPC in the uterotropic effects of E2, including regulation of vascular function and growth. Additionally, we have reported the cell-specific expression of PrPC in epithelia of the uterine endometrium and in trophoblast cells of the FM, and suggest that PrPC may be involved in the adhesion of FM with the endometrium in sheep. These sites of robust PrPC expression in the uterus and placenta are likely those where conversion to PrPSc occurs, and that may provide clues as to how scrapie is spread in sheep during lambing. The novel evidence that E2 increases PrPC expression in uterine tissues may lead to future studies focused on whether E2 increases PrPC expression in the other tissues, and especially brain and nervous system where PrPC may be a key factor in combating AD.
Acknowledgments
Funding
This project was supported by funding from the Animal Disease Research Unit, Animal Research Service, U.S. Department of Agriculture 5348-32000-021-00D, USDA grant (2007-01215) to LPR and ATGB, Hatch Project ND1727, and the INBRE program of the National Center for Research Resources NIH grant number P20RR016741.
The authors acknowledge Dr. Katherine I. O’Rourke, USDA-ARS, Pullman, WA, for funding and leadership in the PrPC studies at North Dakota State University. We acknowledge Dr. Jerzy Bilski, Dr. Pawel Borowicz, Ms. Tammi Neville, Mr. James D. Kirsch, Mr. Kim C. Kraft, Mr. Robert Weigl, Mr. Terry Skunberg and other members of our laboratories and department for their assistance. We also acknowledge the efforts of several of our graduate and undergraduate students in this research project: Roza Yunosova, Mahalakshmi Razdan, Anirudh Gautam, and Mathilde Rupin.
Footnotes
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- Alfaidy N, Chauvet S, Donadio-Andrei S, Salomon A, Saoudi Y, Richaud P, Aude-Garcia C, Hoffmann P, Andrieux A, Moulis JM, et al. Prion protein expression and functional importance in developmental angiogenesis: role in oxidative stress and copper homeostasis. Antioxidants & Redox Signaling. 2013;18:400–11. doi: 10.1089/ars.2012.4637. [DOI] [PubMed] [Google Scholar]
- Barron AM, Pike CJ. Sex hormones, aging, and Alzheimer’s disease. Frontiers in Bioscience (Elite Edition) 2012;4:976–997. doi: 10.2741/e434. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowicz PP, Arnold DR, Johnson ML, Grazul-Bilska AT, Redmer DA, Reynolds LP. Placental growth throughout the last two-thirds of pregnancy in sheep: Vascular development and angiogenic factor expression. Biology of Reproduction. 2007;76:259–67. doi: 10.1095/biolreprod.106.054684. [DOI] [PubMed] [Google Scholar]
- Brandner S, Isenmann S, Raeber A, Fischer M, Sailer A, Kobayashi Y, Marino S, Weissmann C, Aguzzi A. Normal host prion protein necessary for scrapie-induced neurotoxicity. Nature. 1996;379:339–343. doi: 10.1038/379339a0. [DOI] [PubMed] [Google Scholar]
- Cancellotti E, Mahal SP, Somerville R, Diack A, Brown D, Piccardo P, Weissmann C, Manson JC. Post-translational changes to PrP alter transmissible spongiform encephalopathy strain properties. The EMBO Journal. 2013;32:756–69. doi: 10.1038/emboj.2013.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi CJ, Anantharam V, Martin DP, Nicholson EM, Richt JA, Kanthasamy A, Kanthasamy AG. Manganese upregulates cellular prion protein and contributes to altered stabilization and proteolysis: relevance to role of metals in pathogenesis of prion disease. Toxicological Science. 2010;115:535–546. doi: 10.1093/toxsci/kfq049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby DW, Prusiner SB. De novo generation of prion strains. Nature Reviews Microbiology. 2011;11:771–777. doi: 10.1038/nrmicro2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Keukeleire B, Donadio S, Micoud J, Lechardeur D, Benharouga M. Human cellular prion protein hPrPC is sorted to the apical membrane of epithelial cells. Biochem Biochemical and Biophysical Research Communications. 2007;354:949–954. doi: 10.1016/j.bbrc.2007.01.096. [DOI] [PubMed] [Google Scholar]
- Donadio S, Alfaidy N, De Keukeleire B, Micoud J, Feige JJ, Challis JR, Benharouga M. Expression and localization of cellular prion and COMMD1 proteins in human placenta throughout pregnancy. Placenta. 2007;28:907–911. doi: 10.1016/j.placenta.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Dubal DB, Wise PM. Estrogen and neuroprotection: from clinical observations to molecular mechanisms. Dialogues in Clinical Neuroscience. 2002;4:149–161. doi: 10.31887/DCNS.2002.4.2/ddubal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evoniuk JM, Berg PT, Johnson ML, Larson DM, Maddock TD, Stoltenow CL, Schauer CS, O’Rourke KI, Redmer DA. Associations between genotypes at codon 171 and 136 of the prion protein gene and production traits in market lambs. American Journal of Veterinary Research. 2007;68:1073–1078. doi: 10.2460/ajvr.68.10.1073. [DOI] [PubMed] [Google Scholar]
- Feng W, Ye F, Xue W, Zhou Z, Kang YJ. Copper regulation of hypoxia-inducible factor-1 activity. Molecular Pharmacology. 2009;75:174–182. doi: 10.1124/mol.108.051516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forde N, Rogers M, Canty MJ, Lonergan P, Smith GW, Coussens PM, Ireland JJ, Evans AC. Association of the prion protein and its expression with ovarian follicle development in cattle. Molecular Reproduction and Development. 2008;75:243–249. doi: 10.1002/mrd.20807. [DOI] [PubMed] [Google Scholar]
- Grazul-Bilska AT, Borowicz PP, Johnson ML, Minten MA, Bilski JJ, Wroblewski R, Redmer DA, Reynolds LP. Placental development during early pregnancy in sheep: vascular growth and expression of angiogenic factors in maternal placenta. Reproduction. 2010;140:165–174. doi: 10.1530/REP-09-0548. [DOI] [PubMed] [Google Scholar]
- Grazul-Bilska AT, Johnson ML, Borowicz PP, Minten M, Bilski JJ, Wroblewski R, Velimirovich M, Coupe LR, Redmer DA, Reynolds LP. Placental development during early pregnancy in sheep: cell proliferation, global methylation, and angiogenesis in the fetal placenta. Reproduction. 2011;141:529–540. doi: 10.1530/REP-10-0505. [DOI] [PubMed] [Google Scholar]
- Halliday S, Houston F, Hunter N. Expression of PrPC on cellular components of sheep blood. Journal of General Virology. 2005;86:1571–1579. doi: 10.1099/vir.0.80561-0. [DOI] [PubMed] [Google Scholar]
- Harris DA. Cellular biology of prion diseases. Clinical Microbiology Reviews. 1999;12:429–444. doi: 10.1128/cmr.12.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi M, Yamazaki N, Ikeda T, Ishiguro N, Shinagawa M. A cellular form of prion protein (PrPC) exists in many non-neuronal tissues of sheep. Journal of General Virology. 1995;76:2583–2587. doi: 10.1099/0022-1317-76-10-2583. [DOI] [PubMed] [Google Scholar]
- Jeong JK, Seo JS, Moon MH, Lee YJ, Seol JW, Park SY. Hypoxia-inducible factor-1 α regulates prion protein expression to protect against neuron cell damage. Neurobiology of Aging. 2012;33:1006–1026. doi: 10.1016/j.neurobiolaging.2011.09.037. [DOI] [PubMed] [Google Scholar]
- Johnson ML, Grazul-Bilska AT, Redmer DA, Reynolds LP. Effects of estradiol-17beta on expression of mRNA for seven angiogenic factors and their receptors in the endometrium of ovariectomized (OVX) ewes. Endocrine. 2006;30:333–342. doi: 10.1007/s12020-006-0012-5. [DOI] [PubMed] [Google Scholar]
- Johnson ML, Redmer DA, Reynolds LP. Effects of ovarian steroids on uterine growth, morphology, and cell proliferation in ovariectomized, steroid-treated ewes. Biology of Reproduction. 1997;57:588–596. doi: 10.1095/biolreprod57.3.588. [DOI] [PubMed] [Google Scholar]
- Kirk RE. Experimental Design: Procedures for the Behavioral Sciences. 2. Belmont, CA: Brooks/Cole; 1982. [Google Scholar]
- Lacroux C, Corbière F, Tabouret G, Lugan S, Costes P, Mathey J, Delmas JM, Weisbecker JL, Foucras G, Cassard H, et al. Dynamics and genetics of PrPSc placental accumulation in sheep. Journal of General Virology. 2007;88:1056–61. doi: 10.1099/vir.0.82218-0. [DOI] [PubMed] [Google Scholar]
- Laurén J, Gimbel DA, Nygaard HB, Gilbert JW, Strittmatter SM. Cellular prion protein mediates impairment of synaptic plasticity by amyloid-beta oligomers. Nature. 2009;457:1128–1132. doi: 10.1038/nature07761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magness RR, Phernetton TM, Zheng J. Systemic and uterine blood flow distribution during prolonged infusion of 17beta-estradiol. American Journal of Physiology. 1998;275:H731–743. doi: 10.1152/ajpheart.1998.275.3.H731. [DOI] [PubMed] [Google Scholar]
- Mehrpour M, Codogno P. Prion protein: From physiology to cancer biology. Cancer Letters. 2010;290:1–23. doi: 10.1016/j.canlet.2009.07.009. [DOI] [PubMed] [Google Scholar]
- Nicholas O, Gavin R, del Rio JA. New insights into cellular prion protein (PrPC) functions: The “ying and yang” of a relevant protein. Brain Research Reviews. 2009;61:170–184. doi: 10.1016/j.brainresrev.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Peralta OA, Huckle WR, Eyestone WH. Expression and knockdown of cellular prion protein (PrPC) in differentiating mouse embryonic stem cells. Differentiation. 2011;81:68–77. doi: 10.1016/j.diff.2010.09.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peralta OA, Huckle WR, Eyestone WH. Developmental expression of the cellular prion protein (PrP(C) in bovine embryos. Molecular Reproduction and Development. 2012;79:488–498. doi: 10.1002/mrd.22057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds LP, Redmer DA. Angiogenesis in the placenta. Biology of Reproduction 2001. 2001;64:1033–1040. doi: 10.1095/biolreprod64.4.1033. Review. [DOI] [PubMed] [Google Scholar]
- Reynolds LP, Redmer DA. Growth and microvascular development of the uterus during early pregnancy in ewes. Biology of Reproduction. 1992;47:698–708. doi: 10.1095/biolreprod47.5.698. [DOI] [PubMed] [Google Scholar]
- Reynolds LP, Redmer DA. Uteroplacental vascular development and placental function. Journal of Animal Sciences. 1995;73:1839–1851. doi: 10.2527/1995.7361839x. [DOI] [PubMed] [Google Scholar]
- Reynolds LP, Borowicz PP, Caton JS, Vonnahme KA, Luther JS, Buchanan DS, Hafez SA, Grazul-Bilska AT, Redmer DA. Uteroplacental vascular development and placental function: an update. International Journal of Developmental Biology. 2010;54:355–366. doi: 10.1387/ijdb.082799lr. [DOI] [PubMed] [Google Scholar]
- Reynolds LP, Borowicz PP, Vonnahme KA, Johnson ML, Grazul-Bilska AT, Wallace JM, Caton JS, Redmer DA. Animal models of placental angiogenesis. Placenta. 2004;26:689–708. doi: 10.1016/j.placenta.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Reynolds LP, Grazul-Bilska AT, Redmer DA. Angiogenesis in the female reproductive organs: pathological implications. International Journal of Experimental Pathology. 2002;83:151–163. doi: 10.1046/j.1365-2613.2002.00277.x. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds LP, Kirsch JD, Kraft KC, Knutson DL, McClaflin WJ, Redmer DA. Time-course of the uterine response to estradiol-17beta in ovariectomized ewes: uterine growth and microvascular development. Biology of Reproduction. 1998a;59:606–612. doi: 10.1095/biolreprod59.3.606. [DOI] [PubMed] [Google Scholar]
- Reynolds LP, Kirsch JD, Kraft KC, Redmer DA. Time-course of the uterine response to estradiol-17beta in ovariectomized ewes: expression of angiogenic factors. Biology of Reproduction. 1998b;59:613–620. doi: 10.1095/biolreprod59.3.613. [DOI] [PubMed] [Google Scholar]
- Reynolds LP, Vonnahme KA, Lemley CO, Redmer DA, Grazul-Bilska AT, Borowicz PP, Caton JS. Maternal stress and placental vascular function and remodeling. Current Vascular Pharmacology. 2013;11:564–593. doi: 10.2174/1570161111311050003. [DOI] [PubMed] [Google Scholar]
- Rigter A, Langeveld JP, van Zijderveld FG, Bossers A. Prion protein self-interactions: a gateway to novel therapeutic strategies? Vaccine. 2010;28:7810–7823. doi: 10.1016/j.vaccine.2010.09.012. [DOI] [PubMed] [Google Scholar]
- SAS Institute. SAS:User’s Guide, Statistics. 5. Cary, NC: Statistical Analysis System Institute; 2008. [Google Scholar]
- Tang MX, Jacobs D, Stern Y, Marder K, Schofield P, Gurland B, Andrews H, Mayeux R. Effect of oestrogen during menopause on risk and age at onset ofAlzheimer’s disease. Lancet. 1996;348:429–32. doi: 10.1016/S0140-6736(96)03356-9. [DOI] [PubMed] [Google Scholar]
- Tanji K, Saeki K, Matsumoto Y, Takeda M, Hirasawa K, Doi K, Matsumoto Y, Onodera T. Analysis of PrPc mRNA by in situ hybridization in brain, placenta, uterus and testis of rats. Intervirology. 1995;38:309–315. doi: 10.1159/000150457. [DOI] [PubMed] [Google Scholar]
- Thumdee P, Ponsuksili S, Murani E, Nganvongpanit K, Gehrig B, Tesfaye D, Gilles M, Hoelker M, Jennen D, Griese J, et al. Expression of the prion protein gene (PRNP) and cellular prion protein (PrPc) in cattle and sheep fetuses and maternal tissues during pregnancy. Gene Expression Patterns. 2007;13:283–297. doi: 10.3727/000000006780666984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuo W, Zhuang D, Knowles DP, Cheevers WP, Sy MS, O’Rourke KI. Prp-c and Prp-Sc at the fetal-maternal interface. Journal of Biological Chemistry. 2001;276:18229–18234. doi: 10.1074/jbc.M008887200. [DOI] [PubMed] [Google Scholar]
- Wenrich BR, Trumbo TA. Interaction of nucleic acids with Coomassie Blue G-250 in the Bradford assay. Analytical Biochemistry 2012. 2012;428:93–95. doi: 10.1016/j.ab.2012.06.014. [DOI] [PubMed] [Google Scholar]
- Wise PM, Suzuki S, Brown CM. Estradiol: a hormone with diverse and contradictory neuroprotective actions. Dialogues in Clinical Neuroscience. 2009;11:297–303. doi: 10.31887/DCNS.2009.11.3/pmwise. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusa S, Oliveira-Martins JB, Sugita-Konishi Y, Kikuchi Y. Cellular prion protein: from physiology to pathology. Viruses. 2012;4:3109–3131. doi: 10.3390/v4113109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Johnson ML, Redmer DA, Reynolds LP. Estrogen and progesterone receptors, cell proliferation, and c-fos expression in the ovine uterus during early pregnancy. Endocrinology. 1996;137:340–348. doi: 10.1210/endo.137.1.8536633. [DOI] [PubMed] [Google Scholar]
- Zocche Soprana H, Canes Souza L, Debbas V, Martins Laurindo FR. Cellular prion protein (PrP(C)) and superoxide dismutase (SOD) in vascular cells under oxidative stress. Experimental and Toxicologic Pathology. 2011;63:229–236. doi: 10.1016/j.etp.2009.12.004. [DOI] [PubMed] [Google Scholar]