Abstract
The organization of lin genes and IS6100 was studied in three strains of Sphingomonas paucimobilis (B90A, Sp+, and UT26) which degraded hexachlorocyclohexane (HCH) isomers but which had been isolated at different geographical locations. DNA-DNA hybridization data revealed that most of the lin genes in these strains were associated with IS6100, an insertion sequence classified in the IS6 family and initially found in Mycobacterium fortuitum. Eleven, six, and five copies of IS6100 were detected in B90A, Sp+, and UT26, respectively. IS6100 elements in B90A were sequenced from five, one, and one regions of the genomes of B90A, Sp+, and UT26, respectively, and were found to be identical. DNA-DNA hybridization and DNA sequencing of cosmid clones also revealed that S. paucimobilis B90A contains three and two copies of linX and linA, respectively, compared to only one copy of these genes in strains Sp+ and UT26. Although the copy number and the sequence of the remaining genes of the HCH degradative pathway (linB, linC, linD, and linE) were nearly the same in all strains, there were striking differences in the organization of the linA genes as a result of replacement of portions of DNA sequences by IS6100, which gave them a strange mosaic configuration. Spontaneous deletion of linD and linE from B90A and of linA from Sp+ occurred and was associated either with deletion of a copy of IS6100 or changes in IS6100 profiles. The evidence gathered in this study, coupled with the observation that the G+C contents of the linA genes are lower than that of the remaining DNA sequence of S. paucimobilis, strongly suggests that all these strains acquired the linA gene through horizontal gene transfer mediated by IS6100. The association of IS6100 with the rest of the lin genes further suggests that IS6100 played a role in shaping the current lin gene organization.
Hexachlorocyclohexane (HCH) was introduced for the control of agricultural pests and of vector-borne diseases in early 1940s. While this compound was used extensively all over the world, several reports on the persistence of HCH isomers (α, β, γ, and δ) and their toxic effects on nontarget organisms appeared in the 1980s (11). These reports finally resulted in a ban on or restricted use of HCH in most countries. Neither the ban nor the restricted use has, however, reduced the levels of HCH residues in the environment (6, 25, 33), especially in soils that had a previous history of HCH application (2). One serious problem is the uptake of HCH residues from soil by crops, which then enter food products (1, 31). In addition to no further use of HCH, a decontamination program for HCH-polluted soils would diminish the risk posed by HCH residues to human, plant, and animal health. One possibility for decontamination is spontaneous or induced microbial degradation. Unfortunately, spontaneous microbial degradation of HCH isomers proceeds rather slowly (10, 15), although a number of bacteria which can degrade one or more isomers of HCH have been isolated. Thus, addition of naturally occurring microbes to contaminated soils could provide an alternative strategy. Such application could be assisted to a great extent by exploring the potential of such isolates, particularly in order to understand the physiology and genetics of HCH degradation in these strains.
As far as is known, aerobic degradation of HCH is carried out mostly by strains of Sphingomonas paucimobilis and Rhodanobacter lindaniclasticus. HCH-degrading strains have been isolated in different parts of the world; S. paucimobilis SS86 has been isolated in Japan (32, 38), S. paucimobilis B90A has been isolated in India (4, 29), and an R. lindaniclasticus strain has been isolated in France (24, 37). These three strains are remarkably similar and can all degrade α-, γ-, and δ-HCH (9, 14, 29, 37). In addition, S. paucimobilis B90A can also partially degrade β-HCH (10, 14, 29). The metabolic pathway and the genes involved in HCH degradation have been studied in great detail in S. paucimobilis strain UT26 (a mutant of SS86 resistant to nalidixic acid) and to a lesser extent in B90 (Fig. 1). The primary enzyme in γ-HCH degradation is HCH dehydrochlorinase encoded by the linA gene (9, 23). The remaining genes of the γ-HCH degradative pathway are linB (23), linC (20), linD (18), and linE (17, 19), which encode a halidohydrolase, a dehydrogenase, a reductive dechlorinase, and a dioxygenase, respectively. In addition, the linX gene, encoding a protein that has activity similar to that of LinC, was also cloned and characterized (20). A linA gene was also cloned and sequenced from R. lindaniclasticus (37) and was found to be identical to linA from strain UT26 (9) except for one 3-bp insertion, but the remaining genes of the HCH degradative pathway in this strain have still not been described (37). More recently, the lin genes of S. paucimobilis strain B90 were cloned and characterized (14, 21). In contrast to UT26 and R. lindaniclasticus, strain B90 contains two copies of linA, designated linA1 and linA2 (Fig. 1). Both copies of linA produce a functional HCH dehydrochlorinase when they are cloned in Escherichia coli. The amino acid sequences of the products encoded by the linA1 and linA2 genes are 92% identical to each other and 88% (LinA1) and 99% (LinA2) identical to the sequence of LinA of strain UT26 (9). The linB, linC, and linX genes were also cloned from strain B90 and were found to be 99% identical to the corresponding genes of S. paucimobilis UT26 (14). A closer look at the two copies of linA in strain B90 suggested that one of them contains an insertion of an IS element at the 3′ end. This results in a 22-bp difference at the 3′ end between linA1 (462 bp) and linA2 (468 bp). The inserted region exhibits complete sequence identity to IS6100 of Mycobacterium fortuitum (16). In contrast to strain UT26, no linD, linE, and linR genes could be detected by PCR amplification and Southern hybridization in strain B90 (14).
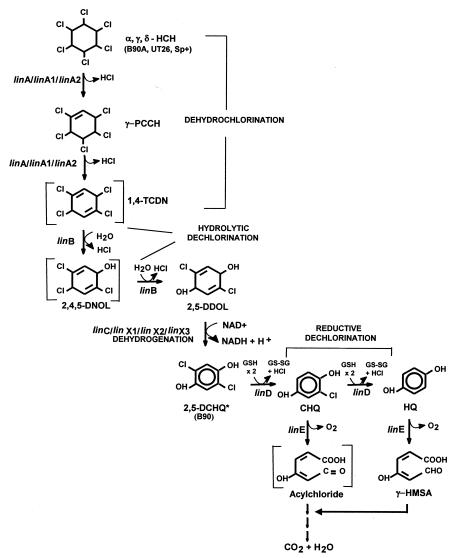
FIG. 1.
Pathway for degradation of HCH isomers in S. paucimobilis strains (data adapted from references 14 and 20). γ-PCCH, gamma-pentachlorocyclohexene; 1,4-TCDN, 1,3,4,6-tetrachloro-1,4-cyclohexadiene; 1,2,4-TCB, 1,2,4 trichlorobenzene; 2,4,5-DNOL, 2,4,5-trichloro-2,5-cyclohexadiene-1-ol; DCP, 2,5-dichlorophenol; 2,5-DDOL, 2,5-dichloro-2,5-cyclohexadiene-1,4-diol; 2,5-DCHQ, 2,5-dichlorohydroquinone; 2-CHQ, 2-chlorohydroquinone; HQ, hydroquinone; γ-HMSA, gamma-hydroxymuconic semialdehyde. The asterisk indicates that in B90 the degradation of α-, γ-, and δ-HCH isomers stops at the level of 2,5-dichlorohydroquinone.
Here we report on the mosaic character of the lin genes in the different S. paucimobilis strains and the close association of the IS6100 element with these genes. By using Southern hybridization, cosmid cloning, and DNA sequencing of strain B90 and an older lab stock of strain B90 (designated B90A) and by comparing these strains with strains UT26 and Sp+, we discovered that multiple copies of IS6100 are present in all of the strains, but they are in different configurations. The consequences of the activity of IS6100 for the stability and distribution of the lin genes among these strains are discussed below.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
Table 1 shows the bacterial strains and plasmids used in this study. S. paucimobilis B90A was obtained from N. Sethunathan (Central Rice Research Institute, Cuttuck, India) in 1992. S. paucimobilis B90, which lacks linD, linE, and linR, is a spontaneous mutant derived from S. paucimobilis B90A (14). S. paucimobilis UT26 and Sp+ were obtained from Y. Nagata (University of Tokyo, Yayoi, Bunkyo-ku, Tokyo, Japan) and Tim Vogel (University of Lyon, Lyon, France), respectively. The non-HCH-degrading strains S. paucimobilis ATCC 29837T and Sphingomonas chlorophenolica DSM 7098T were also used for detecting any IS6100 elements. All of the S. paucimobilis strains and S. chlorophenolica were grown in Luria broth (30) or in mineral salts (SM) medium containing glucose (1%, wt/vol) as described previously (14). E. coli strains were grown in Luria broth at 37°C. Antibiotics, when required, were added to a final concentration of 150 μg/ml (ampicillin) or 50 μg/ml (kanamycin).
TABLE 1.
Bacterial strains and plasmids used
| Strain or plasmid | Description | Source |
|---|---|---|
| E. coli DH5α | F−endA1 hsdR17 supE44 thi-1 recA1 gyrA relA1 Δ(lacZYA-argF)U169 deoR | Lab stock |
| E. coli JM101 | supE thiΔ(lac-proAB) F′[traD36 proAB+lacIqlacZΔM15] | Lab stock |
| S. paucimobilis B90A | Aerobic, nonmotile, rod shaped, degrades all four isomers of HCH (α, β, γ, and δ) | N. Sethunathan, CRRI, Cuttack, India |
| S. paucimobilis B90 | Mutant of S. paucimobilis B90A lacking linD, linE, and linR | Lab stock |
| S. paucimobilis UT26 | Aerobic, nonmotile, rod shaped, degrades α-, γ-, and δ-HCH but not β-HCH | Y. Nagata, University of Tokyo, Tokyo, Japan |
| S. paucimobilis Sp+ | Aerobic, nonmotile, rod shaped, degrades α-, γ-, and δ-HCH but not β-HCH | Tim Vogel, University of Lyon, Lyon, France |
| S. paucimobilis ATCC 29837T | Aerobic, nonmotile, rod shaped, yellow pigmented | M. Hanspal, St. Elizabeth's Medical Center of Boston, Boston, Mass. |
| S. chlorophenolica DSM 7098T | Aerobic, nonsporulating rods, yellow pigmented, degrades pentachlorophenol, and 2,4,6-trichlorophenol | J. Cullum, University of Kaiserslautern, Kaiserslautern, Germany |
| pUC 13/19 | 2.7-kb; Ampr; multiple cloning site internal to lacZ gene | Lab stock |
| pWE15 | 8.2-kb cosmid vector; Ampr; bacteriophage promoter sequences T3 and T7 flanking unique cloning site | Stratagene |
| pLINA57 | pWE15 carrying 41-kb DNA fragment of B90A containing linA1, linC, linX, and IS6100 | This study |
| pLIND33 | pWE15 carrying 5.6-kb DNA fragment of B90A containing linD, linE, linR, and IS6100 | This study |
| pLIND22 | Subclone of pLIND33 in pWE15 (bp 1869 to 6700 of the contiguous ∼8-kb sequence of pLIND33) | This study |
| pLIND22-12 | Subclone of pLIND22 in pUC18 (bp 6701 to 7803 of the contiguous ∼8-kb sequence of pLIND33) | This study |
| pLIND3R24 | Subclone of pLIND33 in pUC18 (bp 1 to 1868 of the contiguous ∼8-kb sequence of pLIND33) | This study |
| pLINB35 | pWE15 carrying DNA fragment of B90A containing linB and IS6100 | This study |
| pLINB23 | Subclone of pLINB35 in pUC18 containing 2.2-kb fragment of pLINB35 | This study |
| pLINUTIS | pUC 18 containing BamHI-digested 3.5-kb fragment of S. paucimobilis UT26 that hybridized with IS6100 | This study |
| pLINSpIS | pUC18 containing HindIII-digested 2.2-kb Sp+ fragment that hybridized with IS6100 | This study |
DNA isolation and hybridization.
Genomic DNA was isolated from S. paucimobilis strains and S. chlorophenolica by using previously described procedures (14). Plasmids were isolated from E. coli by using standard protocols (30). For DNA hybridization, genomic DNA of S. paucimobilis was digested with suitable restriction enzymes and subjected to gel electrophoresis. Separated DNA fragments were transferred from agarose gels to Hybond-N nylon membranes (Pharmacia Amersham Biotech) and probed with [α-32P]dATP (BRIT, Hyderabad, India)-labeled DNA fragments. DNA probes were prepared from plasmid DNA either by restriction enzyme digestion followed by elution of the appropriate DNA fragments from an agarose gel or by PCR amplification. The primers used for amplification of different lin gene fragments, IS6100, and tnpA were based on DNA sequences of the corresponding genes in the GenBank database and can be supplied on request. PCR amplification was performed with a Neugen gene thermocycler (Techne Progeny, Cambridge, United Kingdom) or a Robocycler (Stratagene) by using standard protocols. Hybridization was performed at 68°C. After hybridization, the membranes were washed twice in a solution containing 2× SSC (1× SSC is 150 mM NaCl plus 15 mM sodium citrate, pH 7.0) plus 0.1% sodium dodecyl sulfate at 68°C and once in a solution containing 1× SSC plus 0.1% sodium dodecyl sulfate at room temperature. The membranes were then exposed to X-ray film (Kodak India, Mumbai, India).
Construction and screening of the genomic library of S. paucimobilis B90A.
A genomic library of S. paucimobilis B90A was constructed as described previously for strain B90 (14) in cosmid vector pWE15 (Stratagene). This library was screened specifically for the presence of clones in which lin genes were associated with IS6100. Screening of the library was carried out by using [α-32P]dATP-labeled linA, linB, linC, linD, linE, linX, and IS6100 probes separately. The DNA probes for lin genes were prepared by PCR amplification by using primers designed for amplification from the open reading frames (ORFs) of lin genes. The IS6100 probe was prepared by amplification of the internal fragment of IS6100. The cosmid library (around 2,000 colonies) of B90A was screened by using each probe under stringent conditions (68°C), followed by stringent washing as described above. The tentative clones selected after screening were further confirmed by Southern hybridization. After screening, 15 cosmid clones that gave positive signals with one or more lin genes and/or IS6100 were selected (Table 2).
TABLE 2.
Pattern of hybridization signals in S. paucimobilis B90A cosmid clones with lin genes and IS6100 as probes
| Cosmid | Hybridization signals with the following DNA probes:
|
||||||
|---|---|---|---|---|---|---|---|
| linA | linB | linC | linD | linE | linX | IS6100 | |
| 33 | −a | − | − | + | + | − | + |
| 35 | − | + | − | − | − | − | + |
| 36 | + | − | + | − | − | + | + |
| 39 | + | − | − | − | − | + | − |
| 42 | − | − | − | + | + | − | + |
| 43 | + | − | − | − | − | + | − |
| 45 | − | + | − | + | − | − | + |
| 49 | − | + | − | − | − | − | + |
| 50 | + | − | − | − | − | + | + |
| 52 | − | + | − | − | − | − | + |
| 53 | + | − | − | − | − | + | − |
| 55 | − | − | + | − | − | − | + |
| 56 | − | + | − | − | − | − | + |
| 57 | + | − | + | − | − | + | + |
| 59 | − | − | + | − | − | − | + |
−, no hybridization signal; +, hybridization signal.
Determination of copy numbers of lin genes and IS6100 in the S. paucimobilis strains.
In order to determine the approximate copy numbers of IS6100 and the lin genes, the genomic DNAs of S. paucimobilis B90A, B90, UT26, and Sp+ were digested with restriction enzymes which do not cut within the lin genes or IS6100 and were hybridized by using lin gene and IS6100 fragments as the probes. The maximum numbers of chromosomal hybridizing bands obtained from experiments with different restriction enzymes were considered the copy numbers of the IS6100 and lin genes. In order to detect any IS6100 elements in S. paucimobilis ATCC 29837T and S. chorophenolica DSM7098T (which do not degrade HCH), BamHI-digested DNAs of these strains were hybridized with the [α-32P]dATP-labeled IS6100 probe. To determine the copy number of IS6100 on pLIND33, the plasmid DNA was digested with PstI/EcoRI and hybridized with the internal fragment of IS6100 as the probe. Determination of the copy number from the insert cloned in pLIND33 was necessary to predict the type of recombination leading to spontaneous deletion of a DNA fragment containing linD, linE, and linR from B90A.
DNA sequencing of lin genes and their flanking regions.
DNA sequences were determined by using standard methodologies. Sequences of smaller stretches of DNA fragments cloned into pUC18/19 were determined with an automated DNA sequencer (ABI PRISM model 377, version 3; Applied Biosystems) at the Department of Biochemistry, South Campus, University of Delhi, Delhi, India. DNA sequences of lin genes from strain Sp+ were determined from amplified PCR products directly by using the primers that were used for PCR amplification. A nearly 8-kb insert from cosmid pLIND33 that gave positive hybridization signals with linD, linE, linR, and IS6100 as probes (Table 2) was sequenced, both by making subclones and by primer walking by Microsynth GmbH (Balgach, Switzerland). In a similar manner a 2.2-kb insert that hybridized with linB from HindIII-digested pLINB35 was subcloned in pUC18 (Table 1). The construct pLINB23 was then sequenced by primer walking. The complete sequence of a ∼41-kb insert from cosmid pLINA57 that hybridized with linX, linA, linC, and IS6100 was also determined by primer walking by Microsynth GmbH. One copy of IS6100 from UT26 and one copy of IS6100 from Sp+ from the cloned DNA fragments in pUC18 (pLINUTIS and pLINSpIS, respectively [Table 1]) were sequenced by using M13 primers. The sequences were analyzed by using the DNASIS package (Pharmacia). In order to determine ORFs on the ∼41- and ∼8-kb inserts of cosmids pLINA57 and pLIND33, respectively, the ORF Finder program at the National Center for Biotechnology Information (followed by manual inspection of all potential coding regions and start and stop codons) was used. Potential ribosome sites in front of the start codons of each ORF were also identified manually. The BLAST program (3) was used for homology searches in the GenBank database, and ClustalW (8) was used for multiple alignment of sequences.
Generation of lin mutants and stability of lin genes.
In order to generate mutants that do not degrade HCH and to study the stability of lin genes, cultures of B90A and Sp+ were grown at 28°C to the stationary phase on SM containing 1% glucose (14). A fresh culture was then raised from a 1:100-diluted inoculum from a culture grown overnight (108 cells/ml). Serial dilutions of each stationary-phase culture were plated on SM agar containing glucose (1%, wt/vol), and the number of single colonies was counted after 2 to 3 days. About 200 colonies of each strain were subsequently transferred to liquid SM medium containing 1% glucose and γ-HCH (or γ- and β-HCH in the case of B90A) at a concentration of 5 μg/ml. After the stationary phase was reached (3 to 4 days), γ- or β-HCH was extracted from the samples and analyzed with a gas chromatograph (GC 17A; Shimadzu, Kyoto, Japan). The extraction protocol and conditions used for the gas chromatography analysis have been described previously (14). After analysis for degradation of HCH, cultures that were not able to degrade HCH (mutants) were selected; genomic DNAs were isolated from these mutants and hybridized with [α-32P]dATP-labeled linA or IS6100 gene fragments as probes. In order to attribute the loss of linA of Sp+ to homologous recombination involving IS6100 sequences, genomic DNAs of Sp+ and four mutants were digested with HindIII (a HindIII site is present in IS6100 and not present in linA of Sp+) and BamHI (BamHI does not cut either IS6100 or linA). The DNAs were then hybridized by using [α-32P]dATP-labeled linA and IS6100 probes separately. B90, a spontaneous mutant of B90A, which lacked linD, linE, and linR, was also tested to determine its ability to convert the two possible intermediates, chlorohydroquinone and hydroquinone, in γ-HCH degradation (Fig. 1) by using the method described by Nagata et al. (22).
Nucleotide sequence accession numbers.
The nucleotide sequences of the cloned fragments containing the lin genes and their flanking regions in B90A have been deposited in the National Center for Biotechnology Information database under accession numbers AY331258, AY331259, and AY334273.
RESULTS
Hybridization of a genomic library of strain B90A with IS6100 and lin gene probes.
A genomic library was prepared from S. paucimobilis B90A DNA, an older laboratory stock culture of strain B90, which was described previously (14). This library was screened by hybridization with radioactively labeled probes for all lin genes and for IS6100. From around 2,000 cosmid clones, we selected 15 cosmids which positively hybridized to at least one of the probes (Table 2). Several patterns of cohybridization with different lin markers were observed. Cosmids that hybridized to linC invariably also hybridized with the IS6100 probe. Two of these cosmids (cosmids 36 and 57) hybridized with linA and linX as well. One cosmid (cosmid 50) hybridized to linA, linX, and IS6100 but not to linC. This suggested that this cosmid overlapped the two other cosmids described above. Interestingly, three cosmids that hybridized to linA and linX did not hybridize to IS6100 (cosmids 39, 43, and 53). As argued below, these cosmids might contain the other copies of linA and linX present in strain B90A. All cosmids that hybridized to linB (cosmids 35, 45, 49, 52, and 56) also hybridized to IS6100, but in only one case (number 45) was hybridization detected with one other lin probe, namely, linD. This suggested that linB and linD are relatively close to each other (within 20 to 30 kb). Two cosmids (cosmids 33 and 42) hybridized with linD and linE and also with the IS6100 probe.
To further analyze the patterns of hybridization with linX and linA, digested chromosomal DNAs of strain B90A (and strains B90, UT26, and Sp+) were hybridized with the same probes (Fig. 2). DNA that was digested with HindIII and hybridized with linX resulted in one hybridizing band at ∼4.5-kb for Sp+ and UT26 DNAs (Fig. 2A, lanes 1 and 2). However, with strains B90 and B90A two bands hybridized to linX (Fig. 2A, lanes 3 and 4). This suggested that more than one copy of linX was present in strains B90A and B90. Similarly, when hybridized with a linA probe, DNAs from strains B90A and B90 produced two bands (Fig. 2B, lanes 2 to 5), whereas DNA from UT26 or Sp+ resulted in only one hybridizing band (Fig. 2B, lanes 6 to 9). The presence of two copies of linA in strain B90A was in agreement with previous results for strain B90 (14).
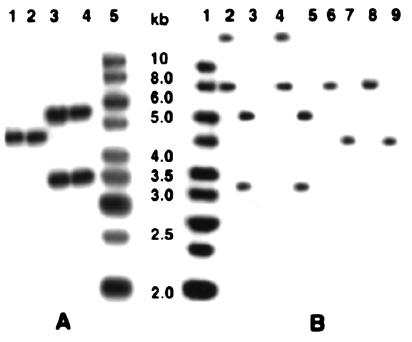
FIG. 2.
(A) Southern blot of total DNAs of S. paucimobilis strains digested with HindIII and hybridized with [α-32P]dATP-labeled linX. Lane 1, S. paucimobilis UT26; lane 2, S. paucimobilis Sp+; lane 3, S. paucimobilis B90; lane 4, S. paucimobilis B90A; lane 5, Gene Ruler DNA ladder mixture (MBI Fermentas). (B) Southern blot of total DNAs of S. paucimobilis strains digested with BamHI and HindIII and hybridized with [α-32P]dATP-labeled linA. Lane 1, Gene Ruler DNA ladder mixture; lane 2, BamHI-digested DNA of S. paucimobilis B90A; lane 3, HindIII-digested DNA of S. paucimobilis B90A; lane 4, BamHI-digested DNA of S. paucimobilis B90; lane 5, HindIII-digested DNA of B90; lane 6, BamHI-digested DNA of S. paucimobilis UT26; lane 7, HindIII-digested DNA of UT26; lane 8, BamHI-digested DNA of S. paucimobilis Sp+; lane 9, HindIII-digested DNA of Sp+.
Sequence analysis of the lin genes and IS6100 in strain B90A.
Several cosmids were selected for further DNA sequencing. The insert of cosmid 57 (pLINA57) was sequenced completely. It contained an approximately 41-kb insert harboring 33 ORFs with clear database homologies (Fig. 3A and Table 3). As expected from the hybridization results, the insert of cosmid 57 contained a linA gene and a linC gene. According to the DNA sequence, the linA copy present in this region is the copy previously cloned from strain B90 and designated linA1 to distinguish it from the slightly different second copy. The linC gene was identical to the gene previously reported for strain B90 (14). In contrast to linA1 and linC, two copies of a linX gene were present; however, these copies were only 66% identical to each other. The linX copies were designated linX1 and linX2 (the linX1 and linX2 genes were represented by a ∼3.5-kb fragment of HindIII-digested DNA that hybridized with the linX probe [Fig. 2A, lane 4]), and linX1 exhibited 99% DNA sequence identity to linX of strain UT26 (22). Apart from the lin genes, three copies of a sequence (IS6100A, IS6100B, and IS6100C) identical to IS6100 were present (Fig. 3A and Table 3), as were many other genes presumably not related to HCH degradation, since the potentially encoded polypeptides had relatively clear homologies to proteins with completely different functions (such as cytochrome c oxidase or Nif proteins). Interestingly, two copies of the IS6100 element flanked the linC gene and the ORFM gene, possibly making a composite transposon. No target site duplication was observed when the boundary sequences of the different IS6100 copies were compared (Fig. 3B).
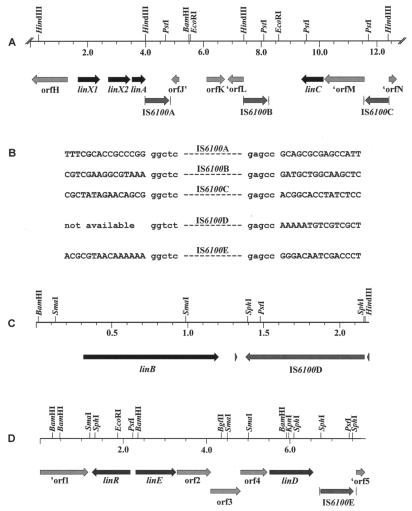
FIG. 3.
(A) Partial physical and genetic map of pLINA57 containing the ∼41-kb fragment from S. paucimobilis B90A. The ORFs deduced from the complete 13-kb nucleotide sequence are indicated by arrows showing the direction of transcription. Details of the coding regions are summarized in Table 3. (B) Comparison of the nucleotide sequences adjacent to five different IS6100 copies, Nucleotide sequences in lowercase letters are common sequences at the terminal ends of each IS6100. IS6100A, IS6100B, and IS6100C are present in pLINA57, and IS6100D and IS6100E are present in pLINB23 and pLIND33, respectively. The sequence of one of the flanking regions of IS6100D could not be determined. (C) Physical and genetic map of the region of pLINB35 containing linB and IS6100. (D) Physical and genetic map of the region of pLIND33 containing linD, linE, linR, and IS6100.
TABLE 3.
Coding regions of the 41-kb insert of pLINA57 from S. paucimobilis B90A
| Protein | Length (amino acids) | % Identity/% similarity | Putative function | Best hit bacterium (accession no.) |
|---|---|---|---|---|
| ORFA | 306 | 39/49 | Hypothetical protein | Novosphingobium aromaticivorans (ZP_00093610) |
| ORFB | 323 | 47/66 | TPP-dependent acetoin dehydrogenase alpha subunit | Clostridium magnum (AAA21744) |
| ORFC | 327 | 47/64 | Acetoin dehydrogenase (TPP dependent) beta subunit | Clostridium magnum (I40791) |
| ORFC1 | 79 | 45/67 | Partial dihydrolipoamide acetyltransferase | Klebsiella pneumoniae (AAC13741) |
| ORFD | 846 | 31/48 | pfam00593, TonB_boxC, TonB-dependent receptor C-terminal region | Novosphingobium aromaticivorans (ZP_00095375) |
| ORFE | 313 | 44/52 | COG1975, xanthine and CO dehydrogenases, maturation factor | Magnetospirillum magnetotacticum (ZP_00054040) |
| ORFF | 330 | 41/59 | COG0303, MoeA, molybdopterin biosynthesis enzyme | Brucella melitensis (AAL53083) |
| ORFG | 448 | 68/80 | COG1249, pyruvate/2-oxoglutarate dehydrogenase complex, dihydrolipoamide dehydrogenase (E3) component | Novosphingobium aromaticivorans (ZP_00094170) |
| ORFH | 412 | 72/82 | COG1960, acyl-coenzyme A dehydrogenases | Novosphingobium aromaticivorans (ZP_00095926) |
| LinX1 | 250 | 99/99 | 2,5-Dichloro-2,5-cyclohexadiene-1,4-diol dehydrogenase (pfam00106, short-chain dehydrogenase) | Sphingomonas paucimobilis UT26 (BAA04939) |
| LinX2 | 250 | 65/81 | 2,5-Dichloro-2,5-cyclohexadiene-1,4-diol dehydrogenase (pfam00106, short-chain dehydrogenase) | Sphingomonas paucimobilis UT26 (BAA04939) |
| LinA1 | 154 | 92/95 | γ-Hexachlorocyclohexane dehydrochlorinase | Sphingomonas paucimobilis UT26 (BAA14369) |
| TnpA1 | 264 | 100/100 | Transposase of IS6100 (COG3316, transposase and inactivated derivatives) | Aeromonas salmonicida (CAD57187) |
| ORFJ | 98 | 80/88 | Putative glutathione S-transferase (COG0625, glutathione S-transferase) | Sphingobium chlorophenolicum (AAM96661) |
| ORFK | 205 | 86/92 | pfam02230, αβ-hydrolase_2, phospholipase/carboxylesterase | Sphingobium chlorophenolicum (AAM96663) |
| ORFL | 175 | 94/96 | Chloromaleylacetate reductase (COG1454, alcohol dehydrogenase, class IV) | Sphingobium chlorophenolicum (AAM96664) |
| TnpA2 | 264 | 100/100 | Transposase of IS6100 (COG3316, transposase and inactivated derivatives) | Aeromonas salmonicida (CAD57187) |
| LinC | 250 | 98/98 | 2,5-Dichloro-2,5-cyclohexadiene-1,4-diol dehydrogenase (pfam00106, short-chain dehydrogenase) | Sphingomonas paucimobilis UT26 (BAA04939) |
| ′ORFM | (451) | 30/49 | pfam00593, TonB-dependent receptor C-terminal region | Novosphingobium aromaticivorans (ZP_00095375) |
| TnpA3 | 264 | 100/100 | Transposase of IS6100 (COG3316, transposase and inactivated derivatives) | Aeromonas salmonicida (CAD57187) |
| ORFN | (86) | 37/59 | Outer membrane protein (COG3047, outer membrane protein W) | Pseudomonas putida KT2440 (AAN66132) |
| ORFO | 254 | 62/72 | Hypothetical regulator (COG0664, cAMP-binding protein-catabolite gene activator) | Novosphingobium aromaticivorans (ZP_00096011) |
| ORFP | 552 | 78/86 | COG3278, cbb3-type cytochrome oxidase, subunit 1 | Novosphingobium aromaticivorans (ZP_00096008) |
| ORFQ | 248 | 81/88 | pfam02433, cytochrome c oxidase, monoheme subunit | Novosphingobium aromaticivorans (ZP_00096007) |
| ORFR | 295 | 59/75 | COG2010, cytochrome c, mono- and diheme variants | Novosphingobium aromaticivorans (ZP_00096005) |
| ORFS | 476 | 69/80 | COG0348, polyferredoxin | Novosphingobium aromaticivorans (ZP_00096004) |
| ORFT | 158 | 46/62 | COG5456, predicted integral membrane protein linked to a cation pump | Novosphingobium aromaticivorans (ZP_00096003) |
| ORFU | 710 | 58/71 | COG2217, cation transport ATPase | Novosphingobium aromaticivorans (ZP_00096002) |
| ORFV | 1,200 | 70/79 | Bifunctional proline dehydrogenase/δ-1-pyrroline-5- carboxylate dehydrogenase (COG4230, δ-1- pyrroline-5-carboxylate dehydrogenase; COG0506, proline dehydrogenase | Sinorhizobium meliloti (CAC41903) |
| ORFW | 233 | 54/64 | COG2755, lysophospholipase L1 and related esterases | Novosphingobium aromaticivorans (ZP_00094852) |
| ORFX | 233 | 68/81 | COG4181, predicted ABC-type transporter involved in lysophospholipase L1 biosynthesis, ATPase component | Novosphingobium aromaticivorans (ZP_00094853) |
| ORFY | 840 | 50/64 | COG3127, predicted ABC-type transporter involved in lysophospholipase L1 biosynthesis, permease component | Novosphingobium aromaticivorans (ZP_00094854) |
| ORFZ | (590) | 43/62 | Two-component hybrid sensor and regulator | Pseudomonas fluorescens (ZP_00084332) |
A 2.2-kb region of cosmid 35 (pLINB35) containing the linB gene was also sequenced. This analysis revealed that linB in strain B90A and the immediate region upstream of linB were 99% similar to the corresponding regions in strain UT26 but that a copy of IS6100 (IS6100D) was located downstream of linB (Fig. 3C).
A third separate 7,808-bp region with the linD, linE, and linR genes was sequenced from cosmid 33 (pLIND33) and several plasmid subclones. The relative position of these genes with respect to linA1 is not known. The nucleotide sequences of linD, linE, and linR were around 99 to 100% identical to those of linD, linE, and linR of strain UT26 (Table 4). Even the ORFs between linD and linE were the same as those described for UT26 (17, 19). Interestingly, however, both the DNA sequences upstream of linR and the DNA sequences downstream of linD were different in strains B90A and UT26. Again, an intact copy of IS6100 (IS6100E) was present downstream of linD (Fig. 3D). The hybridization data showed that yet another copy of IS6100 was upstream of orf1 (data not shown), but its exact location was not determined further.
TABLE 4.
Coding regions of the insert of pLIND33 of S. paucimobilis B90A
| Protein | Length (amino acids) | % Identity/% similarity | Putative function | Best hit Bacterium (accession no.) |
|---|---|---|---|---|
| ORF1 | 385 | 45/60 | Hypothetical TonB-dependent receptor); pfam00593, TonB_dep_Rec, TonB-dependent receptor | Novosphingobium aromaticivorans (gi 23107064) |
| LinR | 303 | 99/99 | LysR-type transcriptional regulator for linD | Sphingomonas paucimobilis UT26 (gi 4092848) |
| LinE | 321 | 100/100 | Hydroquinone meta-cleavage dioxygenase | Sphingomonas paucimobilis UT26 (gi 4587228) |
| ORF2 | 269 | 35/51 | β-Ketoadipate-enol-lactone hydrolase; pfam00561, abhydrolase, alpha/beta hydrolase fold | Ralstonia solanacearum (gi 17427307) |
| ORF3 | 238 | 26/42 | Carboxylesterase; pfam02230, abhydrolase_2, phospholipase/carboxylesterase | Burkholderia fungorum (gi 22989674) |
| ORF4 | 210 | 33/47 | Conserved hypothetical protein; COG2350, uncharacterized protein conserved in bacteria | Ralstonia eutropha (gi 11967267) |
| LinD | 346 | 99/100 | 2,5-Dichlorohydroquinone reductive dechlorinase | Sphingomonas paucimobilis UT26 (gi 1731852) |
| TnpA5 | 264 | 100/100 | Transposase of IS6100 (COG3316, transposase and inactivated derivatives) | Aeromonas salmonicida (CAD57187) |
| ORF5 | 69 | 45/59 | Multidrug resistance protein VceB (truncated, only C-terminal portion) | Vibrio cholerae (gi 2815578) |
Since the three cosmids that hybridized to linA and linX (cosmids 39, 43, and 53) did not hybridize to IS6100 but sequencing of the linA1 region of cosmid 57 showed that IS6100 was located immediately downstream of linA1, we assumed that the second linA copy and perhaps a third copy of linX, designated linX3, were present on cosmids 39, 43, and 53. A comparison of the restriction profiles of cosmids 39, 43, and 53 did not reveal any similarity with the profile of cosmid 57. Hybridization of the digests also confirmed that there was a second signal for the linA and linX probes (data not shown). When the PCR was conducted with conserved linX primers and a ∼5.7-kb eluted fragment of HindIII-digested B90A genomic DNA (Fig. 2A, lane 4), a product was amplified which contained the third copy of linX (linX3), as determined by sequencing. This copy was 100% identical to linX1. Although the functionality of the linX1, linX2, and linX3 genes was not established, the 99% DNA sequence similarity of linX1 and linX3 with linX of UT26 (20) and the intact linX2 ORF (with only 66% similarity with linX of UT26) indicate that these genes (at least linX1 and linX3) should be functional in B90A.
Association of IS6100 copies with the lin genes in S. paucimobilis strains.
Hybridization data for BamHI-digested genomic DNAs of B90A, Sp+, and UT26 revealed the presence of at least 11, 6, and 5 copies of IS6100 in B90A, UT26, and Sp+, respectively (Fig. 4A). However, no signal could be detected with BamHI-digested genomic DNAs of S. paucimobilis ATCC 29837T and S. chlorophenolica DSM 7098T (non-HCH-degrading strains), indicating that IS6100 was not present in these strains (data not shown). Further hybridization data for the cosmids from strain B90A and subsequent DNA sequencing (Fig. 3) demonstrated that several IS6100 copies were associated with the lin genes. All five sequenced copies of IS6100 from B90A, one copy of IS6100 from Sp+, and one copy of IS6100 from UT26 were identical over the complete 880 bp. To further investigate the copy number of IS6100 in each strain and whether IS6100 was actively transposed within S. paucimobilis, Southern hybridizations with digested chromosomal DNAs of strains B90A, B90, Sp+, and UT26 and mutants generated from Sp+ were performed (Fig. 4). S. paucimobilis B90A and B90 were originally derived from the same stock but had been separately maintained for different periods of time during cultivation in the laboratory. When genomic DNAs of strains B90A and B90 were digested with BamHI (an enzyme which does not cut within IS6100) and hybridized with the IS6100 probe, at least 11 different bands were observed (Fig. 4A, lanes 2 and 3). The intensities of the bands were not the same in all cases, suggesting that for some fragments more than one copy of IS6100 was present. Three different hybridizing bands were detected for strains B90A and B90 (Fig. 4A, lanes 2 and 3). For example, for the BamHI digests, the 2.5- and 8.5-kb bands of the B90A digest (Fig. 4A, lane 2) differed from the bands in the B90 digest (Fig. 4A, lane 3). One of these bands could be attributed to the loss of the linD, linE, and linR genes in strain B90. The differences in hybridization signals between B90A and B90 when IS6100 was used as the probe perhaps can be attributed to the loss of one DNA fragment containing linD, linE, linR, and IS6100 (Fig. 3D and 4B). The absence of linD and linE from B90 was also confirmed by Southern blot hybridization (Fig. 4B). This absence could have been due to recombination between two copies of IS6100 flanking linD, linE, and linR in B90A. While both Sp+ and UT26 also contained multiple copies (six and five copies, respectively) (Fig. 4A, lanes 4 and 5) of the IS6100 sequence, the copies were at different chromosomal positions (Fig. 4A). More fragments hybridizing to the IS6100 probe with an apparently similar size were present in the genomic digests of Sp+, B90, and B90A than in the genomic digest of UT26. When the linA gene was amplified by PCR from strain Sp+ and sequenced, it was found that similar to linA1 of strain B90A, the 3′ end of linA of Sp+ was replaced by 22 nucleotides of IS6100. However, the sequence of the region immediately downstream of linA in strain Sp+ did not show a continuation of the IS6100 sequence like that reported for linA1 of B90 (14), but hybridization data revealed the presence of yet another copy of IS6100 further away from the 3′ end of linA in Sp+ (data not shown).
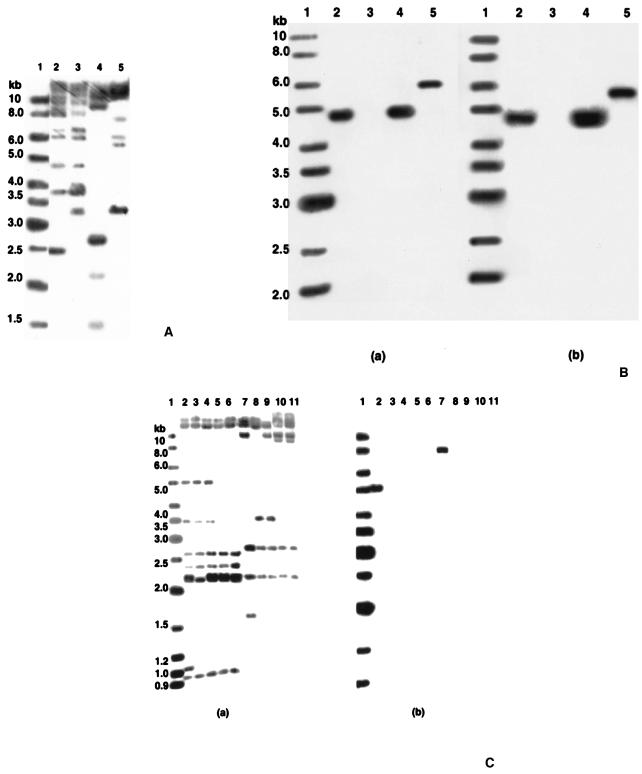
FIG. 4.
(A) Southern blot hybridization of genomic DNAs of S. paucimobilis B90A, B90, Sp+, and UT26 digested with BamHI and hybridized with [α-32P]dATP-labeled IS6100. Lane 1, Gene Ruler DNA ladder mixture; lane 2, B90A; lane 3, B90; lane 4, Sp+; lane 5, UT26. (B) Southern blot hybridization of PstI-digested genomic DNAs of S. paucimobilis B90A, B90, Sp+, and UT26 hybridized with [α-32P]dATP-labeled linD (panel a) and linE (panel b) as probes. Lane 1, Gene Ruler DNA ladder mixture; lane 2, B90A; lane 3, B90; lane 4, Sp+; lane 5, UT26. (C) Southern blot hybridization of genomic DNAs of S. paucimobilis Sp+ mutants digested with HindIII and BamHI and hybridized with [α-32P]dATP-labeled IS6100 (panel a) and linA (panel b). Lane1, Gene Ruler DNA ladder mixture; lanes 2 to 6, HindIII-digested genomic DNAs of Sp+ and mutants 1 to 4; lanes 7 to 11, BamHI-digested genomic DNAs of Sp+ and mutants 1 to 4.
To investigate whether mutants could be obtained from S. paucimobilis in which HCH degradation activity had been lost, which perhaps could be attributed to the activity of IS6100, both strain B90A and strain Sp+ were repeatedly subcultured in SM medium with glucose but without γ-HCH. After about three passages on SM medium, about 200 colonies of both strains were screened for degradation of HCH isomers by gas chromatography analysis. No mutant lacking the ability to degrade HCH isomers was detected with strain B90A, although the spontaneous mutant B90, which lacked linD, linE, and linR, had been obtained previously (unknowingly), probably due to repeated subculturing (14). We presume that the loss of the fragment (as described above) can be attributed to homologous recombination between two copies of IS6100 flanking linD, linE, and linR. B90 was found to accumulate 2,5-dichlorohydroquinone (Holliger, unpublished data), and when resting cells of B90A and B90 were incubated with chlorohydroquinone and hydroquinone, these compounds accumulated in B90, but they were degraded further in B90A (Fig. 1). However, we detected four strain Sp+ mutants that lacked the ability to degrade α-, γ-, and δ-HCH. Genomic DNAs of these Sp+ mutants were isolated, digested with HindIII and BamHI, and hybridized with linA and IS6100 probes (Fig. 4C). In all the mutants linA appeared to have been lost, but IS6100 hybridization patterns different than the wild-type Sp+ pattern were found (Fig. 4C). When BamHI-digested genomic DNAs were used, all the Sp+ mutants lacked at least one copy of IS6100 (1.7-kb BamHI fragment) (Fig. 4C, panel a, lanes 8 to 11), but this did not provide conclusive evidence that there is an association of IS6100 with a deletion of linA through homologous recombination. However, a ∼5.0-kb fragment in a HindIII digest that hybridized with both linA and IS6100 in Sp+ (Fig. 4C, panel a, lane 2) was simultaneously lost in at least two mutants, designated mutants 3 and 4 (Fig. 4C, panel a, lanes 5 and 6). In conclusion, all Sp+ mutants that had lost linA produced different hybridization patterns than the wild type, and at least two of them (mutants 3 and 4) had lost one copy of IS6100 along with linA (Fig. 4C, panel a, lanes 5 and 6). These results, which are consistent with homologous recombination of two direct IS6100 copies and subsequent deletion of a linA fragment, also support the hypothesis that IS6100 is directly associated with the stability of linA in Sp+. No differences were found among three Sp+ mutants (mutants 2, 3, and 4) in terms of the hybridization patterns when the other lin genes (linB, linC, linD, and linE) were used as probes (data not shown). All four mutants contained linC, linD, linE, and linR. However, one of them (mutant 1), in addition to lacking linA, lacked linB as well. This was confirmed by PCR amplification and DNA-DNA hybridization (data not shown). The instability of lin genes in UT26 has been described previously (20). The data on the stability of lin genes and their organization are summarized in Table 5.
TABLE 5.
Comparison of various HCH degradative genes and IS6100 in S. paucimobilis B90A, UT26, and Sp+a
| Gene | No. of nucleotides (no. of amino acids in):
|
G+C content (%) in:
|
Functionb | Stability in:c
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Strain B90A | Strain UT26 | Strain Sp+ | Strain B90A | Strain UT26 | Strain Sp+ | Strain B90A | Strain UT26 | Strain Sp+ | ||
| linA1 | 462 (154) | NDd | 462 (154) | 52.7 | ND | 52.7 | Dehydrochlorinase | ++ | ND | − |
| linA2/linA | 468 (156) | 468 (156) | ND | 53.9 | 53.9 | ND | Dehydrochlorinase | ++ | + | ND |
| linB | 888 (296) | 888 (296) | 888 (296) | 62.5 | 62.5 | 62.5 | Halidohydrolase | ++ | + | − |
| linC | 750 (250) | 750 (250) | 750 (250) | 64.5 | 64.5 | 64.5 | Dehydrogenase | ++ | + | − |
| linD | 1,038 (346) | 1,038 (346) | 1,038 (346) | 61.8 | 61.8 | 61.8 | Reductive dechlorinase | + | + | ++ |
| linE | 963 (321) | 963 (321) | 963 (321) | 60.1 | 60.1 | 60.1 | Ring cleavage dioxygenase | + | + | ++ |
| linR | 909 (303) | 909 (303) | 909 (303) | 60.3 | 61.3 | 60.3 | Transcriptional regulator | + | + | + |
| linX1 | 750 (250) | 750 (250) | 750 (250) | 64.5 | 64.5 | 64.5 | Dehydrogenase | ++ | + | + |
| linX2 | 750 (250) | ND | ND | 64.5 | ND | ND | Dehydrogenase | ++ | ND | ND |
| linX3 | 750 (250) | ND | ND | 64.5 | ND | ND | Dehydrogenase | ++ | ND | ND |
| tnpA | 792 (264) | 792 (264) | 792 (264) | 61 | 61 | 61 | Transposase | + | nde | − |
DISCUSSION
The data presented in this paper show the extraordinary presence of multiple copies of IS6100 in HCH-degrading strains of S. paucimobilis and the complete absence of IS6100 in the non-HCH-degrading strains S. paucimobilis ATCC 29837T and S. chlorophenolica DSM 7098T. We have evidence that acquisition of IS6100 by all HCH- degrading S. paucimobilis strains is important not only for establishment of the linA genes but also for shaping the genetic organization of the lin genes, and we also have evidence that the stability of the lin genotype is strongly influenced by IS6100 activity. We reached this conclusion from the hybridization and sequencing data which showed that most lin genes are associated with IS6100 in B90A, that linD, linE, and linR along with one copy of IS6100 are deleted in the spontaneous mutant strain B90, and that linA and a copy of IS6100 are deleted in at least two Sp+ mutants.
IS6100 is a member of the IS6 family and consequently forms a cointegrate as an end product of transposition (26). In M. fortuitum IS6100 occurs as part of the composite transposon Tn610 that confers resistance to sulfonamides. It seems to be an extremely promiscuous IS element. For instance, the sequenced copies of IS6100 from strains B90A, Sp+, and UT26 (five, one, and one copies, respectively) were 100% identical to those of M. fortuitum (16). The DNA sequences of five IS elements from the nylon oligomer-degrading plasmid pOAD2 of Arthrobacter sp. were identical to the DNA sequence of IS6100 (12). In the Pseudomonas aeruginosa plasmid R1033, IS6100 is located downstream of Tn1696, at the 3′ end of the In4 integron (7). IS6100 is also present within the tnpR gene of transposon Tn5393b from Xanthomonas campestris pv. vesicatoria, where it was shown to increase the expression of the streptomycin resistance genes strA and strB (35). Similarly, IS6100 has been found in Salmonella enterica serovar Typhimurium (5), in plasmid pACM1 from Klebsiella oxytoca (27), in plasmid pTET3 in Corynebacterium glutamicum (36), and in plasmid pRASI in Aeromonas salmonicida (34). There has been no previous report of IS6100 elements in any strain of S. paucimobilis, and using Southern blot hybridization, we were unable to detect IS6100 elements in S. paucimobilis ATCC 29837T and S. chlorophenolica DSM 7098T. Thus, the previously published data on the presence of IS6100 in different bacterial strains and the presence of identical copies of IS6100 only in HCH-degrading S. paucimobilis strains indicate that IS6100 plays a vital role in disseminating genes, including catabolic and antibiotic resistance genes, among different bacteria. These data also indicate that IS6100 has been disseminated both widely and recently among different bacterial species without any change in nucleotide sequence. The presence of IS6100 in distantly related bacterial species, ranging from M. fortuitum to S. paucimobilis, further suggests that IS6100 elements have a very broad host range, and their presence on plasmids (even in strains in which the location has not been ascertained) cannot be ruled out.
The genetic structures of the lin genes in the different S. paucimobilis strains are a remarkable example of rearrangements and pathway evolution, although the implications of the rearrangements in the different strains are not fully understood. For example, data obtained in this work demonstrated that strains B90 and B90A contain two copies of a linA gene, whereas both Sp+ and UT26 carry only one copy. Although the exact specificity differences of linA1 and linA2 have not been unequivocally determined, the fact remains that strains B90A and B90 can (partially) degrade β-HCH, whereas Sp+ and UT26 cannot. In addition, three copies of a linX gene are present in strain B90A. One of the copies, linX2, differs considerably from the other two. Only one copy of linX has been found in strains UT26 and Sp+. While the enzymatic activity of the linX gene (linX1, linX2, and linX3) products for HCH degradation in B90A has not been studied yet, the presence of two copies of linA and three copies of linX might influence HCH degradation rates. In fact, degradation of α-, γ-, and δ-HCH proceeded at a higher rate in B90A than in UT26 and Sp+ (R. Lal, unpublished data).
Despite the different geographic locations at which they were first isolated, the HCH-degrading S. paucimobilis strains have identical lin genes (except linA1 of B90A), even though the lin gene organization is not the same in all of the strains. This supports the hypothesis that the lin genes were distributed relatively recently and by and large have not accumulated strain-specific mutations yet and that they originated from a single source. We have very little information to determine whether the lin genes were assembled once in one microorganism and were subsequently disseminated or whether the lin genes were distributed on a self-transmissible DNA fragment to different suitable (S. paucimobilis) hosts. In two strains which we studied (B90A and Sp+) and in two other strains described previously (9, 37), the five linA genes had a mosaic organization, and at the same time their amino acid and nucleic acid sequences exhibited high levels of similarity. In B90A and B90, the C terminus of the linA1 gene has been replaced by a copy of IS6100, and linA of Sp+ only contains the first 22 nucleotides of the IS6100 sequence towards the C terminus and not a complete copy of IS6100 (like linA1 of B90A). A complete copy of IS6100 appears to be present away from the 3′ end of the linA gene of Sp+ (the exact location of a complete copy of IS6100 in the vicinity of the linA gene of Sp+ has not been determined yet), and the linA gene of UT26 and the linA2 gene of B90A do not appear to be associated with IS6100. In addition, the G+C contents of all of the linA genes reported so far are lower than those of the linB, linC, linD, and linE genes, suggesting that linA might have been acquired by the strains through horizontal gene transfer from an external donor, as proposed previously (14, 20), by IS6100 involvement. Eventually, the association of linA1 with IS6100 might have triggered a duplication process, thereby adding a second copy in B90A. This hypothesis is supported by the presence of IS6100 near linA1. In fact, the current structure of the linA1 and linA2 regions in strain B90A suggests that the genetic organization of linX and linA in strain UT26 was the result of recombination between the 5′ ends of the linA1 and linA2 sequences (14). Additional gene reshuffling followed by further transposition or recombination between IS6100 copies may then have led to activation of rest of the genes (linB, linC, linD, linE) that appear to be indigenous to the strain (20).
In spite of the fact that B90A contains 11 copies of IS6100, compared to the 6 and 5 copies in Sp+ and UT26, respectively, the linA gene was more stable in B90A than in Sp+ (this study) and UT26 (20), indicating that the copy number of IS6100 may not be the only factor that contributes to the stability of lin genes. The stability of genes within a genome is controlled by several underlying mechanisms operating within the genome, including homologous recombination between elements at different genomic locations, duplications, deletions, inversions, translocations, and transductions (13). The loss of the linA gene in Sp+ was always associated with a change in the profile of the IS6100 copies in all the mutants, and the loss in at least two mutants was accompanied by a loss of a copy of IS6100 associated with linA. Likewise, the presence of IS6100 flanking linD and orf1 in B90A also suggests that there was a similar additional deletion event, facilitated by homologous recombination between these two copies, resulting in the formation of B90. This is a very recent step that took place in the laboratory.
In conclusion, the mosaic nature of the linA genes, the association of IS6100 with the lin genes, and the deletions of IS6100 associated with deletions of the lin genes indicate the underlying role that IS6100 elements have played in establishing this pathway. Interestingly, the lin genes are not located in a single operon, like linD, linE, and linR, but are present in several different regions of the genome. This is in contrast to the genes for many catabolic pathways in pseudomonads, for example, which are more closely organized, but it is similar to the genes in other sphingomonads, such as the genes for polycyclic aromatic hydrocarbon degradation in Sphingomonas aromaticivorans (28) and mecocrop degradation in Sphingomonas herbicidovorans (van der Meer, unpublished data). The present study brought up several interesting questions, including questions related to finding the original host containing linA, the evolution of the β-HCH degradation pathway in B90A, and the mechanism of formation and the role of multiple copies of lin genes in some strains, which will require a series of new experimental studies. On the applied side, the occurrence of stable HCH-degrading microorganisms at different locations on the globe suggests that microorganisms have the ability to adapt to this pollutant, which is a hopeful sign for remediation of HCH contamination.
Acknowledgments
We thank Y. Nagata, Department of Biotechnology, University of Tokyo, and Tim Vogel, University of Lyon, for providing S. paucimobilis UT26 and Sp+, respectively. S. paucimobilis ATCC 29837T and S. chlorophenolica DSM 7098T were provided by Manjit Hanspal, St. Elizabeth's Medical Center of Boston, Boston, Mass., and John Cullum, University of Kaiserslautern, Kaiserslautern, Germany, respectively. We also thank R. Eichenlaub for his continuous encouragement in the initiation and continuation of this project.
Part of this work was supported by grants under the Indo-Swiss Collaboration in Biotechnology. C. Dogra and V. Raina acknowledge the Council of Scientific and Industrial Research for providing a doctoral fellowship.
REFERENCES
- 1.Ahuja, A. K., and M. D. Awasthi. 1993. Contamination of rice and wheat grains with residues of HCH and DDT. Pestic. Res. J. 5:83-86. [Google Scholar]
- 2.Akbar, N., A. Aleem, and A. Malik. 2003. Determination of organochlorine pesticides in agricultural soil with reference to γ-HCH degradation by Pseudomonas strains. Bioresource Technol. 88:41-46. [DOI] [PubMed] [Google Scholar]
- 3.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 4.Bhuyan, S., B. Sreedharan, T. K. Adhya, and N. Sethunathan. 1993. Enhanced biological degradation of γ-hexachlorocyclohexane (γ-HCH) in HCH (commercial) acclimated flooded soil. Factors affecting its development and persistence. Pestic. Sci. 38:49-53. [Google Scholar]
- 5.Boyd, D. A., G. A. Peters, L. Ng, and M. R. Mulvey. 2000. Partial characterization of a genomic island associated with the multidrug resistance region of Salmonella enterica typhymurium DT104. FEMS Microbiol. Lett. 189:285-291. [DOI] [PubMed] [Google Scholar]
- 6.Cerkvenik, V., D. Z. Doganoc, and J. Jan. 2000. Evidence of some trace elements, organochlorine pesticides and PCBs in Slovenian cow's milk. Food Technol. Biotechnol. 38:155-160. [Google Scholar]
- 7.Hall, R. M., H. J. Brown, D. E. Brookes, and H. W. Stokes. 1994. Integrons found in different locations have identical 5′ ends but variable 3′ ends. J. Bacteriol. 176:6286-6294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Higgins, D. G., J. D. Thompson, and T. J. Gibson. 1996. Using CLUSTAL for multiple sequence alignments. Methods Enzymol. 266:383-402. [DOI] [PubMed] [Google Scholar]
- 9.Imai, R., Y. Nagata, M. Fukuda, M. Takagi, and K. Yano. 1991. Molecular cloning of a Pseudomonas paucimobilis gene encoding a 17-kilodalton polypeptide that eliminates HCl molecules from γ-hexachlorocyclohexane. J. Bacteriol. 173:6811-6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johri, A. K., M. Dua, D. Tuteja, R. Saxena, D. M. Saxena, and R. Lal. 1996. Genetic manipulation of microorganism for the degradation of hexachlorocyclohexane. FEMS Microbiol. Rev. 19:69-84. [DOI] [PubMed] [Google Scholar]
- 11.Kannan K., S. Tanabe, J. P. Giesy, and R. Tatsukawa. 1997. Organochlorine pesticides and polychlorinated biphenyls in foodstuffs from Asian and Oceanic countries. Rev. Environ. Contam. Toxicol. 152:1-55. [DOI] [PubMed] [Google Scholar]
- 12.Kato, K., K. Ohtsuki, H. Mitsuda, T. Yomo, S. Negoro, and I. Urabe. 1994. Insertion sequence IS6100 on plasmid pOAD2, which degrades nylon oligomers. J. Bacteriol. 176:1197-1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kidwell, M. G., and D. R. Lisch. 2002. Transposable elements as sources of genomic variation, p. 59-90. In N. L Craig, R Craigie, M Gellert, and A. M Lambowitz (ed.), Mobile DNA II. American Society for Microbiology, Washington, D.C.
- 14.Kumari, R., S. Subudhi, M. Suar, G. Dhingra, V. Raina, C. Dogra, S. Lal, J. R. van der Meer, C. Holliger, and R. Lal. 2002. Cloning and characterization of lin genes responsible for the degradation of hexachlorocyclohexane isomers in Sphingomonas paucimobilis B90. Appl. Environ. Microbiol. 68:6021-6028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lal, R., and D. M. Saxena. 1982. Accumulation, metabolism, and effects of organochlorine insecticides on microorganisms. Microbiol. Rev. 46:95-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin, C., J. Timm, J. Rauzler, R. Gomez-Lus, J. Davies, and B. Gicquel. 1990. Transposition of an antibiotic resistance element in mycobacteria. Nature 345:739-743. [DOI] [PubMed] [Google Scholar]
- 17.Miyauchi, K., H. S. Lee, M. Fukuda, M. Takagi, and Y. Nagata. 2002. Cloning and characterization of linR, involved in regulation of downstream pathway for γ-hexachlorocyclohexane degradation in Sphingomonas paucimobilis UT26. Appl. Environ. Microbiol. 68:1803-1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miyauchi, K., S. Suh, Y. Nagata, and M. Takagi. 1998. Cloning and sequencing of a 2,5-dihydrochloroquinone reductive dehalogenase gene whose product is involved in the degradation of γ-hexachlorocyclohexane by Sphingomonas paucimobilis. J. Bacteriol. 180:1354-1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miyauchi, K., Y. Adachi, Y. Nagata, and M. Takagi. 1999. Cloning and sequencing of a novel meta-cleavage dioxygenase gene whose product is involved in degradation of γ-hexachlorocyclohexane in Sphingomonas paucimobilis. J. Bacteriol. 181:6712-6719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagata, Y., K. Miyauchi, and M. Takagi,. 1999. Complete analysis of genes and enzymes for γ-hexachlorocyclohexane degradation in Sphingomonas paucimobilis UT26. J. Ind. Microbiol. Biotechnol. 23:380-390. [DOI] [PubMed] [Google Scholar]
- 21.Nagata, Y., K. Miyauchi, S. Suh, A. Futamura, and M. Takagi. 1996. Isolation and characterization of Tn5-induced mutants of Sphingomonas paucimobilis defective in 2,5-dichlorohydroquinone degradation. Biosci. Biotechnol. Biochem. 60:689-691. [Google Scholar]
- 22.Nagata, Y., R. Ohtomo, K. Miyauchi, M. Fukuda, K. Yano, and M. Takagi. 1994. Cloning and sequencing of a 2,5-dichloro-2,5-cyclohexadiene-1,4-diol dehydrogenase gene involved in the degradation of γ-hexachlorocyclohexane in Pseudomonas paucimobilis. J. Bacteriol. 176:3117-3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagata, Y., T. Nariya, R. Ohtomo, M. Fukuda, K. Yano, and M. Takagi. 1993. Cloning and sequencing of a dehalogenase gene encoding an enzyme with hydrolase activity involved in the degradation of γ-hexachlorocyclohexane in Pseudomonas paucimobilis. J. Bacteriol. 175:6403-6410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nalin, R., P. Simonet, T. M. Vogel, and A. Normand. 1999. Rhodanobacter lindaniclasticus gen. nov., sp. nov., a lindane-degrading bacterium. Int. J. Syst. Bacteriol. 49:19-23. [DOI] [PubMed] [Google Scholar]
- 25.Noren, K., and D. Meironyte. 2000. Organochlorine and organobromine contaminants in Swedish human milk in perspective of past 20-30 years. Chemosphere 44:1111-1123. [DOI] [PubMed] [Google Scholar]
- 26.Pitman, A., P. Herron, and P. Dyson. 2002. Cointegrate resolution following transposition of Tn1792 in Streptomyces avermitilis facilitates analysis of transposon-tagged sequence. J. Microbial Methods 49:89-96. [DOI] [PubMed]
- 27.Preston, K. E., C. C. Radomski, and R. A. Venezia. 1999. The cassettes and 3′ conserved segment of an integron from Klebsiella oxytoca plasmid pACM1. Plasmid 42:104-114. [DOI] [PubMed] [Google Scholar]
- 28.Romine, M. F., L. C. Stillwell, K. K. Wong, S. J. Thurston, E. C. Sisk, C. Sensen, T. Gaasterland, J. K. Fredrickson, and J. D. Saffer. 1999. Complete sequence of a 184-kilobase catabolic plasmid from Sphingomonas aromaticivorans F199. J. Bacteriol. 181:1585-1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sahu, S. K., K. K. Patnaik, M. Sharmila, and N. Sethunathan. 1990. Degradation of alpha-, beta-, gamma-hexachlorocyclohexane by a soil bacterium under aerobic conditions. Appl. Environ. Microbiol. 56:3620-3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 31.Sanghi, R., and V. Tewari. 2001. Monitoring of pesticide residues in summer fruits and vegetables from Kanpur, India. Bull. Environ. Contam. Toxicol. 67:587-593. [DOI] [PubMed] [Google Scholar]
- 32.Senoo, K., and H. Wada. 1989. Isolation and identification of an aerobic γ-HCH decomposing bacterium from soil. Soil Plant Nutr. 35:79-87. [Google Scholar]
- 33.Simonich, S. L., and R. A. Hites. 1995. Global distribution of persistent organochlorine compounds. Science 269:1851-1853. [DOI] [PubMed] [Google Scholar]
- 34.Sorum, H., T. M. L'Abee-Lund, A. Solberg, and A. Wold. 2003. Integron-containing IncU R plasmids pRAS1 and pAr-32 from the fish pathogen Aeromonas salmonicida. Antimicrob. Agents Chemother. 47:1285-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sundin, G. W., and C. L. Bender. 1995. Expression of the strA-strB streptomycin resistance genes in Pseudomonas syringae and Xanthomonas campestris and characterization of IS6100 in X. campestris. Appl. Environ. Microbiol. 61:2891-2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tauch, A., S. Gotker, A. Pühler, J. Kalinowski, and G. Thierbach. 2002. The 27.8-kb R-plasmid pTET3 from Corynebacterium glutamicum encodes the aminoglycoside adenyltransferase gene cassette aadA9 and the regulated tetracycline efflux system Tet33 flanked by active copies of the widespread insertion sequence IS6100. Plasmid 48:117-129. [DOI] [PubMed] [Google Scholar]
- 37.Thomas, J. C., F. Berger, M. Jacquier, D. Bernikkon, F. Baud-Grasset, N. Truffaul, P. Normand, T. M. Vogel, and P. Simonet. 1996. Isolation and characterization of a novel γ-hexachlorocyclohexane-degrading bacterium. J. Bacteriol. 178:6049-6055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wada, H., K. Senoo, and Y. Takai. 1989. Rapid degradation of γ-HCH in upland soil after multiple applications. Soil Sci. Plant Nutr. 35:71-77. [Google Scholar]