Abstract
Leymus chinensis has extensive ecological adaptability and can grow well in saline-alkaline soils. The knowledge about tolerance mechanisms of L. chinensis could be base for utilization of saline-alkaline soils and grassland restoration and rebuilding. Two neutral salts (NaCl : Na2SO4 = 9 : 1) and two alkaline salts (NaHCO3 : Na2CO3 = 9 : 1) with concentration of 0, 100, and 200 mmol/L were used to treat potted 35-day-old seedlings with rhizome growth, respectively. After 10 days, the biomass and number of daughter shoots all decreased, with more reduction in alkali than in salt stress. The rhizome biomass reduced more than other organs. The number of daughter shoots from rhizome was more than from tillers. Under both stresses, Na+ contents increased more in rhizome than in other organs; the reduction of K+ content was more in underground than aerial tissue. Anion ions or organic acids were absorbed to neutralize cations. Na+ content in stem and leaf increased markedly in high alkalinity (200 mmol/L), with accumulation of soluble sugar and organic acids sharply. Rhizomes help L. chinensis to adapt to saline and low alkaline stresses by transferring Na+. However, rhizomes lost the ability to prevent Na+ transport to aerial organs under high alkalinity, which led to severe growth inhibition of L. chinensis.
1. Introduction
The salinization-alkalization of soil is an increasing environmental problem and a limiting factor for plant growth and productivity [1]. The problem is still widespread. Of the world's cultivated land, about 0.34 × 109 ha are saline and other 0.56 × 109 ha are sodic; they occupy 23% and 37% of cultivated lands area, respectively [2]. In many inland areas such as northeastern China, alkalinized meadow covers more than 70% of the land area and is expanding. Neutral salts (NaCl and Na2SO4) and alkaline salts (NaHCO3 and Na2CO3) are the key salt constituents in the soils [3]. Some reports indicated that alkali stress from NaHCO3 and Na2CO3 was more destructive to plants than salt stress from NaCl and Na2SO4 [1, 4]. For example, the water content of halophyte Suaeda glauca shoots was inhibited significantly by 240 mmol/L salt stress or 160 mmol/L alkali stress [5]; the relative growth rate of Kochia sieversiana seedlings was inhibited markedly by 100 mmol/L salt stress or 50 mmol/L alkali stress [1]. The effects of salt stress on plants generally involve osmotic stress and ion injury [6]. Alkali stress causes similar effects, adding extra influence of high pH. This stress usually inhibits the absorption of ions such as NO3 − and SO4 2−, disturbs the ionic balance, and undermines intracellular pH stability [1, 5].
Plants need to maintain osmotic and ion balance by controlling the absorption, transportation, and sequestration of salt ions and synthesizing the organic solutes [7]. Primarily inorganic ions participate in the regulation of ion balance in salt stress, while organic acids are the main components used to balance the excess cations in alkali stress [1, 5, 8]. At the cellular level, plants will lower the uptake and accumulation of Na+ in the cytoplasm to avoid the harmful effect of saline ions (especially Na+) on cytoplasm. This process is mediated by restriction of Na+ influx and/or by active efflux from the cytoplasm to the vacuole and back into the growth medium as well as simultaneous synthesis of micromolecular solutes to balance the osmotic pressure coming from the vacuole [6, 9, 10]. At individual level, plants usually transfer toxic ions (such as Na+) to the root or exclude Na+ from shoots effectively, to reduce the destructive effects on other organs [11]. Some dicotyledonous halophytes excrete Na+ and Cl− using salt glands or bladders [12].
Leymus chinensis (Trin.) Tzvel. is a rhizomatous, perennial grass that is widely distributed in the eastern part of the Eurasian steppes including the Russian Baikal, the northern and eastern parts of the People's Republic of Mongolia, the northern and northeast plain of China, and the Loess Plateau of China [13]. It is a dominant species in saline-alkaline soils of the Songnen Plain in northeast of China. It grows rapidly and is highly tolerant to arid and saline-alkaline soils [14]. L. chinensis is considered one of the most promising grass species for grassland rehabilitation and restoration in northern of China [15, 16]. It has extensive ecological adaptability and plasticity and can grow well in soils with high pH (8.5–11.5) [17]. It also has a high forage value and good palatability, making the grass ideal for rangeland use in arid and semiarid northeastern China [18].
The tolerance mechanisms and adaptive strategies of L. chinensis can be the base for utilization of saline-alkaline soils and grassland restoration and rebuilding. Rhizomes of L. chinensis are special underground organs which function in vegetative reproduction, nutrient storage [19]. and growth adjustment. Genetics and organogenesis of L. chinensis were studied [20, 21]. However, little information is available about physiological changes and saline-alkaline tolerance of L. chinensis. We propose that the existence of rhizome helps L. chinensis to adapt to salt and alkali stresses. To test this hypothesis, the effects of salt and alkali stress on the growth, physiological responses characteristics, and adaptive strategies of L. chinensis seedlings were compared and determined the contribution of rhizome to the saline- or alkaline-tolerance. Based on the previous research of salt stress and alkali stress effects on halophytes, 0–200 mmol/L levels were chosen to treat L. chinensis seedlings in the experiment. Natural saline-alkaline soils are very complex systems; therefore, the physiological responses and adaptive strategies were studied in sandy culture experiments, simulating salt or alkali stresses with adding neutral or alkaline salts.
2. Materials and Methods
2.1. Plant Material
The experiment was performed at Northeast Normal University (Jilin Province, China). Mature L. chinensis seeds (with a thousand-seed weight of 2.35 g) were collected from the Grassland Ecosystem Field Station at the Institute of Grassland Science, Songnen Grassland, Jilin Province, China (123°44′E, 44°44′N, 167 ma.s.l.) in July 2008. The field station is a semiarid, continental monsoonal climate with a frost-free period of about 140 days; annual average temperature is 4.9°C and annual precipitation is 360 mm (1999–2008) [22]. The soil pH was 8.4 [23]. The harvested seeds were then stored in paper bags at 4°C until the experiments were carried out in May 2009.
Seeds with a germination rate of over 80% were sown in 21 cm diameter plastic pots containing washed sand. Seven holes were drilled in each pot and 4–6 seeds were sown in each hole. All pots were watered with half-strength Hoagland's nutrient solution daily after the emergence of seedlings. The Hoagland solution consisted of 5.0 mmol/L Ca2+, 2.0 mmol/L Mg2+, 6.0 mmol/L K+, 22.2 μmol/L (EDTA)-Fe2+, 6.7 μmol/L Mn2+, 3.16 μmol/L Cu2+, 0.77 μmol/L Zn2+, 2.1 mmol/L SO4 2−, 1.0 mmol/L H2PO4 −, 46.3 μmol/L H3BO3, 0.56 μmol/L H2MoO4, and 15.0 mmol/L NO3 − [5].
2.2. Stress Conditions and Treatments
A 9 : 1 molar mixture of two neutral salts (NaCl : Na2SO4) or two alkaline salts (NaHCO3 : Na2CO3) was added to half-strength Hoagland's nutrient solution and used for salt and alkali stress treatment groups, respectively. Two concentrations (100 and 200 mmol/L) were applied to each stress group, that is, S100, S200, A100, and A200, and the control (C) received half-strength Hoagland's nutrient solution without salts. The pH ranges in the control, salt, and alkali stress groups were 6.27, 6.43–6.48, and 9.15–9.17, respectively.
When the seedlings were 20 days old, the seedlings were thinned to one plant per hole and each pot contained a total of seven similar sized seedlings. Fifteen pots (5 groups, 3 replications) were placed outdoors and protected from rain. All plants per pot would be divided into leaves, stems, rhizomes, and roots, for determining inorganic ions and organic solutes after grounding. Stress treatments were applied daily (17.00-18.00 h) to 35-day-old seedlings using a total of 500 mL of nutrient solution containing the appropriate stress salts per pot. Controls were treated with the same volume of nutrient solution. The duration of treatment was 10 days, which was when plant leaves in the highest concentration of alkali treatments began to yellow and wilt.
2.3. Growth and Vegetative Reproduction
All plants were harvested in the morning after final treatment and washed with tap water followed by three washes with distilled water. We recorded the number of daughter shoots from tiller (DST), the number of daughter shoots from rhizome (DSR), the rhizome number (RN), and total number of daughter shoots (TDN) in each pot (Figure 1). The rhizome, root, stem, and leaf tissue were separated and oven-dried at 105°C for 15 min. Samples were then dried at 70°C to reach a constant weight, and the weights were recorded (DW).
Figure 1.
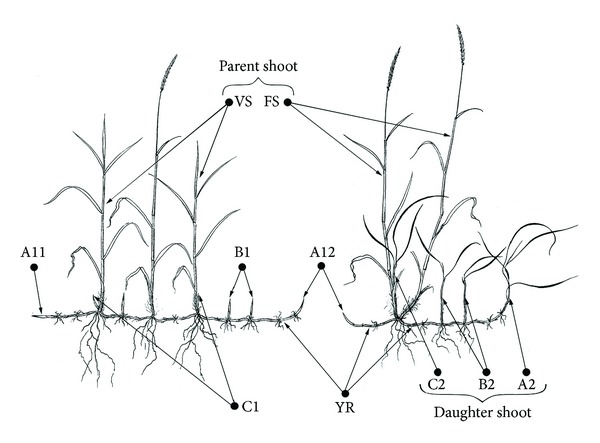
General morphology of Leymus chinensis. Abbreviations: A11, horizontal apical rhizome bud; A12, vertical apical rhizome bud; A2, daughter apical rhizome shoot; B1, axillary rhizome bud; B2, daughter axillary rhizome shoot; C1, shoot bud; C2, daughter axillary shoot; FS, parent flowering shoot; VS, parent vegetative shoot; YR, young rhizome [25]. Here we considered A11, A12, A2, B1, and B2 as the daughter shoots from rhizomes (DSR), C1 and C2 as the daughter shoots from tillers (DST); all these young shoots are included in the total number of daughter shoots (TDN), and YR as rhizome number (RN).
2.4. Inorganic Ions
Dry samples (50 mg of each tissue type) were treated with 10 mL deionized water at 100°C for 1 h, and the extracts were used to determine the inorganic ions and organic solutes.
An atomic absorption spectrophotometer (TAS-990, Purkinje General, Beijing, China) was used to determine the Na+, K+, free Ca2+, and free Mg2+ content. NO3 −, Cl−, SO4 2−, and H2PO4 − were measured by ion chromatography (DX-300 ion chromatographic system, DIONEX, Sunnyvale, USA) using a AS4A-SC ion-exchange column, a CDM-II electrical conductivity detector, and a mobile phase of Na2CO3 : NaHCO3 = 1.7 : 1.8 mM.
2.5. Organic Solutes
Proline and soluble sugar (SS) content were estimated spectrophotometrically using ninhydrin and anthrone, respectively [24]. The organic acid (OA) components (citrate, malate, acetate, succinate, and oxalate) were determined by ion chromatography (DX-300 ion chromatographic system, DIONEX, Sunnyvale, USA) using an ICE-AS6 ion-exclusion column (20°C), a CDM-II electrical conductivity detector, a AMMS-ICE II suppressor, a mobile phase with 0.4 mM heptafluorobutyric acid, a flow rate of 1.0 mL/min, and an injection volume of 50 μL. All sample solutions were filtered through a 0.22 μm filter before analysis. Ultrapure water was used throughout the study. The total OA content was calculated based on cumulative values for the five OA components.
2.6. Statistical Data Analysis
The data were analyzed by one-way factorial ANOVA using the statistical program SPSS 13.0 (SPSS Inc., Chicago, IL, USA). The means and standard errors are reported. Different salt levels were compared using the Least Significant Difference multiple comparison test and different stress treatments were compared by t-tests. Significance level was set at P < 0.05.
3. Results
3.1. Organ Biomass
With increasing solute concentration, the dry weight of all organs decreased significantly (P < 0.05; Table 1) under both stresses. Dry weight reduction was much higher under alkali treatment than under salt stress. Compared to the controls (0.24, 0.16, 0.05, and 0.05 mg/plant), A200 reduced the dry weight of leaves (66.7%), stems (62.5%), rhizomes (60.0%), and roots (60.0%). S200 decreased the dry weight of leaves (33.3%), stems (37.5%), rhizomes (40.0%), and roots (20.0%).
Table 1.
The effects of salt stress (S) and alkali stress (A) on biomass and vegetative reproduction of L. chinensis.
| Stress category | Concentration | Biomass (means ± SE, mg) | Vegetative reproduction (means ± SE,/plant) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Leaf | Stem | Rhizome | Root | DSR | DST | RN | TDN | ||
| Salt stress | C | 0.24 ± 0.003a | 0.16 ± 0.004a | 0.05 ± 0.004a | 0.05 ± 0.007a | 7.57 ± 0.541a | 2.86 ± 0.218a | 3.10 ± 0.048a | 10.43 ± 0.378a |
| S100 | 0.17 ± 0.007b | 0.11 ± 0.005b | 0.04 ± 0.005b | 0.05 ± 0.006a | 5.38 ± 0.497b | 1.90 ± 0.265b | 2.81 ± 0.454a | 7.29 ± 0.756b | |
| S200 | 0.16 ± 0.018b | 0.10 ± 0.012b | 0.03 ± 0.003c | 0.04 ± 0.002b | 4.38 ± 0.172b | 1.90 ± 0.126b | 2.29 ± 0.000b | 6.29 ± 0.286b | |
|
| |||||||||
| Alkali stress | C | 0.24 ± 0.003a | 0.16 ± 0.004a | 0.05 ± 0.004a | 0.05 ± 0.007a | 7.57 ± 0.541a | 2.86 ± 0.218a | 3.10 ± 0.048a | 10.43 ± 0.378a |
| A100 | 0.19 ± 0.012b | 0.12 ± 0.008b | 0.04 ± 0.007b | 0.04 ± 0.004b | 5.86 ± 1.072a | 1.53 ± 0.126b | 2.38 ± 0.290b | 7.38 ± 1.061b | |
| A200 | 0.08 ± 0.008c | 0.06 ± 0.005c | 0.02 ± 0.002c | 0.02 ± 0.003c | 1.90 ± 0.265c | 1.43 ± 0.082b | 1.71 ± 0.143c | 3.33 ± 0.208c | |
The means and standard errors are reported. C: without salt or alkali stresses, S100: salt stress with 100 mmol/L concentration, S200: salt stress with 200 mmol/L concentration, A100: alkali stress with 100 mmol/L concentration, and A200: alkali stress with 200 mmol/L concentration. In the same stress category, within each column, the different letters indicate significant difference among treatments (n = 3, P < 0.05). DSR: daughter shoots from rhizome; DST: daughter shoots from tillers; RN: rhizome number; TDN: total daughter number.
3.2. Vegetative Reproduction
Under both stresses, vegetative reproductive indices were all markedly lower as the concentration increased (P < 0.05; Figure 1 and Table 1). Compared to the controls (10.43, 7.57, 2.86, and 3.10/plant), A200 treatment decreased the TDN (68.0%), DSR (74.8%), DST (50.0%), and RN (44.6%), while S200 decreased the TDN (39.7%), DSR (42.1%), DST (33.3%), and RN (26.2%).
3.3. Inorganic Ions
The Na+ content in all organs increased sharply with the increasing concentration under both stresses (Figure 2). Under salt stress, the Na+ content in leaf, stem, rhizome, and root increased 9.8-, 9.3-, 16.6-, and 12.2-fold compared to the controls (22.03, 27.39, 35.06, and 47.54 μmol/g); Na+ increased more in rhizome and root than in stem and leaf. The concentration of Na+ was higher in rhizome and root than in stem and leaf at A100, while Na+ level was higher in leaf (26.8-fold) and stem (32.6-fold) than in rhizome (18.2-fold) and root (13.8-fold) at A200 (Figure 2). The Na+ content was not significantly different between salt and alkali stresses in any of the organs except the root at 100 mmol/L. But the Na+ content in stem and leaf was much higher at A200 than at S200 (P < 0.05; Figure 2).
Figure 2.
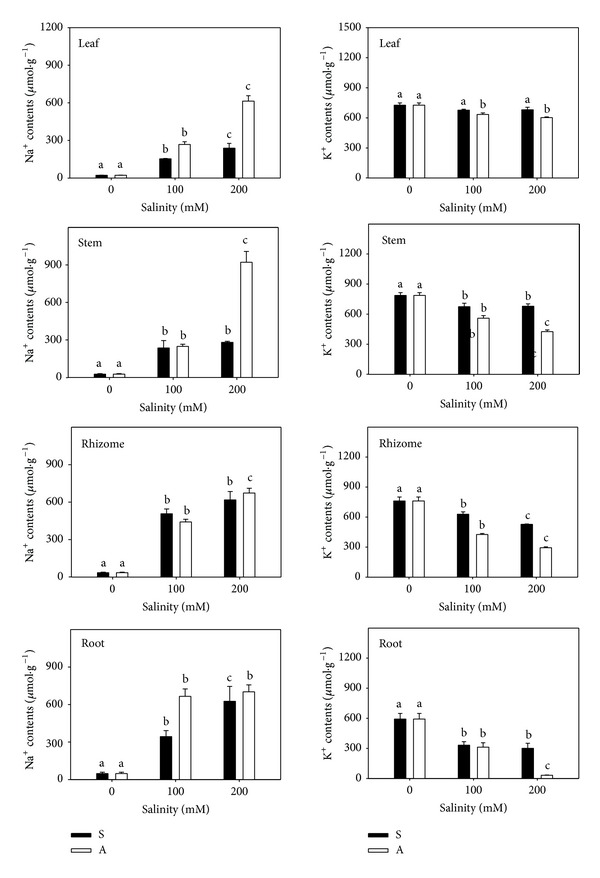
The Na+ and K+ contents. The effects of salt stress (S) and alkali stress (A) on Na+ and K+ contents in rhizomes, stems, roots, and leaves of L. chinensis. There are three levels under both stresses: 0, 100, and 200 mmol/L. The means and standard errors are reported. Within each column, the different letters indicate significant difference among treatments (P < 0.05).
The level of K+ in all organs decreased significantly. Under salt stress, the K+ decrease in root (30.8%) and rhizome (49.2%) was much higher than in stem (13.7%) and leaf (6.3%) compared with the control (726.51, 786.59, 761.56, and 593.40 μmol/g). Under alkali stress, the K+ decrease was notably higher for root (94.6%) and rhizome (61.5%) than stem (45.9%) and leaf (17.1%). There was no significant difference in the K+ level of all organs between S100 and A100, but the decrease of K+ was higher at A200 than at S100 (P < 0.05; Figure 2).
Ca2+ content decreased under salt stress but increased significantly under alkali stress. Compared to the controls (4.04, 1.79, 2.15, and 1.76 μmol/g), the Ca2+ content of leaf, stem, rhizome, and root increased 46.0%, 75.8%, 71.2%, and 700%, respectively. Roots had the greatest accumulation of Ca2+ under alkali stress. The Mg2+ content decreased significantly (Figure 3) in all organs under salt stress, but it only decreased in rhizomes under alkali stress, with no change in leaf and root and a small increase in stems.
Figure 3.
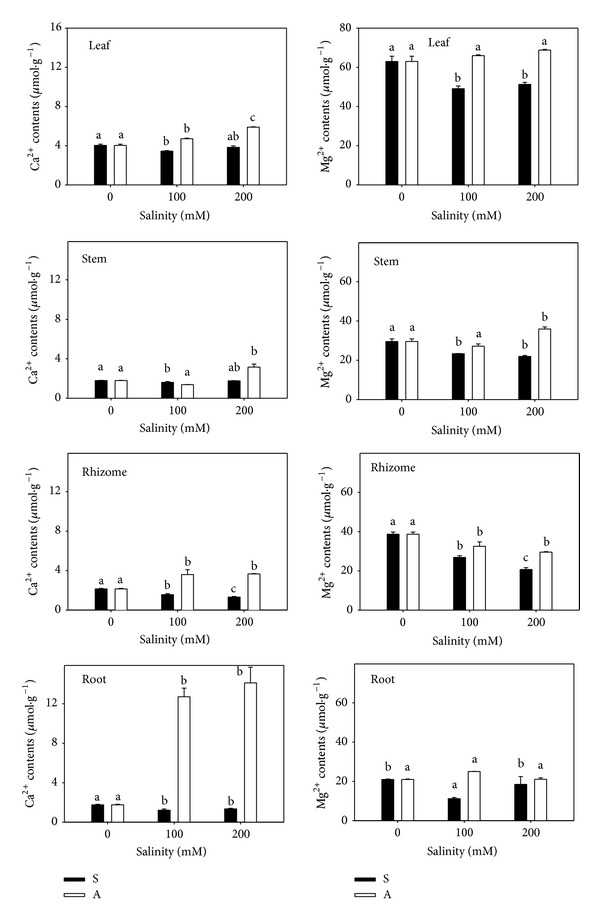
The Ca2+ and Mg2+ contents. The effects of salt stress (S) and alkali stress (A) on the Ca2+ and Mg2+ contents in rhizomes, stems, roots, and leaves of L. chinensis. There are three levels under both stresses: 0, 100, and 200 mmol/L. The means and standard errors are reported. Within each column, the different letters indicate significant difference among treatments (P < 0.05).
With increasing concentration, the Cl− content increased sharply in all organs under salt stress (Figure 4). The increase was higher in root (6.6-fold) and rhizome (7.8-fold) than in leaf (4.7-fold) and stem (4.4-fold). Under alkali stress, the Cl− content changed little compared to salt stress (Figure 4).
Figure 4.
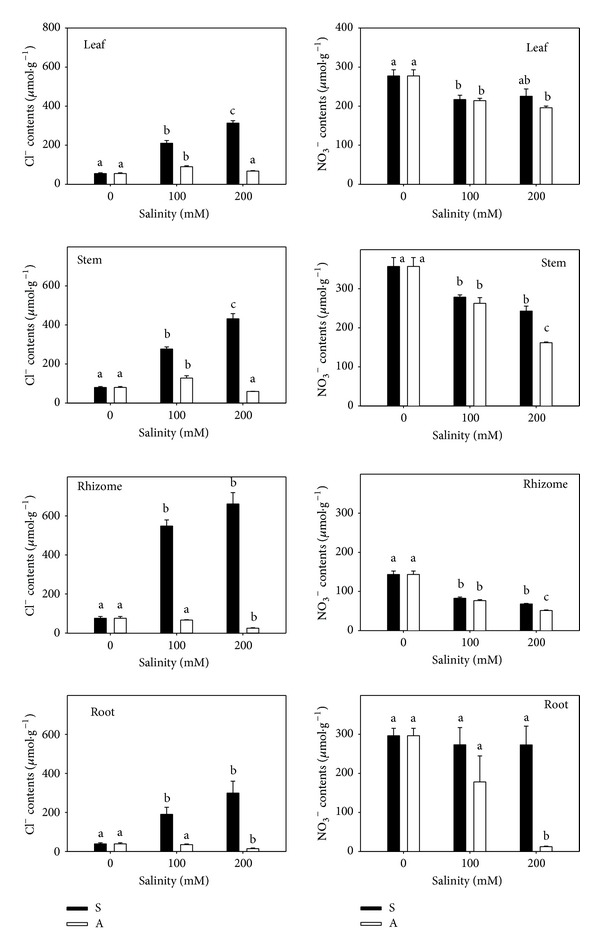
The Cl− and NO3 − contents. The effects of salt stress (S) and alkali stress (A) on the Cl− and NO3 − content in rhizomes, stems, roots, and leaves of L. chinensis. There are three levels under both stresses: 0, 100, and 200 mmol/L. The means and standard errors are reported. Within each column, the different letters indicate significant difference among treatments (P < 0.05).
The NO3 − content decreased significantly under both stresses. Under salt stress, the NO3 − content in rhizome (52.4%) decreased more than in root (7.9%), stem (32.0%), and leaf (18.8%) compared with the control (277.44, 356.84, 143. 43, and 296.47 μmol/g). Under alkali stress, the underground organs root (95.8%) and rhizome (64.2%) had greater reductions in NO3 − than did stem (54.7%) and leaf (29.4%).
The effect on H2PO4 − and SO4 2− differed between the two stresses. H2PO4 − content changed little under salt stress, but it decreased significantly in all organs under alkali stress. The H2PO4 − decrease in root (93.0%) was greater than in rhizome (53.8%), stem (42.6%), and leaf (55.2%) compared with the control (328.04, 355.32, 365.22, and 391.56 μmol/g). The SO4 2− content increased markedly under salt stress (Figure 5), but it was reduced by alkali stress (P < 0.05).
Figure 5.
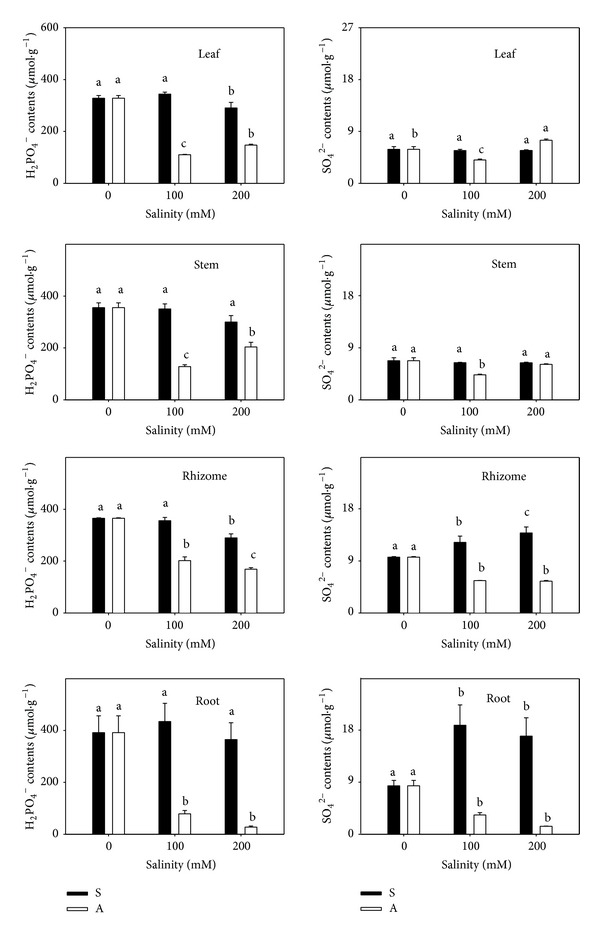
The H2PO4 − and SO4 2− contents. The effects of salt stress (S) and alkali stress (A) on H2PO4 − and SO4 2− content in rhizomes, stems, roots, and leaves of L. chinensis. There are three levels under both stresses: 0, 100, and 200 mmol/L. The means and standard errors are reported. Within each column, the different letters indicate significant difference among treatments (P < 0.05).
3.4. Organic Solutes
The SS contents did not change significantly under salt stress, but they increased slightly under alkali stress (Figure 6). The proline content changed with the stress type, concentration, and plant organ. Neither S100 nor A100 induced the accumulation of proline. However, proline was accumulated in some organs under S200 and A200. Under salt stress, only rhizome and root significantly accumulated proline (P < 0.05; Figure 6), and the increase was 5.2- and 1.9-fold greater than the controls (1.54 and 1.32 μmol/g). Under alkali stress, proline accumulated sharply in leaf, stem, and rhizome, and the increase was 35.2-, 18.0-, and 5.9-fold compared to controls (1.87, 2.42, and 1.54 μmol/g). The proline content under alkali stress was far higher than under salt stress.
Figure 6.
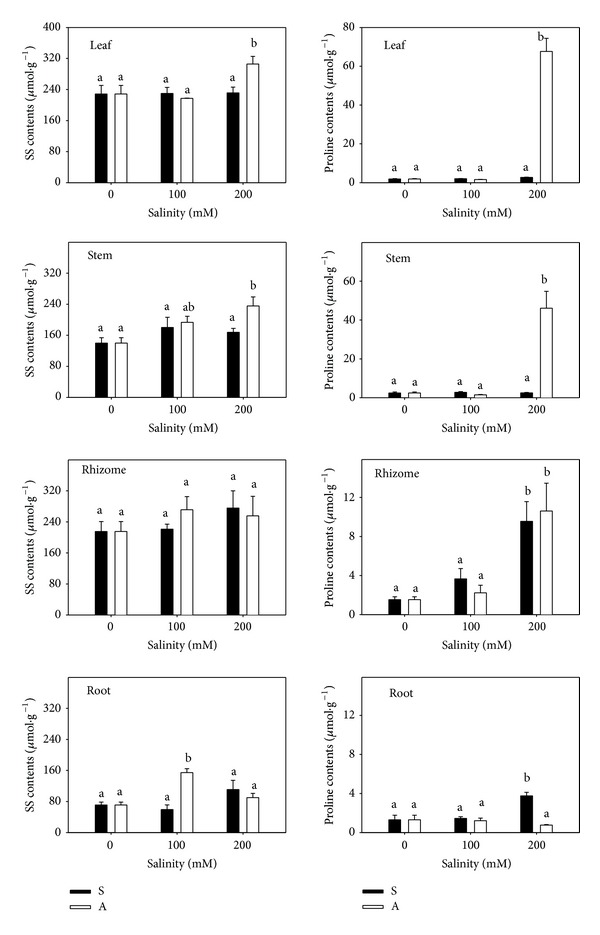
The SS and proline contents. The effects of salt stress (S) and alkali stress (A) on the SS and proline content in rhizomes, stems, roots, and leaves of L. chinensis. There are three levels under both stresses: 0, 100, and 200 mmol/L. The means and standard errors are reported. Within each column, the different letters indicate significant difference among treatments (P < 0.05).
Total OA did not change significantly under salt stress (P > 0.05; Figure 7) but accumulated markedly under alkali stress (P < 0.05; Figure 7). The increase in OAs in leaf, stem, rhizome, and root was 0-, 0.4-, 1.1-, and 7.4-fold at A100 and 2.4-, 5.5-, 3.4-, and 1.5-fold at A200 in comparison to the controls (68.54, 58.74, 60.17, and 33.70 μmol/g). With increasing alkali treatment concentration, the site of OA accumulation changed from root to leaf. In roots, OA accumulation peaked at A100 and decreased at A200. This pattern differed from rhizome, stem, and leaf, which had increased OA accumulation with increased alkalinity. Like total OA, OA components were unchanged under salt stress, but they accumulated significantly under alkali stress (Figures 7 and 8). Malate and citrate were the key OA components in all organs, and their concentration was highest in the A100 treatment in roots.
Figure 7.
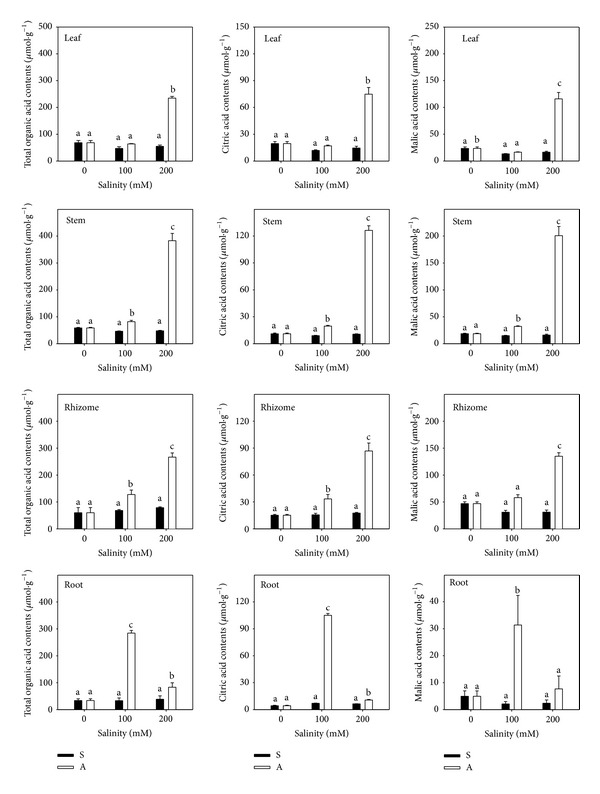
The generous organic acids content. The effect of salt stress (S) and alkali stress (A) on total organic acids, citric acid, and malic acid contents in rhizomes, stems, roots, and leaves of L. chinensis. There are three levels under both stresses: 0, 100, and 200 mmol/L. The means and standard errors are reported. Within each column, the different letters indicate significant difference among treatments (P < 0.05).
Figure 8.
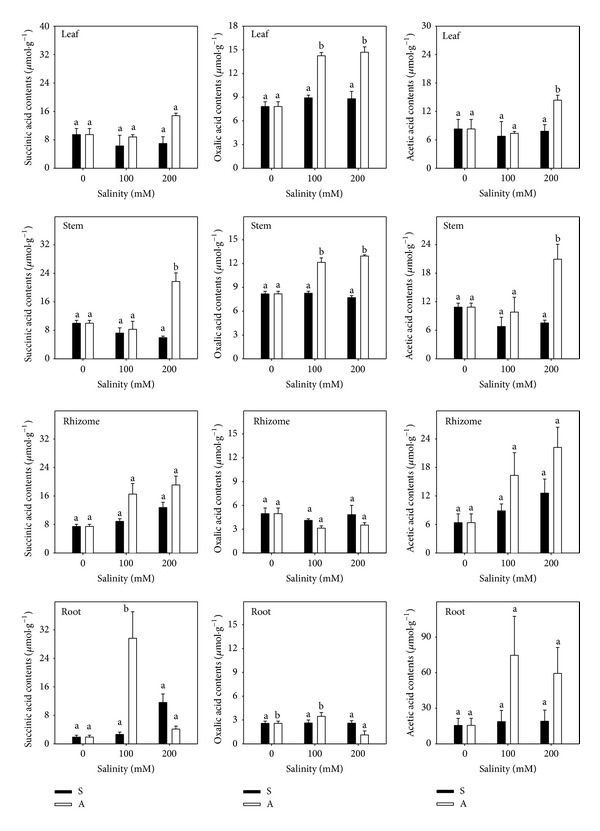
The micro organic acids components contents. The effect of salt stress (S) and alkali stress (A) on the succinic acid, acetic acid, and oxalic acid contents in rhizomes, stems, roots, and leaves of L. chinensis. There are three levels under both stresses: 0, 100, and 200 mmol/L. The means and standard errors are reported. Within each column, the different letters indicate significant difference among treatments (P < 0.05).
4. Discussion
The effects of salt and alkali stresses on organs biomass and vegetative reproduction were different. No significant difference was found between S100 and S200, but the biomass and vegetative reproduction reduced higher at A200 than at A100. The results indicated that the growth tolerances of L. chinensis to salt stress and alkali stresswere similar at 100 mmol/L and separated at 200 mmol/L. The phenomenon was in accordance with the photosynthesis activity in our previous study [26], which proved that there existed more destructive effects of alkali stress than salt stress.
The salt-tolerance of the aerial and underground organs differed. Some studies indicated that the shoot was more sensitive to salt than the root, as was also seen in young umbu plants [27], but in maize [28] and wheat [29], the root was more sensitive than shoot. In our study, both salt stress and alkali stress reduced the organ biomass of L. chinensis significantly. Decrement in rhizome weight was the largest of all organs surveyed, indicating that the rhizome was more sensitive than the other organs. Rhizome in L. chinensis bore the brunt of damage from the alkali or salt, sharing the negative effects of salt and alkali stress on other organs for L. chinensis growing.
L. chinensis was a rhizomatous and perennial forage grass, which formed populations by clonal reproduction of tiller nodes and rhizomes. The daughter plants formed from the tiller nodes and rhizomes were important components of L. chinensis populations. During the growing season, the tiller nodes and rhizomes had buds that germinated, which we designated daughters shoots from tillers (DST) and daughters shoots from rhizomes (DSR) (Figure 1). Salt stress usually caused a considerable reduction in tiller and rhizome number in rhizomatous forage grasses, such as Spartina alterniflora Loisel [30]. A similar result was found in our experiments. DSR, DST, and RN decreased in salt and alkali stress significantly. These results indicated that the clonal organs of L. chinensis were affected by both types of stress, leading to a decrease in vegetative reproduction (Table 1). The reduction of clonal reproduction in L. chinensis might allow an individual to be able to alleviate and resist the harmful effects of salt and alkali stress. The more decrement of DSR than DST indicated that the capacity for L. chinensis population expansion by rhizome was more affected than population expansion by tiller under salt stress.
Na+ was a key toxic cation of salt stress, and neither halophytes nor glycophytes could tolerate large amounts of Na+ in the cytoplasm. Under saline conditions, they either restricted the excess Na+ in the vacuole or compartmentalized the ions into different tissues to facilitate their metabolic functions [31, 32]. The compartmentalization of Na+ was an essential mechanism used by all plants rather than the evolution of tolerance of enzymatic functions in plants from saline environments [11]. Parida and Das (2005) [31] indicated that one of the mechanisms of salt tolerance was the control of ion uptake by roots and transport into leaves. Such as, wheat accumulated Na+ mainly in roots, which reduced the harmful effect of Na+ on shoots [33]. When L. chinensis seedlings grew under salt or low alkali stresses, the Na+ content was higher in root and rhizome than that in stem and leaf. This phenomenon indicated that L. chinensis might retain excess Na+ in the rhizome and root and control Na+ transport to stem and leaf. In saline soils, Na+ that entered root cells in the outer part of root was likely pumped back out again via plasma membrane Na+/H+ antiporters encoded by the gene SOS1 [34]. High sodic salt levels induced the expression of amiloride-resistant Na+/H+ antiporter that could account for the remarkable tolerance to NaCl [35]. High pH condition of alkali stress led to the lacking of H+ in the outer part of root in L. chinensis seedlings. Therefore, the high levels of Na+ and lacking of H+ around L. chinensis root resulted in a high Na+ and H+ concentration gradients existing between intracellular and extracellular parts of root, which made it easier for Na+/H+ antiporters to export H+ and import excess Na+. With increasing alkalinity, the Na+ and H+ concentration gradients increased. Thus, sharp increase of Na+ in L. chinensis root happened (Figure 2). Excess Na+ absorption in root exceeded the carrying capacity of root and rhizome and led to the sharp increases of Na+ in stem and leaf.
Plant accumulation of Na+ can reduce the absorption of other cations. Dudeck and Peacock (1993) reported that Na+, Mg2+, K+, and Ca2+ competed for absorption in several paspalum plants they studied [36]. Nutrient deficiency can be caused by salt stress due to competition between Na+ and other nutrients. In our study, the absorption of Na+ only competed with K+ under both stresses. Ca2+ kept being unchanged under salt stress, even increased under alkali stress. The transient Ca2+ increase potentiated stress signal transduction and led to salt adaptation [37]. L. chinensis accumulated Ca2+ significantly under alkali stress (Figure 3), indicating that Ca2+ might be a unique signal response of L. chinensis to high pH.
Plants usually accumulated inorganic anions (e.g., Cl−, NO3 −, SO4 2−, or H2PO4 −) to balance excess Na+ [38]. Na+ and Cl− were much cheaper source of osmotic solute because ion uptake and accumulation require less energy compared to synthesis of organic solutes [39]. The content and increment of Cl− in rhizomes were far higher than in other organs, indicating that Cl− was one of main physiological response solutes. As the key nutritional sources for plants, NO3 − and H2PO4 − played important roles in plant growth. However, both stresses inhibited their absorption, especially alkali stress. Alkali stress severely restricted the absorption of nutrient elements such as N and P (Figures 4 and 5). Among the vegetative organs, rhizome had the highest accumulation of Na+ and Cl− and the lowest levels of NO3 − and K+, followed by roots. The presence of the rhizome reduced the injury on root, stem, and leaf caused by salt and alkali stress.
A component of the cellular compartmentation model of salt tolerance at the cellular level was the need for the accumulation of metabolically “compatible” (organic) solutes in the cytoplasm to balance the osmotic potential of the Na+ and Cl− accumulated in the vacuole [40]. These compatible solutes included mainly proline, glycine betaine, sugars, and polyols [31]. Under salt and alkali stresses, the SS content did not change significantly, and we therefore concluded that SS were not the most important response solutes for osmotic adjustment in L. chinensis. Proline was one of amino acids that distributed in plants widely and it accumulated in higher amounts than other amino acids in salt-stressed plants [41, 42]. Proline acted as a signalling/regulatory molecule able to activate multiple responses that were components of adaptation to abiotic stress including salt stress [43]. Some reports argued that increased proline under stress is a product of but not an adaptive response to stress [44, 45]. The proline content did not increase at low concentrations, but it increased at high concentrations under salt and alkali stress, indicating that the accumulation of proline did not occur in response to salt stress but was a product of damage from salt stress (Figure 6).
The deficit of negative charge and ion imbalance under alkali stress caused plants to accumulate organic acids to balance the excess cations and supply the negative charge; thus an increase in OA was a physiological response of alkaline-tolerant plants to alkali stress [1, 25, 29]. OA metabolism played a key role in adjusting stable pH, which could decrease the cellular water potential and increased the activity of mechanisms to avoid cell physiological drought [46]. The OA of L. chinensis only increased significantly under alkali stress, especially in stem and leaf at 200 mmol/L. The phenomenon corresponded to excess positive charge caused by sharp increase in Na+ (Figure 7).
Under alkali stress, Chenopodiaceae halophytes such as Suaeda glauca accumulated oxalate, which comprised more than 90% of total OA [5]. Graminaceous halophytes such as Puccinellia tenuiflora accumulated citrate, which comprised more than 90% of total OA [47]. In L. chinensis, malate and citrate were the most abundant OA components in all organs, indicating that plants used different mechanisms of OA metabolism under alkali stress. The proportion of malate and citrate to total OA in leaf, stem, rhizome, and root was 81.3%, 85.5%, 83.2%, and 55.9%, respectively (Figure 8). Similar to total OA, malate and citrate increased then decreased in root but increased in other organs with increasing alkalinity. This change of OA contents in root might be related to the root injury. The OA metabolism of different organs L. chinensis under alkali stress was still not sure and needed further study.
Our research discussed the growth and physiological responses of vegetative organs in L. chinensis seedlings to short time stresses (10 days) caused from salt or alkali conditions. Inorganic ions distribution and organic solutes accumulation in different organs of L. chinensis seedlings showed the tolerance and adaptive strategies, especially in rhizomes. The effect of long exposure to saline or alkaline conditions on productivity and forage quality of L. chinensis should be researched in the future.
5. Conclusion
L. chinensis was considered one of the most promising grass species for grassland rehabilitation and restoration in northern of China. That is because it was a rhizomatous, perennial grass. From the results of our study, the salt tolerance of the aerial and underground organs differed in L. chinensis seedlings. Rhizome was more sensitive than the other organs, which had the highest accumulation of Na+ and Cl− and the lowest levels of NO3 − and K+, followed by roots. At the same time, the more decrement of DSR than of DST indicated that the capacity for L. chinensis population expansion by rhizome was more affected than population expansion by tiller under salt stress. The presence of the rhizome reduced the injury on root, stem, and leaf caused by salt and alkali stress.
SS were not the most important response solutes for osmotic adjustment in L. chinensis. Proline was a product of damage from salt stress. The significant accumulation of OA in stem and leaf at 200 mmol·g−1 corresponded to excess positive charge caused by sharp increase in Na+. Malate and citrate were the most abundant OA components in all organs, indicating that plants used different mechanisms of OA metabolism under alkali stress.
Acknowledgments
The authors thank D. Wang and Y. P. Li for their help in the laboratory. The research was funded by National Natural Science Foundation of China (31172259, 31370432) and the Research Fund for the Doctoral Program of Higher Education (20120043110010).
Abbreviations
- DST:
Daughter shoot from tiller
- DSR:
Daughter shoot from rhizome
- RN:
Rhizome number
- TDN:
Total daughter shoot number
- SS:
Soluble sugar
- OA:
Organic acid
- C:
Control.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Yang CW, Chong JN, Li CY, Kim CM, Shi DC, Wang DL. Osmotic adjustment and ion balance traits of an alkali resistant halophyte Kochia sieversiana during adaptation to salt and alkali conditions. Plant and Soil. 2007;294(1-2):263–276. [Google Scholar]
- 2.Läuchli A, Lüttge U. Salinity in the soil environment. In: Tanji KK, editor. Salinity: Environment—Plants—Molecules. Boston, Mass, USA: Kluwer Academic Publishers; 2002. [Google Scholar]
- 3.Kawanabe S, Zhu TC. Degeneration and conservation of Aneurolepidium chinense grassland in Northern China. Japanese Society of Grassland Science. 1991;37:91–99. [Google Scholar]
- 4.Shi DC, Yin LJ. Difference between salt (NaCl) and alkaline (Na2CO3) stresses on Puccinellia tenuiflora(Griseb.)Scribn. et Merr. plants. Acta Botanica Sinica. 1993;35:144–149. [Google Scholar]
- 5.Yang C, Shi D, Wang D. Comparative effects of salt and alkali stresses on growth, osmotic adjustment and ionic balance of an alkali-resistant halophyte Suaeda glauca (Bge.) Plant Growth Regulation. 2008;56(2):179–190. [Google Scholar]
- 6.Munns R. Comparative physiology of salt and water stress. Plant, Cell and Environment. 2002;25(2):239–250. doi: 10.1046/j.0016-8025.2001.00808.x. [DOI] [PubMed] [Google Scholar]
- 7.Flowers TJ, Colmer TD. Salinity tolerance in halophytes. New Phytologist. 2008;179(4):945–963. doi: 10.1111/j.1469-8137.2008.02531.x. [DOI] [PubMed] [Google Scholar]
- 8.Zhang JT, Mu CS. Effects of saline and alkaline stresses on the germination, growth, photosynthesis, ionic balance and anti-oxidant system in an alkali-tolerant leguminous forage Lathyrus quinquenervius . Soil Science & Plant Nutrition. 2009;55(5):685–697. [Google Scholar]
- 9.Blumwald E. Sodium transport and salt tolerance in plants. Current Opinion in Cell Biology. 2000;12(4):431–434. doi: 10.1016/s0955-0674(00)00112-5. [DOI] [PubMed] [Google Scholar]
- 10.Chinnusamy V, Jagendorf A, Zhu J. Understanding and improving salt tolerance in plants. Crop Science. 2005;45(2):437–448. [Google Scholar]
- 11.Munns R, Tester M. Mechanisms of salinity tolerance. Annual Review of Plant Biology. 2008;59:651–681. doi: 10.1146/annurev.arplant.59.032607.092911. [DOI] [PubMed] [Google Scholar]
- 12.Flowers TJ, Hajibagheri MA, Clipson NJW. Halophytes. Quarterly Review of Biology. 1986;61(3):313–337. [Google Scholar]
- 13.Chen MJ, Jia SX. Fodder Plants of China. Beijing, China: Agricutural Press of China; 2002. (Chinese) [Google Scholar]
- 14.Huang ZH, Zhu JM, Mu XJ, Lin JX. Advances on the mechanism of low sexual reproductivity of Leymus chinensis. Grassland of China. 2002;24:55–60. [Google Scholar]
- 15.Yang YF, Liu GC, Zhang BT. Analysis of age structure and the strategy for asexual propagation of Aneurolepidium chinense (Trin.) Kitag population. Acta Botanica Sinica. 1995;37:147–153. [Google Scholar]
- 16.Liu GS, Qi DM. Research progressonthe biology of Leymus chinensis. Acta Pratacultuae Sinica. 2004;13:6–11. [Google Scholar]
- 17.Jin H, Plaha P, Park JY, et al. Comparative EST profiles of leaf and root of Leymus chinensis, a xerophilous grass adapted to high pH sodic soil. Plant Science. 2006;170(6):1081–1086. [Google Scholar]
- 18.Li JD. Aneurolepidium Chinense grassland in China. Journal of Northeast Normal University. 1978;1:145–159. [Google Scholar]
- 19.Ding X-M, Yang Y-F. Variations of water-soluble carbohydrate contents in different age class modules of leymus chinensis populations in sandy and saline-alkaline soil on the songnen plains of China. Journal of Integrative Plant Biology. 2007;49(5):576–581. [Google Scholar]
- 20.Jin H, Kim HR, Plaha P, et al. Expression profiling of the genes induced by Na2CO3 and NaCl stresses in leaves and roots of Leymus chinensis . Plant Science. 2008;175(6):784–792. [Google Scholar]
- 21.Sun YL, Hong SK. Effects of plant growth regulators and L-glutamic acid on shoot organogenesis in the halophyte Leymus chinensis (Trin.) Plant Cell, Tissue and Organ Culture. 2010;100(3):317–328. [Google Scholar]
- 22.Wang JF, Xie JF, Zhang YT, Gao S, Zhang JT, Mu CS. Methods to improve seed yield of Leymus Chinensis based on nitrogen application and precipitation analysis. Agronomy Journal. 2010;102(1):277–281. [Google Scholar]
- 23.Wang JF, Mu CS, Zhang JT, Gao S, Li XY. Effects of fertilizer on the sexual reproduction of Leymus chinensis. Acta Pratacultuae Sinica. 2008;17(3):53–58. [Google Scholar]
- 24.Zhu GL, Deng XW, Zuo WN. Determination of free proline plants. Plant Physiology Communications. 1983;1:35–37. [Google Scholar]
- 25.Zhang J, Mu C, Wang D, Wang J, Chen G. Shoot population recruitment from a bud bank over two seasons of undisturbed growth of Leymus chinensis . Botany. 2009;87(12):1242–1249. [Google Scholar]
- 26.Li XY, Lin JX, Li XJ, Mu CS. Growth adaptation and Na+ and K+ metabolism responses of Leymus chinensis seedlings under salt and alkali stresses. Acta Prataculturae Sinica. 2013;22(1):201–209. (Chinese with English Abstract) [Google Scholar]
- 27.da Silva EC, Nogueira RJMC, de Araújo FP, de Melo NF, de Azevedo Neto AD. Physiological responses to salt stress in young umbu plants. Environmental and Experimental Botany. 2008;63(1–3):147–157. [Google Scholar]
- 28.Hajlaoui H, Ayeb NE, Garrec JP, Denden M. Differential effects of salt stress on osmotic adjustment and solutes allocation on the basis of root and leaf tissue senescence of two silage maize (Zea mays L.) varieties. Industrial Crops and Products. 2010;31(1):122–130. [Google Scholar]
- 29.Li XY, Liu JJ, Zhang YT, Lin JX, Mu CS. Physiological responses and adaptive strategies of wheat seedlings to salt and alkali stresses. Soil Science & Plant Nutrition. 2009;55(5):680–684. [Google Scholar]
- 30.Li R, Shi F, Fukuda K. Interactive effects of various salt and alkali stresses on growth, organic solutes, and cation accumulation in a halophyte Spartina alterniflora (Poaceae) Environmental and Experimental Botany. 2010;68(1):66–74. [Google Scholar]
- 31.Parida AK, Das AB. Salt tolerance and salinity effects on plants: a review. Ecotoxicology and Environmental Safety. 2005;60(3):324–349. doi: 10.1016/j.ecoenv.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 32.Zhu J. Regulation of ion homeostasis under salt stress. Current Opinion in Plant Biology. 2003;6(5):441–445. doi: 10.1016/s1369-5266(03)00085-2. [DOI] [PubMed] [Google Scholar]
- 33.Saqib M, Zörb C, Rengel Z, Schubert S. The expression of the endogenous vacuolar Na+/H+ antiporters in roots and shoots correlates positively with the salt resistance of wheat (Triticum aestivum L.) Plant Science. 2005;169(5):959–965. [Google Scholar]
- 34.Munns R, James RA, Läuchli A. Approaches to increasing the salt tolerance of wheat and other cereals. Journal of Experimental Botany. 2006;57(5):1025–1043. doi: 10.1093/jxb/erj100. [DOI] [PubMed] [Google Scholar]
- 35.Tartari A, Forlani G. Osmotic adjustments in a psychrophilic alga, Xanthonema sp. (Xanthophyceae) Environmental and Experimental Botany. 2008;63(1–3):342–350. [Google Scholar]
- 36.Dudeck AE, Peacock CH. Salinity effects on growth and nutrient uptake of selected water season turf. International Turfgrass Society Research Journal. 1993;7:680–686. [Google Scholar]
- 37.Knight H, Trewavas AJ, Knight MR. Calcium signalling in Arabidopsis thaliana responding to drought and salinity. The Plant Journal. 1997;12(5):1067–1078. doi: 10.1046/j.1365-313x.1997.12051067.x. [DOI] [PubMed] [Google Scholar]
- 38.Sagi M, Dovrat A, Kipnis T, Lips H. Ionic balance, biomass production, and organic nitrogen as affected by salinity and nitrogen source in annual ryegrass. Journal of Plant Nutrition. 1997;20(10):1291–1316. [Google Scholar]
- 39.Greenway H, Munns R. Interactions between growth, uptake of Cl− and Na+, and water relations of plants in saline environments. Plant Cell and Environment. 1983;6:575–589. [Google Scholar]
- 40.Wyn JG, Gorham J. Intra- and inter-cellular compartmentation of ions. In: Läuchli A, Lüttge U, editors. Salinity: Environment-Plants-Molecules. Dordrecht, The Netherlands: Springer; 2002. [Google Scholar]
- 41.Ashraf M. Breeding for salinity tolerance in plants. Critical Reviews in Plant Sciences. 1994;13(1):17–42. [Google Scholar]
- 42.Ali G, Srivastava PS, Iqbal M. Proline accumulation, protein pattern and photosynthesis in Bacopa monniera regenerants grown under NaCl stress. Biologia Plantarum. 1999;42(1):89–95. [Google Scholar]
- 43.Maggio A, Miyazaki S, Veronese P, et al. Does proline accumulation play an active role in stress-induced growth reduction? Plant Journal. 2002;31(6):699–712. doi: 10.1046/j.1365-313x.2002.01389.x. [DOI] [PubMed] [Google Scholar]
- 44.Lutts S, Majerus V, Kinet JM. NaCl effects on proline metabolism in rice (Oryza sativa) seedlings. Physiologia Plantarum. 1999;105(3):450–458. [Google Scholar]
- 45.de Lacerda CF, Cambraia J, Oliva MA, Ruiz HA, Prisco JT. Solute accumulation and distribution during shoot and leaf development in two sorghum genotypes under salt stress. Environmental and Experimental Botany. 2003;49(2):107–120. [Google Scholar]
- 46.Yang CW, Wang P, Li CY, Shi DC, Wang DL. Comparison of effects of salt and alkali stresses on the growth and photosynthesis of wheat. Photosynthetica. 2008;46(1):107–114. [Google Scholar]
- 47.Shi DC, Yin SJ, Yang GH, Zhao KF. Citric acid accumulation in an alkali-tolerant plant Puccinellia tenuiflora under alkaline stress. Acta Botanica Sinica. 2002;44(5):537–540. [Google Scholar]


