Abstract
In the present study, we demonstrate that, in analogy with the genes encoding ESAT-6 and CFP-10, the genes rv0287 and rv0288 from the ESAT-6 gene family are cotranscribed. Using Western-Western blotting and protein-print overlay methodologies, we demonstrate that ESAT-6 and CFP-10, as well as the protein pair Rv0288/Rv0287, interact pairwise in a highly specific way. Most notably, the ESAT-6 proteins interact directly with Rv3873, a possible cell envelope component of the ESAT-6 secretion pathway.
The 6-kDa early secretory antigenic target (ESAT-6) and the 10-kDa culture filtrate protein (CFP-10) from Mycobacterium tuberculosis are two dominant targets for T cells in the early phases of infection (1, 25, 27). Furthermore, ESAT-6 has recently been demonstrated to induce protective immunity when administered as either a subunit (5) or a DNA (12) vaccine. The genes encoding ESAT-6 and CFP-10 (esx and lhp, respectively) lie next to each other in an operon-like structure (4). A dual knockout of ESAT-6 and CFP-10 in M. bovis results in decreased virulence of the pathogen (29), indicating that the two molecules may play important roles in immunopathogenesis and virulence. Recent genomic and genetic studies have revealed that lhp and esx and their neighboring genes constitute a gene cluster (esx cluster 1), which encodes a cellular function that is fundamental for the virulence of M. tuberculosis (15, 19, 28).
Both ESAT-6 and CFP-10 are low-molecular-mass proteins that belong to a large protein family, the ESAT-6 family, which has 23 members in M. tuberculosis. When M. tuberculosis is grown in broth culture, these proteins are released into the surroundings (2, 4, 23) through a Sec-independent pathway, since none of the proteins contain common signal peptides. The genes encoding ESAT-6 family proteins are arranged in tandem pairs at 11 loci in M. tuberculosis H37Rv and are often preceded by a pe-ppe gene pair. At five loci, the esx-like gene pairs are part of larger gene clusters that have genetic contexts similar to that of the esx cluster 1. These clusters characteristically include a core region, consisting of a pair of esx-like genes next to a pe-ppe gene pair, flanked by open reading frames (ORFs) encoding potential membrane proteins, serine proteinases, and putative ABC transporters. It has been suggested that these gene clusters (the esx clusters) encode novel transporter systems that are responsible for the secretion of ESAT-6-like proteins (9, 18, 28). Experimental evidence that supports this theory is now available (20).
Two of the esx clusters are conserved in M. leprae (9); one is the esx cluster 1, while the other is the esx cluster 3 encoding ESAT-6 family members Rv0287 and Rv0288. As it has been suggested that M. leprae may contain the minimum gene set required by a pathogenic mycobacterium (7), the function represented by the esx cluster 3 may thus also be essential for the pathogenesis of M. tuberculosis. In fact, although large numbers of genetic deletions have been identified in clinical isolates of M. tuberculosis, none of them occurred in the esx cluster 3 (13). Comparing gene expression in virulent M. tuberculosis H37Rv and the avirulent H37Ra, Rindi et al. showed that rv0288 expression was markedly down-regulated in the attenuated strain (22), and the newly reported comprehensive identification of essential genes in M. tuberculosis included the esx cluster 3 in the list of 600 genes that are essential for in vitro growth (24). Recent immunological studies have demonstrated that Rv0288 is a potent T-cell antigen strongly recognized in M. tuberculosis-infected humans, as well as in cattle infected with M. bovis (1, 25, 26). As those to ESAT-6 and CFP-10, the immune response to Rv0288 is fully developed shortly after infection with M. bovis or contact with tuberculosis patients (1, 3), suggesting that this protein is produced and secreted and interacts with the host immune system at the early stages of infection. Information about Rv0287, on the other hand, is very limited, although this protein has been identified in the short-term culture filtrate of M. tuberculosis in proteomic studies (23). The present study demonstrates a close resemblance between ESAT-6/CFP-10 and Rv0287/Rv0288 protein pairs with respect to cotranscription and protein-protein interactions. Furthermore, we demonstrate the existence of direct interactions between the ESAT-6 proteins and the PPE protein encoded by the esx cluster 1.
Coexpression of rv0287 and rv0288 in M. tuberculosis.
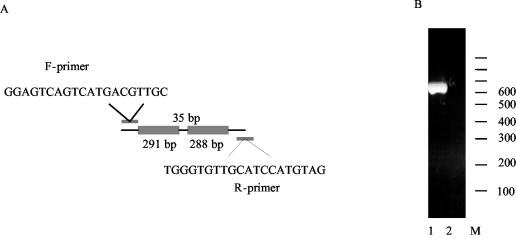
Both Rv0287 and Rv0288 are secreted into the surroundings by M. tuberculosis grown in vitro (23). The ORFs encoding the proteins, rv0287 and rv0288, are 291 and 288 bp, respectively, separated by a 35-bp DNA fragment (Fig. 1A). The short intergenic space suggests that these two genes may be transcribed as one mRNA unit. This was investigated by reverse transcriptase-PCR (RT-PCR) with M. tuberculosis grown in vitro. A specific primer set was designed so that the forward primer (F-primer) carried a sequence from the 5′ region of rv0287 and the reverse primer (R-primer) was 650 bp downstream and had a sequence complementary to that of the 3′ terminus of rv0288 (Fig. 1A). RNA was isolated from M. tuberculosis H37Rv after growth for 1 week in broth culture (17); prior to cDNA synthesis, RNA was treated with RNase-free DNase I (Boehringer Mannheim) at 37°C for 20 min. Reverse transcription was carried out with the R-primer, and the resulting products were amplified by PCR. A single amplicon of the expected size was obtained (Fig. 1B), and it was confirmed by nucleotide sequencing that the amplicon carried the rv0287-rv0288 gene pair (data not shown). No amplicon was obtained in the absence of reverse transcription.
FIG. 1.
Cotranscription of rv0287 and rv0288. (A) Sequences and locations of the primers used for RT-PCR. (B) Lanes 1 and 2, results of RT-PCRs with and without reverse transcriptase, respectively. The 100-bp ladder is indicated (M).
Study of interaction between ESAT-6 proteins by Western-Western blotting.
Recently, using pull-down assays and different spectroscopy techniques, Renshaw et al. observed a strong, direct interaction between ESAT-6 and CFP-10 (21). In the present study, we developed a Western-Western blotting assay to study the interactions between the ESAT-6 family members. In this assay, proteins are electro-separated and immobilized onto a nitrocellulose membrane, which is subsequently overlaid with a solution containing the protein of interest. A specific antibody against the protein in the solution then detects binding to the immobilized proteins. We decided to compare the interaction between ESAT-6 and CFP-10 with that between Rv0287 and Rv0288.
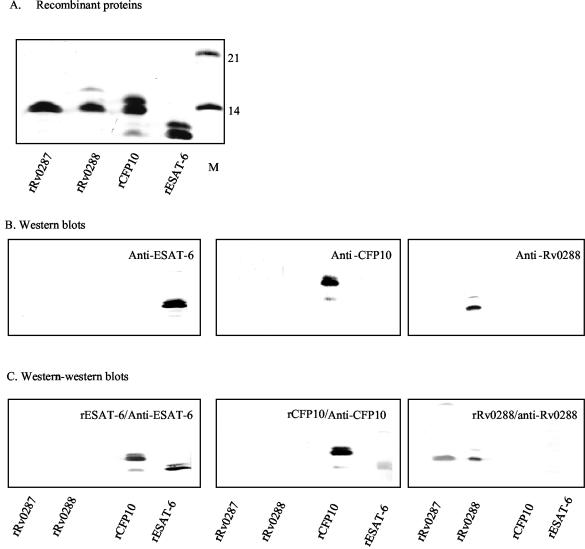
His-tagged recombinant ESAT-6, CFP-10, Rv0287, and Rv0288 had been constructed previously (4, 11, 25; P. Andersen and R. Skjoet, 18 January 2001, international patent application, PCT publication no. WO0104151). The recombinant proteins were purified by a three-step procedure, which has been described elsewhere (17). For Western-Western blotting, 5 μg each of purified His-tagged recombinant Rv0287 (rRv0287), rRv0288, rCFP-10, and rESAT-6 was loaded onto sodium dodecyl sulfate (SDS)-polyacrylamide gels (Fig. 2A), and after electrophoresis the proteins were electro-transferred onto nitrocellulose membranes, which were subsequently overlaid with binding solution (phosphate-buffered saline [pH 7.4] supplemented with 0.1% Tween 20 and 1% skimmed milk) containing 10 μg of rRv0288, rCFP-10, or rESAT-6/ml. The protein-protein interaction complexes were detected by specific antibodies against Rv0288, CFP-10, or ESAT-6, respectively. As shown in Fig. 2C, under the experimental conditions, rESAT-6 and rCFP-10 bound to each other and rRv0288 bound to rRv0287. In the control experiment, neither the primary nor the secondary antibodies showed any cross-reactivity (Fig. 2B). The rCFP-10 preparation contained a minor protein band with a mobility corresponding to about 8 kDa. This band reacted with anti-His antibody (data not shown), indicating that the C-terminal region was missing. Matrix-assisted laser desorption ionization mass spectra analysis suggested that this CFP-10 band lacked the C-terminal 23 residues (P. Højrup, personal communication).
FIG. 2.
Interaction between ESAT-6 proteins. (A) Coomassie-stained SDS-polyacrylamide gel with recombinant ESAT-6 family proteins. The 14- and 21-kDa molecular mass markers are indicated (M). (B) Nitrocellulose membranes overlaid with specific antibodies as indicated. (C) Nitrocellulose membranes which were overlaid first with recombinant ESAT-6 proteins as indicated and subsequently with specific antibodies.
Study of interaction between ESAT-6 proteins by protein-print overlay.
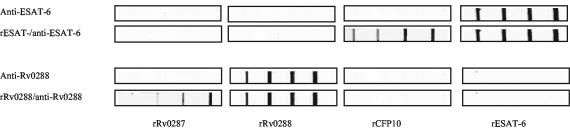
A drawback of Western-Western blotting is that the amounts of proteins transferred onto the membrane are not well defined, as the transfer efficacy varies from experiment to experiment and from protein to protein. We therefore developed a more standardized system using protein-printed membrane strips as a basis for overlay assays. rESAT-6, rCFP-10, rRv0287, and rRv0288 were printed onto the nitrocellulose membrane at concentrations ranging from 0.1 to 1 μg/cm. The membrane was subsequently cut into strips, which were then overlaid with rESAT-6 or rRv0288 at 10 μg/ml. Protein interaction patterns detected by this method were consistent with those obtained by Western-Western blotting, such that ESAT-6 interacted with CFP-10 and Rv0288 interacted with Rv0287. At the protein concentrations applied, the amount of protein captured correlated positively with the amount of immobilized protein (Fig. 3).
FIG. 3.
Interaction between ESAT-6 proteins as demonstrated by protein-print overlay assays. ESAT-6 proteins at 0.1, 0.2, 0.5, and 1 μg/cm were printed onto the nitrocellulose membrane strips by using the Linomat 5 sample application device. The reagents overlaid on the strips are indicated to the left.
Results from both Western-Western blotting and protein-print overlay show that rESAT-6/rCFP-10 and rRv0287/rRv0288 interact in a pairwise and specific way so that rESAT-6 and rCFP-10 do not bind to rRv0287 and rRv0288 and vice versa.
Interaction of ESAT-6 proteins with other proteins encoded by the esx cluster 1.
The genes encoding ESAT-6-like proteins often lie next to a pe-ppe gene pair, which is preceded by an ORF encoding a dimeric membrane-bound ATPase of the FtsK family. In the esx cluster 1, this ATPase is encoded by rv3870 and rv3871 and is required for the optimal transport of ESAT-6 (20). The PPE protein Rv3873 is associated with the cell envelope (17, 19). No data have so far been available as to the presence of the PE protein Rv3872 in M. tuberculosis. However, RNA microarray studies have detected induction of the corresponding RNA in a sigE mutant strain upon exposure to SDS (16). We were interested in exploring whether ESAT-6 and CFP-10 interact directly with Rv3871, Rv3872, and Rv3873. In addition, as these three proteins are homologous to Rv0284, Rv0285, and Rv0286, respectively, of the Rv0287/Rv0288 pathway (Table 1), we were also interested in knowing whether these proteins interact with Rv0287/Rv0288. His-tagged rRv3871, rRv3872, and rRv3873 were purified to high purity and were subsequently tested to determine whether they cross-react with the anti-ESAT-6, anti-CFP-10, and anti-Rv0288 antibodies. This was, unfortunately, the case for rRv3871; all three antibodies cross-reacted with this protein, preventing a meaningful interpretation of the overlay results (data not shown). Therefore, only rRv3872 and rRv3873 were evaluated further in interaction studies with the ESAT-6 proteins. rRv3872 was applied to a nitrocellulose membrane at 1, 0.5, and 0.2 μg/cm. To compensate for the higher molecular weight, rRv3873 was applied at 3.6, 1.8, and 0.72 μg/cm. The membrane strips were overlaid with rESAT-6, rCFP-10, or rRv0288. Interestingly, rRv3873 interacted with all three ESAT-6 proteins, whereas rRv3872 did not bind to any of the proteins tested (Table 2). To investigate whether rRv3873 is sticky so that it would have nonspecific binding to many M. tuberculosis proteins, we performed protein-print overlay assays with other recombinant M. tuberculosis proteins. Screening of a panel of nine monoclonal antibodies (from the Statens Serum Institut monoclonal antibody collections) for high reactivity with recombinant M. tuberculosis proteins showed that four of the antibodies (ST-3 against meromycolate extension acyl carrier protein [AcpM], HBT2 against thiol peroxidase, Hyb 76-5 against heat shock protein HspX, and C24b1 against MPT64 [Rv1980c]) displayed sensitivities comparable to those of the antibodies against the ESAT-6 proteins. HspX has been reported to be a major membrane protein (14), and MPT64 is a secreted protein (10). Under the experimental conditions, none of these proteins bound to rRv3873 in overlay assays (data not shown). Thus, the interactions between the ESAT-6 proteins and rRv3873 are specific.
TABLE 1.
Amino acid sequence homology between components in the CFP-10/ESAT-6 and Rv0287/Rv0288 secretion pathwaysa
| CFP-10/ESAT6 pathway component(s) | Rv0287/Rv0288 pathway component | Description and/or possible function (reference[s]) | % Homology |
|---|---|---|---|
| Rv3868 | Rv0282 | Members of CbxX/CfqX protein family; ATP binding (6, 23) | 38 |
| Rv3869 | Rv0283 | Transmembrane proteins (6, 23) | 37 |
| Rv3870, Rv3871 | Rv0284 | Dimeric membrane-bound ATPases of the FtsK family (6, 23) | 33, 33 |
| Rv3872 | Rv0285 | PE proteins (TubercuList) | 32 |
| Rv3873 | Rv0286 | Cell wall-associated PPE proteins (12, 14) | 35 |
| CFP-10 | Rv0287 | ESAT-6 proteins; secreted antigens (3, 20) | 31 |
| ESAT-6 | Rv0288 | ESAT-6 proteins; secreted antigens (20, 22) | 29 |
| Rv3877 | Rv0290 | Putative transmembrane transporter proteins (23) | 28 |
| Rv3883c | Rv0291 | Serine proteases (6, 8) | 44 |
Protein sequence comparisons were performed by BLASTP at the genome project website (http://genolist.pasteur.fr/TubercuList/).
TABLE 2.
Interaction of the ESAT-6 proteins with Rv3872 and Rv3873
| Protein | Reactivity witha:
|
|
|---|---|---|
| Rv3872 | Rv3873 | |
| CFP-10 | − | + |
| ESAT-6 | − | + |
| Rv0287 | ND | ND |
| Rv0288 | − | + |
+, reactive; −, nonreactive; ND, not determined.
Conclusions.
Our results demonstrating that rv0287 and rv0288 are cotranscribed in M. tuberculosis and the finding that there is direct interaction between rRv0287 and rR0288 support the hypothesis that the esx gene pairs are transcribed as operons and that the proteins interact directly (4, 21). It has been suggested previously that the interaction between ESAT-6 proteins may not be limited to the proteins within a gene pair and that the formation of complexes may also occur between proteins from different ESAT-6 protein pairs (21). Under the present experimental conditions, the recombinant ESAT-6 proteins interacted in a pairwise-specific way and no protein-protein binding was observed across the ESAT-6/CFP-10 and Rv0287/Rv0288 protein pairs.
ESAT-6 and CFP-10 are components of a functional pathway encoded by the esx cluster 1 (Table 1). In the present study, we investigated whether ESAT-6 and CFP-10 interact directly with other protein components of this pathway and we found that Rv3873 interacts directly with ESAT-6 and CFP-10, indicating that Rv3873 may be an essential element of the ESAT-6/CFP-10 transportation system.
Most recently, Pym et al. have demonstrated that optimal secretion of ESAT-6 depends on the presence of an intact esx cluster 1 in M. tuberculosis (20), implying a substrate specificity of the pathway. However, recombinant M. bovis BCG lacking some of the components of the transport machinery was still able to secrete ESAT-6, albeit at a low level (20). This residual transport activity for ESAT-6 may be mediated through transport machineries encoded by other esx gene clusters. If such substrate cross-reactivity exists, one would expect direct protein-protein interactions between ESAT-6 proteins encoded by one esx cluster and components of the transport apparatus encoded by other esx clusters. In this regard, our study demonstrates that in addition to ESAT-6 and CFP10, Rv0288 encoded by esx cluster 3 interacts specifically with Rv3873. In this context, it should be mentioned that, although the overall homology between Rv3873 and its counterpart Rv0286 encoded by esx cluster 3 is only about 35% (Table 1), the homology of the N-terminal halves of the two molecules is much higher (50% identity). Whether this conserved region is directly involved in the interaction with the ESAT-6 proteins remains to be explored.
Acknowledgments
This study was supported by EU X-TB contract number QLK2-CT-2001-02018.
We thank Peter Højrup for matrix-assisted laser desorption ionization mass analysis for the recombinant proteins and Frank Follmann for providing rRv3873. We also thank Kathryn Wattam for excellent technical assistance and Claire Swetman for critical reading of the manuscript.
REFERENCES
- 1.Aagaard, C., M. Movaerts, L. M. Okkels, P. Andersen, and J. M. Pollock. 2003. Genomic approach to identification of Mycobacterium bovis diagnostic antigens in cattle. J. Clin. Microbiol. 41:3719-3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersen, P., A. B. Andersen, A. L. Sorensen, and S. Nagai. 1995. Recall of long-lived immunity to Mycobacterium tuberculosis infection in mice. J. Immunol. 154:3359-3372. [PubMed] [Google Scholar]
- 3.Arend, S. M., A. C. Engelhard, G. Groot, K. de Boer, P. Andersen, T. H. Ottenhoff, and J. T. van Dissel. 2001. Tuberculin skin testing compared with T-cell responses to Mycobacterium tuberculosis-specific and nonspecific antigens for detection of latent infection in persons with recent tuberculosis contact. Clin. Diagn. Lab. Immunol. 8:1089-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berthet, F. X., P. B. Rasmussen, I. Rosenkrands, P. Andersen, and B. Gicquel. 1998. A Mycobacterium tuberculosis operon encoding ESAT-6 and a novel low-molecular-mass culture filtrate protein (CFP-10). Microbiology 144:3195-3203. [DOI] [PubMed] [Google Scholar]
- 5.Brandt, L., M. Elhay, I. Rosenkrands, E. B. Lindblad, and P. Andersen. 2000. ESAT-6 subunit vaccination against Mycobacterium tuberculosis. Infect. Immun. 68:791-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown, G. D., J. A. Dave, N. C. Gey van Pittius, L. Stevens, M. R. Ehlers, and A. D. Beyers. 2000. The mycosins of Mycobacterium tuberculosis H37Rv: a family of subtilisin-like serine proteases. Gene 254:147-155. [DOI] [PubMed] [Google Scholar]
- 7.Cole, S. T., K. Eiglmeier, J. Parkhill, K. D. James, N. R. Thomson, P. R. Wheeler, N. Honore, T. Garnier, C. Churcher, D. Harris, K. Mungall, D. Basham, D. Brown, T. Chillingworth, R. Connor, R. M. Davies, K. Devlin, S. Duthoy, T. Feltwell, A. Fraser, N. Hamlin, S. Holroyd, T. Hornsby, K. Jagels, C. Lacroix, J. Maclean, S. Moule, L. Murphy, K. Oliver, M. A. Quail, M. A. Rajandream, K. M. Rutherford, S. Rutter, K. Seeger, S. Simon, M. Simmonds, J. Skelton, R. Squares, S. Squares, K. Stevens, K. Taylor, S. Whitehead, J. R. Woodward, and B. G. Barrell. 2001. Massive gene decay in the leprosy bacillus. Nature 409:1007-1011. [DOI] [PubMed] [Google Scholar]
- 8.Dave, J. A., N. C. Gey van Pittius, A. D. Beyers, M. R. Ehlers, and G. D. Brown. 7. October 2002, posting date. Mycosin-1, a subtilisin-like serine protease of Mycobacterium tuberculosis, is cell wall-associated and expressed during infection of macrophages. BMC Microbiol. 2:30. [Online.] http://www.biomedcentral.com/1471-2180/2/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gey Van Pittius, N. C., J. Gamieldien, W. Hide, G. D. Brown, R. J. Siezen, and A. D. Beyers. 19. September 2001, posting date. The ESAT-6 gene cluster of Mycobacterium tuberculosis and other high G+C Gram-positive bacteria. Genome Biol. 2:research0044.1-research0044.18. [Online.] http://genomebiology.com/2001/2/10/research/0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harboe, M., S. Nagai, M. E. Patarroyo, M. L. Torres, C. Ramirez, and N. Cruz. 1986. Properties of proteins MPB64, MPB70, and MPB80 of Mycobacterium bovis BCG. Infect. Immun. 52:293-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harboe, M., H. G. Wiker, G. Ulvund, A. S. Malin, H. Dockrell, A. Holm, M. C. Jorgensen, and P. Andersen. 1998. B cell epitopes and quantification of the ESAT-6 protein of Mycobacterium tuberculosis. Infect. Immun. 66:717-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamath, A. T., C. G. Feng, M. Macdonald, H. Briscoe, and W. J. Britton. 1999. Differential protective efficacy of DNA vaccines expressing secreted proteins of Mycobacterium tuberculosis. Infect. Immun. 67:1702-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kato-Maeda, M., J. T. Rhee, T. R. Gingeras, H. Salamon, J. Drenkow, N. Smittipat, and P. M. Small. 2001. Comparing genomes within the species Mycobacterium tuberculosis. Genome Res. 11:547-554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee, B. Y., S. A. Hefta, and P. J. Brennan. 1992. Characterization of the major membrane protein of virulent Mycobacterium tuberculosis. Infect. Immun. 60:2066-2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lewis, K. N., R. Liao, K. M. Guinn, M. J. Hickey, S. Smith, M. A. Behr, and D. Sherman. 2003. Deletion of RD1 from Mycobacterium tuberculosis mimics bacille Calmette-Guérin attenuation. J. Infect. Dis. 187:117-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manganelli, R., M. I. Voskuil, G. K. Schoolnik, and I. Smith. 2001. The Mycobacterium tuberculosis ECF sigma factor sigmaE: role in global gene expression and survival in macrophages. Mol. Microbiol. 41:423-437. [DOI] [PubMed] [Google Scholar]
- 17.Okkels, L. M., I. Brock, F. Follmann, E. M. Agger, S. M. Arend, T. H. M. Ottenhoff, F. Oftung, I. Rosenkrands, and P. Andersen. 2003. PPE protein (Rv3873) from DNA segment RD1 of Mycobacterium tuberculosis: strong recognition of both specific T-cell epitopes and epitopes conserved within the PPE family. Infect. Immun. 71:6116-6123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pallen, M. J. 2002. The ESAT-6/WXG100 superfamily—and a new Gram-positive secretion system? Trends Microbiol. 10:209-212. [DOI] [PubMed] [Google Scholar]
- 19.Pym, A. S., P. Brodin, R. Brosch, M. Huerre, and S. T. Cole. 2002. Loss of RD1 contributed to the attenuation of the live tuberculosis vaccines Mycobacterium bovis BCG and Mycobacterium microti. Mol. Microbiol. 46:709-717. [DOI] [PubMed] [Google Scholar]
- 20.Pym, A. S., P. Brodin, L. Majlessi, R. Brosch, C. Demangel, A. Williams, K. E. Griffiths, G. Marchal, C. Leclerc, and T. Cole. 2003. Recombinant BCG exporting ESAT-6 confers enhanced protection against tuberculosis. Nat. Med. 9:533-539. [DOI] [PubMed] [Google Scholar]
- 21.Renshaw, P. S., P. Panagiotidou, A. Whelan, S. V. Gordon, R. G. Hewinson, R. A. Williamson, and M. D. Carr. 2002. Conclusive evidence that the major T-cell antigens of the Mycobacterium tuberculosis complex ESAT-6 and CFP-10 form a tight, 1:1 complex and characterization of the structural properties of ESAT-6, CFP-10, and the ESAT-6*CFP-10 complex. Implications for pathogenesis and virulence. J. Biol. Chem. 277:21598-21603. [DOI] [PubMed] [Google Scholar]
- 22.Rindi, L., N. Lari, and C. Garzelli. 1999. Search for the genes potentially involved in Mycobacterium tuberculosis virulence by mRNA differential display. Biochem. Biophys. Res. Commun. 258:94-101. [DOI] [PubMed] [Google Scholar]
- 23.Rosenkrands, I., A. King, K. Weldingh, M. Moniatte, E. Moertz, and P. Andersen. 2000. Towards the proteome of Mycobacterium tuberculosis. Electrophoresis 21:3740-3756. [DOI] [PubMed] [Google Scholar]
- 24.Sassetti, C. M., D. H. Boyd, and E. J. Rubin. 2003. Genes required for mycobacterial growth defined by high density mutagenesis. Mol. Microbiol. 48:74-84. [DOI] [PubMed] [Google Scholar]
- 25.Skjøt, R. L., T. Oettinger, I. Rosenkrands, P. Ravn, I. Brock, S. Jacobsen, and P. Andersen. 2000. Comparative evaluation of low-molecular-mass proteins from Mycobacterium tuberculosis identifies members of the ESAT-6 family as immunodominant T-cell antigens. Infect. Immun. 68:214-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Skjøt, R. L. V., I. Brock, S. M. Arend, M. E. Munk, M. Theisen, T. H. M. Ottenhoff, and P. Andersen. 2002. Epitope mapping of the immunodominant antigen TB10.4 and the two homologous proteins TB10.3 and TB12.9, which constitute a subfamily of the esat-6 gene family. Infect. Immun. 70:5446-5453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sorensen, A. L., S. Nagai, G. Houen, P. Andersen, and A. B. Andersen. 1995. Purification and characterization of a low-molecular-mass T-cell antigen secreted by Mycobacterium tuberculosis. Infect. Immun. 63:1710-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tekaia, F., S. V. Gordon, T. Garnier, R. Brosch, B. G. Barrell, and S. T. Cole. 1999. Analysis of the proteome of Mycobacterium tuberculosis in silico. Tuber. Lung Dis. 79:329-342. [DOI] [PubMed] [Google Scholar]
- 29.Wards, B. J., G. W. de Lisle, and D. M. Collins. 2000. An esat6 knockout mutant of Mycobacterium bovis produced by homologous recombination will contribute to the development of a live tuberculosis vaccine. Tuber. Lung Dis. 80:185-189. [DOI] [PubMed] [Google Scholar]