Abstract
Recent studies provide convincing evidence that a combined immunohistochemical or fluorescence in situ hybridization (FISH) score of MYC, BCL2, BCL6 proteins and MYC translocations predicted outcome in diffuse large B-cell lymphoma (DLBCL) patients treated with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP). However, by far, all these researches are based on Western populations. Therefore, we investigate the prognostic relevance of MYC-, BCL2- and BCL6-rearrangements and protein expression by immunohistochemistry and FISH from 336 de novo DLBCL, NOS treated with CHOP or R-CHOP. Breaks in MYC and BCL6, and fusion in IGH/BCL2 were detected in 9.7%, 20.0%, and 11.1% of the cases, respectively, and were not significantly associated with clinical outcomes. Protein overexpression of MYC (≥40%), BCL2 (≥70%) and BCL6 (≥50%) was encountered in 51%, 51% and 36% of the tumors, respectively. On the basis of MYC, BCL2 and BCL6 expression, double-hit scores (DHSs) and triple-hit score (THS) were assigned to all patients with DLBCL. Patients with high MYC/BCL2 DHS, high MYC/BCL6 DHS and high THS had multiple adverse prognostic factors including high LDH level, poor performance status, advanced clinical stage, high International Prognostic Index (IPI) score, and non-germinal center B-cell. In univariate analysis, high MYC/BCL2 DHS, high MYC/BCL6 DHS and high THS were associated with inferior OS and PFS in both CHOP and R-CHOP cohorts (P<0.05). The highly significant correlations with OS and PFS were maintained in multivariate models that controlled for IPI (P<0.05). DLBCLs with high DHSs and high THS share the clinical features and poor prognosis of double-hit lymphoma (P>0.05). These data together suggest that the immunohistochemical DHSs and THS defined a large subset of DLBCLs with double-hit biology and was strongly associated with poor outcome in patients treated with R-CHOP or CHOP.
Introduction
Diffuse large B-cell lymphoma (DLBCL) exhibits various morphologies, immunophenotypes, genetic aberrations, and clinical courses. These features vary across geographic regions, suggesting geographic heterogeneity as a characteristic of this type of lymphoma. DLBCL constitutes 31–34% of all non-Hodgkin lymphomas in Western countries, more than 40% in Asian countries and 45.8% in china [1], [2].
The International Prognostic Index (IPI) has been confirmed to be a valid prognosticator for patients receiving standard chemotherapy [3]. However, there are considerable differences in outcome within each of risk groups, suggesting underlying biologic differences that are not encompassed by the IPI factor [4]. In addition, gene expression profiling has stratified DLBCL into prognostically different molecular subtypes based on cell of origin, including germinal center B-cell (GCB)-like, activated B-cell-like subtypes, and unclassified DLBCL [5], [6]. However, these subtypes do not reliably predict the prognosis of individual patients [7]. Furthermore, gene expression profiling is not available in most clinical laboratories. An immunophenotypical subdivision of DLBCL, not otherwise specified (NOS) into GCB and non-germinal center B-cell (non-GCB) subgroups has been proposed as prognosis predictor by different groups [8]. However, in some studies this immunophenotypic subdivision do not correlate with prognosis [9], [10], and does not currently determine therapy [11].
Recent studies provide convincing evidence that a DLBCL population characterized by the coexpression of MYC and BCL2 proteins by IHC has a poor prognosis with standard rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) immunochemotherapy [12]–[14]. More recently, Heike et al. [15] have reported that a combined immunohistochemical or fluorescence in situ hybridization (FISH)/immunohistochemical score, including MYC, BCL2, BCL6 protein expressions and MYC translocations, predicts outcome in DLBCL patients independent of the IPI following treatment with R-CHOP.
DLBCL in China appears to have many characteristics different from those in Western countries; however, by far, all these researches are based on Western populations. In this study, therefore, we aimed to comprehensively assess the prognostic impact of protein expression patterns of MYC, BCL2, and BCL6 in concert with the chromosomal translocations targeting MYC, BCL2, and BCL6 in a Chinese cohort of 336 de novo DLBCL, NOS patients treated with CHOP or R-CHOP.
Materials and Methods
Patient Selection
We studied 336 cases of de novo DLBCL, NOS from patients who were treated with 6 or 8 cycles of CHOP treatment with or without 8 applications of rituximab. Patients were selected based on the availability of baseline clinical and outcome data, and sufficient formalin-fixed paraffin-embedded (FFPE) tissue from the pre-treatment biopsy sample for representation in tissue microarrays (TMAs). The archived FFPE tissues were obtained from the Department of Pathology, Guangdong General Hospital between January 2000 and October 2012. A consensus diagnosis of DLBCL was confirmed by two expert pathologists according to 2008 World Health Organization (WHO) classification criteria [11]. Median follow-up time was 37 months (range, 1 to 145 months). The Research Ethics Committee of Guangdong General Hospital & Guangdong Academy of Medical Science reviewed and approved the study (No. GDREC2013122H) according to the principles expressed in the Declaration of Helsinki. The Research Ethics Committee specifically waived the need for informed consent for this project.
TMA Construction and Immunohistochemistry (IHC)
TMAs that contained three representative 2.0-mm cores from each tumor of the cases were prepared with a tissue microarrayer (Beecher Instruments, Silver Spring, MD). Immunohistochemical stainings were performed using Real Envision Kit (K5007, DAKO, Carpinteria, CA, USA) on two automated immunostaining instruments (Discovery XT, Ventana Medical Systems, Tucson, AZ, USA; Leica Bond-Max, Leica Biosystems, Germany) according to the manufacturer’s instructions. Internal control cores were present in each TMA. Sections were subjected to staining protocols with the following antibodies: MYC (clone Y69; Epitomics, Burlingame, CA, USA), BCL2 (clone 124; DAKO, Glostrup, Denmark), BCL6 (clone PG-B6p; DAKO), and Ki67 (clone MIB1; DAKO; Table S1). All cases were scored semiquantitatively in 10% increments as previously reported [16] by two observers without knowledge of patient outcome or FISH results. Discrepant scoring of >10% was resolved using a multiheaded microscope to reach a consensus score.
Data were analyzed using MedCalc statistical software to determine the optimal survival cut-off points for dichotomizing expression of MYC protein (≥40%), BCL2 protein (≥70%), BCL6 protein (≥50%) and Ki67 index (≥90%). These cut points correspond to the maximum Chi-Square value of the Kaplan-Meier test for overall survival (OS) between groups above and below the cut-point threshold.
FISH
Interphase FISH was performed on TMAs of 150 cases from the same cohort as previously described [17]. The Vysis LSI MYC dual color, break apart rearrangement probe, the Vysis LSI BCL6 dual color, break apart rearrangement probe and the Vysis LSI IGH/BCL2 dual fusion translocation probe (Abbott Molecular, Abbott Park, IL) were used. FISH signals were analyzed using a fluorescence microscope (Olympus BX51, Tokyo, Japan) equipped with a DP72 camera and DP2-BSW software (Olympus, Tokyo, Japan). Patient cases with break-apart signals in >10% of nuclei were considered positive for the presence of a translocation. The signal distribution was evaluated by two independent observers (Dong-Lan Luo and Jie Cheng). In case of discordant results between the two observers, a third investigator (Jie Xu) was involved.
Statistical Analysis
Statistical analysis was prepared using the Statistical Package of MedCalc statistical software (version 12.7.4; MedCalc, Mariakerke, Belgium) and Social Sciences (SPSS, version 20.0; SPSS, Chicago, IL, USA). A receiver operating characteristic curves were constructed to estimate the optimal cut-off points for of MYC, BCL2 and BCL6 proteins as the predictors for OS. Pearson’s Chi-Square test, Fisher’s exact test, Correction for continuity and Spearman rank correlation analysis were used to determine association and correlation between variables. Survival analyses were plotted using Kaplan-Meier curves and compared using the log-rank test. Univariate and Multivariate survival analyses were analyzed by Cox proportional hazards regression models. The results were considered statistically significant when two-sided P<0.05.
Results
Clinical and Immunophenotypical Characteristics
We studied the series of 336 DLBCL, NOS tumor samples by IHC on the TMAs using antibodies for MYC, BCL2, BCL6 and Ki67 ( Figure 1 ). In addition, ten patient cases which could not be scored for technical reasons were also studied on the corresponding whole tissue sections from the original FFPE tumor blocks. Stainings of the four markers were reliably interpretable in all the 336 samples. The clinical and immunophenotypical characteristics of the DLBCL, NOS are provided in Table 1 . DLBCL, NOS was immunophenotypically subdivided into CD5-positive, GCB and non-GCB subgroups according to the Hans classifier [8] and the 2008 WHO classification [11]. Fifteen DLBCL, NOS (4%) were of CD5-positive-subgroup, 90 (27%) of GCB-subgroup, and 231 (69%) of non-GCB-subgroup.
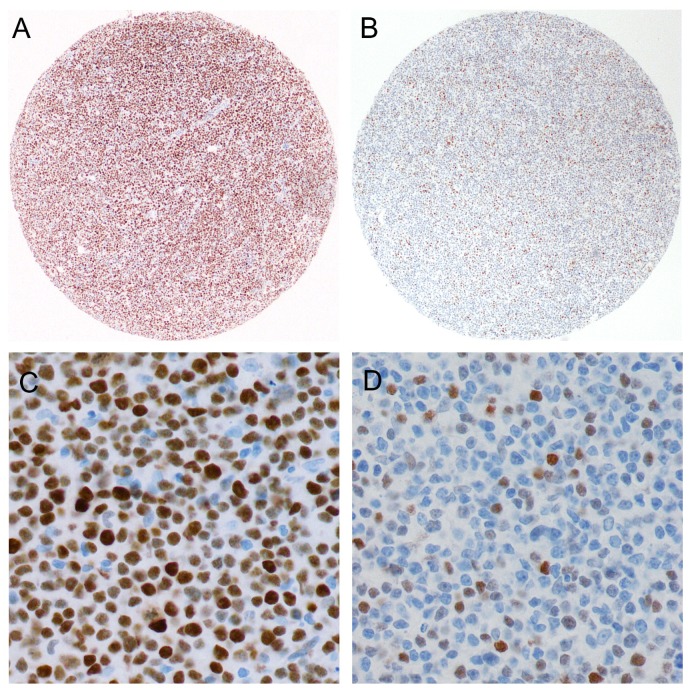
Figure 1. Tissue microarray based representative immunohistochemical analysis of MYC protein expression in DLBCL.
The MYC staining pattern is distinctly nuclear. (A, C) DLBCL scored as having ≥40% MYC-positive lymphoma cells. (B, D) DLBCL scored as having <40% MYC-positive lymphoma cells. A and B original magnification, ×40. C and D original magnification, ×400.
Table 1. Clinical and immunophenotypical characteristics of DLBCL, NOS patients.
| All patients (N = 336) | Patients with FISH data (n = 150) | |||||
| Characteristic | No. | % | No. | % | P | |
| Male | 195/336 | 58 | 86/150 | 57 | 0.885 | |
| Age, years median (range) | 57 (7,87) | 58 (7,86) | ||||
| LDH>upper limit of normal | 129/336 | 38 | 59/150 | 39 | 0.844 | |
| ECOG PS ≥2 # | 51/336 | 15 | 29/150 | 19 | 0.254 | |
| Ann Arbor stage III/IV | 156/336 | 46 | 68/150 | 45 | 0.823 | |
| Extranodal sites ≥2 | 57/336 | 17 | 22/150 | 15 | 0.526 | |
| IPI score $ | ||||||
| 0 or 1 | 170/336 | 51 | 75/150 | 50 | 0.904 | |
| 2 | 83/336 | 25 | 42/150 | 28 | 0.442 | |
| 3 | 59/336 | 18 | 21/150 | 14 | 0.328 | |
| 4 or 5 | 24/336 | 7 | 12/150 | 8 | 0.739 | |
| Extranodal involvement | 235/336 | 70 | 100/150 | 67 | 0.471 | |
| Bone marrow involvement | 36/336 | 11 | 12/150 | 8 | 0.354 | |
| Immunohistochemical subgroups | ||||||
| CD5-positive DLBCL | 15/336 | 4 | 11/150 | 7 | 0.194 | |
| GCB | 90/336 | 27 | 47/150 | 31 | 0.303 | |
| non-GCB | 231/336 | 69 | 92/150 | 61 | 0.110 | |
| High MYC expression | 170/336 | 51 | 88/150 | 59 | 0.100 | |
| High BCL2 expression | 171/336 | 51 | 84/150 | 56 | 0.298 | |
| High BCL6 expression | 121/336 | 36 | 65/150 | 43 | 0.125 | |
| High Ki67 expression | 176/336 | 52 | 92/150 | 61 | 0.067 | |
| R-CHOP | 125/336 | 37 | 64/150 | 43 | 0.254 | |
| CHOP | 211/336 | 63 | 86/150 | 57 | 0.254 | |
| Median follow-up time, months | 37 | 27 | ||||
| 5-year OS | 66 | 70 | ||||
| 5-year PFS | 47 | 40 | ||||
P values were derived from Pearson’s Chi-Square test. ECOG PS, Eastern Cooperative Oncology Group performance status.
ECOG PS ranges from 0 to 4, where higher score indicates greater degree of impairment.
IPI score ranges from 0 to 5, with 0 indicating absence of prognostic factors and 5 indicating presence of all prognostic factors.
High Double-Hit Scores (DHSs) and Triple-Hit Score (THS) are Associated With High-Risk Clinicopathologic Parameters
One hundred and seventy tumors (51%) showed high MYC expression, 171 (51%) showed high BCL2 expression and 121 (36%) showed high BCL6 expression ( Table 1 ). MYC overexpression was associated with high lactate dehydrogenase (LDH) level (P = 0.002) and high IPI score (P = 0.043; Table 2 ). BCL2 overexpression was associated with poor performance status (P = 0.014; Table 2 ). However, no significant differences were observed with regard to clinical characteristics among low and high BCL6 groups.
Table 2. Patient clinical and immunophenotypical characteristics of patients with DLBCL, NOS in relation to protein expressions.
| MYC | BCL2 | BCL6 | ||||||||
| Characteristic | All patients (n = 336) | Low (n = 166) | High (n = 170) | P | Low (n = 165) | High (n = 171) | P | Low (n = 215) | High (n = 121) | P |
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | ||||
| LDH>ULN | 129 (38) | 50 (30) | 79 (46) | 0.002 | 58 (35) | 71 (42) | 0.230 | 84 (39) | 45 (37) | 0.734 |
| ECOG PS ≥2 | 51 (15) | 19 (11) | 32 (19) | 0.060 | 17 (10) | 34 (20) | 0.014 | 34 (16) | 17 (14) | 0.665 |
| Stage III/IV | 156 (46) | 70 (42) | 86 (51) | 0.122 | 75 (45) | 81 (47) | 0.725 | 108 (50) | 48 (40) | 0.062 |
| Extranodal sites ≥2 | 57 (17) | 29 (17) | 28 (16) | 0.807 | 27 (16) | 30 (18) | 0.773 | 39 (18) | 18 (15) | 0.444 |
| IPI score of 3–5 | 83 (25) | 33 (20) | 50 (29) | 0.043 | 36 (20) | 51 (29) | 0.056 | 59 (27) | 24 (20) | 0.121 |
| Immunohistochemical subgroups | ||||||||||
| CD5-positive | 15 (4) | 6 (4) | 9(6) | 0.658* | 5 (2) | 10 (7) | 0.001 * | 12 (6) | 3 (3) | 0.004 * |
| GCB | 90 (27) | 47 (28) | 43 (25) | 0.595# | 59 (36) | 31 (18) | <0.001 # | 45(21) | 45 (37) | 0.002 # |
| non-GCB | 231 (69) | 113 (68) | 118 (69) | 101 (62) | 130 (75) | 158 (73) | 73 (60) | |||
P values were derived from Pearson’s Chi-Square test. Bold font indicates significance. ULN, upper limit of normal.
*P value CD5+ vs. GCB vs. non-GCB.
P value GCB vs. non-GCB.
Using the optimal survival cut-off points as described in Methods section, we assigned each patient a THS that ranged from 0 to 2. Each patient was given one point for each of the two markers (MYC and BCL2) expressed at or above the cut-off points, and one point for BCL6 expressed below the cut-off point. The DHSs of MYC/BCL2, MYC/BCL6 and BCL2/BCL6 were calculated as described previously [16]. Patients with high MYC/BCL2 DHS had multiple adverse clinical factors including high LDH level (P = 0.007), poor performance status (P = 0.010), and high IPI score (P = 0.038; Table 3 ). Similarly, patients with high MYC/BCL2 DHS was associated with high LDH level, high clinical stage, and high IPI score (P>0.05). However, no significant differences were observed with regard to clinical factors included in Table 3 among BCL2/BCL6 DHS 0, 1 and 2. Notably, patients with high THS had multiple adverse prognostic factors including high LDH level (P = 0.024), poor performance status (P = 0.025), high clinical stage (P = 0.008), and high IPI score (P = 0.009; Table 3 ).
Table 3. Patient clinical and immunophenotypical characteristics of patients with DLBCL, NOS in relation to DHS and THS.
| MYC/BCL2 | MYC/BCL6 | BCL2/BCL6 | MYC/BCL2/BCL6 | |||||||||||||||
| Characteristic | All patients(n = 336) | DHS 0(n = 90) | DHS 1(n = 151) | DHS 2(n = 95) | P | DHS 0(n = 57) | DHS 1(n = 173) | DHS 2(n = 106) | P | DHS 0(n = 65) | DHS 1(n = 156) | DHS 2(n = 115) | P | THS 0(n = 27) | THS 1(n = 131) | THS 2(n = 109) | THS 3(n = 69) | P |
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | |||||
| LDH>ULN | 129 (38) | 28 (31) | 52 (34) | 49 (52) | 0.007 | 17 (30) | 61 (35) | 51 (48) | 0.035 | 23 (35) | 57 (37) | 49 (43) | 0.512 | 7 (26) | 47 (36) | 38 (35) | 37 (54) | 0.024 |
| ECOG PS ≥2 | 51 (15) | 8 (9) | 20 (13) | 23 (24) | 0.010 | 6 (11) | 24 (14) | 21 (20) | 0.228 | 4 (6) | 26 (17) | 21 (18) | 0.073 | 2 (7) | 12 (9) | 23 (21) | 14 (20) | 0.025 |
| Stage III/IV | 156 (46) | 36 (40) | 73 (48) | 47 (49) | 0.355 | 19 (33) | 80 (46) | 57 (54) | 0.044 | 28 (43) | 67 (43) | 61 (53) | 0.215 | 12 (44) | 47 (36) | 63 (58) | 34 (49) | 0.008 |
| Extranodal sites ≥2 | 57(17) | 13 (14) | 30 (20) | 14 (15) | 0.440 | 8 (14) | 31 (18) | 18 (17) | 0.795 | 11 (17) | 23 (15) | 23 (20) | 0.522 | 4 (15) | 20 (15) | 22 (20) | 11 (16) | 0.751 |
| IPI score of 3–5 | 83 (25) | 14 (16) | 39 (26) | 30 (32) | 0.038 | 7 (12) | 43 (25) | 33 (31) | 0.029 | 11 (17) | 36 (23) | 36 (31) | 0.081 | 3 (11) | 23 (18) | 36 (33) | 21 (30) | 0.009 |
| Immunohistochemical subgroups | ||||||||||||||||||
| CD5-positive | 15 (4) | 2 (2) | 7 (5) | 6 (6) | NS* | 3 (5) | 3 (2) | 9 (8) | NS* | 0 (0) | 8 (5) | 7 (6) | NS* | 0 (0) | 5 (4) | 4 (4) | 6 (9) | NS* |
| GCB | 90 (27) | 29 (32) | 48 (32) | 13 (14) | 0.004 # | 21 (37) | 50 (29) | 19 (18) | 0.034 # | 33 (51) | 38 (24) | 19 (17) | <0.001 # | 12 (44) | 47 (36) | 21 (19) | 10 (14) | 0.001 # |
| non-GCB | 231 (69) | 59 (66) | 96 (63) | 76 (80) | 33 (58) | 120 (69) | 78 (74) | 32 (49) | 110 (71) | 89 (77) | 15 (56) | 79 (60) | 84 (77) | 53 (77) | ||||
P values were derived using Pearson’s Chi-Square test. Bold font indicates significance.
*NS, not suitable for chi-square test among CD5+ vs. GCB vs. non-GCB, because more than 1/5 of the expected values were less than five.
P value GCB vs. non-GCB.
One hundred and seventy-five tumors (52.1%) showed high Ki67 expression. No significant differences were observed with regard to LDH level, performance status, clinical stage, extranodal sites, IPI score, or immunohistochemical subgroups (GCB vs. non-GCB) among high Ki67 proliferation index and low Ki67 proliferation index groups.
High DHS and THS Show Non-GCB Predominance
The immunophenotypical characteristics of patients with DLBCL, NOS in relation to protein expressions are shown in Table 2 . MYC expression demonstrated no correlation with immunohistochemical subgroups of DLBCL. Considering BCL2 and BCL6 expression individually, the high BCL2 expression group had a significantly higher frequency of non-GCB than the low BCL2 group (75% vs. 62%, P<0.001). However, low BCL6 expression group had a higher frequency of non-GCB than the high BCL6 group (73% vs. 60%, P = 0.002).
MYC/BCL2 coexpression (DHS 2) correlated significantly with the non-GCB immunohistochemical subgroup (P = 0.004; Table 3 ). Of total 336 cases of DLBCL, NOS, with MYC/BCL2 DHS 2, 76 (80%) were of the non-GCB-DLBCL. By contrast, only 96 (63%) of DLBCL with MYC/BCL2 DHS 1 were of the non-GCB-DLBCL, and 59 (66%) of DLBCL with MYC/BCL2 DHS 0 were of the non-GCB-DLBCL ( Table 3 ). Similar results were found for MYC/BCL6, BCL2/BCL6 and MYC/BCL2/BCL6 coexpression.
FISH Studies and Double-Hit Lymphoma (DHL)
Initially, we created a pilot series of 336 DLBCL, NOS tumor samples spotted on TMAs. After IHC analysis, sufficient materials of 150 cases on the TMAs were available for complete FISH analysis. The clinical and immunophenotypical characteristics of the 150 patients are shown in Table 1 . Of 150 DLBCL specimens hybridized, 144 (96%), 140 (93%) and 135 (90%) samples were successfully interpretable for the MYC and BCL6 break-apart probes and the IGH/BCL2 fusion probe used, respectively. MYC, BCL6, and BCL2 gene translocations were observed in 9.7%, 20.0%, and 11.1% of the cases, respectively (Figure S1).
No significant corrections were observed between MYC and BCL6 gene breaks and clinical characteristics, including LDH level, performance status, clinical stage, extranodal sites and IPI. Patients with high IPI score had higher IGH/BCL2 fusion rate than those with low IPI score (28.6% vs. 7.4%; χ 2 test, correction for continuity, P = 0.006). No correlations between gene translocations and immunohistochemical subgroups were seen (Table S2). The lack of significant differences in gene translocations between the GCB and the non-GCB DLBCL subgroups indicates that abnormalities of MYC, BCL6 and BCL2 may be a more global phenomenon in Chinese DLBCL and not restricted to particular immunohistochemical subgroups. Breaks in MYC and BCL6, as well as fusion in IGH/BCL2 did not predict OS and progression-free survival (PFS) in univariate and multivariate analyses in rituximab treated patients. We observed similar results for patients treated without rituximab.
We further investigated the double-hit lymphoma (DHL) in our series. One of 134 DLBCL (0.7%) had concurrent translocation of MYC and BCL2, 1/140 (0.7%) of MYC and BCL6, and 4/131 (3.1%) of BCL2 and BCL6. Thus those were determined to have DHL. No triple-hit lymphoma was detected. Given those low incidences, DHL data sets were pooled for subsequent analyses. These patients with DHL (by FISH) appeared to have more adverse clinical risk factors, including higher levels of LDH, worse performance status, and higher IPI scores than the patients without DHL (non-DHL) (P>0.05; Table S3). The lack of significance is probably due to low statistical power from small group sizes. However, BCL2 protein was expressed in a higher proportion of the DHL patients than the non-DHL patients with THS 0/1 (P = 0.005, Table S3). Three of 6 DHL patients were treated with R-CHOP. The 5-year OS rate of patients with DHL treated with R-CHOP or CHOP in this study (50%) was lower than that of the non-DHL patients (70%), although P>0.05 (Figure S2). Six but too few patient cases with DHL could not preclude any meaningful conclusions. However, the 5-year OS of the DHL patients was poor compared with outcome among non-DHL patients with THS 0/1 (50% vs. 83%; P = 0.048; Figure S2). When comparing the patients with DHL with non-DHL patients in the THS-2/3 group, no significant differences were found between clinical characteristics or survival, implying that patients with DHL and those non-DHL patients in the THS-2/3 group are clinically similar and indicating that they share the same unfavorable double-hit tumor biology (Table S3; Figure S2).
Protein Expressions Predict Corresponding Gene Translocations
To analyze the diagnostic performance of MYC, BCL2 and BCL6 protein expressions for corresponding gene translocations with the highest specificity and sensitivity, we used ROC curve analysis to determine the optimal cut-off values of the percentages of protein positive cells. The optimal cut-off values for MYC, BCL2 and BCL6 were ≥90%, ≥70% (equal to predetermined threshold) and ≥20%. The results signify that immunostaining for MYC, BCL2 and BCL6 appears to be an excellent test with high specificity for the presence of MYC breaks, IGH/BCL2 fusion and BCL6 breaks as detected by FISH (Table S4). High MYC expression showed correlation with MYC breaks in DLBCL, NOS (Spearman rank correlation analysis, Spearman’s rho = 0.481, P<0.001), the GCB subgroup (Spearman’s rho = 0.623, P<0.001) and the non-GCB subgroup (Spearman’s rho = 0.556, P<0.001). High BCL6 expression showed correlation with BCL6 breaks in DLBCL, NOS (Spearman’s rho = 0.223, P = 0.008) and the non-GCB subgroup (Spearman’s rho = 0.277, P = 0.011) but showed no significant correlation in the GCB subgroup. Inversely, high BCL2 expression showed correlation with IGH/BCL2 fusion in and the GCB subgroup (Spearman’s rho = 0.369, P = 0.015) but showed no significant correlation in DLBCL, NOS or the non-GCB subgroup. Chi-Square test for gene translocation and protein expression in are shown in Table S5–S7.
High DHSs and THS Predict Poor Prognosis in DLBCL, NOS
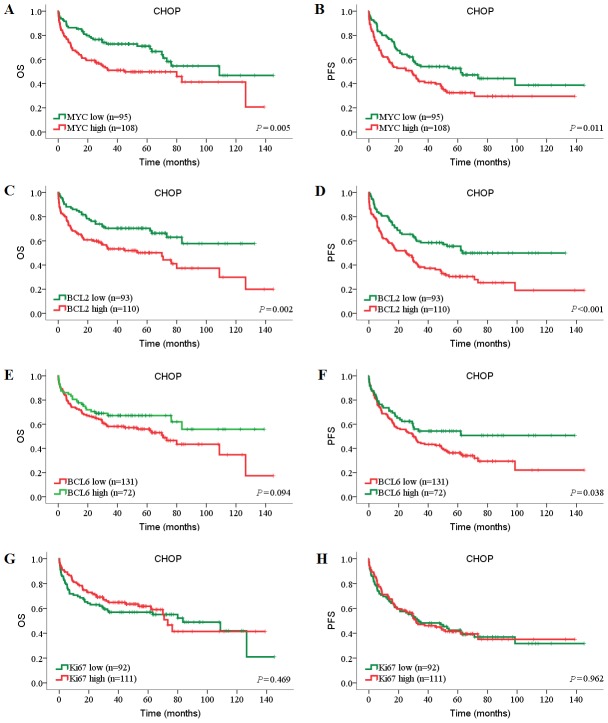
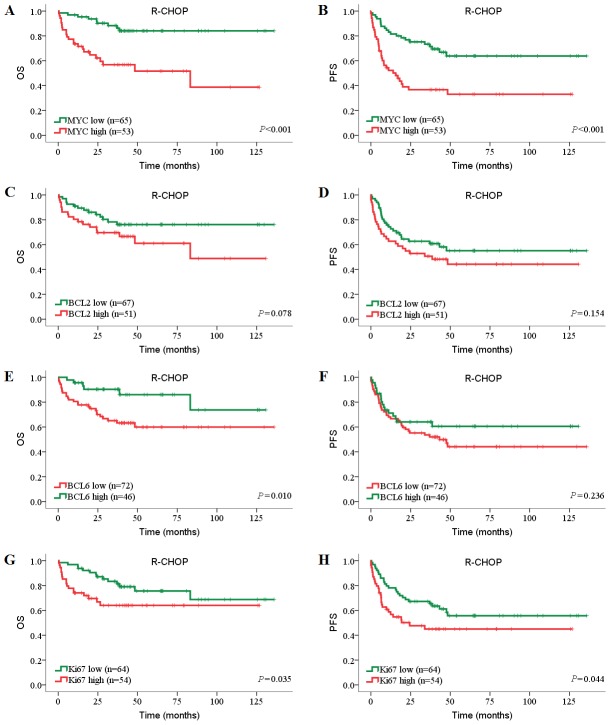
Because several recent studies have shown that prognostic value of biomarkers have changed significantly in rituximab era [18]–[20], we evaluated the candidate prognostic factors separately in the CHOP and R-CHOP cohorts. The survival curves showed that high MYC expression was significantly associated with inferior OS and PFS in both CHOP and R-CHOP cohorts (P<0.05, log-rank tests; Figure 2A–B , Figure 3A–B ). High BCL2 expression, alone, was significantly associated with inferior OS and PFS in CHOP cohort (OS: P = 0.002; PFS, P<0.001) but not in R-CHOP cohort ( Figure 2C–D , Figure 3C–D ). However, low BCL6 expression showed limited prognostic impact on inferior outcome in both CHOP and R-CHOP cohorts (PFS of CHOP cohort: P = 0.038; OS of R-CHOP cohort: P = 0.010; Figure 2E–F , Figure 3E–F ). Interestingly, in contrast with BCL2, Ki67 was significantly associated with inferior OS and PFS in R-CHOP cohort (OS: P = 0.035; PFS, P = 0.044) but not in CHOP cohort ( Figure 2G–H , Figure 3G–H ).
Figure 2. Prognostic impact of MYC, BCL2, BCL6 and Ki67 expression in DLBCL patients treated with CHOP.
Patients’ tumors were stained for (A, B) MYC, (C, D) BCL2, (E, F) BCL6, and (G, H) Ki67. The numbers of patients showing negative or positive stainings (MYC≥40%, BCL2≥70%, BCL6≥50%, Ki67≥90%), cut-off values, and P values are indicated.
Figure 3. Prognostic impact of MYC, BCL2, BCL6 and Ki67 expression in DLBCL patients treated with R-CHOP.
Patients’ tumors were stained for (A, B) MYC, (C, D) BCL2, (E, F) BCL6, and (G, H) Ki67. The numbers of patients showing negative or positive stainings (MYC≥40%, BCL2≥70%, BCL6≥50%, Ki67≥90%), cut-off values, and P values are indicated.
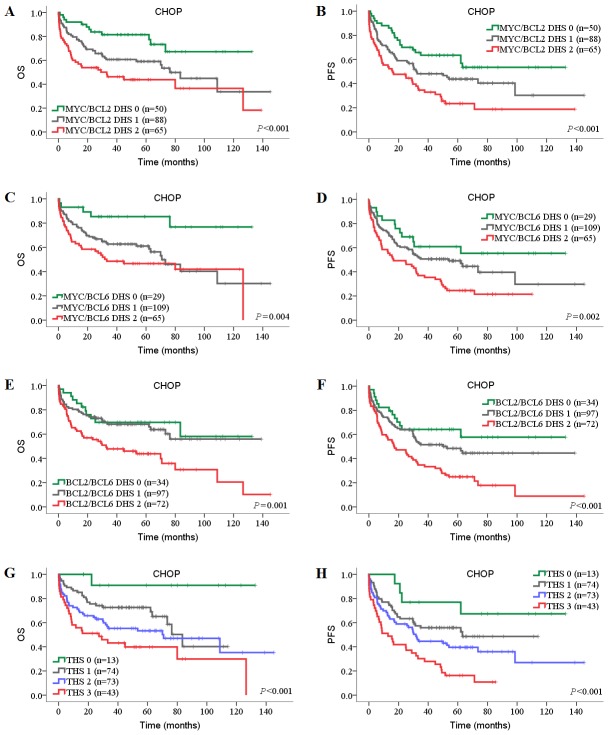
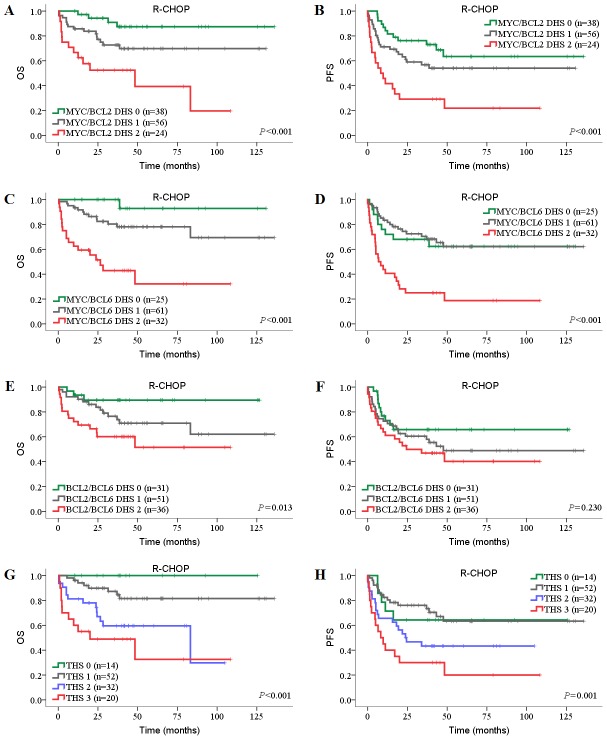
Next, we investigated the prognostic impact of DHSs and THS on DLBCL patients in both CHOP and R-CHOP cohorts. In univariate analysis, high MYC/BCL2 DHS, high MYC/BCL6 DHS and high THS were associated with inferior OS and PFS in both CHOP and R-CHOP cohorts ( Table 4 ; Table 5 ). In the CHOP cohort, compared with individual BCL2 and BCL6 protein, the negative prognostic impact of high BCL2 protein and low BCL6 protein was amplified when BCL2/BCL6 DHS was high ( Table 4 ; Figure 4E–F ). In the R-CHOP cohort, high BCL2 protein expression was only associated with inferior OS and PFS when MYC protein was coexpressed (P<0.001; Table 5 , Figure 5A–B ). BCL2/BCL6 DHS showed limited prognostic impact in R-CHOP cohort (univariate model: OS, P = 0.005, PFS, P = 0.089; multivariate model: OS, P = 0.006, PFS, P = 0.132).
Table 4. Univariate Cox models for DLBCL, NOS patients treated with CHOP.
| OS | PFS | ||||||
| Characteristic | n# | HR | 95% CI | P | HR | 95% CI | P |
| MYC protein low vs. High | 203 | 1.83 | 1.19–2.82 | 0.006 | 1.60 | 1.11–2.31 | 0.012 |
| BCL2 protein low vs. High | 203 | 1.95 | 1.26–3.01 | 0.003 | 1.94 | 1.34–2.82 | 0.001 |
| BCL6 protein low vs. High | 203 | 0.68 | 0.43–1.07 | 0.096 | 0.66 | 0.44–0.98 | 0.040 |
| High Ki67 protein low vs. High | 203 | 0.86 | 0.56–1.30 | 0.470 | 0.99 | 0.69–1.42 | 0.962 |
| MYC/BCL2 DHS 0 vs. 1 vs. 2 | 203 | 1.79 | 1.33–2.40 | <0.001 | 1.67 | 1.30–2.14 | <0.001 |
| MYC/BCL6 DHS 0 vs. 1 vs. 2 | 203 | 1.71 | 1.24–2.37 | 0.001 | 1.63 | 1.23–2.16 | 0.001 |
| BCL2/BCL6 DHS 0 vs. 1 vs. 2 | 203 | 1.70 | 1.24–2.34 | 0.001 | 1.73 | 1.32–2.28 | <0.001 |
| THS 0 vs. 1 vs. 2 vs. 3 | 203 | 1.70 | 1.33–2.18 | <0.001 | 1.65 | 1.33–2.04 | <0.001 |
| GCB vs. Non-GCB | 203 | 1.56 | 0.95–2.56 | 0.082 | 1.75 | 1.12–2.72 | 0.013 |
| IPI score of 0–2 vs. 3–5 | 203 | 2.87 | 1.89–4.36 | <0.001 | 2.21 | 1.52–3.19 | <0.001 |
| MYC break negative vs. positive | 76 | 1.22 | 0.37–4.04 | 0.745 | 1.28 | 0.50–3.25 | 0.604 |
| IGH/BCL2 fusion negative vs. positive | 69 | 0.87 | 0.20–3.67 | 0.844 | 0.70 | 0.22–2.28 | 0.556 |
| BCL6 break negative vs. positive | 72 | 1.50 | 0.60–3.73 | 0.387 | 1.30 | 0.60–2.82 | 0.504 |
Bold font indicates significance. HR, hazard ratio; CI, confidence interval.
Sample sizes differ due to complete data set per cox model.
Table 5. Univariate Cox models for DLBCL, NOS patients treated with R-CHOP.
| OS | PFS | ||||||
| Characteristic | n# | HR | 95% CI | P | HR | 95% CI | P |
| MYC protein low vs. High | 118 | 4.39 | 2.02–9.54 | <0.001 | 2.87 | 1.66–4.99 | <0.001 |
| BCL2 protein low vs. High | 118 | 1.86 | 0.92–3.73 | 0.083 | 1.47 | 0.86–2.51 | 0.157 |
| BCL6 protein low vs. High | 118 | 0.33 | 0.14–0.80 | 0.014 | 0.71 | 0.40–1.26 | 0.238 |
| High Ki67 protein low vs. High | 118 | 2.09 | 1.04–4.23 | 0.040 | 1.72 | 1.01–2.95 | 0.047 |
| MYC/BCL2 DHS 0 vs. 1 vs. 2 | 118 | 2.76 | 1.66–4.60 | <0.001 | 2.03 | 1.39–2.96 | <0.001 |
| MYC/BCL6 DHS 0 vs. 1 vs. 2 | 118 | 4.23 | 2.29–7.79 | <0.001 | 2.25 | 1.47–3.45 | <0.001 |
| BCL2/BCL6 DHS 0 vs. 1 vs. 2 | 118 | 2.11 | 1.26–3.52 | 0.005 | 1.37 | 0.95–1.97 | 0.089 |
| THS 0 vs. 1 vs. 2 vs. 3 | 118 | 2.54 | 1.70–3.81 | <0.001 | 1.72 | 1.28–2.31 | <0.001 |
| GCB vs. non-GCB | 118 | 1.67 | 0.69–4.06 | 0.259 | 1.97 | 0.96–4.03 | 0.064 |
| IPI score of 0–2 vs. 3–5 | 118 | 1.76 | 0.79–3.92 | 0.167 | 1.74 | 0.91–3.30 | 0.093 |
| MYC break negative vs. positive | 57 | 0.52 | 0.07–3.98 | 0.529 | 1.19 | 0.42–3.41 | 0.740 |
| IGH/BCL2 fusion negative vs. positive | 55 | 1.74 | 0.48–6.36 | 0.402 | 0.90 | 0.32–2.59 | 0.851 |
| BCL6 break negative vs. positive | 57 | 1.04 | 0.29–3.81 | 0.948 | 1.27 | 0.59–2.73 | 0.550 |
Bold font indicates significance.
Sample sizes differ due to complete data set per cox model.
Figure 4. Prognostic impact of DHSs and THS in DLBCL patients treated with CHOP.
(A, B) OS (A) and PFS (B) of patients with MYC/BCL2 DHS. (C, D) OS (C) and PFS (D) of patients with MYC/BCL6 DHS. (E, F) OS (E) and PFS (F) of patients with BCL2/BCL6 DHS. (G, H) OS (G) and PFS (H) of patients with MYC/BCL2/BCL6 THS. OS, overall survival; PFS, progression-free survival; DHS, double-hit score; THS, triple-hit score.
Figure 5. Prognostic impact of DHSs and THS in DLBCL patients treated with R-CHOP.
(A, B) OS (A) and PFS (B) of patients with MYC/BCL2 DHS. (C, D) OS (C) and PFS (D) of patients with MYC/BCL6 DHS. (E, F) OS (E) and PFS (F) of patients with BCL2/BCL6 DHS. (G, H) OS (G) and PFS (H) of patients with MYC/BCL2/BCL6 THS. OS, overall survival; PFS, progression-free survival; DHS, double-hit score; THS, triple-hit score.
In the CHOP cohort, multivariate Cox regression model that incorporated IPI, immunohistochemical subgroups (GCB vs. non-GCB), MYC, BCL2, and BCL6 proteins showed that IPI, MYC protein, and BCL2 protein maintained independent prognostic values for OS and PFS (P<0.05; Table 6 ). Also high MYC/BCL2 DHS, high MYC/BCL6 DHS, high BCL2/BCL6 DHS and high THS maintained independent prognostic values for OS and PFS (all P<0.05; Table 6 ).
Table 6. Multivariate Cox models for DBCL, NOS patients treated with CHOP (n = 203).
| Characteristic | OS | PFS | ||||
| HR | 95% CI | P * | HR | 95% CI | P * | |
| IPI score of 0–2 vs. 3–5 | 2.78 | 1.83–4.23 | <0.001 | 2.20 | 1.52–3.18 | <0.001 |
| GCB vs. non-GCB | 1.26 | 0.74–2.13 | 0.397 | 1.49 | 0.94–2.37 | 0.092 |
| MYC protein low vs. High | 1.77 | 1.14–2.73 | 0.010 | 1.67 | 1.15–2.42 | 0.007 |
| BCL2 protein low vs. High | 1.66 | 1.05–2.63 | 0.032 | 1.63 | 1.10–2.41 | 0.015 |
| BCL6 protein low vs. High | 0.67 | 0.42–1.08 | 0.098 | 0.66 | 0.44–0.99 | 0.042 |
| IPI score of 0–2 vs. 3–5 | 2.77 | 1.82–4.20 | <0.001 | 2.20 | 1.52–3.18 | <0.001 |
| MYC/BCL2 DHS 0 vs. 1 vs. 2 | 1.76 | 1.30–2.38 | <0.001 | 1.68 | 1.30–2.16 | <0.001 |
| IPI score of 0–2 vs. 3–5 | 2.86 | 1.88–4.34 | <0.001 | 2.22 | 1.53–3.21 | <0.001 |
| MYC/BCL6 DHS 0 vs. 1 vs. 2 | 1.73 | 1.24–2.41 | 0.001 | 1.66 | 1.24–2.21 | 0.001 |
| IPI score of 0–2 vs. 3–5 | 2.81 | 1.84–4.27 | <0.001 | 2.14 | 1.48–3.11 | <0.001 |
| BCL2/BCL6 DHS 0 vs. 1 vs. 2 | 1.63 | 1.20–2.22 | 0.002 | 1.68 | 1.28–2.20 | <0.001 |
| IPI score of 0–2 vs. 3–5 | 2.79 | 1.84–4.25 | <0.001 | 2.20 | 1.52–3.18 | <0.001 |
| THS 0 vs. 1 vs. 2 vs. 3 | 1.68 | 1.31–2.16 | <0.001 | 1.67 | 1.34–2.06 | <0.001 |
Bold font indicates significance.
*Cox regression enter method.
In the R-CHOP cohort, multivariate Cox regression model that incorporated IPI, MYC, BCL2, and BCL6 proteins showed that only high MYC protein maintained independent prognostic value for both inferior OS (P<0.001) and PFS (P = 0.001), and low BCL6 protein for inferior OS (P = 0.019; Table 7 ). Also high MYC/BCL2 DHS, high MYC/BCL6 DHS, and high THS maintained independent prognostic values for OS and PFS (all P<0.05). High BCL2/BCL6 DHS was independent prognostic value or OS (P = 0.006; Table 7 ).
Table 7. Multivariate Cox models for DBCL, NOS patients treated with R-CHOP (n = 118).
| Characteristic | OS | PFS | ||||
| HR | 95% CI | P * | HR | 95% CI | P * | |
| IPI score of 0–2 vs. 3–5 | 1.24 | 0.55–2.80 | 0.605 | 1.51 | 0.78–2.90 | 0.221 |
| MYC protein low vs. High | 4.12 | 1.86–9.10 | <0.001 | 2.71 | 1.55–4.76 | 0.001 |
| BCL2 protein low vs. High | 1.48 | 0.71–3.07 | 0.293 | 1.35 | 0.77–2.36 | 0.303 |
| BCL6 protein low vs. High | 0.33 | 0.13–0.83 | 0.019 | 0.74 | 0.40–1.37 | 0.336 |
| Ki67 protein low vs. High | 1.71 | 0.83–3.51 | 0.144 | 1.49 | 0.86–2.57 | 0.151 |
| IPI score of 0–2 vs. 3–5 | 1.34 | 0.59–3.03 | 0.483 | 1.56 | 0.81–2.97 | 0.181 |
| MYC/BCL2 DHS 0 vs. 1 vs. 2 | 2.67 | 1.60–4.48 | <0.001 | 1.97 | 1.35–2.88 | <0.001 |
| IPI score of 0–2 vs. 3–5 | 1.22 | 0.54–2.75 | 0.628 | 1.44 | 0.75–2.76 | 0.270 |
| MYC/BCL6 DHS 0 vs. 1 vs. 2 | 4.14 | 2.23–7.68 | <0.001 | 2.19 | 1.42–3.37 | <0.001 |
| IPI score of 0–2 vs. 3–5 | 1.50 | 0.67–3.34 | 0.326 | 1.61 | 0.84–3.07 | 0.151 |
| BCL2/BCL6 DHS 0 vs. 1 vs. 2 | 2.07 | 1.23–3.50 | 0.006 | 1.33 | 0.92–1.94 | 0.132 |
| IPI score of 0–2 vs. 3–5 | 1.30 | 0.58–2.91 | 0.522 | 1.49 | 0.78–2.85 | 0.223 |
| THS 0 vs. 1 vs. 2 vs. 3 | 2.51 | 1.67–3.77 | <0.001 | 1.69 | 1.25–2.29 | 0.001 |
Bold font indicates significance.
*Cox regression enter method.
Immunohistochemical Subtyping and IPI Predict Survival in DLBCL Patients Treated With CHOP but Not With R-CHOP
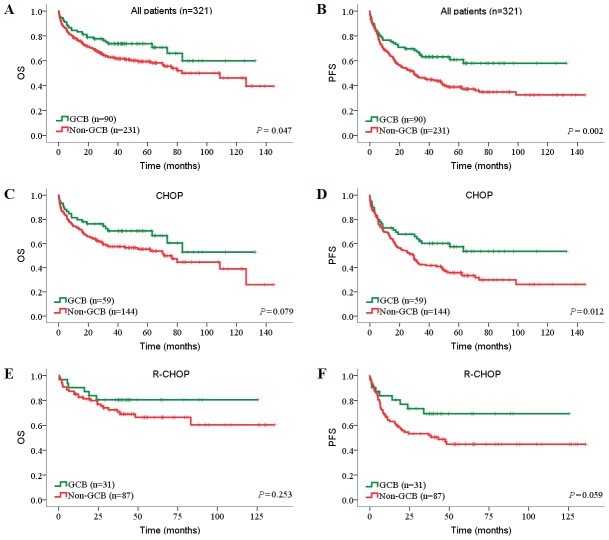
For all DLBCL, NOS patients, rituximab significantly improved OS (72.9% vs. 56.2%, P = 0.011; Figure 6A ) but not PFS, although a trend was seen (54.2% vs. 40.9%, P = 0.135; Figure 6B ). In the GCB subgroup, the OS and PFS showed an increase in the R-CHOP group from survival curves ( Figure 6C–D ), but this was not significant. In the non-GCB subgroup, the patients receiving R-CHOP treatment showed a significantly improved OS than those who received CHOP (70.1% vs. 52.1%, P = 0.020; Figure 6E ). These findings were consistent with the results of previous studies in Chinese patients [20], [21]. Importantly, these results indicated that the use of rituximab conferred a clinical benefit to non-GCB-DLBCL patients, frequently associated with poorer prognosis.
Figure 6. Prognostic impact of treatments in DLBCL risk-stratified according to immunohistochemical subgroups.
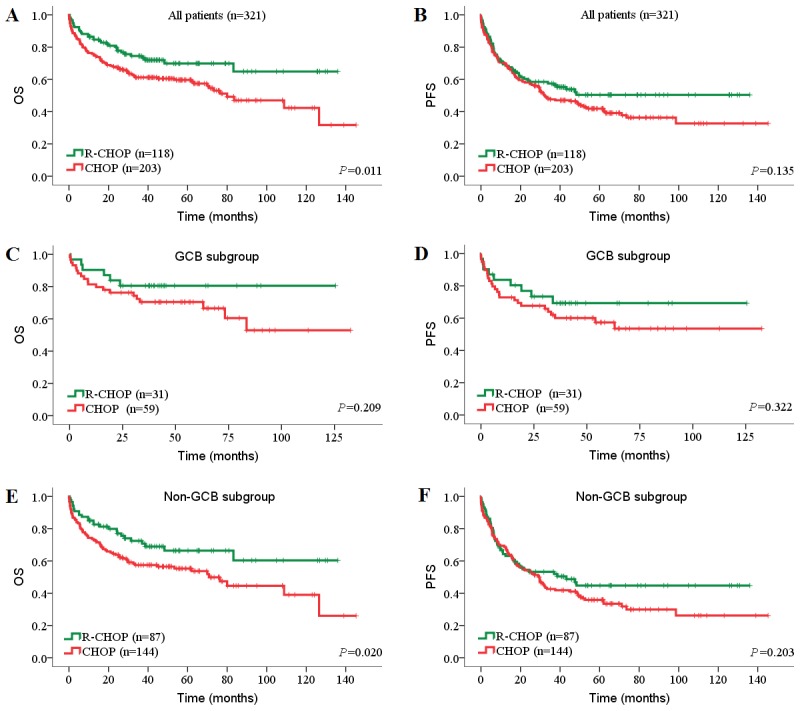
(A, B) OS (A) and PFS (B) of all patients treated with CHOP or R-CHOP. (C, D) OS (C) and PFS (D) of patients treated with CHOP or R-CHOP in GCB subgroup. (E, F) OS (E) and PFS (F) of patients treated with CHOP or R-CHOP in non-GCB subgroup. OS, overall survival; PFS, progression-free survival; GCB, germinal center B-cell.
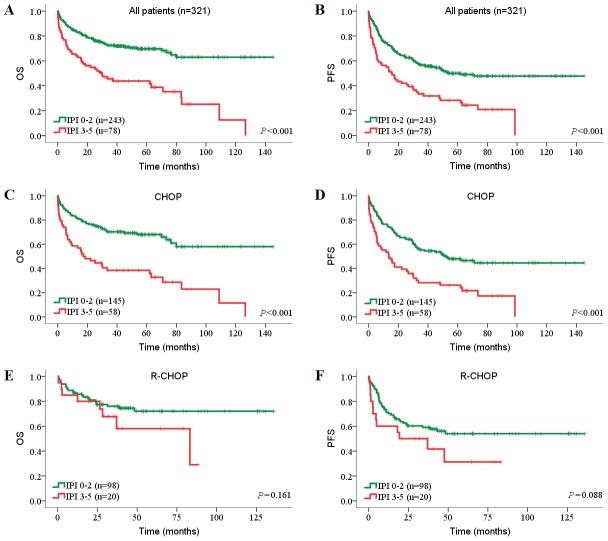
We further investigated the prognostic value of immunohistochemical subtyping in our series undergoing CHOP or R-CHOP treatment. For all patients, the GCB subgroup had superior OS and PFS than the non-GCB subgroup (OS: 71.1% vs. 58.9%, P = 0.047; PFS: 62.2% vs. 39.4%, P = 0.002; Figure 7A–B ). In the CHOP cohort, the GCB subgroup had superior PFS than the non-GCB subgroup (57.6% vs. 34.0%, P = 0.012; Figure 7D ). However, the GCB lost its predictive value in patients treated with rituximab ( Figure 7E–F ; Table 4 ). These findings were consistent with the results of previous studies in both Chinese cohorts [19] and Western cohorts [22]. The IPI proved to be highly valuable for both all patients and CHOP-treated patients ( Figure 8A–D ). However, the IPI lost its predictive value in patients who were treated with rituximab which has improved prognosis significantly ( Figure 8E–F ). This result is consistent with previous studies [11], [19] but inconsistent with some other studies [3], [23].
Figure 7. Prognostic impact of immunohistochemical subtypes in DLBCL risk-stratified according to treatments.
(A, B) OS (A) and PFS (B) of all patients with GCB subtype or non-GCB subtype. (C, D) OS (C) and PFS (D) of CHOP-treated patients with GCB subtype or non-GCB subtype. (E, F) OS (E) and PFS (F) of R-CHOP-treated patients with GCB subtype or non-GCB subtype. OS, overall survival; PFS, progression-free survival; GCB, germinal center B-cell.
Figure 8. Prognostic impact of IPI in DLBCL risk-stratified according to treatments.
(A, B) OS (A) and PFS (B) of all patients with IPI 0–2 or IPI 3–5. (C, D) OS (C) and PFS (D) of CHOP-treated patients with IPI 0–2 or IPI 3–5. (E, F) OS (E) and PFS (F) of R-CHOP-treated patients with IPI 0–2 or IPI 3–5. OS, overall survival; PFS, progression-free survival; IPI, International Prognostic Index.
Discussions
DLBCL, NOS constitutes 25–30% of adult non-Hodgkin lymphomas in Western countries [11] and a higher percentage (37.9%) in Chinese [2]. The poor prognosis of DLBCL patients whose tumors overexpress either MYC or BCL2, or low express BCL6 is well established [24]–[27]. Recently, the negative prognostic impact of coexpression of MYC and BCL2 has been confirmed in DLBCL patients from Western populations treated with R-CHOP [16], [23], [28], [29]. We have confirmed these data in larger series comprising DLBCL, NOS samples from 336 Chinese patients treated with either R-CHOP (n = 125) or CHOP (n = 211). Moreover, we have shown that combined immunohistochemical scores of MYC, BCL2 and BCL6 predict 5-year OS and 5-year PFS in DLBCL patients independent of the IPI following treatment with R-CHOP. Of note, we observed similar results for patients treated with CHOP (data not shown). In contrast, Johnson et al. reported that MYC overexpression predicted poor 3-year OS and event-free survival in R-CHOP-treated patients but not in CHOP-treated patients with unknown mechanism [15]. The contradictory findings may be due to population heterogeneity, different time-to-event end points and different second and/or third line treatments. Different populations treated uniformly within a prospective multicenter trial are needed to further consolidate the prognostic value of MYC combined with BCL2 and BCL6 protein. Importantly, our results indicated that addition of rituximab to standard chemotherapy eliminates the prognostic value of immunohistochemical subgroups (GCB and non-GCB), IPI, BCL2 protein, and BCL2/BCL6 DHS, but enhances the prognostic value of Ki67 in DLBCL.
We here have demonstrated that the analysis of MYC, BCL2 and BCL6 protein expression by IHC is feasible on TMAs in a highly reliable and reproducible manner. High DHSs and THS were associated with many high-risk clinicopathologic features, including high LDH level, poor performance status, advanced stage of disease, multiple extranodal sites of involvement, and high IPI score. Approximately one-third of DLBCL demonstrate MYC/BCL2 DHS 2, in keeping with the 29% and 28% frequency reported by Green et al [16] and Hu et al [29], respectively. By contrast, DHL characterized chromosomal translocations involving MYC, BCL6 and BCL2 is a rare disease, representing approximately 5% of all DLBCL cases in our study, indicating that, in addition to translocations, protein expression could be regulated by other mechanisms including other types of rearrangements, amplifications, mutation, or by miRNA-depandent mechanisms [30]–[32]. Thus, the findings in this study expand the spectrum of DLBCL, defined at the genetic level, by using IHC.
A number of investigators have attempted to use the immunohistochemical expression patterns as prognostic indicators in DLBCL [33], [34]. Hans et al. reported that a combination of CD10, BCL6 and MUM1 expression could subdivide DLBCL patients into long- and short-term survivors [8]. However, contradictory results have been reported on the prognostic role of the Hans classifier [9], [10], [35]. Although non-GCB subgroup was correlated with inferior 5-year OS and PFS in BLBCL, when stratified by treatment non-GCB was only correlated inferior 5-year PFS in the CHOP cohort. Furthermore, non-GCB was not an independent survival predictor for DLBCL patients treated with CHOP, indicating that such algorithm was not a stable survival predictor for DLBCL especially for R-CHOP-treated patients. Consistent with previous reports [10], [12], [36], [37], Chinese patients with DLBCL in our series had a much lower incidence of GCB subtype compared with those reported on Western populations [23], [37], [38]. These data support the notion that immunophenotypic subgroups in DLBCL vary according to the geographic factor.
Translocations of MYC, BCL2 and BCL6 in DLBCL have been consistently reported. Although the prognostic impact of BCL2 and BCL6 translocations has been disputed [13], [15], [22], [39], there is consensus that MYC translocation is a worse prognostic marker in patients with DLBCL treated CHOP, both in combination with and without rituximab [4], [17], [40]. In our study, univariate and multivariate analyses disclosed that breaks in MYC and BCL6, as well as fusion in BCL2 had no impact on survival in CHOP or R-CHOP cohorts.
In our study, BCL6 breaks and IGH/BCL2 fusion were observed in 20.0% and 11.1% of Chinese DLBCL cohort, respectively. While in Western cohorts, t(3;14)(q27;q32) involving BCL6 and t(14;18)(q32;q21) involving IGH and BCL2 have been found in 20–40% and 20–30%, respectively [15], [41]. Consistent with previous studies [14], [36], [42], the incidence of BCL-6 break and IGH/BCL2 fusion were lower in DLBCL in Chinese population compared with Western populations. Similar to what has been previously described [43], we have found a higher frequency of BCL6 translocations in non-GCB subgroup (25%) than in GCB subgroup (13%). In non-GCB subgroup, BCL6 translocations correlated significantly with high BCL6 expression level. However high BCL6 expression is more common in GCB subgroup, indicating that there may be other molecular mechanisms causing BCL6 over-expression in GCB subgroup. Relocation of an IGH transcriptional enhancer next to the BCL2 gene as a result of the t(14;18) translocation is thought to cause constitutive over-expression of BCL2 protein. In this study, high BCL2 expression showed correlation with IGH/BCL2 fusion in GCB subgroup but not no correlation with IGH/BCL2 fusion in GCB subgroup, indicating that BCL2 overexpression in GCB may be due to t(14;18) and in non-GCB is due to other molecular mechanisms. These findings suggest that GCB and non-GCB DLBCL subgroups are two different disease in molecular mechanisms causing the abnormal protein expression. Besides, the low incidence of IGH/BCL2 fusion in Chinese cohort compared to the Western cohorts may be the possible reason for the lower incidence of GCB-DLBCL in China.
Moreover, our study demonstrate that immunostaining for MYC, BCL2 and BCL6, with the optimal cut off of 90%, 70% and 20%, predict the presence of MYC breaks, IGH/BCL2 fusion and BCL6 breaks as detected by FISH in DLBCL with high specificity (>90%). Therefore, all patients with protein aberrant expression should be tested for corresponding gene translocation by FISH. Our findings that high MYC and BCL2 protein expression predict gene translocations are consistent with previous studies [31], [36]. However, Akyurek et al. reported that the level of BCL6 protein expression is not correlated with the presence or absence of BCL6 rearrangement [22]. Different staining and scoring methodologies, cut-off values, and populations may cause this discrepancy. Although MYC, BCL2 and BCL6 translocations can be detected by FISH, FISH fails to detect gene deregulation caused by mechanisms other than translocation [4]. The availability of monoclonal antibodies that target the MYC, BCL2 and BCL6 protein, respectively, has been shown to predict the corresponding gene rearrangements by our and other independent groups and has been validated for use FFPE tissues [44], allowing for the study of large series of archived DLBCL samples for the protein expressions by IHC.
In conclusion, we show DHL in our series is a rare event and dose not predict survival in Chinese DLBCL, NOS. We confirm that DLBCL with high DHSs or THS characterize a subset of DLBCL patients with high-risk clinicopathologic features and inferior survival. Importantly, we report for the first time that high DHSs and THS are poor prognostic predictors independent of the IPI factor in the Chinese cohort that consisted of R-CHOP-treated patients and CHOP-treated patients. We further show that the incidence of non-GCB subtype in our series is higher than that in the Western populations reported by previous studies, and non-GCB subgroup is not a stable survival predictor for DLBCL, especially for R-CHOP-treated patients. Immunostaining for MYC, BCL2 and BCL6 proved to be an excellent test with high specificity (>90%) for the presence of MYC breaks, IGH/BCL2 fusion and BCL6 breaks as detected by FISH. Our data together suggest that the combinations of MYC, BCL2 and BCL6 protein expression assessed by IHC are reliable prognostic predictors and could be used in the future as prognostic markers for stratification of patients with DLBCL for novel therapies.
Supporting Information
Representative FISH analysis of MYC , BCL2 and BCL6 rearrangements in diffuse large B-cell lymphoma (DLBCL). (A) Split signals (orange and green) demonstrating presence of MYC break, and (C) BCL6 break. Fusion signals (orange/green fusion) demonstrating presence of IGH/BCL2 fusion. (A-C original magnification, ×1000). FISH, fluorescence in situ hybridization.
(TIF)
Prognostic impact of DHL in DLBCL. (A) OS and (B) PFS of patients with DHL, other patients without DHL (non-DHL), non-DHL with THS 0/1, and non-DHL with THS 2/3. OS, overall survival; PFS, progression-free survival; DHL, double-hit lymphoma; THS, triple-hit score.
(TIF)
Immunohistochemical assays and methods.
(DOC)
Gene translocation versus immunohistochemical subgroup comparison.
(DOC)
Patient clinical and immunophenotypical characteristics of patients with DLBCL, NOS based on DHL status or THS.
(DOC)
Diagnostic performance of protein candidates for gene translocations in DLBCL, NOS based on ROC curves analysis.
(DOC)
Correlation between MYC protein expression and MYC break in DLBCL, NOS patients.
(DOC)
Correlation between BCL6 protein expression and BCL6 break in DLBCL, NOS patients.
(DOC)
Correlation between BCL2 protein expression and BCL2 break in DLBCL, NOS patients.
(DOC)
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by a National Natural Science Foundation of China (NSFC, http://www.nsfc.gov.cn/) (Grant No. 81172244). YL, FZ, JX, and HZ received the funding. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Krol AD, le Cessie S, Snijder S, Kluin-Nelemans JC, Kluin PM, et al. (2003) Primary extranodal non-Hodgkin’s lymphoma (NHL): the impact of alternative definitions tested in the Comprehensive Cancer Centre West population-based NHL registry. Ann Oncol 14: 131–139. [DOI] [PubMed] [Google Scholar]
- 2. Li XQ, Li GD, Gao ZF, Zhou XG, Zhu XZ (2011) The relative frequencies of lymphoma subtypes in China:a nationwide study of 10002 cases by the Chinese Lymphoma Study Group. Ann Oncol 22: iv141. [Google Scholar]
- 3. Ziepert M, Hasenclever D, Kuhnt E, Glass B, Schmitz N, et al. (2010) Standard International prognostic index remains a valid predictor of outcome for patients with aggressive CD20+ B-cell lymphoma in the rituximab era. J Clin Oncol 28: 2373–2380. [DOI] [PubMed] [Google Scholar]
- 4. Pfreundschuh M (2012) Growing importance of MYC/BCL2 immunohistochemistry in diffuse large B-cell lymphomas. J Clin Oncol 30: 3433–3435. [DOI] [PubMed] [Google Scholar]
- 5. Alizadeh AA, Eisen MB, Davis RE, Ma C, Lossos IS, et al. (2000) Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 403: 503–511. [DOI] [PubMed] [Google Scholar]
- 6. Rosenwald A, Wright G, Chan WC, Connors JM, Campo E, et al. (2002) The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med 346: 1937–1947. [DOI] [PubMed] [Google Scholar]
- 7. Hussain AR, Uddin S, Ahmed M, Al-Dayel F, Bavi PP, et al. (2013) Phosphorylated IkappaBalpha predicts poor prognosis in activated B-cell lymphoma and its inhibition with thymoquinone induces apoptosis via ROS release. PLoS One 8: e60540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hans CP, Weisenburger DD, Greiner TC, Gascoyne RD, Delabie J, et al. (2004) Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood 103: 275–282. [DOI] [PubMed] [Google Scholar]
- 9. Gutierrez-Garcia G, Cardesa-Salzmann T, Climent F, Gonzalez-Barca E, Mercadal S, et al. (2011) Gene-expression profiling and not immunophenotypic algorithms predicts prognosis in patients with diffuse large B-cell lymphoma treated with immunochemotherapy. Blood 117: 4836–4843. [DOI] [PubMed] [Google Scholar]
- 10. Toda H, Sato Y, Takata K, Orita Y, Asano N, et al. (2013) Clinicopathologic analysis of localized nasal/paranasal diffuse large B-cell lymphoma. PLoS One 8: e57677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, et al. (2008) WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues: Lyon, France, IARC Press.
- 12. Shiozawa E, Yamochi-Onizuka T, Takimoto M, Ota H (2007) The GCB subtype of diffuse large B-cell lymphoma is less frequent in Asian countries. Leuk Res 31: 1579–1583. [DOI] [PubMed] [Google Scholar]
- 13. Klapper W, Stoecklein H, Zeynalova S, Ott G, Kosari F, et al. (2008) Structural aberrations affecting the MYC locus indicate a poor prognosis independent of clinical risk factors in diffuse large B-cell lymphomas treated within randomized trials of the German High-Grade Non-Hodgkin’s Lymphoma Study Group (DSHNHL). Leukemia 22: 2226–2229. [DOI] [PubMed] [Google Scholar]
- 14. Chen PM, Yang MH, Yu IT, Lin JT, Lin YC, et al. (2002) Low incidence of BCL-6 gene alterations for diffuse large B-cell lymphomas in Taiwan Chinese. Cancer 94: 2635–2644. [DOI] [PubMed] [Google Scholar]
- 15. Horn H, Ziepert M, Becher C, Barth TF, Bernd HW, et al. (2013) MYC status in concert with BCL2 and BCL6 expression predicts outcome in diffuse large B-cell lymphoma. Blood 121: 2253–2263. [DOI] [PubMed] [Google Scholar]
- 16. Green TM, Young KH, Visco C, Xu-Monette ZY, Orazi A, et al. (2012) Immunohistochemical double-hit score is a strong predictor of outcome in patients with diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol 30: 3460–3467. [DOI] [PubMed] [Google Scholar]
- 17. Savage KJ, Johnson NA, Ben-Neriah S, Connors JM, Sehn LH, et al. (2009) MYC gene rearrangements are associated with a poor prognosis in diffuse large B-cell lymphoma patients treated with R-CHOP chemotherapy. Blood 114: 3533–3537. [DOI] [PubMed] [Google Scholar]
- 18. Feugier P, Van Hoof A, Sebban C, Solal-Celigny P, Bouabdallah R, et al. (2005) Long-term results of the R-CHOP study in the treatment of elderly patients with diffuse large B-cell lymphoma: a study by the Groupe d’Etude des Lymphomes de l’Adulte. J Clin Oncol 23: 4117–4126. [DOI] [PubMed] [Google Scholar]
- 19. Lu HY, Song LX (2012) [Impact of immunochemotherapy on prognostic factors in diffuse large B-cell lymphoma patients]. Zhongguo Shi Yan Xue Ye Xue Za Zhi 20: 315–319. [PubMed] [Google Scholar]
- 20. Huang Y, Ye S, Cao Y, Li Z, Huang J, et al. (2012) Outcome of R-CHOP or CHOP regimen for germinal center and nongerminal center subtypes of diffuse large B-cell lymphoma of Chinese patients. ScientificWorldJournal 2012: 897178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xia ZG, Xu ZZ, Zhao WL, Zhao SQ, Ding F, et al. (2010) The prognostic value of immunohistochemical subtyping in Chinese patients with de novo diffuse large B-cell lymphoma undergoing CHOP or R-CHOP treatment. Ann Hematol 89: 171–177. [DOI] [PubMed] [Google Scholar]
- 22. Akyurek N, Uner A, Benekli M, Barista I (2012) Prognostic significance of MYC, BCL2, and BCL6 rearrangements in patients with diffuse large B-cell lymphoma treated with cyclophosphamide, doxorubicin, vincristine, and prednisone plus rituximab. Cancer 118: 4173–4183. [DOI] [PubMed] [Google Scholar]
- 23.Perry AM, Alvarado-Bernal Y, Laurini JA, Smith LM, Slack GW, et al. (2014) MYC and BCL2 protein expression predicts survival in patients with diffuse large B-cell lymphoma treated with rituximab. Br J Haematol. [DOI] [PubMed]
- 24. Iqbal J, Greiner TC, Patel K, Dave BJ, Smith L, et al. (2007) Distinctive patterns of BCL6 molecular alterations and their functional consequences in different subgroups of diffuse large B-cell lymphoma. Leukemia 21: 2332–2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kluk MJ, Chapuy B, Sinha P, Roy A, Dal Cin P, et al. (2012) Immunohistochemical detection of MYC-driven diffuse large B-cell lymphomas. PLoS One 7: e33813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Visco C, Tzankov A, Xu-Monette ZY, Miranda RN, Tai YC, et al. (2013) Patients with diffuse large B-cell lymphoma of germinal center origin with BCL2 translocations have poor outcome, irrespective of MYC status: a report from an International DLBCL rituximab-CHOP Consortium Program Study. Haematologica 98: 255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Thompson RC, Vardinogiannis I, Gilmore TD (2013) The sensitivity of diffuse large B-Cell lymphoma cell lines to histone deacetylase inhibitor-induced apoptosis is modulated by BCL-2 family protein activity. PLoS One 8: e62822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Johnson NA, Slack GW, Savage KJ, Connors JM, Ben-Neriah S, et al. (2012) Concurrent expression of MYC and BCL2 in diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol 30: 3452–3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu S, Xu-Monette ZY, Tzankov A, Green T, Wu L, et al. (2013) MYC/BCL2 protein coexpression contributes to the inferior survival of activated B-cell subtype of diffuse large B-cell lymphoma and demonstrates high-risk gene expression signatures: a report from The International DLBCL Rituximab-CHOP Consortium Program. Blood 121: 4021–4031; quiz 4250. [DOI] [PMC free article] [PubMed]
- 30. Valentino C, Kendrick S, Johnson N, Gascoyne R, Chan WC, et al. (2013) Colorimetric in situ hybridization identifies MYC gene signal clusters correlating with increased copy number, mRNA, and protein in diffuse large B-cell lymphoma. Am J Clin Pathol 139: 242–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Green TM, Nielsen O, de Stricker K, Xu-Monette ZY, Young KH, et al. (2012) High levels of nuclear MYC protein predict the presence of MYC rearrangement in diffuse large B-cell lymphoma. Am J Surg Pathol 36: 612–619. [DOI] [PubMed] [Google Scholar]
- 32. Leucci E, Cocco M, Onnis A, De Falco G, van Cleef P, et al. (2008) MYC translocation-negative classical Burkitt lymphoma cases: an alternative pathogenetic mechanism involving miRNA deregulation. J Pathol 216: 440–450. [DOI] [PubMed] [Google Scholar]
- 33. de Jong D, Rosenwald A, Chhanabhai M, Gaulard P, Klapper W, et al. (2007) Immunohistochemical prognostic markers in diffuse large B-cell lymphoma: validation of tissue microarray as a prerequisite for broad clinical applications–a study from the Lunenburg Lymphoma Biomarker Consortium. J Clin Oncol 25: 805–812. [DOI] [PubMed] [Google Scholar]
- 34. Veelken H, Vik Dannheim S, Schulte Moenting J, Martens UM, Finke J, et al. (2007) Immunophenotype as prognostic factor for diffuse large B-cell lymphoma in patients undergoing clinical risk-adapted therapy. Ann Oncol 18: 931–939. [DOI] [PubMed] [Google Scholar]
- 35. Mitchell KA, Finn WG, Owens SR (2008) Differences in germinal centre and non-germinal center phenotype in gastric and intestinal diffuse large B-cell lymphomas. Leuk Lymphoma 49: 1717–1723. [DOI] [PubMed] [Google Scholar]
- 36. Chen Y, Han T, Iqbal J, Irons R, Chan WC, et al. (2010) Diffuse large B-cell lymphoma in Chinese patients: immunophenotypic and cytogenetic analyses of 124 cases. Am J Clin Pathol 133: 305–313. [DOI] [PubMed] [Google Scholar]
- 37. Chen Z, Du Z, Chen J, Chen Z, Bao Y, et al. (2011) Prognostic evaluation of immunohistochemical profiles in diffuse large B-cell lymphoma: a Chinese study. Med Oncol 28: 241–248. [DOI] [PubMed] [Google Scholar]
- 38. Gupta M, Maurer MJ, Wellik LE, Law ME, Han JJ, et al. (2012) Expression of Myc, but not pSTAT3, is an adverse prognostic factor for diffuse large B-cell lymphoma treated with epratuzumab/R-CHOP. Blood 120: 4400–4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. van Imhoff GW, Boerma EJ, van der Holt B, Schuuring E, Verdonck LF, et al. (2006) Prognostic impact of germinal center-associated proteins and chromosomal breakpoints in poor-risk diffuse large B-cell lymphoma. J Clin Oncol 24: 4135–4142. [DOI] [PubMed] [Google Scholar]
- 40. Barrans S, Crouch S, Smith A, Turner K, Owen R, et al. (2010) Rearrangement of MYC is associated with poor prognosis in patients with diffuse large B-cell lymphoma treated in the era of rituximab. J Clin Oncol 28: 3360–3365. [DOI] [PubMed] [Google Scholar]
- 41. Hummel M, Bentink S, Berger H, Klapper W, Wessendorf S, et al. (2006) A biologic definition of Burkitt’s lymphoma from transcriptional and genomic profiling. N Engl J Med 354: 2419–2430. [DOI] [PubMed] [Google Scholar]
- 42. Biagi JJ, Seymour JF (2002) Insights into the molecular pathogenesis of follicular lymphoma arising from analysis of geographic variation. Blood 99: 4265–4275. [DOI] [PubMed] [Google Scholar]
- 43. Chen YW, Hu XT, Liang AC, Au WY, So CC, et al. (2006) High BCL6 expression predicts better prognosis, independent of BCL6 translocation status, translocation partner, or BCL6-deregulating mutations, in gastric lymphoma. Blood 108: 2373–2383. [DOI] [PubMed] [Google Scholar]
- 44. Gurel B, Iwata T, Koh CM, Jenkins RB, Lan F, et al. (2008) Nuclear MYC protein overexpression is an early alteration in human prostate carcinogenesis. Mod Pathol 21: 1156–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Representative FISH analysis of MYC , BCL2 and BCL6 rearrangements in diffuse large B-cell lymphoma (DLBCL). (A) Split signals (orange and green) demonstrating presence of MYC break, and (C) BCL6 break. Fusion signals (orange/green fusion) demonstrating presence of IGH/BCL2 fusion. (A-C original magnification, ×1000). FISH, fluorescence in situ hybridization.
(TIF)
Prognostic impact of DHL in DLBCL. (A) OS and (B) PFS of patients with DHL, other patients without DHL (non-DHL), non-DHL with THS 0/1, and non-DHL with THS 2/3. OS, overall survival; PFS, progression-free survival; DHL, double-hit lymphoma; THS, triple-hit score.
(TIF)
Immunohistochemical assays and methods.
(DOC)
Gene translocation versus immunohistochemical subgroup comparison.
(DOC)
Patient clinical and immunophenotypical characteristics of patients with DLBCL, NOS based on DHL status or THS.
(DOC)
Diagnostic performance of protein candidates for gene translocations in DLBCL, NOS based on ROC curves analysis.
(DOC)
Correlation between MYC protein expression and MYC break in DLBCL, NOS patients.
(DOC)
Correlation between BCL6 protein expression and BCL6 break in DLBCL, NOS patients.
(DOC)
Correlation between BCL2 protein expression and BCL2 break in DLBCL, NOS patients.
(DOC)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.