Abstract
Purpose
We have previously reported that older patients with clinical Stage I and II primary cutaneous. melanoma had lower survival rates compared to younger patients We postulated that the incidence of nodal metastasis would therefore be higher among older melanoma patients.
Materials and Methods
The expanded AJCC Melanoma Staging Database contains a cohort of 7756 melanoma patients who presented without clinical evidence of regional lymph node or distant metastasis and who underwent a sentinel node biopsy procedure as a component of their staging workup
Results
Although older patients had primary melanoma features associated with more aggressive biology, we observed paradoxically a significant decrease in the incidence of sentinel node metastasis as patient age increased. Overall, the highest incidence of sentinel node metastasis was 25.8% in patients under 20 years of age, compared to 15.5% in patients 80 years and older (p< 0.001). In contrast, five year mortality rates for Clinical Stage II patients ranged from a low of 20% for those 20–40 years of age up to 38% for those over 70 years of age. Patient age was an independent predictor of sentinel node metastasis in a multifactorial analysis (p<0.001)
Conclusions
Patients with clinical Stage I and II melanoma under 20 years of age had a higher incidence of sentinel lymph node metastasis but, paradoxically, a more favorable survival outcome compared to all other age groups. In contrast, patients >70 years had the most aggressive primary melanoma features and a higher mortality rate compared to all other age groups, but a lower incidence of sentinel lymph node metastasis.
Synopsis
We analyzed the importance of patient age as a component of melanoma staging and in the design and interpretation of clinical trials for patients with localized disease or with regional node metastases.
Introduction
We have previously reported that patient age is a highly significant and independent predictor of survival outcome using the AJCC Melanoma Staging database, even after accounting for the pathological features of the primary melanoma as well as clinical features, including the anatomic site and the gender of the patient [1–3]. Recently, we reported that with increasing age by decade, multiple characteristics of primary melanoma became more advanced: tumors were thicker, exhibited higher mitotic rates, and were more likely to be ulcerated. For patients with localized melanoma (Stage I/II) the mean thickness at presentation for patients over 70 years of age was almost twice that of patients who were 40–60 years of age [3]. From this observation, we postulated that older patients with clinically localized melanoma might have a higher incidence of occult nodal metastases as one explanation of why they exhibited a lower survival rate, and conversely, that younger patients should have a corresponding lower incidence of occult nodal metastases.
METHODS
The AJCC melanoma staging database was originally created in 1999 as a result of an international collaboration that combined prospective melanoma databases from 18 major cancer centers and clinical trial cooperative group [1, 4]. Using a version that was updated in 2008, an evidence-based approach was used to revise the AJCC Melanoma Staging System; these revisions were included in the 7th edition of the AJCC Melanoma Staging Manual published in 2009 [5]. This comprehensive database included 11,088 Stage I and II patients with complete data, including mitotic rate for multivariate analysis of patient age sorted by decade, along with other key clinicopathological prognostic factors. Among these 11,088 patients, a cohort of 7756 melanoma patients who presented without clinical evidence of regional lymph node or distant metastases and who underwent a lymphatic mapping and sentinel node biopsy procedure as a component of their staging workup.. Details of this AJCC melanoma staging database have been published previously [1, 3, 5]). Characteristics of the 28,047 patients partitioned according to age groups have recently been published [3]
Survival times were calculated from the date of initial melanoma diagnosis and censored for patients who were alive at last follow up or who died without evidence of melanoma. Standard statistical methods were used; melanoma-specific survival curves were generated by the Kaplan-Meier product-limit method and compared using the log rank test, and multivariate analyses were based on logistics regression analysis to assess the incidence of sentinel node metastasis [1, 6]. For the Cox multivariate analyses, the relative importance of the prognostic factors was determined according to chi-square values (with associated degrees of freedom (d.f.) and p values) [6].
RESULTS
Among the 7756 patients in the present study, all of whom underwent sentinel node biopsy, 1507 (19.4%) had at least one sentinel lymph node (SLN) metastasis (Table 1).
Table 1.
Clinical and pathological criteria predicting sentinel lymph node metastasis by univariate logistic regression analysis (n= 7,756).
| Variable | No. of Patients (n) | No. of patients with SLN metastases (n) (%) | P-value |
|---|---|---|---|
| Age | 0.0074 | ||
| <40 | 1606 | 342 (21.3) | |
| 40–59 | 3482 | 695 (20.0) | |
| 60- | 2668 | 470 (17.6) | |
|
| |||
| Gender | 0.0009 | ||
| Male | 4521 | 935 (20.7) | |
| Female | 3228 | 570 (17.7) | |
|
| |||
| Location | |||
| Head/neck | 993 | 154 (15.5) | <0.0001 |
| Upper extremity | 1692 | 255 (15.1) | |
| Trunk | 3086 | 656 (21.3) | |
| Lower extremity | 1985 | 442 (22.3) | |
|
| |||
| Tumor thickness | <0.0001 | ||
| ≤1.0 | 1213 | 73 (6.0) | |
| 1.01–2.0 | 3499 | 489 (14.0) | |
| 2.01–4.0 | 2083 | 569 (27.3) | |
| >4.0 | 961 | 376 (39.1) | |
|
| |||
| Ulceration | <0.0001 | ||
| absent | 5688 | 889 (15.6) | |
| present | 2068 | 618 (29.9) | |
|
| |||
| Clark’s Level | <0.0001 | ||
| I/II | 244 | 11 (4.5) | |
| III | 1873 | 223 (11.9) | |
| IV | 5161 | 1111 (21.5) | |
| V | 478 | 162 (33.9) | |
|
| |||
| Lymphovascular invasion | <0.0001 | ||
| Absent | 7212 | 1250 (17.3) | |
| present | 544 | 257 (47.2) | |
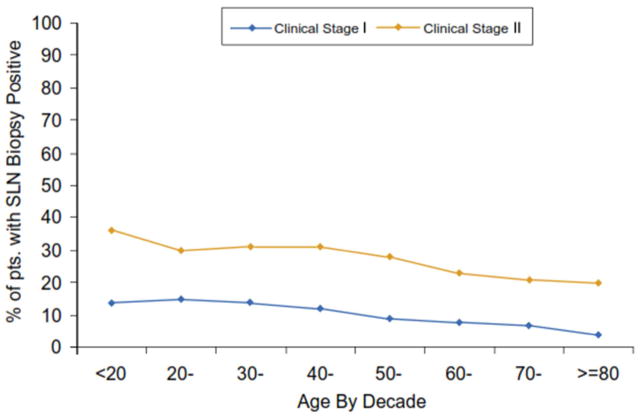
There was a significant decrease in the incidence of sentinel node metastasis as patient age increased (Figure 1). Overall, the highest incidence of SLN metastasis was 25.8% in patients under 20 years of age In contrast, only 15.5% had SLN metastasis among patients 80 years and older (p< 0.001) despite the observation that these older patients had primary tumor features (e.g.: thicker tumors, higher mitotic rate, ulcer) associated with more aggressive biology (data not shown). Among patients with clinical stage I melanoma who underwent a SLN biopsy, the incidence of sentinel node metastases decreased from 14.8% for patients aged 20–30 years to 3.6% for those 80 years of age or older (Fig 1a). The incidence decreased from 35.6% for those 20 years and under who had Clinical Stage II melanoma to 20.2% for those 80 years of age or older (Fig 1a). When the incidence of sentinel node metastasis was stratified by clinical primary tumor substage and age among all patients with primary melanoma who underwent sentinel node biopsy, the incidence of sentinel node metastasis for patients with clinical stage IA melanoma was less than 10% for all age groups; for clinical stage 1B melanoma, the incidence decreased from 20% for those under 20 years of age to 5% for those 80 years of age or older (data not shown). Among patients with clinical stage II melanoma, a significant decrease in SLN metastasis incidence could be seen across all decades of age (Fig. 1b).Among patients with T3/T4 melanomas who underwent a SLN biopsy, the same trends were observed, and were particularly significant for patients with thick, ulcerated (T4b) melanomas)( Fig. 1c).
Figure 1.
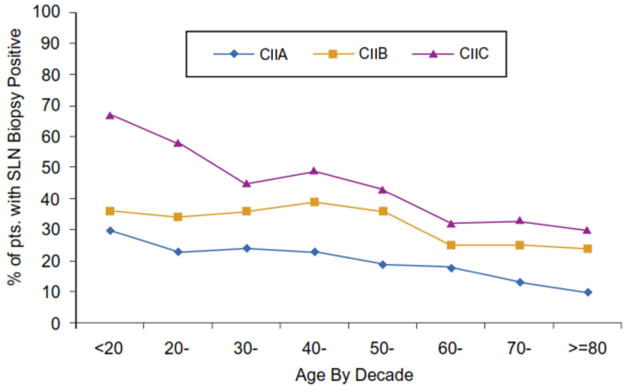
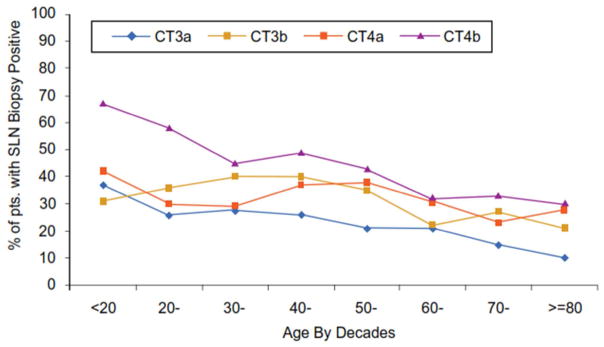
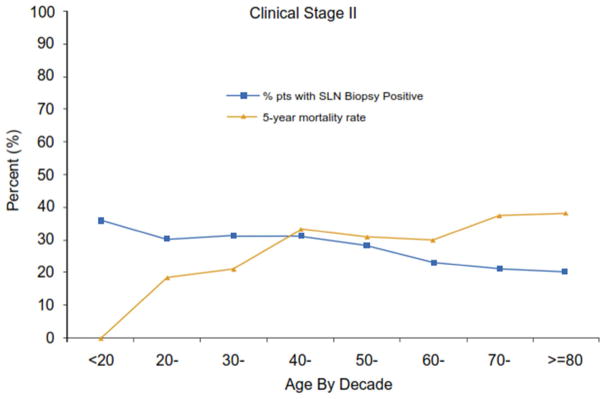
Figure 1A: Incidence of sentinel node metastasis among patients presenting with clinically localized melanoma (Clinical Stage I and II) sorted by patient age in decades. The differences were statistically significant between the clinical stages (p<0.0001)and between the younger and older age groups (p=0.0001)
1B: Incidence of sentinel node metastases among patients presenting with thicker but clinically localized melanoma (Clinical Stage IIA, IIB, and IIC) sorted by patient age in decades. The differences were statistically significant among the clinical substages and between the younger and older age groups (p<0.0001)
Figure 1C: : Incidence on sentinel node metastases among patients presenting with T3 and T4 melanomas with clinically negative nodal disease (Clinical T3a, T3b, T4a, T4b ) with and without tumor ulceration and sorted by patient age in decades. The differences among the age groups were significant (p<0.0001)
Figure 1D: Declining incidence of sentinel node metastases with patient age and increasing mortality among clinical stage II melanoma patients despite increasing five-year melanoma-specific mortality rates (p<0.0001).
Interestingly, an inverse association was observed with increasing age between the incidence of sentinel node metastasis with and melanoma mortality rate for clinical stage II patients (Fig. 1d). The highest incidence of sentinel node metastasis was identified in patients under 20 years of age, yet their five year mortality was only 10%. In contrast, patients aged 20–50 had a 26% incidence of sentinel node metastasis that declined to 24% for those 70 years of age or older, while five year mortality rates ranged from a low of 20% for those 20–40 years of age up to 38% for those over 70 years of age and reached 45% for those age 80 years or older (Fig. 1d).
Patient age was a statistically significant independent predictor of sentinel node metastasis in both the univariate analysis and multivariable analysis. Specifically, the incidence of sentinel node metastasis decreased with increasing patient age by univariate analysis, (p=0.074) (Table 1); by multivariate analysis, patient age was also an independent predictor of sentinel node metastasis (Table 2). The most significant predictors of SLN metastasis (p<0.0001 for each) were: 1) increasing tumor thickness (Chi-square: 223), 2) presence of lymphovascular invasion (Chi-square: 123), 3) younger patient age (Chi-square: 49), 4) trunk or lower extremity location (Chi-square: 48), 5) increasing Clark level of invasion (Chi-square:28) and 6) tumor ulceration (Chi-square: 25).
Table 2.
Clinical and pathological criteria predicting sentinel lymph node metastasis by chi-square analysis (n= 7756).
| Variable | DF | Estimate | SE | Chi-Square | P-value | Odds Ratio | 95% Confidence Interval |
|---|---|---|---|---|---|---|---|
| Thickness (mm) | 3 | 222.8 | <.0001 | ||||
| ≤1.0* | — | — | — | — | — | 1.0 | |
| 1.01–2.0 | 1 | 0.73 | 0.13 | 29.2 | <.0001 | 2.1 | 1.6–2.7 |
| 2.01–4.0 | 1 | 1.45 | 0.14 | 109.2 | <.0001 | 4.3 | 3.3–5.6 |
| >4.0 | 1 | 1.87 | 0.16 | 145.0 | <.0001 | 6.5 | 4.8–8.8 |
|
| |||||||
| Lymphovascular invasion | 1 | 122.9 | <.0001 | ||||
| Absent | — | — | — | — | — | — | |
| Present | 1 | 1.08 | 0.10 | 122.9 | <.0001 | 3.0 | 2.4–3.6 |
|
| |||||||
| Age (year) | 2 | 48.8 | <.0001 | ||||
| ≥60* | — | — | — | — | — | 1.0 | |
| <40 | 1 | 0.58 | 0.09 | 44.5 | <.0001 | 1.8 | 1.5–2.1 |
| 40–59 | 1 | 0.37 | 0.07 | 26.9 | <.0001 | 1.4 | 1.3–1.7 |
|
| |||||||
| Primary site | 3 | 47.7 | <.0001 | ||||
| Head/neck * | — | — | — | — | — | 1.0 | — |
| Upper Extremity | 1 | 0.13 | 0.12 | 1.3 | 0.2554 | 1.1 | 0.9–1.4 |
| Trunk | 1 | 0.53 | 0.10 | 25.6 | <.0001 | 1.7 | 1.4–2.1 |
| Lower Extremity | 1 | 0.57 | 0.11 | 26.2 | <.0001 | 1.8 | 1.4–2.2 |
|
| |||||||
| Clark’s level | 3 | 28.1 | <.0001 | ||||
| II* | — | — | — | — | — | 1.0 | |
| III | 1 | 0.60 | 0.33 | 3.3 | 0.0674 | 1.8 | 1.0–3.4 |
| IV | 1 | 0.98 | 0.32 | 9.3 | 0.0023 | 2.7 | 1.4–5.0 |
| V | 1 | 0.93 | 0.34 | 7.4 | 0.0065 | 2.5 | 1.3–5.0 |
|
| |||||||
| Ulceration | 1 | 25.2 | <.0001 | ||||
| No* | — | — | — | — | — | 1.0 | — |
| Yes | 1 | 0.34 | 0.07 | 25.2 | <.0001 | 1.4 | 1.2–1.6 |
|
| |||||||
| Gender | 1 | ||||||
| Male* | — | — | — | — | — | 1.0 | |
| Female | 1 | −0.13 | 0.07 | 4.2 | 0.0408 | 0.9 | 0.8–1.0 |
Reference group
Discussion
In this AJCC analysis of 7756 stage I and II primary cutaneous melanoma patients who underwent sentinel node biopsy, we observed an inverse relationship between melanoma mortality. In addition, there was an inverse relationship between age and the incidence of sentinel node metastasis when sorted by decades of age. Thus, patients with clinical stage I and II melanoma under 20 years of age had more adverse features in their primary melanoma [3], a higher incidence of sentinel lymph node metastasis and, paradoxically, a more favorable survival when compared to all other age groups. In contrast, patients >70 years had the most adverse primary melanoma features and higher mortality rates compared to all other age groups, but paradoxically a lower incidence of sentinel lymph node metastasis.
Perhaps the most interesting finding from our study, and supported by previous studies, is the lower incidence of sentinel lymph node metastasis despite higher mortality rate among older melanoma patients, and conversely, the higher incidence of sentinel node metastasis despite a lower mortality rate among younger melanoma patients. These data exemplify the heterogeneity of melanoma and the diversity of outcomes that must be accounted for both by clinicians and investigators. The biological implications of this paradox are provocative and support an interesting series of hypotheses to be tested in future studies. Primary melanomas in the very young and in the elderly population may have differing biological features, alternative routes of metastasis, and/or divergent host responses to the metastatic process, especially with regard to immune competence and these differences may contribute to the observed differences mortality rates. It is also possible that there could be a bias to use the SLN biopsy more liberally in young patients, and conversely, more conservatively in older patients.
An inverse relationship between sentinel node metastasis rate and patient age was first described in 2004 by Chao et al (7) and by Sondak et al[8]. Others have reported that age is an independent factor in predicting the risk of SLN metastasis[8–25]. In the Sunbelt Melanoma Trial involving over 3000 patients who had a sentinel node biopsy, the incidence of nodal metastasis for those <30 years was 23% compared to only 12% among patients aged 61 to 70 years (p=0.018) [15]. The inverse relationships between older patients (higher mortality and lower incidence of sentinel node metastasis) and younger patients (lower mortality rates and higher incidence of sentinel node metastasis)as compared to adults patients aged 20 to 60 years suggests that unaccounted features of the host and the disease, such as levels of immune competence, age-related changes in lymphatic function, or other biological features of the primary melanoma, such as hematogenous route of metastases or altered lymphangiogenesis, might be operative in these patients at extremes of age [26, 27]. This may be related to a previously reported age-related decline in the ability of melanoma cells to move radiocolloid from the peri-tumoral dermis to the sentinel node and retain them in that location [26].
It is known that different types of primary melanoma (desmoplastic, lentigo malignant, Spitzoid, etc.) with different prognosis are distributed unequally across age groups; many with more favorable outcome appear to be more prominent in younger patients. One limitation of our AJCC database is that we did not have systematic reporting or central pathology review of melanoma subtype in order to adjust for this variable in our analysis. In addition, frailty among the older population as measured by sarcopenia could adversely influences survival outcome and increases complication rates, especially among elderly melanoma patients [28, 29]. These data might also explain why older patients were less likely to have a survival benefit from lymphadenectomy compared to those <60 years of age [30]. Older patients have a greater preponderance of acral lentiginous melanomas as well as head and neck melanomas, including desmoplastic melanomas, that are associated with a lower incidence of nodal metastasis [31–35]. In addition, overlapping anatomic structures that make it difficult to localize head and neck sentinel lymph nodes on routine lymphoscintigraphy.. The recent use of SPECT/CT scan has substantially increased the accuracy of identifying the sentinel node among head and neck melanoma patients that heretofore may have been understaged [36].
Overall, these observations may help inform evolving indications for performing a sentinel node biopsy to maximize staging in younger patients, and to potentially offer this procedure less frequently in older patients who might be spared the extra morbidity of the procedure, especially in the setting of concomitant risk factors and/or co-morbidities (37).
There is also a great diversity of melanoma presentations among the very young, and a more favorable survival outcome, despite a higher incidence of nodal metastases [32, 37–39]. One explanation is that children or teenagers may have an atypical spitzoid melanocytic tumor or a melanocytic tumor of unknown malignant potential (MTUMP) with sentinel node metastasis; both entities are known to have a more favorable prognosis even in Stage III melanoma [11, 40–45]. Another melanoma presentation among teenagers and young adults with an increased incidence of nodal metastasis and a low potential for metastasis growing at distant sites is the “animal-type melanoma” [46].
These results raise and reiterate some interesting and important hypotheses and emphasize the importance of patient age as a component of melanoma staging and in the design and interpretation of clinical trials for patients with localized disease or with regional node metastases. Just as the genetic profile of acral lentiginous melanomas is distinctly different from melanoma arising in chronic sun-damaged skin [47], so too might melanomas arising in the very young and the very old might have a different cause and natural history from those arising in adults of aged 20 to 60 years. These data will hopefully stimulate a systematic inquiry to examine this possibility. Given the disproportionate rise in incidence among the very young and the disproportionate rise in mortality among older patients with melanoma, these issues are important to address so we can better treat our patients [48, 49]. Further research is also needed to determine if the genetic profile and causative associations are similar or distinct from those of young and middle aged melanoma patients where ultraviolet radiation is thought to be a primary causative agent.
Acknowledgments
The work of the AJCC/UICC Melanoma Staging Committee was supported by a grant from the American Joint Committee on Cancer and by grants from the National Cancer Institute (P30 CA13148 at the University of Alabama at Birmingham and P50 CA93459 SPORE grant in melanoma at The University of Texas M. D. Anderson Cancer Center in Houston, TX). Three meetings held by the Committee were partially supported by an unrestricted educational grant from Schering-Plough (Kenilworth, NJ).
Footnotes
These data have not been reported elsewhere. The authors have no disclaimers to make.
Data Management and Analysis
Seng-jaw Soong, PhD, Professor, University of Alabama at Birmingham, AL (deceased) Shouluan Ding, PhD, Biostatistician, University of Alabama at Birmingham, AL Matthew Dickerson, BS, Programmer Analyst, University of Alabama at Birmingham, AL Rush Elliott, BS, Data Manager, University of Alabama at Birmingham, AL Connie Pitts, Program Coordinator, University of Alabama at Birmingham, AL Marcella Johnson, The University of Texas M. D. Anderson Cancer Center, Houston, TX Carla Warneke, The University of Texas M. D. Anderson Cancer Center, Houston, TX
References
- 1.Balch CM, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27(36):6199–206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balch CM, et al. Multivariate analysis of prognostic factors among 2,313 patients with stage III melanoma: comparison of nodal micrometastases versus macrometastases. J Clin Oncol. 2010;28(14):2452–9. doi: 10.1200/JCO.2009.27.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balch CM, Soong SJ, Gershenwald JE. Age as a prognostic factor in patients with localized melanoma and regional metastases. Ann Surg Oncol. 2013 doi: 10.1245/s10434-013-3100-9. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balch CM, et al. Prognostic factors analysis of 17,600 melanoma patients: validation of the American Joint Committee on Cancer melanoma staging system. J Clin Oncol. 2001;19(16):3622–34. doi: 10.1200/JCO.2001.19.16.3622. [DOI] [PubMed] [Google Scholar]
- 5.Balch CMG, JE, Atkins MB, et al. AJCC Cancer Staging Manual. Springer; New York: 2009. Melanoma of the Skin; pp. 325–44. [Google Scholar]
- 6.Cox DR. Regression Models and Life-Tables. Journal of the Royal Statistical Society Series B. Statistical Methodology. 1972;34(2):187. [Google Scholar]
- 7.Chao C, et al. Correlation between prognostic factors and increasing age in melanoma. Ann Surg Oncol. 2004;11(3):259–64. doi: 10.1245/aso.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 8.Sondak VK, et al. Mitotic rate and younger age are predictors of sentinel lymph node positivity: lessons learned from the generation of a probabilistic model. Ann Surg Oncol. 2004;11(3):247–58. doi: 10.1245/aso.2004.03.044. [DOI] [PubMed] [Google Scholar]
- 9.Balch CM, et al. A multifactorial analysis of melanoma. II. Prognostic factors in patients with stage I (localized) melanoma. Surgery. 1979;86(2):343–51. [PubMed] [Google Scholar]
- 10.Balch CM, et al. A multifactorial analysis of melanoma: III. Prognostic factors in melanoma patients with lymph node metastases (stage II) Ann Surg. 1981;193(3):377–88. doi: 10.1097/00000658-198103000-00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caraco C, et al. Age as predictor in patients with cutaneous melanoma submitted to sentinel lymph node biopsy. Eur J Surg Oncol. 2006;32(9):970–3. doi: 10.1016/j.ejso.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 12.Azimi F, et al. Tumor-infiltrating lymphocyte grade is an independent predictor of sentinel lymph node status and survival in patients with cutaneous melanoma. J Clin Oncol. 2012;30(21):2678–83. doi: 10.1200/JCO.2011.37.8539. [DOI] [PubMed] [Google Scholar]
- 13.Egger ME, et al. Diversity of stage III melanoma in the era of sentinel lymph node biopsy. Ann Surg Oncol. 2013;20(3):956–63. doi: 10.1245/s10434-012-2701-z. [DOI] [PubMed] [Google Scholar]
- 14.Kretschmer L, et al. Age as a key factor influencing metastasizing patterns and disease-specific survival after sentinel lymph node biopsy for cutaneous melanoma. Int J Cancer. 2011;129(6):1435–42. doi: 10.1002/ijc.25747. [DOI] [PubMed] [Google Scholar]
- 15.McMasters KM, et al. Factors that predict the presence of sentinel lymph node metastasis in patients with melanoma. Surgery. 2001;130(2):151–6. doi: 10.1067/msy.2001.115830. [DOI] [PubMed] [Google Scholar]
- 16.Niakosari F, et al. Lymphatic invasion identified by monoclonal antibody D2–40, younger age, and ulceration: predictors of sentinel lymph node involvement in primary cutaneous melanoma. Arch Dermatol. 2008;144(4):462–7. doi: 10.1001/archderm.144.4.462. [DOI] [PubMed] [Google Scholar]
- 17.Rousseau DL, Jr, et al. Revised American Joint Committee on Cancer staging criteria accurately predict sentinel lymph node positivity in clinically node-negative melanoma patients. Ann Surg Oncol. 2003;10(5):569–74. doi: 10.1245/aso.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 18.Sassen S, et al. The complex relationships between sentinel node positivity, patient age, and primary tumor desmoplasia: analysis of 2303 melanoma patients treated at a single center. Ann Surg Oncol. 2008;15(2):630–7. doi: 10.1245/s10434-007-9684-1. [DOI] [PubMed] [Google Scholar]
- 19.Austin PF, et al. Age as a prognostic factor in the malignant melanoma population. Ann Surg Oncol. 1994;1(6):487–94. doi: 10.1007/BF02303614. [DOI] [PubMed] [Google Scholar]
- 20.Thompson JF, Shaw HM. Should tumor mitotic rate and patient age, as well as tumor thickness, be used to select melanoma patients for sentinel node biopsy? Ann Surg Oncol. 2004;11(3):233–5. doi: 10.1245/aso.2004.01.912. [DOI] [PubMed] [Google Scholar]
- 21.Venna SS, et al. Analysis of sentinel lymph node positivity in patients with thin primary melanoma. J Am Acad Dermatol. 2013;68(4):560–7. doi: 10.1016/j.jaad.2012.08.045. [DOI] [PubMed] [Google Scholar]
- 22.White RL, Jr, et al. Factors predictive of the status of sentinel lymph nodes in melanoma patients from a large multicenter database. Ann Surg Oncol. 2011;18(13):3593–600. doi: 10.1245/s10434-011-1826-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ciocan D, et al. Distinctive features of melanoma and its management in elderly patients: a population-based study in france. JAMA Dermatol. 2013;149(10):1150–7. doi: 10.1001/jamadermatol.2013.706. [DOI] [PubMed] [Google Scholar]
- 24.Page AJ, et al. Increasing Age Is Associated with Worse Prognostic Factors and Increased Distant Recurrences despite Fewer Sentinel Lymph Node Positives in Melanoma. Int J Surg Oncol. 2012;2012:456987. doi: 10.1155/2012/456987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lasithiotakis KG, I, Petrakis E, Garbe C. Cutaneous melanoma in the elderly: epidemiology, prognosis and treatment. Melanoma Res. 2010;20(3):163–70. doi: 10.1097/CMR.0b013e328335a8dd. [DOI] [PubMed] [Google Scholar]
- 26.Conway WC, et al. Age-related lymphatic dysfunction in melanoma patients. Ann Surg Oncol. 2009;16(6):1548–52. doi: 10.1245/s10434-009-0420-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pan WR, Suami H, Taylor GI. Senile changes in human lymph nodes. Lymphat Res Biol. 2008;6(2):77–83. doi: 10.1089/lrb.2007.1023. [DOI] [PubMed] [Google Scholar]
- 28.Lange JR, Kang S, Balch CM. Melanoma in the older patient: measuring frailty as an index of survival. Ann Surg Oncol. 2011;18(13):3531–2. doi: 10.1245/s10434-011-2015-6. [DOI] [PubMed] [Google Scholar]
- 29.Sabel MS, et al. Sarcopenia as a prognostic factor among patients with stage III melanoma. Ann Surg Oncol. 2011;18(13):3579–85. doi: 10.1245/s10434-011-1976-9. [DOI] [PubMed] [Google Scholar]
- 30.Balch CM, et al. Long-term results of a prospective surgical trial comparing 2 cm vs. 4 cm excision margins for 740 patients with 1–4 mm melanomas. Ann Surg Oncol. 2001;8(2):101–8. doi: 10.1007/s10434-001-0101-x. [DOI] [PubMed] [Google Scholar]
- 31.Eppsteiner RW, et al. Sentinel node biopsy for head and neck desmoplastic melanoma: not a given. Otolaryngol Head Neck Surg. 2012;147(2):271–4. doi: 10.1177/0194599812439857. [DOI] [PubMed] [Google Scholar]
- 32.Han D, et al. The unique clinical characteristics of melanoma diagnosed in children. Ann Surg Oncol. 2012;19(12):3888–95. doi: 10.1245/s10434-012-2554-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mohebati A, et al. The role of sentinel lymph node biopsy in the management of head and neck desmoplastic melanoma. Ann Surg Oncol. 2012;19(13):4307–13. doi: 10.1245/s10434-012-2468-2. [DOI] [PubMed] [Google Scholar]
- 34.Fadaki N, et al. Is head and neck melanoma different from trunk and extremity melanomas with respect to sentinel lymph node status and clinical outcome? Ann Surg Oncol. 2013;20(9):3089–97. doi: 10.1245/s10434-013-2977-7. [DOI] [PubMed] [Google Scholar]
- 35.Egger ME, et al. Unique prognostic factors in acral lentiginous melanoma. Am J Surg. 2012;204(6):874–9. doi: 10.1016/j.amjsurg.2012.05.013. discussion 879–80. [DOI] [PubMed] [Google Scholar]
- 36.Stoffels I, et al. Association between sentinel lymph node excision with or without preoperative SPECT/CT and metastatic node detection and disease-free survival in melanoma. JAMA. 2012;308(10):1007–14. doi: 10.1001/2012.jama.11030. [DOI] [PubMed] [Google Scholar]
- 37.Chagpar RB, et al. Factors associated with improved survival among young adult melanoma patients despite a greater incidence of sentinel lymph node metastasis. J Surg Res. 2007;143(1):164–8. doi: 10.1016/j.jss.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 38.Howman-Giles R, et al. Sentinel lymph node biopsy in pediatric and adolescent cutaneous melanoma patients. Ann Surg Oncol. 2010;17(1):138–43. doi: 10.1245/s10434-009-0657-4. [DOI] [PubMed] [Google Scholar]
- 39.Mu E, Lange JR, Strouse JJ. Comparison of the use and results of sentinel lymph node biopsy in children and young adults with melanoma. Cancer. 2012;118(10):2700–7. doi: 10.1002/cncr.26578. [DOI] [PubMed] [Google Scholar]
- 40.Berk DR, et al. Melanoma and melanocytic tumors of uncertain malignant potential in children, adolescents and young adults--the Stanford experience 1995–2008. Pediatr Dermatol. 2010;27(3):244–54. doi: 10.1111/j.1525-1470.2009.01078.x. [DOI] [PubMed] [Google Scholar]
- 41.Busam KJ, et al. Atypical spitzoid melanocytic tumors with positive sentinel lymph nodes in children and teenagers, and comparison with histologically unambiguous and lethal melanomas. Am J Surg Pathol. 2009;33(9):1386–95. doi: 10.1097/PAS.0b013e3181ac1927. [DOI] [PubMed] [Google Scholar]
- 42.Ghazi B, et al. Utility of lymph node assessment for atypical spitzoid melanocytic neoplasms. Ann Surg Oncol. 2010;17(9):2471–5. doi: 10.1245/s10434-010-1022-3. [DOI] [PubMed] [Google Scholar]
- 43.Meyers MO, et al. Age and Breslow depth are associated with a positive sentinel lymph node in patients with cutaneous melanocytic tumors of uncertain malignant potential. J Am Coll Surg. 2010;211(6):744–8. doi: 10.1016/j.jamcollsurg.2010.07.020. [DOI] [PubMed] [Google Scholar]
- 44.Mills OL, et al. Sentinel node biopsy in atypical melanocytic neoplasms in childhood: a single institution experience in 24 patients. J Cutan Pathol. 2012;39(3):331–6. doi: 10.1111/j.1600-0560.2011.01853.x. [DOI] [PubMed] [Google Scholar]
- 45.Paradela S, et al. Prognostic factors for melanoma in children and adolescents: a clinicopathologic, single-center study of 137 Patients. Cancer. 2010;116(18):4334–44. doi: 10.1002/cncr.25222. [DOI] [PubMed] [Google Scholar]
- 46.Antony FC, et al. Pigment synthesizing melanoma (so-called animal type melanoma): a clinicopathological study of 14 cases of a poorly known distinctive variant of melanoma. Histopathology. 2006;48(6):754–62. doi: 10.1111/j.1365-2559.2006.02411.x. [DOI] [PubMed] [Google Scholar]
- 47.Curtin JA, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353(20):2135–47. doi: 10.1056/NEJMoa050092. [DOI] [PubMed] [Google Scholar]
- 48.Jemal A, et al. Recent trends in cutaneous melanoma incidence and death rates in the United States, 1992–2006. J Am Acad Dermatol. 2011;65(5 Suppl 1):S17–25. e1–3. doi: 10.1016/j.jaad.2011.04.032. [DOI] [PubMed] [Google Scholar]
- 49.Strouse JJ, et al. Pediatric melanoma: risk factor and survival analysis of the surveillance, epidemiology and end results database. J Clin Oncol. 2005;23(21):4735–41. doi: 10.1200/JCO.2005.02.899. [DOI] [PubMed] [Google Scholar]