Abstract
Purpose
We postulated that the worse prognosis of melanoma with advancing age reflected more aggressive tumor biology and that in younger patients the prognosis would be more favorable.
Materials and Methods
The expanded AJCC melanoma staging database contained 11,088 patients with complete data for analysis, including mitotic rate.
Results
With increasing age by decade, primary melanomas were thicker, exhibited higher mitotic rates, and were more likely to be ulcerated. In a multivariate analysis of patients with localized melanoma, thickness and ulceration were highly significant predictors of outcome at all decades of life (except for patients less than 20 years). Mitotic rate was significantly predictive in all age groups except patients < 20 years and >80 years. For patients with Stage III melanoma, there were four independent variables associated with patient survival: number of nodal metastases, patient age, ulceration, and mitotic rate.
Patients under 20 years of age had primary tumors with slightly more aggressive features, a higher incidence of sentinel lymph node metastasis, but, paradoxically, more favorable survival than all other age groups. In contrast, patients >70 years old had primary melanomas with the most aggressive prognostic features, were more likely to be head and neck primaries, and were associated with a higher mortality rate than the other age groups. Surprisingly, however, these patients had a lower rate of sentinel lymph node metastasis per T stage. Among patients between the two age extremes, clinicopathologic features and survival tended to be more homogeneous.
Conclusions
Melanomas in patients at the extremes of age have a distinct natural history.
Introduction
Patient age has been reported as an independent prognostic factor in melanoma studies spanning over three decades [1–15]. We have previously reported that patient age is a highly significant and powerful predictor of survival using the American Joint Committee on Cancer (AJCC) Melanoma Staging database, even after accounting for adverse prognostic features, such as the anatomic site of the primary melanoma and the patient’s gender [1, 2].
Yet, patient age has not been incorporated into staging systems and is not used consistently as a stratification criterion in early stage melanoma clinical trials. There could be several reasons for this, especially among the older melanoma population. First, there is no reported threshold of patient age that clearly signals a worse prognosis. Second, it is unknown whether age reflects a crude surrogate of declining immune competence, or other co-morbidities, or differences in the biological behavior of melanoma in patients of different age groups. Third, patient age might not be a truly independent predictive factor, but instead may secondarily reflect a combination of adverse characteristics that cannot be accounted for in smaller patient series. Melanoma among teenagers and children is much less common, and as a consequence, few series report on a sufficiently large population to make a valid comparison with prognosis and demographics of melanoma patients <20 years of age with older melanoma population
The AJCC melanoma staging database is comprised of records for more than 25,734 patients with localized melanoma and 2313 patients with nodal metastases. These records include complete clinical and pathological information for all prognostic factors except for mitotic rate which was available in 10,233 patients with localized melanoma and 775 patients with nodal metastases. With such a large dataset, we postulated that we might be able to discern whether melanoma is a different biological entity among younger and older patients, and whether controlling for all the variables in patients at these extremes of age groups might diminish the prognostic significance of patient age if all the other variables were truly accounted for. This was especially true since the important feature of mitotic rate was available in our large sample that could be included in modeling for independent factors.
METHODS
The AJCC melanoma staging database was created in 1999 as a result of an international collaboration that combined prospective melanoma databases from 18 major cancer centers and clinical trial cooperative groups [1, 3]. It was updated in 2008, and the updated information used to revise the AJCC melanoma staging system for inclusion in the 7th edition of the AJCC Melanoma Staging Manual [16]
Survival times were calculated from the date of initial melanoma diagnosis and considered censored for patients who were alive at last follow up or who died without evidence of melanoma. Standard statistical methods were used; melanoma-specific survival curves were generated by the Kaplan-Meier product-limit method and compared using the log rank test, and multivariate analyses were based on the Cox proportional hazards model [3, 16, 17].
RESULTS
Characteristics of the 28, 047 patients grouped by age are shown in the supplemental data files (Supplemental Tables 1 and 2). Mean and median age was 51.8 and 52 years respectively, for patients with localized melanoma (stage I/II), and 51.1 and 51 years, respectively, for patients with regional metastases (stage III).
Primary Melanoma Correlations with Patient Age
Stage I and II
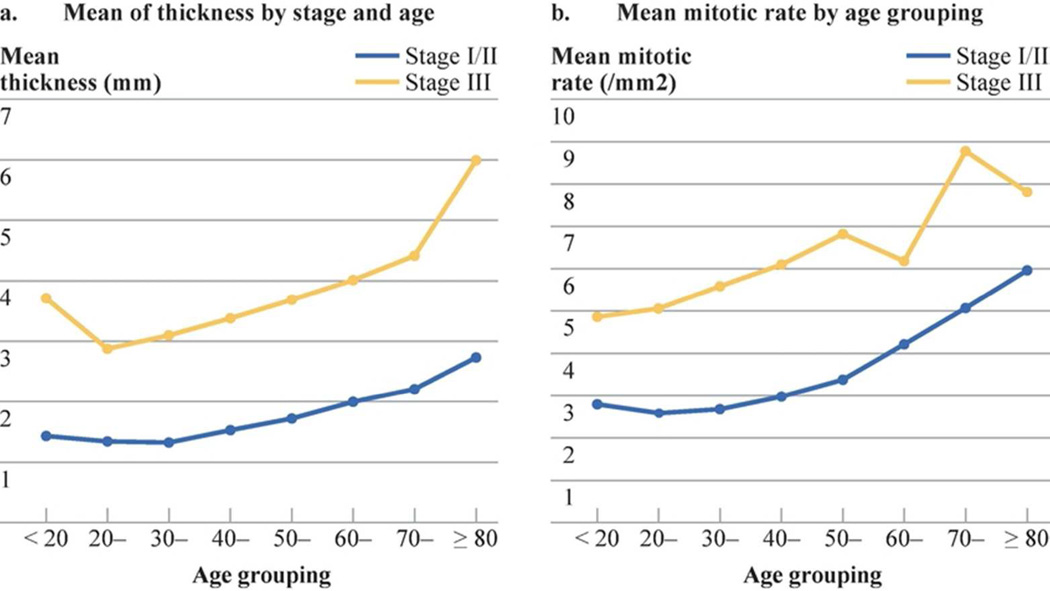
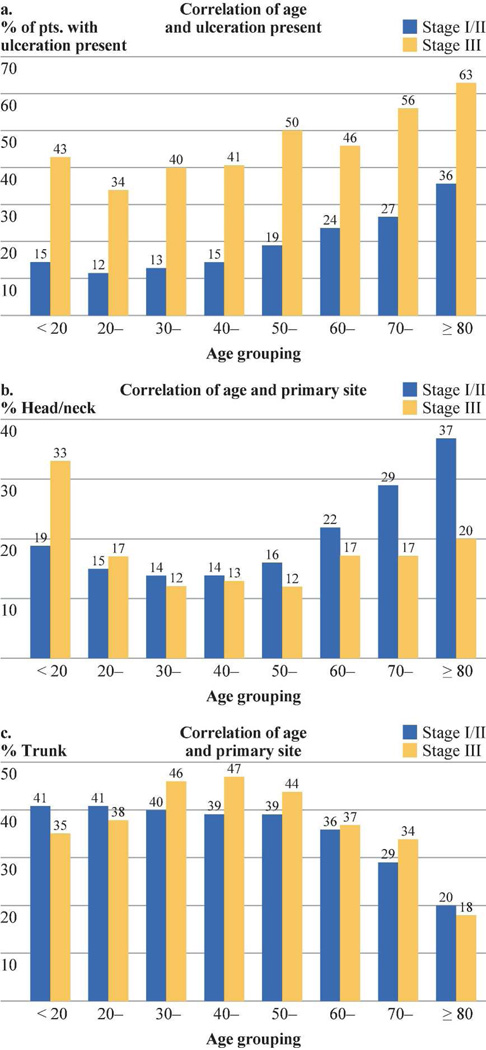
With increasing age by decade, primary melanomas became thicker, (Fig. 1a), exhibited higher mitotic rates (Fig. 1b), and were more likely to be ulcerated (Fig. 2a). Mean thickness of the primary melanoma ranged from 1.4 mm for those younger than 20 years to 2.4 mm in patients older than 70 years The average mitotic rate also increased steadily by decade of age, ranging from 2.5/mm2 for those patients aged 20–40 to 5.8/mm2 for patients older than 80 years (Fig. 1b). Thus, the mitotic rate/mm2 was 2.8 for patients < 20 years, 3.3 for those 20 to 69 years, and 5.3 for those ≥ 70 years (p= 0.0001). Similarly, the incidence of tumor ulceration increased almost threefold by decade of age, ranging from 12% for patients aged 20–30 years up to 36% for those older than 80 years (Fig. 2a).
Figure 1.
A: Mean thickness of the primary melanoma according to age of patients with localized tumors (stages I/II) or regional nodal metastases (stage III)
F-test based on a mixed model: stage difference, F(1,7)=322.2, P<0.0001; Age difference: F(7,7)=19.2, P=0.0005
B: Mean mitotic rate in patients with localized melanoma (Stages I and II) and those with regional nodal metastases (Stage III) sorted by patient age by decade.
F-test based on a mixed model: stage difference, F(1,7)=268.3, P<0.0001; Age difference: F(7,7)=30.4, P=0.0001
Figure 2.
A: Incidence of primary melanoma ulceration in patients with localized melanoma (Stages I and II) and those with regional nodal metastases (Stage III) by patient age.
F-test based on a mixed model: stage difference, F(1,7)=1284.1, P<0.0001; Age difference: F(7,7)=69.6, P<0.0001
B: Incidence of head and neck melanoma in patients with localized disease (Stages I and II) and those with regional nodal metastases (Stage III) by patient age.
F-test based on a mixed model: stage difference, F(1,7)=2.1, P=0.1924; Age difference: F(7,7)=3.9, P=0.0470
C: Incidence of trunk melanoma in patients with localized disease (Stages I and II) and those with regional nodal metastases (Stage III) sorted by patient age.
F-test based on a mixed model: stage difference, F(1,7)=2.2, P=0.1787; Age difference: F(7,7)=23.6, P=0.0002
Stage III
Similar age-related correlations were seen in patients with Stage III nodal metastasis. Mean thickness of the primary melanoma ranged from 2.8 mm in patients aged 20–30 to almost 6 mm for patients 80 years of age and older (Figure 1a). Mitotic rate/mm2 among Stage was 4.9 for patients younger than 20 years, 6.1 for those 20–69 years, and 8.6 for those ≥70 years (p= 0.0001, Fig. 1b). The percentage of patients with a very high mitotic rate of ≥5 /mm2 also increased by decade of age, ranging from 18.8% for those <20 years of age to 40.7% for those 80 years or older. The incidence of ulcerated primary melanoma also increased significantly with increasing patient age, ranging from 34% in patients aged 20–30 years, to 63% in those 80 years of age or older (Fig. 2a). It is interesting to note that among patients under 20 years of age, their average tumor thickness was greater and incidence of ulceration was greater compared to those in the next few decades of life.
Clinical Characteristics
There were fairly dramatic age-related shifts in the distribution of primary tumor sites among patients with stage I/II and stage III melanoma. In patients who were younger than 60 years, the most common anatomic site of the primary was the trunk (Fig. 2c), followed by the lower extremity, upper extremity, and then the head/neck. In contrast, melanoma arising on the trunk was the least common site in patients who were at least 80 years of age,. For patients with stage I and II melanoma, the most common anatomic site was the head and neck, followed by the upper extremity, lower extremity, and then the trunk. For older patients with stage III melanoma, the distribution by anatomic site was lower extremity > upper extremity > head and neck > trunk. As compared with adults, teenagers and children had a higher frequency of head and neck melanomas and a lower frequency of lower-extremity melanomas.
Male patients were slightly older than female patients in stage I/II (39.6% of men versus 28.6 % of women were ≥ 60 years;p<0.01) and stage III groups (33.8% of men versus 27.9 % of women were ≥ 60 years). When analyzed by decade of age, the majority of stage I/II patients between 20 and 50 years of age were women, while the majority of patients over 50 years of age were men (p<0.0001); among stage III patients, female patients were only in the majority for the 20–30 year age group (p<0.0001)
Male patients were slightly older than female patients in stage I/II (39.6% of men versus 28.6 % of women were ≥60 years;p<0.01) and stage III groups (33.8% of men versus 27.9 % of women were ≥60 years p=0.01). When analyzed by decade of age, the majority of stages I/II patients between 20 and 50 years of age were women, while the majority of patients over 50 years were men (p<0.0001); among stage III patients, female patients were only in the majority for the 20–30 year age group (p<0.0001)
Melanoma Specific Survival Rates
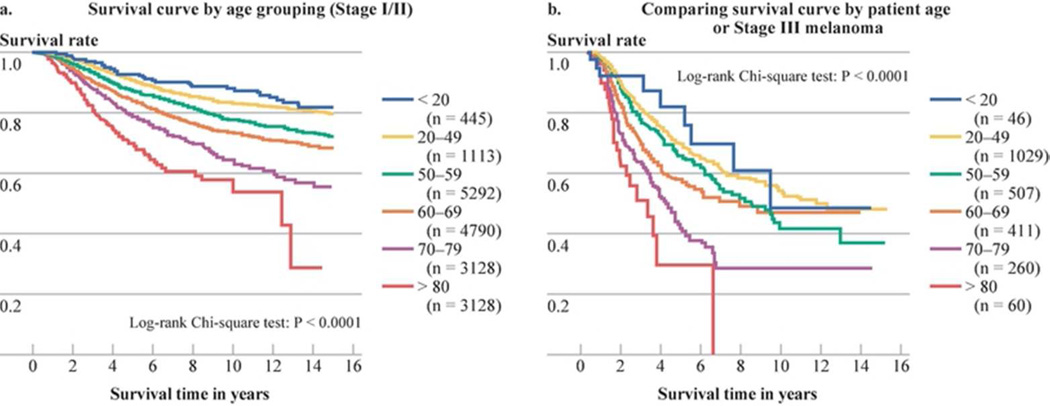
There was a gradual decline in survival rates for Stage I/II patients when sorted by age (Fig. 3a). Patients under 20 years of age had the most favorable survival rates while there was a clustering of patients between 20–50 years of age without discernible survival differences (data not shown). Beyond age 50, there was a statistically significant decline in survival rates based upon decade of age (Fig. 3a). When survival rates were compared by gender and age, female patients < 60 years of age had the best survival rates at 10-years (86%), compared to men <60 years at 78% (p<0.05), women > 60 years at 74% and men > 60 years at 66% (p<0.05). Similarly, there was a gradual decline in survival rates for Stage III patients when sorted by patient age, with patients under 20 years of age having the highest survival and those older than 70 years having the lowest survival (Fig 3b).
Figure 3.
A. Survival curves by age of patients with stage I/II melanoma
B Survival curves by age of patients with stage III melanoma
Multivariate Analysis of Prognosis by Age Groups
Stage I and II
Among 10,233 patients whose records included mitotic rate, six independent clinicopathological characteristics correlated with survival: patient age and sex, and the primary tumor’s thickness, mitotic rate, ulceration status, and anatomic site all (Table 1; p<0.0001). Multivariate analysis within each age group showed that thickness was highly significant at all decades of life, except those under 20 years, and ulceration was significant for patients aged 40 to 80 years. Mitotic rate was significant in all age groups except those < 20 years and > 80 years, while Clark’s level of invasion was not predictive for any age group except patients older than 80 years(Table 1). Patient gender and anatomic site of melanoma were not independent predictive factors in patients older than 70 years.
Table 1.
Multivariate Analysis of Prognosis Within Cohorts of Patient Age by Decade for Localized Melanoma (stage I and II) Chi-square (d.f.=1), P-value
| Age (yr) | Number of patients |
Thickness | Ulceration | Clark’s Level |
Site | Gender | Mitotic Rate |
|---|---|---|---|---|---|---|---|
| <20 ** | 185 | 1.4 P=0.2401 | 3.2 (df=1) P=0.0759 | 0.1 (df=1) P=0.8125 | 5.6 (df=1) P=0.0177* | 4.3 (df=1) P=0.0386* | 0.1 (df=1) P=0.7947 |
| 20- | 796 | 7.8 (df=3) P=0.0505* | 1.7 (df=1) P=0.1926 | 0.2 (df=1) P=0.6610 | 0.6 (df=1) P=0.4497 | 3.8 (df=1) P=0.0524 | 17.3 (df=3) P=0.0006* |
| 30- | 1509 | 14.6 (df=3) P=0.0022* | 2.2 (df=1) P=0.1346 | 1.9 (df=1) P=0.1725 | 6.1 (df=1) P=0.0136* | 8.7 (df=1) P=0.0031* | 17.1 (df=3) P=0.0007* |
| 40- | 1903 | 25.4 (df=3) P<0.0001* | 10.6 (df=1) P=0.0011* | 0.3 (df=1) P=0.6019 | 6.8 (df=1) P=0.0089* | 7.7(df=1) P=0.0055* | 8.6 (df=3) P=0.0344* |
| 50- | 2073 | 32.2 (df=3) P<0.0001* | 8.4 (df=1) P=0.0038* | 1.7 (df=1) P=0.1936 | 3.1 (df=1) P=0.0784 | 4.2 (df=1) P=0.0406* | 21.7 (df=3) P<0.0001* |
| 60- | 1924 | 25.9 (df=3) P<0.0001* | 22.0 (df=1) P<0.0001* | 0.3 (df=1) P=0.5544 | 8.4 (df=1) P=0.0038* | 7.9 (df=1) P=0.0050* | 15.3 (df=3) P=0.0016* |
| 70- | 1392 | 13.6 (df=3) P=0.0034* | 7.9 (df=1) P=0.0049* | 1.4(df=1) P=0.2429 | 3.5 (df=1) P=0.0629 | 0.3 (df=1) P=0.5819 | 11.7 (df=3) P=0.0083* |
| ≥80** | 451 | 9.2 (df=1) P=0.0024* | 1.0 (df=1) P=0.3258 | 4.9 (df=1) P=0.0272* | 3.3 (df=1) P=0.0692 | 0.1 (df=1) P=0.7141 | 1.6 (df=1) P=0.2110 |
P<0.05
For patients <20 or ≥80 years of age, tumor thickness was categorized as ≤2.0 or >2.0 mm, and mitotic rate was categorized as <5.0 or ≥5.0/mm2, due to the smaller sample size. For other patients, tumor thickness was categorized as 0–1.00, 1.01–2.00, 2.01–4.00, or >4.00 mm; mitotic rate was categorized as <1, 1-, 5.0- or ≥10.0/mm2.
Stage III
Among 775 Stage III patients whose records included mitotic rate, only four independent variables were associated with survival: number of nodal metastases (p<0.0001), patient age (p=0.0014), tumor ulceration (p=0.0038), and mitotic rate (p= 0.0319). The multivariate analysis compared patients at a threshold of age 60 due to sample size considerations. For 501 patients younger than 60 years, the independent factors in a multivariate analysis were the number of nodal metastases (p< 0.0001), and primary tumor ulceration (p-0.0245); while tumor thickness, Clark’s level of invasion, gender, anatomic site, tumor burden (microscopic vs. macroscopic) and mitotic rate were not significant. In contrast, for 274 patients ≥60 years of age, the only independent factor was the number of nodal metastases (p=0.0423).
Discussion
In this large multi-institutional study, we correlated prognostic significance and survival with all the known independent clinicopathologic features in patients with AJCC/UICC Stage I, II and III melanoma for the whole range of patient age groups. This is the first such major study to include mitotic rate in relation to age. After accounting for all the dominant prognostic factors, the patient’s age is still a very strong and independent predictor of survival outcome.
Analysis of this data set involving over 28,000 patients displays the diversity of melanoma outcomes across the age spectrum. Patients under 20 years of age have melanomas with slightly more aggressive features but, paradoxically, a more favorable survival outcome compared to all other age groups. Patients >70 years have melanomas with the most aggressive prognostic features, a unique distribution according to anatomic site, and, higher lethality compared to all other age groups. Finally, patients 20–70 years of age have few discernible differences in the natural history of their disease, anatomic site of melanoma presentation or survival rates within their age grouping. Thus, it seems apparent from this data analysis and review of the literature, that melanomas at the extremes of age have a distinct natural history. Interestingly, the incidence and mortality of melanoma in the older population has increased significantly, especially among older men. In contrast, the incidence of melanoma among children and teenagers is also increasing and yet, based upon national statistics in the United States [18–20]. their mortality rates seem to be decreasing
Understanding the causes and the unique natural history of melanomas in the elderly is vitally important [21, 22]. Compared to other cancers in the United States of America, there is a disproportionate increase in mortality in melanoma patients 65 years of age or older, particularly among men [18, 23]. Mortality among older men increased by over 157% from 1969 to 1999, while their incidence increased by five-fold compared to a three-fold for women of the same age group [24]. From a policy standpoint, melanoma care presents a significant economic burden among the elderly population in America[25].
The features of the primary melanoma among older patients are, in general, those of more locally advanced melanomas (thicker and more ulcerated) with a much greater proliferative activity as calibrated by the mitotic rate[9, 10, 26, 27]. The anatomic location of melanomas in the elderly population is clearly distinct from that of younger and middle age adults with an increase in the proportion of head and neck melanomas (due to an increase in melanomas on the face and scalp in some series) and a decline in the proportion of trunk melanomas [9, 10, 26]. These results could not be explained by gender distribution. Whether or not this feature of increased risk for head and neck melanomas contributes to the increased mortality among elderly patients with melanomas is unknown. Our analysis showed that men over 60 have the worst prognosis, as others have reported [9, 10, 27]
Natural history and survival outcomes are clearly different for patients under 20 years of age, both from our analysis and from prior reports in the literature [28–30]. The characteristics of primary melanomas are somewhat more advanced in this age group, although not to the same extent as in older patients. Interestingly, this is the only age group where tumor thickness is not an independent predictor of survival, an observation also made in a study based on the National Cancer Data base (Lange, 2007)[19]. Despite higher risk primary features and an increase in the proportion of patients with SLN involvement in this age group relative to older patients, the mortality rate is, lower than other age groups [8, 20, 28, 31–35]. One explanation is that some children or teenagers may be more likely to have an atypical spitzoid melanocytic tumor or a melanocytic tumor of unknown malignant potential (MELTUMP) with sentinel node metastasis; both entities are known to have a relatively favorable prognosis [31, 36, 37]. There is evidence that the genetic phenotype in children is distinctive, with a more frequent loss of INK4A and gain of KIT [38]. The contribution of these genetic features, if any, to the observed improved survival remains to be determined. National statistics in the United States show an increase in the incidence of melanomas among children and teenagers [20, 39], but in contrast to older melanoma patients, mortality rates among children and teenagers with melanomas seem to be falling [39].
The variable behavior of melanoma at the extremes of age might be due to differences in the aggressiveness of the tumor, and/or altered host response to the disease, changes in lymphatic flow, co-morbidities or a combination of factors. Elderly patients have a higher incidence of scalp melanomas, desmoplastic melanomas, and acral lentginous melanomas [9, 26], lesions that have more spindle cell appearance and which are less likely to contain BRAF mutations. [30, 31, 36, 37]. Furthermore host immune function, and lymphatic flow have been shown to decrease with advanced age. Research addressing host responses to melanoma can detect alterations in host immunity that might further explain why melanoma is more likely to be fatal in older patients
With all of these different clinical and pathological features that are used to determine melanomas stage and predict metastatic risk it is becoming increasing difficult for the clinician to integrate them into a unified estimate of survival for an individual patients? Through the AJCC, we have developed a mathematical predictive model that estimates actuarial survival rates of melanoma patients with any given combination of clinical and pathological features of their melanoma [40]. This predictive model can be accessed at www.melanomaprognosis.org. This model has been updated to include patient age. For example: a 25 year old with a 2.9 mm non-ulcerated localized melanoma of the trunk has a predicted 10 year survival of 74%%, whereas a 75 year old patient with the same characteristics has a predicted 10 year survival of 60% if the T3 melanoma is not ulcerated and 42% if it is ulcerated.
There is some limitation on interpreting survival data across age groups in a study like this. We cannot examine age-related bias in the survival data related to “death from other causes” where some patients dying of “other causes” may have died from occult metastatic melanoma; so it is difficult to know accurately about the incidence of melanoma specific deaths without autopsy verification. Fatalities among younger melanoma patients would almost always have been from metastatic melanoma, whereas older patients often have co-morbidities that may have directly or indirectly been a cause of death. Misattribution of “cause of death” when calculating melanoma-specific survival rates will likely have affected the older age group asymmetrically, but it is not possible account for this potential bias in our study.
Melanomas in patients at the extremes of age have a distinct natural history. These results generate interesting hypotheses and emphasize the importance of accounting for patient age in both the design and interpretation of melanoma clinical trials for patients with localized disease and regional metastases. BRAF mutations have been associated with younger age and poor prognosis, but alone could not account for our findings [41]. Our data will hopefully stimulate a systemic inquiry to examine these age correlations at a molecular level. Given the disproportionate rise in incidence of melanoma among very young and the disproportionate rise in melanoma mortality among older patients, these issues are important to permit better tailoring of treatment to match the biology of each patient’s tumors.
Supplementary Material
Acknowledgment
The work of the AJCC/UICC Melanoma Staging Committee was supported by a grant from the American Joint Committee on Cancer and by grants from the National Cancer Institute (P30 CA13148 at the University of Alabama at Birmingham and P50 CA93459 SPORE grant in melanoma at The University of Texas M. D. Anderson Cancer Center in Houston, TX). Three meetings held by the Committee were partially supported by an unrestricted educational grant from Schering-Plough (Kenilworth, NJ).
The authors thank Ms Gwen Berry at the John Wayne Cancer Institute for her editorial assistance.
Footnotes
These data have not been reported elsewhere. The authors have no disclaimers to make.
Data Management and Analysis
Seng-jaw Soong, PhD, Professor, University of Alabama at Birmingham, AL
Shouluan Ding, PhD, Biostatistician, University of Alabama at Birmingham, AL
Matthew Dickerson, BS, Programmer Analyst, University of Alabama at Birmingham, AL
Rush Elliott, BS, Data Manager, University of Alabama at Birmingham, AL
Connie Pitts, Program Coordinator, University of Alabama at Birmingham, AL
Marcella Johnson, The University of Texas M. D. Anderson Cancer Center, Houston, TX
Carla Warneke, The University of Texas M. D. Anderson Cancer Center, Houston, TX
REFERENCES
- 1.Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balch CM, Gershenwald JE, Soong SJ, et al. Multivariate analysis of prognostic factors among 2,313 patients with stage III melanoma: comparison of nodal micrometastases versus macrometastases. J Clin Oncol. 2010;28:2452–2459. doi: 10.1200/JCO.2009.27.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balch CM, Soong SJ, Gershenwald JE, et al. Prognostic factors analysis of 17,600 melanoma patients: validation of the American Joint Committee on Cancer melanoma staging system. J Clin Oncol. 2001;19:3622–3634. doi: 10.1200/JCO.2001.19.16.3622. [DOI] [PubMed] [Google Scholar]
- 4.Balch CM, Soong SJ, Milton GW, et al. A comparison of prognostic factors and surgical results in 1,786 patients with localized (stage I) melanoma treated in Alabama, USA, New South Wales, Australia. Ann Surg. 1982;196:677–684. doi: 10.1097/00000658-198212001-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balch CM, Soong SJ, Murad TM, et al. A multifactorial analysis of melanoma. II. Prognostic factors in patients with stage I (localized) melanoma. Surgery. 1979;86:343–351. [PubMed] [Google Scholar]
- 6.Balch CM, Soong SJ, Murad TM, et al. A multifactorial analysis of melanoma: III. Prognostic factors in melanoma patients with lymph node metastases (stage II) Ann Surg. 1981;193:377–388. doi: 10.1097/00000658-198103000-00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrone CR, Panageas KS, Busam K, et al. Multivariate prognostic model for patients with thick cutaneous melanoma: importance of sentinel lymph node status. Ann Surg Oncol. 2002;9:637–645. doi: 10.1007/BF02574479. [DOI] [PubMed] [Google Scholar]
- 8.Kretschmer L, Starz H, Thoms KM, et al. Age as a key factor influencing metastasizing patterns and disease-specific survival after sentinel lymph node biopsy for cutaneous melanoma. Int J Cancer. 2011;129:1435–1442. doi: 10.1002/ijc.25747. [DOI] [PubMed] [Google Scholar]
- 9.Lasithiotakis K, Leiter U, Meier F, et al. Age and gender are significant independent predictors of survival in primary cutaneous melanoma. Cancer. 2008;112:1795–1804. doi: 10.1002/cncr.23359. [DOI] [PubMed] [Google Scholar]
- 10.Macdonald JB, Dueck AC, Gray RJ, et al. Malignant melanoma in the elderly: different regional disease and poorer prognosis. J Cancer. 2011;2:538–543. doi: 10.7150/jca.2.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thompson JF, Soong SJ, Balch CM, et al. Prognostic significance of mitotic rate in localized primary cutaneous melanoma: an analysis of patients in the multi-institutional American Joint Committee on Cancer melanoma staging database. J Clin Oncol. 2011;29:2199–2205. doi: 10.1200/JCO.2010.31.5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wiener M, Acland KM, Shaw HM, et al. Sentinel node positive melanoma patients: prediction and prognostic significance of nonsentinel node metastases and development of a survival tree model. Ann Surg Oncol. 2010;17:1995–2005. doi: 10.1245/s10434-010-1049-5. [DOI] [PubMed] [Google Scholar]
- 13.Balch CM, Murad TM, Soong SJ, et al. A multifactorial analysis of melanoma: prognostic histopathological features comparing Clark's and Breslow's staging methods. Ann Surg. 1978;188:732–742. doi: 10.1097/00000658-197812000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caraco C, Marone U, Botti G, et al. Age as predictor in patients with cutaneous melanoma submitted to sentinel lymph node biopsy. Eur J Surg Oncol. 2006;32:970–973. doi: 10.1016/j.ejso.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 15.Egger ME, Callender GG, McMasters KM, et al. Diversity of stage III melanoma in the era of sentinel lymph node biopsy. Ann Surg Oncol. 2013;20:956–963. doi: 10.1245/s10434-012-2701-z. [DOI] [PubMed] [Google Scholar]
- 16.Balch CMGJE, Atkins MB, et al. Melanoma of the Skin. In: Edge SB, Byrd DR, Compton CC, et al., editors. AJCC Cancer Staging Manual. Edition. New York: Springer; 2009. pp. 325–344. [Google Scholar]
- 17.Cox DR. Regression Models and Life-Tables. Journal of the Royal Statistical Society Series B-Statistical Methodology. 1972;34:187. -&. [Google Scholar]
- 18.Jemal A, Saraiya M, Patel P, et al. Recent trends in cutaneous melanoma incidence and death rates in the United States, 1992–2006. J Am Acad Dermatol. 2011;65:S17–S25. e11–e13. doi: 10.1016/j.jaad.2011.04.032. [DOI] [PubMed] [Google Scholar]
- 19.Lange JR, Palis BE, Chang DC, et al. Melanoma in children and teenagers: an analysis of patients from the National Cancer Data Base. J Clin Oncol. 2007;25:1363–1368. doi: 10.1200/JCO.2006.08.8310. [DOI] [PubMed] [Google Scholar]
- 20.Strouse JJ, Fears TR, Tucker MA, Wayne AS. Pediatric melanoma: risk factor and survival analysis of the surveillance, epidemiology and end results database. J Clin Oncol. 2005;23:4735–4741. doi: 10.1200/JCO.2005.02.899. [DOI] [PubMed] [Google Scholar]
- 21.Testori A, Soteldo J, Sances D, et al. Cutaneous melanoma in the elderly. Melanoma Res. 2009;19:125–134. doi: 10.1097/CMR.0b013e328329fe95. [DOI] [PubMed] [Google Scholar]
- 22.Tsai S, Balch C, Lange J. Epidemiology and treatment of melanoma in elderly patients. Nature Reviews Clinical Oncology. 2010;7:148–152. doi: 10.1038/nrclinonc.2010.1. [DOI] [PubMed] [Google Scholar]
- 23.Simard EP, Ward EM, Siegel R, Jemal A. Cancers with increasing incidence trends in the United States: 1999 through 2008. CA Cancer J Clin. 2012 doi: 10.3322/caac.20141. [DOI] [PubMed] [Google Scholar]
- 24.Geller AC, Miller DR, Annas GD, et al. Melanoma incidence and mortality among US whites, 1969–1999. JAMA. 2002;288:1719–1720. doi: 10.1001/jama.288.14.1719. [DOI] [PubMed] [Google Scholar]
- 25.Seidler AM, Pennie ML, Veledar E, et al. Economic Burden of Melanoma in the Elderly Population Population-Based Analysis of the Surveillance, Epidemiology, and End Results (SEER)-Medicare Data. Arch Dermatol. 2010;146:249–256. doi: 10.1001/archdermatol.2009.389. [DOI] [PubMed] [Google Scholar]
- 26.Chao C, Martin RC, 2nd, Ross MI, et al. Correlation between prognostic factors and increasing age in melanoma. Ann Surg Oncol. 2004;11:259–264. doi: 10.1245/aso.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 27.Pollack LA, Li J, Berkowitz Z, et al. Melanoma survival in the United States, 1992 to 2005. J Am Acad Dermatol. 2011;65:S78–S86. doi: 10.1016/j.jaad.2011.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chagpar RB, Ross MI, Reintgen DS, et al. Factors associated with improved survival among young adult melanoma patients despite a greater incidence of sentinel lymph node metastasis. J Surg Res. 2007;143:164–168. doi: 10.1016/j.jss.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 29.Sassen S, Shaw HM, Colman MH, et al. The complex relationships between sentinel node positivity, patient age, primary tumor desmoplasia: analysis of 2303 melanoma patients treated at a single center. Ann Surg Oncol. 2008;15:630–637. doi: 10.1245/s10434-007-9684-1. [DOI] [PubMed] [Google Scholar]
- 30.Moore-Olufemi S, Herzog C, Warneke C, et al. Outcomes in Pediatric Melanoma Comparing Prepubertal to Adolescent Pediatric Patients. Ann Surg. 2011;253:1211–1215. doi: 10.1097/SLA.0b013e318217e852. [DOI] [PubMed] [Google Scholar]
- 31.Berk DR, LaBuz E, Dadras SS, et al. Melanoma and melanocytic tumors of uncertain malignant potential in children, adolescents and young adults--the Stanford experience 1995–2008. Pediatr Dermatol. 2010;27:244–254. doi: 10.1111/j.1525-1470.2009.01078.x. [DOI] [PubMed] [Google Scholar]
- 32.Niakosari F, Kahn HJ, McCready D, et al. Lymphatic invasion identified by monoclonal antibody D2–40, younger age, ulceration: predictors of sentinel lymph node involvement in primary cutaneous melanoma. Arch Dermatol. 2008;144:462–467. doi: 10.1001/archderm.144.4.462. [DOI] [PubMed] [Google Scholar]
- 33.Paradela S, Fonseca E, Pita-Fernandez S, et al. Prognostic factors for melanoma in children and adolescents: a clinicopathologic, single-center study of 137 Patients. Cancer. 2010;116:4334–4344. doi: 10.1002/cncr.25222. [DOI] [PubMed] [Google Scholar]
- 34.Howman-Giles R, Shaw HM, Scolyer RA, et al. Sentinel Lymph Node Biopsy in Pediatric and Adolescent Cutaneous Melanoma Patients. Ann Surg Oncol. 2010;17:138–143. doi: 10.1245/s10434-009-0657-4. [DOI] [PubMed] [Google Scholar]
- 35.Livestro DP, Kaine EM, Michaelson JS, et al. Melanoma in the young: Differences and similarities with adult melanoma - A case-matched controlled analysis. Cancer. 2007;110:614–624. doi: 10.1002/cncr.22818. [DOI] [PubMed] [Google Scholar]
- 36.Busam KJ, Murali R, Pulitzer M, et al. Atypical spitzoid melanocytic tumors with positive sentinel lymph nodes in children and teenagers, and comparison with histologically unambiguous and lethal melanomas. Am J Surg Pathol. 2009;33:1386–1395. doi: 10.1097/PAS.0b013e3181ac1927. [DOI] [PubMed] [Google Scholar]
- 37.Meyers MO, Yeh JJ, Deal AM, et al. Age and Breslow Depth Are Associated with a Positive Sentinel Lymph Node in Patients with Cutaneous Melanocytic Tumors of Uncertain Malignant Potential. Journal of the American College of Surgeons. 2010;211:744–748. doi: 10.1016/j.jamcollsurg.2010.07.020. [DOI] [PubMed] [Google Scholar]
- 38.Daniotti M, Ferrari A, Frigerio S, et al. Cutaneous melanoma in childhood and adolescence shows frequent loss of INK4A and gain of KIT. J Invest Dermatol. 2009;129:1759–1768. doi: 10.1038/jid.2008.422. [DOI] [PubMed] [Google Scholar]
- 39.Lewis KG. Trends in pediatric melanoma mortality in the United States, 1968 through 2004. Dermatol Surg. 2008;34:152–159. doi: 10.1111/j.1524-4725.2007.34032.x. [DOI] [PubMed] [Google Scholar]
- 40.Soong SJ, Ding S, Coit D, et al. Predicting survival outcome of localized melanoma: an electronic prediction tool based on the AJCC Melanoma Database. Ann Surg Oncol. 2010;17:2006–2014. doi: 10.1245/s10434-010-1050-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Long GV, Menzies AM, Nagrial AM, et al. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J Clin Oncol. 2011;29:1239–1246. doi: 10.1200/JCO.2010.32.4327. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.