Abstract
Toll like receptor (TLR)-stimulated dendritic cells (DCs) are able to overcome the inhibitory activity of regulatory T cells (Tregs) and induce the proliferation of effector T cells. TLR-activated DCs secrete a soluble factor and act directly on Tregs to convert them into interferon γ-secreting TH1-like cells that express the transcription factor T-bet.
Keywords: FOXP3, T-bet, dendritic cells, interferon γ, regulatory T cells
Tumors have developed elaborate mechanisms to avoid recognition and subsequent elimination by the immune system. One of such evasion mechanisms consist in the recruitment and expansion of regulatory T cells (Tregs). While there are both regulatory CD4+ and CD8+ T cells, most studies performed so far have focused on CD4+ Tregs, which can be distinguished from conventional CD4+ T cells by the expression of CD25 at high levels and the transcription factor forkhead box P3 (FOXP3). Cancer patients exhibit increased numbers of circulating Tregs, which actively inhibit the proliferation and activation of effector immune cells. The importance of Tregs in cancer is such that the ratio of tumor-infiltrating CD8+ T cells to Tregs has been proposed as a prognostic indicator of disease progression.1
Dendritic cells (DCs) are key components of the immune system and can determine the extent, specificity, and type of T-cell response elicited by both microbial and neoplastic stimuli.2,3 The robust ability of DCs to take-up antigenic material, process it and present it in the form of peptide: MHC molecules to direct T-cell responses has allowed for the development of DC-based anticancer therapeutics. The infusion of DCs loaded with tumor-associated antigens, be them provided in the form of purified peptides or apoptotic/necrotic cancer cells, can indeed generate potent tumor-specific immune responses. Of note, the induction of TH1 T cell responses requires the production of bioactive interleukin (IL)-12 by DCs.4 We and others have developed maturation protocols for the generation of TH1-polarized DCs (DC1s) that produce high levels of biologically active IL-12. Mature monocyte-derived DC1s can be obtained by the exposure of immature DCs to inflammatory cytokines and Toll-like receptor (TLR) agonists.5
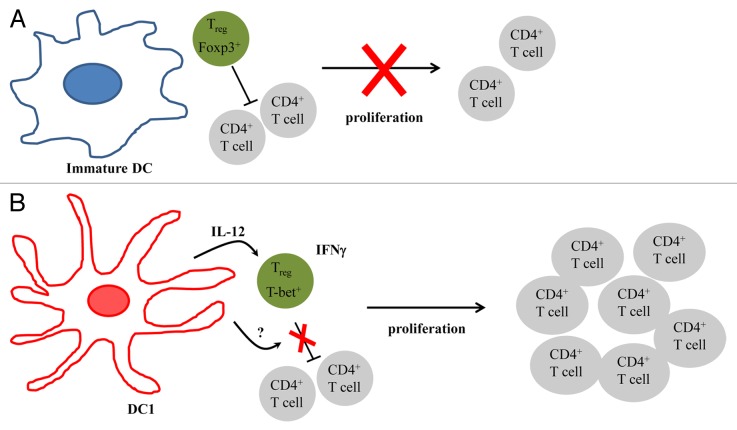
It has been shown that some TLRs cannot only activate DCs, but also impair the inhibitory activity of Tregs.6 We have recently examined whether DCs matured in the presence of TLR agonists can overcome the inhibitory activity of Tregs.7 Carboxyfluorescein succinimidyl ester (CFSE)-labeled T cells were stimulated with anti-CD3 antibodies to proliferate in the presence of autologous Tregs and either immature DCs (iDCs) or DCs matured by lipopolysaccharide (LPS) and interferon γ (IFNγ), i.e., DC1s. CD4+ and CD8+ T-cell proliferation was inhibited by the presence of Tregs, a phenomenon that was unaffected by the presence of iDCs. In contrast, DC1s were able to overcome the suppressive effect of Tregs. Experiments involving the physical separation of responder T cells and Tregs from DCs by a semi-permeable membrane revealed that DC1s produce a soluble factor that impairs the inhibitory functions of Tregs. The addition of neutralizing antibodies specific for IL-12, which is produced in large amounts by DC1s, or IL-6, which is involved in the LPS-mediated inhibition of Tregs, minimally affected the proliferation of responder cells in the presence of Tregs and DC1s, excluding a major role for IL-12 and IL-6 in this setting. The exposure of Tregs for 24 h to cell-free DC1-conditioned culture medium partly blocked their ability to inhibit the proliferation of responder cells, suggesting that the soluble factor produced by DC1s directly act on the Tregs. When co-cultured with DC1s, but not with iDCs, Tregs produced IFNγ. Furthermore, a considerable portion of FOXP3+ cells expressed IFNγ. The presence of DC1s stimulated the expression of the transcription factor T-box 21 (TBX21, best known as T-bet), which is associated with TH1 immune responses, pointing to a conversion of Tregs into TH1-like cells. The expression of T-bet by Tregs was inhibited upon the neutralization of DC1-derived IL-12, suggesting that distinct DC1-derived factors regulate the inhibitory functions of Tregs and their conversion into IFNγ-producing cells.
DCs have been tested in clinical trials for cancer therapy with variable success. One reason for the lack of consistent clinical efficacy of this approach is the use of differentially matured DCs. The conventional maturation protocol (which includes prostaglandin E2, PGE2) results indeed in DCs that do not produce IL-12 and reportedly favor the expansion of FOXP3+ cells, which would be an undesirable outcome.8 In contrast, we and others have shown that the maturation of iDCs in the presence of TLR agonists and inflammatory cytokines yields DC1s, which secrete high levels of bioactive IL-12 and are able to promote TH1 immune responses.9 Our findings suggest that, in contrast to iDCs, DC1s cannot only induce antitumor TH1/TC1 responses, but might also be able to abolish the inhibitory activity of Tregs and convert Tregs into TH1-like effector cells, which could significantly enhance the overall antineoplastic potential of DC-based immunotherapy (Fig. 1). Furthermore, our data highlights the plasticity of the Treg population and the possibility to modulate their function. We are currently attempting to identify the soluble DC-derived factor(s) that influence(s) the immunosuppressive functions of Tregs, a discovery that will improve our understanding of the mechanism by which DC1s can modulate the activity of this immunosuppressive cell population.
Figure 1. Superior immunostimulatory activity of TLR-activated dendritic cells. (A) In the presence of immature dendritic cells (iDCs), regulatory T cells (Tregs) inhibit the proliferation of conventional T cells. (B) DCs matured in the presence of Toll-like receptor (TLR) agonists (DC1s) secrete an unknown soluble factor that abolish the immunosuppressive functions of Tregs, hence allowing for T-cell proliferation. DC1s also secrete biologically active interleukin-12 (IL-12), which converts Tregs into T-bet+ interferon γ (IFNγ)-secreting TH1-like cells.
Disclosure of Potential Conflict of Interest
No potential conflicts of interest were disclosed.
Citation: Berk E, Xu S, Czerniecki BJ. Dendritic cells matured in the presence of TLR ligands overcome the immunosuppressive functions of regulatory T cells. OncoImmunology 2013; 2:e27617; 10.4161/onci.27617
References
- 1.Suzuki H, Chikazawa N, Tasaka T, Wada J, Yamasaki A, Kitaura Y, Sozaki M, Tanaka M, Onishi H, Morisaki T, et al. Intratumoral CD8(+) T/FOXP3 (+) cell ratio is a predictive marker for survival in patients with colorectal cancer. Cancer Immunol Immunother. 2010;59:653–61. doi: 10.1007/s00262-009-0781-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 3.Kaliński P, Hilkens CM, Wierenga EA, Kapsenberg ML. T-cell priming by type-1 and type-2 polarized dendritic cells: the concept of a third signal. Immunol Today. 1999;20:561–7. doi: 10.1016/S0167-5699(99)01547-9. [DOI] [PubMed] [Google Scholar]
- 4.Manetti R, Parronchi P, Giudizi MG, Piccinni MP, Maggi E, Trinchieri G, Romagnani S. Natural killer cell stimulatory factor (interleukin 12 [IL-12]) induces T helper type 1 (Th1)-specific immune responses and inhibits the development of IL-4-producing Th cells. J Exp Med. 1993;177:1199–204. doi: 10.1084/jem.177.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mailliard RB, Wankowicz-Kalinska A, Cai Q, Wesa A, Hilkens CM, Kapsenberg ML, Kirkwood JM, Storkus WJ, Kalinski P. alpha-type-1 polarized dendritic cells: a novel immunization tool with optimized CTL-inducing activity. Cancer Res. 2004;64:5934–7. doi: 10.1158/0008-5472.CAN-04-1261. [DOI] [PubMed] [Google Scholar]
- 6.Peng G, Guo Z, Kiniwa Y, Voo KS, Peng W, Fu T, Wang DY, Li Y, Wang HY, Wang RF. Toll-like receptor 8-mediated reversal of CD4+ regulatory T cell function. Science. 2005;309:1380–4. doi: 10.1126/science.1113401. [DOI] [PubMed] [Google Scholar]
- 7.Lee MK, 4th, Xu S, Fitzpatrick EH, Sharma A, Graves HL, Czerniecki BJ. Inhibition of CD4+CD25+ Regulatory T Cell Function and Conversion into Th1-Like Effectors by a Toll-Like Receptor-Activated Dendritic Cell Vaccine. PLoS One. 2013;8:e74698. doi: 10.1371/journal.pone.0074698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banerjee DK, Dhodapkar MV, Matayeva E, Steinman RM, Dhodapkar KM. Expansion of FOXP3high regulatory T cells by human dendritic cells (DCs) in vitro and after injection of cytokine-matured DCs in myeloma patients. Blood. 2006;108:2655–61. doi: 10.1182/blood-2006-03-011353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roses RE, Xu S, Xu M, Koldovsky U, Koski G, Czerniecki BJ. Differential production of IL-23 and IL-12 by myeloid-derived dendritic cells in response to TLR agonists. J Immunol. 2008;181:5120–7. doi: 10.4049/jimmunol.181.7.5120. [DOI] [PubMed] [Google Scholar]