Abstract
Objective
Informal caregivers (family and friends) of people with cancer are often unprepared for their caregiving role, leading to increased burden or distress. CHESS (Comprehensive Health Enhancement Support System) is a web-based lung cancer information, communication and coaching system for caregivers. This randomized trial reports the impact on caregiver burden, disruptiveness and mood of providing caregivers access to CHESS versus the Internet with a list of recommended lung cancer websites.
Methods
285 informal caregivers of patients with advanced non-small cell lung cancer were randomly assigned to a comparison group that received Internet or a treatment group that received Internet and CHESS. Caregivers were provided a computer and Internet service if needed. Written surveys were completed at pretest and during the intervention period bimonthly for up to 24 months. ANCOVA analyses compared the intervention’s effect on caregivers’ disruptiveness and burden (CQOLI-C), and negative mood (combined Anxiety, Depression, and Anger scales of the POMS) at six months, controlling for blocking variables (site, caregiver’s race, and relationship to patient) and the given outcome at pretest.
Results
Caregivers randomized to CHESS reported lower burden [t (84) = 2.36, p = .021, d= .39] and negative mood [t (86) = 2.82, p = .006, d= .44] than those in the Internet group. The effect on disruptiveness was not significant.
Conclusions
Although caring for someone with a terminal illness will always exact a toll on caregivers, eHealth interventions like CHESS may improve caregivers’ understanding and coping skills and, as a result, ease their burden and mood.
Keywords: cancer, telemedicine, oncology, quality of life, caregiver, mood
Introduction
Despite advances in cancer therapies, the prognosis for non-small cell lung cancer patients remains poor, with a median survival period of eight months (NSCLC Meta-Analyses Collaborative Group, 2008; Schiller et al., 2002; Spiro et al., 2004). About 580,350 US residents will die of cancer in 2013; with an estimated 27% (159,480) from lung cancer in 2013 (American Cancer Society, 2013). The diagnosis of advanced cancer often inflicts fear, despair, and hopelessness on patients and their families. Changes in medical and economic practices increasingly put family caregivers at the center of care throughout the course of the patient’s disease (Glajchen, 2004; Mitnick, Leffler, & Hood, 2010) without preparation for this role (Bee, Barnes, & Luker, 2009; Mehta, Cohen, Carnevale, Ezer, & Ducharme, 2010) As terminal illnesses evoke rapidly changing physical, psychological, and existential challenges (DuBenske, et al., 2008b), effective caregiver coping and communication with the health care system are vital. This randomized comparison study examines the impact of an eHealth information, communication and coaching system on caregivers’ disruptiveness, burden and negative mood, experienced as anxiety, depression, and anger.
The Caregiver Experience
Families are important in gathering information, making decisions, and giving emotional as well as instrumental support to terminally ill patients. Cancer places demands on caregivers that can disrupt their lifestyle. For example, time spent accompanying the patient to appointments or monitoring the patient’s symptoms and side effects may interrupt the caregiver’s ability to work or attend social engagements. As activities of caregiving increase, so too can disruptiveness. Burden arises when caregivers feel ill-equipped to handle the activities of caregiving, particularly with unmet information, communication or resource needs (Kim, Duberstein, Sorensen, & Larson, 2005; Sharpe, Butow, Smith, McConnell, & Clarke, 2005), and most caregivers have such unmet needs (Osse, Vernooij-Dassen, Schade, & Grol, 2006; Coleman, Smith, Frank, Min, Parry, & Kramer, 2004; Shelby, Taylor, Kerner, Coleman, & Blum, 2002). Accordingly, caregivers’ physical and mental health may decline, relationships and finances deteriorate, and social activities drop off (Haley, 2003; Northouse, Williams, Given, & McCorkle, 2012). Caregivers often neglect their own needs, increasing their risk for secondary morbidity from caregiving and for anxiety and depression (Bevans & Sternberg, 2012; Given et al., 2004). In fact, caregivers can experience similar and sometimes greater distress than patients undergoing cancer treatment (Carmack Taylor et al., 2008; Grunfeld et al., 2004; Hodges, Humphris, & Macfarlane, 2005). However, meeting these caregiver needs for information, communication and resources reduces caregiver distress (Northouse et al., 2012). Providing opportunities for information, support, and coping skills training to caregivers increases the likelihood of positive outcomes for caregivers and patients (Harding, List, Epiphaniou, & Jones, 2012; Northouse et al., 2012).
eHealth System: CHESS
Interventions that focus on cancer education, problem-solving, and personal support services have been found to be effective in reducing caregiver burden (Badr & Krebs, 2013; Northouse, Katapodi, Song, Zhang, & Mood, 2010; Sorensen, Pinquart, & Duberstein, 2002). In a meta-analysis, Sorenson et al. demonstrated that psychoeducational interventions significantly reduced burden and depression and increased well-being and knowledge. Multi-component interventions, those that included educational, supportive, and psychotherapeutic components, increased wellbeing and knowledge and eased burden more effectively than any single psychoeducational, supportive, psychotherapeutic, or respite intervention alone (Sorensen et al., 2002). Also, one-on-one interventions had greater impact than group interventions (Sorensen et al., 2002), though they can be time-consuming and expensive (Klemm & Wheeler, 2005). Support groups may be difficult for caregivers to attend because of the demands of caregiving (Given, Given, & Kozachik, 2001). eHealth interventions may address these problems, offering information when and where the caregiver needs it. eHealth can provide resources from a variety of disciplines and build a team-care approach, while making tailored support available to the patient and/or caregiver as long as it is needed (Tixier & Lewkowicz, 2011; Chou, Hunt, Beckjord, Moser, & Hesse, 2009; DuBenske, Gustafson, Shaw, & Cleary, 2010; Gustafson et al., 2002). In addition, an eHealth system can give clinicians timely information about the patient and caregiver, potentially producing an even greater positive effect on the patient, caregiver, and health system (Cleeland et al., 2011; DuBenske, Chih, Dinauer, Gustafson, & Cleary, 2008a).
Kinsella et al (1998), Glasgow (2007) and others (e.g.,Pingree, Hawkins, Baker, DuBenske, Roberts & Gustafson 2010; Atienza, Hesse, Gustafson & Croyle, 2010) call for a theory-based approach to designing eHealth systems. Of relevance to caregiving, Lazarus and Folkman’s (1984) model of coping is built around one’s appraisal of susceptibility to and controllability of stressors (e.g., patient symptom monitoring, balancing work with medical appointments) and coping mechanisms applied to perceived stressors. More than the stressors themselves, appraisals of the stressors influence physical, emotional, and social well-being (Folkman & Moskowitz, 2000; Lawton, Kleban, Moss et al., 1989; Oberst, Gass & Ward, 1989). Even in advanced cancer, caregivers can control stressors (e.g., by knowing and using palliative care options to reduce pain and suffering, by leveraging support networks to distribute care tasks) and greatly improve their quality of life (Folkman, 2008; Haley, LaMonde, Han, Burton & Schonwetter, 2003; Folkman, 2001). But many caregivers of people with advanced cancer lack the knowledge, skills or resources to make appropriate coping choices. Furthermore, the clinical team’s ability to help control cancer-related stressors is limited by caregivers’ and patients’ reluctance to communicate distress to clinicians between visits (Gaugler et al., 2009; Docherty et al., 2008; van den Beuken-van Everdingen, de Rijke, Kessels, Schouten, van Kleef, & Patijn,.2007).
The eHealth system in this study was a version of the Comprehensive Health Enhancement Support System (CHESS), a non-commercial, home-based system created by clinical, communication, and decision scientists at the University of Wisconsin. CHESS is distinguished by the quality and depth of its information and resources and by ease of use. CHESS is an extensively studied eHealth system, having been (over its evolutions) the subject of numerous needs assessments (DuBenske et al., 2008a; DuBenske et al., 2008b; Wen & Gustafson, 2004) randomized clinical trials (Gustafson et al., 2001, 2008), and field tests (Gustafson et al., 2005; Gustafson, McTavish, Schubert, & Johnson, 2012). These studies demonstrated that CHESS can be widely accepted and used, improve patient quality of life and information competence, and in some cases lead to more efficient use of health services (Gustafson et al., 2001; Shaw et al., 2007; Shaw, McTavish, Hawkins, Gustafson, & Pingree, 2000). This study applied CHESS in a new way – for advanced lung cancer, rather than earlier stage disease, targeting caregiver rather than solely patient outcomes, and sharing critical information with clinicians via a Clinician Report. The Clinician Report gave caregivers (and patients) a way to report their needs and the patient’s symptoms to the clinical team via the website. At CHESS check-in, caregivers rated the patient’s symptoms on a 0–10 scale; scores of 7 or greater initiated an alert email to the patient’s treating clinician and/or mid-level provider. Caregivers also reported their confidence with meeting caregiver needs and could post any questions they had for the clinician. Clinician Reports, which included the caregiver’s symptom assessment, were then available to the clinical team at the time of the patient’s clinical appointment.
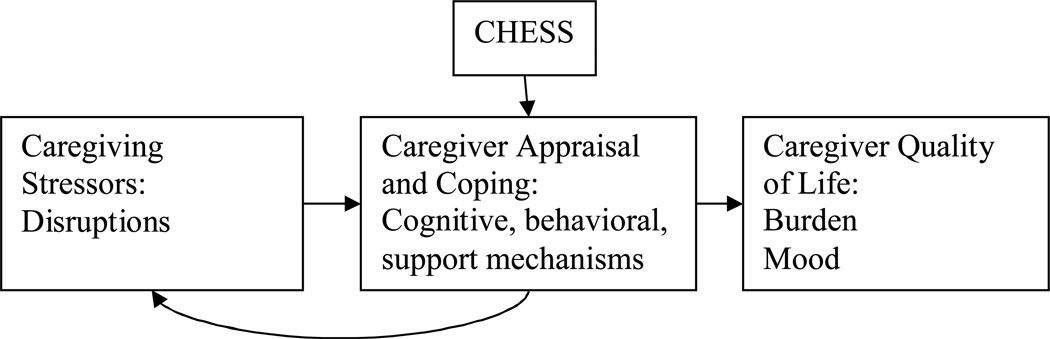
Built upon a model of coping self-efficacy, CHESS is designed to help users appraise the controllability of cancer-related stressors, and improve cognitive, behavioral and supportive coping skills (see Figure 1). For example, caregivers may learn to distribute caregiving responsibilities and prioritize, therefore reducing the degree of caregiving disruption in their lives. They may also learn to control stressors through cognitions (e.g., knowledge, reframing), behaviors (e.g., asking for help, informing the clinical team, taking respite, relaxation exercises), or support (e.g. emotional support such as validation or bonding with others to reduce isolation), in turn reducing burden and negative mood. Toward these ends, CHESS is a comprehensive system offering a variety of information, communication and coaching services appropriate to the contexts of cancer and caregiving, from which caregivers self-select according the their needs and preferences.
Figure 1.
Application of CHESS to Caregiving Model of Stress and Coping
Study Overview and Hypotheses
This randomized comparison trial compared the impact on caregiver burden, distruptiveness and mood of providing caregivers access to CHESS versus the Internet with list of recommended lung cancer and palliative care websites. It was hypothesized that at six-month follow-up caregivers in the CHESS group would have less burden, disruptiveness and negative mood than those in the Internet group.
Methods
Participants and Recruitment
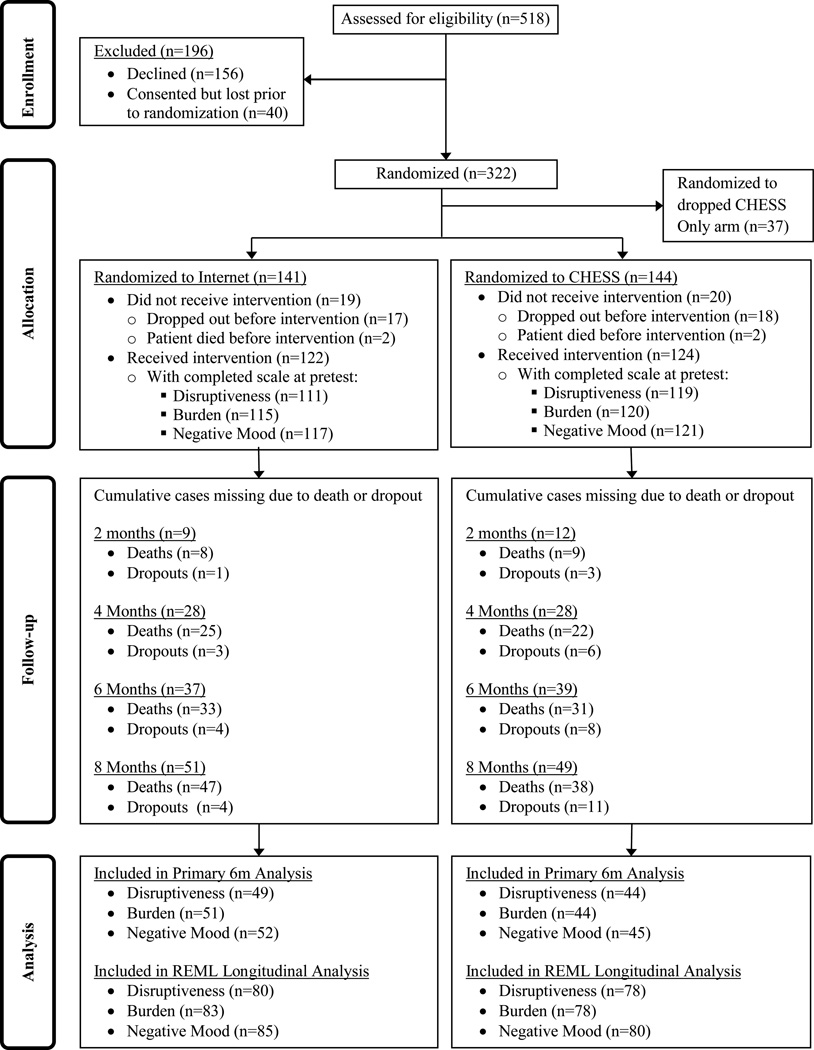
While caregivers of lung-cancer patients were our target population, we recruited patients and their caregivers together because the CHESS intervention required communicating with the patient’s clinical team. Caregiver-patient dyads were recruited from four major cancer centers in the Northeastern, Midwestern, and Southwestern United States between January 2005 and April 2007. Eligible lung cancer patients were English-speaking adults with non-small cell lung cancer at stage IIIA, IIIB, or IV, with a clinician-perceived life expectancy of at least four months and a patient-identified primary caregiver willing to participate in the study. Depending on disease statuses, patients were receiving standard care including curative or palliative treatment. Patients may or may not have had a hospitalization during the course of the treatment, but our intervention was targeted to the outpatient setting. Participation in this research did not exclude patients from other treatments or clinical trials that may have been involved in their cancer care. 322 cancer patient-caregiver dyads enrolled in the study. The study protocol called for 3 groups consisting of standard care plus (1) CHESS with the Clinician Report, (2) CHESS without the Clinician Report, and (3) the Internet (randomization ration 1:1:1). Because of low study accrual across groups, group 2 (CHESS only) was dropped after only a few months. Reducing the study to only two groups (randomization ratio 1:1) facilitated retaining the power needed for analysis and completing the study within the funded timeframe. 37 dyads were in the CHESS only group and they continued to participate in the study although their data is not included in the current analysis. Of those in the remaining two study groups, 246 received their intervention (either CHESS or Internet) after completing pretests and being randomized to either the Internet (122) or CHESS (124) group. Figure 2 shows the reasons for study attrition.
Figure 2.
Participant Flow Diagram.
At recruitment, caregivers in both groups were encouraged to log into the computer regularly (weekly, if able). Although encouraged, using the computer was not required of participants, though, because we wanted to see how CHESS is naturally used, similar to how the intervention would be delivered via broad dissemination models where a medical system or insurer might offer access to the eHealth resource. Furthermore, given the severity of illness of this population, we were conscious of not wanting to tax already burdened caregivers with a demanding protocol, thus voluntary rather than assigned computer use reduced the perception of study burden that might have otherwise been a barrier to accrual.
Study Administration
This study was approved by the Institutional Review Boards at each site. Because the research included sharing information with oncology clinicians via CHESS, clinicians first had to consent to participate in the study and agree to receive this information via the website in order for their patients to be eligible. Only patients with consented physicians were approached for participation. After completing the consent form and pretest, dyads were randomly assigned to the Internet or CHESS treatment group (1:1 ratio). Participants were stratified by study site, caregiver's race (Caucasian vs. non-Caucasian), and caregiver-patient relationship (spouse/partner vs. other relationship). A random number generator was used to create blocks of size six or nine. The project director assigned participants to treatment arms within these blocks as they were enrolled. The intervention period was up to 2 years, or 13 months after patient death, whichever came first.
Internet Comparison Group
The comparison group received standard care plus a laptop computer with Internet access (if needed) and a list of lung cancer and palliative care websites based on clinician recommendations of high-quality websites representative of websites they would offer their patients in clinical encounters (e.g., cancer.gov and alcase.org).
CHESS Treatment Group
In addition to standard care, participants in the treatment group received a laptop computer and Internet access (if needed) and access to the CHESS lung cancer website, “Coping with Lung Cancer: A Network of Support.” Access to CHESS is password protected. In an easy-to-use, Internet-based format, CHESS integrates services to facilitate coping by: (1) providing ready and organized access to information; (2) serving as a channel for communication and support with peers, experts, and users’ social networks; and (3) acting as an interactive coach by gathering information from the user, applying algorithms or decision rules, and providing feedback specifically relevant to the user. Table 1 lists and describes CHESS services. Given the variety of caregiver needs, CHESS is designed to be user-driven, making all services available at all times and allowing caregivers to self-direct to content that suits their needs. That said, use is also facilitated by content suggestions based on assessment of caregiver topic needs at Check-in, website search via keywords, and suggestions for content viewing via Ask an Expert.
Table 1.
CHESS Services
| Information services | |
| Frequently Asked Questions (FAQs) | Brief answers to many questions about the physical, emotional, practical, social, and spiritual aspects of the disease |
| Instant Library | Links to relevant full-text articles from the scientific and popular press |
| Resource Directory | Descriptions of local and national services and ways to contact them |
| Web links | Connections to other high-quality clinician-approved websites |
| Cancer News | Summaries of recent cancer-related news and research |
| Personal Stories | First-person narratives of patient and caregiver experiences |
| Caregiver Tips | Brief tips on topics developed by experts or added by CHESS users |
| Communication services | |
| Discussion Groups | Limited-access bulletin boards monitored by a professional facilitator; Separate groups exist for patients, caregivers, and bereaved caregivers |
| Ask an Expert | One-on-one confidential question-and-answer service with a cancer information specialist |
| Personal Web page | Personal bulletin board and interactive calendar with user’s own family and friends to share updates and messages, and request help (e.g., transportation to clinic visits, meals, errands) |
| Clinician Report | Summaries of health status reported to the clinical team in
three ways:
|
| Coaching/training services | |
| Health Status | Data about the patient’s health, including graphs illustrating changes, created by prompting the user to enter data |
| Decision Aids | Structured analysis to help patients and caregivers learn about options, clarify values, understand consequences, and implement decisions |
| Easing Distress | Cognitive-Behavioral Therapy principles help users identify emotional distress and use coping techniques to manage it |
| Healthy Relating | Instruction and exercises on communication techniques to increase closeness and decrease conflict |
| Action Plan | Guidance to help users plan behavior changes by identifying goals, resources, and ways to overcome obstacles |
To track the use of CHESS and/or the Internet, participants were given a choice of either a laptop computer or a study browser to install on their own computer. The study arranged and paid for the Internet service for the period of the study. Computers and user manuals were mailed to the caregiver, along with instructions and labels for easy set-up. Technical support was available by telephone if needed. Study staff provided brief (15–40 minute) training via telephone on using the Internet and, for those in the CHESS group, using CHESS. Participants could opt out of training if they felt they did not need it: 59% of those who received the intervention (CHESS or Internet) were trained. Caregivers who completed the study were allowed to keep the laptop.
Caregivers received access to the intervention for up to 24 months and posttest surveys were sent every two months during the period of intervention. Posttests were mailed to caregivers and returned in pre-addressed stamped envelopes. A follow-up call was made to remind caregivers to return a survey if we had not received it within two weeks of mailing. The complete study protocol is available by request from the corresponding author (LD).
Measures
Caregiver characteristics
Caregiver demographic characteristics (e.g., age, education) were assessed on the pretest survey.
CHESS Use
Although browsers were installed with the intention to track user navigation of any Internet website, this data was determined to be unreliable as users were accessing the Internet in many different ways: using a different computer, a different browser. Therefore, only CHESS website use is available, as this was tracked separately by configuring CHESS web servers to log every click on a link requesting a CHESS page (all identified with a unique URL). To do this, a special module was installed on the web server to log any incoming request for a page (click on a link) with the user name, date/time and the unique URL requested. Use can be measured by number of logins, number of pages viewed, or time spent on the system. Time is a controversial metric for use due to the potential for participants to walk away from the computer. Given this, for each CHESS service (i.e, information, discussion group), individual cut-off times were preset based on the format and type of content, ranging from 5 minutes (on services such as news) to 15 minutes (pages requiring the user to write, like discussion group messaging). If time on a given page exceeded the cut-off, then the time was marked as the cut-off for analysis purposes.
Disruptiveness
Disruptiveness is one primary outcome and refers to the degree to which caregiving tasks interfere with the caregiver’s regular daily routine of life activities. This was measured at pretest and posttests using the Caregiver Quality of Life – Cancer Scale (CQOLC) Disruptiveness Subscale (7 Likert-type items). This has demonstrated good internal consistency reliability (Cronbach’s α of .83; from a written communication with Michael Weitzner in 2004) and criterion validity (Weitzner, Jacobsen, Wagner, Friedland, & Cox, 1999; Weitzner, McMillan, & Jacobsen, 1999). Responses are selected from a scale of 0–4 and scale scores are calculated as the mean across items multiplied by the number of items. Higher scores indicate greater Disruptiveness. Cronbach’s α at pretest matched Weitzner’s (2004 written communication) data at α=.83.
Burden
Caregiver burden, a primary outcome, refers to the caregiver’s subjective impact of caregiving, and was measured on the pretest and posttests using the Caregiver Quality of Life – Cancer Scale (CQOLC) Burden Subscale (10 Likert-type items).This has demonstrated good internal consistency reliability (Cronbach’s α of .89; from a written communication with Michael Weitzner in 2004) and criterion validity (Weitzner, Jacobsen, Wagner, Friedland, & Cox, 1999; Weitzner, McMillan, & Jacobsen, 1999). Responses are scored as described above for Disruptiveness, with higher scores indicating greater Burden. . Cronbach’s α of .89 at pretest matched Weitzner’s (2004 written communication) data.
Negative Mood
Negative mood was also a primary outcome, measured both at pretest and bimonthly posttests. To reduce participant survey burden, a 16-item subset of negative mood items from the Short Version Profile of Mood States (SV-POMS) (Dilorenzo, Bovbjerg, Montgomery, Valdimarsdottir, & Jacobsen, 1999) was used to assess caregiver negative mood. Items were selected to be representative of the three negative mood sub-scales: (a) Tension-Anxiety (tense, on edge, uneasy, nervous, anxious); (b) Anger-Hostility (annoyed, angry, grouchy, furious, bitter); and (c) Depression-Dejection (discouraged, helpless, hopeless, sad, unhappy, worthless) and, using data from a previous CHESS study (Gustafson, McTavish, Stengle, et al., 2005), demonstrated reliability with similar overall scale alphas compared to the original measures.
In the present study, internal consistency reliability for these subscales calculated at pretest was high (α of .91, .91, and .89, respectively) and consistent with that of the original SV-POMS and the full-length POMS (Dilorenzo, et al., 1999). Likert-type items are rated on scales from 0–4 and scale scores are calculated as means across items. These three scales are highly correlated to one another, e.g., the inter-item correlation of Anxiety and Depression is .81. To avoid multicollinearity, we combined the items from all three mood states into one scale of negative mood by calculating total item mean. Alpha reliability of the negative mood scale was .95 at pretest. Greater scores indicate higher levels of negative mood.
Patient symptom distress
Caregiver perception of patient’s symptom distress was measured at pretest and bimonthly posttests using a modified Edmonton Symptom Assessment Scale (ESAS) (Bruera, Kuehn, Miller, Selmser, & Macmillan, 1991), and used in secondary analysis. The ESAS measures distress arising from 9 physical and psychological problems and 1 overall “distress” item on a 0–10 scale, with 10 indicating highest symptom burden. This modified scale included 6 original symptoms: 4 physical (pain, nausea, appetite, and shortness of breath) and 2 psychological (depression, anxiety). Based on feedback from study oncologists, 3 other physical symptoms (activity, drowsy, and well-being) were replaced with symptoms common in lung cancer: fatigue, constipation, and diarrhea. The overall symptom distress scale score was calculated by summing the scores of the 9 symptom items (pretest α = .79).
Analysis
The primary analysis examined caregiver disruptiveness, burden, and negative mood at the 6-month intervention access time point, as this gives those in the CHESS group a reasonable amount of exposure to CHESS, in order to allow CHESS to have an impact, but at the same time it is sensitive to the average 8-month lifespan of advanced lung cancer patients, to power analyses with the number of remaining current caregivers at that time point. Group differences on the primary outcomes were examined using ordinary least-squares ANCOVA, with pretest values of the given outcome as a covariate. Each analysis also controlled for the design factors of caregiver race, relationship to patient, site, and the interaction of treatment and site (Moher et al., 2010). All tests were non-directional with an allotted Type I error rate of .05. It was estimated that (with α = .05) approximately 100 caregivers per group would provide adequate (≥.80) power to detect the moderately small (.35σ) covariate-adjusted intervention effect of interest. Exploratory analysis repeated the primary analysis with the addition of the ESAS as a covariate, as patient symptom distress may impact caregiver disruptiveness, burden, and mood.
Secondary analyses used restricted maximum likelihood estimation to examine the group differences controlling for time (caregiver report at 2, 4, 6, and 8 months after intervention). Proper analysis of repeated measurements from the same individuals must account for the within-person correlation of observations. Maximum likelihood estimation accounts for the within-person correlations while allowing all available data to be used in the analysis and so was the preferred method to examine the longitudinal effect of group. The analysis was conducted using Linear Mixed Models in IBM SPSS 20, with a first-order autoregressive covariance structure for the repeated measure and restricted maximum likelihood estimation (REML) to calculate fixed effects. In addition to the design factors and covariates from the primary analysis, the effects of month and the group by month interaction were also included in this model.
Although surveys were administered to dyads for up to 24 months, the analysis was limited to only the first 8 months on study. People diagnosed with lung cancer have an average life expectancy of 8 months post-diagnosis, therefore the available sample size was expected to be no greater than half at 8 months and unreturned surveys were expected given the likelihood of diminishing patient health status over time. The simple effects of group at survey months 2, 4, 6, and 8 were examined in an exploratory analysis.
Results
Caregiver Demographic and Disease Characteristics at Baseline
Table 2 provides descriptive statistics for demographic variables, comfort with the Internet and caregiver report of patient symptom distress at baseline (pretest) by group. For the combined sample, caregivers were predominantly female (68.3%), with mean age of 55.56 years (range 18–84 years). Most caregivers (72%) were spouse/partners of the patient. This was a highly educated sample, with 53.1% of caregivers having earned the equivalent of an Associate’s degree or a more advanced degree.
Table 2.
Characteristics of lung cancer caregivers at pretest
| Characteristics | Internet | CHESS | ||
|---|---|---|---|---|
| N | Mean (SD) | N | Mean (SD) | |
| Age | 117 | 54.57 (12.21) | 117 | 56.56 (12.86) |
| Comfort with the Internet | 118 | 2.69 (1.23) | 123 | 2.37 (1.26) |
| Caregiver reported patient ESAS | 111 | 29.31 (14.72) | 118 | 28.37 (14.69) |
| N | % | N | % | |
| Gender (Female) | 86 | 70.5 | 82 | 66.1 |
| Do Patient and Caregiver live together? (Yes) | 94 | 78.3 | 96 | 78.0 |
| Is Caregiver the Patient’s spouse/partner? (Yes) | 86 | 70.5 | 91 | 73.4 |
| Highest Level of Education: Caregiver | ||||
| High School Diploma or Less | 27 | 22.5 | 24 | 19.9 |
| Some College Courses | 34 | 28.3 | 28 | 23.1 |
| Advanced Degree (Assoc., BS, MS, PhD) | 59 | 49.2 | 69 | 57.0 |
| Any other major illness or health condition? (Yes) | 56 | 46.7 | 52 | 42.6 |
Internet comfort was measured on a 0 to 4 scale with 0 being “not comfortable at all” and 4 being “extremely comfortable”.
Table 3 provides descriptive statistics for disease characteristics of time since diagnosis, stage of lung cancer, and whether the patient had additional major health issues (e.g, heart disease, diabetes, arthritis). For the combined sample, most of the patients (160, 66.4%) had stage IV lung cancer. Over half of the patients (139, 57.7%) had an additional major illness or health condition. The mean time from diagnosis to study intervention was approximately one year. As the one-year relative survival rate for lung cancer is 44% (American Cancer Society, 2013), this population lived longer than patients with advanced lung cancer generally do. To be eligible, patients needed a clinician-anticipated survival of at least 4 months. This excluded those who were extremely ill and likely had very short survival times. We also recruited all eligible patients regardless of time since diagnosis, rather than restricting criteria to a time point close to diagnosis, favoring accrual of patients who had been alive longer rather than those who were diagnosed with significantly advanced disease and may have died shortly thereafter.
Table 3.
Disease characteristics of Lung Cancer Patients at Pretest
| Characteristics | Internet | CHESS | ||
|---|---|---|---|---|
| N | Mean (SD): Min/Max |
N | Mean (SD): Min/Max |
|
| Days from Diagnosis to Intervention | 121 | 370.47 (470.01): 19/2800 | 122 | 342.94 (440.66): 8/2337 |
| N | % | N | % | |
| Cancer Stage | ||||
| IIIA | 16 | 13.3 | 21 | 17.4 |
| IIIB | 23 | 19.2 | 21 | 17.4 |
| IV | 81 | 67.5 | 79 | 65.3 |
| Any other major illness or health condition? (Yes) | 73 | 61.9 | 66 | 55.5 |
Figure 2 depicts the rates of attrition caused by withdrawing from the study, the death of the patient, or non-response to the six-month survey. To examine whether sample composition affected attrition, caregivers were divided into groups based on whether they completed the six-month survey. There were no significant differences on study variables at baseline between caregivers who completed the six-month survey and those who did not (all p values ≥ .30).
CHESS Use
Ninety-one of the 124 (73%) caregivers assigned to the CHESS group logged onto the CHESS website at least one time during the first 6 months of the study. During this time, average CHESS use for these caregivers was 14.6 logins (SD=25.1), 293.0 pages (SD=482.1), and 177.7 minutes (SD=245.0). CHESS users averaged 50.0 minutes per month (SD=42.9) on this system. It is noteworthy that time on the system is significantly and nearly perfectly correlated with number of pages, r=0.95, p< 0.00.
Intervention Effects
Table 3 provides descriptive statistics for outcome variables by group for pretest and each follow-up survey time point. Ordinary least-squares ANCOVA was used to test for effects on disruptiveness, burden, and negative mood six-months after the start of intervention while controlling for the previously described design factors and the pretest measure of the given outcome. Results are shown in Table 4. Caregivers in the CHESS group had significantly lower levels of caregiver Burden [CHESS: M=12.97, SE=1.42; Internet: M=16.37, SE=1.43; P =.021] and Negative Mood [CHESS: M=.56, SE=.13; Internet: M=.92, SE=.13; P =.006] than those in the Internet group. There was no significant difference between groups on Disruptiveness [CHESS: M=3.17, SE=.95; Internet: M=4.58, SE=.97; P =.150]. Results were consistent after adding caregiver’s pretest report of patient ESAS as an additional covariate.
Table 4.
Descriptive statistics for outcomes by group and survey time point.
| Internet | CHESS | |||||
|---|---|---|---|---|---|---|
| Outcome/Time Point | N | Mean | SD | N | Mean | SD |
| Disruptiveness | ||||||
| Pretest | 111 | 5.26 | 4.91 | 119 | 5.39 | 4.91 |
| Month 2 | 62 | 5.63 | 5.07 | 58 | 5.24 | 4.82 |
| Month 4 | 51 | 5.85 | 4.73 | 60 | 5.51 | 5.28 |
| Month 6 | 49 | 5.27 | 5.55 | 44 | 4.58 | 5.13 |
| Month 8 | 38 | 4.61 | 3.99 | 42 | 5.50 | 4.71 |
| Burden | ||||||
| Pretest | 115 | 19.59 | 9.08 | 120 | 17.55 | 8.50 |
| Month 2 | 64 | 18.91 | 9.67 | 58 | 16.15 | 8.26 |
| Month 4 | 51 | 17.36 | 9.56 | 60 | 15.87 | 8.43 |
| Month 6 | 51 | 18.03 | 10.86 | 44 | 13.48 | 7.63 |
| Month 8 | 40 | 17.22 | 9.98 | 42 | 16.34 | 6.86 |
| Negative Mood | ||||||
| Pretest | 117 | 1.06 | 0.88 | 121 | .88 | .77 |
| Month 2 | 65 | 0.96 | 0.88 | 58 | .92 | .74 |
| Month 4 | 53 | 0.95 | 0.80 | 60 | .88 | .72 |
| Month 6 | 52 | 1.00 | 0.92 | 45 | .65 | .60 |
| Month 8 | 41 | 0.85 | 0.85 | 42 | .92 | .81 |
Note: In order to minimize case deletion due to item missingness, scale scores were calculated if at least 75% of scale items were answered for four-item scales and if at least 80% of scale items were answered for scales with five or more items.
Similar results were found for the analysis of group differences controlling for time in an ANCOVA using restricted maximum likelihood estimation. Specifically, significant group differences between model-estimated means existed for Burden [CHESS: M=15.19, SE=.94; Internet: M=17.15, SE=.99; P =.048] and Negative Mood [CHESS: M=.78, SE=.08; Internet: M=.95, SE=.08; P =.050] but not for Disruptiveness [CHESS: M=4.32, SE=.54; Internet: M=5.37, SE=.58]. Within this model, an exploratory analysis of the simple effects of group at each survey month (see Table 5) showed that group differences were significant only at 6 months for both Burden [ P =.011] and Negative Mood [P =.007] and at no month for Disruptiveness.
Table 5.
Tests for group differences on caregiver outcomes for primary (6 months) and secondary (longitudinal) analyses
| Outcome | Internet M (SE) |
CHESS M (SE) |
Mean Difference (95% CI) |
t(df) | P | dc |
|---|---|---|---|---|---|---|
| At 6 Monthsa | ||||||
| Disruptiveness | 4.58 (.97) | 3.17 (.95) | 1.41 (-.52 to 3.34) | 1.45 (82) | .150 | 0.287 |
| Burden | 16.37 (1.43) | 12.97 (1.42) | 3.40 (.54 to 6.26) | 2.36 (84) | .021 | 0.387 |
| Negative Mood | .92 (.13) | .56 (.13) | .36 (.11 to .62) | 2.82 (86) | .006 | 0.436 |
| Longitudinalb | ||||||
| Disruptiveness | ||||||
| Main Effect | 5.37 (.58) | 4.32 (.54) | 1.05 (-.07 to 2.17) | 1.86 (16.22) | .065 | 0.214 |
| Simple Effects | ||||||
| Month 2 | 4.78 (0.65) | 4.01 (0.63) | 0.77 (−0.65 to 2.2) | 1.07 (298.44) | .286 | 0.157 |
| Month 4 | 5.66 (0.67) | 4.54 (0.61) | 1.12 (−0.29 to 2.53) | 1.56 (310.52) | .119 | 0.228 |
| Month 6 | 5.24 (0.68) | 3.89 (0.65) | 1.35 (−0.16 to 2.86) | 1.76 (333.22) | .080 | 0.275 |
| Month 8 | 5.81 (0.74) | 4.84 (0.69) | 0.97 (−0.70 to 2.63) | 1.14 (338.15) | .254 | 0.198 |
| Burden | ||||||
| Main Effect | 17.15 (.99) | 15.19 (.94) | 1.96 (.02 to 3.91) | 1.99 (149.61) | .048 | 0.223 |
| Simple Effects | ||||||
| Month 2 | 17.50 (1.10) | 15.55 (1.10) | 1.94 (−0.52 to 4.40) | 1.55 (293.64) | .121 | 0.221 |
| Month 4 | 16.22 (1.15) | 15.19 (1.07) | 1.02 (−1.43 to 3.47) | 0.82 (309.04) | .413 | 0.116 |
| Month 6 | 16.97 (1.16) | 13.56 (1.13) | 3.40 (0.80 to 6.01) | 2.57 (330.37) | .011 | 0.387 |
| Month 8 | 17.92 (1.26) | 16.44 (1.21) | 1.48 (−1.38 to 4.34) | 1.02 (336.06) | .310 | 0.168 |
| Negative Mood | ||||||
| Main Effect | .95 (.08) | .78 (.08) | .17 (.00 to .34) | 1.98 (134.08) | .050 | 0.206 |
| Simple Effects | ||||||
| Month 2 | 0.89 (0.10) | 0.85 (0.10) | 0.04 (−0.19 to 0.27) | 0.37 (318.12) | .711 | 0.048 |
| Month 4 | 0.92 (0.10) | 0.82 (0.10) | 0.10 (−0.13 to 0.32) | 0.83 (342.05) | .407 | 0.121 |
| Month 6 | 0.96 (0.10) | 0.62 (0.10) | 0.34 (0.09 to 0.58) | 2.73 (357.81) | .007 | 0.412 |
| Month 8 | 1.04 (0.11) | 0.84 (0.11) | 0.20 (−0.07 to 0.46) | 1.43 (352.54) | .153 | 0.242 |
Used ordinary least squares (OLS) estimation with covariates for each respective outcome evaluated at the following values: CQOLC Disruptiveness at pretest = 5.086; CQOLC Burden at pretest = 18.044; Negative Mood at pretest = .926.
Used restricted maximum likelihood (REML) estimation (ordinary least-squares ANCOVA would have substantially reduced the sample size as only cases with data at all time points could have been included in the analysis) with covariates for each respective outcome evaluated at the following values: CQOLC Disruptiveness at pretest = 5.257; CQOLC Burden at pretest = 17.950; Negative Mood at pretest = .910.
Cohen's d calculated as the mean difference divided by the pooled standard deviation at pretest: 4.910 for CQOLC Disruptiveness; 8.789 for CQOLC Burden; and 0.826 for Negative Mood.
Discussion
CHESS was designed within a stress and coping theoretical framework to help caregivers by either facilitating ways to reduce the stressors (e.g., distribution of caregiving responsibilities) or by improving appraisal and coping via cognitive (e.g., education), behavioral (e.g. stress symptom and side effect monitoring and communication) or support (e.g., caregiver peer communication) mechanisms. Consistent with the model, caregivers who had access to CHESS had lower burden and negative mood after six months than those with access to the Internet alone. These group differences persisted when controlling for the caregiver’s pretest report of patient symptom distress (ESAS), as well as when examining the longitudinal effect over the first eight months (the median survival time for patients with advanced stage lung cancer) of the intervention.
CHESS is a comprehensive system of information, communication and coaching services in which users self-direct to a variety of services to fit their individual needs at any given point in the cancer caregiving trajectory. Accordingly, no individual service is seen as more important than the others, and all serve a role within the stress and coping model. While speculation, generally speaking, one explanation of the impact of CHESS may be that exposure to the multi-component resources of CHESS may have helped caregivers understand what to expect from lung cancer and its treatment and therefore feel better prepared and better able to cope with caregiving. On the other hand, it is possible that connecting caregivers with others in the same situation also may have helped CHESS caregivers feel less alone in this often isolating set of circumstances and to solve problems by learning from others. Or, to further speculate, clinicians reported elsewhere that the Clinician Report helped caregivers be more involved during the clinical encounter (DuBenske et al., 2008a). The Clinician Report enabled caregivers to describe the patient’s condition and their own concerns directly to the clinical staff, helping them feel “a part of the team.” In turn, it is possible that caregivers felt more competent and better supported in their caregiving, which may have lessened their sense of burden and negative mood. Regardless of the specific services individual caregivers used, CHESS was designed with the intention to meet specific needs as they arise, and address coping in a variety of formats. The finding that CHESS caregivers had less burden and negative mood at 6 months indicates that access to CHESS services can ease caregiving, potentially through a variety of mechanisms that enhance coping. However, further research is needed to understand how the specific services are utilized and to what benefit, to illuminate the more specific mechanism for CHESS’s effect on burden and negative mood.
The clinical significance of the CHESS benefit on burden is difficult to assess given that the measure does not have clinical reference scores. The magnitude of difference between CHESS and Internet on negative mood is comparable to the difference seen between a healthy population and a cancer population (Dilorenzo, Bovbjerg, Montgomery, Valdimarsdottir, & Jacobsen, 1999), suggesting this is a clinically meaningful difference. Using effect size as a gauge of clinical significance, the effect sizes for CHESS’s benefit over Internet on caregiver burden and negative mood were small to medium. The selection of the Internet as a comparison group may underestimate the effect of CHESS itself, as the resources available on the Internet, particularly those on the recommended website list provided, may have met some of the caregiver’s needs reducing the measurable impact that might have otherwise been seen had the comparison been to a pure control in which some caregivers would not have had Internet access. Furthermore, 27% of caregivers in the CHESS group did not use CHESS, or used it minimally. While this intent-to-treat analysis shows that a broadly disseminated eHealth system like CHESS may have a small to medium effect on the caregiver population, individuals who use the system more may potentially find greater benefit.
Although caregivers using CHESS felt less burdened, there was no significant difference in disruptiveness. Disruptiveness refers to the interference, or stressors, posed by caregiving responsibilities in the caregiver’s life. While some CHESS services aimed to help caregivers reduce the tasks of caregiving by sharing responsibilities among their support network, it appears this had limited impact on reducing the tasks of caregiving and/or the time needed to accomplish these responsibilities. Therefore, caregivers experienced equal levels of disruption.
Why might CHESS have reduced the sense of burden but not disruption? “Disruptiveness” seems to denote an objective degree to which daily life is different, whereas “burden” is a subjective appraisal of caregiving’s impact. .CHESS appears unable to alter the daily changes caused by caregiving (disruptiveness) as much as it can alter how caregivers perceive/appraise these activities (burden).
Alternatively, finding no significant effect on disruptiveness may reflect measurement factors rather than the constructs. Given the possible range was 0–25 for the Disruptiveness scale, the low means (consistently around a value of 5) may indicate a potential floor effect. The scale’s items include language such as “It bothers me that …” and “My daily life is imposed upon.” Despite the disruption in their daily lives, caregivers may hesitate to agree with such statements because they feel glad to help or guilty for feeling bothered or imposed upon given what their loved one with advanced cancer is experiencing. In contrast, items in the Burden scale give more socially acceptable reactions to caregiving, such as worry, fear, sadness, and guilt. Accordingly, caregivers may have more readily agreed with items on the Burden scale than the Disruptiveness scale, offering statistical variance that allowed for effects on burden to be detected.
Limitations
This study, like all intervention studies, may have sample bias resulting from the self-selection of participants. Our research presented a study of a lung cancer information, communication and coaching website for caregivers . Caregivers who were not interested in working with computers or accessing information and support were more likely to decline participation (Buss et al., 2008). Although we attempted to reduce the study burden and concern for having to use a computer by not requiring a set amount of computer use, those who consented may have been more inclined to benefit from an information and support intervention than the general population, which would inflate the effects of CHESS compared to its effects in a broad dissemination. On the other hand, those disinclined to participate may also be those who most need the resources. Previous CHESS research has shown greater effectiveness for disadvantaged participants (Gustafson et al., 2005). Future analysis will examine whether amount of use, or types of services used within CHESS (i.e, information, communication, coaching) have differing benefits. For example, we are interested in whether some people may benefit more from information versus communication resources. It remains to be determined what the essential ingredients in such a comprehensive system are, and what amount and pattern of use would generate the most benefit with minimal user burden.
The design of our study also may have diluted the effects of CHESS. Caregivers in the control group received a computer, training on using the Internet, and a list of high-quality websites on lung cancer. This may have been enough support for some control-group caregivers to meet their need for information and relieve some distress from caregiving. Unfortunately, Internet website use data was not reliable enough to monitor the types of material participants were accessing via other websites to make estimates of comparable exposure. Nevertheless, this study supports that those who are interested in receiving information and support are likely to benefit from an eHealth system like CHESS.
The study also had a high level of attrition. For example, 22% of patients had died and 3% of dyads had dropped within the first 6 months of intervention. Of the remaining 185 dyads, just over 50% of caregivers returned their 6 month survey. This study focuses on caregiving for advanced stage terminal cancer. Accordingly, we expected attrition due to patient death across the duration of the 24 month intervention when lung cancer has a median survival of 8 months. Survey burden is also a barrier for caregivers facing the loss of their loved one. Accordingly, this level of attrition is not unusual for longitudinal studies of patients or caregivers with advanced illness (Buss et al., 2008). But it is possible that the attrition is not random and that caregivers who did not complete the survey suffered from more distress, leading them not to complete the survey. For example, caregivers in the CHESS arm who did not use CHESS were more likely not to return follow-up surveys than those who used the website, indicating a general lack of participation in study activities. However, attrition was similar across arms, and there were no significant differences on pretest levels of caregiver disruptiveness, burden, or negative mood between those who did and did not complete the six-month survey. Strategies to balance research persistence and study burden with sensitivity to the context of the caregiver situation are critical considerations for further research in advanced disease and end of life caregiving.
Conclusions/Future Directions
With increasing reliance on informal caregivers and documentation of the physical and emotional burden of caregiving, developing theoretical and evidenced-based interventions to support these caregivers is essential. Within the model of stress and coping, CHESS aimed to improve the caregiving experience by either reducing the stressors of caregiving to reduce disruption or improving caregiver coping with those stressors to reduce burden and negative mood. While the impact on disruptiveness was not supported, this study does demonstrate the potential of eHealth interventions to improve the caregiver burden and mood within the context of advanced cancer. This study supports the developing foundation of evidence for eHealth’s positive role in facilitating informal caregiving. Even so, the mechanism by which CHESS relieved caregiver burden and negative mood is not clear. Analysis of moderating psychological constructs, such as improved preparedness and coping skills, are needed in order to understand the mechanism. Furthermore, how an individual uses the CHESS system may likely influence the individual’s results. Because CHESS is a self-guided system, individuals choose different components on their own. For example, those who used the discussion group for support may have different outcomes from those who used CHESS solely to gain information about the side effects of treatment. Future analysis will examine CHESS use patterns to determine how use affects outcomes.
Acknowledgments
Funding and Registration
This research was made possible by Grant P50 CA095817-01A1 from the National Cancer Institute. This study is registered at ClinicalTrials.gov with registration number NCT00365963.
References
- American Cancer Society. Cancer Facts & Figures 2013. Atlanta, GA: American Cancer Society; 2013. [Google Scholar]
- Atienza AA, Hesse BW, Gustafson DH, Croyle RT. e-Health Research and Patient-Centered Care: Examining Theory, Methods, and Application. American journal of preventive medicine. 2010;38(1):85–88. doi: 10.1016/j.amepre.2009.10.027. [DOI] [PubMed] [Google Scholar]
- Badr H, Krebs P. A systematic review and meta-analysis of psychosocial interventions for couples coping with cancer. Psycho-Oncology, epub ahead of print. 2013 doi: 10.1002/pon.3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bee PE, Barnes P, Luker KA. A systematic review of informal caregivers’ needs in providing home-based end-of-life care to people with cancer. Journal of Clinical Nursing. 2009;18(10):1379–1393. doi: 10.1111/j.1365-2702.2008.02405.x. [DOI] [PubMed] [Google Scholar]
- Bevans M, Sternberg EM. Caregiving burden, stress, and health effects among family caregivers of adult cancer patients. JAMA : The Journal of the American Medical Association. 2012;307(4):398–403. doi: 10.1001/jama.2012.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruera E, Kuehn N, Miller MJ, Selmser P, Macmillan K. The Edmonton Symptom Assessment System (ESAS): a simple method for the assessment of palliative care patients. Journal of Palliative Care. 1991;7(2):6–9. [PubMed] [Google Scholar]
- Buss MK, DuBenske LL, Dinauer S, Gustafson DH, McTavish F, Cleary JF. Patient/Caregiver influences for declining participation in supportive oncology trials. The Journal of Supportive Oncology. 2008;6(4):168–174. [PubMed] [Google Scholar]
- Carmack Taylor CL, Badr H, Lee JH, Fossella F, Pisters K, Gritz ER, Schover L. Lung cancer patients and their spouses: psychological and relationship functioning within 1 month of treatment initiation. Annals of Behavioral Medicine. 2008 Oct; doi: 10.1007/s12160-008-9062-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleeland CS, Wang XS, Shi Q, Mendoza TR, Wright SL, Berry MD, Malveaux D, et al. Automated symptom alerts reduce postoperative symptom severity after cancer surgery: a randomized controlled clinical trial. Journal of Clinical Oncology. 2011;29(8):994–1000. doi: 10.1200/JCO.2010.29.8315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou WS, Prestin A, Lyons C, Wen K. Web 2.0 for health promotion: reviewing the current evidence. American Journal of Public Health. 2012;103(1):9–18. doi: 10.2105/AJPH.2012.301071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman E, Smith J, Frank J, Min S, Parry C, KIramer A. Preparing patients and caregivers to participate in care delivered across settings: the Care Transitions Intervention. Jouranl of the American Geriatrics Society. 2004;52(11):1817–1825. doi: 10.1111/j.1532-5415.2004.52504.x. [DOI] [PubMed] [Google Scholar]
- Dilorenzo TA, Bovbjerg DH, Montgomery GH, Valdimarsdottir H, Jacobsen PB. The application of a shortened version of the profile of mood states in a sample of breast cancer chemotherapy patients. British Journal of Health Psychology. 1999;4(4):315–325. British Psychological Society. [Google Scholar]
- Docherty A, Owens A, Asadi-Lari M, Petchey R, Williams J, Carter YH. Knowledge and information needs of informal caregivers in palliative care: a qualitative systematic review. Palliative Medicine. 2008;22(2):153–171. doi: 10.1177/0269216307085343. Worcestershire PCT. [DOI] [PubMed] [Google Scholar]
- DuBenske LL, Gustafson DH, Shaw BR, Cleary JF. Web-based cancer communication and decision making systems: connecting patients, caregivers, and clinicians for improved health outcomes. Medical Decision Making. 2010;30(6):732–744. doi: 10.1177/0272989X10386382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuBenske LL, Chih M-Y, Dinauer S, Gustafson DH, Cleary JF. Development and implementation of a clinician reporting system for advanced stage cancer: initial lessons learned. Journal of the American Medical Informatics Association. 2008a;15(5):679–686. doi: 10.1197/jamia.M2532. BMJ Publishing Group Ltd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuBenske LL, Wen K-Y, Gustafson DH, Guarnaccia CA, Cleary JF, Dinauer SK, McTavish FM. Caregivers’ differing needs across key experiences of the advanced cancer disease trajectory. Palliative & Supportive Care. 2008b;6(3):265–272. doi: 10.1017/S1478951508000400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman S. Revised coping theory and the process of bereavement. In: Stroebe MS, Hansson RO, Stroebe W, Scut H, editors. Handbook of bereavement research. Washington DC: American Psychological Association; 2001. pp. 563–584. [Google Scholar]
- Folkman S. The case for positive emotions in the stress process. Anxiety, Stress, & Coping. 2008;21(1):3–14. doi: 10.1080/10615800701740457. [DOI] [PubMed] [Google Scholar]
- Folkman S, Moskowitz JT. Positive affect and the other side of coping. American Psychologist. 2000;55(6):647–654. doi: 10.1037//0003-066x.55.6.647. [DOI] [PubMed] [Google Scholar]
- Gaugler JE, Linder J, Given CW, Kataria R, Tucker G, Regine WF. Family cancer caregiving and negative outcomes: the direct and mediational effects of psychosocial resources. Journal of Family Nursing. 2009;15(4):417–444. doi: 10.1177/1074840709347111. [DOI] [PubMed] [Google Scholar]
- Given BA, Given CW, Kozachik S. Family support in advanced cancer. CA: A Cancer Journal for Clinicians. 2001;51(4):213–231. doi: 10.3322/canjclin.51.4.213. [DOI] [PubMed] [Google Scholar]
- Given B, Wyatt G, Given C, et al. Burden and Depression Among Caregivers of Patients With Cancer at the End of Life. Oncology Nursing Forum. 2004;31:1105–1117. doi: 10.1188/04.ONF.1105-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glajchen M. The emerging role and needs of family caregivers in cancer care. The Journal of Supportive Oncology. 2004;2(2):145–155. [PubMed] [Google Scholar]
- Glasgow RE. eHealth evaluation and dissemination research. American journal of preventive medicine. 2007;32(5 Suppl):S119–S126. doi: 10.1016/j.amepre.2007.01.023. [DOI] [PubMed] [Google Scholar]
- Grunfeld E, Coyle D, Whelan T, Clinch J, Reyno L, Earle CC, Willan A, et al. Family caregiver burden: results of a longitudinal study of breast cancer patients and their principal caregivers. Canadian Medical Association Journal. 2004;170(12):1795–1801. doi: 10.1503/cmaj.1031205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson DH, Hawkins R, McTavish F, Pingree S, Chen WC, Volrathongchai K, Stengle W, et al. Internet-Based Interactive Support for Cancer Patients: Are Integrated Systems Better? The Journal of Communication. 2008;58(2):238–257. doi: 10.1111/j.1460-2466.2008.00383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson DH, Hawkins RP, Boberg EW, McTavish F, Owens B, Wise M, Berhe H, et al. CHESS: 10 years of research and development in consumer health informatics for broad populations, including the underserved. International Journal of Medical Informatics. 2002;65(3):169–177. doi: 10.1016/s1386-5056(02)00048-5. [DOI] [PubMed] [Google Scholar]
- Gustafson DH, Hawkins R, Pingree S, McTavish F, Arora NK, Mendenhall J, Cella DF, et al. Effect of computer support on younger women with breast cancer. Journal of General Internal Medicine. 2001;16(7):435–445. doi: 10.1046/j.1525-1497.2001.016007435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson DH, McTavish FM, Schubert CJ, Johnson Ra. The Effect of a Computer-based Intervention on Adult Children of Alcoholics. Journal of Addiction Medicine. 2012;6(1):24–28. doi: 10.1097/ADM.0b013e31822b80ca. [DOI] [PubMed] [Google Scholar]
- Gustafson DH, McTavish FM, Stengle W, Ballard D, Hawkins R, Shaw BR, Jones E, et al. Use and Impact of eHealth System by Low-income Women with Breast Cancer. Journal of Health Communication. 2005;10(Suppl 1):195–218. doi: 10.1080/10810730500263257. [DOI] [PubMed] [Google Scholar]
- Haley WE. Family caregivers of elderly patients with cancer: understanding and minimizing the burden of care. Journal of Supportive Oncology. 2003;1(4 Suppl 2):25–29. [PubMed] [Google Scholar]
- Haley WE, LaMonde LA, Han B, Burton AM, Schonwetter R. Predictors of depression and life satisfaction among spousal caregivers in hospice: application of a stress process model. Journal of palliative medicine. 2003;6(2):215–224. doi: 10.1089/109662103764978461. [DOI] [PubMed] [Google Scholar]
- Harding R, List S, Epiphaniou E, Jones H. How can informal caregivers in cancer and palliative care be supported? An updated systematic literature review of interventions and their effectiveness. Palliative Medicine. 2012;26(1):7–22. doi: 10.1177/0269216311409613. [DOI] [PubMed] [Google Scholar]
- Hodges LJ, Humphris GM, Macfarlane G. A meta-analytic investigation of the relationship between the psychological distress of cancer patients and their carers. Social Science & Medicine (1982) 2005;60(1):1–12. doi: 10.1016/j.socscimed.2004.04.018. [DOI] [PubMed] [Google Scholar]
- Kim Y, Duberstein PR, Sörensen S, Larson MR. Levels of Depressive Symptoms in Spouses of People with Lung Cancer: Effects of Personality, Social Support, and Caregiving Burden. Psychosomatics. 2005;46:123–130. doi: 10.1176/appi.psy.46.2.123. [DOI] [PubMed] [Google Scholar]
- Kinsella G, Cooper B, Picton C, Murtaugh D. A review of the measurement of caregiver and family burden in SOPCare. J. Palliative Care. 1998;14(2):37–45. [PubMed] [Google Scholar]
- Klemm P, Wheeler E. Cancer caregivers online: hope, emotional roller coaster, and physical/emotional/psychological responses. Computers, Informatics, and Nursing. 2005;23(1):38–45. doi: 10.1097/00024665-200501000-00008. [DOI] [PubMed] [Google Scholar]
- Lawton M, Kleban M, Moss M, et al. Measuring caregiving appraisal. J. Gerontology. 1989;44(3):61–71. doi: 10.1093/geronj/44.3.p61. [DOI] [PubMed] [Google Scholar]
- Lazarus R, Folkman S. Stress, appraisal and coping. New York: Springer; 1984. [Google Scholar]
- Lobchuk M, Degner L. Symptom experiences: perceptual accuracy between advanced-stage cancer patients and family caregivers in the home care setting. Journal of Clinical Oncology. 2002;20:3495–3507. doi: 10.1200/JCO.2002.01.153. [DOI] [PubMed] [Google Scholar]
- Mehta A, Cohen SR, Carnevale Fa, Ezer H, Ducharme F. Strategizing a game plan: family caregivers of palliative patients engaged in the process of pain management. Cancer Nursing. 2010;33(6):461–469. doi: 10.1097/NCC.0b013e3181de72cc. [DOI] [PubMed] [Google Scholar]
- Mitnick S, Leffler C, Hood VL. Family caregivers, patients and physicians: ethical guidance to optimize relationships. Journal of General Internal Medicine. 2010;25(3):255–260. doi: 10.1007/s11606-009-1206-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux PJ, et al. CONSORT 2010: Explanation and Elaboration: updated guidelines for reporting parallel group randomised trials. Journal of Clinical Epidemiology. 2010;63:e1–ee37. doi: 10.1016/j.jclinepi.2010.03.004. [DOI] [PubMed] [Google Scholar]
- NSCLC Meta-Analyses Collaborative Group. Chemotherapy in addition to supportive care improves survival in advanced non-small-cell lung cancer: a systematic review and meta-analysis of individual patient data from 16 randomized controlled trials. Journal of Clinical Oncology. 2008;26(28):4617–4625. doi: 10.1200/JCO.2008.17.7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northouse L, Williams A-L, Given B, McCorkle R. Psychosocial Care for Family Caregivers of Patients With Cancer. Journal of Clinical Oncology. 2012;30:11. doi: 10.1200/JCO.2011.39.5798. [DOI] [PubMed] [Google Scholar]
- Northouse LL, Katapodi MC, Song L, hang L, Mood DW. Interventions with family caregivers of cancer patients: Meta-analysis of randomized controlled trials. CA: A Cancer Journal for Clinicians. 2010;60(5):317–339. doi: 10.3322/caac.20081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberst J, Thomas S, Gass K, Ward S. Caregiving demands and appraisal of stress among family caregivers. Cancer Nursing. 1989;12:209–215. [PubMed] [Google Scholar]
- Osse BH, Vernooij-Dassen MJ, Schade E, Grol RP. Problems experienced by informal caregivers of cancer patients and their needs for support. Cancer Nursing. 2006;29(5):378–388. doi: 10.1097/00002820-200609000-00005. [DOI] [PubMed] [Google Scholar]
- Pingree S, Hawkins R, Baker T, DuBenske L, Roberts LJ, Gustafson DH. The value of theory for enhancing and understanding e-health interventions. American journal of preventive medicine. 2010;38(1):103–109. doi: 10.1016/j.amepre.2009.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnizky S, Bentur N. Can family caregivers of terminally ill patients be a reliable source of information about the severity of patient symptoms? American Journal of Hospice and Palliative Care. 2007 Jan 1;:447–456. doi: 10.1177/1049909106294825. [DOI] [PubMed] [Google Scholar]
- Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, Zhu J, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. The New England Journal of Medicine. 2002;346(2):92–98. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- Sharpe L, Butow P, Smith C, McConnell D, Clarke S. The relationship between available support, unmet needs and caregiver burden in patients with advanced cancer and their carers. Psycho-oncology. 2005;14(2):102–114. doi: 10.1002/pon.825. [DOI] [PubMed] [Google Scholar]
- Shaw BR, Han JY, Hawkins RP, Stewart J, McTavish F, Gustafson DH. Doctor-patient relationship as motivation and outcome: examining uses of an Interactive Cancer Communication System. International Journal of Medical Informatics. 2007;76(4):274–282. doi: 10.1016/j.ijmedinf.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Shaw BR, McTavish F, Hawkins R, Gustafson DH, Pingree S. Experiences of women with breast cancer: exchanging social support over the CHESS computer network. Journal of Health Communication. 2000;5(2):135–159. doi: 10.1080/108107300406866. [DOI] [PubMed] [Google Scholar]
- Shelby Ra, Taylor KL, Kerner JF, Coleman E, Blum D. The role of community-based and philanthropic organizations in meeting cancer patient and caregiver needs. CA: A Cancer Journal for Clinicians. 2002;52(4):229–246. doi: 10.3322/canjclin.52.4.229. [DOI] [PubMed] [Google Scholar]
- Sorensen S, Pinquart M, Duberstein P. How effective are interventions with caregivers? An updated meta-analysis. The Gerontologist. 2002;42(3):356–372. doi: 10.1093/geront/42.3.356. [DOI] [PubMed] [Google Scholar]
- Spiro SG, Rudd RM, Souhami RL, Brown J, Fairlamb DJ, Gower NH, Maslove L, et al. Chemotherapy versus supportive care in advanced non-small cell lung cancer: improved survival without detriment to quality of life. Thorax. 2004;59(10):828–836. doi: 10.1136/thx.2003.020164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tixier M, Lewkowicz M. Design and evaluation of an online social support application for family caregivers. In: Ozok AA, Zaphiris P, editors. Online Communities and Social Computing. Berlin, Germany: Springer; 2011. pp. 267–276. [Google Scholar]
- van den Beuken-van Everdingen MH, de Rijke JM, Kessels AG, Schouten HC, van Kleef M, Patijn J. Prevalence of pain in patients with cancer: a systematic review of the past 40 years. Annals of Oncology: Official Journal of the European Society for Medical Oncology/ESMO. 2007;18(9):1437–1449. doi: 10.1093/annonc/mdm056. [DOI] [PubMed] [Google Scholar]
- Weitzner MA, Jacobsen PB, Wagner H, Friedland J, Cox C. The Caregiver Quality of Life Index-Cancer (CQOLC) scale: development and validation of an instrument to measure quality of life of the family caregiver of patients with cancer. Quality of Life Research. 1999;8(1–2):55–63. doi: 10.1023/a:1026407010614. [DOI] [PubMed] [Google Scholar]
- Weitzner MA, McMillan SC, Jacobsen PB. Family caregiver quality of life: differences between curative and palliative cancer treatment settings. Journal of pain and symptom management. 1999;17(6):418–428. doi: 10.1016/s0885-3924(99)00014-7. [DOI] [PubMed] [Google Scholar]
- Wen KY, Gustafson DH. Needs assessment for cancer patients and their families. Health and Quality of Life Outcomes. 2004;2:11. doi: 10.1186/1477-7525-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]