Abstract
Polymorphonuclear leukocytes (PMNs) are recruited to sites of injury and biomaterial implants. Once activated, PMNs can exocytose their granule subsets to recruit monocytes (MCs) and mediate MC/macrophage activation. We investigated the release of myeloperoxidase (MPO), a primary granule marker, and matrix metalloproteinase-9 (MMP-9), a tertiary granule marker, from human blood-derived PMNs cultured on poly(ethylene glycol) (PEG) hydrogels, polydimethylsiloxane (PDMS), tissue culture polystyrene (TCPS) and gelatin-PEG (GP) hydrogels, with and without the presence of the bacterial peptide formyl-Met-Leu-Phe. Supernatants from PMN cultures on PEG-containing hydrogels (i.e., PEG and GP hydrogels) had higher concentrations of MPO than those from PMN cultures on PDMS or TCPS at 2 hours. PMNs on all biomaterials released comparable levels of MMP-9 at 2 hours, indicating that PMNs cultured on PEG-containing hydrogels have different mechanisms of release for primary and tertiary granules. Src family kinases were involved in the release of MPO from PMNs cultured on PEG hydrogels, TCPS and GP hydrogels and in the release of MMP-9 from PMNs cultured on all four materials. The increased release of primary granules from PMNs on PEG-containing hydrogels did not significantly increase MC chemotaxis, indicating that additional co-effectors in the dynamic inflammatory milieu in vivo modulate PMN-mediated MC recruitment.
Keywords: Neutrophil, acute inflammation, degranulation, myeloperoxidase, matrix metalloproteinase-9, poly(ethylene glycol)
INTRODUCTION
Implantation of a biomaterial initiates a series of inflammatory and wound healing events collectively termed the foreign body response (FBR), during which polymorphonuclear leukocytes (PMNs; neutrophils) extravasate to the implant site in response to injury to vascular tissue.1,2 PMNs contain four subsets of granule proteins, formed sequentially based on the biosynthetic capabilities of the cell at the given stage of myelopoeisis (Supplementary Figure 1).3 These subsets include primary granules, secondary granules, tertiary granules and secretory vesicles.4 There are nearly 300 granule proteins, including antimicrobial proteins, proteases, β2-integrin receptors, chemotactic receptors and soluble mediators of inflammation.5,6 Upon receptor-mediated activation, PMNs can release their granule subsets to facilitate monocyte (MC) chemotaxis and mediate MC/macrophage activation and function,7–18 thereby influencing subsequent inflammatory or wound healing events in the FBR.
To better understand how biomaterials modulate PMN-MC paracrine interactions, in a previous study, we cultured human blood-derived MCs on poly(ethylene glycol) (PEG) hydrogels, polydimethylsiloxane (PDMS) and tissue culture polystyrene (TCPS) and primed the MCs for 24 hours with conditioned media from cultures of autologous PMNs pre-activated with matching biomaterials (PMN-conditioned media; PCM).19 PCM significantly increased MC viability/adhesion on PEG hydrogels.19 We hypothesized that biomaterial-dependent PMN degranulation may contribute to the observed increase in MC viability/adhesion upon treatment with PCM from PMN cultures on PEG hydrogels. Therefore, in this study, we sought to determine how biomaterials influence PMN degranulation events, as PMN granule proteins have implications for downstream inflammatory response. We focused on the release of primary and tertiary granules to investigate both early and late degranulation events. PMN primary granules contain toxic antimicrobial reagents such as myeloperoxidase (MPO), human neutrophil peptides 1–3 (HNP1–3), cathepsin G and elastase, and are generally released only after PMN extravasation to the site of inflammation.16 PMN tertiary granules contain matrix-degrading enzymes such as matrix metalloproteinase-9 (MMP-9) and are released when PMNs transmigrate through the endothelium on their journey to the site of inflammation.16 Proteases such as elastase, cathepsin G and MMP-9 are involved in tissue remodeling in inflammation but are also involved in tissue pathology in inflammatory diseases.20–22 In addition to the biomaterials we used in our previous study (PEG hydrogels, PDMS and TCPS),19 we also investigated gelatin-PEG (GP) hydrogels, which are a hybrid biomatrix developed in our group based on the thiol-ene reaction of PEG diacrylate and cysteine/PEG-modified gelatin.23 The incorporation of gelatin in the hydrogel introduces cell-binding motifs such as RGD (arginine-glycineaspartic acid) peptides and could possibly regulate MMP expression.
MATERIALS AND METHODS
PEG hydrogel, GP hydrogel, PDMS and TCPS preparation
PEG diacrylate (molecular weight 3350) and cysteine/PEG-modified gelatin (gel-PEG-cys) were synthesized as previously described23 and characterized by proton NMR (Varian Unity-Inova 400 MHz; Varian Inc., Palo Alto, CA). The proton NMR spectrum of PEG diacrylate in deuterated chloroform showed peaks at 3.6, 3.7 and 4.3 ppm (methylene protons), and 5.8, 6.2 and 6.4 ppm (acrylate protons). PEG diacrylate products were routinely found to have > 85% acrylation based on proton NMR peak area measurements. The proton NMR spectrum of gel-PEG-cys in deuterated water showed peaks at 1.3 ppm (d, 2H, -CH2SH), 2.9 ppm (t, 1H, -CHCH2SH), 3.7 ppm (m, -CH2- from PEG backbone) and broad gelatin peaks at 1.7, 1.8, 1.9, 3.2 and 4.2 ppm. PEG hydrogels were prepared by dissolving 10 weight percent PEG diacrylate (molecular weight 3350) and 0.1 weight percent 2-hydroxy-4'-(2-hydroxyethoxy)-2-methylpropiophenone (Sigma-Aldrich, St. Louis, MO) in water with heat (37°C). GP hydrogels were prepared by dissolving 10 weight percent PEG diacrylate (molecular weight 3350), 0.1 weight percent 2-hydroxy-4'-(2-hydroxyethoxy)-2-methylpropiophenone and 10 weight percent gel-PEG-cys in water with heat (37°C). The PEG and GP hydrogel solutions were injected into Teflon molds held between two, pre-cleaned glass slides and polymerized by placing the assemblies under a UV light (UV CF1000 LED, λmax = 365 nm; Clearstone Technologies, Minneapolis, MN) for 3 minutes per side. The PEG and GP hydrogel films were cold sterilized in 70% ethanol (Decon Labs, Inc., King of Prussia, PA) for 45 minutes and equilibrated overnight in Dulbecco's phosphate-buffered saline (DPBS; Cellgro, Manassas, VA). The PEG and GP hydrogel films were cut into 8-mm diameter disks with biopsy punches (Miltex, Inc., York, PA), placed into 48-well culture plates (Corning Inc., Corning, NY) and cold sterilized in 70% ethanol for 45 minutes.
PDMS (0.762 mm thickness, non-reinforced vulcanized gloss/gloss; Specialty Manufacturing Inc., Saginaw, MI) was cut into 8-mm diameter disks with biopsy punches and sonicated for 10 minutes in a 0.5 weight percent solution of sodium dodecyl sulfate (Amresco, Solon, OH). The PDMS disks were rinsed with deionized water, placed into 48-well culture plates and cold sterilized in 70% ethanol for 45 minutes. Sterilized PEG hydrogels, GP hydrogels and PDMS disks were washed three times with DPBS and equilibrated in RPMI-1640 basal medium (Cellgro, Manassas, VA) prior to seeding with cells. TCPS wells (Corning Inc., Corning, NY) were also washed three times with DPBS and equilibrated in RPMI-1640 medium prior to cell seeding.
Culture of human blood-derived PMNs and MCs
Peripheral whole blood was obtained from consenting healthy adult donors in accordance with protocols approved by the University of Wisconsin-Madison Institutional Review Board. PMNs and MCs were isolated as previously described using a gradient method.24 A small percentage (<1%) of basophils and eosinophils are possible in the PMN isolation. The MC isolation procedure routinely results in approximately 80–90% MC purity with lymphocytes as the primary impurities. PMNs were adjusted to 1 × 106 cells/ml in RPMI-1640 medium supplemented with 10% autologous human serum (AHS). The PMN suspension was split into two groups where one group received 100 nM of the bacterial peptide formyl-Met-Leu-Phe (fMLP; Sigma-Aldrich, St. Louis, MO). This concentration of fMLP is used to stimulate PMN degranulation.25 PMNs were statically seeded onto PEG hydrogel, PDMS, TCPS and GP hydrogel surfaces in 48-well plates (0.5 ml/well for a density of approximately 5.3 × 103 PMNs/mm2) and were incubated in a humidified atmosphere at 37°C with 5% CO2 for between 2 and 24 hours. MCs were adjusted to 1.7 × 106 cells/ml in RPMI-1640 medium supplemented with 10% AHS and were seeded as described below for the MC chemotaxis assay.
Analysis of cell adhesion and viability
At pre-determined time points, the culture media was aspirated from PMN cultures and replaced with a solution containing 4 μM green-fluorescent calcein AM and 2 μM red-fluorescent ethidium homodimer-1 (reagents from LIVE/DEAD Viability/Cytotoxicity Kit; Molecular Probes, Eugene, OR) in DPBS. The PMN cultures were then incubated for 45 minutes in a humidified atmosphere at 37°C with 5% CO2. Three representative fields per well of a 48-well plate were imaged at 10× magnification with a digital camera attached to an inverted fluorescent microscope (Nikon Eclipse TE 300; Nikon Instruments Inc., Melville, NY). Viable (green) and necrotic (red) cells were counted in an area of 0.62 mm2 per field to determine adherent viable and necrotic cell densities (cells/mm2). Metabolic capacity was measured using CellTiter-Blue® Reagent (Promega Corporation, Madison, WI) as an indicator of cell viability; CellTiter-Blue® Reagent contains a redox dye (resazurin) that can be converted into a fluorescent product (resofurin) by metabolically active cells. At 2 and 4 hours, the culture media was aspirated from PMN cultures (seeded at a density of approximately 5.3 × 103 PMNs/mm2) and replaced with a solution containing 10% CellTiter-Blue® Reagent in RPMI-1640 medium supplemented with 10% AHS. PMN cultures containing the CellTiter-Blue® Reagent were incubated for 4 hours in a humidified atmosphere at 37°C with 5% CO2. The culture media was then transferred to wells in a 96-well plate (Corning Inc., Corning, NY) and the fluorescent signal (584 nm excitation, 620 nm emission) was measured using a plate reader (FLUOstar OPTIMA; BMG Labtech, Cary, NC). Fluorescent intensities (FI) of PMN samples were normalized to biomaterial control samples that did not contain cells.
Measurement of MPO and MMP-9
After 2 and 4 hours, supernatants (i.e., PCM) from PMN cultures on the biomaterials (seeded at a density of approximately 5.3 × 103 PMNs/mm2) were collected and centrifuged at 2500 × g for 10 minutes at 4°C to remove cells and cellular debris. These two time points were selected for measurement of MPO and MMP-9 based on an initial experiment in which adherent viable and necrotic cell densities were quantified after PMNs were cultured on the same four biomaterials with and without 100 nM fMLP for 1, 2, 4, 6, 12 and 24 hours. Compared to the other time points, at 2 and 4 hours, adherent viable cell densities on the biomaterials were high and necrotic adherent cell densities were low (Supplementary Figure 2).
MPO in supernatants from PMN cultures was measured using an enzyme-linked immunosorbent assay (ELISA) kit (Hycult Biotech, Plymouth Meeting, PA) or a Milliplex® bead-based immunoassay kit (EMD Millipore, Billerica, MA) according to manufacturer's instructions. MPO is a primary granule protein that catalyzes the formation of hypochlorous acid from hydrogen peroxide and chloride anion. MMP-9 in supernatants from PMN cultures was measured using a Milliplex® bead-based immunoassay kit (EMD Millipore, Billerica, MA) according to manufacturer's instructions. MMP-9 (gelatinase) is a tertiary granule protein that is involved in the degradation of extracellular matrix proteins such as gelatin and collagen type IV. PCM samples were diluted between 4- and 200-fold to stay within the measureable range of the MPO and MMP-9 assays.
PMN pre-treatment with Src family kinase inhibitor PP1
After isolation, PMNs were suspended at a concentration of 2 × 106 cells/ml in RPMI-1640 medium supplemented with 10% AHS and 10 μM Src family kinase inhibitor PP1 (4-amino-5-(4-methylphenyl)-7-(t-butyl)pyrazolo-d-3,4-pyrimidine; EMD Millipore, Billerica, MA) or an equivalent volume of dimethyl sulfoxide (Sigma-Aldrich, St. Louis, MO). The PMNs were then statically seeded onto PEG hydrogel, PDMS, TCPS and GP hydrogel surfaces in 48-well plates (0.25 ml/well for a density of approximately 5.3 × 103 PMNs/mm2). The plates were incubated in a humidified atmosphere at 37°C with 5% CO2 for 10 minutes. After 10 minutes, an additional 250 μl of RPMI-1640 medium supplemented with 10% AHS was added to the wells and the well plates were incubated for 2 hours in a humidified atmosphere at 37°C with 5% CO2. After 2 hours, cell adhesion and viability, MPO, and MMP-9 were measured as described above.
MC chemotaxis assay
At 2 hours, PCM from PMN cultures on the biomaterials (seeded at a density of approximately 5.3 × 103 PMNs/mm2) were collected and centrifuged at 2500 × g for 10 minutes at 4°C to remove cells and cellular debris. This time point was selected for collection of PCM for the MC chemotaxis assay due to the increased concentrations of MPO in PCM from PMN cultures on PEG and GP hydrogels at 2 hours. The PCM chemoattractant solutions were added to the bottom wells of 96-well ChemoTx® chemotaxis plates (Neuro Probe, Inc., Gaithersburg, MD). Control chemoattractant solutions (i.e., 10% AHS in RPMI-1640 medium without fMLP and 10% AHS in RPMI-1640 medium with 50 or 100 nM fMLP) were also added to the bottom wells of the chemotaxis plates. A polycarbonate filter with 5-μm diameter pores was placed on top of the wells and approximately 4.2 × 104 autologous MCs were placed in each well site on the top of the filter for a density of approximately 5.3 × 103 MCs/mm2. The chemotaxis plate was covered and incubated for 90 minutes in a humidified atmosphere at 37°C with 5% CO2, during which time MCs could transmigrate to the underside of the filter in response to the chemoattractant solutions in the bottom wells. After 90 minutes, the filter was removed and non-migrated MCs were scraped from the top side of the filter. The filter was then fixed and stained using a modified Wright-Giemsa stain (HEMA 3® Stain Set; Fisher Scientific Company, Middletown, VA). One representative field per filter site was imaged at 10× magnification with a digital camera attached to an optical microscope (Leica DMLB Microscope Type 11888010; Leica Microsystems GmbH, Wetzlar, Germany). Transmigrated MCs were counted in an area of 0.13 mm2 per field.
Statistical analysis
Unless otherwise indicated, each experiment was performed four times, using four different blood donors (n = 4) and data are shown as mean ± standard deviation. Statistical analysis was performed using one- or two-way analysis of variance (ANOVA) combined with Bonferroni's multiple comparison post tests (GraphPad Prism, San Diego, CA). Values of p ≤ 0.05 were considered statistically significant.
RESULTS
PMN-biomaterial interactions: cell adhesion and viability
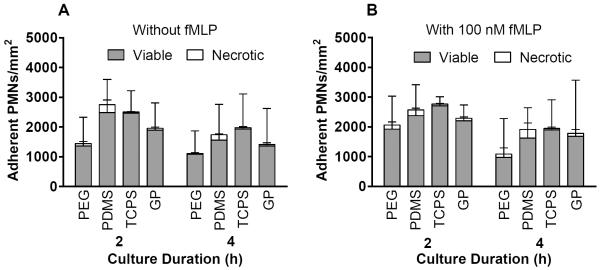
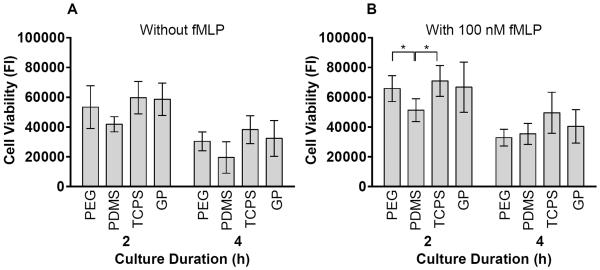
There were no significant differences in viable or necrotic adherent cell densities due to the addition of 100 nM fMLP in the culture medium (Figure 1). However, the addition of 100 nM fMLP to the culture medium significantly increased cell viability based on metabolic capacity for PMNs on PDMS and TCPS at 2 hours (p = 0.01–0.05) (Figure 2). At 4 hours without fMLP, necrotic cell densities were significantly higher on PDMS than on PEG hydrogels (p = 0.01–0.05) (Figure 1A). In agreement, cell viability on PDMS at 4 hours without fMLP was lower than on the other materials (Figure 2A). Furthermore, at 2 hours with 100 nM fMLP, cell viability on PDMS was significantly lower than that on PEG hydrogels or TCPS (p = 0.01–0.05) (Figure 2B). There were no significant differences in total (viable + necrotic) adherent cell densities among materials at 2 or 4 hours with or without the addition of fMLP (Figure 1).
Figure 1.
Viable and necrotic adherent cell densities in PMN cultures A) without fMLP and B) with 100 nM fMLP on PEG hydrogels, PDMS, TCPS and GP hydrogels at 2 and 4 hours as measured using calcein AM and ethidium homodimer-1 fluorescent stains. Results represent the mean + standard deviation, n = 4.
Figure 2.
Cell viability in PMN cultures A) without fMLP and B) with 100 nM fMLP on PEG hydrogels, PDMS, TCPS and GP hydrogels at 2 and 4 hours as measured by fluorescent intensity (FI) of cell metabolic capacity using CellTiter-Blue® Reagent. Results represent the mean ± standard deviation, n = 4. *: significant difference between materials, p = 0.01–0.05.
PEG-containing hydrogels promote the release of MPO, but not MMP-9 from PMNs
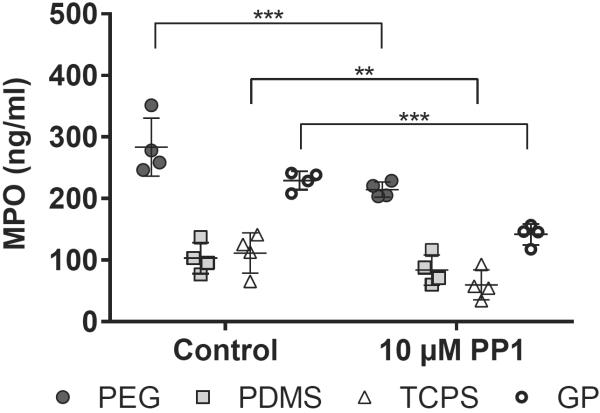
At 2 hours, supernatants from PMN cultures on PEG hydrogels had significantly higher MPO concentrations than those from PMN cultures on PDMS or TCPS (p = 0.001–0.05) (Figure 3). There were also higher MPO concentrations in supernatants from PMN cultures on GP hydrogels than in those from PMN cultures on PDMS or TCPS. The addition of 100 nM fMLP to the culture medium significantly increased MPO concentrations in supernatants from PMN cultures on TCPS at 2 hours (from 64 ± 29 ng/ml on TCPS without fMLP to 325 ± 51 ng/ml on TCPS with 100 nM fMLP) (p = 0.01–0.05). MPO concentrations in supernatants from PMNs cultures without fMLP on PEG and GP hydrogels decreased significantly between 2 and 4 hours (p = 0.0001–0.001). MPO concentrations in supernatants from PMN cultures with 100 nM fMLP on PEG hydrogels, PDMS, TCPS and GP hydrogels also decreased significantly between 2 and 4 hours (p < 0.01). This reduction in MPO between 2 and 4 hours suggests that MPO in the culture media may degrade, adhere to the biomaterials or complex with serum proteins.
Figure 3. PEG-containing hydrogels promote the release of primary granules from PMNs.
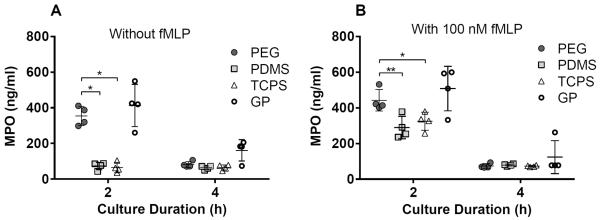
MPO concentrations in supernatants from PMN cultures on PEG hydrogels, PDMS, TCPS and GP hydrogels at 2 and 4 hours. Results represent the mean ± standard deviation with each data point representing a different donor, n = 4. *: significant difference between materials, p = 0.01–0.05; **: significant difference between materials, p = 0.001–0.01.
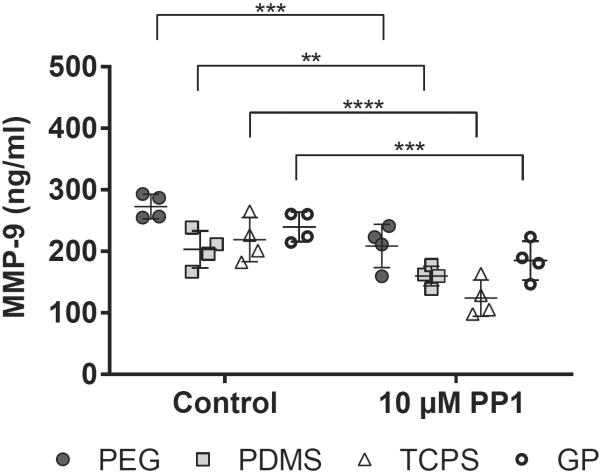
The release of MMP-9 from PMNs cultured on the biomaterials did not follow the same trend as the release of MPO: at 2 hours there were no significant differences in MMP-9 concentrations among biomaterials (Figure 4). However, in PMN cultures on GP hydrogels with 100 nM fMLP, MMP-9 concentrations in supernatants decreased significantly between 2 and 4 hours (p = 0.01–0.05), resulting in significantly lower MMP-9 concentrations in supernatants from PMNs cultures on GP hydrogels than in those from PMN cultures on PEG hydrogels, PDMS or TCPS at 4 hours (p = 0.0001–0.01). Because gelatin is a substrate of MMP-9, MMP-9 released from PMNs may have been actively cleaving the gelatin in the GP hydrogels and therefore was not as freely available in the culture media to be detected by immunoassay.
Figure 4.
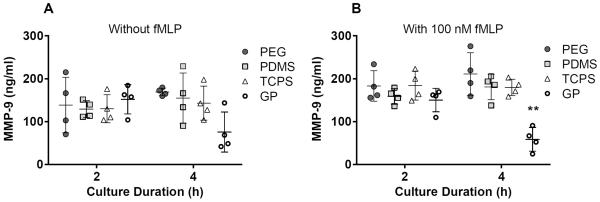
MMP-9 concentrations in supernatants from PMN cultures on PEG hydrogels, PDMS, TCPS and GP hydrogels at 2 and 4 hours. Results represent the mean ± standard deviation with each data point representing a different donor, n = 4. **: significant difference from PEG hydrogel, PDMS and TCPS at the same time point, p = 0.0001–0.01.
Pre-treatment with Src family kinase inhibitor PP1 decreases MPO and MMP-9 release from PMNs
To determine the role of Src family kinases in biomaterial-mediated PMN degranulation, we pre-treated PMNs cultured on the biomaterials with PP1, a Src family kinase inhibitor. The addition of 10 μM PP1 to the culture medium significantly decreased MPO release from PMNs cultured on PEG hydrogels, TCPS and GP hydrogels (p = 0.0001–0.01) (Figure 5) and significantly decreased MMP-9 release from PMNs cultured on all four materials (p < 0.01) (Figure 6). To confirm that the PP1-mediated decreases in MPO and MMP-9 release were not due to changes in cell adhesion or viability, we also measured adherent viable and necrotic cell densities and cell viability based on metabolic capacity. The addition of 10 μM PP1 to the culture medium did not significantly influence adherent viable PMN densities (Supplementary Figure 3A), but did decrease adherent necrotic PMN densities on PDMS (p < 0.0001) (Supplementary Figure 3B), suggesting that the addition of PP1 decreased PMN death on PDMS. The addition of 10 μM PP1 to the culture medium decreased PMN viability based on metabolic capacity on GP hydrogels (p = 0.001–0.01), but did not affect PMN viability on PEG hydrogels, PDMS or TCPS (Supplementary Figure 4). Overall, these results suggest that PP1 minimally influences PMN adhesion and viability. Therefore, it is likely that the PP1-mediated decreases in primary and tertiary granule release are not a consequence of PP1-mediated changes in cell viability or adhesion.
Figure 5.
MPO concentrations in supernatants from PMN cultures pre-treated with 10 _M PP1 on PEG hydrogels, PDMS, TCPS and GP hydrogels at 2 hours. Results represent the mean ± standard deviation with each data point representing a different donor, n = 4. **: significant difference with PP1 pre-treatment, p = 0.001–0.01; ***: significant difference with PP1 pre-treatment, p = 0.0001–0.001.
Figure 6.
MMP-9 concentrations in supernatants from PMN cultures pre-treated with 10 _M PP1 on PEG hydrogels, PDMS, TCPS and GP hydrogels at 2 hours. Results represent the mean ± standard deviation with each data point representing a different donor, n = 4. **: significant difference with PP1 pre-treatment, p = 0.001–0.01; ***: significant difference with PP1 pre-treatment, p = 0.0001–0.001; ****: significant difference with PP1 pre-treatment, p < 0.0001.
Effect of PMN granular content on MC chemotaxis
There were no significant differences in MC transmigration in response to autologous PCM from PMN cultures on any of the four biomaterials with or without fMLP (Supplementary Figure 5). Additionally PCM did not significantly increase MC transmigration as compared with the 10% AHS culture medium baseline. However, compared to the other materials and the 10% AHS baseline, there were slight increases in MC transmigration in response to PCM from PMN cultures on PEG hydrogels with and without fMLP. There were also slight increases in MC transmigration due to the presence of fMLP in chemoattractant solutions from PMN cultures on GP hydrogels. Lastly, compared with the 10% AHS baseline, there were slight elevations in MC transmigration in response to 10% AHS with 50 nM fMLP.
DISCUSSION
PEG-containing hydrogels promote primary granule release from PMNs
PMN degranulation generally occurs in a stepwise manner, with secretory vesicles exocytosed most readily, followed by tertiary, secondary, and finally, primary granules, which are the most tightly regulated due to their abundance of toxic microbicidal substances.26 Therefore, a major finding in our study was that PEG and GP hydrogels were sufficiently activating to induce the release of primary granules from PMNs at 2 hours. Furthermore, PMNs on PEG hydrogels had significantly greater release of primary granules than PMNs on PDMS or TCPS at 2 hours. Similarly, in a previous study we found that at 2 hours, supernatants from PMN cultures on PEG hydrogels had significantly higher concentrations of HNP1–3 than those from PMN cultures on PDMS or TCPS.19 The enhanced release of primary granule proteins from PMNs on PEG hydrogels may account for the significant increase in MC viability/adhesion that we previously observed upon addition of PCM to MC cultures on PEG hydrogels,19 perhaps by modifying protein adsorption on PEG hydrogels or by acting as soluble signals for MCs to increase viability and adhesion. While we do see lower viable adherent PMN densities on PEG and GP hydrogels, we do not believe that leakage of cell contents from nonadherent PMNs is responsible for the increased primary granule release from PMNs on PEG and GP hydrogels. We previously observed that secondary necrosis (as measured by caspase 3/7 activity in cell culture supernatants) of PMNs on PEG hydrogels was comparable to that of PMNs on PDMS at 2 hours19, thereby suggesting that secondary necrosis of nonadherent PMNs (and thus leakage of cell contents) does not contribute to the observed primary granule release from PMNs on PEG and GP hydrogels.
PEG is traditionally considered to be protein resistant and bioinert.27–30 However, studies from our group and others have shown that MC/macrophages have increased pro-inflammatory cytokine expression (e.g., IL-1β and TNF) when cultured on PEG hydrogels in vitro.19,24,31,32 Furthermore, Lynn et al. demonstrated that implantation of PEG hydrogels in mice resulted in a robust inflammatory reaction characterized by a dense layer of macrophages at the biomaterial surface.31 The results from our study suggest that like MC/macrophages, PMNs may also undergo an inflammatory response to PEG-containing hydrogels.
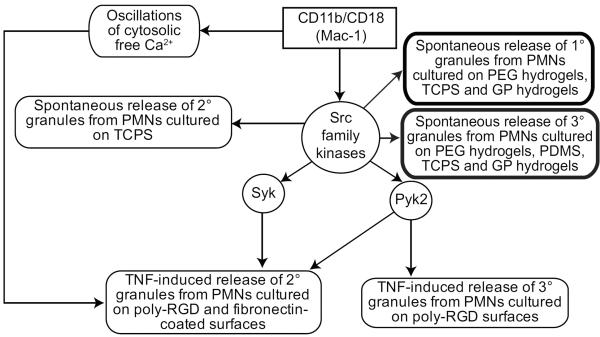
It is plausible that post-integrin-ligation signaling events regulate the release of primary granules from PMNs adherent on PEG and GP hydrogels. While much research on PMN degranulation involves PMNs in suspension, some reports focused on adhesion-dependent degranulation (graphically summarized in Figure 7). Ligation of the β2-integrin CD11b/CD18 (Mac-1) is responsible for spontaneous oscillations of cytosolic free Ca2+ in adherent PMNs33 and these oscillations were shown to be involved in TNF-induced release of secondary granules from adherent PMNs.34 Tyrosine kinases Syk and Pyk2 and Src family kinases have also been implicated in integrin-mediated degranulation of adherent PMNs. Wild-type murine PMNs adherent on fibrinogen-coated surfaces released secondary granules in response to TNF stimulation, whereas Syk-deficient murine PMNs on fibrinogen-coated surfaces did not release secondary granules in response to TNF stimulation.35 Pyk2-deficient murine PMNs plated on poly-RGD surfaces and treated with TNF had reduced release of secondary and tertiary granules when compared with wild-type PMNs.36 Additionally, Src family kinases were required for the release of secondary granules from PMNs adherent on fibrinogen-coated surfaces and treated with TNF.37 Based on these reports, we hypothesized that Src family kinases and their downstream tyrosine kinases may also be involved in the release of primary granules from PMNs adherent on PEG and GP hydrogels. In experiments using the Src family kinase inhibitor PP1, we determined that Src family kinases are involved in primary granule release from PMNs on PEG hydrogels, TCPS and GP hydrogels, but not in primary granule release from PMNs on PDMS. Because Src family kinases were involved in primary granule release from PMNs on TCPS, it does not appear that differences in Src family kinase-mediated signaling are responsible for the increased primary granule release from PMNs on PEG and GP hydrogels.
Figure 7. Adhesion-dependent PMN degranulation.
In Mac-1-mediated signaling, Src family kinases activate non-receptor tyrosine kinases Syk and Pyk2 by tyrosine phosphorylation.52–54 From top left to bottom right: TNF-induced release of secondary granules from adherent human PMNs is dependent on Mac-1-integrin-triggered oscillations of cytosolic free Ca2+.34 Src family kinases are involved in the spontaneous release of secondary granules from human PMNs adherent on TCPS.37 Syk is involved in the release of secondary granules from murine PMNs adherent on fibrinogen-coated surfaces in response to TNF stimulation.35 Pyk2 is involved in the TNF-induced release of secondary and tertiary granules from murine PMNs adherent on poly-RGD surfaces.36 Bold boxes: In this study, we found that Src family kinases are involved in the spontaneous release of primary granules from PMNs cultured on PEG hydrogels, TCPS and GP hydrogels and that Src family kinases are also involved in the spontaneous release of tertiary granules from PMNs cultured on PEG hydrogels, PDMS, TCPS, and GP hydrogels.
Differing serum protein adsorption on PEG and GP hydrogels compared to that on PDMS and TCPS may lead to the increased primary granule release from PMNs cultured on PEG and GP hydrogels. However, we did not observe a correlation between PMN adhesion and release of primary granules, suggesting that if there are unique serum protein adsorption profiles on PEG and GP hydrogels, they are not reflected in PMN adhesion on these materials. PMNs cultured on fibronectin-, fibrinogen- and albumin-coated surfaces showed minimal spontaneous primary granule release,38 suggesting that PMN adhesion to fibronectin, fibrinogen or albumin adsorbed on PEG and GP hydrogels is not responsible for the primary granule release observed in our study. In addition, Wang et al. showed that PEG hydrogels had significantly lower adsorption of vitronectin, thrombin, fibrinogen, and complement component C3 than TCPS,28 suggesting that these proteins are also not involved in the release of primary granules from PMNs cultured on PEG hydrogels. Differences in adsorption of PMN granule proteins or less abundant serum proteins on PEG-containing hydrogels may contribute to the increased primary granule release from PMNs on these materials. Unfortunately, studies investigating the adsorption of these less abundant proteins are challenging due to the interference of albumin and other highly abundant serum proteins.
PMNs cultured on PEG-containing hydrogels have different mechanisms of release for primary and tertiary granules
PEG-containing hydrogels (PEG and GP hydrogels) enhanced the release of MPO, but not MMP-9 from PMNs, suggesting that primary granule release from PMNs on PEG and GP hydrogels may be regulated differently than tertiary granule release. This is not surprising, as different signaling pathways have been implicated in degranulation of different granule subsets. For example, compared with wild-type murine PMNs in suspension, Rac2-deficient murine PMNs were unable to release primary granules in response to cytochalasin B (an inhibitor of actin filament polymerization) and fMLP treatment, but had normal secondary and tertiary granule release.39 In another example, Chakrabarti et al. demonstrated that blocking β2 integrins inhibited the release of secondary granules from TNF-stimulated human PMNs in suspension, but only partially attenuated the release of tertiary granules, suggesting that different pathways regulate secondary and tertiary granule release from suspended PMNs and that tertiary granule release uses β2-integrin-independent signaling pathways.40 If tertiary granule release in adherent PMNs also occurs independently of β2-integrin signaling pathways, we would not expect to observe major differences in MMP-9 release among the biomaterials due to their main differences involving PMN adhesion via β2 integrins.
Adhesion-dependent PMN activation may be more likely to induce the release of primary granules than tertiary granules due to the involvement of the actin cytoskeleton in both adhesion and primary granule release. Mitchell et al. showed that stabilization of filamentous actin in PMNs with jasplakinolide, an actin filament stabilizing drug, inhibited primary granule release, but did not affect secondary granule release.41 Additionally, another study showed that disruption of the cytoskeleton with cytochalasin B allowed for the release of primary granules from fMLP-treated PMNs in suspension.39 Rac2, a Rho GTPase involved in actin remodeling, has also been implicated in the release of primary granules from PMNs, but not in the release of secondary or tertiary granules.39,41–43 These data suggest that tertiary granule release is regulated by a different pathway than primary granule release, which does not involve Rac2-mediated signaling or actin cytoskeletal remodeling. Pathways involving protein kinase C α and δ (PKCα and PKCδ) have been shown to be involved in tertiary granule release from TNF-stimulated human PMNs in suspension.40
fMLP does not enhance degranulation from PMNs cultured on PEG-containing hydrogels or PDMS
fMLP is a bacterial peptide that activates PMNs through the fMLP receptor, a pertussis toxin-sensitive G-protein coupled receptor.44 We included fMLP treatment in the current study because others have suggested that full activation of PMNs requires two signals: 1) a signal generated from ligation of integrins and 2) a soluble pro-inflammatory stimulus (e.g., fMLP or TNF) or a signal from ligation of nonintegrin Fc receptors.35,45 For example, fMLP-induced primary granule release from human PMNs in suspension was increased in the presence of soluble fibrinogen, which binds to Mac-1, the main adhesion receptor in human PMNs.46 Additionally, Rainger et al. showed that fMLP induced the release of primary granules from surface-adherent PMNs, whereas no degranulation was observed from fMLP-treated PMNs in suspension.47 In agreement with the theory that two signals are needed for full activation of PMNs, in our studies, the addition of 100 nM fMLP to the culture medium significantly increased the release of primary granules from PMNs on TCPS at 2 hours. In contrast, at 2 hours on PEG hydrogels, PDMS and GP hydrogels and at 4 hours on all materials, we did not observe a significant increase in the release of primary granules upon addition of 100 nM fMLP to the culture medium, suggesting that maximal primary granule release from PMNs was reached independently of secondary fMLP-mediated signaling. Furthermore, fMLP did not enhance the release of tertiary granules from PMNs on any of the four biomaterials studied. Therefore, overall, our results indicate that biomaterial identity (i.e., physiochemical properties and/or associated proteins) largely dictates PMN activation and degranulation in lieu of the conventional “two-signal” activation mechanism.
Increased release of primary granules does not significantly enhance MC chemotaxis
We hypothesized that the increased release of primary granules from PMNs on PEG-containing hydrogels may enhance MC chemotaxis due to the presence of MC chemotactic proteins in primary granules (e.g., azurocidin, cathepsin G, HNP1–3).7,10,13,18 However, the increased release of primary granules from PEG-containing hydrogels did not promote significant MC chemotaxis, although slight elevations in MC chemotaxis were observed in response to PCM from PMN cultures on PEG hydrogels when compared with the 10% AHS culture medium baseline. In our previous studies, we observed only selective, modest increases in pro-inflammatory cytokine release from MCs treated with PCM from biomaterial-activated PMN cultures,19 despite other studies showing that PMN granule proteins enhance pro-inflammatory cytokine release from MCs.11,12,48 Taken together, these data suggest that serum antiproteases such as α1 antichymotrypsin in the 10% AHS culture medium may inactivate certain primary granules proteins such as HNP1–3, elastase and cathepsin G,13,22,49 preventing these granule proteins from promoting MC chemotaxis or enhancing pro-inflammatory cytokine release from MCs. Nonetheless, it is possible that the inflammatory effects of PMN primary granule proteins may exist in vivo at areas of close contact between PMNs and MCs that exclude serum antiproteases. Furthermore, in vivo, additional co-effectors in the complex inflammatory milieu are likely involved in modulating PMN-mediated MC recruitment.15 For example, PMN granule proteins can activate endothelial cells to express cell adhesion molecules that enhance MC adhesion and can also induce endothelial cells to produce MC-attracting chemokines.50,51
CONCLUSIONS
We have demonstrated that PEG-containing hydrogels promote the release of primary granules, but not tertiary granules from human blood-derived PMNs. Primary granules contain toxic microbicidal proteins, some of which are implicated in chronic inflammation. Divergent post-ligation signaling events of PMN integrin families and/or differing protein adsorption on the biomaterials may be responsible for the observed differences in the release of primary granules from PMNs cultured on PEG-containing hydrogels, as compared with PDMS or TCPS.
Supplementary Material
Supplementary Figure 1. PMN granule subsets. PMNs contain four subsets of granules: primary granules, secondary granules, tertiary granules and secretory vesicles, which are formed sequentially during myelopoeisis. Secretory vesicles are generally exocytosed most readily, followed by tertiary granules, secondary granules and lastly, primary granules.26 Characteristic proteins are used as markers of the granule subsets. For example, myeloperoxidase (MPO), elastase, and human neutrophil peptides 1–3 (HNP1–3) are markers of primary granules, lactoferrin is a marker of secondary granules, matrix metalloproteinase-9 (MMP-9) is a marker of tertiary granules, and CD35 (complement receptor 1) is a marker of secretory vesicles. Figure adapted from Amulic et al.55
Supplementary Figure 2. Viable and necrotic adherent cell densities in PMN cultures A) without fMLP and B) with 100 nM fMLP on PEG hydrogels, PDMS, TCPS and GP hydrogels at 1, 2, 4, 6, 12 and 24 hours as measured using calcein AM and ethidium homodimer-1 fluorescent stains. Results represent the mean + standard deviation, n = 2.
Supplementary Figure 3. Viable (A) and necrotic (B) adherent cell densities in PMN cultures pre-treated with 10 _M PP1 on PEG hydrogels, PDMS, TCPS and GP hydrogels at 2 hours as measured using calcein AM and ethidium homodimer-1 fluorescent stains. Results represent the mean ± standard deviation, n = 4.
Supplementary Figure 4. Cell viability in PMN cultures pre-treated with 10 _M PP1 on PEG hydrogels, PDMS, TCPS and GP hydrogels at 2 hours as measured by fluorescent intensity (FI) of cell metabolic capacity using CellTiter-Blue® Reagent. Results represent the mean ± standard deviation, n = 4. **: significant difference with PP1 pre-treatment, p = 0.001–0.01.
Supplementary Figure 5. PCM does not promote significant MC chemotaxis. Density of MCs (cells/mm2) that transmigrated across a polycarbonate filter with 5-_m diameter pores in 90 minutes in response to PCM from 2-hour cultures of autologous PMNs on PEG hydrogels, PDMS, TCPS and GP hydrogels without or with 100 nM fMLP. Negative and positive controls were 10% AHS in RPMI-1640 medium without fMLP and 10% AHS in RPMI-1640 medium with 50 or 100 nM fMLP, respectively. The dashed horizontal line indicates the mean density of transmigrated MCs in response to 10% AHS in RPMI-1640 medium. Results represent the mean ± standard deviation with each data point representing a different donor, n = 3.
ACKNOWLEDGMENTS
This work was supported in part by the Department of Defense through a National Defense Science and Engineering Graduate fellowship awarded to H.C. Cohen and through NIH HL115482. The authors thank David A. Cantu for his assistance with phlebotomy procedures and the University of Wisconsin Carbone Comprehensive Cancer Center for use of the Bio-Plex® suspension array system to complete this research.
REFERENCES
- 1.Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin. Immunol. 2008;20(2):86–100. doi: 10.1016/j.smim.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson JM. Inflammatory response to implants. ASAIO J. 1988;34(2):101–107. doi: 10.1097/00002480-198804000-00005. [DOI] [PubMed] [Google Scholar]
- 3.LeCabec V, Cowland JB, Calafat J, Borregaard N. Targeting of proteins to granule subsets is determined by timing and not by sorting: The specific granule protein NGAL is localized to azurophil granules when expressed in HL-60 cells. Proc. Natl. Acad. Sci. U. S. A. 1996;93(13):6454–6457. doi: 10.1073/pnas.93.13.6454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borregaard N, Lollike K, Kjeldsen L, Sengelov H, Bastholm L, Nielsen MH, Bainton DF. Human neutrophil granules and secretory vesicles. Eur. J. Haematol. 1993;51(4):187–198. doi: 10.1111/j.1600-0609.1993.tb00629.x. [DOI] [PubMed] [Google Scholar]
- 5.Lominadze G, Powell DW, Luerman GC, Link AJ, Ward RA, McLeish KR. Proteomic analysis of human neutrophil granules. Mol. Cell. Proteomics. 2005;4(10):1503–1521. doi: 10.1074/mcp.M500143-MCP200. [DOI] [PubMed] [Google Scholar]
- 6.Jethwaney D, Islam MR, Leidal KG, de Bernabe DBV, Campbell KP, Nauseef WM, Gibson BW. Proteomic analysis of plasma membrane and secretory vesicles from human neutrophils. Proteome Sci. 2007:5. doi: 10.1186/1477-5956-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soehnlein O, Zernecke A, Eriksson EE, Rothfuchs AG, Pham CT, Herwald H, BidzhekoV K, Rottenberg ME, Weber C, Lindbom L. Neutrophil secretion products pave the way for inflammatory monocytes. Blood. 2008;112(4):1461–1471. doi: 10.1182/blood-2008-02-139634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soehnlein O, Kai-Larsen Y, Frithiof R, Sorensen OE, Kenne E, Scharffetter-Kochanek K, Eriksson EE, Herwald H, Agerberth B, Lindbom L. Neutrophil primary granule proteins HBP and HNP1-3 boost bacterial phagocytosis by human and murine macrophages. J. Clin. Invest. 2008;118(10):3491–3502. doi: 10.1172/JCI35740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaly YV, Paleolog EM, Kolesnikova TS, Tikhonov II, Petratchenko EV, Voitenok NN. Human neutrophil alpha-defensin modulates cytokine production in human monocytes and adhesion molecule expression in endothelial cells. Eur. Cytokine Network. 2000;11(2):257–266. [PubMed] [Google Scholar]
- 10.Territo MC, Ganz T, Selsted ME, Lehrer R. Monocyte-chemotactic activity of defensins from human-neutrophils. J. Clin. Invest. 1989;84(6):2017–2020. doi: 10.1172/JCI114394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rasmussen PB, Bjorn S, Hastrup S, Nielsen PF, Norris K, Thim L, Wiberg FC, Flodgaard H. Characterization of recombinant human HBP/CAP37/azurocidin, a pleiotropic mediator of inflammation-enhancing LPS-induced cytokine release from monocytes. FEBS Lett. 1996;390(1):109–112. doi: 10.1016/0014-5793(96)00639-4. [DOI] [PubMed] [Google Scholar]
- 12.Heinzelmann M, Mercer-Jones MA, Flodgaard H, Miller FN. Heparin-binding protein (CAP37) is internalized in monocytes and increases LPS-induced monocyte activation. J. Immunol. 1998;160(11):5530–5536. [PubMed] [Google Scholar]
- 13.Chertov O, Ueda H, Xu LL, Tani K, Murphy WJ, Wang JM, Howard OMZ, Sayers TJ, Oppenheim JJ. Identification of human neutrophil-derived cathepsin G and azurocidin/CAP37 as chemoattractants for mononuclear cells and neutrophils. J. Exp. Med. 1997;186(5):739–747. doi: 10.1084/jem.186.5.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soehnlein O, Weber C, Lindbom L. Neutrophil granule proteins tune monocytic cell function. Trends Immunol. 2009;30(11):546–556. doi: 10.1016/j.it.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 15.Soehnlein O, Lindbom L, Weber C. Mechanisms underlying neutrophil-mediated monocyte recruitment. Blood. 2009;114(21):4613–4623. doi: 10.1182/blood-2009-06-221630. [DOI] [PubMed] [Google Scholar]
- 16.Soehnlein O, Zernecke A, Weber C. Neutrophils launch monocyte extravasation by release of granule proteins. Thromb. Haemostasis. 2009;102(2):198–205. doi: 10.1160/TH08-11-0720. [DOI] [PubMed] [Google Scholar]
- 17.Soehnlein O. Direct and alternative antimicrobial mechanisms of neutrophil-derived granule proteins. J. Mol. Med. (Heidelberg, Ger.) 2009;87(12):1157–1164. doi: 10.1007/s00109-009-0508-6. [DOI] [PubMed] [Google Scholar]
- 18.Soehnlein O, Lindbom L. Neutrophil-derived azurocidin alarms the immune system. J. Leukocyte Biol. 2009;85(3):344–351. doi: 10.1189/jlb.0808495. [DOI] [PubMed] [Google Scholar]
- 19.Cohen HC, Joyce EJ, Kao WJ. Biomaterials selectively modulate interactions between human blood-derived polymorphonuclear leukocytes and monocytes. Am. J. Pathol. 2013;182(6):2180–2190. doi: 10.1016/j.ajpath.2013.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doring G. The role of neutrophil elastase in chronic inflammation. Am. J. Respir. Crit. Care Med. 1994;150(6):S114–S117. doi: 10.1164/ajrccm/150.6_Pt_2.S114. [DOI] [PubMed] [Google Scholar]
- 21.Ahrens D, Koch AE, Pope RM, Stein-Picarella M, Niedbala MJ. Expression of matrix metalloproteinase 9 (96-kd gelatinase B) in human rheumatoid arthritis. Arthritis Rheum. 1996;39(9):1576–1587. doi: 10.1002/art.1780390919. [DOI] [PubMed] [Google Scholar]
- 22.Janusz MJ, Doherty NS. Degradation of cartilage matrix proteoglycan by human neutrophils involves both elastase and cathepsin-G. J. Immunol. 1991;146(11):3922–3928. [PubMed] [Google Scholar]
- 23.Xu KD, Fu Y, Chung WJ, Zheng XX, Cui YJ, Hsu IC, Kao WJ. Thiol-ene-based biological/synthetic hybrid biomatrix for 3-D living cell culture. Acta Biomater. 2012;8(7):2504–2516. doi: 10.1016/j.actbio.2012.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Waldeck H, Wang XT, Joyce E, Kao WJ. Active leukocyte detachment and apoptosis/necrosis on PEG hydrogels and the implication in the host inflammatory response. Biomaterials. 2012;33(1):29–37. doi: 10.1016/j.biomaterials.2011.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takafuji S, Ishida A, Miyakuni Y, Nakagawa T. Matrix metalloproteinase-9 release from human leukocytes. J. Invest. Allergol. Clin. Immunol. 2002;13(1):50–55. [PubMed] [Google Scholar]
- 26.Faurschou M, Sorensen OE, Johnsen AH, Askaa J, Borregaard N. Defensin-rich granules of human neutrophils: characterization of secretory properties. Biochim. Biophys. Acta, Mol. Cell Res. 2002;1591(1–3):29–35. doi: 10.1016/s0167-4889(02)00243-4. [DOI] [PubMed] [Google Scholar]
- 27.Kingshott P, Griesser HJ. Surfaces that resist bioadhesion. Curr. Opin. Solid State Mater. Sci. 1999;4(4):403–412. [Google Scholar]
- 28.Wang XT, Schmidt DR, Joyce EJ, Kao WJ. Application of MS-based proteomics to study serum protein adsorption/absorption and complement C3 activation on poly(ethylene glycol) hydrogels. J. Biomater. Sci., Polym. Ed. 2011;22(10):1343–1362. doi: 10.1163/092050610X508400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alcantar NA, Aydil ES, Israelachvili JN. Polyethylene glycol-coated biocompatible surfaces. J. Biomed. Mater. Res. 2000;51(3):343–351. doi: 10.1002/1097-4636(20000905)51:3<343::aid-jbm7>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 30.Lee JH, Lee HB, Andrade JD. Blood compatibility of polyethylene oxide surfaces. Prog. Polym. Sci. 1995;20(6):1043–1079. [Google Scholar]
- 31.Lynn AD, Kyriakides TR, Bryant SJ. Characterization of the in vitro macrophage response and in vivo host response to poly(ethylene glycol)-based hydrogels. J. Biomed. Mater. Res., Part A. 2010;93A(3):941–953. doi: 10.1002/jbm.a.32595. [DOI] [PubMed] [Google Scholar]
- 32.Schmidt DR, Kao WJ. Monocyte activation in response to polyethylene glycol hydrogels grafted with RGD and PHSRN separated by interpositional spacers of various lengths. J. Biomed. Mater. Res., Part A. 2007;83A(3):617–625. doi: 10.1002/jbm.a.31270. [DOI] [PubMed] [Google Scholar]
- 33.Ngsikorski J, Andersson R, Patarroyo M, Andersson T. Calcium signaling capacity of the CD11b/CD18 integrin on human neutrophils. Exp. Cell Res. 1991;195(2):504–508. doi: 10.1016/0014-4827(91)90402-g. [DOI] [PubMed] [Google Scholar]
- 34.Richter J, Ngsikorski J, Olsson I, Andersson T. Tumor necrosis factor-induced degranulation in adherent human neutrophils is dependent on CD11b/CD18-integrin-triggered oscillations of cytosolic free Ca2+ Proc. Natl. Acad. Sci. U. S. A. 1990;87(23):9472–9476. doi: 10.1073/pnas.87.23.9472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mocsai A, Zhou MJ, Meng FY, Tybulewicz VL, Lowell CA. Syk is required for integrin signaling in neutrophils. Immunity. 2002;16(4):547–558. doi: 10.1016/s1074-7613(02)00303-5. [DOI] [PubMed] [Google Scholar]
- 36.Kamen LA, Schlessinger J, Lowell CA. Pyk2 is required for neutrophil degranulation and host defense responses to bacterial infection. J. Immunol. 2011;186(3):1656–1665. doi: 10.4049/jimmunol.1002093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mocsai A, Ligeti E, Lowell CA, Berton G. Adhesion-dependent degranulation of neutrophils requires the Src family kinases Fgr and Hck. J. Immunol. 1999;162(2):1120–1126. [PubMed] [Google Scholar]
- 38.Xu X, Hakansson L. Degranulation of primary and secondary granules in adherent human neutrophils. Scand. J. Immunol. 2002;55(2):178–188. doi: 10.1046/j.1365-3083.2002.01041.x. [DOI] [PubMed] [Google Scholar]
- 39.Abdel-Latif D, Steward M, Macdonald DL, Francis GA, Dinauer MC, Lacy P. Rac2 is critical for neutrophil primary granule exocytosis. Blood. 2004;104(3):832–839. doi: 10.1182/blood-2003-07-2624. [DOI] [PubMed] [Google Scholar]
- 40.Chakrabarti S, Zee JM, Patel KD. Regulation of matrix metalloproteinase-9 (MMP-9) in TNF-stimulated neutrophils: novel pathways for tertiary granule release. J. Leukocyte Biol. 2006;79(1):214–222. doi: 10.1189/jlb.0605353. [DOI] [PubMed] [Google Scholar]
- 41.Mitchell T, Lo A, Logan MR, Lacy P, Eitzen G. Primary granule exocytosis in human neutrophils is regulated by Rac-dependent actin remodeling. Am. J. Physiol.: Cell Physiol. 2008;295(5):C1354–C1365. doi: 10.1152/ajpcell.00239.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ambruso DR, Knall C, Abell AN, Panepinto J, Kurkchubasche A, Thurman G, Gonzalez-Aller C, Hiester A, deBoer M, Harbeck RJ, et al. Human neutrophil immunodeficiency syndrome is associated with an inhibitory Rac2 mutation. Proc. Natl. Acad. Sci. U. S. A. 2000;97(9):4654–4659. doi: 10.1073/pnas.080074897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abdel-Latif D, Steward M, Lacy P. Neutrophil primary granule release and maximal superoxide generation depend on Rac2 in a common signalling pathway. Can. J. Physiol. Pharmacol. 2005;83(1):69–75. doi: 10.1139/y04-123. [DOI] [PubMed] [Google Scholar]
- 44.Walker BA, Hagenlocker BE, Stubbs EB, Sandborg RR, Agranoff BW, Ward PA. Signal transduction events and Fc gamma R engagement in human neutrophils stimulated with immune complexes. J. Immunol. 1991;146(2):735–741. [PubMed] [Google Scholar]
- 45.Jakus Z, Berton G, Ligeti E, Lowell CA, Mocsai A. Responses of neutrophils to anti-integrin antibodies depends on costimulation through low affinity Fc gamma Rs: Full activation requires both integrin and nonintegrin signals. J. Immunol. 2004;173(3):2068–2077. doi: 10.4049/jimmunol.173.3.2068. [DOI] [PubMed] [Google Scholar]
- 46.Tuluc F, Garcia A, Bredetean O, Meshki J, Kunapuli SP. Primary granule release from human neutrophils is potentiated by soluble fibrinogen through a mechanism depending on multiple intracellular signaling pathways. Am. J. Physiol.: Cell Physiol. 2004;287(5):C1264–C1272. doi: 10.1152/ajpcell.00177.2004. [DOI] [PubMed] [Google Scholar]
- 47.Rainger GE, Rowley AF, Nash GB. Adhesion-dependent release of elastase from human neutrophils in a novel, flow-based model: Specificity of different chemotactic agents. Blood. 1998;92(12):4819–4827. [PubMed] [Google Scholar]
- 48.Chaly YV, Paleolog EM, Kolesnikova TS, Tikhonov II, Petratchenko EV, Voitenok NN. Human neutrophil alpha-defensin modulates cytokine production in human monocytes and adhesion molecule expression in endothelial cells. Eur. Cytokine Network. 2000;11(2):257–266. [PubMed] [Google Scholar]
- 49.Panyutich AV, Hiemstra PS, Vanwetering S, Ganz T. Human neutrophil defensing and serpins form complexes and inactivate each other. Am. J. Respir. Cell Mol. Biol. 1995;12(3):351–357. doi: 10.1165/ajrcmb.12.3.7873202. [DOI] [PubMed] [Google Scholar]
- 50.Taekema-Roelvink MEJ, Van Kooten C, Van der Kooij S, Heemskerk E, Daha MR. Proteinase 3 enhances endothelial monocyte chemoattractant protein-1 production and induces increased adhesion of neutrophils to endothelial cells by upregulating intercellular cell adhesion molecule-1. J. Am. Soc. Nephrol. 2001;12(5):932–940. doi: 10.1681/ASN.V125932. [DOI] [PubMed] [Google Scholar]
- 51.Lee TD, Gonzalez ML, Kumar P, Grammas P, Pereira HA. CAP37, a neutrophil-derived inflammatory mediator, augments leukocyte adhesion to endothelial monolayers. Microvasc. Res. 2003;66(1):38–48. doi: 10.1016/s0026-2862(03)00010-4. [DOI] [PubMed] [Google Scholar]
- 52.Mocsai A, Abram CL, Jakus Z, Hu YM, Lanier LL, Lowell CA. Integrin signaling in neutrophils and macrophages uses adaptors containing immunoreceptor tyrosine-based activation motifs. Nat. Immunol. 2006;7(12):1326–1333. doi: 10.1038/ni1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schymeinsky J, Mocsai A, Walzog B. Neutrophil activation via beta(2) integrins (CD11/CD18): Molecular mechanisms and clinical implications. Thromb. Haemostasis. 2007;98(2):262–273. [PubMed] [Google Scholar]
- 54.Lowell CA. Src-family and Syk kinases in activating and inhibitory pathways in innate immune cells: Signaling cross talk. Cold Spring Harbor Perspect. Biol. 2011;3(3) doi: 10.1101/cshperspect.a002352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Amulic B, Cazalet C, Hayes GL, Metzler KD, Zychlinsky A. Neutrophil function: From mechanisms to disease. Annu. Rev. Immunol. 2012;30:459–489. doi: 10.1146/annurev-immunol-020711-074942. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. PMN granule subsets. PMNs contain four subsets of granules: primary granules, secondary granules, tertiary granules and secretory vesicles, which are formed sequentially during myelopoeisis. Secretory vesicles are generally exocytosed most readily, followed by tertiary granules, secondary granules and lastly, primary granules.26 Characteristic proteins are used as markers of the granule subsets. For example, myeloperoxidase (MPO), elastase, and human neutrophil peptides 1–3 (HNP1–3) are markers of primary granules, lactoferrin is a marker of secondary granules, matrix metalloproteinase-9 (MMP-9) is a marker of tertiary granules, and CD35 (complement receptor 1) is a marker of secretory vesicles. Figure adapted from Amulic et al.55
Supplementary Figure 2. Viable and necrotic adherent cell densities in PMN cultures A) without fMLP and B) with 100 nM fMLP on PEG hydrogels, PDMS, TCPS and GP hydrogels at 1, 2, 4, 6, 12 and 24 hours as measured using calcein AM and ethidium homodimer-1 fluorescent stains. Results represent the mean + standard deviation, n = 2.
Supplementary Figure 3. Viable (A) and necrotic (B) adherent cell densities in PMN cultures pre-treated with 10 _M PP1 on PEG hydrogels, PDMS, TCPS and GP hydrogels at 2 hours as measured using calcein AM and ethidium homodimer-1 fluorescent stains. Results represent the mean ± standard deviation, n = 4.
Supplementary Figure 4. Cell viability in PMN cultures pre-treated with 10 _M PP1 on PEG hydrogels, PDMS, TCPS and GP hydrogels at 2 hours as measured by fluorescent intensity (FI) of cell metabolic capacity using CellTiter-Blue® Reagent. Results represent the mean ± standard deviation, n = 4. **: significant difference with PP1 pre-treatment, p = 0.001–0.01.
Supplementary Figure 5. PCM does not promote significant MC chemotaxis. Density of MCs (cells/mm2) that transmigrated across a polycarbonate filter with 5-_m diameter pores in 90 minutes in response to PCM from 2-hour cultures of autologous PMNs on PEG hydrogels, PDMS, TCPS and GP hydrogels without or with 100 nM fMLP. Negative and positive controls were 10% AHS in RPMI-1640 medium without fMLP and 10% AHS in RPMI-1640 medium with 50 or 100 nM fMLP, respectively. The dashed horizontal line indicates the mean density of transmigrated MCs in response to 10% AHS in RPMI-1640 medium. Results represent the mean ± standard deviation with each data point representing a different donor, n = 3.