Abstract
Aging is a common risk factor of many disorders. With age, the level of insoluble extracellular matrix increases leading to increased stiffness of a number of tissues. Matrix accumulation can also be observed in fibrotic disorders, such as systemic sclerosis (SSc). Although the intrinsic aging process in skin is phenotypically distinct from SSc, here we demonstrate similar behavior of aged and SSc skin fibroblasts in culture. We have used quantitative proteomics to characterize the phenotype of dermal fibroblasts from healthy subjects of various ages and from patients with SSc. Our results demonstrate that proteins involved in DNA and RNA processing decrease with age and in SSc, while those involved in mitochondrial and other metabolic processes behave the opposite. Specifically, mini-chromosome maintenance (MCM) helicase proteins are less abundant with age and SSc, and they exhibit an altered subcellular distribution. We observed that lower levels of MCM7 correlate with reduced cell proliferation, lower autophagic capacity and higher intracellular protein expression phenotypes of aged and SSc cells. Additionally, we show that SSc fibroblasts exhibit higher levels of senescence than their healthy counterparts, suggesting further similarities between the fibrotic disorder and the aging process. Hence, at the molecular level, SSc fibroblasts exhibit intrinsic characteristics of fibroblasts from aged skin.
Keywords: systemic sclerosis, aging, skin fibrosis, autophagy, fibroblasts, mass spectrometry, proteomics
Introduction
Aging is the common, predisposing feature of numerous diseases, including diabetes, cardiovascular dysfunction, cancer, dementia and Alzheimer’s disease, and it afflicts all individuals in a society and the society itself at large (Grayson, 2012). In the last century, with an increase in average life expectancy by ~30 years (Niccoli and Partridge, 2012), a change of attitude towards aging has been noted: The understanding of the aging process is fundamental to promote health. Cutaneous aging, which has both genetic and environmental components, has been classified into intrinsic and extrinsic aging (Jenkins, 2002; Uitto, 2008). Intrinsic aging of the skin proceeds similarly to that of most internal organs (Jenkins, 2002), and respective research is likely to be transferable and even directly applicable to aging phenomena in general.
Although aged skin is characterized by a loss of collagens and other extracellular matrix (ECM) components, which leads to its thinning (Papakonstantinou et al., 2012), most other organs display increased levels of insoluble ECM with age, resulting in progressive stiffening and loss of elasticity of the tissue (Monnier et al., 2005). These symptoms are also a key feature of cutaneous fibrotic diseases, systemic sclerosis (SSc) being the prototype of such disorders. A characteristic feature of SSc is the presence of fibroblasts with an activated phenotype, which display an increased expression of genes coding for ECM proteins and actin, while the expression of genes encoding ECM degrading proteins is reduced (Rosenbloom et al., 2010). As a consequence of these changes, excessive deposition of collagen and other ECM components typically occurs in the skin and other organs, primarily in the lung and kidneys, and accumulation of connective tissue leads to subsequent organ dysfunction (Varga et al., 1994). However, the signals responsible for fibroblast activation and the mechanisms involved in the propagation of the disease phenotype remain largely unknown (Gerber et al., 2013). In fibroblasts, senescence may also lead to activation accompanied by the senescence-associated secretory phenotype (SASP) (Freund et al., 2010), which leads to the production and release of pro-inflammatory factors (Newgard & Sharpless, 2013). Despite the unclear connection between SASP and SSc, chronically inflamed tissue is characterized, among other features, by fibrosis (Freund et al., 2010).
Increased protein deposition in fibrosis could reflect increased protein synthesis, decreased protein degradation, or a combination of these events. A distinct characteristic of aging cells is the sustained accumulation of defective macromolecules and organelles, and deterioration of intracellular degradative processes with age is well documented (Vellai, 2009). Thus, it has been suggested that enhancing the mechanisms responsible for clearance of damaged macromolecules may mitigate the consequences of aging (Ravikumar et al., 2010). A key system in cellular degradative processes is autophagy, a constitutive, homeostatic mechanism responsible for controlled degradation and recycling of cytoplasmic components, macromolecules, and organelles within lysosomes (Dumit and Dengjel, 2012). Cell stresses, such as nutrient starvation, stimulate autophagy by inhibiting the mammalian target of rapamycin (MTOR) (Choi et al., 2013). Double-membrane vesicles, known as autophagosomes, sequester macromolecules and organelles targeted for degradation, and subsequent fusion with lysosomes leads to degradation of the autophagosomal content. Dysregulation of this catabolic mechanism has been implicated in aging as well as in several age-dependent pathologies, including cancer and neurodegenerative, infectious, cardiovascular, metabolic and pulmonary diseases. Further evidence in support of a connection between aging and autophagy is the plethora of environmental factors influencing both processes. Conditions, such as starvation, hyperthermia and hypoxia, which have proven to extend life span of model organisms, are also inducers of autophagy (Bellot et al., 2009, Chen et al., 2008), suggesting a causative connection between autophagy and aging (Vellai, 2009). Additional studies on model organisms have pointed out that activation of autophagy extends the life-span while its inhibition produces opposite results (Vellai, 2009; Ravikumar et al., 2010).
To identify potentially causative proteins involved in skin aging and SSc, we performed unbiased, quantitative mass spectrometry (MS)-based proteomics analyses of proteomes of fibroblasts cultured from skin of SSc patients and from healthy control individuals of varying ages. MS is the method of choice to study protein dynamics in a global manner (Engelke et al., 2012; Zimmermann et al., 2010). Depending on the experimental setup, MS allows the determination of differences in protein abundance (Küttner et al., 2013; Sprenger et al., 2013b), organellar proteomes (Dengjel et al., 2012), and posttranslational modification states (Akimov et al., 2011). By employing the method of Stable Isotope Labeling by Amino acids in Cell culture (SILAC) to metabolically label primary human skin fibroblasts (Sprenger et al., 2013a, 2010) we identified proteins and cellular pathways that were deregulated in aged and SSc fibroblasts, with focus on autophagy.
Results
Proteomic changes in aging fibroblasts
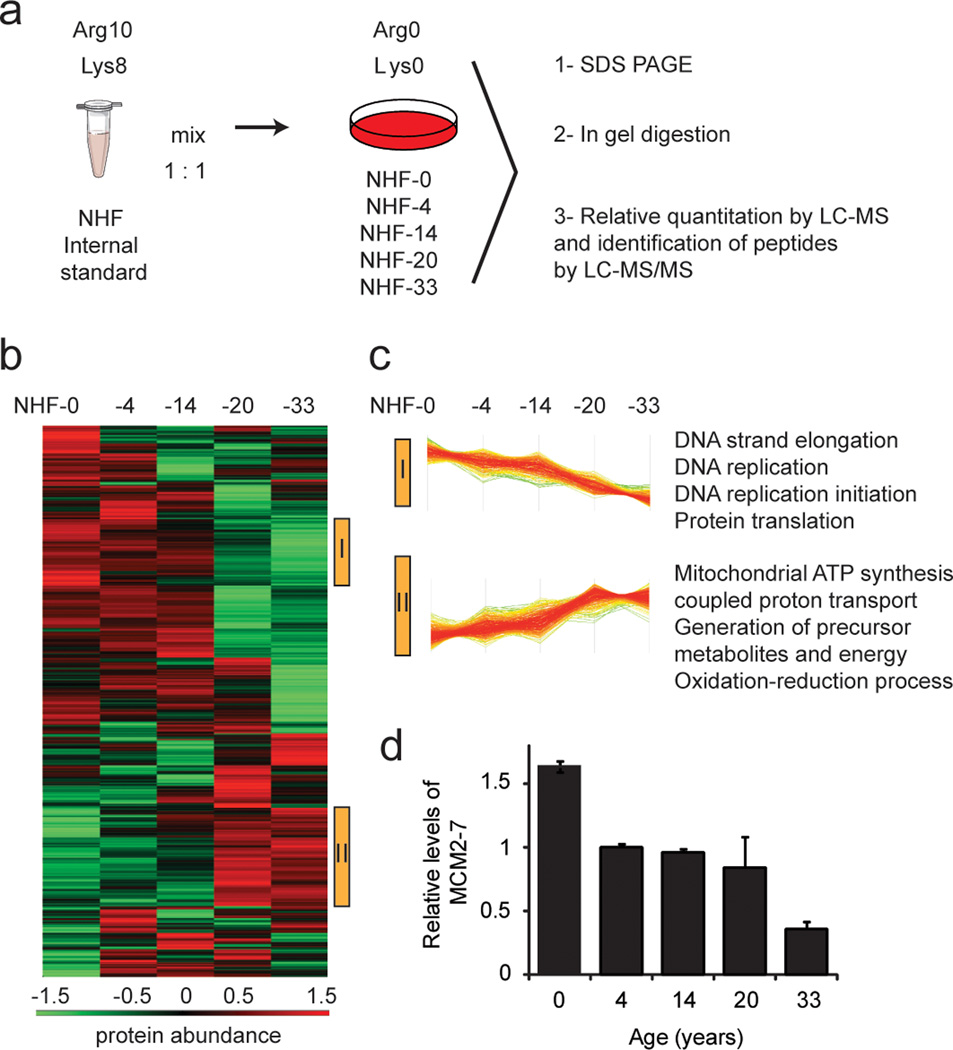
We first analyzed the intracellular proteome of primary, normal human dermal fibroblasts (NHF) from five healthy donors of 0, 4, 14, 20 and 33 years of age (see Table 1) employing SILAC-MS. In this procedure, fibroblasts of the same passage number were lysed and combined with cell lysate of a fully, “heavy-labeled” fibroblast control as an internal standard (Figure 1-a, Table 1). The common internal control allowed us to compare protein abundance differences between all five primary cells. In total, we quantified 3,082 proteins, of which 1,542 were quantified in all five samples (Supplementary Table 1, Supplementary Figure 1), by a minimum of one unique peptide with a false discovery rate being <1% on protein and peptide level. Protein ratios were analyzed by hierarchical clustering to identify proteins exhibiting abundance dynamics correlating with age (Figure 1-b). We identified two clusters in which proteins constantly decreased (cluster I) or increased (cluster II) with advancing age from 0 to 33 years, comprising 186 and 277 proteins, respectively. Figure 1-c shows selected, enriched GO terms in the respective clusters relative to the total dataset (see also Supplementary Table 2). Proteins which increased with age were components of different organelles, such as the endoplasmic reticulum, lysosomes and peroxisomes. Metabolic pathways, such as mitochondrial ATP synthesis, proton transport, purine ribonucleoside triphosphate biosynthetic pathway, protein glycosylation and protein folding were upregulated with age. On the other hand, proteins whose abundance consistently decreased with age carried mostly nuclear annotations, particularly nucleosome, chromatin and telomeres, and they were components of processes involving nucleic acids, such as DNA replication, RNA metabolism and mRNA translation. These observations suggest a lower proliferative capacity of older cells. Among proteins that declined with age (cluster I) we found all six members of the MCM helicase complex (Figure 1-d; Supplementary Table 1), a complex important for DNA replication during cell cycle, and we subsequently focused on studies to examine their involvement in the aging process.
Table 1.
Fibroblasts used in this study.
| Cells used to study chronological aging | Cells used to study SSc | ||||||
|---|---|---|---|---|---|---|---|
| Cell ID |
Donor’s age |
Body part |
Gender | Cell ID |
Donor’s age |
Bodypart | Gender |
| NHF-0 | 0 | foreskin | male | SSc-A | 52 | extensor forearm | female |
| NHF-4 | 4 | foreskin | male | SSc-B | 47 | extensor forearm | female |
| NHF-14 | 14 | axilla | female | SSc-C | 49 | extensor forearm | female |
| NHF-20 | 20 | left thigh | female | NHF-A | 47 | extensor forearm | female |
| NHF-33 | 33 | genital ridge | female | NHF-B | 29 | extensor forearm | female |
| NHF-44 | 44 | abdomen | male | NHF-C | 33 | extensor forearm | female |
| NHF-49 | 49 | back | female | ||||
| NHF-56 | 56 | abdomen | female | ||||
| NHF-60 | 60 | gluteus | n.d. | ||||
| NHF-69 | 69 | neck | male | ||||
Figure 1. Proteomics analysis of skin fibroblasts from donors of different ages: 0, 4, 14, 20 and 33 years.
(a) Work-flow followed to investigate proteome changes of fibroblasts. A “heavy” (Arg10, Lys8) labeled primary human fibroblast standard was spiked in 1:1 ratios to “light” (Arg0, Lys0) labeled samples. Lysates were separated by SDS-PAGE, proteins were digested by trypsin in gel, and the resulting peptide mixtures were analyzed by reversed-phase LC-MS/MS. (b) Hierarchical clustering of protein ratios. Samples were ordered according to the age of the donors. Protein ratios were log2 transformed and z score normalized prior to hierarchical clustering. Two clusters were identified with proteins that consistently decreased (cluster I) or increased (cluster II) with age, which are marked by yellow bars on the right side of the heat map. Bottom: color scale indicates relative protein abundance values. (c) Line diagrams of identified clusters, each line representing one protein. GO terms of proteins enriched more than 1.5 fold in the highlighted clusters are explicitly mentioned (p<0.05). (d) Average relative levels of MCM complex proteins (MCM2-MCM7), as quantified by MS. Primary cells were compared to internal standard. Error bars indicate S.E.M. of all protein complex members (n = 5).
Characterization of the aging phenotype in skin fibroblasts
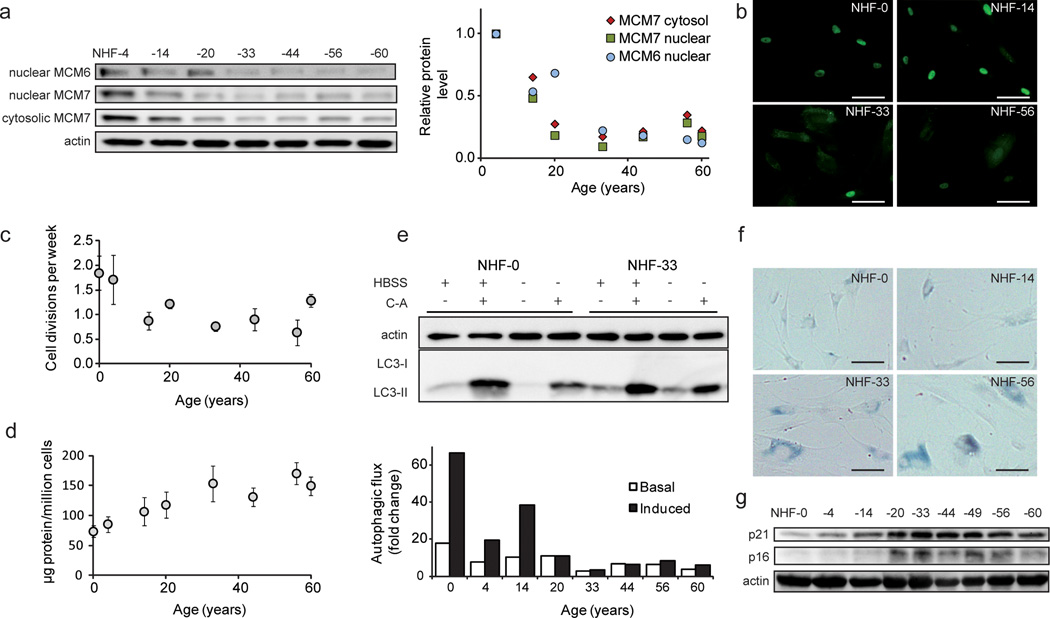
As a follow-up of the SILAC-MS results (Figure 1d), we analyzed MCM6 and MCM7 levels in NHF from donors of 4 to 60 years of age by Western blot (Figure 2-a). MCM7 protein levels decreased up to 30 years of age and remained essentially stable until the age of 60 years, both in the cytosolic and nuclear fractions (Figure 2-a). MCM6 remained undetected in the cytosolic fraction and followed the MCM7 levels in the nuclear fraction. Thus, consistent with the SILAC-MS data, Western analyses confirmed that the cellular/nuclear levels of MCM proteins displayed a consistent, age-dependent decrease in abundance. Additionally, MCM7 immunostaining (Figure 2-b and Supplementary Figure 2) was in agreement with the Western blots, and suggested that MCM7 shifts its localization from the nucleus to the cytosol with increasing age. Since MCM proteins distribute in a cell cycle dependent manner, entering the nucleus during the S-phase and leaving shortly after DNA replication (Nguyen et al., 2000), the observed heterogeneity in subcellular localization may be linked to different cell cycle states of respective cells.
Figure 2. Phenotype of skin fibroblasts from donors of varying ages.
(a) Relative levels of MCM6 and MCM7 as detected by Western blot. Samples were normalized to protein amount, and actin is shown as loading control. The diagram shows quantitation relative to NHF-4. (b) Microscopy pictures of MCM7 immunostaining of cells of different ages. Scale bars = 100 µm. (c) Proliferation rates, calculated as cell divisions per week (n = 3). (d) Total protein concentration in whole cell lysates. (e) Analysis of autophagic fluxes under amino acid starvation (induced) and control conditions (basal). LC3-II levels of samples treated with and without concanamycin A (C-A) were quantified and normalized to actin. A representative Western blot is depicted (see Supplementary Figure 3 for further analyses). Fibroblasts exhibit increased senescence with age, as detected by (f) β-galactosidase activity, and (g) p16 and p21 levels.
Since the MCM proteins are involved in cellular proliferation (Freeman et al., 1999), we evaluated the proliferative capacity of primary fibroblasts from individuals of different ages. As expected, with increasing age the cells divided less frequently, consistent with reduced levels of MCM proteins (Figure 2-c), particularly in the nucleus (Figure 2-b). We determined protein levels in whole cell lysates of NHF obtained from donors of different ages, and calculated protein concentrations per million cells. Figure 2-d shows the total protein concentration of fibroblasts in culture, highlighting a clear increase of protein content in cells from older donors. According to our SILAC-MS results, mRNA translation mechanisms were down-regulated (Figure 1-c), suggesting that accumulation of proteins reflected an impairment of degradative processes, although the presence of proteins of lytic organelles, e.g. lysosomes and peroxisomes, was also augmented (Supplementary Table 2).
To assess the autophagic capacities of the cells, we assigned the fibroblasts from individuals of different ages to four different treatments: no nutritional restriction (normal growth medium), or amino acid starvation conditions (HBSS medium), both with and without concanamycin A (C-A), an inhibitor of the lysosomal H+-ATPase that blocks lysosomal degradation and thus enables the analysis of autophagic flux (Klionsky et al., 2012). Amino acid starvation stimulates autophagy inducing degradation of proteins to compensate for the lack of nutrients (Zimmermann et al., 2010). Figure 2-e and Supplementary Figure 3 depict the age-dependent decrease of autophagic flux under normal conditions and, to a greater extent, under amino acid starvation. Notably, the responsiveness to nutritional restriction was remarkably high in cells from young donors compared to cells from older donors. Older cells responded less to nutritional stress and exhibited an increased basal level of LC3-II under normal conditions, which indicates a partial block of autophagy. This could also be shown by image analyses employing LC3 immunostaining (Supplementary Figure 4). As expected, fibroblasts of increasing age also exhibited boosted cellular senescence: β-galactosidase activity (Figure 2-f) and the levels of the cell cycle regulating proteins, p21 (CDKN1A) and p16 (CDKN2A) (Figure 2-g), were higher in older fibroblasts compared to younger cells.
Collectively, we conclude that the aging phenotype of fibroblasts in culture involves lower abundance of MCM proteins, a redistribution of MCM proteins, lower proliferation rates, general protein accumulation, a lower autophagic capacity, and an increasing level of senescence.
Characterization of the molecular phenotype of systemic sclerosis in skin fibroblasts
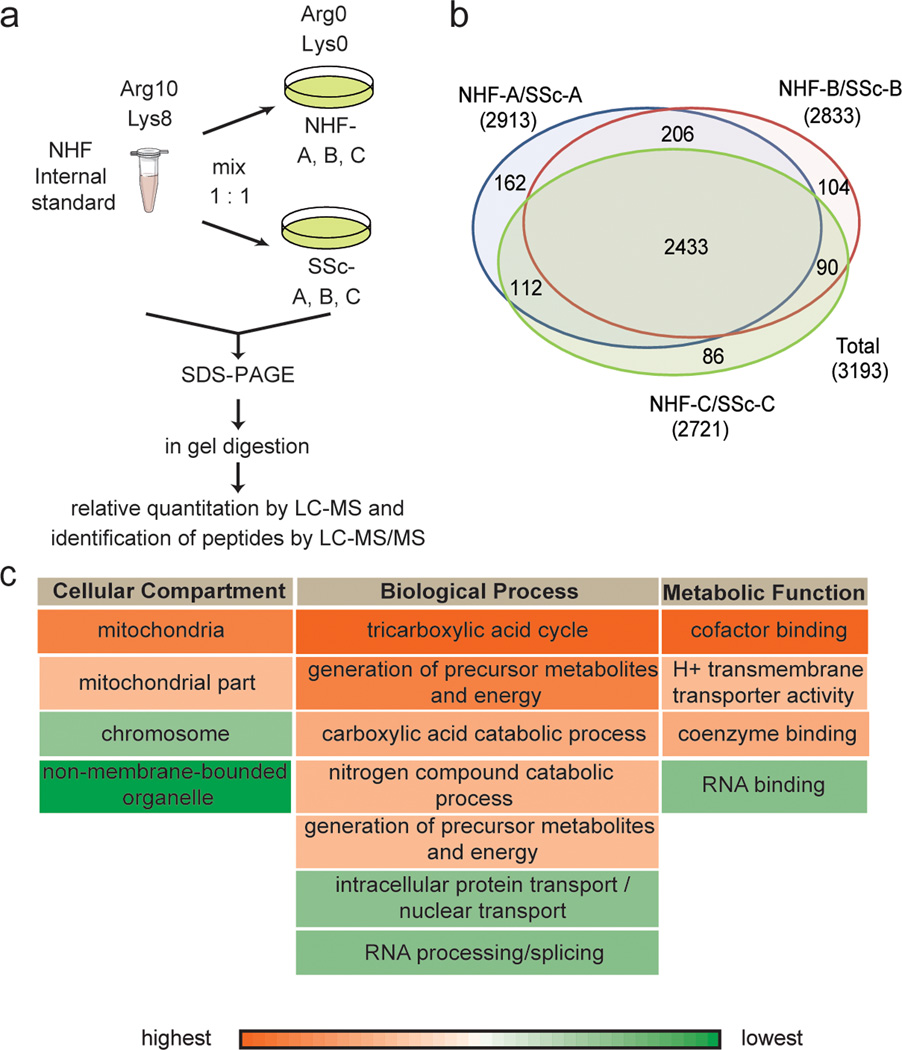
To investigate molecular characteristics of SSc, the following primary fibroblasts were included in this study: SSc-A, SSc-B and SSc-C from individuals with SSc, while NHF-A, NHF-B and NHF-C were age, gender and biopsy site matched control fibroblasts, respectively (Table 1 and Supplementary Information). To compare intracellular proteomes of SSc and control fibroblasts, we followed a procedure similar to that described above. Specifically, we mixed whole cell lysate of SSc or control fibroblasts to a fully labeled NHF internal standard in 1:1 ratio (Figure 3-a).
Figure 3. Proteomics analysis of SSc fibroblasts by MS.
(a) Work-flow followed to compare proteomes of SSc and control fibroblasts. Fully labeled control NHF were used as an internal standard to combine abundance values. (b) Venn Diagram represents the number of detected proteins in each experiment (two biological replicates each). (c) Selected GO terms of significantly altered proteins in SSc fibroblasts as detected by DAVID are shown (p<0.05). Red highlights up-regulated and green down-regulated protein abundance.
In two biological replicates each (see Supplementary Figure 5), giving rise to a set of 12 experiments with fibroblasts from three patients and three matching controls, we quantified almost 3,200 proteins. Of these, 2,433 could be quantified in all the three matching pairs, i.e., over 75% of protein ratios were calculated for the three pairs (Figure 3-b and Supplementary Table 3). An analysis of variance (ANOVA) was performed to identify the differentially expressed proteins. This disclosed 66 up-regulated and 82 down-regulated proteins between the patient and control samples (p<0.05; see Supplementary Table 4). Significantly changed proteins were processed by DAVID Bioinformatics resources 6.7 (Huang et al., 2009a, 2009b) to detect enriched GO terms by functional annotation clustering (Figure 3-c; enrichment score >1.5; p<0.05). Proteins up-regulated in SSc were involved in energy metabolism, while those down-regulated belong to DNA/RNA processes. Hence, there was a similarly altered pattern of cellular pathways in both SSc and aging fibroblasts, as represented in Figure 3-c and Figure 1-c, respectively.
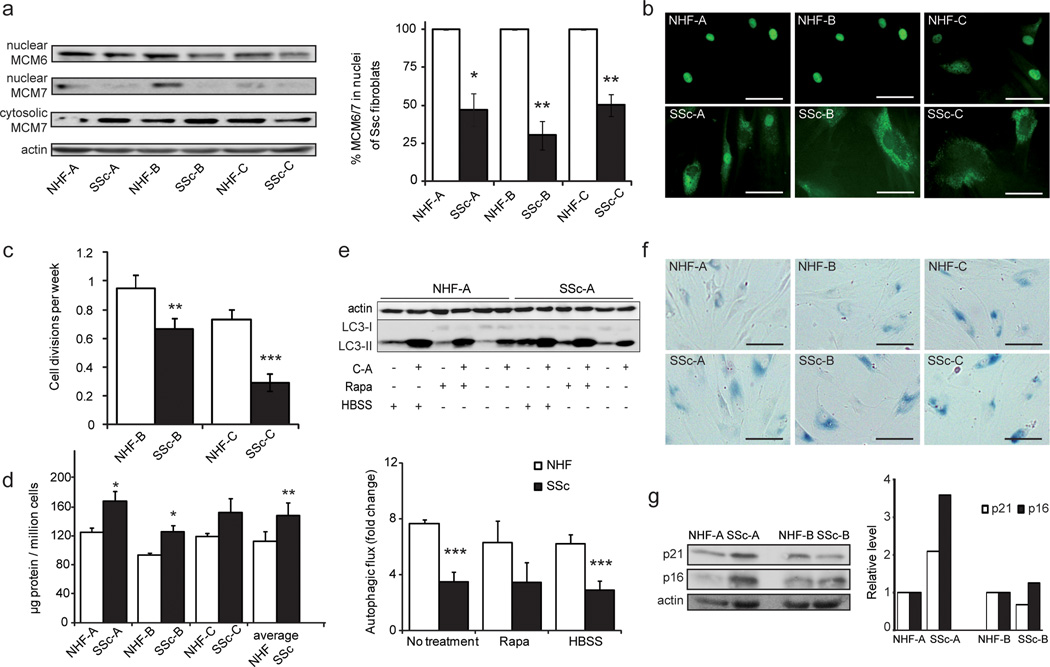
To characterize the molecular phenotype of SSc fibroblasts, we measured MCM protein levels, total protein concentration, cell proliferation, autophagic capacity and cellular senescence of SSc vs. control cells. MCM6 and MCM7 levels in the nuclear fraction of SSc fibroblasts were significantly lower than in control cells (Figure 4-a). However, cytosolic levels of MCM7 proteins were similar in SSc and control fibroblasts. Figure 4-b shows immunostaining of MCM7 and corroborates the differential distribution of MCM7 in SSc and control cells, with less MCM7 in nuclei of SSc cells. The same tendency was exhibited by older fibroblasts compared to younger cells (Figure 2-b). As depicted in Figure 3-c, proteins involved in nuclear transport were down-regulated in SSc cells (see Supplementary Table 4), which may result in the lower levels of MCM proteins detected in nuclei. Lower proliferation rates of SSc fibroblasts were in agreement with changes in nuclear MCM protein levels (Figure 4-c). Total protein concentrations were increased in SSc fibroblasts, compatible with the fibrotic characteristics of the disorder (Figure 4-d). To determine if protein accumulation was due to diminished autophagic capacity of SSc fibroblasts, we followed a similar procedure as described above, and challenged the cells with amino acid starvation (HBSS) and rapamycin (Rapa) treatment, an inhibitor of MTOR and therefore inducer of autophagy. Treatments were applied with and without C-A to calculate autophagic fluxes. We observed that the autophagic capacity of SSc cells was impaired relative to controls, not only under control conditions but also under amino acid starvation and rapamycin treatment (Figure 4-e and Supplementary Figure 6). LC3 immunostaining (Supplementary Figure 7) confirmed the lack of responsiveness to nutritional challenge of SSc cells relative to control fibroblasts. It is tempting to speculate that hindrance in autophagic degradation pathways in SSc cells leads to the increased abundance of mitochondrial proteins detected by MS (Figure 3-c).
Figure 4. Molecular phenotype of SSc affected fibroblasts.
(a) Levels of MCM6 and MCM7 in nuclear fractions, as detected by Western blot, are shown. Bar diagram represents quantification of nuclear MCM6 and MCM7 by Western blot analysis. Samples were normalized to protein content, and actin is shown as loading control. (b) Microscopy pictures of MCM7 immunostaining indicate a change in subcellular localization. (c) Proliferation rates, calculated as cell divisions per week of two matching pairs, are highlighted. (d) Protein concentration in whole cell lysate is depicted. (e) Autophagic fluxes under amino acid starvation, rapamycin treatment and control conditions are shown (see Figure 2; * = P < 0.05; ** = P < 0.01; *** = P < 0.001). A representative Western blot is depicted (see Supplementary Figure 7 for further analyses). Senescence levels in SSc and control fibroblasts as detected by (f) β-galactosidase activity, and (g) p16 and p21 levels.
Interestingly, SSc cells proved to be more senescent compared to population doubling level matched controls, as evidenced by β-galactosidase activity (Figure 4-f) and, in case of SSc-A, by increased p21 and p16 levels (Figure 4-g). Collectively, with respect to the presence of MCM proteins, the SSc fibroblasts behaved similarly to fibroblasts derived from older healthy donors. Also, the proliferation rate, the protein concentration, the autophagic capacity and the senescence level of SSc fibroblasts were similar to those of cells from older donors.
Behavior of MCM7 knock-down cells
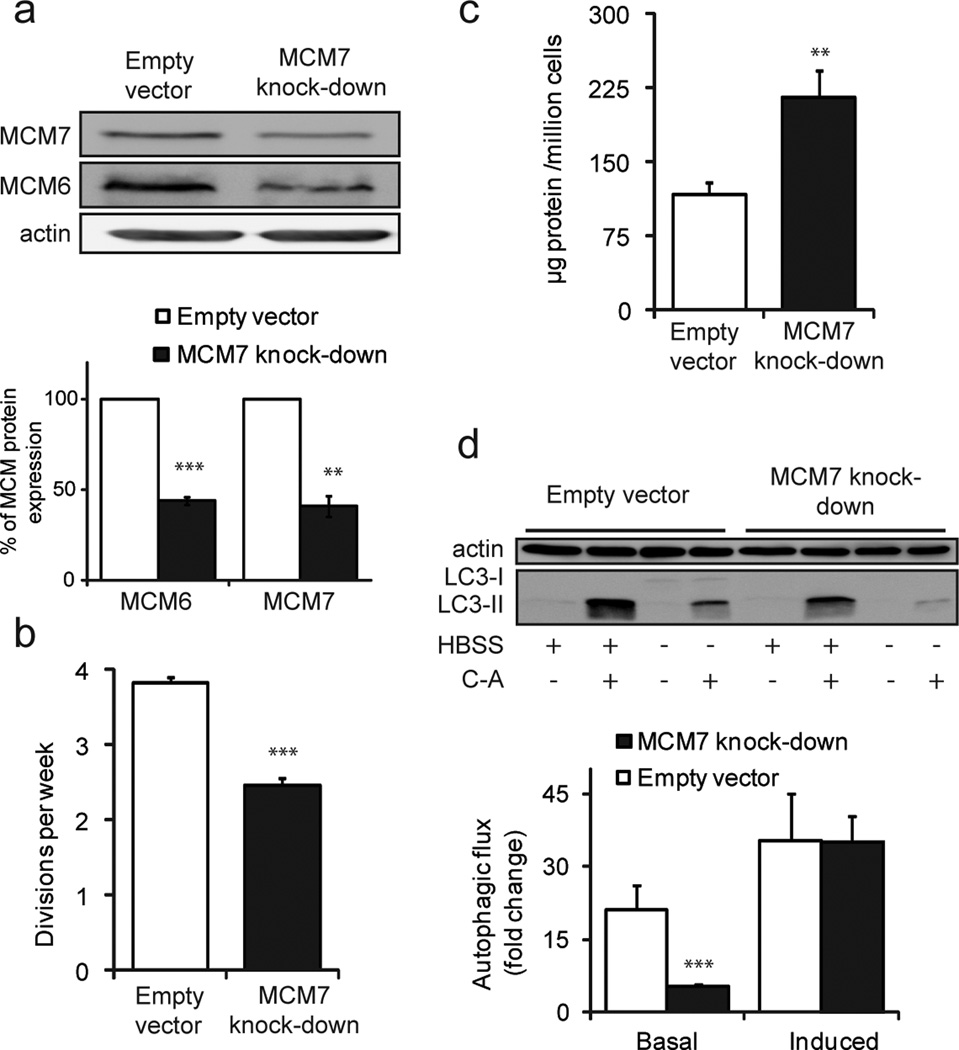
We next analyzed whether the observed phenotypes were a direct result of lower MCM protein abundance. For this purpose we generated doxycycline (DOX) inducible MCM7 knock-down fibroblasts and challenged them under the conditions utilized above to characterize the aging and SSc primary fibroblast phenotypes. MCM7 knock-down fibroblasts were generated by transfecting NHF-4 with a shRNA specific to MCM7 using a human TRIPZ lentiviral inducible shRNAmir target gene. After 6 days of induction with DOX, knock-down fibroblasts showed a more than 50% decrease of MCM7 in control cells (Figure 5-a). Notably, levels of MCM6 were reduced to a similar extent as MCM7 in these knock-down cells.
Figure 5. Behavior of MCM7 knock-down fibroblasts.
(a) Abundance of MCM proteins in whole cell lysate of MCM7 knock-down and mock treated control fibroblasts. Bar diagram depicts protein ratios normalized to actin. (b) Proliferation rates calculated as cell divisions per week. (c) Protein concentration in whole cell lysate. (d) Autophagic fluxes under amino acid starvation (induced) and control conditions (basal) (see Figure 2; ** = P < 0.01; *** = P < 0.001).
DOX treatment increased cell proliferation, and specifically, NHF-4 cells without DOX showed a division rate of 1.7 times per week, while under DOX treatment, control and MCM7 knock-down cells divided 3.8 and 2.4 times, respectively (Figure 5-b). Assuming that DOX affects control and knock-down cells in the same manner, it is reasonable to assume that their behavior is comparable under identical culture conditions. Thus, we conclude that a decrease in the levels of MCM7 and MCM6 results in a marked reduction in proliferation. Additionally, upon decrease of MCM7 levels in fibroblasts, an increase of total protein of almost 100% could be observed (Figure 5-c). This result indicates that by influencing the rate of proliferation, cells alter their protein content, in agreement with the notion that low proliferating cells exhibit an increased protein abundance in culture. Also, autophagic flux proved to be impaired in MCM7 knock-down cells under basal conditions (Figure 5-d). However, lower levels of MCM7 did not have a significant effect on the autophagic capacity of cells under amino acid starvation. Supplementary Figure 8 shows corresponding LC3 immunostaining. Although it has been documented that senescent cells display lower levels of MCM proteins (Harada et al., 2008), we did not detect any change in the level of senescence in MCM7 knock-down fibroblasts (data not shown), which might be due to the short timeframe of DOX treatment.
Collectively, behavior of MCM7 knock-down cells reproduced most phenotypic aspects of cells from older individuals and from patients with SSc. Lower levels of MCM proteins relate to lower proliferation rates, elevated protein abundance, and autophagy impairment, at least under basal conditions. Autophagy induced by amino acid starvation was not altered in knock-down cells. Hence, we conclude that reduced MCM protein levels contribute to the fibrotic and aging phenotypes, which can be observed in primary human skin fibroblasts.
Discussion
Aging of skin fibroblasts
In the recent years, concomitantly with a remarkable increase in life expectancy, incidence of age-dependent diseases has increased. However, despite the interest in this topic, the processes of aging are not fully understood. A comprehensive, in-depth characterization of the aging phenotype is needed as a starting point to elucidate the underlying biochemical mechanisms, in order to promote healthy aging. As an aging phenotype can readily be observed in human skin, such as alterations in the ECM proteins, collagen and elastin, coupled with physiochemical alterations (Uitto and Bernstein, 1998; Uitto, 2008), we decided to study molecular mechanisms involved in age-associated processes in primary human skin fibroblasts from individuals of varying ages by MS-based proteomics. With this approach we intended to detect relevant protein biomarkers as well as molecular players associated with the aging phenotype. Proteins that were up-regulated in age mainly belonged to mitochondria and were involved in energy metabolism, as well as in homeostatic processes, such as transport vesicles and lysosomes (Hwang et al., 2009). However, the latter appeared to be not functional as indicated by impaired constitutive and stress-induced autophagy in the current study.
Proteins which were down-regulated with donor age mostly participated in processes involving DNA- and RNA and carried, amongst others, GO terms: nuclear pore, telomere, chromatin, chromosome or ribonucleoproteins (RNP). RNPs are a combination of ribonucleic acids and proteins, e.g., ribosome, telomerease, and vault RNPs. Telomerease is of interest in the context of telomere shortening as a natural consequence of the aging process (Oulton and Harrington, 2000). Our results support the notion that aging is linked to altered RNA pathways (Malatesta et al., 2004). Among the nuclear proteins that decrease with age, we detected all components of the MCM helicase complex. MCM proteins form a hexamer, which functions as replicative helicase (Labib et al., 2000), and they are considered to be proliferation markers in cell lines, their levels directly reflecting cell division rates (Freeman et al., 1999; Kikuchi et al., 2011; Guida et al., 2005). It has been described that proliferation rates of fibroblasts decrease with increasing age of the donor until the age of 80 years (Takeda et al., 1992). Correspondingly, we found that less frequent divisions of fibroblasts with increasing age correlate with MCM protein levels.
In the present study, we showed that cells from older individuals proliferating at lower rates accumulate higher amounts of protein and become more senescent. Accumulation of damaged DNA, proteins, lipids and organelles during the aging process, resulting for example from the action of reactive oxygen species (ROS) and from a lower activity of degradative mechanisms, is well documented (Vellai, 2009; Ravikumar et al., 2010). Autophagy has been directly implicated in aging in model organisms (Ravikumar et al., 2010). Here we showed that also in primary human skin fibroblasts autophagic activity correlates inversely with the donor age, possibly linking age-dependent increase in protein concentration to impaired autophagic degradation. Thus, we provide evidence in support of the connection between four processes: proliferation rate, senescence, accumulation of total protein, and autophagic capacity.
Although lower levels of MCM proteins have been involved in senescence (Harada et al., 2008), MCM proteins have so far not been implicated in regulation of protein abundance and autophagy, and it is not clear if they exert direct effects on the underlying molecular machinery itself, or if lower proliferation rates due to decreased MCM levels indirectly modulate these processes. Furthermore, the specific role of MCM proteins in aging remains unclear. Recently, MTOR kinase, a negative master regulator of autophagy, was shown not only to control cell growth but also to play a role in cell proliferation (Robitaille et al., 2013). It can be hypothesized then that MCM proteins influence MTOR activity by controlling cell proliferation and thereby indirectly interfere with autophagy, possibly when they localize in the cytosol. In addition to MTOR, MCM proteins can be linked to autophagy via the autophagy promoting transcription factor hypoxia inducible factor 1 (HIF-1) (Hubbi et al., 2011, 2013). MCM7 enhances HIF-1 ubiquitination and consequent proteosomal degradation, which may also reduce the cellular autophagic capacity.
Molecular phenotype of SSc fibroblasts
Alterations in the protein composition of the ECM can be observed not only in aging tissues but also in fibrotic disorders. Although these processes differ substantially in many respects, we decided to study specific phenotypic alterations in the prototypic fibrotic disease, SSc, with respect to cell proliferation, senescence, protein deposition, protein degradation, as well as the influence of MCM proteins, in comparison to age matched controls. As the secretome of SSc dermal fibroblasts has already been studied (Del Galdo et al., 2010), the primary aim of the present work was to assess differences in the intracellular proteome to identify upstream effects, which may ultimately lead to alterations in the ECM. We recently showed that the abundance of intra- and extracellular proteins correlates only weakly (Küttner et al., 2013), which might explain the fact that proteins known to accumulate in the ECM in SSc, such as collagens type I, III, VI, and VII (Peltonen et al., 1990; Rudnicka et al., 1994; Jimenez et al., 1996), were not detected as regulated proteins in the current study. In addition, the SILAC approach used here yielded only relative protein abundance differences and did not address absolute protein levels. The MS analysis revealed cellular proteins involved in nuclear and RNA-related processes to be down-regulated, in agreement with what was observed for the aging process. A decrease in the nuclear levels of MCM protein was identified, which can be linked to the lower proliferation rates of SSc cells. However, whether this relationship is causative of the SSc phenotype remains to be tested.
Interestingly, we showed the same relation between protein accumulation and autophagy hindrance in SSc fibroblasts as in aging cells. The list of pathological conditions, which exhibit an imbalance in the autophagic capacity, is rapidly increasing (Choi et al., 2013). Several fibrotic disorders appear to exhibit defects in protein degradation (Del Principe et al., 2011) and it is not farfetched to speculate, as indicated by the current study, that impaired autophagy may contribute to accumulation of ECM proteins. Interestingly, in a mouse model of SSc increased autophagy could be observed and an inhibition of autophagy was suggested as potential therapeutic strategy (Castello-Cros et al., 2011). It appears that autophagy could have both beneficial and deleterious effects on SSc disease progression, similar to other disorders (Sridhar et al., 2012). Further research is needed to be able to decide whether an inhibition or induction of autophagy is desirable therapeutic approach.
Collectively, although fibrotic disorders and aging show distinct clinical phenotypes, we identified molecular similarities and overlapping cellular pathways being deregulated in both situations. Thus, deregulated proteins might represent promising therapeutic molecular targets in both settings.
Materials and Methods
Cell culture and SILAC labeling
Unless otherwise stated, primary dermal fibroblasts were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (high glucose) supplemented with 10% fetal calf serum (FBS), penicillin/streptomycin (100 units/ml, 100 µg/ml), and 2 mM L-glutamine. For the labeling of NHF-4 used as an internal standard in MS, cells were cultured in SILAC-DMEM supplemented with 10% dialyzed FBS, penicillin/streptomycin (100 units/ml, 100 µg/ml), 2 mM L-glutamine, 42 mg/L L-arginine 13C6-15N4, 73 mg/L L-lysine 13C6-15N2 (Arg10-Lys8), and 82 mg/L proline.
Mass spectrometry sample preparation, measurements and data analysis
A detailed description is given in the Supplementary Information.
Protein concentration determination and determination of cell proliferation rates
See Supplementary Information for details.
Detection of autophagic levels
8 105 fibroblasts per 10 cm plate were challenged to different treatments, with and without Concanamycin A: medium HBSS to simulate amino acid starvation conditions, normal growth medium, and normal growth medium supplemented with rapamycin. After 4 hours incubation at 37°C and 5% CO2, cells were washed with ice cold DPBS with protease inhibitors, and harvested with cell scraper. For fibroblast lysis, cell pellets were incubated for 30 min in 80 µL NP-40 lysis buffer on ice, with three rounds of vortexing. After centrifugation at 16.000 rcf for 10 min at 4°C, the supernatant was transferred into a new tube. We determined protein concentration using the BCA Protein Assay Kit and loaded 30 µg protein per gel well to detect LC3 levels by western blot.
Immunoblotting and staining
Proteins were resolved by SDS-PAGE, transferred onto a PVDF (semi-dry blot) or nitrocellulose (wet blot) membranes. Antibodies against MCM7 (Santa Cruz, (0.N.194): sc-71550), MCM6 (Santa Cruz, (B-4): sc-55576), LC3 (5F10, nanoTools, Teningen, Germany), p21 (Santa Cruz, (H-164): sc-756), p16 (Santa Cruz, (H-156): sc-759) and actin (sc-47778, Santa Cruz) were used according to the supplier’s manual, followed by appropriate horseradish peroxidase-conjugated secondary antibodies. Enhanced chemiluminescent method was used for detection on a Fujifilm LAS-4000. Immunofluorescence staining and β-Galactosidase activity staining are described in the Supplementary Information.
Knock-down of MCM7 in skin fibroblasts
NHF-4 cells were used to generate a MCM7 knock-down cell line by transfection with a shRNA specific to MCM7 using a human TRIPZ lentiviral inducible shRNAmir target gene set from Thermo Scientific, as previously published (Martins et al., 2009). Adequate controls were obtained following the same procedure. Cells were seeded at 50% confluence and subjected to transfection after 16 hours. MCM7 expression was analyzed by immunoblotting. Experiments utilizing MCM7 knock-down cells were performed after a 6 day induction adding DOX at a concentration of 1:500 to normal growth medium.
Supplementary Material
Acknowledgements
This research was supported by funding from the Excellence Initiative of the German Federal and State Governments through FRIAS, School of Life Sciences - LifeNet and the excellence cluster BIOSS, by grants from the Deutsche Forschungsgemeinschaft (GZ DE1757/2-1; BR1475/12-1), and from the Federal Ministry of Education and Research through GerontoSys II – NephAge (031 5896 A), and by NIH/NIAMS (to S.A.J.). We thank Mostafa Zarei for MS support. V.I.D. is grateful to Stefan Strohmeier for useful suggestions in statistical data analysis.
Abbreviations
- ECM
extracellular matrix
- MCM
mini-chromosome maintenance
- ROS
reactive oxygen species
- MTOR
mammalian target of rapamycin
- MS
mass spectrometry
- SILAC
stable isotope labeling by amino acids in cell culture
- NHF
normal human fibroblasts
- DOX
doxycyclinee
- Rapa
rapamycin
- C-A
concanamycin A
- RNP
ribonucleoproteins
Footnotes
Conflict of Interest
The authors declare that there are no conflicts of interest.
References
- Akimov V, Rigbolt KT, Nielsen MM, et al. Characterization of ubiquitination dependent dynamics in growth factor receptor signaling by quantitative proteomics. Mol Biosyst. 2011;7:3223–3233. doi: 10.1039/c1mb05185g. [DOI] [PubMed] [Google Scholar]
- Bellot G, Garcia-Medina R, Gounon P, et al. Hypoxia-induced autophagy is mediated through hypoxia-inducible factor induction of BNIP3 and BNIP3L via their BH3 domains. Mol Cell Biol. 2009;29:2570–2581. doi: 10.1128/MCB.00166-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castello-Cros R, Whitaker-Menezes D, Molchansky A, et al. Scleroderma-like properties of skin from caveolin-1-deficient mice: implications for new treatment strategies in patients with fibrosis and systemic sclerosis. Cell Cycle. 2011;10:2140–2150. doi: 10.4161/cc.10.13.16227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Wang CC, Kim E, Harrison LE. Hyperthermia in combination with oxidative stress induces autophagic cell death in HT-29 colon cancer cells. Cell Biol Int. 2008;32:715–723. doi: 10.1016/j.cellbi.2008.02.010. [DOI] [PubMed] [Google Scholar]
- Choi AM, Ryter SW, Levine B. Autophagy in human health and disease. N Engl J Med. 2013;368:651–662. doi: 10.1056/NEJMra1205406. [DOI] [PubMed] [Google Scholar]
- Del Galdo F, Shaw MA, Jimenez SA. Proteomic analysis identification of a pattern of shared alterations in the secretome of dermal fibroblasts from systemic sclerosis and nephrogenic systemic fibrosis. Am J Pathol. 2010;177:1638–1646. doi: 10.2353/ajpath.2010.091095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Principe D, Vona R, Giordani L, et al. Defective autophagy in fibroblasts may contribute to fibrogenesis in autoimmune processes. Curr Pharm Des. 2011;17:3878–3887. doi: 10.2174/138161211798357791. [DOI] [PubMed] [Google Scholar]
- Dengjel J, Hoyer-Hansen M, Nielsen MO, et al. Identification of autophagosome-associated proteins and regulators by quantitative proteomic analysis and genetic screens. Mol Cell Proteomics. 2012:11. doi: 10.1074/mcp.M111.014035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumit VI, Dengjel J. Autophagosomal protein dynamics and influenza virus infection. Front Immunol. 2012;3:43. doi: 10.3389/fimmu.2012.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelke R, Becker AC, Dengjel J. The degradative inventory of the cell: proteomic insights. Antioxid Redox Signal. 2012;17:803–812. doi: 10.1089/ars.2011.4393. [DOI] [PubMed] [Google Scholar]
- Freeman A, Morris LS, Mills AD, et al. Minichromosome maintenance proteins as biological markers of dysplasia and malignancy. Clin Cancer Res. 1999;5:2121–2132. [PubMed] [Google Scholar]
- Freund A, Orjalo AV, Desprez PY, Campisi J. Inflammatory networks during cellular senescence: causes and consequences. Trends Mol Med. 2010;16:238–246. doi: 10.1016/j.molmed.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson M. Ageing. Nature. 2012;492:S1. doi: 10.1038/492S1a. [DOI] [PubMed] [Google Scholar]
- Gerber EE, Gallo EM, Fontana SC, et al. Integrin-modulating therapy prevents fibrosis and autoimmunity in mouse models of scleroderma. Nature. 2013 doi: 10.1038/nature12614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guida T, Salvatore G, Faviana P, et al. Mitogenic effects of the up-regulation of minichromosome maintenance proteins in anaplastic thyroid carcinoma. J Clin Endocrinol Metab. 2005;90:4703–4709. doi: 10.1210/jc.2004-2459. [DOI] [PubMed] [Google Scholar]
- Harada H, Nakagawa H, Takaoka M, et al. Cleavage of MCM2 licensing protein fosters senescence in human keratinocytes. Cell Cycle. 2008;7:3534–3538. doi: 10.4161/cc.7.22.7043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang DAW, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009a;37:1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang DAW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009b;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- Hubbi ME, Hu H, Kshitiz, et al. Chaperone-mediated Autophagy Targets Hypoxia-inducible Factor-1a (HIF-1a) for Lysosomal Degradation. J Biol Chem. 2013;288:10703–10714. doi: 10.1074/jbc.M112.414771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbi ME, Luo W, Baek JH, et al. MCM proteins are negative regulators of hypoxia-inducible factor 1. Mol Cell. 2011;42:700–712. doi: 10.1016/j.molcel.2011.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang ES, Yoon G, Kang HT. A comparative analysis of the cell biology of senescence and aging. Cell Mol Life Sci. 2009;66:2503–2524. doi: 10.1007/s00018-009-0034-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins G. Molecular mechanisms of skin ageing. Mech Ageing Dev. 2002;123:801–810. doi: 10.1016/s0047-6374(01)00425-0. [DOI] [PubMed] [Google Scholar]
- Jimenez SA, Hitraya E, Varga J. Pathogenesis of scleroderma. Collagen. Rheum Dis Clin North Am. 1996;22:647–674. doi: 10.1016/s0889-857x(05)70294-5. [DOI] [PubMed] [Google Scholar]
- Kikuchi J, Kinoshita I, Shimizu Y, et al. Minichromosome maintenance (MCM) protein 4 as a marker for proliferation and its clinical and clinicopathological significance in non-small cell lung cancer. Lung Cancer. 2011;72:229–237. doi: 10.1016/j.lungcan.2010.08.020. [DOI] [PubMed] [Google Scholar]
- Klionsky DJ, Abdalla FC, Abeliovich H, et al. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy. 2012;8:445–544. doi: 10.4161/auto.19496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Küttner V, Mack C, Rigbolt KT, et al. Global remodelling of cellular microenvironment due to loss of collagen VII. Mol Syst Biol. 2013;9:657. doi: 10.1038/msb.2013.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labib K, Tercero JA, Diffley JF. Uninterrupted MCM2-7 function required for DNA replication fork progression. Science. 2000;288:1643–1647. doi: 10.1126/science.288.5471.1643. [DOI] [PubMed] [Google Scholar]
- Malatesta M, Bertoni-Freddari C, Fattoretti P, et al. Aging and vitamin E deficiency are responsible for altered RNA pathways. Ann N Y Acad Sci. 2004;1019:379–382. doi: 10.1196/annals.1297.067. [DOI] [PubMed] [Google Scholar]
- Monnier VM, Mustata GT, Biemel KL, et al. Cross-linking of the extracellular matrix by the maillard reaction in aging and diabetes: an update on "a puzzle nearing resolution". Ann N Y Acad Sci. 2005;1043:533–544. doi: 10.1196/annals.1333.061. [DOI] [PubMed] [Google Scholar]
- Newgard CB, Sharpless NE. Coming of age: molecular drivers of aging and therapeutic opportunities. J Clin Invest. 2013;123:946–950. doi: 10.1172/JCI68833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen VQ, Co C, Irie K, Li JJ. Clb/Cdc28 kinases promote nuclear export of the replication initiator proteins Mcm2-7. Curr Biol. 2000;10:195–205. doi: 10.1016/s0960-9822(00)00337-7. [DOI] [PubMed] [Google Scholar]
- Niccoli T, Partridge L. Ageing as a risk factor for disease. Curr Biol. 2012;22:741–752. doi: 10.1016/j.cub.2012.07.024. [DOI] [PubMed] [Google Scholar]
- Oulton R, Harrington L. Telomerees, telomerease, and cancer: life on the edge of genomic stability. Curr Opin Oncol. 2000;12:74–81. doi: 10.1097/00001622-200001000-00013. [DOI] [PubMed] [Google Scholar]
- Papakonstantinou E, Roth M, Karakiulakis G. Hyaluronic acid: A key molecule in skin aging. Dermatoendocrinol. 2012;4:253–258. doi: 10.4161/derm.21923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltonen J, Kähäri L, Uitto J, et al. Increased expression of type VI collagen genes in systemic sclerosis. Arthritis Rheum. 1990;33:1829–1835. doi: 10.1002/art.1780331211. [DOI] [PubMed] [Google Scholar]
- Ravikumar B, Sarkar S, Davies JE, et al. Regulation of mammalian autophagy in physiology and pathophysiology. Physiol Rev. 2010;90:1383–1435. doi: 10.1152/physrev.00030.2009. [DOI] [PubMed] [Google Scholar]
- Robitaille AM, Christen S, Shimobayashi M, et al. Quantitative phosphoproteomics reveal mTORC1 activates de novo pyrimidine synthesis. Science. 2013;339:1320–1323. doi: 10.1126/science.1228771. [DOI] [PubMed] [Google Scholar]
- Rosenbloom J, Castro SV, Jimenez SA. Narrative review: fibrotic diseases: cellular and molecular mechanisms and novel therapies. Ann Intern Med. 2010;152:159–166. doi: 10.7326/0003-4819-152-3-201002020-00007. [DOI] [PubMed] [Google Scholar]
- Rudnicka L, Varga J, Christiano AM, et al. Elevated expression of type VII collagen in the skin of patients with systemic sclerosis. Regulation by transforming growth factor-beta. J Clin Invest. 1994;93:1709–1715. doi: 10.1172/JCI117154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprenger A, Küttner V, Biniossek ML, et al. Comparative quantitation of proteome alterations induced by aging or immortalization in primary human fibroblasts and keratinocytes for clinical applications. Mol Biosyst. 2010;6:1579–1582. doi: 10.1039/c003962d. [DOI] [PubMed] [Google Scholar]
- Sprenger A, Küttner V, Bruckner-Tuderman L, et al. Global proteome analyses of SILAC-labeled skin cells. Methods Mol Biol. 2013a;961:179–191. doi: 10.1007/978-1-62703-227-8_10. [DOI] [PubMed] [Google Scholar]
- Sprenger A, Weber S, Zarei M, et al. Consistency of the proteome in primary human keratinocytes with respect to gender, age, and skin localization. Mol Cell Proteomics. 2013b;12:2509–2521. doi: 10.1074/mcp.M112.025478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridhar S, Botbol Y, Macian F, Cuervo AM. Autophagy and disease: always two sides to a problem. J Pathol. 2012;226:255–273. doi: 10.1002/path.3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K, Gosiewska A, Peterkofsky B. Similar, but not identical, modulation of expression of extracellular matrix components during in vitro and in vivo aging of human skin fibroblasts. J Cell Physiol. 1992;153:450–459. doi: 10.1002/jcp.1041530303. [DOI] [PubMed] [Google Scholar]
- Uitto J. The role of elastin and collagen in cutaneous aging: intrinsic aging versus photoexposure. J Drugs Dermatol. 2008;7:12–16. [PubMed] [Google Scholar]
- Uitto J, Bernstein EF. Molecular mechanisms of cutaneous aging: connective tissue alterations in the dermis. J Invest Dermatol Symp Proc. 1998;3:41–44. [PubMed] [Google Scholar]
- Varga J, Rudnicka L, Uitto J. Connective tissue alterations in systemic sclerosis. Clin Dermatol. 1994;12:387–396. doi: 10.1016/0738-081x(94)90291-7. [DOI] [PubMed] [Google Scholar]
- Vellai T. Autophagy genes and ageing. Cell Death Differ. 2009;16:94–102. doi: 10.1038/cdd.2008.126. [DOI] [PubMed] [Google Scholar]
- Zimmermann AC, Zarei M, Eiselein S, et al. Quantitative proteomics for the analysis of spatio-temporal protein dynamics during autophagy. Autophagy. 2010;6:1009–1016. doi: 10.4161/auto.6.8.12786. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.