Abstract
The increased prevalence and high comorbidity of metabolic syndrome and mental health disorders have prompted investigation into the potential contributing mechanisms. There is a bidirectional association between metabolic syndrome and mental health disorders including schizophrenia, bipolar disorder, depression, anxiety, attention deficit/hyperactivity disorder, and autism spectrum disorders. Medication side effects and social repercussions are contributing environmental factors, but there are a number of shared underlying neurological and physiological mechanisms that explain the high comorbidity between these two disorders. Inflammation is a state shared by both disorders, and it contributes to disruptions of neuroregulatory systems, including the serotonergic, dopaminergic, and neuropeptide Y systems, as well as dysregulation of the hypothalamic-pituitary-adrenal axis. Metabolic syndrome in pregnant women also exposes the developing fetal brain to inflammatory factors that predispose the offspring to metabolic syndrome and mental health disorders. Due to the shared nature of these conditions, treatment should address aspects of both mental health and metabolic disorders. Additionally, interventions need to be developed that can interrupt the transfer of increased risk of the disorders to the next generation.
Keywords: obesity, metabolic syndrome, diabetes, schizophrenia, bipolar disorder, depression
Introduction
Interest in the common mechanisms between metabolic and mental health disorders (MHDs) is rising due to increasing prevalence and comorbidity of both. Metabolic syndrome (MetS) is both preventable and deadly. It is currently defined as a set of chronic and associated features that increase risk of cardiovascular disease and type 2 diabetes mellitus, including central obesity, atherogenic dyslipidemia, insulin resistance, and endothelial dysfunction [1, 2]. There are several definitions of childhood MetS, but all contain features of obesity, dyslipidemia, high blood pressure, and impaired glucose metabolism [3]. These childhood features are better correlated with waist circumference than BMI, and the cardiovascular risk factors persist to adulthood unless changes in nutrition and physical activity are made [4]. Metabolic and mental health conditions are both impacted by numerous environmental and genetic factors, but this review will focus on the overlapping mechanisms between MetS and MHDs that contribute to the recent increases in prevalence and may explain their comorbidity.
Increased Prevalence and Common Occurrence of both Metabolic Diseases and Mental Health Disorders
The prevalence of MetS and its components is widespread and continuing to rise. Overweight and obese people have an increased risk of developing MetS [1]. In 2010, every state in America had a prevalence of obesity above 20% [5], and one third of the nation is obese [6]. Though rates are plateauing in women, they continue to increase in men and adolescents [7, 8]. Type 2 diabetes is the seventh leading cause of death in the United States [9], and if current trends persist, its incidence will increase to 1 in every 3 by 2050 [10]. Evidence also indicates that maternal obesity and high fat diet consumption during the perinatal period predispose offspring to MetS [11].
MHDs are common: approximately 25% of American adults have a mental health disorder [12]. When delineated further, about 7% of adults suffer from major depressive disorder, about 3% have generalized anxiety disorder, and approximately 4% have attention deficit/hyperactivity disorder (ADHD) [12]. In children, developmental disabilities have increased dramatically (17%) in the last decade, driven largely by increases in ADHD and autism spectrum disorders (ASD) [13]. This increased prevalence has lead to numerous investigations into the environmental risk factors contributing to this recent and rapid rise in childhood neurodevelopmental disorders. Interestingly, the rise in the prevalence of childhood developmental disabilities parallels the increase in adult obesity and several lines of evidence suggest that maternal obesity increases offspring risk for both MetS and MHDs [11, 14, 15].
Metabolic Syndrome and Mental Health Comorbidity
Both MetS and obesity are comorbid with MHDs in 45% of cases [16]. Individuals with schizophrenia, bipolar disorder, depression, anxiety, ADHD, and ASD have a higher prevalence of both obesity and MetS compared to the general population [17, 18]. Evidence linking MetS to specific MHDs will be further outlined in the following sections.
Schizophrenia and Bipolar Disorder
MetS is more prevalent in patients with bipolar disorder or schizophrenia than in the general population. Individuals with bipolar disorder have the highest rates of MetS [17, 19] as well as increased risk for obesity [20] and other metabolic complications [21]. This association is controversial as both typical [22] and atypical [22, 23] antipyschotics are reported to contribute to the increased body weight and MetS. These medications are likely not fully responsible for the association because increased weight and adiposity is also seen in drug-naïve individuals [24] and patients diagnosed with their first-episode of psychosis were also reported to have increased frequencies of hypertension, diabetes and metabolic syndrome [25].
Depression and Anxiety
Childhood [26, 27] and adult obesity are associated with an increased risk of depression [28–32] and anxiety [28, 29, 31]. Though body weight is a stronger predictor of depression than diabetes [33], evidence shows that diabetes, independent of weight status, is linked with higher rates of depression [33, 34]; some studies report a four-fold risk increase in diabetic patients [35] that increases with symptom severity [34, 36, 37]. Interestingly, a recent study indicates that obesity may only be linked to depression in individuals with a higher socioeconomic status, and depressive symptoms were associated with increased BMI only in Hispanic women [38]. This report and other conflicting reports of the association between affective disorders and obesity indicate that the association is quite complex and is influenced by factors such as socioeconomic status and ethnicity.
ADHD
Children [39], adolescents [39], and adults [40] with ADHD are more likely to be overweight or obese than the general population. Similarly, ADHD is more common in obese teenagers [27, 41, 42]. Bariatric surgery is prescribed to promote weight loss in morbidly obese individuals, and pre-operative evaluations showed rates of ADHD double that of the general population [43].
ASD
Several studies show that obesity is twice as likely in adolescents with ASD [42, 44]. However, others report that the lower nutrient intake experienced by children with ASD is severe enough to counter their obesity and may eventually result in underweight status [45].
Disordered Eating
Obesity and mental health issues are often comorbid in compulsive eating disorders such as night-time eating syndrome and binge eating. Binge eating disorder [46] and night eating syndrome [47] are widespread in the obese population, and night eating syndrome is associated with obesity [48], anxiety [49], and depression [46, 48–50]. Furthermore, obese individuals with ADHD display abnormal eating habits compared to obese patients without ADHD [51], and this is also observed in children with ADHD [41] and ASD [52].
Sex-Dependent Evidence
Many relationships between MetS and MHDs are sex-dependent. There is a stronger association between obesity and psychopathologies in women [29, 53]; only morbidly obese men display an increased risk of depression [54]. Overweight and obese females have increased prevalence of anxiety [53], major depressive disorder [53, 55–62], and both childhood and adult ADHD [63], while obese men did not show the same trends [53, 56, 58, 61]. The risk for generalized anxiety disorder and major depressive disorder is increased six-fold in obese females [64]. Morbidly obese women seeking bariatric surgery were also more likely to have a history of mood and anxiety disorders [65], and women with type 2 diabetes have higher risk of depression than men [33, 66].
Metabolic Syndrome Increases Risk of Psychopathologies
Beyond comorbidity, several studies highlight that MetS increases the likelihood of developing affective disorders [53], such as depression [67–72] and bipolar disorder [69], due to similar underlying mechanisms. Obesity is not found to increase risk of depression in the general population, but the subset of obese individuals with high socioeconomic status have a doubled risk of depression [38]. The relationship between diabetes and later depressive symptoms has been reported to be modest [73] or weak [74], but the strength of the relationship depends on whether the study used self-reported or diagnostic depressive symptoms [75].
Societal discrimination and stigma against obesity may also increase risk of MHDs [57]. Diabetes may contribute to depression through the fear and lifestyle restriction potentially associated with receiving this diagnosis [73, 75], as well as symptoms like hyperglycemia-induced fatigue [75]. Indeed, there is a peak in antidepressant use after a diagnosis of diabetes [76] and when treatment begins [77].
Psychopathologies Increase Risk of Metabolic Syndrome
Evidence supports the bidirectionality of this association between MetS and MHDs. Adults with ASD have higher risk of developing diabetes [78], and male children with ADHD have a higher risk for adult obesity [79]. Depression similarly increases likelihood of developing diabetes [73] and obesity [68]. Men with depressive symptoms had higher likelihood of developing obesity or MetS in the next decade [80]. Depression during adolescence predicts a higher BMI later in life [61]. This data is not conclusive, however, as other studies do not see an increased risk of obesity with depression [67].
Explanations for this relationship are often accredited to medication side effects or disease-induced lifestyle modifications. Typical and atypical antipsychotics and antidepressants cause dyslipidemia [17], weight gain [22, 81], and glucose dysregulation [81]. Correspondingly, individuals with schizophrenia and bipolar disorder have higher risk for components of MetS [17]. Selective serotonin reuptake inhibitors (SSRIs) do have short-term benefits for glucose regulation, but tricyclics and noradrenergic antidepressants worsen the metabolic state [82]. Indeed, female patients taking antidepressants have higher risk of developing type 2 diabetes than unmedicated patients [66].
Environmental factors increasing risk of MetS may be specific to populations with high risk of MHDs. Reduced access to healthcare in patients with schizophrenia or bipolar disorder may contribute to the development of MetS [17]. The increased sugar and saturated fat intake seen in women with depression and obesity [83] increases the likelihood of weight gain and MetS. Furthermore, depression and emotional dysregulation commonly accompany a preference for sweet and fatty food [84], higher caloric consumption [85], and sedentary behavior [85]. Therefore, MHDs may contribute to developing and maintaining an obese state and may also increase resistance to treatment.
Changes in Metabolic Status Impact Mental Health
Psychopathologies are observed in over half of individuals seeking bariatric surgery [86], but symptomology ratings improve after successful surgery in adults [62, 87, 88] and adolescents [89]. These improvements continue years afterward and are greater in women [90]. An inpatient weight loss program also reported improvements in depression after successful weight loss [55].
In stark contrast, a systematic review of bariatric surgery found an increased risk for suicide completion in patients compared to the general public [91]. Another systematic review found that obese people have lower rates of suicide completion despite higher reports of suicidal ideation [58, 92] and attempts in obese women [92]. Thus, it may be that many bariatric surgery patients have improvements in depressive symptoms, but those that do not are more likely to act on their intrusive thoughts.
Conversely, there is limited evidence that successful treatment of psychiatric disorders improves metabolic functioning. One study reports that glucose metabolism improves after treatment for depression [81].
Maternal Metabolic Disorders and Offspring Mental Health
Maternal metabolic status impacts the neurophysiology of developing offspring; predictably, a relationship between maternal obesity and offspring psychopathology has been observed. Obese mothers are 67% more likely to have a child with ASD [93]. Several studies also identify maternal diabetes as a risk factor for ASD [94, 95] and developmental delays [93]. Additionally, maternal obesity is associated with affective problems in children [96, 97] and adolescents [97], as well as increased ADHD behaviors [98, 99]. Similarly, children with ADHD are twice as likely to have a mother who is obese [100].
Diet-induced maternal obesity in animals shows similar metabolic and behavioral impairments in offspring. Perinatal consumption of a high fat diet leads to mouse offspring with deficits in spatial learning and memory [101] as well as increased aggression and hyperactivity [102]. Rodent [103, 104] and non-human primate [105] offspring from mothers fed a high fat diet show increases in anxious behavior.
Similarly, mouse offspring of depressed mothers have compromised memory, higher emotionality, and decreased neurogenesis [106]. This data provides compelling evidence that maternal metabolic status, and potentially maternal mental health, impacts the outcome for offspring.
Potential Mechanisms for Comorbidity of Mental Health Disorders and Metabolic Syndrome Inflammation
High fat diet consumption and consequent obesity elicit an inflammatory response [107], and key inflammatory cytokines, such as C-reactive protein (CRP), tumor necrosis factor-α (TNF-α), interferon-γ, and interleukin (IL)-6 and -8, are also involved with mood disorders. IL-6, for example, influences both stress and feeding behaviors [108] and inhibits hippocampal neurogenesis [109], which is involved in schizophrenia and depression [110].
Schizophrenia and Bipolar Disorder
A hypothesis of cytokine-stimulated immune response leading to abnormal brain development is generally accepted for schizophrenia [111]. IL-6 and TNF-α levels are markedly increased in patients with schizophrenia [112–114], and TNF-α is considered a trait marker of the disease [114]. Pathways regulating inflammatory response show alterations in 40% of people with schizophrenia [115]. The cytokine release induced by a toll-like receptor agonist was higher in whole blood from patients with schizophrenia and bipolar disorder [113]. Bipolar patients also show an elevation in IL-6 levels [113].
Inflammation plays such an important role in schizophrenia that this disease is often modeled in rodents by dispensing cytokines and other inflammatory agents to neonates; this results in behavioral abnormalities consistent with human schizophrenia symptoms, and these symptoms respond to antipsychotics [116, 117].
Depression
Increased levels of IL-6 correspond with symptoms of major depressive disorder [112, 118]. Male patients with a history of depression [119] or currently in a depressive episode [120] had higher levels of CRP; this association remained after correction for BMI [120].
Studies examining elderly patients report an association between depression and increased levels of TNF-α [121], CRP [121], and IL-6 [122, 123], though the relationship with IL-6 is stronger in men [121]. Risk for depression in elderly individuals is associated with elevated levels of two of the following pro-inflammatory factors: TNF-α, CRP, and IL-6 [121].
ADHD
Many studies have identified inflammation as a contributing factor for increased risk of ADHD [124]. The brains of individuals with ADHD show higher rates of T cell-induced apoptosis, which is activated by exposure to pro-inflammatory cytokines [125].
ASD
Individuals with ASD present elevated levels of TNF-α [52], IL-1 [52], IL-6 [52, 126], and interferon-γ [127]. Animal studies reveal that overexposure to IL-6 in the brain results in both cellular abnormalities (poor adhesion and migration [126], over-formation of excitatory synapses [126, 128], and abnormal dendritic spines [128]) and behavioral disturbances (learning deficits, low social interaction, and abnormal features of anxiety and habituation [128]) consistent with ASD.
Brain-derived Neurotrophic Factor
Similar to inflammatory cytokines, growth factors such as brain-derived neurotrophic factor (BDNF) are potential mediators of the comorbidity between MetS and MHDs. BDNF is critical in neuron development, differentiation, synaptogenesis, regulation, and survival in systems critical in regulating cognition and behavior (dopamine and serotonin [129]) and food intake and body weight (pro-opiomelanocortin and agouti-related protein [130, 131] (discussed in the next section). In humans, polymorphisms of BDNF are associated with schizophrenia [132–134], depression [135], anxiety [135, 136], and other mood disorders [136] as well as with obesity [137–140]. Moreover, mice deficient in BDNF display behavioral abnormalities including increased aggression and hyperactivity [141] in addition to obesity [142]. Interestingly, in a mouse model, maternal obesity was associated with decreased hippocampal BDNF and impaired spatial cognitive function [101].
Perturbations in Pathways that Regulate Behavior and Metabolic Status
Perturbations in common neuroregulatory pathways likely contribute to the comorbidity of MetS and MHDs. Neuropeptide systems involved in regulating mental health and metabolic status, such as the serotonin, dopamine, neuropeptide Y (NPY), corticotropin-releasing hormone (CRH), and endocannabinoid systems [143], are likely candidates.
Serotonin
Serotonin is best known for regulating mood and behavior. A suppression of central serotonin synthesis is consistently reported in humans with anxiety [144], depression [145, 146], ADHD [147], and ASD [148, 149]. Serotonin 1A receptors have been identified to play a role in anxiety [144, 150]. Mood disorders are commonly treated with SSRIs, which increase the levels of available serotonin.
The brain serotonin system has also received substantial attention for regulating energy balance. Reduction of serotonin system activity increases food intake [151–154], while drugs that stimulate serotonin release reduce food intake in rats [155, 156], baboons [157], and humans [158, 159]. Of the fourteen subtypes of serotonin receptors, the 1B and 2C subtypes are most strongly implicated in modulating feeding and body weight. These receptors are expressed in hypothalamic regions involved in food intake regulation [160–163]. Furthermore, both 1B and 2C receptor agonists suppress feeding in rodents [164–166], and 2C receptor knockout mice display chronic hyperphagia and obesity [167, 168]. On the other hand, obesity is associated with alterations in the metabolism of tryptophan, the precursor for serotonin synthesis [169].
Dopamine
Recent neuroimaging studies indicate that dopamine synthesis and release is altered in individuals with schizophrenia [170], depression [171, 172], social anxiety [173], ADHD [174], and ASD [175, 176]. Polymorphisms in dopamine transporter are associated with depression [177], social anxiety [178], ADHD [179, 180], and ASD [181]. Also, a polymorphism in dopamine 3 receptors is associated with repetitive behavior in children with ASD [182]. Pharmacological treatment of many MHDs involves modulation of the dopamine system: typical antipsychotic drugs work by blocking dopamine 2 receptors (D2Rs), ADHD is treated using psychostimulants that increase dopamine levels [183], and treatment with a dopamine agonist produces antidepressant effects in treatment-resistant patients with major depressive disorder [184].
Neuroimaging studies provide compelling evidence of the dopamine system’s involvement in eating behavior and obesity [185] particularly via dopaminergic projections from the ventral tegmental area to the nucleus accumbens [186]. Additional routes of food intake regulation are dopaminergic projections from the nucleus accumbens to the hypothalamus [187] and dopaminergic neurons in the ventral tegmental area that are impacted by the hormone leptin, which exerts a neurotrophic influence in the development of hypothalamic circuits regulating food intake [186]. Cues associated with food increase dopamine levels [185]. Obese subjects exhibit reduced D2R availability, which likely increases eating in these individuals in order to acutely stimulate underactive reward circuits [188]. This reduction of D2Rs is associated with suppressed metabolism in brain areas involved in self-control and increased metabolism in regions involved in sensory processing of palatability [185, 189]. Interestingly, a recent imaging study of patients recovered from anorexia nervosa indicates that their eating-induced dopamine release may produce anxiety instead of the typical pleasurable response [190].
Mice that lack the gene encoding tyrosine hydroxylase, the enzyme responsible for dopamine synthesis, initially gain weight and feed normally, but, unless dopamine is supplemented, they will stop feeding and die from starvation [191]. Dysfunctional processing of reward-based feeding through the dopaminergic system is a potential contributor to the obesity epidemic and likely contributes to the comorbidity of metabolic and psychiatric disorders.
Hypothalamic Neurotransmitters
Neurons producing NPY/ agouti-related protein and alpha-melanocyte stimulating hormone in the arcuate nucleus are key regulators of body weight and food intake [192]. NPY is also implicated in behavioral regulation, and NPY expression is reduced in schizophrenia [193, 194], bipolar disorder [193–195], and depression [193, 196] and elevated in children with ADHD [197]. Application of a NPY 1 receptor antagonist in rats produces increased anxiety and decreased social interaction [198], and mice given central administration of NPY [199, 200] or lacking NPY 2 receptors display decreased anxiety.
Dysregulation of the Hypothalamic-Pituitary-Adrenal Axis
The role of the HPA axis in MetS is well established. Obese humans with insulin resistance exhibit elevated cortisol [201]. Furthermore, hyperactivation of the HPA axis may increase adiposity by promoting hyperphagia and consumption of palatable foods [84]. Consumption of these “comfort foods” inhibits the HPA axis; thus, overeating may be a compensatory response to temporarily reduce chronic stress [202]. Animal studies demonstrate an acute reduction in anxiety and depressive-like behavior after consumption of palatable food [203]. Cortisol exposure may also impact the reward value of a food item by influencing factors such as leptin, insulin, and NPY [202].
It is also well documented that MHDs are associated with dysregulation of the HPA axis. CRH expressing paraventricular neurons [204] and cerebrospinal fluid CRH levels are increased in individuals with depression [205, 206]. Postmortem analysis of suicide victims reveals a reduction in CRH receptor density [207], which occurs via negative feedback to compensate for CRH overexposure. There is also evidence that depressed individuals have chronically elevated cortisol that is responsible for increasing MetS symptoms [61, 85].
Mice that overexpress CRH display symptoms of MHDs and MetS including hyperphagia, insulin resistance, increased anxiety, and impaired coping to stress [208–210]. Pharmacological agents used to treat both MetS and MHDs modulate HPA axis activity [211].
Reduction of the Heart Rate Variability
Heart rate variability (HRV) is a non-invasive measurement of cardiac autonomic function that has been considered a valid tool for diagnosis and management of cardiovascular disease [212]. As a number of studies suggest that MetS negatively affects autonomic cardiac control [213, 214], autonomic dysfunction could contribute to an increased risk of subsequent cardiovascular events in individuals with MetS. In general, MetS patients have reduced HRV, suggesting decreased parasympathetic and/or increased sympathetic modulation of the heart [212, 214–217].
This autonomic dysregulation has also been suggested as a possible contributor to the increased cardiovascular risk in patients with psychiatric disorders [218, 219]. Decreased HRV values were found in subjects with depression [220, 221] or schizophrenia [222]. Decreased vagal stimulation was also found in children with ADHD [223]. However, it remains unclear whether mood disorders or medications are driving the autonomic dysregulation found in patients with MHDs. Tricyclic medications and SSRIs were also associated with reduced HRV [224, 225] while non-pharmacological therapies such as physical exercise, meditation, smoking cessation, and dietary changes are associated with increased HRV [224].
Heart rate variability is not the only measurement that highlights the link between components of MetS and MHDs; non-invasive brain stimulation strategies can improve depressive symptoms in patients with depression or bipolar disorder [226] and may be effective in reducing HPA activity [227].
Maternal Metabolic Health Programs Offspring
The mechanisms previously discussed are compounded by the effect of maternal metabolic status. The placenta transfers maternal inflammation to the developing fetal brain, so maternal MetS has additional consequences for offspring metabolic and mental health. In fact, in animal models, perinatal exposure to maternal obesity and a high fat diet has been demonstrated to alter the serotonergic [105] and dopaminergic [228] systems of offspring.
Placental Dysfunction
The placenta is highly sensitive to maternal metabolic status; gestational diabetes [149, 229, 230] and obesity are associated with an inflammatory response in this organ [149, 229–231]. Large animal studies report negative effects of obesity and over-nourishment on the placenta: decreased mass [232], reduced capillary density [232], and reduced uterine blood flow [232, 233].
Poor placental functioning is compounded by the transmission of inflammatory factors. Pregnant women who are obese have system-wide inflammation [229], and increased circulating cytokines further impair placental function [234, 235].
Gestational Exposure to Inflammation
Fetal brain development and neurotransmitter systems essential for behavioral regulation are sensitive to elevated circulating cytokines [236]. Pro-inflammatory factors initiate extensive neuronal plasticity and growth in the fetal brain and contribute to a state of chronic fetal inflammation [237]. Many symptoms of ASD are proposed to result from this early exposure to elevated inflammatory cytokines [237].
High fat diet-induced inflammatory factors are present in both obesity and MHDs. The structural differences of fetal brains exposed to high levels of IL-8 correspond with brain alterations seen in patients with schizophrenia [238]. Additionally, IL-6 has a critical influence on ASD risk in offspring [239]. IL-4 and IL-5 are elevated in mothers of children with ASD [240]. Over-nutrition results in increased levels of inflammatory factors that are also elevated in children with ASD [241, 242].
Alternate Mechanisms of Maternal Programming
Offspring exposed to maternal obesity and high fat diet consumption are exposed to excess levels of nutrients and hormones that are postulated to impact fetal brain development [11]. Maternal glucose passes through the placenta [243], and the pancreatic beta cells of the fetus respond by increasing insulin secretion. As insulin is a critical neural growth factor [244], this hyperinsulinemia during development of neural pathways may predispose the offspring of obese mothers to MetS. For example, rodent studies indicate that insulin administration during development produces obesity [245–247] and risk for diabetes [247] in offspring. In addition, offspring of obese mothers are exposed to elevated levels of leptin [244].
Conclusion
Recent scientific research has identified an association between components of MetS and MHDs, and common underlying mechanisms are credited. A number of lifestyle factors and neurological alterations increase vulnerability to both MetS and MHDs, but the overlapping neurological mechanisms that are implicated in both conditions include changes to neuroregulatory brain pathways, dysregulation of the HPA axis, and a chronic state of inflammation. Additionally, placental dysfunction allows mothers with MetS to transfer the inflammatory state and consequent brain alterations to their developing fetuses.
Psychopathologies have a high comorbidity with obesity and MetS, especially in women, and thus treatment for high body weight should include a therapeutic aspect that is specific to the presented disturbances in the patient. Beyond this holistic approach, it is imperative to prevent transferring these syndromes to the next generation by developing intervention strategies.
Fig. 1.
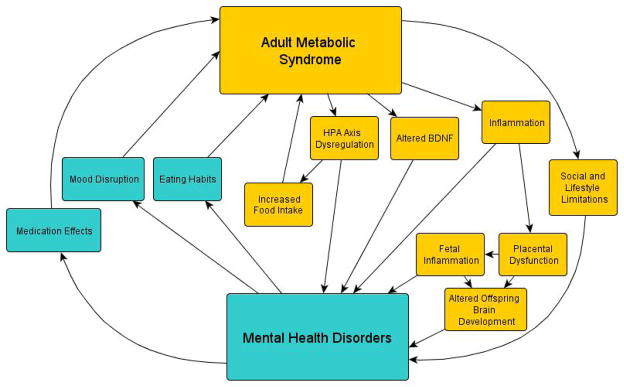
The bidirectional relationship and overlapping mechanisms of MetS and MHDs.
Acknowledgments
This publication was supported by the Murdock Charitable Trust, Murdock College Research Program for Life Science, grant number 2011273:HVP and Oregon Clinical and Translational Research Institute (OCTRI), grant number (UL1TR000128) from the National Center for Advancing Translational Sciences (NCATS) at the National Institutes of Health (NIH).
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References
- 1.Alberti KG, et al. The metabolic syndrome--a new worldwide definition. Lancet. 2005;366(9491):1059–62. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 2.Huang PL. A comprehensive definition for metabolic syndrome. Dis Model Mech. 2009;2(5–6):231–7. doi: 10.1242/dmm.001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weiss R. Childhood metabolic syndrome: must we define it to deal with it? Diabetes Care. 2011;34(Suppl 2):S171–6. doi: 10.2337/dc11-s214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cook S, et al. Prevalence of a metabolic syndrome phenotype in adolescents: findings from the third National Health and Nutrition Examination Survey, 1988–1994. Arch Pediatr Adolesc Med. 2003;157(8):821–7. doi: 10.1001/archpedi.157.8.821. [DOI] [PubMed] [Google Scholar]
- 5.Prevention, C.f.D.C.a; U.S.D.o.H.a.H. Services, editor Obesity Trends Among US Adults Between 1985 and 2010. [Google Scholar]
- 6.Flegal KM, et al. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307(5):491–7. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 7.Duncan GE, Li SM, Zhou XH. Prevalence and trends of a metabolic syndrome phenotype among u.s. Adolescents, 1999–2000. Diabetes Care. 2004;27(10):2438–43. doi: 10.2337/diacare.27.10.2438. [DOI] [PubMed] [Google Scholar]
- 8.Ogden CL, et al. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295(13):1549–55. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 9.Prevention, C.f.D.C.a; U.S.D.o.H.a.H. Services, editor. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. Atlanta, GA: 2011. [Google Scholar]
- 10.Boyle JP, et al. Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Popul Health Metr. 2010;8:29. doi: 10.1186/1478-7954-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sullivan EL, Grove KL. Metabolic imprinting in obesity. Forum Nutr. 2010;63:186–94. doi: 10.1159/000264406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kessler RC, et al. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):617–27. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boyle CA, et al. Trends in the prevalence of developmental disabilities in US children, 1997- 2008. Pediatrics. 2011;127(6):1034–42. doi: 10.1542/peds.2010-2989. [DOI] [PubMed] [Google Scholar]
- 14.Sullivan EL, Smith MS, Grove KL. Perinatal exposure to high-fat diet programs energy balance, metabolism and behavior in adulthood. Neuroendocrinology. 2011;93(1):1–8. doi: 10.1159/000322038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sullivan EL, Nousen EK, Chamlou KA. Maternal high fat diet consumption during the perinatal period programs offspring behavior. Physiol Behav. 2012 doi: 10.1016/j.physbeh.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carpiniello B, et al. Mental disorders in patients with metabolic syndrome. The key role of central obesity. Eat Weight Disord. 2012;17(4):e259–66. doi: 10.3275/8809. [DOI] [PubMed] [Google Scholar]
- 17.Newcomer JW. Metabolic syndrome and mental illness. Am J Manag Care. 2007;13(7 Suppl):S170–7. [PubMed] [Google Scholar]
- 18.Stanley SH, Laugharne JD. Obesity, cardiovascular disease and type 2 diabetes in people with a mental illness: a need for primary health care. Aust J Prim Health. 2012;18(3):258–64. doi: 10.1071/PY11045. [DOI] [PubMed] [Google Scholar]
- 19.Grover S, et al. Comparative study of prevalence of metabolic syndrome in bipolar disorder and schizophrenia from North India. Nord J Psychiatry. 2013 doi: 10.3109/08039488.2012.754052. [DOI] [PubMed] [Google Scholar]
- 20.Ahmed AT, Blair TR, McIntyre RS. Surgical treatment of morbid obesity among patients with bipolar disorder: a research agenda. Adv Ther. 2011;28(5):389–400. doi: 10.1007/s12325-011-0015-3. [DOI] [PubMed] [Google Scholar]
- 21.Mansur RB, et al. Selfish brain and neuroprogression in bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2013;43:66–71. doi: 10.1016/j.pnpbp.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 22.Allison DB, et al. Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry. 1999;156(11):1686–96. doi: 10.1176/ajp.156.11.1686. [DOI] [PubMed] [Google Scholar]
- 23.Almandil NB, et al. Weight gain and other metabolic adverse effects associated with atypical antipsychotic treatment of children and adolescents: a systematic review and meta-analysis. Paediatr Drugs. 2013;15(2):139–50. doi: 10.1007/s40272-013-0016-6. [DOI] [PubMed] [Google Scholar]
- 24.Allison DB, et al. Obesity among those with mental disorders: a National Institute of Mental Health meeting report. Am J Prev Med. 2009;36(4):341–50. doi: 10.1016/j.amepre.2008.11.020. [DOI] [PubMed] [Google Scholar]
- 25.Bensenor IM, et al. Cardiovascular risk factors in patients with first-episode psychosis in Sao Paulo, Brazil. Gen Hosp Psychiatry. 2012;34(3):268–75. doi: 10.1016/j.genhosppsych.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 26.Rofey DL, et al. A longitudinal study of childhood depression and anxiety in relation to weight gain. Child Psychiatry Hum Dev. 2009;40(4):517–26. doi: 10.1007/s10578-009-0141-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Halfon N, Larson K, Slusser W. Associations between obesity and comorbid mental health, developmental, and physical health conditions in a nationally representative sample of US children aged 10 to 17. Acad Pediatr. 2013;13(1):6–13. doi: 10.1016/j.acap.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 28.Maddi SR, et al. Psychosocial correlates of psychopathology in a national sample of the morbidly obese. Obes Surg. 1997;7(5):397–404. doi: 10.1381/096089297765555377. [DOI] [PubMed] [Google Scholar]
- 29.Scott KM, et al. Obesity and mental disorders in the general population: results from the world mental health surveys. Int J Obes (Lond) 2008;32(1):192–200. doi: 10.1038/sj.ijo.0803701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao G, et al. Waist circumference, abdominal obesity, and depression among overweight and obese U.S. adults: National Health and Nutrition Examination Survey 2005–2006. BMC Psychiatry. 2011;11:130. doi: 10.1186/1471-244X-11-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petry NM, et al. Overweight and obesity are associated with psychiatric disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Psychosom Med. 2008;70 (3):288–97. doi: 10.1097/PSY.0b013e3181651651. [DOI] [PubMed] [Google Scholar]
- 32.Zhong W, et al. Obesity and depression symptoms in the Beaver Dam Offspring Study population. Depress Anxiety. 2010;27(9):846–51. doi: 10.1002/da.20666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nichols GA, Brown JB. Unadjusted and adjusted prevalence of diagnosed depression in type 2 diabetes. Diabetes Care. 2003;26(3):744–9. doi: 10.2337/diacare.26.3.744. [DOI] [PubMed] [Google Scholar]
- 34.Bajaj S, et al. Association of depression and its relation with complications in newly diagnosed type 2 diabetes. Indian J Endocrinol Metab. 2012;16(5):759–63. doi: 10.4103/2230-8210.100670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mezuk B, et al. Is ignorance bliss? Depression, antidepressants, and the diagnosis of prediabetes and type 2 diabetes. Health Psychol. 2013;32(3):254–63. doi: 10.1037/a0029014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pouwer F, et al. Rates and risks for co-morbid depression in patients with Type 2 diabetes mellitus: results from a community-based study. Diabetologia. 2003;46(7):892–8. doi: 10.1007/s00125-003-1124-6. [DOI] [PubMed] [Google Scholar]
- 37.Peyrot M, Rubin RR. Levels and risks of depression and anxiety symptomatology among diabetic adults. Diabetes Care. 1997;20(4):585–90. doi: 10.2337/diacare.20.4.585. [DOI] [PubMed] [Google Scholar]
- 38.Fowler-Brown AG, Ngo LH, Wee CC. The relationship between symptoms of depression and body weight in younger adults. Obesity (Silver Spring) 2012;20(9):1922–8. doi: 10.1038/oby.2011.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Waring ME, Lapane KL. Overweight in children and adolescents in relation to attention-deficit/hyperactivity disorder: results from a national sample. Pediatrics. 2008;122(1):e1–6. doi: 10.1542/peds.2007-1955. [DOI] [PubMed] [Google Scholar]
- 40.Pagoto SL, et al. Association between adult attention deficit/hyperactivity disorder and obesity in the US population. Obesity (Silver Spring) 2009;17(3):539–44. doi: 10.1038/oby.2008.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Erhart M, et al. Examining the relationship between attention-deficit/hyperactivity disorder and overweight in children and adolescents. Eur Child Adolesc Psychiatry. 2012;21(1):39–49. doi: 10.1007/s00787-011-0230-0. [DOI] [PubMed] [Google Scholar]
- 42.Chen AY, et al. Prevalence of obesity among children with chronic conditions. Obesity (Silver Spring) 2010;18(1):210–3. doi: 10.1038/oby.2009.185. [DOI] [PubMed] [Google Scholar]
- 43.Alfonsson S, Parling T, Ghaderi A. Screening of adult ADHD among patients presenting for bariatric surgery. Obes Surg. 2012;22(6):918–26. doi: 10.1007/s11695-011-0569-9. [DOI] [PubMed] [Google Scholar]
- 44.Rimmer JH, et al. Obesity and obesity-related secondary conditions in adolescents with intellectual/developmental disabilities. J Intellect Disabil Res. 2010;54(9):787–94. doi: 10.1111/j.1365-2788.2010.01305.x. [DOI] [PubMed] [Google Scholar]
- 45.Hyman SL, et al. Nutrient intake from food in children with autism. Pediatrics. 2012;130(Suppl 2):S145–53. doi: 10.1542/peds.2012-0900L. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Zwaan M. Binge eating disorder and obesity. Int J Obes Relat Metab Disord. 2001;25(Suppl 1):S51–5. doi: 10.1038/sj.ijo.0801699. [DOI] [PubMed] [Google Scholar]
- 47.Cleator J, et al. Night eating syndrome: implications for severe obesity. Nutr Diabetes. 2012;2:e44. doi: 10.1038/nutd.2012.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Milano W, et al. Night eating syndrome: an overview. J Pharm Pharmacol. 2012;64(1):2–10. doi: 10.1111/j.2042-7158.2011.01353.x. [DOI] [PubMed] [Google Scholar]
- 49.Vander Wal JS. Night eating syndrome: a critical review of the literature. Clin Psychol Rev. 2012;32(1):49–59. doi: 10.1016/j.cpr.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 50.Grilo CM, et al. Night eating in obese treatment-seeking Hispanic patients with and without binge eating disorder. Int J Eat Disord. 2012;45(6):787–91. doi: 10.1002/eat.22011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Docet MF, et al. Attention deficit hyperactivity disorder increases the risk of having abnormal eating behaviours in obese adults. Eat Weight Disord. 2012;17(2):e132–6. doi: 10.1007/BF03325337. [DOI] [PubMed] [Google Scholar]
- 52.Al-Ayadhi LY. Pro-inflammatory cytokines in autistic children in central Saudi Arabia. Neurosciences (Riyadh) 2005;10(2):155–8. [PubMed] [Google Scholar]
- 53.Lopez-Pantoja JL, et al. Personality profiles between obese and control subjects assessed with five standardized personality scales. Actas Esp Psiquiatr. 2012;40(5):266–74. [PubMed] [Google Scholar]
- 54.Zhao G, et al. Depression and anxiety among US adults: associations with body mass index. Int J Obes (Lond) 2009;33(2):257–66. doi: 10.1038/ijo.2008.268. [DOI] [PubMed] [Google Scholar]
- 55.Czegledi E, Urban R. Risk factors and alteration of depression among participants of an inpatient weight loss program. Psychiatr Hung. 2012;27(5):361–78. [PubMed] [Google Scholar]
- 56.McLaren L, et al. The relationship between body mass index and mental health. A population-based study of the effects of the definition of mental health. Soc Psychiatry Psychiatr Epidemiol. 2008;43(1):63–71. doi: 10.1007/s00127-007-0269-x. [DOI] [PubMed] [Google Scholar]
- 57.Pickering RP, et al. Temporal relationships between overweight and obesity and DSM-IV substance use, mood, and anxiety disorders: results from a prospective study, the National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry. 2011;72(11):1494–502. doi: 10.4088/JCP.10m06077gry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Carpenter KM, et al. Relationships between obesity and DSM-IV major depressive disorder, suicide ideation, and suicide attempts: results from a general population study. Am J Public Health. 2000;90(2):251–7. doi: 10.2105/ajph.90.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Heo M, et al. Depressive mood and obesity in US adults: comparison and moderation by sex, age, and race. Int J Obes (Lond) 2006;30(3):513–9. doi: 10.1038/sj.ijo.0803122. [DOI] [PubMed] [Google Scholar]
- 60.Onyike CU, et al. Is obesity associated with major depression? Results from the Third National Health and Nutrition Examination Survey. Am J Epidemiol. 2003;158(12):1139–47. doi: 10.1093/aje/kwg275. [DOI] [PubMed] [Google Scholar]
- 61.Stunkard AJ, Faith MS, Allison KC. Depression and obesity. Biol Psychiatry. 2003;54(3):330–7. doi: 10.1016/s0006-3223(03)00608-5. [DOI] [PubMed] [Google Scholar]
- 62.Papageorgiou GM, et al. Pre- and postoperative psychological characteristics in morbidly obese patients. Obes Surg. 2002;12(4):534–9. doi: 10.1381/096089202762252307. [DOI] [PubMed] [Google Scholar]
- 63.Fleming JP, Levy LD, Levitan RD. Symptoms of attention deficit hyperactivity disorder in severely obese women. Eat Weight Disord. 2005;10(1):e10–3. doi: 10.1007/BF03354661. [DOI] [PubMed] [Google Scholar]
- 64.Kasen S, et al. Obesity and psychopathology in women: a three decade prospective study. Int J Obes (Lond) 2008;32(3):558–66. doi: 10.1038/sj.ijo.0803736. [DOI] [PubMed] [Google Scholar]
- 65.Black DW, Goldstein RB, Mason EE. Prevalence of mental disorder in 88 morbidly obese bariatric clinic patients. Am J Psychiatry. 1992;149(2):227–34. doi: 10.1176/ajp.149.2.227. [DOI] [PubMed] [Google Scholar]
- 66.Pan A, et al. Bidirectional association between depression and type 2 diabetes mellitus in women. Arch Intern Med. 2010;170(21):1884–91. doi: 10.1001/archinternmed.2010.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Roberts RE, et al. Prospective association between obesity and depression: evidence from the Alameda County Study. Int J Obes Relat Metab Disord. 2003;27(4):514–21. doi: 10.1038/sj.ijo.0802204. [DOI] [PubMed] [Google Scholar]
- 68.Luppino FS, et al. Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Arch Gen Psychiatry. 2010;67(3):220–9. doi: 10.1001/archgenpsychiatry.2010.2. [DOI] [PubMed] [Google Scholar]
- 69.Simon GE, et al. Association between obesity and psychiatric disorders in the US adult population. Arch Gen Psychiatry. 2006;63(7):824–30. doi: 10.1001/archpsyc.63.7.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Koponen H, et al. Metabolic syndrome predisposes to depressive symptoms: a population-based 7-year follow-up study. J Clin Psychiatry. 2008;69(2):178–82. doi: 10.4088/jcp.v69n0202. [DOI] [PubMed] [Google Scholar]
- 71.Aarts S, et al. Diabetes mellitus type II as a risk factor for depression: a lower than expected risk in a general practice setting. Eur J Epidemiol. 2009;24(10):641–8. doi: 10.1007/s10654-009-9385-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.O’Connor PJ, et al. Does diabetes double the risk of depression? Ann Fam Med. 2009;7(4):328–35. doi: 10.1370/afm.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mezuk B, et al. Depression and type 2 diabetes over the lifespan: a meta-analysis. Diabetes Care. 2008;31(12):2383–90. doi: 10.2337/dc08-0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bisschop MI, et al. The longitudinal relation between chronic diseases and depression in older persons in the community: the Longitudinal Aging Study Amsterdam. J Clin Epidemiol. 2004;57(2):187–94. doi: 10.1016/j.jclinepi.2003.01.001. [DOI] [PubMed] [Google Scholar]
- 75.Nouwen A, et al. Type 2 diabetes mellitus as a risk factor for the onset of depression: a systematic review and meta-analysis. Diabetologia. 2010;53(12):2480–6. doi: 10.1007/s00125-010-1874-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kivimaki M, et al. Antidepressant use before and after the diagnosis of type 2 diabetes: a longitudinal modeling study. Diabetes Care. 2010;33(7):1471–6. doi: 10.2337/dc09-2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Knol MJ, et al. Antidepressant use before and after initiation of diabetes mellitus treatment. Diabetologia. 2009;52(3):425–32. doi: 10.1007/s00125-008-1249-8. [DOI] [PubMed] [Google Scholar]
- 78.Tyler CV, et al. Chronic disease risks in young adults with autism spectrum disorder: forewarned is forearmed. Am J Intellect Dev Disabil. 2011;116(5):371–80. doi: 10.1352/1944-7558-116.5.371. [DOI] [PubMed] [Google Scholar]
- 79.Cortese S, et al. Obesity in Men With Childhood ADHD: A 33-Year Controlled, Prospective, Follow-up Study. Pediatrics. 2013 doi: 10.1542/peds.2012-0540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Valtonen MK, et al. Low-grade inflammation and depressive symptoms as predictors of abdominal obesity. Scand J Public Health. 2012;40(7):674–80. doi: 10.1177/1403494812461730. [DOI] [PubMed] [Google Scholar]
- 81.Hasnain M, WVRV, Hollett B. Weight gain and glucose dysregulation with second-generation antipsychotics and antidepressants: a review for primary care physicians. Postgrad Med. 2012;124(4):154–67. doi: 10.3810/pgm.2012.07.2577. [DOI] [PubMed] [Google Scholar]
- 82.Deuschle M. Effects of antidepressants on glucose metabolism and diabetes mellitus type 2 in adults. Curr Opin Psychiatry. 2013;26(1):60–5. doi: 10.1097/YCO.0b013e32835a4206. [DOI] [PubMed] [Google Scholar]
- 83.Appelhans BM, et al. Depression severity, diet quality, and physical activity in women with obesity and depression. J Acad Nutr Diet. 2012;112(5):693–8. doi: 10.1016/j.jand.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gibson EL. The psychobiology of comfort eating: implications for neuropharmacological interventions. Behav Pharmacol. 2012;23(5–6):442–60. doi: 10.1097/FBP.0b013e328357bd4e. [DOI] [PubMed] [Google Scholar]
- 85.Champaneri S, et al. Biological basis of depression in adults with diabetes. Curr Diab Rep. 2010;10 (6):396–405. doi: 10.1007/s11892-010-0148-9. [DOI] [PubMed] [Google Scholar]
- 86.Kinzl JF, Maier C, Bosch A. Morbidly obese patients: psychopathology and eating disorders - Results of a preoperative evaluation. Neuropsychiatr. 2012;26(4):159–65. doi: 10.1007/s40211-012-0036-4. [DOI] [PubMed] [Google Scholar]
- 87.Karlsson J, et al. Psychosocial functioning in the obese before and after weight reduction: construct validity and responsiveness of the Obesity-related Problems scale. Int J Obes Relat Metab Disord. 2003;27(5):617–30. doi: 10.1038/sj.ijo.0802272. [DOI] [PubMed] [Google Scholar]
- 88.Maddi SR, et al. Reduction in psychopathology following bariatric surgery for morbid obesity. Obes Surg. 2001;11(6):680–5. doi: 10.1381/09608920160558605. [DOI] [PubMed] [Google Scholar]
- 89.Sysko R, et al. Psychological outcomes and predictors of initial weight loss outcomes among severely obese adolescents receiving laparoscopic adjustable gastric banding. J Clin Psychiatry. 2012;73(10):1351–7. doi: 10.4088/JCP.12m07690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dixon JB, Dixon ME, O’Brien PE. Depression in association with severe obesity: changes with weight loss. Arch Intern Med. 2003;163(17):2058–65. doi: 10.1001/archinte.163.17.2058. [DOI] [PubMed] [Google Scholar]
- 91.Peterhansel C, et al. Risk of completed suicide after bariatric surgery: a systematic review. Obes Rev. 2013 doi: 10.1111/obr.12014. [DOI] [PubMed] [Google Scholar]
- 92.Klinitzke G, et al. Obesity and suicide risk in adults--a systematic review. J Affect Disord. 2013;145(3):277–84. doi: 10.1016/j.jad.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 93.Krakowiak P, et al. Maternal metabolic conditions and risk for autism and other neurodevelopmental disorders. Pediatrics. 2012;129(5):e1121–8. doi: 10.1542/peds.2011-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Leonard H, et al. Maternal health in pregnancy and intellectual disability in the offspring: a population-based study. Ann Epidemiol. 2006;16(6):448–54. doi: 10.1016/j.annepidem.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 95.Hamlyn J, et al. Modifiable risk factors for schizophrenia and autism - Shared risk factors impacting on brain development. Neurobiol Dis. 2013;53:3–9. doi: 10.1016/j.nbd.2012.10.023. [DOI] [PubMed] [Google Scholar]
- 96.Rodriguez A. Maternal pre-pregnancy obesity and risk for inattention and negative emotionality in children. J Child Psychol Psychiatry. 2010;51(2):134–43. doi: 10.1111/j.1469-7610.2009.02133.x. [DOI] [PubMed] [Google Scholar]
- 97.MR, et al. Pre-pregnancy maternal overweight and obesity increase the risk for affective disorders in offspring. J Dev Origins of Health and Disease. 2013;4(1):42–48. doi: 10.1017/S2040174412000578. [DOI] [PubMed] [Google Scholar]
- 98.Buss C, et al. Impaired executive function mediates the association between maternal pre-pregnancy body mass index and child ADHD symptoms. PLoS One. 2012;7(6):e37758. doi: 10.1371/journal.pone.0037758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rodriguez A, et al. Maternal adiposity prior to pregnancy is associated with ADHD symptoms in offspring: evidence from three prospective pregnancy cohorts. Int J Obes (Lond) 2008;32(3):550–7. doi: 10.1038/sj.ijo.0803741. [DOI] [PubMed] [Google Scholar]
- 100.Ray GT, Croen LA, Habel LA. Mothers of children diagnosed with attention-deficit/hyperactivity disorder: health conditions and medical care utilization in periods before and after birth of the child. Med Care. 2009;47(1):105–14. doi: 10.1097/MLR.0b013e31817e18c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tozuka Y, et al. Maternal obesity impairs hippocampal BDNF production and spatial learning performance in young mouse offspring. Neurochem Int. 2010;57(3):235–47. doi: 10.1016/j.neuint.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 102.Raygada M, Cho E, Hilakivi-Clarke L. High maternal intake of polyunsaturated fatty acids during pregnancy in mice alters offsprings’ aggressive behavior, immobility in the swim test, locomotor activity and brain protein kinase C activity. J Nutr. 1998;128(12):2505–11. doi: 10.1093/jn/128.12.2505. [DOI] [PubMed] [Google Scholar]
- 103.Peleg-Raibstein D, Luca E, Wolfrum C. Maternal high-fat diet in mice programs emotional behavior in adulthood. Behav Brain Res. 2012;233(2):398–404. doi: 10.1016/j.bbr.2012.05.027. [DOI] [PubMed] [Google Scholar]
- 104.Bilbo SD, Tsang V. Enduring consequences of maternal obesity for brain inflammation and behavior of offspring. FASEB J. 2010;24(6):2104–15. doi: 10.1096/fj.09-144014. [DOI] [PubMed] [Google Scholar]
- 105.Sullivan EL, et al. Chronic consumption of a high-fat diet during pregnancy causes perturbations in the serotonergic system and increased anxiety-like behavior in nonhuman primate offspring. J Neurosci. 2010;30(10):3826–30. doi: 10.1523/JNEUROSCI.5560-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gong Y, et al. Female early adult depression results in detrimental impacts on the behavioral performance and brain development in offspring. CNS Neurosci Ther. 2012;18(6):461–70. doi: 10.1111/j.1755-5949.2012.00324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Deng T, et al. Class II Major Histocompatibility Complex Plays an Essential Role in Obesity-Induced Adipose Inflammation. Cell Metab. 2013;17(3):411–22. doi: 10.1016/j.cmet.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bauer S, Kerr BJ, Patterson PH. The neuropoietic cytokine family in development, plasticity, disease and injury. Nat Rev Neurosci. 2007;8(3):221–32. doi: 10.1038/nrn2054. [DOI] [PubMed] [Google Scholar]
- 109.Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302(5651):1760–5. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- 110.Lagace DC, Noonan MA, Eisch AJ. Hippocampal neurogenesis: a matter of survival. Am J Psychiatry. 2007;164(2):205. doi: 10.1176/ajp.2007.164.2.205. [DOI] [PubMed] [Google Scholar]
- 111.Watanabe Y, Someya T, Nawa H. Cytokine hypothesis of schizophrenia pathogenesis: evidence from human studies and animal models. Psychiatry Clin Neurosci. 2010;64(3):217–30. doi: 10.1111/j.1440-1819.2010.02094.x. [DOI] [PubMed] [Google Scholar]
- 112.Sasayama D, et al. Increased cerebrospinal fluid interleukin-6 levels in patients with schizophrenia and those with major depressive disorder. J Psychiatr Res. 2013;47(3):401–6. doi: 10.1016/j.jpsychires.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 113.McKernan DP, et al. Enhanced peripheral toll-like receptor responses in psychosis: further evidence of a pro-inflammatory phenotype. Transl Psychiatry. 2011;1:e36. doi: 10.1038/tp.2011.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kubistova A, Horacek J, Novak T. Increased interleukin-6 and tumor necrosis factor alpha in first episode schizophrenia patients versus healthy controls. Psychiatr Danub. 2012;24(Suppl 1):S153–6. [PubMed] [Google Scholar]
- 115.Fillman SG, et al. Increased inflammatory markers identified in the dorsolateral prefrontal cortex of individuals with schizophrenia. Mol Psychiatry. 2013;18(2):206–14. doi: 10.1038/mp.2012.110. [DOI] [PubMed] [Google Scholar]
- 116.Nawa H, Yamada K. Experimental schizophrenia models in rodents established with inflammatory agents and cytokines. Methods Mol Biol. 2012;829:445–51. doi: 10.1007/978-1-61779-458-2_28. [DOI] [PubMed] [Google Scholar]
- 117.Tohmi M, et al. Perinatal inflammatory cytokine challenge results in distinct neurobehavioral alterations in rats: implication in psychiatric disorders of developmental origin. Neurosci Res. 2004;50(1):67–75. doi: 10.1016/j.neures.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 118.Alesci S, et al. Major depression is associated with significant diurnal elevations in plasma interleukin-6 levels, a shift of its circadian rhythm, and loss of physiological complexity in its secretion: clinical implications. J Clin Endocrinol Metab. 2005;90(5):2522–30. doi: 10.1210/jc.2004-1667. [DOI] [PubMed] [Google Scholar]
- 119.Danner M, et al. Association between depression and elevated C-reactive protein. Psychosom Med. 2003;65(3):347–56. doi: 10.1097/01.psy.0000041542.29808.01. [DOI] [PubMed] [Google Scholar]
- 120.Vogelzangs N, et al. Association of depressive disorders, depression characteristics and antidepressant medication with inflammation. Transl Psychiatry. 2012;2:e79. doi: 10.1038/tp.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Penninx BW, et al. Inflammatory markers and depressed mood in older persons: results from the Health, Aging and Body Composition study. Biol Psychiatry. 2003;54(5):566–72. doi: 10.1016/s0006-3223(02)01811-5. [DOI] [PubMed] [Google Scholar]
- 122.Bremmer MA, et al. Inflammatory markers in late-life depression: results from a population-based study. J Affect Disord. 2008;106(3):249–55. doi: 10.1016/j.jad.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 123.Dentino AN, et al. Association of interleukin-6 and other biologic variables with depression in older people living in the community. J Am Geriatr Soc. 1999;47(1):6–11. doi: 10.1111/j.1532-5415.1999.tb01894.x. [DOI] [PubMed] [Google Scholar]
- 124.Donev R, Thome J. Inflammation: good or bad for ADHD? Atten Defic Hyperact Disord. 2010;2(4):257–66. doi: 10.1007/s12402-010-0038-7. [DOI] [PubMed] [Google Scholar]
- 125.Fredriksson A, Archer T. Neurobehavioural deficits associated with apoptotic neurodegeneration and vulnerability for ADHD. Neurotox Res. 2004;6(6):435–56. doi: 10.1007/BF03033280. [DOI] [PubMed] [Google Scholar]
- 126.Wei H, et al. IL-6 is increased in the cerebellum of autistic brain and alters neural cell adhesion, migration and synaptic formation. J Neuroinflammation. 2011;8:52. doi: 10.1186/1742-2094-8-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.El-Ansary A, Al-Ayadhi L. Neuroinflammation in autism spectrum disorders. J Neuroinflammation. 2012;9:265. doi: 10.1186/1742-2094-9-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wei H, et al. Brain IL-6 elevation causes neuronal circuitry imbalances and mediates autism-like behaviors. Biochim Biophys Acta. 2012;1822(6):831–42. doi: 10.1016/j.bbadis.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 129.Hall FS, et al. Reduced behavioral effects of cocaine in heterozygous brain-derived neurotrophic factor (BDNF) knockout mice. Neuropsychopharmacology. 2003;28(8):1485–90. doi: 10.1038/sj.npp.1300192. [DOI] [PubMed] [Google Scholar]
- 130.Byerly MS, et al. Effects of BDNF, T3, and corticosterone on expression of the hypothalamic obesity gene network in vivo and in vitro. Am J Physiol Regul Integr Comp Physiol. 2009;296(4):R1180–9. doi: 10.1152/ajpregu.90813.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Unger TJ, et al. Selective deletion of Bdnf in the ventromedial and dorsomedial hypothalamus of adult mice results in hyperphagic behavior and obesity. J Neurosci. 2007;27(52):14265–74. doi: 10.1523/JNEUROSCI.3308-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Li W, et al. Association of BDNF gene polymorphisms with schizophrenia and clinical symptoms in a Chinese population. Am J Med Genet B Neuropsychiatr Genet. 2013 doi: 10.1002/ajmg.b.32183. [DOI] [PubMed] [Google Scholar]
- 133.Watanabe Y, Nunokawa A, Someya T. Association of the BDNF C270T polymorphism with schizophrenia: updated meta-analysis. Psychiatry Clin Neurosci. 2013;67(2):123–5. doi: 10.1111/pcn.12018. [DOI] [PubMed] [Google Scholar]
- 134.Favalli G, et al. The role of BDNF in the pathophysiology and treatment of schizophrenia. J Psychiatr Res. 2012;46(1):1–11. doi: 10.1016/j.jpsychires.2011.09.022. [DOI] [PubMed] [Google Scholar]
- 135.Sun MM, et al. BDNF Val66Met polymorphism and anxiety/depression symptoms in schizophrenia in a Chinese Han population. Psychiatr Genet. 2013;23(3):124–9. doi: 10.1097/YPG.0b013e328360c866. [DOI] [PubMed] [Google Scholar]
- 136.Ernst C, et al. Highly penetrant alterations of a critical region including BDNF in human psychopathology and obesity. Arch Gen Psychiatry. 2012;69(12):1238–46. doi: 10.1001/archgenpsychiatry.2012.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hallden S, et al. Smoking and obesity associated BDNF gene variance predicts total and cardiovascular mortality in smokers. Heart. 2013;99(13):949–53. doi: 10.1136/heartjnl-2013-303634. [DOI] [PubMed] [Google Scholar]
- 138.Hotta K, et al. Association between obesity and polymorphisms in SEC16B, TMEM18, GNPDA2, BDNF, FAIM2 and MC4R in a Japanese population. J Hum Genet. 2009;54(12):727–31. doi: 10.1038/jhg.2009.106. [DOI] [PubMed] [Google Scholar]
- 139.Beckers S, et al. Association of the BDNF Val66Met variation with obesity in women. Mol Genet Metab. 2008;95(1–2):110–2. doi: 10.1016/j.ymgme.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 140.Gray J, et al. Hyperphagia, severe obesity, impaired cognitive function, and hyperactivity associated with functional loss of one copy of the brain-derived neurotrophic factor (BDNF) gene. Diabetes. 2006;55(12):3366–71. doi: 10.2337/db06-0550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Coppola V, Tessarollo L. Control of hyperphagia prevents obesity in BDNF heterozygous mice. Neuroreport. 2004;15(17):2665–8. doi: 10.1097/00001756-200412030-00022. [DOI] [PubMed] [Google Scholar]
- 142.Sha H, et al. Disruption of a novel regulatory locus results in decreased Bdnf expression, obesity, and type 2 diabetes in mice. Physiol Genomics. 2007;31(2):252–63. doi: 10.1152/physiolgenomics.00093.2007. [DOI] [PubMed] [Google Scholar]
- 143.Faludi G, et al. Pharmaco- and therapygenetic aspects in the treatment of anxiety disorders beyond the serotonergic system: a brief review. Neuropsychopharmacol Hung. 2012;14(4):221–9. [PubMed] [Google Scholar]
- 144.Spindelegger C, et al. Influence of escitalopram treatment on 5-HT 1A receptor binding in limbic regions in patients with anxiety disorders. Mol Psychiatry. 2009;14(11):1040–50. doi: 10.1038/mp.2008.35. [DOI] [PubMed] [Google Scholar]
- 145.Sullivan GM, et al. Low cerebrospinal fluid transthyretin levels in depression: correlations with suicidal ideation and low serotonin function. Biol Psychiatry. 2006;60(5):500–6. doi: 10.1016/j.biopsych.2005.11.022. [DOI] [PubMed] [Google Scholar]
- 146.Kiyohara C, Yoshimasu K. Molecular epidemiology of major depressive disorder. Environ Health Prev Med. 2009;14(2):71–87. doi: 10.1007/s12199-008-0073-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Oades RD, et al. The influence of serotonin- and other genes on impulsive behavioral aggression and cognitive impulsivity in children with attention-deficit/hyperactivity disorder (ADHD): Findings from a family-based association test (FBAT) analysis. Behav Brain Funct. 2008;4:48. doi: 10.1186/1744-9081-4-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Chugani DC, et al. Developmental changes in brain serotonin synthesis capacity in autistic and nonautistic children. Ann Neurol. 1999;45(3):287–95. doi: 10.1002/1531-8249(199903)45:3<287::aid-ana3>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 149.Challier JC, et al. Obesity in pregnancy stimulates macrophage accumulation and inflammation in the placenta. Placenta. 2008;29(3):274–81. doi: 10.1016/j.placenta.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Andrews N, et al. 5-HT1A receptors in the median raphe nucleus and dorsal hippocampus may mediate anxiolytic and anxiogenic behaviours respectively. Eur J Pharmacol. 1994;264(3):259–64. doi: 10.1016/0014-2999(94)00473-0. [DOI] [PubMed] [Google Scholar]
- 151.Ghosh MN, Parvathy S. The effect of cyproheptadine on water and food intake and on body weight in the fasted adult and weanling rats. Br J Pharmacol. 1973;48(2):328P–329P. [PMC free article] [PubMed] [Google Scholar]
- 152.Blundell JE, Leshem MB. Central action of anorexic agents: effects of amphetamine and fenfluramine in rats with lateral hypothalamic lesions. Eur J Pharmacol. 1974;28(1):81–8. doi: 10.1016/0014-2999(74)90115-0. [DOI] [PubMed] [Google Scholar]
- 153.Geyer MA, et al. Behavioral studies following lesions of the mesolimbic and mesostriatal serotonergic pathways. Brain Res. 1976;106(2):257–69. doi: 10.1016/0006-8993(76)91024-6. [DOI] [PubMed] [Google Scholar]
- 154.Saller CF, Stricker EM. Hyperphagia and increased growth in rats after intraventricular injection of 5,7-dihydroxytryptamine. Science. 1976;192(4237):385–7. doi: 10.1126/science.1257774. [DOI] [PubMed] [Google Scholar]
- 155.Blundell JE, Leshem MB. The effect of 5-hydroxytryptophan on food intake and on the anorexic action of amphetamine and fenfluramine. J Pharm Pharmacol. 1975;27(1):31–7. doi: 10.1111/j.2042-7158.1975.tb09374.x. [DOI] [PubMed] [Google Scholar]
- 156.Grinker JA, et al. Effects of d-amphetamine and fenfluramine on feeding pattens and activity of obese and lean Zucker rats. Pharmacol Biochem Behav. 1980;12(2):265–75. doi: 10.1016/0091-3057(80)90367-6. [DOI] [PubMed] [Google Scholar]
- 157.Foltin RW, Moran TH. Food intake in baboons: effects of a long-acting cholecystokinin analog. Appetite. 1989;12(2):145–52. doi: 10.1016/0195-6663(89)90103-7. [DOI] [PubMed] [Google Scholar]
- 158.Rogers PJ, Blundell JE. Effect of anorexic drugs on food intake and the micro-structure of eating in human subjects. Psychopharmacology (Berl) 1979;66(2):159–65. doi: 10.1007/BF00427624. [DOI] [PubMed] [Google Scholar]
- 159.McGuirk J, et al. Differential effects of d-fenfluramine, l-fenfluramine and d-amphetamine on the microstructure of human eating behaviour. Behav Pharmacol. 1991;2(2):113–119. [PubMed] [Google Scholar]
- 160.Hoffman BJ, Mezey E. Distribution of serotonin 5-HT1C receptor mRNA in adult rat brain. FEBS Lett. 1989;247(2):453–62. doi: 10.1016/0014-5793(89)81390-0. [DOI] [PubMed] [Google Scholar]
- 161.Wright DE, et al. Comparative localization of serotonin1A, 1C, and 2 receptor subtype mRNAs in rat brain. J Comp Neurol. 1995;351(3):357–73. doi: 10.1002/cne.903510304. [DOI] [PubMed] [Google Scholar]
- 162.Pasqualetti M, et al. Distribution and cellular localization of the serotonin type 2C receptor messenger RNA in human brain. Neuroscience. 1999;92(2):601–11. doi: 10.1016/s0306-4522(99)00011-1. [DOI] [PubMed] [Google Scholar]
- 163.Pazos A, Palacios JM. Quantitative autoradiographic mapping of serotonin receptors in the rat brain. I. Serotonin-1 receptors. Brain Res. 1985;346(2):205–30. doi: 10.1016/0006-8993(85)90856-x. [DOI] [PubMed] [Google Scholar]
- 164.Kennett GA, Curzon G. Evidence that hypophagia induced by mCPP and TFMPP requires 5-HT1C and 5-HT1B receptors; hypophagia induced by RU 24969 only requires 5-HT1B receptors. Psychopharmacology (Berl) 1988;96(1):93–100. doi: 10.1007/BF02431539. [DOI] [PubMed] [Google Scholar]
- 165.Halford JC, Blundell JE. The 5-HT1B receptor agonist CP-94,253 reduces food intake and preserves the behavioural satiety sequence. Physiol Behav. 1996;60(3):933–9. doi: 10.1016/0031-9384(96)00073-x. [DOI] [PubMed] [Google Scholar]
- 166.Lee MD, Simansky KJ. CP-94, 253: a selective serotonin1B (5-HT1B) agonist that promotes satiety. Psychopharmacology (Berl) 1997;131(3):264–70. doi: 10.1007/s002130050292. [DOI] [PubMed] [Google Scholar]
- 167.Tecott LH, et al. Eating disorder and epilepsy in mice lacking 5-HT2c serotonin receptors. Nature. 1995;374(6522):542–6. doi: 10.1038/374542a0. [DOI] [PubMed] [Google Scholar]
- 168.Nonogaki K, et al. Leptin-independent hyperphagia and type 2 diabetes in mice with a mutated serotonin 5-HT2C receptor gene. Nat Med. 1998;4(10):1152–6. doi: 10.1038/2647. [DOI] [PubMed] [Google Scholar]
- 169.Mangge H, et al. Obesity-related dysregulation of the tryptophan-kynurenine metabolism: Role of age and parameters of the metabolic syndrome. Obesity (Silver Spring) 2013 doi: 10.1002/oby.20491. [DOI] [PubMed] [Google Scholar]
- 170.Dean B. Neurochemistry of schizophrenia: the contribution of neuroimaging postmortem pathology and neurochemistry in schizophrenia. Curr Top Med Chem. 2012;12(21):2375–92. doi: 10.2174/156802612805289935. [DOI] [PubMed] [Google Scholar]
- 171.Wu H, et al. SPECT imaging of dopamine transporters with (99m)Tc-TRODAT-1 in major depression and Parkinson’s disease. J Neuropsychiatry Clin Neurosci. 2011;23(1):63–7. doi: 10.1176/jnp.23.1.jnp63. [DOI] [PubMed] [Google Scholar]
- 172.Felicio AC, et al. Higher dopamine transporter density in Parkinson’s disease patients with depression. Psychopharmacology (Berl) 2010;211(1):27–31. doi: 10.1007/s00213-010-1867-y. [DOI] [PubMed] [Google Scholar]
- 173.Cervenka S, et al. Changes in dopamine D2-receptor binding are associated to symptom reduction after psychotherapy in social anxiety disorder. Transl Psychiatry. 2012;2:e120. doi: 10.1038/tp.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Spencer TJ, et al. Functional Genomics of Attention-Deficit/Hyperactivity Disorder (ADHD) Risk Alleles on Dopamine Transporter Binding in ADHD and Healthy Control Subjects. Biol Psychiatry. 2012 doi: 10.1016/j.biopsych.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Nakamura K, et al. Brain serotonin and dopamine transporter bindings in adults with high-functioning autism. Arch Gen Psychiatry. 2010;67(1):59–68. doi: 10.1001/archgenpsychiatry.2009.137. [DOI] [PubMed] [Google Scholar]
- 176.Sun X, Yue J, Zheng C. Study of dopamine transporter imaging on the brain of children with autism. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2008;25(2):327–30. [PubMed] [Google Scholar]
- 177.Pattarachotanant N, et al. Association of C/T polymorphism in intron 14 of the dopamine transporter gene (rs40184) with major depression in a northeastern Thai population. Genet Mol Res. 2010;9(1):565–72. doi: 10.4238/vol9-1gmr757. [DOI] [PubMed] [Google Scholar]
- 178.van der Wee NJ, et al. Increased serotonin and dopamine transporter binding in psychotropic medication-naive patients with generalized social anxiety disorder shown by 123I-beta-(4-iodophenyl)-tropane SPECT. J Nucl Med. 2008;49(5):757–63. doi: 10.2967/jnumed.107.045518. [DOI] [PubMed] [Google Scholar]
- 179.Oh KS, et al. Dopamine transporter genotype influences the attention deficit in Korean boys with ADHD. Yonsei Med J. 2003;44(5):787–92. doi: 10.3349/ymj.2003.44.5.787. [DOI] [PubMed] [Google Scholar]
- 180.El-Tarras AE, et al. Association study between the dopamine-related candidate gene polymorphisms and ADHD among Saudi Arabia population via PCR technique. Mol Biol Rep. 2012;39(12):11081–6. doi: 10.1007/s11033-012-2012-2. [DOI] [PubMed] [Google Scholar]
- 181.Gadow KD, et al. Association of ADHD, tics, and anxiety with dopamine transporter (DAT1) genotype in autism spectrum disorder. J Child Psychol Psychiatry. 2008;49(12):1331–8. doi: 10.1111/j.1469-7610.2008.01952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Staal WG, de Krom M, de Jonge MV. Brief report: the dopamine-3-receptor gene (DRD3) is associated with specific repetitive behavior in autism spectrum disorder (ASD) J Autism Dev Disord. 2012;42(5):885–8. doi: 10.1007/s10803-011-1312-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Wu J, et al. Role of dopamine receptors in ADHD: a systematic meta-analysis. Mol Neurobiol. 2012;45(3):605–20. doi: 10.1007/s12035-012-8278-5. [DOI] [PubMed] [Google Scholar]
- 184.Hori H, Kunugi H. The efficacy of pramipexole, a dopamine receptor agonist, as an adjunctive treatment in treatment-resistant depression: an open-label trial. ScientificWorldJournal. 2012;2012:372474. doi: 10.1100/2012/372474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Wang GJ, et al. Imaging of brain dopamine pathways: implications for understanding obesity. J Addict Med. 2009;3(1):8–18. doi: 10.1097/ADM.0b013e31819a86f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Narayanan NS, Guarnieri DJ, DiLeone RJ. Metabolic hormones, dopamine circuits, and feeding. Front Neuroendocrinol. 2010;31(1):104–12. doi: 10.1016/j.yfrne.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Baldo BA, Kelley AE. Discrete neurochemical coding of distinguishable motivational processes: insights from nucleus accumbens control of feeding. Psychopharmacology (Berl) 2007;191(3):439–59. doi: 10.1007/s00213-007-0741-z. [DOI] [PubMed] [Google Scholar]
- 188.Wang GJ, et al. Brain dopamine and obesity. Lancet. 2001;357(9253):354–7. doi: 10.1016/s0140-6736(00)03643-6. [DOI] [PubMed] [Google Scholar]
- 189.Volkow ND, et al. Low dopamine striatal D2 receptors are associated with prefrontal metabolism in obese subjects: possible contributing factors. Neuroimage. 2008;42(4):1537–43. doi: 10.1016/j.neuroimage.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Bailer UF, et al. Amphetamine induced dopamine release increases anxiety in individuals recovered from anorexia nervosa. Int J Eat Disord. 2012;45(2):263–71. doi: 10.1002/eat.20937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Zhou QY, Palmiter RD. Dopamine-deficient mice are severely hypoactive, adipsic, and aphagic. Cell. 1995;83(7):1197–209. doi: 10.1016/0092-8674(95)90145-0. [DOI] [PubMed] [Google Scholar]
- 192.Morrison CD, Berthoud HR. Neurobiology of nutrition and obesity. Nutr Rev. 2007;65(12 Pt 1):517–34. doi: 10.1301/nr.2007.dec.517-534. [DOI] [PubMed] [Google Scholar]
- 193.Raghanti MA, et al. Neuropeptide Y-immunoreactive Neurons in the Cerebral Cortex of Humans and Other Haplorrhine Primates. Am J Primatol. 2013;75(5):415–24. doi: 10.1002/ajp.22082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Kuromitsu J, et al. Reduced neuropeptide Y mRNA levels in the frontal cortex of people with schizophrenia and bipolar disorder. Brain Res Gene Expr Patterns. 2001;1(1):17–21. doi: 10.1016/s1567-133x(01)00003-5. [DOI] [PubMed] [Google Scholar]
- 195.Caberlotto L, Hurd YL. Reduced neuropeptide Y mRNA expression in the prefrontal cortex of subjects with bipolar disorder. Neuroreport. 1999;10(8):1747–50. doi: 10.1097/00001756-199906030-00022. [DOI] [PubMed] [Google Scholar]
- 196.Morales-Medina JC, Dumont Y, Quirion R. A possible role of neuropeptide Y in depression and stress. Brain Res. 2010;1314:194–205. doi: 10.1016/j.brainres.2009.09.077. [DOI] [PubMed] [Google Scholar]
- 197.Oades RD, Daniels R, Rascher W. Plasma neuropeptide-Y levels, monoamine metabolism, electrolyte excretion and drinking behavior in children with attention-deficit hyperactivity disorder. Psychiatry Res. 1998;80(2):177–86. doi: 10.1016/s0165-1781(98)00064-x. [DOI] [PubMed] [Google Scholar]
- 198.Kask A, Rago L, Harro J. NPY Y1 receptors in the dorsal periaqueductal gray matter regulate anxiety in the social interaction test. Neuroreport. 1998;9(12):2713–6. doi: 10.1097/00001756-199808240-00005. [DOI] [PubMed] [Google Scholar]
- 199.Sajdyk TJ, Shekhar A, Gehlert DR. Interactions between NPY and CRF in the amygdala to regulate emotionality. Neuropeptides. 2004;38(4):225–34. doi: 10.1016/j.npep.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 200.Heilig M, et al. Centrally administered neuropeptide Y (NPY) produces anxiolytic-like effects in animal anxiety models. Psychopharmacology (Berl) 1989;98(4):524–9. doi: 10.1007/BF00441953. [DOI] [PubMed] [Google Scholar]
- 201.Bjorntorp P. Endocrine abnormalities of obesity. Metabolism. 1995;44(9 Suppl 3):21–3. doi: 10.1016/0026-0495(95)90315-1. [DOI] [PubMed] [Google Scholar]
- 202.Adam TC, Epel ES. Stress, eating and the reward system. Physiol Behav. 2007;91(4):449–58. doi: 10.1016/j.physbeh.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 203.Prasad A, Prasad C. Short-term consumption of a diet rich in fat decreases anxiety response in adult male rats. Physiol Behav. 1996;60(3):1039–42. doi: 10.1016/0031-9384(96)00135-7. [DOI] [PubMed] [Google Scholar]
- 204.Raadsheer FC, et al. Increased numbers of corticotropin-releasing hormone expressing neurons in the hypothalamic paraventricular nucleus of depressed patients. Neuroendocrinology. 1994;60 (4):436–44. doi: 10.1159/000126778. [DOI] [PubMed] [Google Scholar]
- 205.Arborelius L, et al. The role of corticotropin-releasing factor in depression and anxiety disorders. J Endocrinol. 1999;160(1):1–12. doi: 10.1677/joe.0.1600001. [DOI] [PubMed] [Google Scholar]
- 206.Nemeroff CB, et al. Elevated concentrations of CSF corticotropin-releasing factor-like immunoreactivity in depressed patients. Science. 1984;226(4680):1342–4. doi: 10.1126/science.6334362. [DOI] [PubMed] [Google Scholar]
- 207.Nemeroff CB, et al. Reduced corticotropin releasing factor binding sites in the frontal cortex of suicide victims. Arch Gen Psychiatry. 1988;45(6):577–9. doi: 10.1001/archpsyc.1988.01800300075009. [DOI] [PubMed] [Google Scholar]
- 208.Stenzel-Poore MP, et al. Development of Cushing’s syndrome in corticotropin-releasing factor transgenic mice. Endocrinology. 1992;130(6):3378–86. doi: 10.1210/endo.130.6.1597149. [DOI] [PubMed] [Google Scholar]
- 209.Coste SC, Murray SE, Stenzel-Poore MP. Animal models of CRH excess and CRH receptor deficiency display altered adaptations to stress. Peptides. 2001;22(5):733–41. doi: 10.1016/s0196-9781(01)00386-2. [DOI] [PubMed] [Google Scholar]
- 210.Muller MB, et al. Hypothalamic-pituitary-adrenocortical system and mood disorders: highlights from mutant mice. Neuroendocrinology. 2004;79(1):1–12. doi: 10.1159/000076041. [DOI] [PubMed] [Google Scholar]
- 211.Bornstein SR, et al. Approaching the shared biology of obesity and depression: the stress axis as the locus of gene-environment interactions. Mol Psychiatry. 2006;11(10):892–902. doi: 10.1038/sj.mp.4001873. [DOI] [PubMed] [Google Scholar]
- 212.Soares-Miranda L, et al. Metabolic syndrome, physical activity and cardiac autonomic function. Diabetes Metab Res Rev. 2012;28(4):363–9. doi: 10.1002/dmrr.2281. [DOI] [PubMed] [Google Scholar]
- 213.Soares-Miranda L, et al. Trans-fatty acid consumption and heart rate variability in 2 separate cohorts of older and younger adults. Circ Arrhythm Electrophysiol. 2012;5(4):728–38. doi: 10.1161/CIRCEP.111.966259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Assoumou HG, et al. Metabolic syndrome and short-term and long-term heart rate variability in elderly free of clinical cardiovascular disease: the PROOF study. Rejuvenation Res. 2010;13(6):653–63. doi: 10.1089/rej.2010.1019. [DOI] [PubMed] [Google Scholar]
- 215.Lee K, et al. Heart rate variability and metabolic syndrome in hospitalized patients with schizophrenia. J Korean Acad Nurs. 2011;41(6):788–94. doi: 10.4040/jkan.2011.41.6.788. [DOI] [PubMed] [Google Scholar]
- 216.Koskinen T, et al. Metabolic syndrome and short-term heart rate variability in young adults. The cardiovascular risk in young Finns study. Diabet Med. 2009;26(4):354–61. doi: 10.1111/j.1464-5491.2009.02686.x. [DOI] [PubMed] [Google Scholar]
- 217.Min KB, et al. The impact of the components of metabolic syndrome on heart rate variability: using the NCEP-ATP III and IDF definitions. Pacing Clin Electrophysiol. 2008;31(5):584–91. doi: 10.1111/j.1540-8159.2008.01045.x. [DOI] [PubMed] [Google Scholar]
- 218.Nemeroff C, Goldschmidt-Clermont P. Heartache and heartbreak--the link between depression and cardiovascular disease. Nat Rev Cardiology. 2012 Sep;9(9):526–39. doi: 10.1038/nrcardio.2012.91. [DOI] [PubMed] [Google Scholar]
- 219.Thayer JF, Yamamoto SS, Brosschot JF. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int J Cardiol. 2010;141(2):122–31. doi: 10.1016/j.ijcard.2009.09.543. [DOI] [PubMed] [Google Scholar]
- 220.Valiengo L, et al. The sertraline versus electrical current therapy for treating depression clinical study (select-tdcs): results of the crossover and follow-up phases. Depress Anxiety. 2013;30(7):646–53. doi: 10.1002/da.22079. [DOI] [PubMed] [Google Scholar]
- 221.Kemp AH, et al. Impact of depression and antidepressant treatment on heart rate variability: a review and meta-analysis. Biol Psychiatry. 2010;67(11):1067–74. doi: 10.1016/j.biopsych.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 222.Ieda M, et al. Evaluation of autonomic nervous system by salivary alpha-amylase level and heart rate variability in patients with schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2013 doi: 10.1007/s00406-013-0411-6. [DOI] [PubMed] [Google Scholar]
- 223.Tonhajzerova I, et al. Changes in the cardiac autonomic regulation in children with attention deficit hyperactivity disorder (ADHD) Indian J Med Res. 2009;130(1):44–50. [PubMed] [Google Scholar]
- 224.Kemp AH, Quintana DS. The relationship between mental and physical health: Insights from the study of heart rate variability. Int J Psychophysiol. 2013 doi: 10.1016/j.ijpsycho.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 225.Licht CM, et al. Longitudinal evidence for unfavorable effects of antidepressants on heart rate variability. Biol Psychiatry. 2010;68(9):861–8. doi: 10.1016/j.biopsych.2010.06.032. [DOI] [PubMed] [Google Scholar]
- 226.Brunoni AR, et al. Transcranial direct current stimulation (tDCS) in unipolar vs. bipolar depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(1):96–101. doi: 10.1016/j.pnpbp.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 227.Sampaio LA, et al. A systematic review of non-invasive brain stimulation therapies and cardiovascular risk: implications for the treatment of major depressive disorder. Front Psychiatry. 2012;3:87. doi: 10.3389/fpsyt.2012.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Vucetic Z, et al. Maternal high-fat diet alters methylation and gene expression of dopamine and opioid-related genes. Endocrinology. 2010;151(10):4756–64. doi: 10.1210/en.2010-0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Basu S, et al. Pregravid obesity associates with increased maternal endotoxemia and metabolic inflammation. Obesity (Silver Spring) 2011;19(3):476–82. doi: 10.1038/oby.2010.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Roberts KA, et al. Placental structure and inflammation in pregnancies associated with obesity. Placenta. 2011;32(3):247–54. doi: 10.1016/j.placenta.2010.12.023. [DOI] [PubMed] [Google Scholar]
- 231.Radaelli T, et al. Gestational diabetes induces placental genes for chronic stress and inflammatory pathways. Diabetes. 2003;52(12):2951–8. doi: 10.2337/diabetes.52.12.2951. [DOI] [PubMed] [Google Scholar]
- 232.Wallace JM, et al. Serial measurement of uterine blood flow from mid to late gestation in growth restricted pregnancies induced by overnourishing adolescent sheep dams. Placenta. 2008;29(8):718–24. doi: 10.1016/j.placenta.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 233.Frias AE, et al. Maternal high-fat diet disturbs uteroplacental hemodynamics and increases the frequency of stillbirth in a nonhuman primate model of excess nutrition. Endocrinology. 2011;152(6):2456–64. doi: 10.1210/en.2010-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Madan I, et al. The frequency and clinical significance of intra-amniotic infection and/or inflammation in women with placenta previa and vaginal bleeding: an unexpected observation. J Perinat Med. 2010;38(3):275–9. doi: 10.1515/JPM.2010.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Toker S, et al. The association between burnout, depression, anxiety, and inflammation biomarkers: C-reactive protein and fibrinogen in men and women. J Occup Health Psychol. 2005;10 (4):344–62. doi: 10.1037/1076-8998.10.4.344. [DOI] [PubMed] [Google Scholar]
- 236.Das UN. Is obesity an inflammatory condition? Nutrition. 2001;17(11–12):953–66. doi: 10.1016/s0899-9007(01)00672-4. [DOI] [PubMed] [Google Scholar]
- 237.Buehler MR. A proposed mechanism for autism: an aberrant neuroimmune response manifested as a psychiatric disorder. Med Hypotheses. 2011;76(6):863–70. doi: 10.1016/j.mehy.2011.02.038. [DOI] [PubMed] [Google Scholar]
- 238.Ellman LM, et al. Structural brain alterations in schizophrenia following fetal exposure to the inflammatory cytokine interleukin-8. Schizophr Res. 2010;121(1–3):46–54. doi: 10.1016/j.schres.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Smith SE, et al. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci. 2007;27(40):10695–702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Goines PE, et al. Increased midgestational IFN-gamma, IL-4 and IL-5 in women bearing a child with autism: A case-control study. Mol Autism. 2011;2:13. doi: 10.1186/2040-2392-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Ashwood P, et al. Altered T cell responses in children with autism. Brain Behav Immun. 2011;25 (5):840–9. doi: 10.1016/j.bbi.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Ashwood P, et al. Associations of impaired behaviors with elevated plasma chemokines in autism spectrum disorders. J Neuroimmunol. 2011;232(1–2):196–9. doi: 10.1016/j.jneuroim.2010.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Oken E, Gillman MW. Fetal origins of obesity. Obes Res. 2003;11(4):496–506. doi: 10.1038/oby.2003.69. [DOI] [PubMed] [Google Scholar]
- 244.Simerly RB. Hypothalamic substrates of metabolic imprinting. Physiol Behav. 2008;94(1):79–89. doi: 10.1016/j.physbeh.2007.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Jones AP, et al. Maternal hormonal manipulations in rats cause obesity and increase medial hypothalamic norepinephrine release in male offspring. Brain Res Dev Brain Res. 1995;88(2):127–31. doi: 10.1016/0165-3806(95)00078-r. [DOI] [PubMed] [Google Scholar]
- 246.Jones AP, Dayries M. Maternal hormone manipulations and the development of obesity in rats. Physiol Behav. 1990;47(6):1107–10. doi: 10.1016/0031-9384(90)90359-c. [DOI] [PubMed] [Google Scholar]
- 247.Plagemann A, et al. Lifelong enhanced diabetes susceptibility and obesity after temporary intrahypothalamic hyperinsulinism during brain organization. Exp Clin Endocrinol. 1992;99(2):91–5. doi: 10.1055/s-0029-1211143. [DOI] [PubMed] [Google Scholar]


