Abstract
Regulatable transgene expression in human pluripotent stem cells (hPSCs) and their progenies is often necessary to dissect gene function in a temporal and spatial manner. However, hPSC lines with inducible transgene expression, especially in differentiated progenies, have not been established due to silencing of randomly inserted genes during stem cell expansion and/or differentiation. Here, we report the use of TALEN (transcription activator–like effector nucleases)-mediated targeting to AAVS1 site to generate versatile conditional hPSC lines. Transgene (both GFP and a functional gene) expression in hPSCs and their derivatives was not only sustained but also tightly regulated in response to doxycycline both in vitro and in vivo. We modified the donor construct so that any gene of interest can be readily inserted to produce hPSC lines with conditional transgene expression. This technology will substantially improve the way we study human stem cells.
Keywords: stem cells, gene editing, gene regulation, neural differentiation
Introduction
Human pluripotent stem cells (hPSCs), including embryonic stem cells (ESCs) and induced PSCs (iPSCs), offer a model for unveiling cellular and molecular events underlying normal and abnormal human development as well as for understanding disease pathogenesis under the human genetic background. Like model animals of which genetic alteration has greatly advanced biomedical research, an ability to genetically modify hPSCs will empower the model system to bridge animal studies to clinics. Unlike their mouse counterparts, hPSCs are difficult to modify genetically, especially in a regulatable manner. The chief reasons include (1) low transfection efficiency, (2) uneven transgene expression in cells established by random insertion (Du et al., 2009; Zwaka and Thomson, 2003), (3) silencing of transgenes in human stem cells in a promoter-dependent manner (Xia et al., 2007), and (4) down regulation of transgenes upon stem cell differentiation, especially to neurons.
Recent technological development, including TALEN (transcription activator–like effector nucleases), enables targeted genetic manipulation of hPSCs (Gaj et al., 2013; Mali and Cheng, 2012). Targeted insertion of transgenes into an envy site by TALEN offers an opportunity for stable transgene expression (Hockemeyer et al., 2011). The AAVS1 locus, located within the first intron of PPP1R12C gene, has an open chromatin structure that is flanked by insulator elements that shield the integrated cassette from trans-activation or repression (Ogata et al., 2003). Although gene insertion at AAVS1 locus may affect PPP1R12C gene expression, disruption of AAVS1 allele in transgenic mice and in human hPSCs appears to have no adverse effects (DeKelver et al., 2010; Hockemeyer et al., 2009). Hence, transgenes integrated in AAVS1 site exhibit robust and persistent expression.
Transgenic hPSCs, established through traditional random transgene insertion, often do not sustain transgene expression upon stem cell differentiation, especially to functional neurons (Irion et al., 2007; Xia et al., 2007). It is presently not known if transgene expression persists when transgenic hPSCs are established via TALEN and differentiate to functional mature cells. More importantly, it is often necessary to regulate transgene expression in human stem cell progenies, especially in post-mitotic neurons and glia, in order for dissection of gene function in a temporal and spatial manner. This remains a technical challenge for the hPSC field.
By integrating our inducible construct (Du et al., 2009) with the TALEN-mediated targeting to AAVS1 site (Lombardo et al., 2011) we generated versatile conditional hPSC lines. Transgene (both GFP and a functional gene) expression in hPSCs and their derivatives was tightly regulated in response to doxycycline both in vitro and in vivo. We also modified the donor construct so that any gene of interest may be inserted to produce inducible transgene expression in hPSCs and their derivatives.
Results
Establishment of hESC Lines with Inducible GFP Expression by TALEN-Mediated Homologous Recombination
To construct a Tet-inducible expression system, pTRE3G cassette and Tet-On 3G cassette were inserted into a donor plasmid. The CAG promoter and pTRE3G promoter were used to drive Tet3G and exogenous gene expression, respectively (Fig. 1A). We also introduced a sequence recognized by Sal1 and Mlu1 into the multiple cloning site (MCS) of the pTRE3G vector by PCR cloning (Fig. 1A) for simple and convenient insertion of target genes. To establish inducible cell lines, we inserted EGFP to the donor plasmid through digestion with Sal1 and Mlu1 (Fig. 1A). Human ESCs (H9, passage 20, 1×107 cells) were co-transfected with the donor plasmid and one pair of AAVS1-TALEN plasmids by electroporation. After 14 days of puromycin selection, 30 clones were obtained. To screen positive clones, PCR were performed with a pair of primers, one on PPP1R12C locus and the other on donor puromycin vector (See method). We identified 20 clones that were positive based on correct insertion of the construct in PPP1R12C locus. Thus, the efficiency of positive clones with inducible gene expression from antibiotics-resistant clones is 70%. Analysis of transgene expression kinetics indicated that upon treatment with 1μg/ml DOX, GFP appeared within 12 hours and reached a plateau by 48 hours. After DOX withdrawal, GFP intensity decreased gradually until it completely disappeared by 96 hours (Fig. 1B, 1C). As a comparison, we used a traditional method to infect hESCs with lentivirus carrying an inducible EGFP construct and selected stable clones using both G418 and puromycin (Zhang et al., 2010). We obtained 80 clones and 7 clones (9%) exhibited inducible EGFP expression without detectable leakage. However, most of clones expressed EGFP unevenly, i.e., in a mosaic form, while in TALEN targeted clones, EGFP expression was homogeneous (Table 1). Hence, while more clones are obtained using lentivirus, few clones exhibit regulatable transgene expression and hardly any show homogeneous transgene expression as compared to those produced with TALEN.
Figure 1. Establishment of inducible GFP expressing hESC lines.
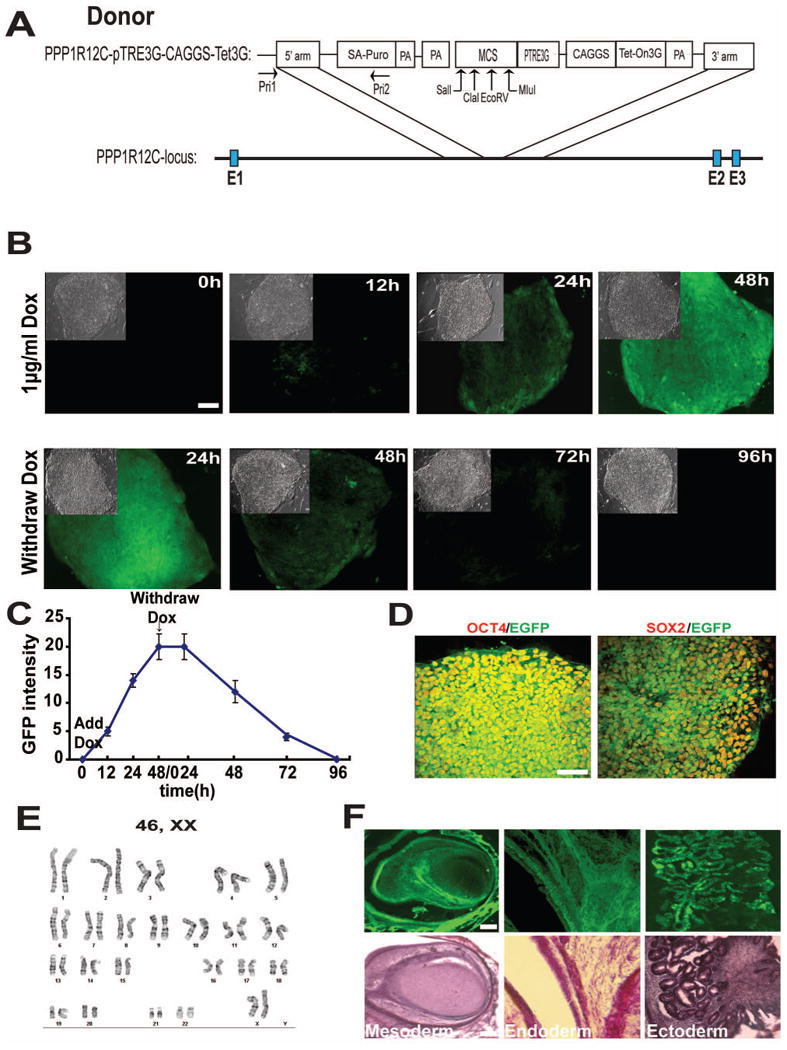
(A) Schematic diagram of inducible gene expression construct targeting AAVS1 site. Upward arrows indicate cloning sites.
(B) GFP expression in hESCs after treatment with 1 μg/ml doxycycline (DOX) and withdrawal of DOX. Upper-left insets are corresponding phase contrast images.
(C) Quantification of the EGFP fluorescent intensity after DOX application and withdrawal.
(D) Immunofluorescent images of SOX2 and OCT4 expression in the cell line.
(E) Karyotyping of the cell line.
(F) Inducible EGFP expression in teratomas formed by TALEN-targeted hESCs.
Scale bar = 50μm.
Table 1. Comparison between TALEN- and Lentivirus-mediated transgenesis.
| TALEN | Lentivirus | |
|---|---|---|
| cells transfected | 1.0×107 | 1.0×107 |
| clone survived | 30 | 80 |
| Clones with inducible GFP expression | 27 | 7 |
| Efficiency | 70% | 9% |
| In vitro silence | No | in neuron linage |
| In vivo silence | No | in neuron linage |
The selected clones were cultured and passaged by mechanical dissection (Reubinoff et al., 2000) continuously for 3 months as their parental hESCs. They retained a uniform expression of OCT4 and SOX2 (Fig. 1D), suggesting their pluripotency. The cells also exhibited a normal karyotype after being passaged for 3 months (Fig. 1E). By analyzing sites that have sequence similarity with AAVS1-TALEN targeting site, the Jaenisch group has shown that AAVS1-TALEN pair generally has little off-target mutation (Hockemeyer et al., 2011). Only one of the off-target sites, OT10, shows higher incidence of mutation. Using PCR to amplify the OT10 site from our transgenic PSCs followed by sequencing of the PCR product, we found that the OT10 site had no mutation, verifying the integrity of our genetically modified cell lines.
To further confirm pluripotency of the cell lines and determine inducibility of transgene expression in three germ layers in vivo, we injected the transgenic hESCs to produce teratomas. Teratomas were observed two months later. The animals were then fed with DOX (500 μg/ml) in drinking water for 10 days before teratomas were removed. Hematoxylin and eosin staining revealed mesoderm, ectoderm, and endoderm tissues in teratoma sections and all tissues representing the three germ layers expressed EGFP (Fig. 1F).
hESC derivatives sustain inducible GFP expression in vitro
Transgene expression is often silenced during human stem cell expansion and/or following differentiation. To determine if transgene can still be induced after long-term expansion, we passaged the transgenic cells for 3 months. As shown in Fig. 2A, hESCs from the TALEN-engineered group retained uniform expression of GFP in the presence of DOX whereas those in the lentivirus-engineered group exhibited a mosaic pattern of GFP expression. Upon differentiation as embryoid bodies, a first step toward differentiation to three germ layers, a similar uniform GFP expression pattern was present in the TALEN group but the lentivirus group showed a mosaic expression pattern (Fig. 2B). This result suggests a gradual loss of transgene expression/inducibility over expansion of lentivirus-generated hPSCs.
Figure 2. EGFP expression in transgenic hESCs and their derivatives in vitro.
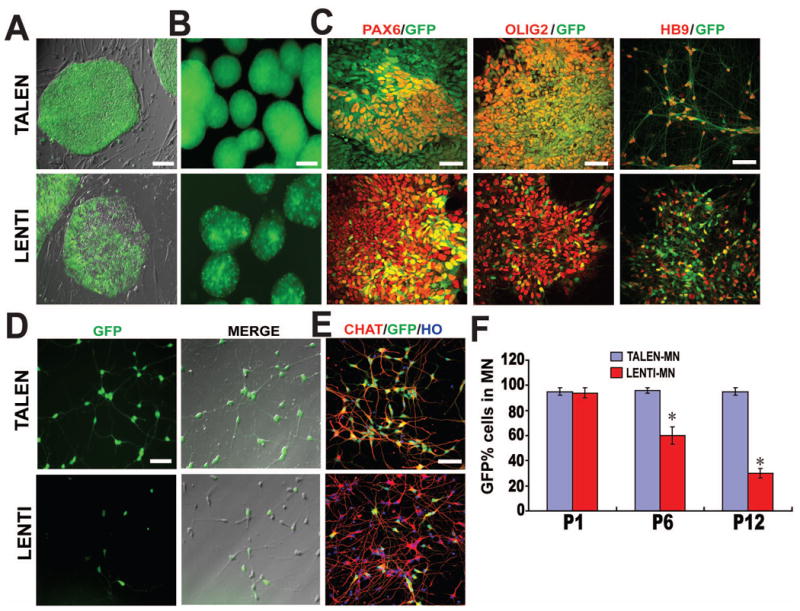
(A) EGFP fluorescent and phase contrast images of hESCs established by TALEN (upper) and Lentivirus (lower).
(B) EGFP fluorescent images of EBs.
(C) Immunofluorescent images of PAX6+ neuroepithelial cells, OLIG2+ MN progenitors, MNX1+ (HB9) postmitotic MNs from hESCs established by TALEN (upper) and Lentivirus (lower).
(D) EGFP fluorescent and phase contrast images of CHAT+ MNs derived from hESCs that were established by TALEN (upper) and Lentivirus (lower).
(E) Immunofluorescent images of CHAT+ maturing MNs derived from hESCs that were established by TALEN (upper) and Lentivirus (lower).
(F) Quantification of EGFP-expressing cells among CHAT+ MNs derived from hESCs that were established by TALEN and Lentivirus. * P<0.05.
Scale bar = 50μm.
Transgene silencing occurs more frequently during differentiation to the neural lineage. We further differentiated the hESCs to neuroepithelial cells (NE), motor neuron progenitors (MNP), and motor neurons (MN) using our established protocols. As shown in Fig. 2C-E, GFP was induced in Pax6-expressing NE, Olig2-expressing MNPs, MNX1+ (HB9+) positive MNs, and ChAT-expressing mature MN in the TALEN group. In contrast, fewer motor neurons displayed GFP in the Lentivirus group. Quantification of GFP-expressing cells among ChAT-expressing motor neurons indicated a progressive loss of GFP induction over passaging, from 95% of MNs displayed GFP in the presence of DOX in the first passage to less than 30% by passage 12. In contrast, MNs derived from TALEN-established transgenic hESCs retained GFP expression in response to DOX throughout the period (Fig. 2C-F and Table 1).
EGFP is induced in neurons and astrocytes in vivo
Transgenic hPSCs, established by random insertion, often downregulate transgenes following differentiation to functional neurons in vivo although transgene expression is often retained in astrocytes (Han et al., 2013; Windrem et al., 2008). To investigate whether EGFP is regulated in astrocytes and neurons in vivo, we differentiated transgenic PSCs to spinal neural progenitors for 35 days (Li et al., 2005; Li et al., 2008) and transplanted the progenitors into the adult mouse cervical spinal cord. Three months after transplantation, neural progenitors from both the lentivirus and TALEN groups differentiated to MAP2 positive neurons in the (ventral) grey matter. Interestingly, the majority of MAP2+ human (hNu+) neurons in the TALEN group (85%) were positive for EGFP, whereas few human neurons from the Lentivirus group expressed EGFP (Fig. 3A-B and Table 1). When quantified among EGFP+/hGFAP+ astrocytes, EGFP was retained in human astrocytes that were derived from TALEN- or Lentivirus-established hESCs (Fig. 3C-D). These results indicate that transgene is readily silenced in neurons when the transgenic lines are established through random insertion via lentivirus but it lasts much longer from targeted transgenic lines via TALEN. Transgene expression is retained in astrocytes that are derived from both lentivirus- and TALEN-established hPSCs.
Figure 3. EGFP induction in neurons and astrocytes in vivo.
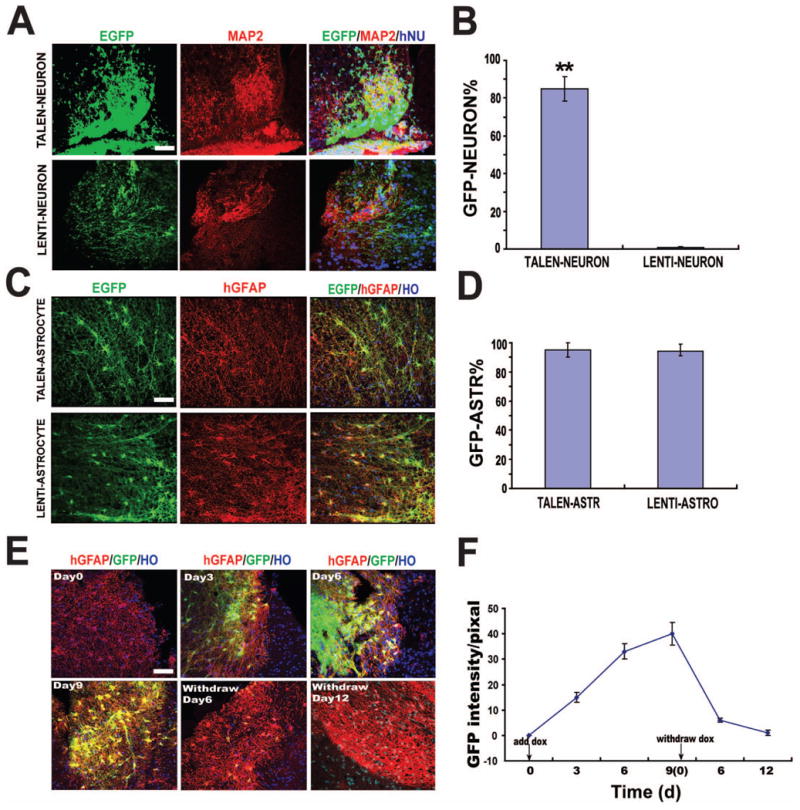
(A) Immunofluorescent images of EGFP expression in MAP2+ neurons derived from TALEN- or Lentivirus-established hESCs.
(B) Quantification of EGFP positive cells among MAP2+ neurons. ** P<0.01
(C) Immunofluorescent images of EGFP expression in hGFAP+ astrocytes derived from TALEN- or Lentivirus-established hESCs.
(D) Quantification of the EGFP positive cells among hGFAP+ astrocytes.
(E) Temporal course of EGFP expression during DOX intake and withdrawal.
(F) Quantification of EGFP fluorescent intensity after DOX application and withdrawal in vivo.
Scale bar = 50μm.
To determine if transgene expression is regulatable in differentiated neural cells, mice were fed with DOX five months after cell transplantation. In the spinal cord, the vast majority of transplanted cells become astrocytes by 5 months. EGFP expression was observed in hGFAP-positive cells after 3 days of oral DOX intake. The maximum number of GFP-positive cells was detected 9 days after DOX application. The EGFP expression gradually decreased after DOX withdrawal. It completely disappeared 12 days later (Fig. 3E, 3F). Thus, GFP is regulatable in differentiated human neural cells in vivo.
Functional gene is induced in iPSC-derived neurons
Expression and regulation of GFP does not always translate to conditional expression of a functional gene in human cells. In this study, we chose neurofilament-low molecular weight (NEF-L) gene to demonstrate the simplicity and versatility of the transgenic system. We first inserted the NEF-L-EGFP fusion cassette into the donor plasmid to replace the EGFP cassette (Fig. 1A). Together with the identical TALEN pairs, the donor plasmid was transfected to iPSCs (IMR-90-4). The established transgenic iPSCs were differentiated to spinal neurons as descried in materials and methods. Forty-eight hours after DOX treatment, NEF-L was induced in neurons, especially neurites, as shown by GFP in live cultures (Fig. 4A). It should be noted that not all cells are maturing neurons at this stage (day-23). Hence, some cells, especially progenitors, did not display bright GFP signal (Fig. 4A, Suppl. Fig. 1). Western blotting and densitometry analysis indicated a dose-dependent induction of NEF-L-EGFP expression in response to increasing doses of DOX (Fig. 4B, 4C). Thus, a functional gene like NEF-L can be readily regulated in human neurons.
Figure 4. NEF-L-EGFP is induced in neurons in a dose-dependent manner.
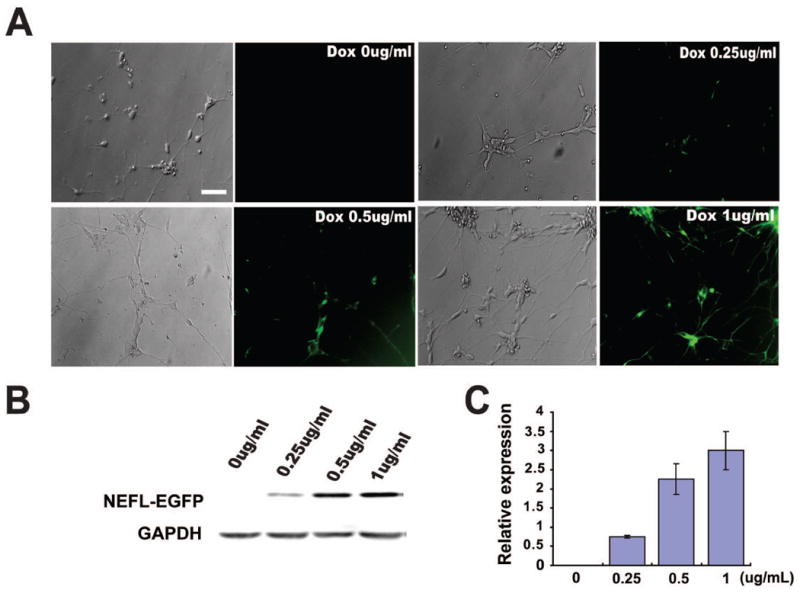
(A) Phase contrast and corresponding NEF-L-EGFP fluorescent images of MN cultures in the presence of gradient concentrations of DOX.
(B) Western blot showing expression of NEF-L-EGFP fusion protein after treatment with increasing doses of DOX.
(C) Quantification of NEF-L-EGFP fusion protein levels.
Scale bar = 50μm.
Discussion
Transgenesis of hPSCs is typically achieved by random insertion of transgenes due to inefficiency in homologous recombination/cellular cloning, and such transgenes are often downregulated upon stem cell differentiation, especially to functional neurons (Cherry et al., 2000; Ellis, 2005; Liew et al., 2007; Smith et al., 2008; Yao et al., 2004). The TALEN-based homologous recombination overcomes inefficiency in gene targeting, and insertion of transgenes in AAVS1 locus could potentially prevent transgene silencing (Hockemeyer et al., 2011). Indeed, transgene expression persists in cells that are differentiated from transgenic hPSCs established via TALEN-mediated gene targeting into AAVS1 site, including neurons in vitro and in vivo. The advantage of using TALEN-mediated gene targeting is also corroborated by our parallel observation that transgene expression is down regulated when lentivirus-established hPSCs are differentiated to neural cells, similar to our previous observation (Xia et al., 2008; Xia et al., 2007). It is possible that the use of different promoters, CAG vs. EF-1α, may contribute to differential down regulation of transgenes in our system. Nevertheless, we have previously shown that GFP expression under ubiquitous promoters, especially EF-1α and CAG promoters, is significantly downregulated in hESCs that are established by lentivirus (Xia et al., 2007). An interesting observation is that transgene expression is retained in glial cells derived from both TALEN-mediated and lentivirus-established cell lines, suggesting that randomly inserted genes continue to be expressed after hPSCs differentiate to glial cells and that transgene silencing appears to be cell type-specific. This phenomenon may have some implication. The level of transgene expression mediated by TALEN is generally low because of a single copy of transgenes whereas Lentinvirus-mediated transgenesis allows multiple inserting copies. Hence, when a high level of transgene expression is sought, including expression in glia, lentivirus infection may be an option. Nevertheless, random insertion comes with another drawback, i.e., uneven expression among cell populations as observed in the present study as well as by others (Cherry et al., 2000; Ellis, 2005; Laker et al., 1998; Yao et al., 2004). This is likely due to varied degrees of silencing depending on insertion sites.
Precise spatial and temporal regulation of transgenes is often necessary for most biomedical application. Efforts have been made to produce transgenic hPSC lines with regulatable gene expression (Du et al., 2009; Vigna et al., 2002; Xia et al., 2008; Zhou et al., 2007). While expression of transgenes (often GFP) can be regulated in stem cells and their differentiated progenies at an early stage, more than often transgenes are no longer expressed and regulatable in mature cells, including neurons (Cherry et al., 2000; Ellis, 2005; Laker et al., 1998; Yao et al., 2004). In that regard, our present study demonstrates unequivocally that transgene expression in hPSC-differentiated progenies, including neurons, can be regulated by DOX in a dose-dependent manner. Transgene regulation can also be achieved in mature neurons and glia in vivo 3-5 months post-transplantation. Such a technology opens new possibilities to interrogate gene functions in human cells in vitro and in vivo. As an example, we were able to regulate the expression levels of NEF-L protein in neurons, which is critical for neuronal function and pathogenesis in neurodegenerative diseases. Because we target the transgene into a “safe harbor”, the risk of inserting mutagenesis is minimal, and so is off-target effects (Ding et al., 2013), as indicated by our genomic integrity assay. TLAEN-mediated homologous recombination is also much more efficient than traditional homologous recombination. Hence, much less cellular cloning is needed. Thus, TALEN-established hPSC lines are stable and they retain consistent differentiation potency. Furthermore, the ability to regulate transgene expression in mature human cells that are derived from TALEN-established hPSCs makes it possible for potential clinical applications.
The TALEN-mediated transgenesis is also simple and highly efficient. Our parallel comparison with lentivirus mediated transgenesis indicates that even though lentivirus infection results in a substantially more number of clones selected by antibiotics, the efficiency of producing correct clones with inducible transgene expression is significantly lower than that targeted by TALEN. If we target the same site (AAVS1), the TALEN pair and the donor plasmid are essentially the same, making it easy to produce transgenic hPSC lines. In particular, we have modified the donor construct to incorporate convenient restriction sites for easy swap of a gene of interest. Therefore, hPSC lines with inducible expression of any gene of interest can be made using the same set of plasmids.
We have engineered a convenient inducible transgene expression vector and targeted it to AAVS1 site of both human ESCs and iPSCs via TALEN-mediated homologous recombination. We have demonstrated unequivocally, by parallel comparison with lentivirus-mediated transgenesis, that transgene can be exquisitely regulated in both stem cells and their differentiated progenies, including neurons, in vitro and in vivo. We further demonstrated that the transgenic hPSC lines are stable over expansion and functional genes such as neurofilament can be regulated in differentiated post-mitotic neurons. This technology will enable production of versatile conditional transgenic human PSC lines and substantially improve our ability to dissect gene functions in human development and disease pathogenesis.
Materials and Methods
Inducible vector construction and TALEN assembly
The original donor plasmid AAV-CAGGS-EGFP (Addgene, Cambridge, MA, www.addgene.org/) was modified by removing the Spe1 site via digestion with a low concentration of Spe1, followed by dephosphorylation, blunting and self-ligation. For simple and convenient insertion of target genes, Sal1 and Mlu1 digestion sites were introduced into the multiple cloning site (MCS) of pTRE3G vector. For expression of Tet-On 3G, the sequence was cloned by PCR using pEF1α-TeT3G (Clontech, Mountain View, CA, http://www.clontech.com/) as a template, and inserted into the donor plasmid by Xho1 and Asc1 digestion. In order to construct Tet-inducible expression systems, pTRE3G-SV40-polyA cassette was inserted into the Spe1 site of donor plasmid by PCR cloning. TALEN pairs targeting AAVS1 locus were designed as described (Hockemeyer et al., 2011). With Joung lab REAL assembly TALEN kit (Addgene, Cambridge, MA, http://www.addgene.org/), TALE repeat arrays were constructed using standard restriction digest and ligation reactions. TALEN activity was assayed via surveyor nuclease (Transgenomic, Omaha, NE, http://www.transgenomic.com/).
Production of transgenic hPSCs by TALEN and lentivirus
Human iPSCs (IMR-90) or ESCs (H9) were cultured in 1μM Rho Kinase (ROCK)-inhibitor (Calbiochem, USA) for 24 hours prior to electroporation. Cells (1×107) were electroporated with 40 μg of donor plasmids and 5 μg of each of the TALEN encoding plasmids (Bio-Rad, Hercules, CA: 250 V, 500μF, 4-mm cuvettes). Transfected cells were plated on MEF in the MEF-conditioned medium supplemented with ROCK-inhibitor for the first 24 hours. Individual colonies were selected with puromycin (1 μg/ml) and expanded in the same media without ROCK inhibitor for 10 to 14 days. Positive clones were identified by PCR using the following primers: Forward: 5′ACCAACGCCGACGGTATCAG3′; Reverse: 5′CAGACCCTTGCCCTGGTGGT3′. Construction of lentiviral vectors, production of lentivirus, and transduction of hPSCs were described previously (Zhang et al., 2010).
Off-target analysis of TALEN-established hPSCs
The off-target site, OT10 for AAVS1-TALEN was determined by Jaenisch lab's previous study (Hockemeyer et al., 2011). To determine potential off-target integration, we genotyped OT10, by PCR with primers: F-gaatggatgaatgagtgaatgt, R-tcctgagttctcggttctttg and Sanger sequencing.
Neural Differentiation of hPSCs
Human ESCs and iPSCs were first differentiated to neuroepithelia in a neural medium consisting of Dulbecco's modified Eagle's medium/Ham's F-12 medium (Gibco, Grand Island, NY, http://www.invitrogen.com), non-essential amino acids, N2 supplement, 2μM SB431542, 300nM LDN193189 and 3μM CHIR99021 (all from Stemgent) for 7 days. At day 8, the neuroepithelia were treated with 0.1 μM retinoic acid (RA) and 0.5μM Purmorphamine (Stemgent) for 7 days to induce ventral spinal progenitors, including those for motor neurons. At day-14, neural progenitors were expanded as floating clusters in suspension in the same medium but without SB431542, LDN193189 and CHIR99021.
Teratoma Formation
Severe combined immunodeficient (SCID) mice (Jackson Laboratory, Bar Harbor, ME, http://www.jax.org) were injected subcutaneously on the back with human ESCs and iPSCs (Xia et al., 2008). Animals were given 500μg/ml doxycycline dissolved in drinking water containing 3% sucrose after a teratoma forms (about two months after injection). Ten days later, mice were perfused with 4% paraformaldehyde in PBS and teratomas were processed for hematoxylin and eosin staining.
Cell Transplantation
Total 30 adult SCID mice were used. Mice were anesthetized with 1.5% isoflurane, and the C5-C6 spinal cord was exposed by laminectomy. Using a glass micropipette with a tip-diameter of 50μm that was attached to a stereotaxic device, about 5 ×104 neural progenitors (in 0.5 μl) were transplanted into the right site of C5-C6 cervical spinal cord. Before the wound was sutured the injection site was marked with charcoal. At 3 months after transplantation mice were overdosed using pentobarbital (250 mg/kg, i.p.) and perfused transcardially with 0.9% sodium chloride followed by 4% paraformaldehyde. The spinal cord was removed and post fixed in 4% paraformaldehyde for 4 hours, then dehydration in 20% and 30% sucrose solution. The tissues were cut into 20-μm thick free-floating cross sections with microtome. To determine the kinetics of GFP expression in differentiated astrocytes, mice were treated with DOX at 5 months after transplantation and perfused at day-0, 3, 6, and 9 after induction and at day-6 and 12 after DOX was withdrawn. The spinal cord was processed as described above.
Immunohistochemistry and Quantification
Immunocytochemical staining was performed following the protocol described before (Zhang et al., 2001). Antibodies used included rabbit anti-Oct4 (1:200, Abcam, Cambridge, MA, http://www.abcam.com), goat anti-Sox2 (1:1,000, R&D, Minneapolis, MN, http://www.rndsystems.com/), mouse anti-Pax6 (1:5,000, DSHB, Iowa City, IA, http://dshb.biology.uiowa.edu/), rabbit anti Olig2 (1:500, Chemicon & Millipore, Billerica, MA, http://www.millipore.com), mouse anti Hb9 (1:50, DSHB), goat anti ChAT (1:300, Chemicon & Millipore), Rabbit GFAP (1:5000, DAKO, Carpinteria, CA, http://www.dako.com), rat anti MAP2 (1:1000, Chemicon & Millipore), mouse anti hGFAP (1:500, Stem Cells Inc, Newark, CA, http://www.stemcellsinc.com/).
Images were collected with a Nikon TE600 fluorescence microscope (Nikon Instruments, Melville, NY) or a Nikon C1 laser-scanning confocal microscope (Nikon, Tokyo, Japan). The populations of MAP2/GFP, hGFAP /GFP positive cells in the spinal cord were counted in fields chosen by an automated stage movement operated by Stereo Investigator software (MicroBrightField Inc.) or using Z-section images analyzed using image J software. GFP positive neurons or GFP positive astrocytes were counted every six sections as described (Ma et al., 2012). For cell quantification in vitro, 150-200 neurons were counted from 3 repeated experiments. Data are presented as mean ± SEM.
Western blot
Cells were lysed using RIPA buffer (Zhang et al., 2010). Lysates were resolved by SDS-PAGE. The following primary antibodies were used: mouse anti Neurofilament-68 (1:200, Sigma, St. Louis, MO, http://www.sigmaaldrich.com/) and GAPDH. Western blotting was carried out using horseradish peroxidase-conjugated IgG as a secondary antibody and the ECL system (Thermo Scientific, Rockford, IL, http://www.piercenet.com) for detection. The level of NEF-L-EGFP was calculated as a ratio to corresponding GAPDH by densitometry in three separate experiments.
Statistical analysis
The results were expressed as mean ± SEM. The difference between TALEN group and LENTI group were tested by paired t-test. One way ANOVA analysis of variance was performed on dose-dependent induction of NEF-L-EGFP expression in response to DOX, followed by SNK post hoc test. P<0.05 was considered significant.
Supplementary Material
Supplementary Figure 1. NEF-L-EGFP is induced in neurons in a dose-dependent manner (A) Phase contrast image and (B) corresponding fluorescent image of MN cultures derived from NEF-L-EGFP iPSCs in the presence of 1μg/mL of DOX. Scale bar = 50μm.
Acknowledgments
This study was supported in part by the NIH-NINDS (NS045926, NS076352, MH099587), the Bleser Family Foundation, the Busta Foundation, the NICHD (P30 HD03352), and NSFC (81170583 and 81070986).
Footnotes
Kun Qian: Collection and assembly of data; Cindy Huang, Hong Chen, Lisle W. Blackbourn IV, Yuejun Chen, Jingyuan Cao, Lin Yao and Cornall Sauvey2: Data collection and analysis
Zhongwei Du: Conception and design, Su-Chun Zhang: Conception, design, final approval of manuscript and financial support.
References
- Cherry SR, Biniszkiewicz D, van Parijs L, Baltimore D, Jaenisch R. Retroviral expression in embryonic stem cells and hematopoietic stem cells. Mol Cell Biol. 2000;20:7419–7426. doi: 10.1128/mcb.20.20.7419-7426.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeKelver RC, Choi VM, Moehle EA, Paschon DE, Hockemeyer D, Meijsing SH, Sancak Y, Cui X, Steine EJ, Miller JC, et al. Functional genomics, proteomics, and regulatory DNA analysis in isogenic settings using zinc finger nuclease-driven transgenesis into a safe harbor locus in the human genome. Genome Res. 2010;20:1133–1142. doi: 10.1101/gr.106773.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Q, Lee YK, Schaefer EA, Peters DT, Veres A, Kim K, Kuperwasser N, Motola DL, Meissner TB, Hendriks WT, et al. A TALEN genome-editing system for generating human stem cell-based disease models. Cell Stem Cell. 2013;12:238–251. doi: 10.1016/j.stem.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle EL, Booher NJ, Standage DS, Voytas DF, Brendel VP, Vandyk JK, Bogdanove AJ. TAL Effector-Nucleotide Targeter (TALE-NT) 2.0: tools for TAL effector design and target prediction. Nucleic Acids Res. 2012;40:W117–122. doi: 10.1093/nar/gks608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du ZW, Hu BY, Ayala M, Sauer B, Zhang SC. Cre recombination-mediated cassette exchange for building versatile transgenic human embryonic stem cells lines. Stem Cells. 2009;27:1032–1041. doi: 10.1002/stem.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis J. Silencing and variegation of gammaretrovirus and lentivirus vectors. Hum Gene Ther. 2005;16:1241–1246. doi: 10.1089/hum.2005.16.1241. [DOI] [PubMed] [Google Scholar]
- Gaj T, Gersbach CA, Barbas CF., 3rd ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol. 2013;31:397–405. doi: 10.1016/j.tibtech.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Chen M, Wang F, Windrem M, Wang S, Shanz S, Xu Q, Oberheim NA, Bekar L, Betstadt S, et al. Forebrain engraftment by human glial progenitor cells enhances synaptic plasticity and learning in adult mice. Cell Stem Cell. 2013;12:342–353. doi: 10.1016/j.stem.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockemeyer D, Soldner F, Beard C, Gao Q, Mitalipova M, DeKelver RC, Katibah GE, Amora R, Boydston EA, Zeitler B, et al. Efficient targeting of expressed and silent genes in human ESCs and iPSCs using zinc-finger nucleases. Nat Biotechnol. 2009;27:851–857. doi: 10.1038/nbt.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockemeyer D, Wang H, Kiani S, Lai CS, Gao Q, Cassady JP, Cost GJ, Zhang L, Santiago Y, Miller JC, et al. Genetic engineering of human pluripotent cells using TALE nucleases. Nat Biotechnol. 2011;29:731–734. doi: 10.1038/nbt.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irion S, Luche H, Gadue P, Fehling HJ, Kennedy M, Keller G. Identification and targeting of the ROSA26 locus in human embryonic stem cells. Nat Biotechnol. 2007;25:1477–1482. doi: 10.1038/nbt1362. [DOI] [PubMed] [Google Scholar]
- Laker C, Meyer J, Schopen A, Friel J, Heberlein C, Ostertag W, Stocking C. Host cis-mediated extinction of a retrovirus permissive for expression in embryonal stem cells during differentiation. J Virol. 1998;72:339–348. doi: 10.1128/jvi.72.1.339-348.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XJ, Du ZW, Zarnowska ED, Pankratz M, Hansen LO, Pearce RA, Zhang SC. Specification of motoneurons from human embryonic stem cells. Nat Biotechnol. 2005;23:215–221. doi: 10.1038/nbt1063. [DOI] [PubMed] [Google Scholar]
- Li XJ, Hu BY, Jones SA, Zhang YS, Lavaute T, Du ZW, Zhang SC. Directed differentiation of ventral spinal progenitors and motor neurons from human embryonic stem cells by small molecules. Stem Cells. 2008;26:886–893. doi: 10.1634/stemcells.2007-0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew CG, Draper JS, Walsh J, Moore H, Andrews PW. Transient and stable transgene expression in human embryonic stem cells. Stem Cells. 2007;25:1521–1528. doi: 10.1634/stemcells.2006-0634. [DOI] [PubMed] [Google Scholar]
- Lombardo A, Cesana D, Genovese P, Di Stefano B, Provasi E, Colombo DF, Neri M, Magnani Z, Cantore A, Lo Riso P, et al. Site-specific integration and tailoring of cassette design for sustainable gene transfer. Nat Methods. 2011;8:861–869. doi: 10.1038/nmeth.1674. [DOI] [PubMed] [Google Scholar]
- Mali P, Cheng L. Concise review: Human cell engineering: cellular reprogramming and genome editing. Stem Cells. 2012;30:75–81. doi: 10.1002/stem.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata T, Kozuka T, Kanda T. Identification of an insulator in AAVS1, a preferred region for integration of adeno-associated virus DNA. J Virol. 2003;77:9000–9007. doi: 10.1128/JVI.77.16.9000-9007.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reubinoff BE, Pera MF, Fong CY, Trounson A, Bongso A. Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nat Biotechnol. 2000;18:399–404. doi: 10.1038/74447. [DOI] [PubMed] [Google Scholar]
- Smith JR, Vallier L, Lupo G, Alexander M, Harris WA, Pedersen RA. Inhibition of Activin/Nodal signaling promotes specification of human embryonic stem cells into neuroectoderm. Dev Biol. 2008;313:107–117. doi: 10.1016/j.ydbio.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Vigna E, Cavalieri S, Ailles L, Geuna M, Loew R, Bujard H, Naldini L. Robust and efficient regulation of transgene expression in vivo by improved tetracycline-dependent lentiviral vectors. Mol Ther. 2002;5:252–261. doi: 10.1006/mthe.2002.0542. [DOI] [PubMed] [Google Scholar]
- Windrem MS, Schanz SJ, Guo M, Tian GF, Washco V, Stanwood N, Rasband M, Roy NS, Nedergaard M, Havton LA, et al. Neonatal chimerization with human glial progenitor cells can both remyelinate and rescue the otherwise lethally hypomyelinated shiverer mouse. Cell Stem Cell. 2008;2:553–565. doi: 10.1016/j.stem.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia X, Ayala M, Thiede BR, Zhang SC. In vitro- and in vivo-induced transgene expression in human embryonic stem cells and derivatives. Stem Cells. 2008;26:525–533. doi: 10.1634/stemcells.2007-0710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia X, Zhang Y, Zieth CR, Zhang SC. Transgenes delivered by lentiviral vector are suppressed in human embryonic stem cells in a promoter-dependent manner. Stem Cells Dev. 2007;16:167–176. doi: 10.1089/scd.2006.0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao S, Sukonnik T, Kean T, Bharadwaj RR, Pasceri P, Ellis J. Retrovirus silencing, variegation, extinction, and memory are controlled by a dynamic interplay of multiple epigenetic modifications. Mol Ther. 2004;10:27–36. doi: 10.1016/j.ymthe.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Zhang SC, Wernig M, Duncan ID, Brustle O, Thomson JA. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat Biotechnol. 2001;19:1129–1133. doi: 10.1038/nbt1201-1129. [DOI] [PubMed] [Google Scholar]
- Zhang X, Huang CT, Chen J, Pankratz MT, Xi J, Li J, Yang Y, Lavaute TM, Li XJ, Ayala M, et al. Pax6 is a human neuroectoderm cell fate determinant. Cell Stem Cell. 2010;7:90–100. doi: 10.1016/j.stem.2010.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou BY, Ye Z, Chen G, Gao ZP, Zhang YA, Cheng L. Inducible and reversible transgene expression in human stem cells after efficient and stable gene transfer. Stem Cells. 2007;25:779–789. doi: 10.1634/stemcells.2006-0128. [DOI] [PubMed] [Google Scholar]
- Zwaka TP, Thomson JA. Homologous recombination in human embryonic stem cells. Nat Biotechnol. 2003;21:319–321. doi: 10.1038/nbt788. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. NEF-L-EGFP is induced in neurons in a dose-dependent manner (A) Phase contrast image and (B) corresponding fluorescent image of MN cultures derived from NEF-L-EGFP iPSCs in the presence of 1μg/mL of DOX. Scale bar = 50μm.


