Abstract
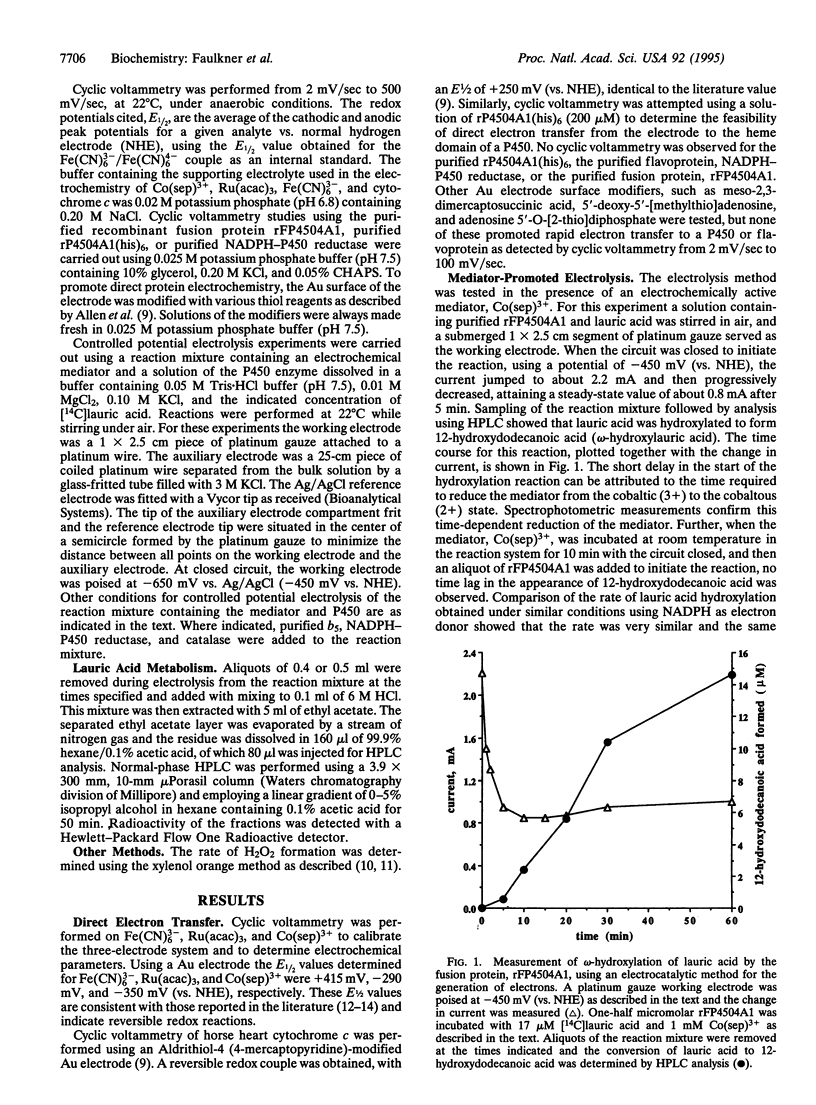
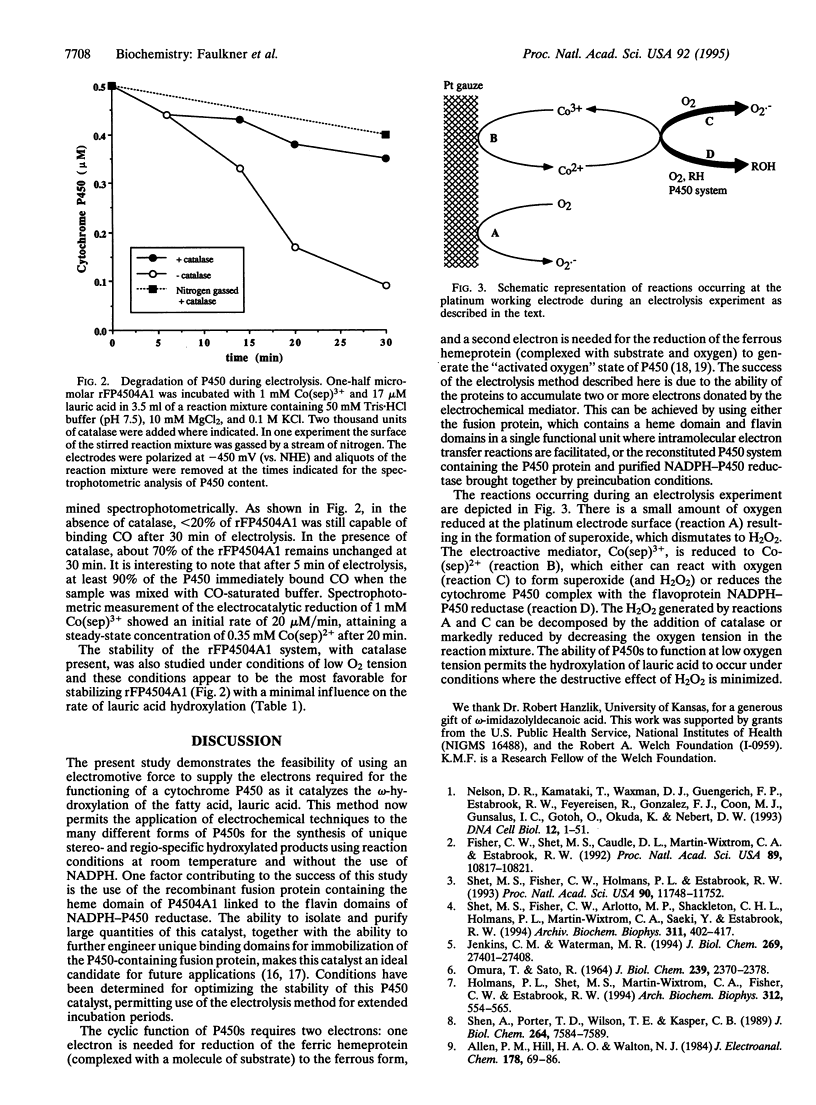
The cyclic enzymatic function of a cytochrome P450, as it catalyzes the oxygen-dependent metabolism of many organic chemicals, requires the delivery of two electrons to the hemeprotein. In general these electrons are transferred from NADPH to the P450 via an FMN- and FAD-containing flavoprotein (NADPH-P450 reductase). The present paper shows that NADPH can be replaced by an electrochemically generated reductant [cobalt(II) sepulchrate trichloride] for the electrocatalytically driven omega-hydroxylation of lauric acid. Results are presented illustrating the use of purified recombinant proteins containing P450 4A1, such as the fusion protein (rFP450 [mRat4A1/mRatOR]L1) or a system reconstituted with purified P450 4A1 plus purified NADPH-P450 reductase. Rates of formation of 12-hydroxydodecanoic acid by the electrochemical method are comparable to those obtained using NADPH as electron donor. These results suggest the practicality of developing electrocatalytically dependent bioreactors containing different P450s as catalysts for the large-scale synthesis of stereo- and regio-selective hydroxylation products of many chemicals.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achtnich U. R., Tiefenauer L. X., Andres R. Y. Covalent immobilization of avidin on glassy carbon electrodes as the basis for multivalent biosensors. Biosens Bioelectron. 1992;7(4):279–290. doi: 10.1016/0956-5663(92)87006-b. [DOI] [PubMed] [Google Scholar]
- Fisher C. W., Shet M. S., Caudle D. L., Martin-Wixtrom C. A., Estabrook R. W. High-level expression in Escherichia coli of enzymatically active fusion proteins containing the domains of mammalian cytochromes P450 and NADPH-P450 reductase flavoprotein. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10817–10821. doi: 10.1073/pnas.89.22.10817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmans P. L., Shet M. S., Martin-Wixtrom C. A., Fisher C. W., Estabrook R. W. The high-level expression in Escherichia coli of the membrane-bound form of human and rat cytochrome b5 and studies on their mechanism of function. Arch Biochem Biophys. 1994 Aug 1;312(2):554–565. doi: 10.1006/abbi.1994.1345. [DOI] [PubMed] [Google Scholar]
- Jenkins C. M., Waterman M. R. Flavodoxin and NADPH-flavodoxin reductase from Escherichia coli support bovine cytochrome P450c17 hydroxylase activities. J Biol Chem. 1994 Nov 4;269(44):27401–27408. [PubMed] [Google Scholar]
- Jiang Z. Y., Hunt J. V., Wolff S. P. Ferrous ion oxidation in the presence of xylenol orange for detection of lipid hydroperoxide in low density lipoprotein. Anal Biochem. 1992 May 1;202(2):384–389. doi: 10.1016/0003-2697(92)90122-n. [DOI] [PubMed] [Google Scholar]
- Nelson D. R., Kamataki T., Waxman D. J., Guengerich F. P., Estabrook R. W., Feyereisen R., Gonzalez F. J., Coon M. J., Gunsalus I. C., Gotoh O. The P450 superfamily: update on new sequences, gene mapping, accession numbers, early trivial names of enzymes, and nomenclature. DNA Cell Biol. 1993 Jan-Feb;12(1):1–51. doi: 10.1089/dna.1993.12.1. [DOI] [PubMed] [Google Scholar]
- Shen A. L., Porter T. D., Wilson T. E., Kasper C. B. Structural analysis of the FMN binding domain of NADPH-cytochrome P-450 oxidoreductase by site-directed mutagenesis. J Biol Chem. 1989 May 5;264(13):7584–7589. [PubMed] [Google Scholar]
- Shet M. S., Faulkner K. M., Holmans P. L., Fisher C. W., Estabrook R. W. The effects of cytochrome b5, NADPH-P450 reductase, and lipid on the rate of 6 beta-hydroxylation of testosterone as catalyzed by a human P450 3A4 fusion protein. Arch Biochem Biophys. 1995 Apr 20;318(2):314–321. doi: 10.1006/abbi.1995.1235. [DOI] [PubMed] [Google Scholar]
- Shet M. S., Fisher C. W., Arlotto M. P., Shackleton C. H., Holmans P. L., Martin-Wixtrom C. A., Saeki Y., Estabrook R. W. Purification and enzymatic properties of a recombinant fusion protein expressed in Escherichia coli containing the domains of bovine P450 17A and rat NADPH-P450 reductase. Arch Biochem Biophys. 1994 Jun;311(2):402–417. doi: 10.1006/abbi.1994.1255. [DOI] [PubMed] [Google Scholar]
- Shet M. S., Fisher C. W., Holmans P. L., Estabrook R. W. Human cytochrome P450 3A4: enzymatic properties of a purified recombinant fusion protein containing NADPH-P450 reductase. Proc Natl Acad Sci U S A. 1993 Dec 15;90(24):11748–11752. doi: 10.1073/pnas.90.24.11748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R. E., Coon M. J. Oxygen activation by cytochrome P-450. Annu Rev Biochem. 1980;49:315–356. doi: 10.1146/annurev.bi.49.070180.001531. [DOI] [PubMed] [Google Scholar]



