Abstract
The function of middle hepatitis B surface protein C-terminally truncated at amino acid position 167 (MHBst167) is not currently clear. This study aimed to screen and identify the proteins that interact with MHBst167 in hepatocytes using a yeast two-hybrid system, and to explore the effects of MHBst167 in the development of hepatocellular carcinoma and precancerous diseases of the liver. The MHBst167 gene was amplified by polymerase chain reaction (PCR) and cloned into a pGEM-T vector. The target region was sequenced and the constructed bait plasmid, pGBKT7-MHBst167, was transformed into AH109 yeast cells. The transformed AH109 cells were then mated with Y187 yeast cells containing the fetal liver cDNA library plasmid using a yeast two-hybrid system. The false positives were eliminated and the true positive clones were selected by PCR and sequencing analysis. The pGBKT7-MHBst167 bait plasmid was successfully constructed and 66 clones grew in the selective synthetic defined media lacking leucine, tryptophan, histidine and adenine. Fifty-two clones were identified following X-α-Gal selection and segregation analysis. Seven proteins were found to be expressed that could interact with MHBst167 in hepatocytes by the yeast two-hybrid system. These results have provided novel insights into the biological functions of MHBst167.
Keywords: hepatitis B virus, MHBst167, yeast two-hybrid
Introduction
Previous studies have indicated that numerous proteins are associated with the development of hepatocellular carcinoma (HCC) (1–8). Hepatitis B virus (HBV) encodes three envelope proteins in the pre-S/S open reading frame that are termed large, middle and small (major) surface proteins (9). Following the integration of viral sequences into the genome, the translation of C-terminally truncated surface proteins of HBV frequently occurs. It has been suggested that the expression of certain genes, activated by these translated viral sequences, may contribute to hepatocarcinogenesis (10).
In a previous study (10), the transactivating potential of middle hepatitis B surface protein C-terminally truncated at amino acid 167 (MHBst167) on the HBV regulatory element was investigated. The data indicated that MHBst167 is a pleiotropic, non-liver-specific transactivator which can modulate ubiquitous transcription factors that are associated with proliferation and inflammation (10). In order to further reveal the biological roles of MHBst167, the present study investigated cellular proteins interacting with the carboxyl terminus of MHBst167, an important functional region. To identify these proteins, a GAL4-based yeast two-hybrid system (Clontech Laboratories, Inc., Mountain View, CA, USA), using the MHBst167 cDNA as bait, was utilized to screen a human fetal liver cDNA library. The aim of the study was to provide novel insights into the biological functions of MHBst167.
Materials and methods
Yeast strains and plasmids
The Matchmaker® GAL4-based two-hybrid system 3 and vector pACT2 containing a human fetal liver cDNA library were obtained from Clontech Laboratories, Inc. The yeast strain AH109 (MATα, trp1–901, leu2–3, 112, ura3–52, his3–200, gal4Δ, gal80Δ, LYS2:GAL1UAS-GAL1TATA-HIS3, GAL2UAS-GAL2TATA-ADE2, URA3:MEL1UAS-MEL1TATA-LacZ) contained the pGBKT7–53 plasmid, encoding the DNA-binding domain (DNA-BD) mouse p53 fusion protein. The yeast strain Y187 (MATα, ura3–52, his3–200, ade2–101, trp1–901, leu2–3, 112, gal4, gal80, met-, URA3::GAL1UAS-GAL1TATA-lacZ, MEL1) contained the pTD1-1 plasmid, in which vector pACT2 encoded the AD/simian virus 40 large T antigen fusing protein. AH109 was used for cloning bait plasmids and Y187 was used for cloning library plasmids. The yeast-Escherichia coli shuttle plasmids pGBKT7 DNA-BD cloning plasmid, pGADT7 DNA-activation domain (DNA-AD) cloning plasmid, pGBKT7–53 control plasmid, pGADT7, pGBKT7-Lam control plasmid and pCL1 were obtained from Clontech Laboratories, Inc.
Chemical agents and culture media
The pGEM-T vector and Taq DNA polymerase were purchased from Promega Corp. (Madison, WI, USA). T4 DNA ligase, EcoRI and BamHI restriction endonucleases were purchased from Takara (Shiga, Japan), and c-Myc monoclonal antibody and goat anti-mouse immunoglobulin G antibody conjugated with horseradish peroxidase were obtained from Zhongshan Company (Zhongshan, China). Tryptone and yeast extracts were obtained from Oxoid (Thermo Fisher Scientific, Inc., Waltham, MA, USA). X-α-Gal and yeast peptone dextrose adenine, synthetic defined (SD)/-tryptophan (Trp), SD/-leucine (Leu), SD/-Trp/-Leu, SD/-Trp/-Leu/-histidine (His) and SD/-Trp/-Leu/-His/-adenine (Ade) media were purchased from Clontech Laboratories, Inc. Glass beads were purchased from Sigma (St. Louis, MO, USA), while lysis buffer [20 g/l Triton X-100, 10 g/l SDS, 10 mmol/l NaCl, 10 mmol/l Tris-HCl (pH 8.0) and 1 mmol/l Na2EDTA] and phenol, chloroform and isoamyl alcohol (volume fraction 25:24:1) were also obtained from Clontech Laboratories, Inc.
Construction of bait plasmid and expression of MHBst167
The extracted MHBst167 DNA was amplified by polymerase chain reaction (PCR) from the A7 plasmid (HBV strain subtype adr). The primer sequences, which contained EcoRI and BamHI sites, respectively, for cloning were as follows: Sense, 5′-gaattcatggtcaccttgaggtgg-3′; antisense, 5′-ggatcccaaacaggagatgaaggtcct-3′. The PCR conditions were as follows: 94°C for 4 min, denaturation at 94°C for 50 sec, annealing at 58°C for 50 sec, extension at 72°C for 1 min, 35 cycles. The PCR product was cloned into the pGEM-T vector and the primary structure of the insert was confirmed by direct sequencing. The auto-sequencing assay was performed by the Shanghai Hua Nuo Biological Corporation (Shanghai, China). The fragment encoding the MHBst167 was digested from the pGEM-T-MHBst167 using EcoRI and BamHI restriction enzymes, and ligated into the pGBKT7 vector. The pGBKT7 vector expressed proteins fused to amino acids 1–147 of the GAL4 DNA-BD and the pGADT7 vector expressed proteins fused to amino acids 768–881 of the GAL4 DNA-AD. The MHBst167 gene was inserted into the pGBKT7 multiple cloning site. The resulting plasmid, pGBKT7-MHBst167 (Fig. 1), containing the full-length MHBst167 gene, could direct expression of the DNA-BD, c-Myc and MHBst167 fusion protein. The plasmid was transformed into yeast strain AH109 using a lithium acetate method as previously described (11).
Figure 1.
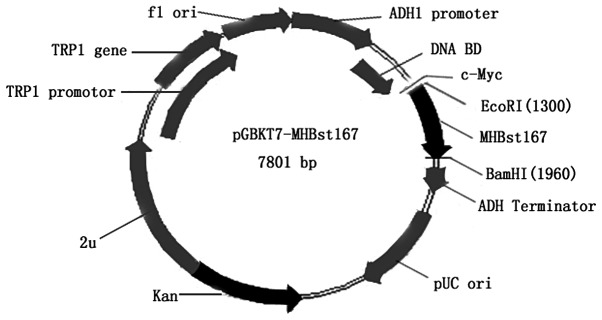
Structure of the bait plasmid pGBKT7- MHBst167.
Yeast two-hybrid screen
The yeast two-hybrid screening procedure used was a modification of the method described by Gietz et al (11). The pACT2-cDNA plasmid genome was isolated following the standard protocol. AH109 yeast cells containing pGBKT7-MHBst167 were transformed into the Y187 yeast strain containing the pACT2-cDNA liver library (Clontech Laboratories, Inc.) by the lithium acetate method, in accordance with the manufacturer’s instructions. Cells were plated and selected for on quadruple dropout (QDO) media lacking leucine, tryptophan, histidine and adenine. After 6–18 days of growth at 30°C, the yeast colonies were transferred onto plates containing X-α-Gal to check for the expression of the MEL1 reporter gene. Positive interactions were detected by the appearance of blue-colored colonies. Segregation analysis and mating experiments were performed to exclude the false positives, ensuring that only true positive colonies were selected. Following the sequencing of the positive colonies, the sequences were BLASTed with GenBank (blast.ncbi.nlm.nih.gov/Blast.cgi) to analyze the function of the genes.
Results
Identification of the plasmid
The 501-bp fragment of MHBst167 was generated by PCR amplification of the HBV plasmid (subtype adr), sequenced and analyzed using Vector NTI® 6 (Life Technologies, Carlsbad, CA, USA) and BLAST database homology. The PCR product was cloned with the pGEM-T vector. Following digestion with EcoRI/BamHI restriction enzymes, the fragments were ligated in-frame into the pGBKT7 and pGADT7 EcoRI/BamHI sites. Restriction enzyme analysis of pGBKT7-MHBst167 and pGADT7-MHBst167, respectively, with EcoRI/BamHI yielded two products: 7,300 bp empty pGBKT7 and 501 bp MHBst167, and 7,900 bp empty pGADT7 and 501 bp MHBst167. Analysis of the PCR products by agarose gel electrophoresis (Fig. 2) showed the products with the expected size (501 bp, MHBst167). The pGEM-T-MHBst167 sequence was confirmed by direct sequencing (Fig. 3).
Figure 2.
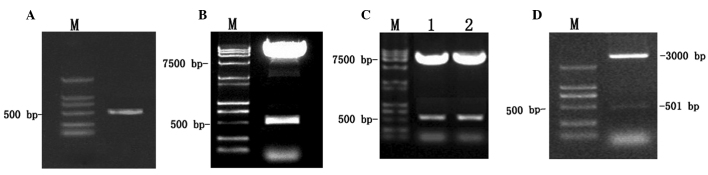
Construction and identification of plasmids by agarose gel electrophoresis. (A) Polymerase chain reaction product MHBst167 (501 bp). (B) Restriction enzyme digestion of pGBKT7-MHBst167 plasmids by EcoRI/BamHI (pGBKT7, 7,300 bp; MHBst167, 501 bp). (C) Restriction enzyme digestion of (Lane 1) pGBKT7-MHBst167 and (Lane 2) pGADT7-MHBst167 by EcoRI/BamHI. (D) Restriction enzyme digestion of pGEM-T-MHBst167 plasmids by EcoRI/BamHI. M, DL2000 DNA marker; bp, base pairs.
Figure 3.
Sequence of the pGEM-T-MHBst167 by direct sequencing.
Screening of the fetal liver cell cDNA library
Plasmids from the blue-colored colonies containing only pGBKT7-MHBst167, as the bait for screening the human fetal liver cell cDNA library, were isolated. Sixty-six clones grew on the QDO media. The clones were further selected for by X-α-Gal assay and, again, blue colonies were picked. Following elimination, 52 positive clones were further tested by the specificity of the selective SD/-Trp-Leu-His-Ade/X-α-Gal media and expression (Fig. 4). Since pACT2-cDNA plasmids contained two restriction endonuclease sites for recognition by BglII either side of the multiple cloning site, the gene fragments of the fetal liver cell cDNA library (pACT2-cDNA) screened were released by BglII digestion (Fig. 5).
Figure 4.
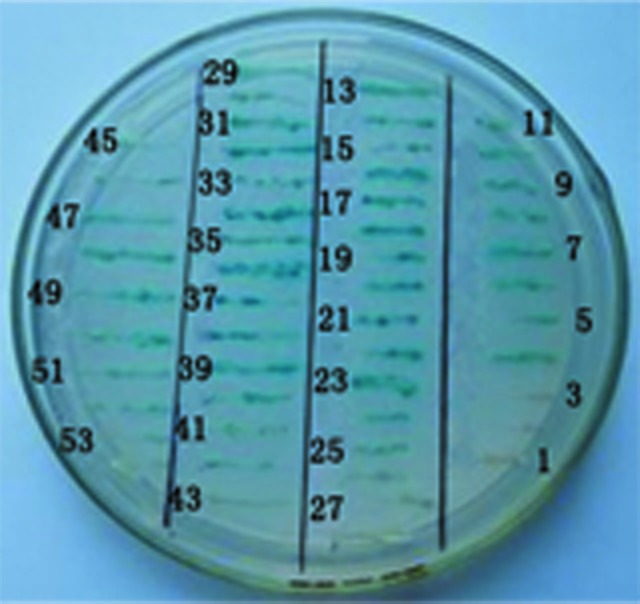
Screening of blue colonies containing only the MEL1 reporter gene on the X-α-Gal medium lacking leucine, tryptophan, histidine and adenine.
Figure 5.
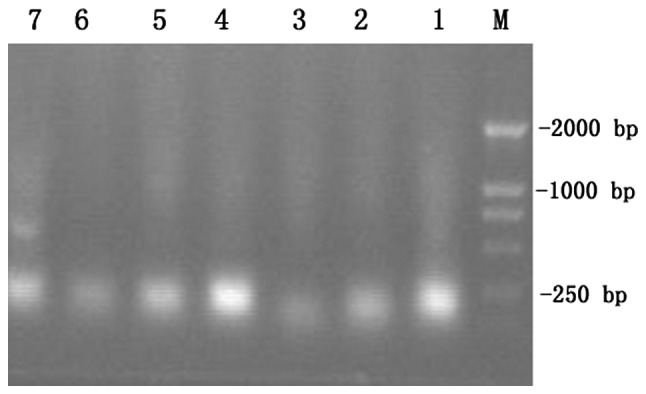
Identification of different colonies with BglII digestion. M, DL2000 DNA marker; bp, base pairs.
Analysis of cDNA sequencing and homology
Seven positive colonies grew on the selective SD/-Trp-Leu-His-Ade/X-α-Gal media. These colonies were prescreened by BglII digestion to ensure that only colonies with different inserts were subjected to sequencing. The seven colonies from the cDNA library were sequenced and analyzed using BLAST software provided by the National Center for Biotechnology Information. The seven sequences had high similarity to known genes. A summary of the identified genes is shown in Table I.
Table I.
Comparison between positive clones and similar sequences in GenBank.
| High similarity to known genes | No. of similar clones | Homology (%) |
|---|---|---|
| Homo sapiens ADP-ribosylation factor 1, mRNA (cDNA clone IMAGE: 4024984) | 2 | 98 |
| Full-length cDNA clone CS0DM004YC15 of fetal liver of Homo sapiens | 1 | 100 |
| Homo sapiens ALDOB gene, virtual transcript | 1 | 100 |
| Homo sapiens complement component 3, mRNA | 1 | 99 |
| Homo sapiens BAC clone GS1–306C12 from 7 | 1 | 100 |
| Homo sapiens vitronectin (serum spreading factor, somatomedin B, complement S-protein), mRNA | 1 | 98 |
IMAGE, integrated molecular analysis of genomes and their expression; BAC, bacterial artificial chromosome.
Discussion
Genetic alterations associated with human HCC have been reported for numerous genes; however, at present these are not sufficient to distinguish between HCC and precancerous liver diseases, including hepatitis, hepatic fibrosis and cirrhosis (12). It has previously been suggested that the expression of certain genes, activated by translated HBV sequences, may contribute to hepatocarcinogenesis (10). Large HBV surface protein and MHBst belong to the HBV PreS2 activator protein family. PreS2 activators have been suggested to act in a similar manner to promoters of tumorigenesis, modulating the control of proliferation through the activation of certain key enzymes (13). The ability of PreS2 activator proteins to activate transcription is associated with the cytoplasmic orientation of the PreS2 domain. While MHBst represents a standard for PreS2 activator proteins, the full-length MHBs exhibits no transcriptional activator activity, since the PreS2 domain is oriented into the lumen of the endoplasmic reticulum. For functional MHBst to be generated from the full-length MHBs, the 3′ end of the preS2/S gene, which encodes the last 70 amino acids in the sequence, must be deleted during the integration process. This sequence of amino acids corresponds to the third hydrophobic region of the S domain of the protein (14).
The potential of MHBst167 to activate transcription is mediated via various ubiquitous transcription factors, which are involved in the activation of proto-oncogenes or genes associated with inflammation. The overexpression of proto-oncogenes induced by MHBst167 may be causative of over-proliferation or may maintain mitogenesis; as such, it has been proposed that an association exists between transactivation by integrated HBV sequences and the development of HCC (10).
The present study used a yeast two-hybrid system as an approach for detecting protein-protein interactions. By this method, weak protein interactions may be identified which may not be observed using other in vitro assays, such as immunoprecipitation. The yeast two-hybrid system 3 utilized in this study was commercially available from Clontech Laboratories, Inc. (15–17). This system was selected since the promoters modulating HIS3, ADE2 and MEL1 reporter gene expression in the AH109 yeast strain result in significantly fewer false positives. Furthermore, the simple mating protocol is time- and labor-efficient. As such, the system facilitates the identification of rare protein-protein interactions and produces more reproducible results (18,19).
Following the screening of a fetal liver cDNA library, this study identified seven putative clones associated with MHBst167. The proteins identified were as follows: i) and ii) Homo sapiens ADP-ribosylation factor 1; iii) full-length cDNA clone CS0DM004YC15 of the fetal liver of Homo sapiens; iv) Homo sapiens ALDOB gene; v) Homo sapiens complement component 3 (C3); vi) Homo sapiens bacterial artificial chromosome (BAC) clone GS1–306C12 from chromosome 7; and vii) serum spreading factor (SF).
The first interacting protein identified in this study, ADP-ribosylation factor 1, is a GTPase required for the exocytosis of cytotoxic T-lymphocyte antigen 4 (CTLA-4), a process that is also dependent on phospholipase D activity. It has been suggested that ADP-ribosylation factor 1 and phospholipase D act to stimulate the rapid translocation of specialized compartments containing CTLA-4 in regulatory T cells to the plasma membrane (20). Poly(ADP-ribose) polymerase (PARP) is an enzyme predominantly localized in the nucleus and is responsible for catalyzing poly-ADP-ribosylation. The activity of PARP is associated with numerous biological processes, including DNA repair, cell proliferation and malignant transformation. It has been shown that the expression of PARP in HCC is increased in patients with liver cirrhosis, with higher expression levels in less-differentiated tumors (21).
The present study showed that MHBst167 also interacted with the full-length cDNA clone CS0DM004YC15 of the fetal liver of Homo sapiens and the Homo sapiens ALDOB gene. ALDOB is an enzyme involved in glycolysis and gluconeogenesis and is the only isoenzyme of aldolase expressed in differentiated hepatocytes. The impaired function of ALDOB has been associated with hereditary fructose intolerance, a recessively inherited disorder of carbohydrate metabolism. To date, 29 mutations have been identified in the ALDOB gene that impair the functioning of the enzyme (22). ALDOB has additionally been associated with HCC. In a study by Kinoshita et al (12), ALDOB was shown to be downregulated in primary HCC tissues in 90% of a cohort of 20 patients, as compared with healthy controls. Therefore, the underexpression of key ALDOB gene products may be important in the development and/or progression of HCC.
C3 is a key protein involved in the complement immune system. In a previous study of patients with chronic liver disease caused by HBV infection, C3 expression levels were revealed to be lower in the serum of patients than those in the serum of healthy controls. Furthermore, the levels of circulating immune complexes (CICs) in the patients were increased (23). It was suggested that the decreased C3 levels may have been a result of decreased synthesis and/or an increased consumption by the CICs (23). Results from a study by Takezaki et al (24) indicated that C3 levels may exhibit diagnostic power in the detection of HCC in patients with liver cirrhosis.
The present study showed that MHBst167 also interacted with the Homo sapiens BAC clone GS1–306C12 from chromosome 7.
Another important protein interacting with MHBst167 from the fetal liver cDNA library was serum SF (Homo sapiens vitronectin). Human serum SF is a secreted glycoprotein that is involved in the promotion of cell adhesion and spreading. SF has been shown to be synthesized and secreted into culture by HepG2 human HCC cells (25). A study by Inuzuka et al (26) analyzed plasma vitronectin levels in patients with liver disease and compared these levels with various parameters of liver function and the severity of liver cirrhosis, graded according to Child’s criteria. The plasma vitronectin level was low in all liver disease groups as compared with that in the healthy controls, although the difference was only significant between the controls and patients with HCC and decompensated cirrhosis. Furthermore, the vitronectin level was inversely correlated with the severity of cirrhosis. These results suggested that the plasma vitronectin level may be used as an indicator of the synthetic function of the liver in patients with liver diseases and that it may also be a marker of the severity of cirrhosis. Immunoelectron microscopy in the same study revealed the presence of vitronectin in the rough endoplasmic reticulum of hepatocytes, as well as around certain cells close to sites of necrosis and on collagen fibers (26).
These interacting proteins, identified by the yeast-two hybrid system, are closely associated with carbohydrate metabolism, tumor development/progression and immunoregulation. These data may provide novel insights into the functions of MHBst167, the pathogenesis of HBV-related diseases and malignant transformation. Further studies are required to investigate how the interactions between MHBst167 and the proteins identified in this study affect the occurrence and development of chronic hepatitis B, hepatic fibrosis and HCC.
Acknowledgements
This study was previously published as a poster: Zhi Qun Li, Song Zhang, Yang Zhu and Jun Cheng: Screening of hepatocyte proteins binding with C-terminally truncated surface antigen middle protein of hepatitis B virus (MHBst167) by yeast two-hybrid system. International Journal of Infectious Diseases 2010.07: 98. The authors wish to thank Chinese PLA 302 Hospital for the supply of financial and technological assistance in carrying out this research.
Abbreviations
- HBV
hepatitis B virus
- MHBst167
middle hepatitis B surface protein C-terminally truncated at amino acid position 167
- PCR
polymerase chain reaction
- DNA-BD
DNA binding domain
- DNA-AD
DNA activation domain
- HCC
hepatocellular carcinoma
- QDO
quadruple dropout medium lacking leucine, tryptophan, histidine and adenine
- CIC
circulating immune complex
- BAC
bacterial artificial chromosome
- SF
spreading factor
References
- 1.Kong XB, Yang ZK, Liang LJ, Huang JF, Lin HL. Overexpression of P-glycoprotein in hepatocellular carcinoma and its clinical implication. World J Gastroenterol. 2000;6:134–135. doi: 10.3748/wjg.v6.i1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun JJ, Zhou XD, Liu YK, Zhou G. Phase tissue intercellular adhesion molecule-1 expression in nude mice human liver cancer metastasis model. World J Gastroenterol. 1998;4:314–316. doi: 10.3748/wjg.v4.i4.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xiao CZ, Dai YM, Yu HY, Wang JJ, Ni CR. Relationship between expression of CD44v6 and nm23-H1 and tumor invasion and metastasis in hepatocellular carcinoma. World J Gastroenterol. 1998;4:412–414. doi: 10.3748/wjg.v4.i5.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feng T, Zeng ZC, Zhou L, Chen WY, Zuo YP. Detection of human liver-specific F antigen in serum and its preliminary application. World J Gastroenterol. 1999;5:175–176. doi: 10.3748/wjg.v5.i2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin GY, Chen ZL, Lu CM, Li Y, Ping XJ, Huang R. Immunohistochemical study on p53, H-rasp21, c-erbB-2 protein and PCNA expression in HCC tissues of Han and minority ethnic patients. World J Gastroenterol. 2000;6:234–238. doi: 10.3748/wjg.v6.i2.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang XF, Wang CM, Dai XW, et al. Expressions of chromogranin A and cathepsin D in human primary hepatocellular carcinoma. World J Gastroenterol. 2000;6:693–698. doi: 10.3748/wjg.v6.i5.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mei MH, Xu J, Shi QF, Yang JH, Chen Q, Qin LL. Clinical significance of serum intercellular adhesion molecule-1 detection in patients with hepatocellular carcinoma. World J Gastroenterol. 2000;6:408–410. doi: 10.3748/wjg.v6.i3.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao Cai Yan, Li Yue Lin, Liu Su Xia, Feng Zhong Jun. Changes of IL-6 and relevant cytokines in patients with hepatocellular carcinoma and their clinical significance. World J Gastroenterol. 2000;4(Suppl 2):33. [Google Scholar]
- 9.Wang HC, Wu HC, Chen CF, et al. Different types of ground glass hepatocytes in chronic hepatitis B virus infection contain specific pre-S mutants that may induce endoplasmic reticulum stress. Am J Pathol. 2003;163:2441–2449. doi: 10.1016/S0002-9440(10)63599-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caselmann WH, Renner M, Schlüter V, Hofschneider PH, Koshy R, Meyer M. The hepatitis B virus MHBst167protein is a pleiotropic transactivator mediating its effect via ubiquitous cellular transcription factors. J Gen Virol. 1997;78:1487–1495. doi: 10.1099/0022-1317-78-6-1487. [DOI] [PubMed] [Google Scholar]
- 11.Gietz RD, Triggs-Raine B, Robbins A, Graham KC, Woods RA. Identification of proteins that interact with a protein of interest: applications of the yeast two-hybrid system. Mol Cell Biochem. 1997;172:67–69. [PubMed] [Google Scholar]
- 12.Kinoshita M, Miyata M. Underexpression of mRNA in human hepatocellular carcinoma focusing on eight loci. Hepatology. 2002;36:433–438. doi: 10.1053/jhep.2002.34851. [DOI] [PubMed] [Google Scholar]
- 13.Hildt E, Munz B, Saher G, Reifenberg K, Hofschneider PH. The PreS2 activator MHBs(t) of hepatitis B virus activates c-raf-1/Erk2 signaling in transgenic mice. EMBO J. 2002;21:525–535. doi: 10.1093/emboj/21.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lauer U, Weiss L, Hofschneider PH, Kekulé AS. The hepatitis B virus pre-S/S(t) transactivator is generated by 3′ truncations within a defined region of the S gene. J Virol. 1992;66:5284–5289. doi: 10.1128/jvi.66.9.5284-5289.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fields S, Song O. A novel genetic system to detect protein-protein interactions. Nature. 1989;340:245–246. doi: 10.1038/340245a0. [DOI] [PubMed] [Google Scholar]
- 16.Evangelista C, Lockshon D, Fields S. The yeast two-hybrid system: prospects for protein linkage maps. Trends Cell Biol. 1996;6:196–199. doi: 10.1016/0962-8924(96)40002-2. [DOI] [PubMed] [Google Scholar]
- 17.Kharel Y, Takahashi S, Yamashita S, Koyama T. In vivo interaction between the human dehydrodolichyl diphosphate synthase and the Niemann-Pick C2 protein revealed by a yeast two-hybrid system. Biochem Biophys Res Commun. 2004;318:198–203. doi: 10.1016/j.bbrc.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 18.Vidalain PO, Boxem M, Ge H, Li S, Vidal M. Increasing specificity in high-throughput yeast two-hybrid experiments. Methods. 2004;32:363–370. doi: 10.1016/j.ymeth.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 19.von Mering C, Krause R, Snel B, et al. Comparative assessment of large-scale data sets of protein-protein interactions. Nature. 2002;417:399–403. doi: 10.1038/nature750. [DOI] [PubMed] [Google Scholar]
- 20.Mead KI, Zheng Y, Manzotti CN, et al. Exocytosis of CTLA-4 is dependent on phospholipase D and ADP ribosylation factor-1 and stimulated during activation of regulatory T cells. J Immunol. 2005;174:4803–4811. doi: 10.4049/jimmunol.174.8.4803. [DOI] [PubMed] [Google Scholar]
- 21.Shimizu S, Nomura F, Tomonaga T, et al. Expression of poly(ADP-ribose) polymerase in human hepatocellular carcinoma and analysis of biopsy specimens obtained under sonographic guidance. Oncol Rep. 2004;12:821–825. [PubMed] [Google Scholar]
- 22.Esposito G, Santamaria R, Vitagliano L, et al. Six novel alleles identified in Italian hereditary fructose intolerance patients enlarge the mutation spectrum of the aldolase B gene. Hum Mutat. 2004;24:534. doi: 10.1002/humu.9290. [DOI] [PubMed] [Google Scholar]
- 23.Joshi N, Ayesha Q, Habibullah CM. Immunological studies in HBV-related chronic liver diseases. Indian J Pathol Microbiol. 1990;33:351–354. [PubMed] [Google Scholar]
- 24.Takezaki E, Murakami S, Nishibayashi H, Kagawa K, Ohmori H. A clinical study of complements as a marker of a hepatocellular carcinoma. Gan No Rinsho. 1990;36:2119–2122. (In Japanese) [PubMed] [Google Scholar]
- 25.Barnes DW, Reing J. Human spreading factor: synthesis and response by HepG2 hepatoma cells in culture. J Cell Physiol. 1985;125:207–214. doi: 10.1002/jcp.1041250206. [DOI] [PubMed] [Google Scholar]
- 26.Inuzuka S, Ueno T, Torimura T, et al. Vitronectin in liver disorders: biochemical and immunohistochemical studies. Hepatology. 1992;15:629–636. doi: 10.1002/hep.1840150413. [DOI] [PubMed] [Google Scholar]


