Introduction
Radiosurgery, in the mind of its creator Lars Leksell, was clearly intended to mimic the lesional effects of a surgeon’s knife, hence the name “gamma knife surgery” (GKS) being given to the first completed instrument. The high doses initially selected for the thalamotomies [1], capsulotomies [2], or benign tumors [3, 4] were rapidly identified as being unnecessarily toxic. Since its creation by Leksell in the 1950’s, clinical and experimental radiosurgery experiences have demonstrated that, for classical indications like arteriovenous malformations and benign tumors, radiosurgery is effective owing to its specific histological effects of thrombotic endothelial proliferation and apoptosis, not frank coagulative necrosis. In functional neurosurgery, the strategy is either to target a small volume of normal tissue [i.e., ventrointermediate nucleus (VIM), capsulotomy, trigeminal neuralgia (TN), etc.] with a high dose (maximum 80–140 Gy) or to target a large volume of tissue (i.e., 5–9 cc in epilepsy radiosurgery) with a moderate dose (17–24 Gy at the marginal isodose).
Initially, these procedures have been proposed, technically performed, and evaluated based on the hypothesis that their mechanism of action was purely destructive. However, modern neurophysiological, radiological, and histological studies are leading us to question this assumption. Tissue destruction is turning out to be either absent or minimal and in almost all cases insufficient to explain the clinical effects obtained. Therefore, one possibility is that radiosurgery induces changes in the functioning of the neural tissue by inducing remodeling of the glial environment, leading to the modulation of function while preserving basic processing. Thus, the majority of radiosurgery procedures may induce the desired biological effect without requiring a destructive histological effect for the completion of the therapeutic goal. Therefore the concept of “lesional” radiosurgery may be partially incorrect, and a completely hidden world of neuromodulatory effects may remain to be discovered [5, 6].
TN
In 1951, Leksell performed the first radiosurgical procedure for TN by targeting the gasserian ganglion, as identified on X-rays [7]. Gamma Knife Surgery (GKS) was implemented in 1968, and gained further interest for the treatment of TN in the early 1990s, with the use of magnetic resonance imaging (MRI) and limitations of other more invasive techniques [8, 9]. In 1993, Rand et al. [9] proposed to move the target from the gasserian ganglion to the cisternal segment of the nerve. For a short time afterwards, Lindquist [10] promoted the idea of targeting the nerve at its emergence from the brainstem [dorsal root entry zone (DREZ)], with a dose of 70 Gy. We then reported the first 5 cases successfully treated in Marseille with a more anterior target (plexus triangularis target) and a higher dose of 90 Gy [11]. In 1996, the multicentric trial published by Kondziolka et al. [8] demonstrated the safety and efficacy of GKS with a maximum dose between 70 and 90 Gy. Although this remains a subject of controversy in the literature, experience has oriented the use of an optimal distance between 7.5 and 8 mm, as it has shown clear benefit in terms of efficacy, toxicity, and long-lasting pain relief [12]. In 2006, what is still the only prospective trial in the field of TN radiosurgery was published [13]. This prospective controlled trial, with an independent neurological assessment, demonstrated a high efficacy/safety rate using an average high dose of 85 Gy and the anterior cisternal target, comparing favorably with the series using a DREZ target and lower radiation doses.
Long-term results have been reported in two series, with 22 % of patients pain-free at 7 years [14] and 30 % of patients pain-free at 10 years [15] , respectively, both using the DREZ and lower radiation doses of 75 and 80 Gy (median) instead of 85 Gy. The large Marseille series (Régis J, Tuleasca C, Resseguier N, Carron R, Donnet A, Gaudart J, Yomo S, Levivier M., submitted) is the only study to report the long-term results with an anterior cisternal target. From a series of 737 consecutive patients, we analyzed 497 with more than § year of follow-up: 45.3 % remained pain free at 10 years; the hypoesthesia rate was low, with only 21.1 % being “somewhat bothersome” in only 8 patients (1.6 %) and “very bothersome” in 3 (0.6 %). The majority of patients with good efficacy of radiosurgery have no hypoesthesia. At the opposite end of the lesional percutaneous spectrum, for example techniques like thermocoagulation, a sensory deficit is not necessary in order to obtain a therapeutic effect. Therefore, the mechanism of action of the gamma knife is unclear. The different clinical pattern of efficacy are indicates a possible dual mechanism [16]. Nowadays, gamma knife radiosurgery is clearly recognized as the least invasive neurosurgical technic for TN [17]. Very little is known about the mechanism of action of radiosurgery. Radiosurgery has been demonstrated to induce demyelination both in experimental models [18] and in autopsy [19]. This finding has been confirmed by tractography examinations [20].
Nuances in the radiosurgical technique result in significant differences in clinical results being obtained [21]. For example, Flickinger et al. [22] demonstrated that increasing the volume of the treated nerve leads to a dramatic increase in the risk of toxicity, with no clear benefit in efficacy: we call this phenomenon the Flickinger effect [22]. Our work comparing treatment variables between 2 centers (Marseille and Brussels) demonstrated a dramatic increase of toxicity when using channel blocking. More recently, the dose received by the brainstem has been demonstrated to be a negative prognostic factor for trigeminal function injury [23].
Even if microvascular decompression (MVD) remains the reference technique for the treatment of refractory TN [24], the current evidence for the long-term safety-efficacy of the GKS is sufficient to propose this method of treatment as a first intervention [21]. Technical nuances should be taken into account, as they have a major impact on the results of this approach.
Movement Disorders
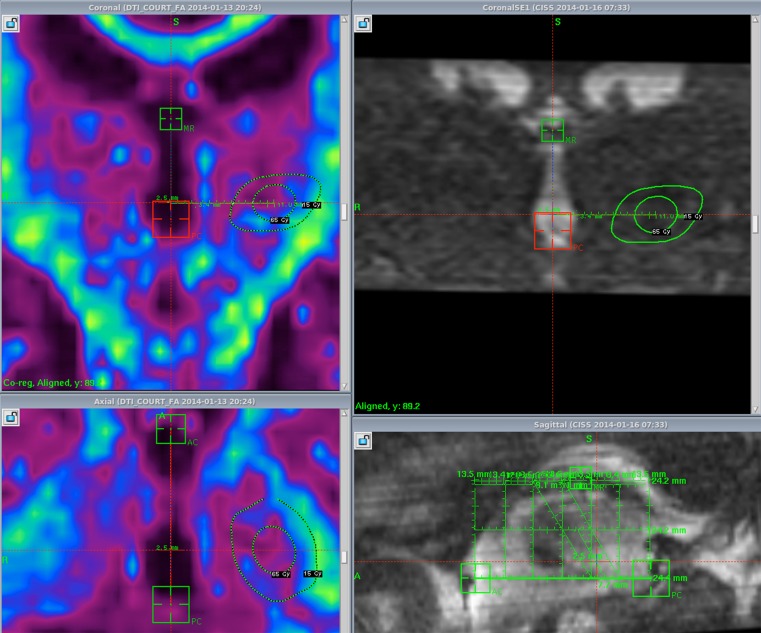
VIM thalamotomies are classically performed using a maximum dose of 130–140 Gy, inducing a small area of necrosis after several months that can be clearly seen at the 12-month follow-up MRI (Fig. 1). This is one of the infrequent indications for radiosurgery where the intended effect is to mimic the histologically destructive effect produced by thermocoagulation. However, in 2000, Ohye et al. [25] proposed that the clinical effect on tremor was not only the result of the necrotic lesion, but also that the size of the lesion induced by radiosurgery was too small to account for the clinical effect seen (it is interesting to note that the limit of the MRI lesion corresponds, in our experience, to the volume of the 90-Gy isodose line). Some experimental observations [26] support this hypothesis, but further studies are still necessary in order to better understand the nature and respective role of nonlesional and lesional mechanisms in VIM radiosurgery. In 2008, Terao et al. [27] reported that the somatotopic distribution of kinaesthetic cells was modified by GKS of the VIM, raising the possibility that the specific properties of the neurons change in response to GKS. The “cocade” theory (Fig. 2) is an original concept that we proposed some years ago summarizing the effect of a radiosurgery dose on normal brain by artificially separating it into 4 concentric zones [5]. When sufficient dose/volume parameters are used, the central zone shows evidence of histologic necrosis. Around this “necrotic core” is the “subnecrotic area” where cellular death is observed without coagulative necrosis. Typically, the subnecrotic area is the area in which cellular differential effects of radiosurgery are observed with considerable washout of glial cells and very few noncycling cells (neurons) dying. Outside of the “subnecrotic area” is the “neuromodulatory area”. More subtle changes are visible without significant increase in cell death. Inflammatory compounds produced in the subnecrotic area are likely to account for a significant number of the changes observed in this area. Outside of this “neuromodulatory area”, no effect is observed. The demonstration of the existence of this “neuromodulatory area” requires further experimental demonstration.
Fig. 1.
Doseplanning of a left VIM Gamma Knife Surgery
Fig. 2.
Doseplanning of radiosurgery for mesial temporal lobe epilepsy (MTLE)
In thalamotomies, the treatment is completely standard in terms of the volume of the target, location, and dose. Although the tissue reaction to radiosurgery is reportedly very focal in some series [28, 29], up to 10 % of patients can have a much larger radiographic reaction, as seen on MRI, and these imaging changes may be associated with a hemiparesis, which is usually transient (Table 1). Kondziolka et al. [30, 31] demonstrated the radioprotective effect of the 21-aminosteroid U-74389G in an experimental study in rats. They report that this drug reduces the cytokine expression normally seen after radiation injury and which may be overexpressed in the patients having a greater reaction to radiosurgery seen clinically. Rahn et al. [32] have shown in a group of patients with brain metastases that those patients treated in the morning were doing substantially better than those treated in the afternoon, suggesting that some sort of cellular circadian rhythm affects the response of tumor and normal tissues to radiosurgery. The genetic profile of the individual is certainly crucial. The main series in the literature report a good safety efficacy of GKS thalamotomy for tremor when practiced using a state-of-the-art set up (a single 4-mm shot, maximum dose between 130 and 160 Gy). Some series have reported less satisfactory results [33, 34]. Okun et al. [34] reported a high rate of toxicity; however the very high dose (maximum 200 Gy) used in these patients is much higher than that recommended in modern literature (120–140 Gy), which easily explains such an unusual rate of toxicity [34]. In a prospective, multicentric trial, Ohye et al. [35] demonstrated the reproducibility of a good safety efficacy when radiosurgery follows strict technical rules. Our series confirms this finding (Witjas T, Carron R, Krack P, Azulay JP, Régis J., submitted). Of 99 patients (60 with more than 1 year of follow-up), prospectively selected, operated on (GKS, maximum 130 Gy in a single 4-mm shot), and followed, the improvement in activities of daily life was 73 % and the outcome of tremor was good in 87 % of patients. On MRI, 3 patients demonstrated an unusually large reaction. Only 1/3 demonstrated a transient hemiparesis. Even though a good safety of bilateral VIM gamma knife thalamotomy has been reported [35, 36], a strict, prospective evaluation of bilateral treatments by GKS is still lacking in the literature. In particular, the risk of significant gait disturbance induced by bilateral surgery of the VIM must not be underestimated.
Table 1.
Main series in the literature (reporting > 20 patients, with > 1 year of follow-up)
| First author, year [ref.] | No. of patients | % ET | Dose at max. (Gy) | FU (months) | Efficacy (%) | Complication type | Complication rate (%) |
|---|---|---|---|---|---|---|---|
| Young, 1998 [81] | 27 (73,3) | – | 120–160 | 22.3 (12–44) | 89 | None | 0 |
| Duma, 1999 [82] | 38 (60–84) | – | 120–160 | 30 (6–72) | 90 | Dysarthria | 2.6 |
| Young, 2000 [29] | 158 (72) | 33 | 120–160 | 12–96 | PD 88 ET 92 | Balance, paresthesia, weakness, dysphasia. | 1.3 |
| Kondziolka, 2008 [77] | 31 (52–92) | – | 130–140 | 36 (4–96) | 92 | Hemiparesis, dysphasia, dysarthria | 7.7 |
| Young, 2010 [36] | 161 (18–93) | – | 141–152 | 44 ± 33 | Dr 81 Wr 77 | Sensory loss, motor impairments, dysarthria | 8.4 |
| Ohye, 2012 [35] | 72 | 18 | 130 | > 24 | 81 (Ex or G) | Transient hemiparesis | 1.4 |
| Witjas, submitted | 60 (72) | 72 | 130 | 12–96 | 87 % (Ex or G) | Transient hemiparesis | 1.6 |
ET = essential tremor; PD = Parkinson disease; max. = maximum; FU = follow-up; Dr = drawing; Wr = writing; Ex = excellent result; G = good result; Ref = reference; Gy = gray
Epilepsy
Until the 1990s, curative epilepsy surgery was limited to open microsurgery (MS), where the abnormal epileptogenic tissue was physically removed. Stimulation techniques [vagus nerve stimulation or deep brain stimulation (DBS)] and disconnection surgery (callosotomies or subpial transection) are palliative techniques usually reserved for very severe cases of drug-resistant epilepsies where there is no hope left for a curative resective procedure [37].
The clinical observation of seizure cessation after radiosurgery of arterio-venous malformation (AVM) in a highly functional area without any deficit has led us to hypothesize the existence of some kind of differential biological effect of radiosurgery in tissues, namely that a low-dose radiosurgery applied to normal neuronal tissue—relying on subtle, but specific, biological changes—may affect some processes, producing the desired clinical effect while sparing others [38]. In 1994, the very first paper was published demonstrating that the existence of such a differential effect was manifest at the biochemical level [38]. This differential effect has recently been found at the cellular level in hippocampus of animal epilepsy models [39]. In epileptic rats irradiated with 40 Gy to the medial temporal lobe, immunohistochemical findings suggest that at least 1 subtype of hippocampal interneurons are selectively vulnerable to GKS. Thus, this work suggests a selective vulnerability of certain neuronal subtypes to our proposed “neuromodulative” effect. However, the clinically safe and efficient implementation of radiosurgery to effect some form of neuromodulation still requires further basic science work [40] to allow for a better understanding of the influence of dose, volume, target topography, and dose distribution homogeneity on the modulation of specific biological systems [5, 38].
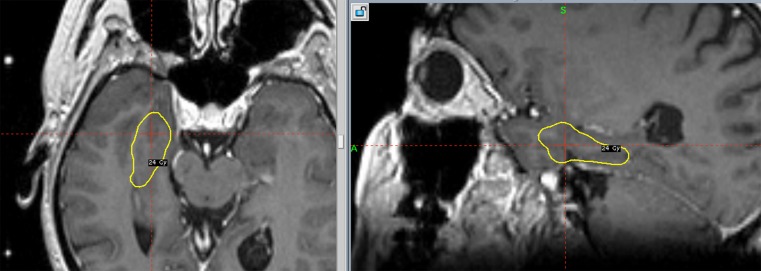
The first cases of mesial temporal lobe epilepsy (MTLE) treated with GKS in Marseille in 1993 [41, 42] were encouraging, with short-term results demonstrating the safety and efficacy of this approach. Seizure control rates were similar to those of resective surgery, and these early results are now confirmed to be durable responses on medium- [43, 44] and long-term [45] follow-up. Other groups are attempting to confirm the potential of GKS as a new, noninvasive treatment for MTLE in comparison with standard open temporal lobe surgery (personal communication, Nicholas Barbaro). The impressive MRI findings observed at roughly 1 year after radiosurgery led us to speculate initially that a necrotic lesional effect was responsible for the clinical effect [46]. MRI changes may sometimes be misleading [47]. Typically, the extent of the white matter changes are more related to an increase in extracellular fluid than the locally delivered dose affecting the role of secondary messengers. For example, in MTLE, the major white matter changes extend far from the target laterally following association fiber tracts, but those fiber tracts within the brainstem, which receive similar energies, are absolutely not modified on follow-up imaging studies (Fig. 3). Obviously, these distant changes are due to propagation of inflammatory small molecules through the white matter tracts and are not at all induced directly by ionizing radiation—not dissimilar to the diffuse white matter changes sometimes observed after the treatment of small midline meningiomas.
Fig. 3.
Doseplanning of radiosurgery for hypothalamic hamartoma (HH)
However, medium-term disappearance of these MRI findings—leaving the medial structures of the temporal lobe either similar to, or only slightly more atrophic than the preoperative status—suggests a more subtle effect of radiosurgery than initially thought (Fig. 4), and shows a much more limited volume of the temporal lobe being involved compared with the effect of MS [44]. Further studies correlating the efficacy of the quality of the coverage of each structure of the medial temporal lobe area on seizure outcome have demonstrated the importance of the targeting of the anterior parahippocampal cortex and, in particular, the perirhinal and entorhinal cortex [48]. These finding are perfectly consistent with those of Wieser et al. [49] in their series of microsurgical amygdalohippocampectomies. Perirhinal and entorhinal cortex play a major role in memory processing [50]. Globally, it is estimated that 40 % of patients with MTLE operated on microsurgically on the dominant side have a significant postoperative short-term verbal memory deficit [51, 52]. Clusmann et al. [53] have shown, in their series of 285 patients, that this deficit was slightly more common (43.4 % vs 30.9 %) when an anterior temporal lobectomy was performed instead of an amygdalohippocampectomy. Our first prospective trial of GKS for MTLE found that in 65 % of those who had dominant temporal lobe treatment there was no evidence of any neuropsychological deficit [44, 45]. Since that time, our subsequent experience has confirmed this observation. Nowadays, we consider this memory-sparing benefit of GKS to be the major advantage of radiosurgery over MS in patients with dominant temporal lobe MTLE. Thus, nowadays the MTLE patients selected for GKS instead of MS are those who may suffer more and longer if an additional memory deficit is produced, namely young patients, with a high level of functioning, socially adapted, working, concerned by the risk of MS and the time off from work, presenting with risk factors for verbal memory loss in case of resection (no atrophy, dominant side, few neuropsychological deficits before surgery) [54]. This group of patients are more often highly functioning individuals with an IQ sufficient to allow them to understand the peculiarities and nuances of radiosurgery. In addition, they frequently do not have more severe epilepsies, which affords them the luxury of waiting. The San Diego group has observed in patients tested during the “acute phase” (when the acute MRI signs are still present) a neuropsychological worsening in some patients [55], but this group has not reported the long-term results of their neuropsychological testing after the resolution of the acute MRI signs. We have observed the same verbal memory sparing in long-term MTLE patients treated on the dominant side. This same observation has been recently confirmed by a multicenter, phase I-II trial in the USA [43]. The mechanism of this functional preservation is still a matter of speculation. It may be that at this dose regimen we are not lesional at all! It may also be that the cell loss is selective, affecting primarily the glial environment. Perhaps a certain degree of neuronal and astrocytic damage is being induced, but the process is so slow and delayed that the brain has the sufficient time to functionally reorganize. In an elegant study, Maesawa et al. [56, 57] tested both the efficacy of radiosurgery and the sparing of memory in a rat kainic acid model of epilepsy. The untreated control group continued to have seizures, while those rats treated with 30 Gy (maximum dose) had a reduction in seizure frequency. The group treated with 60 Gy (at the maximum) had cessation of seizure activity between 5 and 11 weeks after GKS. The Morris water maze test, used for spatial memory testing, was found to be highly abnormal in the control group, but the radiosurgically treated 30-Gy group had a normal spatial memory [56, 57].
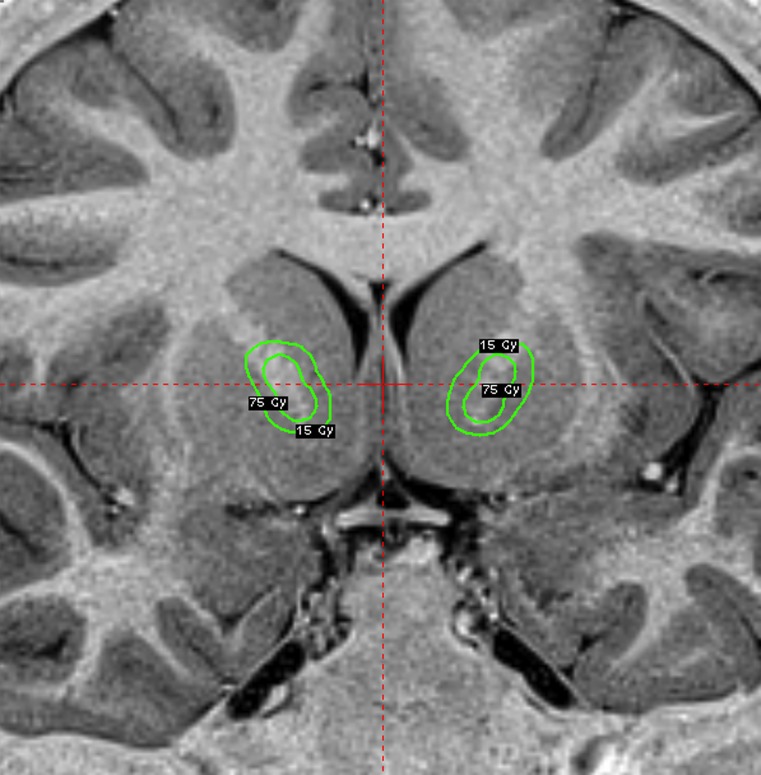
Fig. 4.
Doseplanning of a capsulotomy: On each side two shots of 4 mm are positionned at the anterior limb of the internal capsule, 8-10 mm anterior to the commissure and the 50 % isodose line is covering the ventral half of theinternal capsule. A dose of 180 is delivered at the 100 % and a plug pattern used to shape thedoseplanning
Jenrow et al. [58] reported that the selective reduction of densities in the dentate granular cell layer and medial CA3 pyramidal cell layer was prevented or reversed in epileptic rats (kindling model) by irradiation at 25 Gy, but not at 18 Gy. These experimental studies tended to support the dose effect found in humans [59]. Several magnetic resonance spectroscopy studies show, at around 12 months (the usual delay for major clinical and radiological changes), a strong reduction in choline, creatine, and N-acetylaspartate, with an elevation in lactate, pointing out the lack of normal oxidative metabolism (ischemia) [60–65].
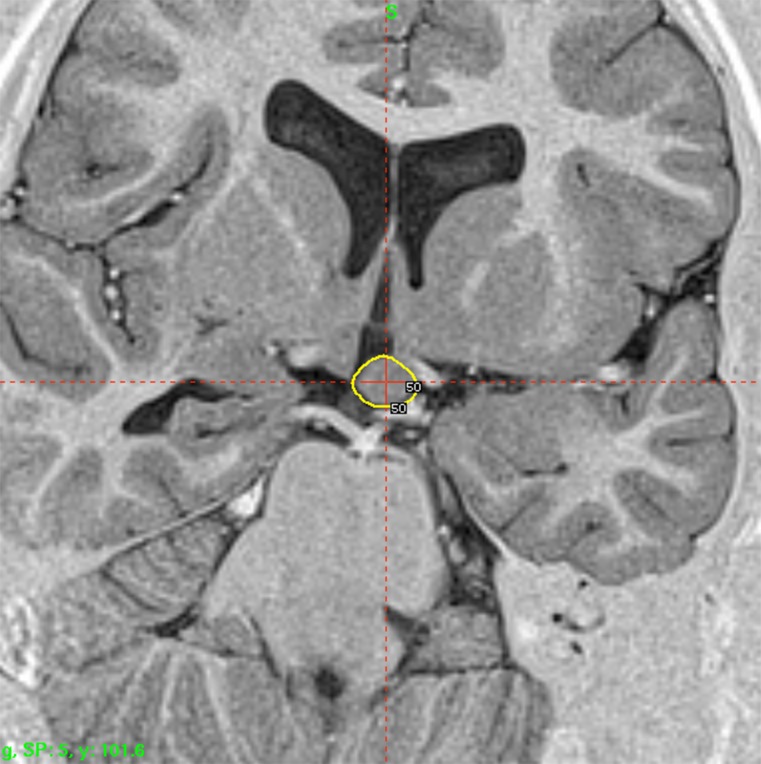
Hypothalamic hamartoma radiosurgery for epilepsy control is an even more convincing example of the functional, nonlesional effect in GKS. As an international referral center for this rare pathology, we have treated more than 80 patients. From a theoretical point of view, the question of the definition of the epileptogenic zone (EZ) is straightforward. The hamartoma itself, usually quite well delineated on high-resolution MRI, is supposed to be the EZ [66, 67] and defines the limits of the target, thus making the targeting simpler than that compared to MTLE. After the first multicentered retrospective trial [68], a prospective trial was organized [67, 69, 70]. The 64 patients operated on between 1999 and 2007 have been all followed-up for > 3 years (36–107 months). Owing to significant, but partial, efficacy 25 patients (62.5 %) were treated twice. The preoperative cognitive deficits, behavioral disturbances, and investigated relationship of seizure severity and anatomical type to cognitive abilities were characterized [71, 72]. Low peripheral doses were used because of the close relationship with optic pathways and hypothalamus (median 17 Gy; range 13–26 Gy). The lesions treated are generally small (median 9.5 mm; range 5–26 mm). Special attention is paid to the dose delivered to the mamillary body and to the fornix, and the dose plan for each patient is tailored based on the use of a single run of shots with the 4-mm collimator. Results demonstrate that in 65 % of these patients Engel I + II is achieved. Engel III is observed in 20 % of the patients. The frequency of seizures before radiosurgery was 92 per month (mean 427 ± 1009; minimum 3.3) and dropped afterwards to six per month (mean 34.6 ± 78.0; minimum 0, maximum 425). Dramatic behavioral and cognitive improvement was observed in the majority of these patients. Psychiatric and cognitive comorbidity was cured in 28 %, improved in 56 % and stabilized in 8 % of the patients. Globally, a very good result has been obtained in 60 % of patients. A microsurgical approach has been performed in 6 patients (9.3 %) with quite large hypothalamic hamartoma (HH) and poor efficacy of radiosurgery. After MS in 6 patients, 1 (17 %) has been cured (Engel I), 2 (33 %) have improved (Engel III), and 3 (50 %) have not improved (Engel IV). No permanent or even transient neurological deficit has been reported. A transient increase of seizure was observed in 7 patients (17.5 %). A transient, nondisabling poïkilothermia was observed in 3 patients. The effect on the comorbidities usually occurs prior to the effect on the seizure control and in absence of any changes in the MRI. The clinical observation of profound therapeutic effect with absolutely no histological necrosis induced by radiosurgery is encouraging and again suggests some neuromodulatory effect of radiosurgery, and shows that low doses can lead to cessation of neuronal firing [67].
Psychiatric Disease
Radiosurgery for psychiatric disorders has been performed for over 50 years, mainly for obsessive–compulsive disorder (OCD). OCD is marked by recurrent unwanted and intrusive thoughts or repetitive behaviors. These “rituals” reflect the patient’s anxiety or attempts to control it, and can be greatly exaggerated in stressful situations and, in some cases, can be incapacitating [73]. In 1949, the French neurosurgeon Jean Talairach (Saint-Anne Hospital, Paris) was the first to selectively lesion the anterior limb of the internal capsule for the treatment of psychiatric disorders [74]. Talairach developed the technique of anterior capsulotomy in an attempt to obtain the same benefits provided by extensive procedures like transorbital lobotomy or prefrontal lobotomy through the creation of a much smaller lesion incurring fewer side effects. Initially, he reported good results in patients with anxiety disorders, and soon the procedure was tested by other neurosurgeons around the world. The original reason for targeting the internal capsule was that fibers that connect the orbitofrontal cortex to the other limbic structures pass through the anterior limb of the internal capsule. Like the cingulotomy or subcaudate tractotomy, the results were poor in patients with psychosis but better in patients with anxiety and OCD disorders. The Swedish neurosurgeon Lars Leksell modernized the anterior capsulotomy in the 1950s, with the development of a procedure producing radiofrequency thermocoagulative lesions and gamma knife radiosurgery [75]. The first radiosurgical capsulotomy was performed in 1953 using 300-kV X-rays [76]. With at least 55 % of patients responding to gamma knife capsulotomy, it is an effective treatment for severe OCD. More recent series have confirmed the results of these historical series [77]. I believe that, given the encouraging clinical results, gamma knife radiosurgery should be evaluated further in patients with severe and disabling behavioral disorders. Convincing demonstration of the safety efficacy of GKS capsulotomy and more precise definition of the ideal dose and target will require strict, rigorous, prospective, double-blinded randomized trials with rigorous neuropsychological evaluation. Similarly, given the good clinical results of thermocingulotomy, it would be interesting to evaluate the efficacy of radiosurgery with this target. Studies that directly compare DBS with stereotactic radiosurgery would also be beneficial. In our center, we favor DBS for psychiatric surgery because, in contrast to stereotactic ablative techniques, it is both adjustable and, in most cases, reversible. However, we want to stay open-minded. Gamma knife capsulotomy should be regarded as a part of a multidisciplinary therapeutic approach to these challenging patients. We recently participated in the preparation of a consensus document, designed to enhance patient safety, on the ethical and scientific conduct of psychiatric surgery worldwide [78]. In line with this guideline document, we would like to highlight that “the nature of these and many other procedures in neurosurgery, including DBS for psychiatric disorders, remains at a ‘proof-of-principle’ investigational stage of development” [78, 79].
Conclusion
With appropriate management of radiosurgical techniques, radiosurgery can be used either as a lesional instrument or to induce a precise biological effect in a specific brain structure. As a lesional tool, particularly for movement disorders, radiosurgery has the following advantages over thermolesion: absence of hemorrhagic or infectious complication; higher stereotactic accuracy; better control of the extent of the lesion; lower interindividual variability of the effect. Disadvantages include the absence of physiological control and a delayed efficacy. The safety efficacy of VIM radiosurgery, in experienced hands, for disabling, drug-resistant unilateral tremor (essential or Parkinson), has been shown in large prospective series [35]. I consider this indication of radiosurgery in psychiatric disorders to still be investigational (see, for a review [80]).
Consequently, based on experimental data, it has been clearly demonstrated that radiosurgery can modify and modulate the functioning of a neural system without inducing significant histological necroses. This kind of effect is particularly interesting for epilepsy, and is well demonstrated in hypothalamic hamartomas. Thus, I think it makes sense to use the term “neuromodulation”, but this will certainly remain—at least for a while—a matter of controversy. From a clinical perspective this allows the achievement of therapeutic modification of a neural system in a highly functional area without compromising the underlying function of the brain (e.g., epilepsy). This potential nonlesional effect distinguishes radiosurgery from lesional procedures based on a thermocoagulation mechanism (radiofrequency, laser, focused ultrasound, etc.). The possibility of a curative treatment of the EZ, even in very highly functional areas, makes radiosurgery a critical complement in the armamentarium of epilepsy surgery. Drug-resistant TN radiosurgery is now current practice and undoubtedly the least invasive technic for tic, with a lower rate of complications [17].
References
- 1.Steiner L, Forster D, Leksell L. Gammathalamotomy in intractable pain. Acta Neurochir (Wien) 1980;52:173–184. doi: 10.1007/BF01402072. [DOI] [PubMed] [Google Scholar]
- 2.Cecconi JP, Lopes AC, Duran FL, et al. Gamma ventral capsulotomy for treatment of resistant obsessive-compulsive disorder: a structural MRI pilot prospective study. Neurosci Lett. 2008;447:138–142. doi: 10.1016/j.neulet.2008.09.061. [DOI] [PubMed] [Google Scholar]
- 3.Noren G. Gamma knife radiosurgery of acoustic neurinomas. A historic perspective. Neurochirurgie. 2004;50:253–256. [PubMed] [Google Scholar]
- 4.Thoren M, Rahn T, Hall K, Backlund EO. Treatment of pituitary dependent Cushing's syndrome with closed stereotactic radiosurgery by means of 60Co gamma radiation. Acta Endocrinol (Copenh) 1978;88:7–17. doi: 10.1530/acta.0.0880007. [DOI] [PubMed] [Google Scholar]
- 5.Regis J, Bartolomei F, Hayashi M, Chauvel P. Gamma knife surgery, a neuromodulation therapy in epilepsy surgery! Acta Neurochir Suppl. 2002;84:37–47. doi: 10.1007/978-3-7091-6117-3_4. [DOI] [PubMed] [Google Scholar]
- 6.Regis J, Carron R, Park M. Is radiosurgery a neuromodulation therapy? A 2009 Fabrikant award lecture. J Neurooncol. 2010;98:155–162. doi: 10.1007/s11060-010-0226-5. [DOI] [PubMed] [Google Scholar]
- 7.Leksell L. Sterotaxic radiosurgery in trigeminal neuralgia. Acta Chir Scand. 1971;137:311–314. [PubMed] [Google Scholar]
- 8.Kondziolka D, Lunsford LD, Flickinger JC, et al. Stereotactic radiosurgery for trigeminal neuralgia: a multiinstitutional study using the gamma unit. J Neurosurg. 1996;84:940–945. doi: 10.3171/jns.1996.84.6.0940. [DOI] [PubMed] [Google Scholar]
- 9.Rand RW, Jacques DB, Melbye RW, Copcutt BG, Levenick MN, Fisher MR. Leksell gamma knife treatment of tic douloureux. Stereotact Funct Neurosurg. 1993;61(Suppl.1):1–188. doi: 10.1159/000100663. [DOI] [PubMed] [Google Scholar]
- 10.Alexander E, Lindquist C. Special indications: Radiosurgery for functional neurosurgery and epilepsy. In: Alexander E III, Loeffler J, Lunsford L, editors. Stereotactic radiosurgery. New York: McGraw-Hill; 1993. pp. 221–225. [Google Scholar]
- 11.Régis J, Manera L, Dufour H, Porcheron D, Sedan R, Peragut JC. Effect of the gamma knife on trigeminal neuralgia. Stereotact Funct Neurosurg. 1994;64(Suppl. 1):182–192. doi: 10.1159/000098778. [DOI] [PubMed] [Google Scholar]
- 12.Régis J, Bartolomei F, Metellus P, et al. Radiosurgery for trigeminal neuralgia and epilepsy. Neurosurg Clin N Am. 1999;10:359–377. [PubMed] [Google Scholar]
- 13.Régis J, Metellus P, Hayashi M, Roussel P, Donnet A, Bille-Turc F. Prospective controlled trial of gamma knife surgery for essential trigeminal neuralgia. J Neurosurg. 2006;104:913–924. doi: 10.3171/jns.2006.104.6.913. [DOI] [PubMed] [Google Scholar]
- 14.Dhople AA, Adams JR, Maggio WW, et al. Long-term outcomes of gamma knife radiosurgery for classic trigeminal neuralgia: implications of treatment and critical review of the literature. Clinical article. J Neurosurg. 2009;111:351–358. doi: 10.3171/2009.2.JNS08977. [DOI] [PubMed] [Google Scholar]
- 15.Kondziolka D, Zorro O, Lobato-Polo J, et al. Gamma knife stereotactic radiosurgery for idiopathic trigeminal neuralgia. J Neurosurg. 2010;112:758–765. doi: 10.3171/2009.7.JNS09694. [DOI] [PubMed] [Google Scholar]
- 16.Tuleasca C, Carron R, Resseguier N, et al. Patterns of pain-free response in 497 cases of classic trigeminal neuralgia treated with gamma knife surgery and followed up for least 1 year. J Neurosurg. 2012;117(Suppl):181–188. doi: 10.3171/2012.8.GKS121015. [DOI] [PubMed] [Google Scholar]
- 17.Gronseth G, Cruccu G, Alksne J, et al. Practice parameter: the diagnostic evaluation and treatment of trigeminal neuralgia (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology and the European Federation of Neurological Societies. Neurology. 2008;71:1183–1190. doi: 10.1212/01.wnl.0000326598.83183.04. [DOI] [PubMed] [Google Scholar]
- 18.Kondziolka D, Lacomis D, Niranjan A, et al. Histological effects of trigeminal nerve radiosurgery in a primate model: implications for trigeminal neuralgia radiosurgery. Neurosurgery. 2000;46:971–976. doi: 10.1097/00006123-200004000-00038. [DOI] [PubMed] [Google Scholar]
- 19.György T, Szeifert IS, Lorenzoni J, Massager N, Levivier M. Pathological findings following trigeminal neuralgia radiosurgery. In: Karger (ed.) Radiosurgery and pathological fundamentals. Karger, Basel, 2007, pp. 244–248. [DOI] [PubMed]
- 20.Hodaie M, Chen DQ, Quan J, Laperriere N. Tractography delineates microstructural changes in the trigeminal nerve after focal radiosurgery for trigeminal neuralgia. PLoS One. 2012;7:e32745. doi: 10.1371/journal.pone.0032745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Regis J, Tuleasca C. Fifteen years of gamma knife surgery for trigeminal neuralgia in the Journal of Neurosurgery: history of a revolution in functional neurosurgery. J Neurosurg. 2011;115(Suppl):2–7. [PubMed] [Google Scholar]
- 22.Flickinger JC, Pollock BE, Kondziolka D, et al. Does increased nerve length within the treatment volume improve trigeminal neuralgia radiosurgery? A prospective double-blind, randomized study. Int J Radiat Oncol Biol Phys. 2001;51:449–454. doi: 10.1016/S0360-3016(01)01606-6. [DOI] [PubMed] [Google Scholar]
- 23.Massager N, Murata N, Tamura M, Devriendt D, Levivier M, Regis J. Influence of nerve radiation dose in the incidence of trigeminal dysfunction after trigeminal neuralgia radiosurgery. Neurosurgery. 2007;60:681–687. doi: 10.1227/01.NEU.0000255393.77538.75. [DOI] [PubMed] [Google Scholar]
- 24.Linskey ME, Ratanatharathorn V, Penagaricano J. A prospective cohort study of microvascular decompression and gamma knife surgery in patients with trigeminal neuralgia. J Neurosurg. 2008;109(Suppl):160–172. doi: 10.3171/JNS/2008/109/12/S25. [DOI] [PubMed] [Google Scholar]
- 25.Ohye C, Shibazaki T, Ishihara J, Zhang J. Evaluation of gamma thalamotomy for parkinsonian and other tremors: survival of neurons adjacent to the thalamic lesion after gamma thalamotomy. J Neurosurg. 2000;93(Suppl. 3):120–127. doi: 10.3171/jns.2000.93.supplement. [DOI] [PubMed] [Google Scholar]
- 26.Zerris VA, Zheng Z, Noren G, Sungarian A, Friehs GM. Radiation and regeneration: behavioral improvement and GDNF expression after gamma knife radiosurgery in the 6-OHDA rodent model of hemi-parkinsonism. Acta Neurochir Suppl. 2002;84:99–105. doi: 10.1007/978-3-7091-6117-3_12. [DOI] [PubMed] [Google Scholar]
- 27.Terao T, Yokochi F, Taniguchi M, et al. Microelectrode findings and topographic reorganisation of kinaesthetic cells after gamma knife thalamotomy. Acta Neurochir (Wien) 2008;150:823–827. doi: 10.1007/s00701-008-1606-x. [DOI] [PubMed] [Google Scholar]
- 28.Kondziolka D. Gamma knife thalamotomy for disabling tremor. Arch Neurol. 2002;59:1660. doi: 10.1001/archneur.59.10.1660. [DOI] [PubMed] [Google Scholar]
- 29.Young RF, Jacques S, Mark R, et al. Gamma knife thalamotomy for treatment of tremor: long-term results. J Neurosurg. 2000;93(Suppl. 3):128–135. doi: 10.3171/jns.2000.93.supplement. [DOI] [PubMed] [Google Scholar]
- 30.Kondziolka D, Mori Y, Martinez A, McLaughlin M, Flickinger J, Lunsford L. Beneficial effects of the radioprotectant 21-aminosteroid U-74389G in a radiosurgery rat malignant glioma model. Int J Radiat Oncol Biol Phys. 1999;44:179–184. doi: 10.1016/S0360-3016(98)00552-5. [DOI] [PubMed] [Google Scholar]
- 31.Kondziolka D, Somaza S, Martinez A, et al. Radioprotective effects of the 21-aminosteroid U-74389G for stereotactic radiosurgery. Neurosurgery. 1997;41:203–208. doi: 10.1097/00006123-199707000-00032. [DOI] [PubMed] [Google Scholar]
- 32.Rahn D, Dibyendu K, Schlesinger D, et al. Gamma knife radiosurgery for brain metastasis of non small cell lung cancer: Is there a difference in outcome between morning and afternoon treatment ? Cancer. 2011;117:414–420. doi: 10.1002/cncr.25423. [DOI] [PubMed] [Google Scholar]
- 33.Lim SY, Hodaie M, Fallis M, Poon YY, Mazzella F, Moro E. Gamma knife thalamotomy for disabling tremor: a blinded evaluation. Arch Neurol. 2010;67:584–588. doi: 10.1001/archneurol.2010.69. [DOI] [PubMed] [Google Scholar]
- 34.Okun MS, Stover NP, Subramanian T, et al. Complications of gamma knife surgery for Parkinson disease. Arch Neurol. 2001;58:1995–2002. doi: 10.1001/archneur.58.12.1995. [DOI] [PubMed] [Google Scholar]
- 35.Ohye C, Higuchi Y, Shibazaki T, et al. Gamma knife thalamotomy for Parkinson’s disease and essential tremor: A prospective multicenter study. Neurosurgery. 2012;70:526–535. doi: 10.1227/NEU.0b013e3182350893. [DOI] [PubMed] [Google Scholar]
- 36.Young RF, Li F, Vermeulen S, Meier R. Gamma knife thalamotomy for treatment of essential tremor: Long-term results. J Neurosurg 2010;112:1311–1317. [DOI] [PubMed]
- 37.Schramm J, Clusmann H. The surgery of epilepsy. Neurosurgery. 2008;62(Suppl. 2):463–481. doi: 10.1227/01.neu.0000316250.69898.23. [DOI] [PubMed] [Google Scholar]
- 38.Régis J, Kerkerian-Legoff L, Rey M, et al. First biochemical evidence of differential functional effects following gamma knife surgery. Stereotact Funct Neurosurg. 1996;66:29–38. doi: 10.1159/000099698. [DOI] [PubMed] [Google Scholar]
- 39.Tsuchitani S, Drummond J, Kamiryo T, et al. Selective vulnerability of interneurons to low dosage radiosurgery. Presented at Society for Neuroscience Annual Meeting, New Orleans, LA, USA, 2003 [abstract].
- 40.Quigg M, Rolston J, Barbaro NM. Radiosurgery for epilepsy: Clinical experience and potential antiepileptic mechanisms. Epilepsia. 2012;53:7–15. doi: 10.1111/j.1528-1167.2011.03339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Régis J, Bartolomei F, Rey M, et al. Gamma knife surgery for mesial temporal lobe epilepsy. Epilepsia. 1999;40:1551–1556. doi: 10.1111/j.1528-1157.1999.tb02039.x. [DOI] [PubMed] [Google Scholar]
- 42.Régis J, Peragut JC, Rey M, et al. First selective amygdalohippocampic radiosurgery for mesial temporal lobe epilepsy. Stereotact Funct Neurosurg. 1994;64:191–201. doi: 10.1159/000098779. [DOI] [PubMed] [Google Scholar]
- 43.Barbaro NM, Quigg M, Broshek DK, et al. A multicenter, prospective pilot study of gamma knife radiosurgery for mesial temporal lobe epilepsy: seizure response, adverse events, and verbal memory. Ann Neurol. 2009;65:167–175. doi: 10.1002/ana.21558. [DOI] [PubMed] [Google Scholar]
- 44.Regis J, Rey M, Bartolomei F, et al. Gamma knife surgery in mesial temporal lobe epilepsy: a prospective multicenter study. Epilepsia. 2004;45:504–515. doi: 10.1111/j.0013-9580.2004.07903.x. [DOI] [PubMed] [Google Scholar]
- 45.Bartolomei F, Hayashi M, Tamura M, et al. Long-term efficacy of gamma knife radiosurgery in mesial temporal lobe epilepsy. Neurology. 2008;70:1658–1663. doi: 10.1212/01.wnl.0000294326.05118.d8. [DOI] [PubMed] [Google Scholar]
- 46.Regis J, Semah F, Bryan R, et al. Early and delayed MR and PET changes after selective temporomesial radiosurgery in mesial temporal lobe epilepsy. AJNR Am J Neuroradiol. 1999;20:213–216. [PMC free article] [PubMed] [Google Scholar]
- 47.Lunsford L, Kondziolka D, Maitz A, Flickinger J. Black holes, white dwarfs and supernovas: imaging after radiosurgery. Stereotact Funct Neurosurg. 1998;70(Suppl. 1):2–10. doi: 10.1159/000056401. [DOI] [PubMed] [Google Scholar]
- 48.Hayashi M, Bartolomei F, Rey M, Farnarier P, Chauvel P, Regis J. MR changes after gamma knife radiosurgery for mesial temporal lobe epilepsy: an evidence for the efficacy of subnecrotic doses. In: Kondziolka D, editor. Radiosurgery. Basel: Karger; 2002. pp. 192–202. [Google Scholar]
- 49.Wieser HG, Siegel AM, Yasargil GM. The Zurich amygdalo-hippocampectomy series: a short up-date. Acta Neurochir Suppl (Wien) 1990;50:122–127. doi: 10.1007/978-3-7091-9104-0_24. [DOI] [PubMed] [Google Scholar]
- 50.Bartolomei F, Barbeau E, Gavaret M, et al. Cortical stimulation study of the role of rhinal cortex in deja vu and reminiscence of memories. Neurology. 2004;63:858–864. doi: 10.1212/01.WNL.0000137037.56916.3F. [DOI] [PubMed] [Google Scholar]
- 51.Pilcher WH, Roberto DW, Flanigin HF, et al. Complications of epilepsy surgery. In: Engel J, et al., editors. Surgical treatment of epilepsies. New York: Raven Press; 1993. pp. 565–581. [Google Scholar]
- 52.Stroup E, Langfitt J, Berg M, McDermott M, Pilcher W, Como P. Predicting verbal memory decline following anterior temporal lobectomy (ATL) Neurology. 2003;60:1266–1273. doi: 10.1212/01.WNL.0000058765.33878.0D. [DOI] [PubMed] [Google Scholar]
- 53.Clusmann H, Schramm J, Kral T, et al. Prognostic factors and outcome after different types of resection for temporal lobe epilepsy. J Neurosurg. 2002;97:1131–1141. doi: 10.3171/jns.2002.97.5.1131. [DOI] [PubMed] [Google Scholar]
- 54.Helmstaedter C, Reuber M, Elger CC. Interaction of cognitive aging and memory deficits related to epilepsy surgery. Ann Neurol. 2002;52:89–94. doi: 10.1002/ana.10260. [DOI] [PubMed] [Google Scholar]
- 55.McDonald CR, Norman MA, Tecoma E, Alksne J, Iragui V. Neuropsychological change following gamma knife surgery in patients with left temporal lobe epilepsy: a review of three cases. Epilepsy Behav. 2004;5:949–957. doi: 10.1016/j.yebeh.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 56.Maesawa S, Kondziolka D, Balzer J, Fellows W, Dixon E, Lunsford LD. The behavioral and electroencephalographic effects of stereotactic radiosurgery for the treatment of epilepsy evaluated in the rat kainic acid model. Stereotact Funct Neurosurg. 1999;73:115. doi: 10.1159/000029766. [DOI] [PubMed] [Google Scholar]
- 57.Maesawa S, Kondziolka D, Dixon C, Balzer J, Fellows W, Lunsford L. Subnecrotic stereotactic radiosurgery controlling epilepsy produced by kainic acid injection in rats. J Neurosurg. 2000;93:1033–1040. doi: 10.3171/jns.2000.93.6.1033. [DOI] [PubMed] [Google Scholar]
- 58.Jenrow KA, Ratkewicz AE, Lemke NW, et al. Effects of kindling and irradiation on neuronal density in the rat dentate gyrus. Neurosci Lett. 2004;371:45–50. doi: 10.1016/j.neulet.2004.08.036. [DOI] [PubMed] [Google Scholar]
- 59.Regis J, Levivier MMH. Radiosurgery for intractable epilepsy. Techniques Neurosurg. 2003;9:191–203. doi: 10.1097/00127927-200309030-00012. [DOI] [Google Scholar]
- 60.Hajek M, Dezortova M, Liscak R, Vymazal J, Vladyka V. 1H MR spectroscopy of mesial temporal lobe epilepsies treated with Gamma knife. Eur Radiol. 2003;13:994–1000. doi: 10.1007/s00330-002-1723-5. [DOI] [PubMed] [Google Scholar]
- 61.Hoggard N, Wilkinson ID, Griffiths PD, Vaughan P, Kemeny AA, Rowe JG. The clinical course after stereotactic radiosurgical amygdalohippocampectomy with neuroradiological correlates. Neurosurgery. 2008;62:336–344. doi: 10.1227/01.neu.0000316000.96140.12. [DOI] [PubMed] [Google Scholar]
- 62.Omary RA, Berr SS, Kamiryo T, et al. 1995 AUR Memorial Award. Gamma knife irradiation-induced changes in the normal rat brain studied with 1H magnetic resonance spectroscopy and imaging. Acad Radiol. 1995;2:1043–1051. doi: 10.1016/S1076-6332(05)80511-2. [DOI] [PubMed] [Google Scholar]
- 63.Rabinov JD, Cheng LL, Lee PL, et al. MR spectroscopic changes in the rat hippocampus following proton radiosurgery. Stereotact Funct Neurosurg. 2006;84:147–154. doi: 10.1159/000094862. [DOI] [PubMed] [Google Scholar]
- 64.Tokumaru O, Kitano T, Takei H, et al. Effects of gamma ray irradiation on energy metabolism in the rat brain: a 31P nuclear magnetic resonance spectroscopy study. J Neurosurg. 2006;105(Suppl):202–207. doi: 10.3171/sup.2006.105.7.202. [DOI] [PubMed] [Google Scholar]
- 65.Toyran N, Zorlu F, Severcan F. Effect of stereotactic radiosurgery on lipids and proteins of normal and hypoperfused rat brain homogenates: a Fourier transform infrared spectroscopy study. Int J Radiat Biol. 2005;81:911–918. doi: 10.1080/09553000600571022. [DOI] [PubMed] [Google Scholar]
- 66.Munari C, Kahane P, Francione S, et al. Role of the hypothalamic hamartoma in the genesis of gelastic fits (a video-stereo-EEG study) Electroencephalogr Clin Neurophysiol. 1995;95:154–160. doi: 10.1016/0013-4694(95)00063-5. [DOI] [PubMed] [Google Scholar]
- 67.Regis J, Scavarda D, Tamura M, et al. Epilepsy related to hypothalamic hamartomas: surgical management with special reference to gamma knife surgery. Childs Nerv Syst. 2006;22:881–895. doi: 10.1007/s00381-006-0139-y. [DOI] [PubMed] [Google Scholar]
- 68.Regis J, Bartolomei F, de Toffol B, et al. Gamma knife surgery for epilepsy related to hypothalamic hamartomas. Neurosurgery. 2000;47:1343–1351. doi: 10.1097/00006123-200012000-00014. [DOI] [PubMed] [Google Scholar]
- 69.Regis J, Hayashi M, Eupierre LP, et al. Gamma knife surgery for epilepsy related to hypothalamic hamartomas. Acta Neurochir Suppl. 2004;91:33–50. doi: 10.1007/978-3-7091-0583-2_4. [DOI] [PubMed] [Google Scholar]
- 70.Regis J, Scavarda D, Tamura M, et al. Gamma knife surgery for epilepsy related to hypothalamic hamartomas. Semin Pediatr Neurol. 2007;14:73–79. doi: 10.1016/j.spen.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 71.Frattali CM, Liow K, Craig GH, et al. Cognitive deficits in children with gelastic seizures and hypothalamic hamartoma. Neurology. 2001;57:43–46. doi: 10.1212/WNL.57.1.43. [DOI] [PubMed] [Google Scholar]
- 72.Weissenberger AA, Dell ML, Liow K, et al. Aggression and psychiatric comorbidity in children with hypothalamic hamartomas and their unaffected siblings. J Am Acad Child Adolesc Psychiatry. 2001;40:696–703. doi: 10.1097/00004583-200106000-00015. [DOI] [PubMed] [Google Scholar]
- 73.Jenike MA, Rauch SL. Managing the patient with treatment-resistant obsessive compulsive disorder: current strategies. J Clin Psychiatry. 1994;55(Suppl):11–17. [PubMed] [Google Scholar]
- 74.Talairach J, Hecaen H, David M. Lobotomies prefrontal limitee par electrocoagulation des fibres thalamo-frontales a leur emergence dubras anterieur de la capsule interne. Revue Neurologique, IV Congres Neurologique International 83, 1949.
- 75.Leksell L. Stereotactic radiosurgery. J Neurol Neurosurg Psychiatry. 1983;46:797–803. doi: 10.1136/jnnp.46.9.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Leksell LH, Herner T, Liden K. Stereotaxic radiosurgery of the brain: report of a case. Kungl Fysiogr Sallsk Lund Forhandl. 1955;25:1–10. [Google Scholar]
- 77.Kondziolka D, Hudak R. Management of obsessive-compulsive disorder-related skin picking with gamma knife radiosurgical anterior capsulotomies: a case report. J Clin Psychiatry. 2008;69:1337–1340. doi: 10.4088/JCP.v69n0819c. [DOI] [PubMed] [Google Scholar]
- 78.Nuttin B, Wu H, Mayberg H, et al. Consensus on guidelines for stereotactic neurosurgery for psychiatric disorders. J Neurol Neurosurg Psychiatry 2014 Jan 20 [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 79.Rabins P, Appleby BS, Brandt J, et al. Scientific and ethical issues related to deep brain stimulation for disorders of mood, behavior, and thought. Arch Gen Psychiatry. 2009;66:931–937. doi: 10.1001/archgenpsychiatry.2009.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lévêque M, Régis J. Radiosurgery for the treatment of psychiatric disorders – A review World Neurosurg 2013;80:S32.e1–9. [DOI] [PubMed]
- 81.Young RF, Shumway-Cook A, Vermeulen SS, Grimm P, Blasko J, Posewitz A, Burkhart WA, Goiney RC. Gamma knife radiosurgery as a lesioning technique in movement disorder surgery. J Neurosurg 1998;89:183–193. [DOI] [PubMed]
- 82.Duma CM, Jacques D, Kopyov OV. The treatment of movement disorders using Gamma Knife stereotactic radiosurgery. Neurosurg Clin N Am 1999;10:379–389. [PubMed]