Abstract
Currently, the mechanism of action of vagus nerve stimulation (VNS) is not fully understood, and it is unclear which factors determine a patient’s response to treatment. Recent preclinical experiments indicate that activation of the locus coeruleus noradrenergic system is critical for the antiepileptic effect of VNS. This study aims to evaluate the effect of VNS on noradrenergic signaling in the human brain through a noninvasive marker of locus coeruleus noradrenergic activity: the P3 component of the event-related potential. We investigated whether VNS differentially modulates the P3 component in VNS responders versus VNS nonresponders. For this purpose, we recruited 20 patients with refractory epilepsy who had been treated with VNS for at least 18 months. Patients were divided into 2 groups with regard to their reduction in mean monthly seizure frequency: 10 responders (>50 %) and 10 nonresponders (≤50 %). Two stimulation conditions were compared: VNS OFF and VNS ON. In each condition, the P3 component was measured during an auditory oddball paradigm. VNS induced a significant increase of the P3 amplitude at the parietal midline electrode, in VNS responders only. In addition, logistic regression analysis showed that the increase of P3 amplitude can be used as a noninvasive indicator for VNS responders. These results support the hypothesis that activation of the locus coeruleus noradrenergic system is associated with the antiepileptic effect of VNS. Modulation of the P3 amplitude should be further investigated as a noninvasive biomarker for the therapeutic efficacy of VNS in patients with refractory epilepsy.
Electronic supplementary material
The online version of this article (doi:10.1007/s13311-014-0272-3) contains supplementary material, which is available to authorized users.
Keywords: Vagus nerve stimulation, epilepsy, event-related potentials, P3, biomarker, norepinephrine.
Introduction
Vagus nerve stimulation (VNS) is a well-tolerated adjunctive therapy for patients with medically or surgically refractory epilepsy [1, 2]. VNS consists of a programmable pulse generator, which is implanted subclavicularly and connected to 2 spiral electrodes. The electrodes are wound around the left vagus nerve at the cervical level, and deliver chronic, intermittent electrical stimulation. Since the first human VNS implantation in 1989, more than 100,000 patients with epilepsy worldwide have been treated with VNS. Two randomized, double-blind clinical trials have shown a statistically significant decrease in seizure frequency by VNS [3–5]. Several long-term follow-up studies have further established the efficacy and safety of VNS [6–9]. A meta-analysis of VNS efficacy has shown that seizure reduction ranges from 0 to 100 % (mean 45 %), and varies considerably across patients [10]. In general, VNS reduces seizure frequency by at least 50 % in a third of patients; one-third experience a seizure frequency reduction of 30–50 %; and the remaining third show little to no response [1]. Therefore, at this moment, 1 of 3 patients undergoing the invasive and costly VNS surgical procedure does not experience any benefit from it.
The exact mechanism by which VNS exerts its antiepileptic effect is not yet fully understood. Therefore, prior to implantation of the VNS device, we are currently unable to predict which patients will be responders or nonresponders. Further elucidation of the mechanism of action is necessary for the identification of predictive biomarkers for positive therapeutic response and a more rational setting of stimulation parameters. Over the last 20 years, there has been an increasing amount of evidence indicating that activation of the locus coeruleus (LC) norepinephrine (NE) system is critical for the antiepileptic effect of VNS [11]. In addition, a recent preclinical study carried out in our laboratory has demonstrated a strong positive correlation between the VNS-induced increase in NE levels in the brain and the seizure-suppressing effect of VNS [12]. These results suggest that the degree of NE release in the brain can be a useful biomarker for the therapeutic efficacy of VNS in epileptic patients.
Currently, there are no techniques available for direct measurement of NE levels in the human brain. Fortunately, changes in NE levels can be indirectly inferred from parameters that are modulated by the amount of noradrenergic signaling in the brain. One of these parameters is the P3 component of the event-related potential (ERP). The P3 is a broad positive component with an onset latency of 300–900 ms that can be measured using the auditory oddball paradigm. In this cognitive paradigm, low-probability target stimuli (“oddballs”, e.g., low frequency tones) are embedded in a train of high-probability nontarget stimuli (“standards”, e.g., high-frequency tones). After the presentation of the infrequent target stimuli a large P3 can be measured at parietal midline electrodes [13, 14].
Converging evidence from animal, genetic, and pharmacological studies suggests that the P3 component of the scalp-recorded ERP reflects the phasic activity of the neuromodulatory LC–NE system [15, 16]. The hypothesis that the LC–NE system contributes to P3 generation during a target detection task is consistent with the allocation of attentional resources and arousal effects in humans [13, 17–19]. Monkey studies with the oddball paradigm have shown that the conditions for generating the P3 are highly similar to those for the LC phasic response: both are preferentially elicited by attended, task-relevant, arousing, and salient stimuli that are important for goal-directed behavior [15, 20]. Furthermore, the timing and topographic distribution of intracranial and scalp-recorded P3 activity are consistent with the anatomical and physiological activation of temporo-parietal areas by the LC–NE system [13, 15]. Finally, lesions of the LC cell bodies reduce the auditory P3 amplitude in monkeys, indicating that the LC nucleus and its ascending fibers are important in the generation and modulation of the P3 [21].
In this study, we assessed the effect of VNS on the NE signaling in patients with epilepsy by investigating VNS-induced modulation of the P3 ERP component recorded during a standard auditory oddball paradigm. In light of the important role of the noradrenergic system in the therapeutic effect of VNS, and given the association between the P3 component and this specific neurotransmitter system, we hypothesized that we would find a different modulation of the P3 component in VNS responders versus VNS nonresponders. The goal of this study was to investigate whether VNS-induced modulation of the P3 ERP component could be used as a noninvasive biomarker for the treatment response to VNS.
Methods
Patients
Twenty patients with epilepsy were included (8 men and 12 women, mean age 44 years). The study took place during a video-electroencephalogram (EEG) monitoring session in the Reference Center for Refractory Epilepsy, Ghent University Hospital, Ghent, Belgium. Patients were included in the study if they met the following criteria: 1) at least 18 months of treatment with VNS for refractory epilepsy; 2) older than 18 years; 3) full-scale IQ score ≥ 70 on the Wechsler Adult Intelligence Scale, Third Edition. Only patients who were treated with VNS for at least 18 months were included because current reports suggest that VNS efficacy has a tendency to improve up to 18 months after surgery, after which a plateau is usually reached [22, 23]. Patients were divided into 2 groups depending on their reduction in mean monthly seizure frequency: 10 responders (>50 % reduction) and 10 nonresponders (≤50 % reduction). Mean monthly seizure frequency was defined as the mean seizure frequency during the 3 consecutive months before implantation and before testing. The mean monthly seizure frequency before VNS was not significantly different between both groups [nonresponders: 53.2 ± 54.4 seizures/month; responders: 39.3 ± 48.4 seizures/month; t(18) = 0.61, p = 0.55]. Conversely, the mean monthly seizure frequency reduction post-VNS was significantly higher in the group of responders (86.7 %) than in the group of nonresponders (14.2 %) [t(18) = 8.53, p < 0.001]. The main clinical characteristics of patients and habitual VNS parameters are summarized in Table 1. The study was approved by the ethics committee of Ghent University Hospital. After a full description of the procedure was provided and explained, all patients gave written informed consent.
Table 1.
Patient characteristics
| Patient ID | Sex | Age (years) | Seizure reduction (%) | Year of VNS implantation | VNS parameters | HEZ | AEDs | P3 absolute amplitude (μV) | P3 relative amplitude (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Output (mA) | Frequency (Hz) | Pulsewidth (μs) | Lobe | Side | OFF | ON | ON–OFF | (ON–OFF)/OFF | ||||||
| Responders | ||||||||||||||
| R_1 | M | 52 | 100.0 | 1995 | 2.00 | 30 | 500 | TL | Bilateral | VPA, VGB, CBZ | 7.41 | 11.77 | 4.36 | 58.81 |
| R_2 | F | 57 | 100.0 | 1997 | 1.50 | 30 | 500 | FL + TL + PL | Right | LTG | 3.94 | 5.58 | 1.64 | 41.78 |
| R_3 | F | 52 | 100.0 | 2003 | 2.50 | 30 | 500 | TL | Right | LEV, CBZ | 3.77 | 3.63 | −0.14 | −3.61 |
| R_4 | M | 22 | 100.0 | 2007 | 0.75 | 20 | 500 | General | Bilateral | VPA, LTG | 3.96 | 6.21 | 2.26 | 57.09 |
| R_5 | M | 36 | 95.6 | 2010 | 2.25 | 20 | 250 | FL | Right | LEV, PGB, CZP | 2.37 | 2.04 | −0.34 | −14.15 |
| R_6 | F | 66 | 95.0 | 2003 | 2.50 | 20 | 500 | General | Bilateral | LEV, LTG, CZP | 3.15 | 4.48 | 1.32 | 41.92 |
| R_7 | F | 55 | 85.0 | 2002 | 3.00 | 20 | 500 | TL | Right | LEV, LTG, CBZ | 7.30 | 8.88 | 1.58 | 21.65 |
| R_8 | F | 45 | 73.3 | 1997 | 2.75 | 20 | 250 | TL | Right | LEV, LTG, CZP | 4.34 | 7.44 | 3.10 | 71.48 |
| R_9 | F | 30 | 63.3 | 2005 | 2.50 | 25 | 500 | General | Bilateral | VPA, LEV, PGB, CZP | 4.99 | 7.06 | 2.07 | 41.45 |
| R_10 | F | 21 | 54.5 | 2009 | 3.00 | 30 | 500 | General | Bilateral | VPA, LTG, PGB, LCZ | 2.45 | 2.68 | 0.23 | 9.55 |
| Mean | 44 | 86.7 | 2003 | 2.28 | 25 | 450 | 4.37 | 5.98 | 1.61 | 32.60 | ||||
| Nonresponders | ||||||||||||||
| NR_1 | F | 62 | 50.0 | 2008 | 3.00 | 30 | 130 | General | Bilateral | PHT, LEV, LTG, CZP, RG | 5.30 | 5.94 | 0.64 | 11.98 |
| NR_2 | M | 55 | 46.9 | 2003 | 2.75 | 30 | 500 | FL + TL | Bilateral | PHT, LCZ | 16.45 | 16.29 | −0.16 | −0.98 |
| NR_3 | M | 61 | 33.3 | 1999 | 2.50 | 20 | 500 | FL | Bilateral | CBZ, PB, LEV, PGB, CZP | 6.50 | 5.56 | −0.93 | −14.38 |
| NR_4 | M | 53 | 9.1 | 2007 | 2.75 | 30 | 500 | FL + PL | Bilateral | VPA, LTG | 2.32 | 1.77 | −0.55 | −23.75 |
| NR_5 | M | 23 | 3.1 | 2007 | 2.75 | 30 | 500 | FL + PL | Bilateral | LTG, CZP, OXC, LCZ | 3.35 | 3.82 | 0.46 | 13.83 |
| NR_6 | M | 25 | 0.0 | 2008 | 2.00 | 30 | 500 | OL | Left | LEV, CZP, CBZ | 5.90 | 7.80 | 1.89 | 32.08 |
| NR_7 | F | 32 | 0.0 | 2007 | 2.00 | 20 | 250 | FL + OL | Right | LEV, CZP, CBZ | 10.06 | 5.95 | −4.11 | −40.89 |
| NR_8 | F | 30 | 0.0 | 2011 | 0.75 | 20 | 250 | FL + TL + PL | Left | CLB, CZP, OXC | 6.61 | 6.09 | −0.52 | −7.94 |
| NR_9 | F | 54 | 0.0 | 2010 | 2.75 | 20 | 500 | FL | Left | CZP, CBZ, RG, LCZ | 5.74 | 6.71 | 0.98 | 17.06 |
| NR_10 | F | 48 | 0.0 | 2010 | 1.75 | 30 | 500 | FL | Left | VPA, LEV, PB, LCZ | 4.14 | 4.46 | 0.32 | 7.82 |
| Mean | 44 | 14.2 | 2007 | 2.30 | 26 | 413 | 6.64 | 6.44 | −0.20 | −0.52 | ||||
VNS vagus nerve stimulation; HEZ hypothesized epileptogenic zone; AEDs antiepileptic drugs; R responder; NR nonresponder; M male; F female; TL temporal lobe; FL frontal lobe; PL parietal lobe; OL occipital lobe; VPA valproic acid; VGB vigabatrin; CBZ carbamazepine; LTG lamotrigine; LEV levetiracetam; PGB pregabalin; CZP clonazepam; PHT phenytoin; RG retigabine; LCZ lacosamide; PB phenobarbital; OXC oxcarbazepine; CLB clobazam
VNS Parameters and Procedure
All patients had a chronically implanted VNS device (Cyberonics, Houston, TX, USA), comprising a programmable pulse generator placed subcutaneously under the left clavicle and 2 helical electrodes wound around the left vagus nerve in the neck. Time since start of VNS treatment varied between 1.5 and 16.2 years. During this period, the stimulation parameters were individually adjusted by a previously described standard ramping-up scheme [6, 8]. Stimulation parameters were gradually changed in order to achieve maximal therapeutic effect with minimal side effects. At the start of the study VNS parameters, battery voltage and lead impedance were checked with a handheld computer and programmable wand.
Patients performed the task during 2 stimulation conditions—VNS OFF and VNS ON—in a randomized, counterbalanced order. During the VNS ON condition the duty cycle was 7 s ON/18 s OFF. Other stimulation parameters during the VNS ON condition were patient-specific (see Table 1), with output current ranging between 0.75 and 3.00 mA, a frequency of 20 or 30 Hz, and a pulse width of 250 or 500 μs. These values were the habitual therapeutic parameters of each patient that had optimal clinical efficacy. There were no significant differences in stimulation parameters between the group of responders and nonresponders. After switching the VNS device ON or OFF, there was a pause of 20 min in order to allow habituation and achieve a stable baseline condition before the oddball task was initiated.
Auditory Oddball Paradigm
Patients performed a standard auditory oddball task [14]. This task requires participants to press a predefined button with the index finder of the dominant hand in response to “target” tones (low frequency), but not to respond to “nontarget” tones (high frequency). Participants were given a practice session in order to become familiar with the target and nontarget tones. The target tones were presented with a probability of 10 %. During 4 blocks of 140 trials (total = 560 trials), participants listened to a series of tones consisting of 504 nontarget and 56 target tones that were presented in a random order with an interstimulus interval of 1 s. Both speed and accuracy of the response to the infrequent target tone were emphasized. To reduce ocular artifacts, participants were instructed to fixate their gaze on a cross on the monitor while listening to the stimuli. Stimulus presentation and response time recording were controlled using E-Prime software 2.0 (Psychology Software Tools, Pittsburgh, PA, USA) on a Dell (Round Rock, TX, USA) desktop computer.
Electrophysiological Recordings
The EEG was recorded with a Micromed System Plus (Micromed, Mogliano, Italy) using Ag/AgCl electrodes placed at 59 standard locations according to the extended International 10-20 System using a precabled electrode cap (WaveGuard EEG cap system; Eemagine, Berlin, Germany). The online reference electrode was placed on CPz and the ground electrode on AFz. The vertical electro-oculogram was monitored using 2 facial electrodes placed on inferior and superior areas of the left orbit. The electrocardiogram (ECG) was recorded with 2 ECG electrodes placed above the heart. Two additional electrodes were placed in the neck cranial and caudal to the vagus nerve electrode to monitor the VNS artifact. The EEG, electro-oculogram, ECG, and VNS signals were digitized online with a sampling frequency rate of 1024 Hz, antialiasing filter of 250 Hz, gain of 50 dB, and a resolution of 16 bits. Electrode impedance was maintained below 10 kΩ. ERPs of interest were computed offline following a standard sequence of data transformations [24]. Using an independent component analysis that subtracts artifact components from each electrode, the EEG was corrected for vertical and horizontal eye movements, blinks, heartbeat, and VNS artifacts. The EEG signal was then re-referenced to the average of all 59 recorded channels. The continuous EEG was first digitally filtered with a 50-Hz notch filter and a half-power band-pass filter between 0.1 and 30 Hz, and a roll-off of 12 dB/octave and then down-sampled to 256 Hz. The EEG was segmented into epochs from −200 ms to +1000 ms relative to the onset of the target and standard stimuli. Baseline correction was performed on the 200-ms prestimulus interval, and epochs with a voltage exceeding ±75 μV were excluded from averaging. Artifact-free epochs were averaged separately for standard and target stimuli, for each condition and each individual. To isolate the P3 component, we created a classical target-standard difference waveform; P3 amplitude and latency were measured from the resulting difference waves. For each patient, the P3 peak was determined using automatic local peak detection in the 300–900 ms interval poststimulus onset at the parietal midline electrode Pz. The latency of the P3 was measured as the time point at which the voltage reached 50 % of the peak amplitude. This is because in averaged waveforms the absolute onset latency will reflect the trials with the earliest onsets rather than the average of the single trial onset latencies. Therefore, the 50 % peak latency measure is a much more accurate and sensitive measure of the relative onset time of an ERP component [25, 26]. The P3 amplitude was calculated as the mean amplitude during the 100-ms interval round the peak detected by the automatic peak detection relative to baseline. Mean amplitude measurements capture more of a component than just the extreme value, and are less sensitive to noise than peak amplitude measurements [25]. ERP amplitude measurements can be influenced by individual differences in nuisance variables [27], such as skull thickness [28] and cortical folding patterns [29]. To minimize the impact of these nuisance factors, we calculated the percentage difference in P3 amplitude of the ON condition relative to the OFF condition with following formula: P3 amplitude (ON–OFF)/OFF.
Statistical Analysis
All statistical analyses were conducted with SPSS version 20.0 (SPSS, Chicago, IL, USA). The level of statistical significance was set at 0.05. The behavioral and electrophysiological data were analyzed using a mixed model analysis of variance (ANOVA) with between-subject factor group (responders vs nonresponders) and within-subject factor condition (ON vs OFF). Two-tailed paired t tests were also computed as post hoc analyses. Logistic regression analysis was used to test whether VNS-induced modulation of the P3 amplitude is a good indicator to differentiate between responders and nonresponders. Odds ratios (OR) were calculated with 95 % confidence intervals (CI). Receiver operating characteristic (ROC) curve analysis was used to assess the optimal cut-off value with maximal sensitivity and specificity. Correlation between the relative P3 amplitude and behavioral responses (reaction time and accuracy) were tested using Pearson’s correlation coefficient.
Results
Behavioral Results
The behavioral results are summarized in Table 2: mean accuracy (percentage correct responses) and reaction times are shown, along with F- and p-values of the statistical analyses. During the auditory oddball task patients detected the target stimuli with a very high accuracy: mean performance was 97 % correct. The high accuracy confirms that patients were paying attention to the stimuli and could easily discriminate between target and nontarget stimuli. Patients had significantly greater accuracy and faster reaction times during VNS ON than during VNS OFF condition. The accuracy and reaction time measures indicate that both groups of patients were generally better in detecting the targets during VNS ON condition, leading to a significant main effect of condition, but no group with condition interaction.
Table 2.
Behavioral results and P3 measures (mean ± SD), with F- and p-values for the statistical analyses
| Dependent variable | Responders | Nonresponders | Statistics | ||||
|---|---|---|---|---|---|---|---|
| OFF | ON | OFF | ON | Group df = 1,18 | Condition df = 1,18 | Group × condition df = 1,18 | |
| Accuracy (%) | 95.71 ± 2.82 | 98.21 ± 2.06 | 96.61 ± 5.29 | 98.21 ± 2.66 |
F = 0.12 p = 0.729 |
F = 5.58 p = 0.030 |
F = 0.26 p = 0.615 |
| Reaction time (ms) | 417.52 ± 59.85 | 408.98 ± 78.40 | 419.34 ± 68.17 | 394.54 ± 62.24 |
F = 0.05 p = 0.831 |
F = 4.53 p = 0.047 |
F = 1.07 p = 0.313 |
| Amplitude (μV) | 4.37 ± 1.77 | 5.98 ± 2.97 | 6.64 ± 4.04 | 6.44 ± 3.84 |
F = 0.92 p = 0.351 |
F = 4.22 p = 0.055 |
F = 5.39 p = 0.017 |
| Latency (ms) | 439.84 ± 107.14 | 437.11 ± 1 49.59 | 420.32 ± 82.99 | 400.39 ± 80.08 |
F = 0.35 p = 0.561 |
F = 1.26 p = 0.276 |
F = 0.73 p = 0.405 |
df degrees of freedom
Electrophysiological Results
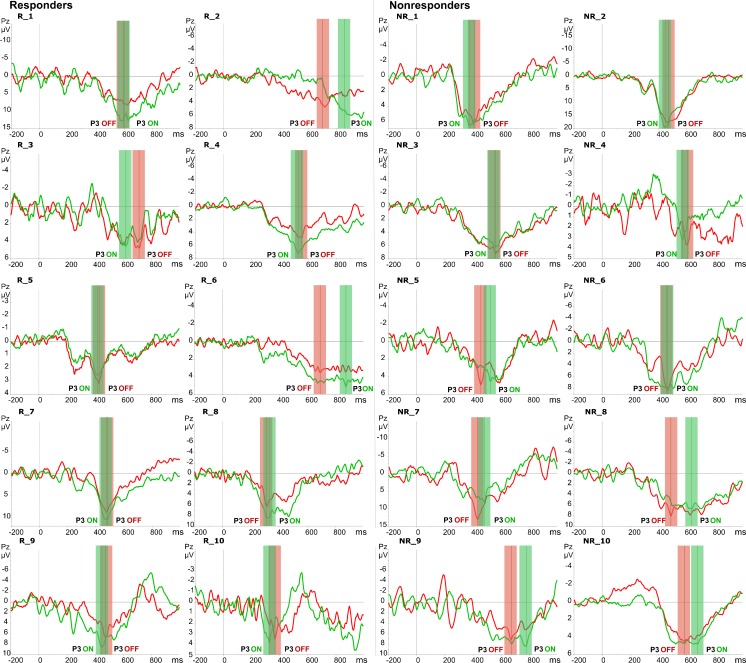
Consistent with many previous ERP studies [16, 30–36], the P3 component of the auditory ERP was recorded at the parietal midline electrode Pz. During the auditory oddball task, the processing of deviant auditory stimuli was associated with the generation of this well-characterized P3 component. Table 1 summarizes the P3 amplitudes of each individual patient in the responder and nonresponder groups. Owing to the large interindividual variability in P3 latency and amplitude in the heterogeneous patient population, visualization of the effect of VNS on the P3 was optimized by plotting the separate difference waves of each individual patient (Fig. 1).
Fig. 1.
Target-standard event-related potential difference waveforms at the parietal midline electrode Pz displayed separately for each patient: R_1–10 responders and NR_1–10 nonresponders. P3 amplitude was measured as the mean of the 100-ms marked interval round the peak in the OFF condition (red) and ON condition (green)
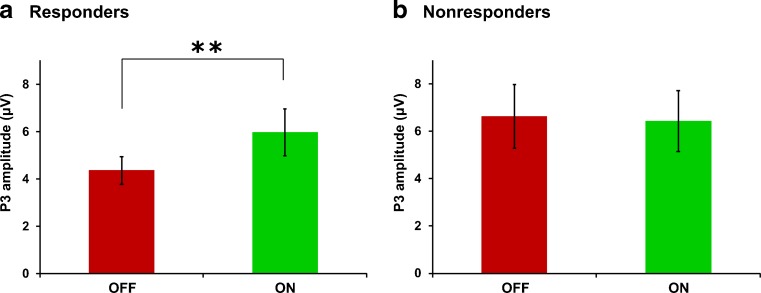
Table 2 summarizes the means of the P3 latency and amplitude and F and p-values of the statistical analysis. Mixed-model ANOVA of the latency of the P3 component revealed no significant effects of condition, group or the group × condition interaction. Post hoc tests confirmed that there were no significant differences in the latency of the P3 component between the ON and OFF conditions in both groups: responders [t(9) = 0.16, p = 0.875] and nonresponders [t(9) = 1.81, p = 0.105]. However, the mixed-model ANOVA of the amplitude of the P3 component revealed a significant interaction between group and condition [F(1,18) = 5.39, p = 0.017]. The main effects of group and condition were not significant. Post hoc analysis comparing the VNS ON and OFF conditions revealed that the amplitude of the P3 was significantly increased in responders: amplitude OFF 4.4 ± 1.8 μV and ON 6.0 ± 3.0 μV [t(9) = 3.48, p = 0.007], while in nonresponders this increase was not observed: amplitude OFF 6.6 ± 4.0 μV and ON 6.4 ± 3.8 μV [t(9) = 0.39, p = 0.706] (Fig. 2). VNS induced an average P3 amplitude increase of 32.6 % in responders, while in nonresponders there was an average decrease of 0.5 % when the ON condition was compared with the OFF condition (see Table 1). In conclusion, VNS induces a significant increase of the oddball P3 amplitude at the parietal midline electrode in VNS responders only.
Fig. 2.
Bar plots showing the average and standard error of the P3 peak amplitude, displayed separately for each group: (a) responders and (b) nonresponders. Only in the responder group was the amplitude of the P3 significantly larger for the vagus nerve stimulation (VNS) ON condition compared with the VNS OFF condition. **p < 0.01
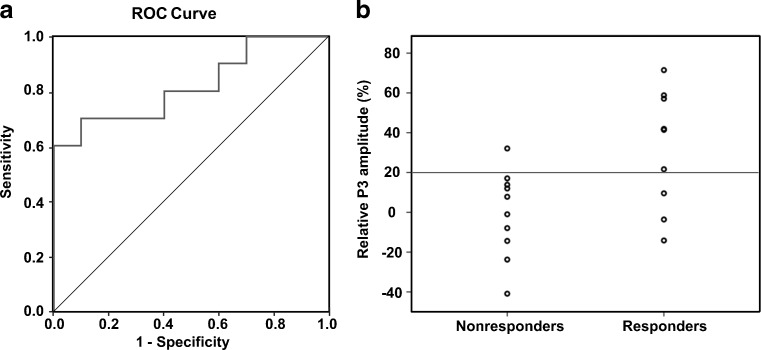
Logistic regression analysis with VNS responder as the dependent variable showed that a 1 % VNS-induced increase of the P3 amplitude has an OR of 1.056 (95 % CI 1.005–1.109; p = 0.030). This OR means that with each percentage of increase of the P3 amplitude the odds of being a VNS responder will be 5.6 % higher. The ROC analysis revealed an area under the curve of 0.82 (95 % CI 0.63–1.00; p = 0.016) (Table 3, Fig. 3). This indicates that the measured variable has a very good predictability for VNS responders vs nonresponders. ROC analysis of the VNS-induced P3 amplitude increase shows that a cut-off score of > 20 % has the optimal trade-off between sensitivity and specificity, and the highest predictive values. Sensitivity and specificity for a P3 amplitude cut-off score of >1.0 μV were 70 % and 90 %, respectively. The positive predictive value for this cut-off score was 88 %; the negative predictive value was 75 %.
Table 3.
Receiver operating characteristic and predictive values of P3 amplitude for vagus nerve stimulation responders
| Cut-off score (%) | NPV | PPV | Specificity | Sensitivity | AUC | SE | 95 % CI | p |
|---|---|---|---|---|---|---|---|---|
| >15 | 0.73 | 0.78 | 0.80 | 0.70 | – | – | – | – |
| >20 | 0.75 | 0.88 | 0.90 | 0.70 | 0.82 | 0.097 | 0.63–1.00 | 0.016 |
| >25 | 0.69 | 0.86 | 0.90 | 0.60 | – | – | – | – |
| >35 | 0.71 | 1.00 | 1.00 | 0.60 | – | – | – | – |
NPV negative predictive value; PPV positive predictive value; AUC area under curve; SE standard error; CI confidence interval
Fig. 3.
(a) Receiver operating characteristic (ROC) curve of the relative P3 amplitude for vagus nerve stimulation (VNS) responders/nonresponders. (b) Scatter plot of the group of responders and nonresponders. Logistic regression analysis with VNS responder as the dependent variable and cut-off score of >20 % (horizontal line) has a sensitivity of 70 % and specificity of 90 %
To examine whether the effect of VNS on the P3 amplitude might be related to the behavioral responses, we performed a correlation analysis using a 2-tailed Pearson coefficient. There was no significant correlation between the relative P3 amplitude and the response accuracy (r = 0.41, p = 0.071) or the reaction time (r = 0.20, p = 0.394).
Discussion
This study provides novel evidence that supports the hypothesis that VNS-induced activation of the LC–NE system is associated with the therapeutic response to VNS in patients with epilepsy. Our ERP results show that VNS induces a significant increase of the oddball P3 amplitude at the parietal midline electrode in VNS responders only. In addition, logistic regression analysis revealed that the increase of the P3 amplitude can be used as a noninvasive indicator for VNS responders and nonresponders with high sensitivity and specificity when a cut-off value of >20 % amplitude increase is used.
Our new ERP results are consistent with the research of Neuhaus et al. [36], who examined the effect of VNS on the P3 component of the auditory ERP in patients with major depressive disorder. Their study reported that after 10 weeks of VNS the amplitude of the P3 was significantly increased, but only in VNS responders, that is in patients with a significant reduction of the depressive symptoms, as measured using the Hamilton Depression Rating Scale [36]. So far, only 2 ERP studies focusing on the effect of VNS on the P3 have been conducted in patients with refractory epilepsy [30, 34]. Hammond et al. [30] found no effect of either acute or chronic VNS on the latency and amplitude of the auditory P3 component. Brázdil et al. [34] showed that VNS had no effect on auditory ERP components. In contrast, VNS induced higher visual N2/P3 peak-to-peak amplitude on visual ERPs [34]. However, both studies had rather small sample sizes (9 and 10 patients, respectively) and did not distinguish between VNS responders and nonresponders, which might explain these discrepant results. The results of the previous ERP studies of VNS in epilepsy patients are not inconsistent with our study or the study by Neuhaus et al. [36], as in both the latter studies clear effects at the level of the P3 were evidenced only when separating responders and nonresponders to VNS therapy.
Early observations in patients with epilepsy have shown that VNS has mood-enhancing effects [4]. Prospective studies using standard depression rating scales confirmed that VNS is associated with statistically significant mood improvements [37, 38]. Today, VNS is used as a safe and effective therapy for treatment-resistant depression [39, 40]. Depression is the most frequent psychiatric comorbidity in epilepsy [41]. Converging evidence indicates that mood disorders and epilepsy have a complex bidirectional relationship with the existence of common pathogenic mechanisms that are operant in both conditions [42]. At the neurotransmitter level, reduced noradrenergic signaling has been proposed as one of the underlying mechanism giving rise to epilepsy and depression [43]. In line with this assumption several antidepressive medications, such as selective noradrenaline reuptake inhibitors, work by increasing the NE levels in the brain. Therefore, the antidepressive effects of VNS can also be caused by activation of the neuromodulatory LC–NE system [43]. This hypothesis is supported by the study of Neuhaus et al. [36], which demonstrated that the P3 is enhanced in responders to VNS for treatment of major depressive disorder. This suggests that the P3 can be used as an indirect measure for VNS-induced activation of the LC–NE system to detect VNS responders in both refractory epilepsy and depression. As such, our new results confirm that ERPs may provide a valuable tool as potential biomarkers for several psychiatric and neurologic disorders (for a review, see [27]).
In this study, we found that in VNS responders, the ON condition led to an increase of the P3 amplitude compared with the OFF condition, which suggests, though indirectly, a mediation of this antiepileptic effect by the LC–NE system. Our new ERP results add to the existing literature showing that electrical stimulation of the vagus nerve activates the LC–NE system and that this activation is critical for the antiepileptic effect of VNS [11]. This hypothesis is supported by several lines of converging evidence in the literature. First, the vagus nerve afferent fibers project to the nucleus of the solitary tract; in turn, the nucleus of the solitary tract projects both directly and indirectly to the LC [44]. Acute electrical stimulation of the vagus nerve induces an increase in the discharge rate of LC noradrenergic neurons [45]. In addition, the basal firing rate in the LC is significantly increased after long-term treatment with VNS [46]. In rats, VNS induces an increase of NE concentration in the hippocampus [47], the amygdala [48], and the cerebral cortex [47, 49]. Moreover, lesions of the LC block the anticonvulsant effect of VNS [50]. Furthermore, a strong positive correlation was found between the VNS-induced increase in NE levels and the seizure-suppressing effects of VNS [12]. Intrahippocampal application of an alpha2 adrenoreceptor antagonist in a hippocampal seizure model blocked the anticonvulsant effect of VNS [12]. Together, these results bolster the assumption that the degree of NE release in the brain can be a useful biomarker for the therapeutic efficacy of VNS in epileptic patients.
To our surprise, we found a general improvement in behavioral response (i.e., faster reaction times and greater accuracy) during VNS in all patients, regardless of the therapeutic efficacy of VNS. These results are consistent with previous evidence demonstrating VNS-induced cognitive and behavioral improvements that were not related to the changes in seizure frequency [51–54]. It seems contradictory that VNS improves the behavioral performance in all patients, while it increases the P3 amplitude only in responders. However, it is possible that the threshold for “functional” activation of the LC–NE system (reflected by the behavioral improvement) is much lower than the threshold for the antiepileptic effect of the LC–NE system (reflected by the P3). The P3 component is thought to originate from neuronal inhibitory signals that inhibit unrelated neural activity to promote processing efficiency of task-relevant stimuli, thereby yielding large P3 amplitudes [13]. Correspondingly, activation of the LC–NE system in response to an important stimulus sends a “network reset” signal to the brain that interrupts the activity of the ongoing functional networks and facilitates their reorganization to promote rapid behavioral adaptation [18, 55]. It is probable that only in the group of responders, because of differences in network connectivity and interactions with other neuromodulators, the VNS-induced activation of the LC–NE system results in a stronger inhibition and network reorganization that causes the antiepileptic effect. The P3 amplitude is proposed to reflect the LC–NE inhibition [13] and this could explain why the amplitude is increased only in responders. Nonetheless, this remains a hypothetical explanation and further research is necessary to clarify the different effects of VNS on cognition and seizure control.
The current results should be interpreted with caution because epilepsy is a complex, heterogeneous, and variable neurological condition, and various potential confounding factors need to be taken into account, such as type of epilepsy, type and frequency of seizures, age of onset and duration of epilepsy, brain lesions, side and localization of the hypothesized epileptogenic zone, and antiepileptic drugs (AEDs) [33, 35]. The strength of our approach was to compare responder and nonresponder patients with epilepsy while controlling as much as possible for potential confounding factors stemming from the heterogeneous clinical parameters and the AEDs. It is conceivable that the differential P3 modulation in responders versus nonresponders could be a consequence of different AEDs. However, this seems unlikely given the extensive evidence that AED can increase the latency of P3 components, but are not responsible for P3 amplitude modulation in both healthy adults [32] and epilepsy patients [31, 33, 35]. In our study, both groups took a comparable range of AEDs, and no reliable modulation of the latency of the P3 component was found depending on the experimental condition. Only the P3 amplitude varied systematically with the protocol in the responder group exclusively. None of the patients’ AEDs were tapered after they became VNS responders, which excludes that the VNS-induced effects on P3 amplitude are caused by reduced AED-related side effects after drug tapering.
Our analysis has several other limitations. First, there is considerable overlap between the P3 amplitude values of the responder and nonresponder groups. Although a cut-off of P3 amplitude increase of >20 % has reasonably good sensitivity (70 %) and specificity (90 %), this biomarker is definitely not perfect, because 30 % of responders and 10 % of nonresponders are misclassified with this cut-off. The patients in our study had very heterogeneous ERP waveshapes and clinical characteristics. It is highly probable that because of these interindividual differences VNS will not have the same effect on the P3 in all patients. The P3 component of the ERP is only an indirect measure of LC–NE activity, and other confounding factors could influence the amplitude of the P3. We have tried to control for these factors by comparing the P3 amplitude between the ON and OFF condition within the same subject and by calculating the relative amplitude differences. Second, the vagus nerve has widespread projections to nuclei in the brainstem and to all cortical regions, as well as to the thalamus, hippocampus, and amygdala, where they modulate the activity of target cells and networks [1, 2]. In addition, there are strong reciprocal connections between the different neuromodulatory systems (noradrenaline, dopamine, serotonin, and acetylcholine), which makes it very hard to delineate the role of one single system [18]. Consequently, the LC–NE system does not act alone, but interacts with other modulatory neurotransmitter pathways that also play an important contributive role in the antiepileptic effect of VNS. A third limitation is that we were unable to determine causality in our study. We found a significant increase of the P3 in the group of responders, but we cannot be certain what caused the seizure reduction. The P3 is probably an epiphenomenon of the LC–NE activation and subsequent inhibition. These limitations notwithstanding, we believe that the significant difference that was found in our analysis indicates that the P3 has potential as a noninvasive biomarker for VNS responders.
In the present study, we have recruited patients who received VNS therapy for at least 18 months. Presumably, this chronic stimulation has led to long-term changes in the neuronal networks and neurotransmitter systems [1]. Therefore, it remains unclear whether these P3-effects will also be observed in epilepsy patients prior to initiation of VNS therapy. Future longitudinal prospective studies are needed to resolve this issue. Patients with epilepsy should be tested prior to implantation of the VNS device as well as 12–18 months afterwards in order to compare the acute versus chronic effects of VNS. These longitudinal studies are required to assess whether we can predict, based on the baseline measurement (phasic effects), which patients will eventually become responders to the VNS therapy (chronic effects) and whether modulation of P3 amplitude can be used as a prospective measure to predict the therapeutic efficacy of VNS.
In conclusion, our novel ERP results support the hypothesis that VNS-induced activation of the LC noradrenergic signaling is associated with the antiepileptic effect of VNS. Amplitude modulations of the P3 should be further investigated as a noninvasive biomarker to predict the treatment response to VNS in patients with refractory epilepsy. A biomarker for the efficacy of VNS could help neurologists to choose the optimal stimulation parameters in a more objective way. In combination with a noninvasive technique to deliver VNS, such as transcutaneous VNS (t-VNS) [56], responders could be identified prior to surgery. Hence, the biomarker could avoid unnecessary implantations of a VNS device in nonresponders and consequently improve the clinical efficacy of VNS.
Electronic supplementary material
(PDF 1225 kb)
Acknowledgments
L. De Taeye is supported by a Fonds Wetenschappelijk Onderzoek (FWO) aspirant grant. K. Vonck is supported by a Bijzonder Onderzoeksfonds (BOF) grant from Ghent University Hospital. P. Boon is supported by grants from FWO, grants from BOF, and by the Clinical Epilepsy Grant from Ghent University Hospital. M. van Bochove, L. Mollet, and T. Verguts are supported by grants from FWO. R. Raedt is supported by a BOF Tenure Track grant from Ghent University.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
References
- 1.Boon P, Vonck K, De Reuck J, Caemaert J. Vagus nerve stimulation for refractory epilepsy. Seizure. 2001;10:448–455. doi: 10.1016/S1059-1311(01)90626-0. [DOI] [PubMed] [Google Scholar]
- 2.Ben-Menachem E. Vagus-nerve stimulation for the treatment of epilepsy. Lancet Neurol. 2002;1:477–482. doi: 10.1016/S1474-4422(02)00220-X. [DOI] [PubMed] [Google Scholar]
- 3.George R, Sonnen A, Upton A, et al. A randomized controlled trial of chronic vagus nerve-stimulation for treatment of medically intractable seizures. Neurology. 1995;45:224–230. doi: 10.1212/WNL.45.6.1244-b. [DOI] [PubMed] [Google Scholar]
- 4.Handforth A, DeGiorgio CM, Schachter SC, et al. Vagus nerve stimulation therapy for partial-onset seizures: a randomized active-control trial. Neurology. 1998;51:48–55. doi: 10.1212/WNL.51.1.48. [DOI] [PubMed] [Google Scholar]
- 5.Privitera MD, Welty TE, Ficker DM, Welge J. Vagus nerve stimulation for partial seizures. Cochrane Database Syst Rev 2002:CD002896. [DOI] [PubMed]
- 6.Vonck K, Boon P, D’Have N, Vandekerckhove T, O’Connor S, de Reuck J. Long-term results of vagus nerve stimulation in refractory epilepsy. Seizure. 1999;8:328–334. doi: 10.1053/seiz.1999.0299. [DOI] [PubMed] [Google Scholar]
- 7.DeGiorgio CM, Schachter SC, Handforth A, et al. Prospective long-term study of vagus nerve stimulation for the treatment of refractory seizures. Epilepsia. 2000;41:1195–1200. doi: 10.1111/j.1528-1157.2000.tb00325.x. [DOI] [PubMed] [Google Scholar]
- 8.De Herdt V, Boon P, Ceulemans B, et al. Vagus nerve stimulation for refractory epilepsy: A Belgian multicenter study. Eur J Paediatr Neurol. 2007;11:261–269. doi: 10.1016/j.ejpn.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 9.Elliott RE, Morsi A, Kalhorn SP, et al. Vagus nerve stimulation in 436 consecutive patients with treatment-resistant epilepsy: Long-term outcomes and predictors of response. Epilepsy Behav. 2011;20:57–63. doi: 10.1016/j.yebeh.2010.10.017. [DOI] [PubMed] [Google Scholar]
- 10.Englot DJ, Chang EF, Auguste KI. Vagus nerve stimulation for epilepsy: a meta-analysis of efficacy and predictors of response A review. J Neurosurg. 2011;115:1248–1255. doi: 10.3171/2011.7.JNS11977. [DOI] [PubMed] [Google Scholar]
- 11.Fornai F, Ruffoli R, Giorgi FS, Paparelli A. The role of locus coeruleus in the antiepileptic activity induced by vagus nerve stimulation. Eur J Neurosci. 2011;33:2169–2178. doi: 10.1111/j.1460-9568.2011.07707.x. [DOI] [PubMed] [Google Scholar]
- 12.Raedt R, Clinckers R, Mollet L, et al. Increased hippocampal noradrenaline is a biomarker for efficacy of vagus nerve stimulation in a limbic seizure model. J Neurochem. 2011;117:461–469. doi: 10.1111/j.1471-4159.2011.07214.x. [DOI] [PubMed] [Google Scholar]
- 13.Polich J. Updating p300: An integrative theory of P3a and P3b. Clin Neurophysiol. 2007;118:2128–2148. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duncan CC, Barry RJ, Connolly JF, et al. Event-related potentials in clinical research: Guidelines for eliciting, recording, and quantifying mismatch negativity, P300, and N400. Clin Neurophysiol. 2009;120:1883–1908. doi: 10.1016/j.clinph.2009.07.045. [DOI] [PubMed] [Google Scholar]
- 15.Nieuwenhuis S, Aston-Jones G, Cohen JD. Decision making, the p3, and the locus coeruleus-norepinephrine system. Psychol Bull. 2005;131:510–532. doi: 10.1037/0033-2909.131.4.510. [DOI] [PubMed] [Google Scholar]
- 16.Murphy PR, Robertson IH, Balsters JH, O’Connell RG. Pupillometry and P3 index the locus coeruleus-noradrenergic arousal function in humans. Psychophysiology. 2011;48:1532–1543. doi: 10.1111/j.1469-8986.2011.01226.x. [DOI] [PubMed] [Google Scholar]
- 17.Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Rev. 2003;42:33–84. doi: 10.1016/S0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- 18.Sara SJ. The locus coeruleus and noradrenergic modulation of cognition. Nat Rev Neurosci. 2009;10:211–223. doi: 10.1038/nrn2573. [DOI] [PubMed] [Google Scholar]
- 19.Nieuwenhuis S, De Geus EJ, Aston-Jones G. The anatomical and functional relationship between the P3 and autonomic components of the orienting response. Psychophysiology. 2011;48:162–175. doi: 10.1111/j.1469-8986.2010.01057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- 21.Pineda JA, Foote SL, Neville HJ. Effects of locus coeruleus lesions on auditory, long-latency, event-related potentials in monkey. J Neurosci. 1989;9:81–93. doi: 10.1523/JNEUROSCI.09-01-00081.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boon P, De Herdt V, Vonck K, Van Roost D. Clinical experience with vagus nerve stimulation and deep brain stimulation in epilepsy. Acta Neurochir Suppl. 2007;97:273–280. doi: 10.1007/978-3-211-33081-4_30. [DOI] [PubMed] [Google Scholar]
- 23.Shahwan A, Bailey C, Maxiner W, Harvey AS. Vagus nerve stimulation for refractory epilepsy in children: More to VNS than seizure frequency reduction. Epilepsia. 2009;50:1220–1228. doi: 10.1111/j.1528-1167.2008.01940.x. [DOI] [PubMed] [Google Scholar]
- 24.Picton TW, Bentin S, Berg P, et al. Guidelines for using human event-related potentials to study cognition: Recording standards and publication criteria. Psychophysiology. 2000;37:127–152. doi: 10.1111/1469-8986.3720127. [DOI] [PubMed] [Google Scholar]
- 25.Luck SJ. An introduction to the event-related potential technique. Harvard, MA: MIT Press; 2005. [Google Scholar]
- 26.Kappenman ES, Kaiser ST, Robinson BM, et al. Response activation impairments in schizophrenia: Evidence from the lateralized readiness potential. Psychophysiology. 2012;49:73–84. doi: 10.1111/j.1469-8986.2011.01288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luck SJ, Mathalon DH, O’Donnell BF, et al. A roadmap for the development and validation of event-related potential biomarkers in schizophrenia research. Biol Psychiatry. 2011;70:28–34. doi: 10.1016/j.biopsych.2010.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frodl T, Meisenzahl EM, Muller D, et al. The effect of the skull on event-related P300. Clin Neurophysiol. 2001;112:1773–1776. doi: 10.1016/S1388-2457(01)00587-9. [DOI] [PubMed] [Google Scholar]
- 29.Ahlfors SP, Han J, Lin FH, et al. Cancellation of EEG and MEG signals generated by extended and distributed sources. Hum Brain Mapp. 2010;31:140–149. doi: 10.1002/hbm.20851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hammond EJ, Uthman BM, Reid SA, Wilder BJ. Electrophysiologic studies of cervical vagus nerve stimulation in humans: II. Evoked potentials. Epilepsia. 1992;33:1021–1028. doi: 10.1111/j.1528-1157.1992.tb01753.x. [DOI] [PubMed] [Google Scholar]
- 31.Enoki H, Sanada S, Oka E, Ohtahara S. Event-related potentials in epileptic children treated with monotherapy. J Epilepsy. 1995;8:219–226. doi: 10.1016/0896-6974(95)00036-D. [DOI] [Google Scholar]
- 32.Meador KJ, Loring DW, Moore EE, et al. Comparative cognitive effects of phenobarbital, phenytoin, and valproate in healthy adults. Neurology. 1995;45:1494–1499. doi: 10.1212/WNL.45.8.1494. [DOI] [PubMed] [Google Scholar]
- 33.Kubota F, Kifune A, Shibata N, Akata T, Takeuchi K, Takahashi S. Study on the P300 of adult epileptic patients (unmedicated and medicated patients) J Epilepsy. 1998;11:325–331. doi: 10.1016/S0896-6974(98)00040-1. [DOI] [Google Scholar]
- 34.Brázdil M, Chadim P, Daniel P, et al. Effect of vagal nerve stimulation on auditory and visual event-related potentials. Eur J Neurol. 2001;8:457–461. doi: 10.1046/j.1468-1331.2001.00262.x. [DOI] [PubMed] [Google Scholar]
- 35.Caravaglios G, Natale E, Ferraro G, Fierro B, Raspanti G, Daniele O. Auditory event-related potentials (P300) in epileptic patients. Neurophysiol Clin. 2001;31:121–129. doi: 10.1016/S0987-7053(01)00252-0. [DOI] [PubMed] [Google Scholar]
- 36.Neuhaus AH, Luborzewski A, Rentzsch J, et al. P300 is enhanced in responders to vagus nerve stimulation for treatment of major depressive disorder. J Affect Disorders. 2007;100:123–128. doi: 10.1016/j.jad.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 37.Elger G, Hoppe C, Falkai P, Rush AJ, Elger CE. Vagus nerve stimulation is associated with mood improvements in epilepsy patients. Epilepsy Res. 2000;42:203–210. doi: 10.1016/S0920-1211(00)00181-9. [DOI] [PubMed] [Google Scholar]
- 38.Harden CL, Pulver MC, Ravdin LD, Nikolov B, Halper JP, Labar DR. A Pilot study of mood in epilepsy patients treated with vagus nerve stimulation. Epilepsy Behav. 2000;1:93–99. doi: 10.1006/ebeh.2000.0046. [DOI] [PubMed] [Google Scholar]
- 39.Nahas Z, Marangell LB, Husain MM, et al. Two-year outcome of vagus nerve stimulation (VNS) for treatment of major depressive episodes. J Clin Psychiatry. 2005;66:1097–1104. doi: 10.4088/JCP.v66n0902. [DOI] [PubMed] [Google Scholar]
- 40.Daban C, Martinez-Aran A, Cruz N, Vieta E. Safety and efficacy of vagus nerve stimulation in treatment-resistant depression. A systematic review. J Affect Disorders. 2008;110:1–15. doi: 10.1016/j.jad.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 41.Kanner A, Schachter SC. Psychiatric controversies in epilepsy. Elsevier Science, Academic Press, Amsterdam, The Netherlands, 2010.
- 42.Kanner AM. Depression and epilepsy: A bidirectional relation? Epilepsia. 2011;52:21–27. doi: 10.1111/j.1528-1167.2010.02907.x. [DOI] [PubMed] [Google Scholar]
- 43.Jobe PC. Common pathogenic mechanisms between depression and epilepsy: an experimental perspective. Epilepsy Behav. 2003;4:S14–S24. doi: 10.1016/j.yebeh.2003.08.020. [DOI] [PubMed] [Google Scholar]
- 44.Van Bockstaele EJ, Peoples J, Telegan P. Efferent projections of the nucleus of the solitary tract to peri-locus coeruleus dendrites in rat brain: Evidence for a monosynaptic pathway. J Comp Neurol. 1999;412:410–428. doi: 10.1002/(SICI)1096-9861(19990927)412:3<410::AID-CNE3>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 45.Groves DA, Bowman EM, Brown VJ. Recordings from the rat locus coeruleus during acute vagal nerve stimulation in the anaesthetised rat. Neurosci Lett. 2005;379:174–179. doi: 10.1016/j.neulet.2004.12.055. [DOI] [PubMed] [Google Scholar]
- 46.Dorr AE, Debonnel G. Effect of vagus nerve stimulation on serotonergic and noradrenergic transmission. J Pharmacol Exp Ther. 2006;318:890–898. doi: 10.1124/jpet.106.104166. [DOI] [PubMed] [Google Scholar]
- 47.Roosevelt RW, Smith DC, Clough RW, Jensen RA, Browning RA. Increased extracellular concentrations of norepinephrine in cortex and hippocampus following vagus nerve stimulation in the rat. Brain Res. 2006;1119:124–132. doi: 10.1016/j.brainres.2006.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hassert DL, Miyashita T, Williams CL. The effects of peripheral vagal nerve stimulation at a memory-modulating intensity on norepinephrine output in the basolateral amygdala. Behav Neurosci. 2004;118:79–88. doi: 10.1037/0735-7044.118.1.79. [DOI] [PubMed] [Google Scholar]
- 49.Follesa P, Biggio F, Gorini G, et al. Vagus nerve stimulation increases norepinephrine concentration and the gene expression of BDNF and bFGF in the rat brain. Brain Res. 2007;1179:28–34. doi: 10.1016/j.brainres.2007.08.045. [DOI] [PubMed] [Google Scholar]
- 50.Krahl SE, Clark KB, Smith DC, Browning RA. Locus coeruleus lesions suppress the seizure-attenuating effects of vagus nerve stimulation. Epilepsia. 1998;39:709–714. doi: 10.1111/j.1528-1157.1998.tb01155.x. [DOI] [PubMed] [Google Scholar]
- 51.Clark KB, Naritoku DK, Smith DC, Browning RA, Jensen RA. Enhanced recognition memory following vagus nerve stimulation in human subjects. Nat Neurosci. 1999;2:94–98. doi: 10.1038/4600. [DOI] [PubMed] [Google Scholar]
- 52.Aldenkamp AP, Majoie HJM, Berfelo MW, et al. Long-term effects of 24-month treatment with vagus nerve stimulation on behaviour in children with Lennox-Gastaut syndrome. Epilepsy Behav. 2002;3:475–479. doi: 10.1016/S1525-5050(02)00517-6. [DOI] [PubMed] [Google Scholar]
- 53.Sackeim HA, Keilp JG, Rush AJ, et al. The effects of vagus nerve stimulation on cognitive performance in patients with treatment-resistant depression. Neuropsychiatry Neuropsychol Behave Neurol. 2001;14:53–62. [PubMed] [Google Scholar]
- 54.Sjogren MJC, Hellstrom PTO, Jonsson MAG, Runnerstam M, Silander HC, Ben-Menachem E. Cognition-enhancing effect of vagus nerve stimulation in patients with Alzheimer’s disease: A pilot study. J Clin Psychiat. 2002;63:972–980. doi: 10.4088/JCP.v63n1103. [DOI] [PubMed] [Google Scholar]
- 55.Sara SJ, Bouret S. Orienting and reorienting: The locus coeruleus mediates cognition through arousal. Neuron. 2012;76:130–141. doi: 10.1016/j.neuron.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 56.Stefan H, Kreiselmeyer G, Kerling F, et al. Transcutaneous vagus nerve stimulation (t-VNS) in pharmacoresistant epilepsies: a proof of concept trial. Epilepsia. 2012;53:e115–e118. doi: 10.1111/j.1528-1167.2012.03492.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 1225 kb)