Abstract
The ability to focus acoustic energy through the intact skull on to targets millimeters in size represents an important milestone in the development of neurotherapeutics. Magnetic resonance-guided focused ultrasound (MRgFUS) is a novel, noninvasive method, which—under real-time imaging and thermographic guidance—can be used to generate focal intracranial thermal ablative lesions and disrupt the blood–brain barrier. An established treatment for bone metastases, uterine fibroids, and breast lesions, MRgFUS has now been proposed as an alternative to open neurosurgical procedures for a wide variety of indications. Studies investigating intracranial MRgFUS range from small animal preclinical experiments to large, late-phase randomized trials that span the clinical spectrum from movement disorders, to vascular, oncologic, and psychiatric applications. We review the principles of MRgFUS and its use for brain-based disorders, and outline future directions for this promising technology.
Electronic supplementary material
The online version of this article (doi:10.1007/s13311-014-0281-2) contains supplementary material, which is available to authorized users.
Keywords: MR-guided focused ultrasound, essential tremor, Parkinson’s disease, blood–brain barrier
Introduction
Ultrasound technology has been a mainstay of medical treatment for decades [1]. As early as the 1940s, researchers have attempted to focus ultrasound waves on body tissue, and on the brain in particular, as an alternative to resective and ablative procedures [1–4]. However, the requirement for a craniotomy, owing to poor transmission of ultrasound waves through bone, limited the clinical applications of early attempts [1, 5]. But the last two decades have seen several advances in the fields of imaging, physics, and engineering that now make it possible to focus ultrasound onto targets virtually anywhere in the body. As a result, magnetic resonance-guided focused ultrasound (MRgFUS) has proven to be an attractive modality for noninvasive thermal ablation of soft tissue, and has been used to treat thousands of patients globally with uterine fibroids, breast carcinoma, and bone metastases [6–10].
The application of MRgFUS to brain disorders has only recently been explored, in large part owing to the persistent challenges posed by the intact human skull to the passage of acoustic power. However, several advances have now permitted the development of transcranial MRgFUS. First, was the design of a spherical, phased array, multielement transducer helmet that permits a focusing of ultrasound energy [11] coupled to software that that compensates for skull-induced wavefront distortions as the ultrasound energy passes through the skull. Second, was preclinical animal models that demonstrated the safety, validity, and efficacy of this technology in generating brain lesions and disrupting the blood–brain barrier (BBB) [12, 13]. Finally, the development of sophisticated MR imaging (MRI) sequences that permit high-resolution visualization of brain targets, as well as real-time tissue temperature maps [14–17]. These technical accomplishments have resulted in a noninvasive and image-guided approach to brain surgery that obviates the need for open neurosurgical procedures.
General Principles of Intracranial MRgFUS
The steps involved in an MRgFUS procedure are designed to achieve 3 primary objectives: 1) to transmit efficiently ultrasound energy through the intact skull, accounting for energy dispersion due to inhomogeneities in bone morphology and density; 2) monitor effectively tissue disruption, whether ablation or vascular permeability, using real-time imaging and thermometry; and 3) confirm the clinical effect of sonication, through patient and imaging feedback.
Among the advances that have made intracranial focused ultrasound possible is the ability to overcome the significant acoustic absorption, reflection, and distortion associated with the bony skull. To accomplish this, a specially designed MRgFUS helmet is used, which contains > 1000 individual transducer elements (Fig. 1). Using preoperative bone window computed tomography, treatment software can then make phase/amplitude corrections to compensate for aberrations along the acoustic path from each individual element, such that acoustic energy arrives at the target in phase. The result is a highly focused delivery of ultrasound energy, leading to temperature increase, and if the thermal exposure is high enough then it causes tissue necrosis and/or apoptosis [18, 19]. BBB disruption using MRgFUS is mediated by mechanical forces, rather then thermal, and its mechanisms are discussed below. To date, the feasibility of intracranial MRgFUS has been demonstrated in multiple animal models, including mouse, rabbit, pig, and primate [12, 20–22]. These models have helped inform the mechanisms of MRgFUS, its relative safety, and its effects on the brain compared with other ablative modalities. For example, a recent study in a swine model compared lesions following MRgFUS, radiofrequency, and radiosurgical ablation using MRI and histological examination [18]. The study found that MRgFUS and radiofrequency (RF) lesions evolve similarly on imaging and microscopic examination in the acute, subacute, and long-term periods. However, lesions following radiosurgery evolved differently, with little radiographic evidence of a lesion at 3 months, and with histologic examination demonstrating a relatively less circumscribed lesion. There was also evidence for effects beyond the treated zone with radiosurgery, such as edema and macrophage infiltration, that were not seen with RF and MRgFUS lesions [23]. The spatial and temporal resolution of MR thermometry coupled to focused ultrasound has also been demonstrated in animal models, which have shown high degrees of precision, ensuring that temperature rises are tightly restricted to the volume of interest [24–27]. Although a detailed review of the physics of MRgFUS and the transmission of acoustic energy through the intact skull are outside the scope of this review, additional information can be found in [12, 13, 20, 28 and 29].
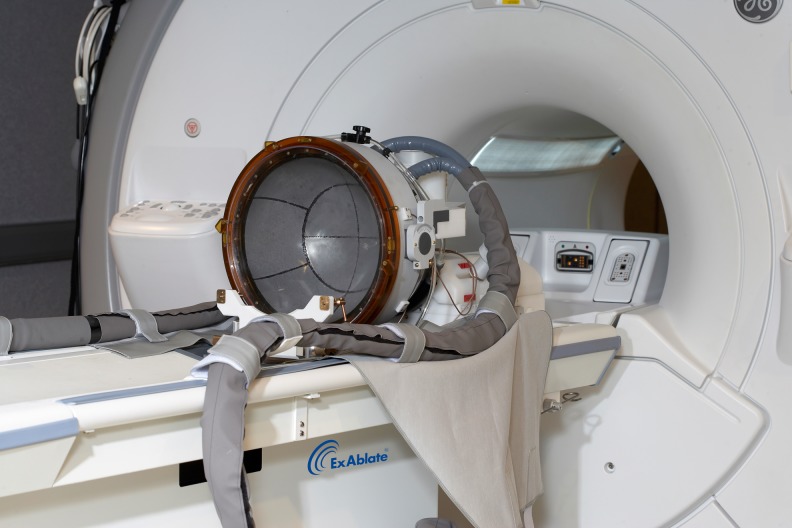
Fig. 1.
Magnetic resonance-guided focused ultrasound transducer helmet on a magnetic resonance imaging table. The model shown is ExAblate Neuro (InSightec, Haifa, Israel). The hose to the helmet carries cooled, degassed water that will provide the medium through which acoustic energy passes
In addition to the thermal interactions described above, ultrasound can induce mechanical effects on the tissue [30]. These mechanical effects are most often mediated by interactions of the pressure wave with microbubbles that are either generated in tissue by the pressure wave or are preformed and injected in the blood stream. The former requires very high exposures and is often associated with blood vessel rupture or occlusion [31], but may be controlled to induce complete tissue disintegration [32] or vaporization [33, 34]. Preformed microbubbles are routinely injected in the bloodstream to enhance ultrasound signals from the blood for diagnostic imaging. These bubbles are very effective energy concentrators and can mediate ultrasound bioeffects at power levels that are < 0.1 % of that required for thermal coagulation. In brain, ultrasound-induced microbubble interactions can open the BBB transiently and without any observable permanent effect on the brain tissue [21]. This method can then be used to facilitate MRI-guided local drug delivery into the brain, as will be discussed later..
The following describes the basic components of a MRgFUS thermal coagulation procedure, adapted from our current protocol to treat patients with treatment-refractory essential tremor. The device at our institution (Sunnybrook Health Sciences Center, Toronto, Canada) is manufactured by InSightec (Haifa, Israel).
Patient Preparation
On the morning of surgery patients undergo a complete head shave. Their scalp is inspected for scars and other lesions that could compromise the passage of ultrasound. A standard 4-pin stereotactic frame is installed on the patient’s head under local anesthetic. The pins and frame are placed as low as possible on the head to ensure that the center of the brain is within the treatment envelope. Once the frame is in place, a silicone membrane with a central hole is placed over the patient’s head. This membrane will contain cooled, degassed water, which serves as the medium through which ultrasound will travel. All required preoperative checks are then performed. Vital signs are measured, monitoring devices—including pulse oximetry—are applied, and an intravenous line inserted. The patient is then led to the MRI machine. While lying on the MRI table, the patient’s head is coupled to the MRgFUS helmet. The helmet contains a large, phased array transducer system that is composed of 1024 individual transducer elements [35]. The space between the patient’s head and the transducers is filled with cooled, degassed water. The water is chilled at constant temperature (approximately 18 ºC) so that the skull bone temperature remains within safe limits.
Treatment Procedures
Treatment begins by acquiring 3-T MR sequences through the entire brain and target region. Images are aligned and the target area examined. The surgeon reviews the images on the system workstation, identifies a target volume and location, delineates the treatment contours on the images, and reviews the treatment plan. Therapy-planning software calculates the parameters (phase and amplitude corrections to focus through the skull) required to treat effectively the defined region. Once the target and parameters are decided, treatment can begin (Fig. 2).
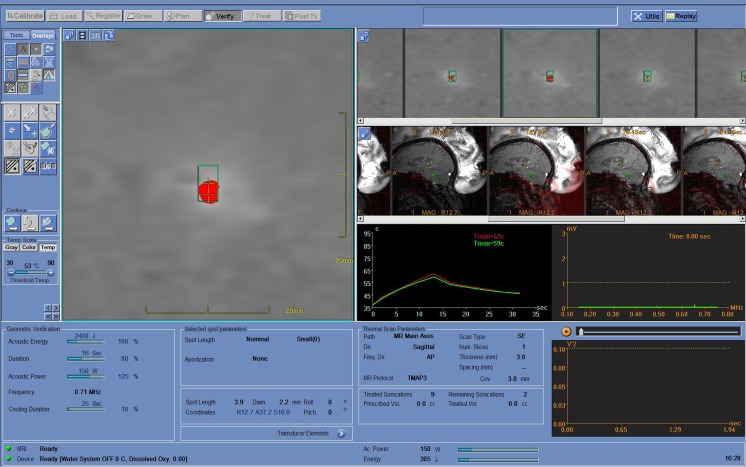
Fig. 2.
Representative screen shot from the planning station during a magnetic resonance-guided focused ultrasound treatment. On the left is a thermographic map indicating the target volume and shape as it is heated (shown in red). In the middle of the figure (red and green lines) is a temperature time curve displaying a single sonication of just over 30 s duration. During this sonication it took approximately 13 s to reach the maximal temperature of 59 ºC. The serial sagittal images of the brain allow the treating surgeon to view in real-time the location and development of the lesion
During treatment, an ultrasound transducer generates a point of focused ultrasound energy, called a “sonication”. First, a subablative power level is used to elevate the brain tissue at the ultrasound beam focus for a few degrees while mapping the temperature elevation distribution using MRI thermometry. The location of the maximum temperature elevation will be identified from these temperature maps and compared with the target location. Adjustments in the beam targeting will be used to align the temperature maximum to overlap with the target location. For ablative procedures, the sonication raises the tissue temperature within the target region to a prescribed maximum, causing a thermal coagulation effect. MR images acquired during sonication provide a quantitative, real-time temperature map of the entire field-of-view around the target area to confirm the location of the sonication and the size of the coagulated region. Thermoablative lesions are done sequentially in small increments to verify efficacy, and monitoring for adverse effects between sonications. The sonication process is repeated at multiple adjacent points, as required, to cover a prescribed treatment volume. Brain tissue temperatures are monitored in real-time using MR thermometry. A thermal map of the treatment volume confirms the therapeutic radiologic effect and is used to confirm that the ablation is proceeding according to plan.
Throughout treatment, following each sonication the patient is examined for both clinical and adverse events. At all times, the patient maintains remote control access with the ability to abort any sonication(s). Raising sonication temperatures to sublesional levels permits “mapping” of the target region, wherein neuronal activity is temporarily affected, but not abolished. In the thalamus, for example, sensory or motor disturbances following sublesional sonications, may alter targeting and guide optimal positioning for the definitive lesion.
Treatment ends once the clinical and radiologic effects are deemed satisfactory by the surgeon. For example, for essential tremor (ET), treatment is concluded once a complete or significant tremor reduction has been achieved while in the scanner, and after a lesion in the Vim nucleus of the thalamus has been demonstrated on T2-weighted imaging. The patient is then decoupled from the transducer helmet, removed from the MRI machine, and examined.
Post-treatment Procedures
Following the procedure, the patient undergoes a neurological examination in the recovery room. They are transferred to a neurosurgical unit and observed overnight. Structural MRI is obtained the following morning. If clinically well, patients can be discharged the day after their procedure. Clinical and radiologic follow-up is then arranged. Serial MRI will demonstrate the evolution of the lesion over time (Fig. 3), and clinical follow-up is required to monitor for adverse events and recurrence, as well as to characterize the clinical effect. At our center, an MRI scan is performed on the first postoperative day, as well as at 1 week, and 1 and 3 months after treatment (Fig. 3).
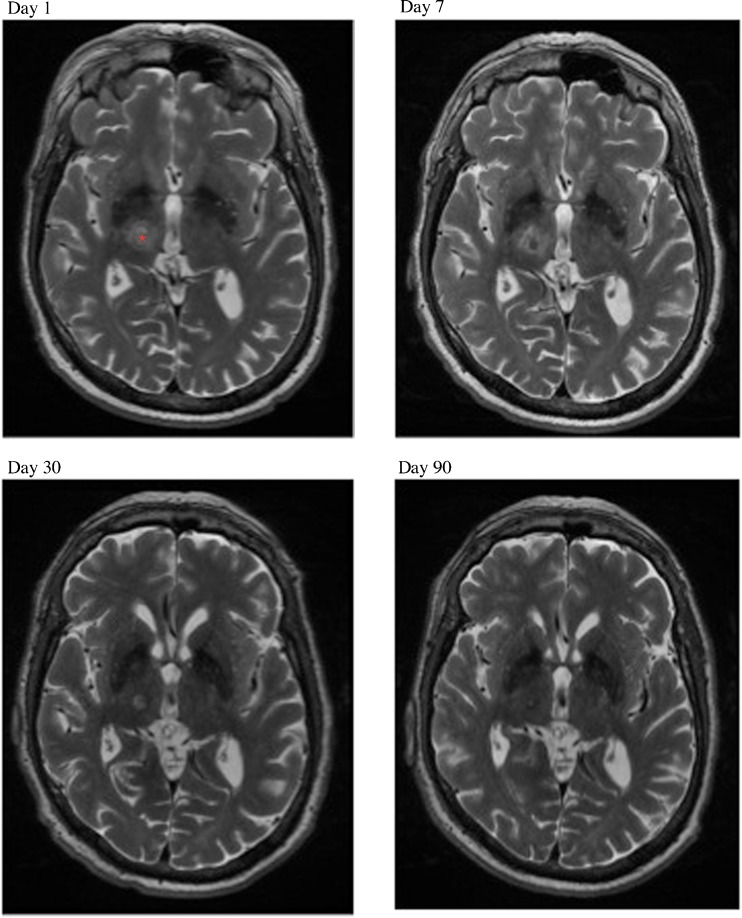
Fig. 3.
Evolution of magnetic resonance-guided focused ultrasound thalamotomy lesion over time. On the day 1 scan, the center of the lesion in the right ventro-intermediate (Vim) nucleus of the thalamus is marked with a red asterisk. The lesion in the same location is shown at 1 week, and 1 and 3 months following treatment. Over time, edema surrounding the ablation site subsides leaving a well-circumscribed lesion within the Vim thalamus by 3 months
Current Applications
Currently, MRgFUS can be used in 2 ways: 1) to focally ablate neural tissue or 2) to temporarily disrupt vessel permeability, also known as BBB disruption. Studies investigating both applications are in various stages of development, ranging from early, preclinical animal models to multicenter, late-phase clinical trials. Here, we review each of the emerging indications for MRgFUS for both ablation and BBB disruption, and highlight future directions for this promising means of treating brain-based diseases (Table 1).
Table 1.
Current magnetic resonance-guided focused ultrasound (MRgFUS) indications under investigation and their status
| Indication | Stage | Status |
|---|---|---|
| ET | Phase II/III | 2 phase I studies completed. Randomized, double-blind, sham-controlled study currently being conducted [39, 46] |
| PD | Phase I | Phase I trial now recruiting for MRgFUS pallidotomy for levodopa-induced dyskinesias of PD and thalamotomy for tremor-dominant PD |
| Brain tumor—ablation | Phase I | Phase I study now recruiting patients |
| Brain tumor—BBB disruption | Phase I | Phase I study now recruiting patients |
| Depression/anxiety | Phase I | Phase I trial now in development for MRgFUS cingulotomy in patients with treatment-refractory OCD and major depression |
| Pain syndromes | Phase I | Models in cadaveric models completed and have shown feasibility of trigeminal nerve root entry zone lesions [56]. Phase I study under development |
| Open-label studies of centromedian thalamotomy for neuropathic pain published and continue to recruit [35, 52] | ||
| Epilepsy | Preclinical | Models investigating the feasibility of MRgFUS-mediated amygdalohippocampectomy now in process |
| AD | Preclinical | Models in transgenic mice have shown that MRgFUS BBB disruption results in influx of anti-Aβ antibodies and subsequent reduction of plaque burden [22, 91] |
| Thrombolysis/intracerebral hemorrhage | Preclinical | Swine and human cadaveric models demonstrated feasibility of ICH liquefaction. Rabbit carotid occlusion model demonstrated feasibility of this model for vascular recanalization [61, 75–77] |
| CSF diversion | Preclinical | Preclinical study performed, providing proof-of-principle of MRgFUS third ventriculostomy [92] |
ET=essential tremor; PD=Parkinson’s disease; BBB=blood–brain barrier; AD=Alzheimer’s disease; CSF=cerebrospinal fluid; OCD=obsessive–compulsive disorder; Aβ=amyloid beta; ICH=intracerebral hemorrhage
ET
ET is the most common movement disorder, affecting between 0.4 and 4.0 % of the general population [36]. Most commonly affecting the upper extremities, and the dominant arm in particular, the tremor is characteristically postural and exacerbated by movement. Progressive over time, ET leads in many patients to significant disability and functional impairment. Medical treatments are available for the majority of patients, but for up to 25–30 %, these are either ineffective or not tolerated [37–42]. Such patients are unable to eat, drink, write, or otherwise engage in routine activities of daily living without caregiver or other support. For these treatment-refractory patients, neurosurgical options may be appropriate.
All surgical approaches to ET target the ventral intermediate (Vim) nucleus of the thalamus, a key cerebello-motor relay structure, where neuronal oscillations have been linked to the tremor of ET [43]. Disruption of Vim activity can be achieved in 2 ways: 1) by ablating the nucleus, using RF thalamotomy, or 2) electrically stimulating it, using deep brain stimulation (DBS). Thalamotomy involves the introduction, under stereotactic guidance, of a probe through the brain and into the Vim, where its tip is heated to 75–80 ºC, leading to permanent destruction of the nucleus. DBS is a nonablative alternative to thalamotomy, and involves the insertion of stimulating electrodes into the Vim, which are then connected to an implanted pulse generator. Both RF thalamotomy and DBS are highly effective in treating ET, with tremor control rates approaching 80–90 % [37, 38, 44, 45]. DBS can be performed bilaterally, whereas thalamotomy is typically restricted to one side. Both procedures are invasive, requiring cranial access and passage through the brain, with attendant surgical risks, including intracranial hemorrhage and infection.
MRgFUS has been developed as a noninvasive alternative to RF thalamotomy, allowing the generation of a thalamic lesion with real-time image and thermometry guidance, thus obviating the need to pass electrodes through the brain. In a feasibility study published in 2013, our group reported the first experience with MRgFUS in 4 patients with chronic, medically refractory ET [39]. All 4 patients underwent noninvasive thalamotomy, and experienced an immediate reduction in tremor during the procedure. At the 3-month follow-up, patients had a mean reduction in tremor of 81 %, as measured by the Clinical Rating Scale for Tremor, with functional impairment secondary to tremor reduced by an average of 40 %. The procedure was well tolerated, with one patient experiencing, at 3 months, permanent, but nondisabling, parasthesias in the fingertips of the treated hand; the other patients reported no serious adverse events. Serial MRI scans at 1 week, and 1 and 3 months, demonstrated a gradual evolution of the thalamic lesion with resolution of perilesional edema over time, which is in keeping with what is currently known about the progression of similar lesions following RF thalamotomy [23] (Fig. 3). These results were replicated in a larger phase I study from the University of Virginia, in which 15 patients with ET also underwent MRgFUS thalamotomy [46]. At 1-year follow-up, patients saw a mean reduction of 75 % in their dominant arm tremor score, using the Clinical Rating Scale for Tremor, as well as an 85 % reduction in functional disability. In this series, 4 patients (27 %) experienced persistent parasthesias, a figure in keeping with our own results, as well as the literature on sensory side effects following conventional RF thalamotomy and DBS [38]. Currently, only unilateral MRgFUS thalamotomy is being performed for ET. This is owing to the recognized risks of bilateral thalamotomy, including an up to 20–30 % risk of postoperative speech disturbances and ataxia [47–50].
The results of both phase I trials suggest that MRgFUS is safe, and can produce radiologic and clinical results similar to open thalamotomy. Long-term follow-up is required to determine the durability of the clinical response, and whether rates of recurrence are similar to those with RF thalamotomy. Definitive evidence of efficacy also awaits the results of a randomized, double-blind, sham-controlled trial, which is currently being conducted.
Chronic Pain
Chronic pain is among the most challenging conditions to treat. The mainstay of pain management is pharmacological, often consisting of diverse combinations of different classes of medications. When patients remain symptomatic despite optimal medical management, surgical procedures become an option. Neurosurgeons have been involved in the management of chronic pain for > 75 years, and several operations have been developed to treat both central- and peripheral-type pain syndromes [51]. Most of these are ablative, targeting either the sensory or affective component of the condition [51]. Central procedures, such as thalamotomy, cingulotomy, and dorsol root entry zone-otomy, target the brain and spinal cord and ascending pain pathways, while peripheral procedures, such as ganglionectomy and rhizotomy, target specific nerves or nerve bundles [51].
The first study to examine MRgFUS for chronic, neuropathic pain was published in 2009 [35]. In this study, 9 patients with chronic, medication-resistant neuropathic pain underwent central lateral thalamotomy using MRgFUS. Therapy resistance was defined as no effective response to an adequate course of antiepileptic and antidepressant medications. The site of ablation was targeted to the posterior part of the thalamic central lateral nucleus. For all patients, the treatment was well tolerated and did not result in any side effects or neurological deficits with all patients experiencing some level of pain relief during the procedure. At 48 h after the treatment, patients reported pain relief ranging from 30 % to 100 % (mean 68 %). MRI at 48 h post-treatment showed lesions of 3–5 mm in diameter that were located at the target site as determined by preoperative stereotactic coordinates and the Morel stereotactic atlas. This proof-of-concept study was expanded in a more recent publication, in which 11 patients with chronic pain underwent MRgFUS and were followed for 1-year [52]. Five patients underwent a unilateral procedure, and 6 a bilateral procedure. As a group, patients experienced a mean pain reduction of 49 % and 57 % at 3 and 12 months, respectively. Six patients experienced immediate pain relief following their procedure. One patient experienced a small hemorrhage in the area of the motor thalamus, resulting in post-operative neurologic deficits. These resolved over time, with only minor impairment present at 1-year follow-up.
Trigeminal Neuralgia
Trigeminal neuralgia (TN), with an estimated prevalence of 20 per 100,000 population, is a common and challenging pain syndrome, characterized by sharp, stabbing electric pain in the distribution of the trigeminal nerve [53]. Surgical treatments for TN are reserved for patients in whom medical management, typically with antiepileptic medications, is insufficient or not tolerated. Surgery for TN focuses on the trigeminal nerve, at some point in it’s course from skin to pons, and can include intentionally damaging the nerve and/or it’s divisions, as well as nondestructive procedures that relieve vascular compression of the nerve at it’s root entry zone (microvascular decompression) [54]. Gamma knife radiosurgery (GKRS) is now also frequently used in the treatment of TN, where a dose of 80 Gy is administered to the mid-cisternal portion of the nerve. It appears that one-third of patients treated with radiosurgery enjoy pain relief off medication. Another third of patients are pain free, but remain on medication, and an additional third derive only partial benefit from radiosurgery [55]. Risks of GKRS include the variation in susceptibility to radiation between patients, as well as the development of radiation-associated complications, such as secondary malignancy.
MRgFUS has also been proposed for the noninvasive management of refractory TN. However, there are important technical challenges associated with the generation of thermal injury next to the trigeminal nerve due to proximity of the petrous bone. Energy from ultrasound beams is efficiently absorbed by bone and could lead to excessive heating. For this reason, current treatment regimens require that the “treatment envelope” for sonications be at least 2.5 cm away from the inner table of the skull, to prevent heating of the bone and underlying dura. Therefore, optimal targets are those that are in the center of the brain, including periventricular structures such as the thalamus and basal ganglia. One study, performed in cadavers, examined the feasibility of making a trigeminal root entry zone lesion with MRgFUS and found that the petrous bone got as hot as the nerve but nonetheless concluded that the treatment could be done [56]. Using imaging data sets from 5 patients treated for ET at our center, we have recently modeled whether a similar lesion of the trigeminal root entry zone could be achieved with modification of focused ultrasound parameters. These preliminary data found that it would, indeed, be possible to focus on the very proximal trigeminal nerve at its root entry zone without significantly heating the petrous bone and the structures in and around it.
Brain Tumors
Treatment of malignant brain tumors is aimed at enhancing and prolonging quality of life. Unfortunately, the last 50 years have seen few treatment advances for the most malignant, and common, brain tumor, glioblastoma multiforme. Treatment of glioblastoma multiforme is multidisciplinary and typically involves radiologic and/or tissue diagnosis, followed by as safe as possible maximal surgical resection, and chemoradiation therapy. Important advances in the genetics of gliomas have further led to treatments being tailored to the individual genetic profile of a patient’s tumor. Although there are innumerable challenges in brain cancer management, 2 of them include the infiltrative nature of the tumor, and hence diffuse spread throughout the brain at time of diagnosis, as well as the inability of current chemotherapy regiments to adequately cross the BBB. In clinical and preclinical trials, MRgFUS has been be used to address both of these challenges, namely to focally ablate lesions seen on MRI and to facilitate the passage of chemotherapy through BBB disruption.
Ablation
In 2010, McDannold et al. [57] published their experience with MRgFUS ablation in 3 patients with high-grade glioma. The purpose of the study was to evaluate the safety of MRgFUS thermal ablation of brain tumors performed through the intact human skull and to examine the radiologic effect of thermal ablation in the target tumor with contrast-enhanced MRI. All 3 patients underwent the procedure under conscious sedation and tolerated the procedure well. Significant tissue temperature increases were achieved within the tumor in all patients; however, thermal coagulation and permanent ablation was not achieved. Technical adjustments to the system, including a doubling of transducer elements, decreasing the frequency from 650 kHz to 230 k Hz, and increasing the power capability, were subsequently made and a fourth patient treated. Although the patient tolerated the procedure well, and the tumor was successfully ablated, the patient suffered a large brain hemorrhage 5 days after the procedure and died. The cause of death was linked to a possible underlying coagulopathy, and changes were made to exclude such patients from subsequent MRgFUS trials. In addition, only the higher frequency devices are currently used for thermal ablation in order to reduce a risk of inertial cavitation (formation and collapse of gas bubbles) that has been associated with bleeding [31]. Additional trials investigating MRgFUS ablation for metastatic brain cancer, a condition in which resection may have a more substantial impact on the natural history of the condition, are currently underway.
BBB Disruption
The BBB is composed of tightly bound capillary endothelial cells and is the brain’s primary defense against large and molecularly heavy toxins. This dense capillary network, which is lined by a continuous layer of epithelial cells, provides a broad barrier system, prohibiting the passive and active transport of large and potentially harmful molecules [28]. Tight junctions between capillary endothelial cells further limit the transport of molecules between cells, ensuring that access to the central nervous system is transcellular. Therefore, passage across the BBB is by diffusion for small molecules or receptor-mediated for larger molecules [28]. This rigid, size, charge and biochemically mediated barrier has hampered the development of neurotherapeutics for brain cancer, and also for other neurodegenerative conditions such as Alzheimer’s disease (AD) and Parkinson’s disease (PD) [58]. A potentially promising application of MRgFUS is the ability to temporarily disrupt the BBB, thus facilitating the passage of compounds too large to otherwise pass into the brain. Such compounds can include chemotherapy agents, as well as monoclonal antibodies. The last decade has seen significant advances in this area, with preclinical models demonstrating 1) that MRgFUS can open the BBB temporarily while not generating a lesion or irreversible damage [21]; 2) that chemotherapy agents can get into the brain in concentrations that correlate with the time that the BBB was open [59, 60]; 3) that temporary BBB opening is safe in small- and medium-sized animals [20, 21, 61]; and 4) that it is possible to achieve significant levels of concentration in various types of tumors, including gliomas and brain metastases [62].
BBB disruption can be achieved using a combination of MRgFUS at low frequencies and the simultaneous administration of contrast agents containing microbubbles. The interaction of acoustic energy and microbubbles at the capillary endothelial cells results in temporary disruption of the BBB and diffusion of large molecules [28]. The precise mechanisms underlying MRgFUS-mediated BBB disruption are under active investigation. Preclinical models suggest that temporary disruption of the BBB is likely the result of stable bubble oscillations, induced by the interaction of low acoustic power with preinjected microbubbles at the surface of capillary endothelial cells. Bubble oscillation and growth results in stretching of endothelial cell membranes, thus permitting transient opening of the BBB within seconds of the start of the sonications [28, 58, 63]. The opening is healed within approximately 6 h, although longer openings of 24 h and longer have been reported and are presumably associated with more serious tissue effects [64]. In animal models, a wide range of molecules, varying in size, have been transported across the BBB following MRgFUS-mediated disruption (see below). In general, these long-term studies have shown that the effects of MRgFUS BBB disruption on brain tissue is minimal, with negligible neuronal damage, no evidence of ischemia or apoptosis, no extravasation of erythrocytes, and no damage to sonicated tissue [12, 65].
Using these approaches, several groups have shown that MRgFUS-mediated BBB disruption can achieve significant intratumoral and tissue concentrations of several key chemotherapeutic agents, including Herceptin (Roche, Basel, Switzerland), doxorubin and temozolamide (TMZ). In one study, Kinoshita et al. [60] used a mouse model to show Herceptin concentrations in target tissue were significantly correlated with the extent and duration of MRgFUS-mediated BBB disruption. Wei et al. [66] used a glioma rat model to study concentrations of TMZ and tumor progression after MRgFUS-mediated BBB disruption. The authors found that compared with TMZ administration alone, chemotherapy plus MRgFUS resulted in greater cerebrospinal fluid concentrations of TMZ and reduced 7-day tumor progression rates. Using doxorubicin and MRgFUS BBB disruption in a rat model, Treat et al. [67] also found similar effects on glioma progression, with modest effects on survival. These results provide proof of concept that MRgFUS-mediated BBB disruption is possible, and can achieve significant brain tissue concentrations of complex and large biologic agents. Such findings suggest that MRgFUS may be used either alone or as adjunctive therapy for patients undergoing surgical resection of primary or secondary brain malignancy. As a result, several human trials are currently underway to explore the use of MRgFUS for brain tumor therapy.
Neurodegenerative Disorders
PD
PD is a heterogeneous motor disorder, characterized by progressive tremor, rigidity, akinesia, and postural instability [68]. Among the pathologic hallmarks of the illness is cell death and subsequent loss of dopaminergic cells in the substantia nigra. Medications aim to restore dopamine reserves, but, over time, motor symptoms fail to respond even to dopamine replacement, and symptoms become difficult to predict and control. Neurophysiologic and preclinical primate models have shown that surgical disruption of key motor nuclei is associated with significant improvement in motor symptoms [69]. Several clinical trials have since established the role of neurosurgery for treatment-resistant PD [69]. As with brain tumors, MRgFUS can be used in 2 possible ways to manage PD. The first is leveraging the decades-long experience with ablative surgery and investigating MRgFUS-mediated lesioning. This can include targeting the globus pallidus, with pallidotomy, in patients with disabling dyskinesias, as well as the ventral intermediate thalamus, or Vim thalamotomy, in patients with tremor-dominant disease. Phase I trials exploring both of these indications are currently underway. In addition, MRgFUS BBB disruption can potentially be used to facilitate the passage of neuronal growth factors, such as brain-derived neurotropic factor, or immune therapies, should these prove promising in the treatment of PD. For example, in a mouse model, Kinoshita et al. [70] demonstrated that, following MRgFUS BBB disruption, dopamine receptor antibodies were able to cross the BBB and find their antigens. The implication is that immunotherapies for neurodegenerative conditions, including PD and AD, may be facilitated by focal and temporary disruption of the BBB.
AD
AD is the most common neurodegenerative disorder. Primarily affecting those > 60 years of age, the next 2 decades will see an exponential rise in cases. The pathologic hallmarks of AD include tau tangles and amyloid plaques, as well as a marked degeneration in structures and pathways subserved by cholinergic transmission, with medical management aimed at boosting cholinergic reserves and preventing the breakdown of acetylcholine. Unfortunately, these approaches have provided little, if any, meaningful benefit to patients. More recent trials, investigating immunobiologic agents targeting amyloid-beta (Aβ) peptide, also failed to demonstrate significant effects on cognitive and memory declines. The failure of these trials may relate, at least in part, to the recognized difficulty for large compounds to cross the BBB, with some studies showing that only 0.1 % of intravenously administrated anti-Aβ antibodies reaching the brain [22, 71]. Several studies using transgenic animal models of AD have now demonstrated that MRgFUS-mediated BBB disruption can be an effective means of introducing antibodies and reducing plaque burden. In one study, a single session of four MRgFUS sonications to 1 hemisphere in transgenic AD mice resulted in significant BBB disruption, delivery and binding of Aβ antibody to plaques, and a reduction in the number and size of plaques compared with the contralateral hemisphere [22]. Notably, antibody concentrations in the brain were similar to those achieved with surgical transplantation, as well as with intravenous infusion, but with 10 times less the dose. The same group also demonstrated the successful translocation of significant concentrations of neural stem cells, as well as adenoassociated virus type 9, into the brain using MRgFUS BBB disruption [72, 73]. These promising findings suggest that MRgFUS may offer a gateway into the brain for previously impassable compounds. Whether plaque reductions translate into improvements in behavioral outcomes, and whether these results can be translated to human trials, remains open to investigation.
Stroke and Thrombolysis
Ischemic and hemorrhagic stroke are major causes of mortality and morbidity. Current management strategies include early administration of thrombolytics for the former, and the option of surgical evacuation for large, accessible bleeds, in cases of intracerebral hemorrhage (ICH). Outcomes are influenced by patient factors, such as comorbidites, as well as the timing of medical and surgical intervention. Large trials comparing surgical with medical management for accessible ICH lesions have shown that early surgery does not increase risk and may be associated with improved long-term outcomes [74]. MRgFUS has been proposed as an additional tool for the management of patients with ICH, with the rationale that the procedure can offer a safe and efficient means of liquefying blood to facilitate MR-guided aspiration. This would help reduce clot burden and mass effect, and avoid a craniotomy. The mechanisms underlying MRgFUS-mediated clot lysis is mechanical and, more specifically, involves inertial cavitation with the generation and collapse of gas bubbles under high-pressure amplitude waves. In this approach, the ultrasound peak powers are much higher than in any of the above applications and there is no need to inject preformed microbubbles. Preclinical work in animals and human cadavers has utilized lower frequencies for intraclot lysis, with the objective of disrupting the clot matrix and to generate a thin lysate, suitable for MR-guided aspiration. For example, Monteith et al. [75] used a swine model of ICH to demonstrate that MRgFUS can achieve liquefaction of ICH within seconds. The authors then used human cadaveric heads, injected with 40 cc of ICH, and showed that MRgFUS can successfully liquefy > 95 % of the clot, allowing almost complete aspiration of blood contents. Thus, MRgFUS may offer a noninvasive alternative to craniotomy in these cases, and, in particular, for deep-seated, dominant hemisphere bleeds, as well as intraventricular hemorrhages, where transcortical approaches may increase the risk of postoperative neurologic sequelae. Additional trials are now required to examine the feasibility of this intervention in human patients.
MRgFUS-mediated vascular recanalization is also an area of active investigation. Studies in rabbit models of carotid occlusion have shown that it may be feasible to use MRgFUS to recanalize blocked blood vessels [61, 76, 77]. Although very much in the early stages, such models can provide valuable information about the influence of acoustic energy on blood vessels and flow dynamics. Additional intriguing vascular applications include vascular and cavernous malformations, where the ablative properties of MRgFUS can be used to thrombose and exclude these lesions from the circulation.
Major Depression and Obsessive–Compulsive Disorder
Ablative procedures have been used to treat refractory psychiatric disease for > 70 years [78–80]. Although these procedures have changed dramatically over time, the central premise, namely the disruption of limbic pathways connecting frontal with subcortical structures, remains the same. The management of mood and anxiety disorders is pharmacologic and psychotherapeutic, and for the majority of patients these approaches are effective. However, up to a third remain symptomatic despite optimal care, and are eligible for neuromodulation, including electroconvulsive-therapy, transcranial magnetic stimulation, DBS, and ablative or lesional procedures [81]. The most common ablative procedures for depression and obsessive–compulsive disorder (OCD) are cingulotomy and anterior capsulotomy. Cingulotomy involves the generation of typically bilateral lesions in the anterior cingulate gyrus, approximately 2 cm posterior to the edge of the corpus callosum [82, 83]. The procedure has been shown to be very safe, and an effective treatment option for patients with refractory mood and anxiety symptoms. In one prospective series, Dougherty et al. [82] followed 44 patients with OCD for a mean of 32 months and found that nearly half of the patients were at least partially improved following their operation. Capsulotomy involves a lesion in the anterior limb of the internal capsule, hence severing fronto-striatal and limbic projections. A recent study of 8 patients with treatment-refractory depression found that bilateral anterior capsulotomy resulted in complete or partial remission in 4 patients at the 2–3 year follow-up [84].
Both the anterior cingulate and anterior limb of the internal capsule are within current “treatment envelopes” for MRgFUS. For patients who have reached the limits of conventional psychiatric treatment, as well as for those who cannot tolerate or are not interested in other neuromodulation approaches, MRgFUS may offer a potential alternative. Real-time MR guidance and the ability to noninvasively re-treat patients who have a recurrence, are additional advantages over traditional open surgical approaches to ablative psychiatric surgery. Phase I trials exploring cingulotomy for depression and OCD are currently underway, and will provide valuable information about the safety of the procedure and it’s clinical efficacy.
Risks of MRgFUS
In North America, MRgFUS is currently approved only for the treatment of painful bone metastases and uterine fibroids. Transcranial MRgFUS is not approved by the US Food and Drug Administration or Health Canada for any indication, and remains investigational. In Europe, the machine manufactured by InSightec (Haifa, Israel), known as the ExAblate Neuro System, recently received the European CE mark for noninvasive treatment of neurological disorders. This designation indicates the growing interest, and demonstrated safety, of the device for intracranial use. Approval by North American healthcare agencies will await the results of larger, multicenter, sham-controlled trials that are currently being conducted.
Critically, the enthusiasm for MRgFUS should be tempered by the relative infancy of the field, and it’s as yet unknown long-term effects. With tremor, for example, questions remain about the long-term durability of both clinical and radiologic effects. Therefore, it may be too soon to draw conclusions or comparisons with open surgical approaches. Further, MRgFUS remains a neurosurgical procedure, with potentially life-threatening risks, that should not be minimized or trivialized. Indeed, the early experience with brain tumors showed that patients can, and do, die after a FUS procedure. It is therefore important to discuss the nature of the procedure with patients, and to disabuse them of the notion that this “noninvasive” approach does not involve direct, often irreversible, damage to the brain.
In addition to the potential risk of hemorrhage, there is also a risk of imprecise targeting of the focal point, and ablation of an area outside the planned treatment volume. Although this risk is mitigated by direct visualization of the target with MRI, patient movement and anatomical variations may, nevertheless, influence the final target position. MRgFUS currently allows surgeons to perform target position checks by applying sublesional sonications, checking for both adverse and clinical effects prior to the delivery of lesional temperatures. The ability to “map” thalamic regions, for example, is not unlike similar procedures performed in the course of radiofrequency thalamotomy and DBS. As with every new surgical technology, the initial costs of MRgFUS remain high. As a result, MRgFUS trials are currently being conducted only in centers with the necessary human, operational, and technical resources to study systematically the technology and it’s applications.
Benefits of MRgFUS and Future Directions
The development of MRgFUS is most commonly compared to GKRS. There are certainly some similarities, namely that both are noninvasive, frame-based systems, which permit treatment within a prescribed and physician-contoured “treatment envelope”. However, there are important differences. Unlike GKRS, MRgFUS does not use ionizing radiation, thus permitting multiple treatments, if necessary, without concern for cumulative radiation exposure. With GKRS there is also concern regarding variable biological responses to radiation and the development of secondary malignancy [85], the risk of which has been estimated to be 0.04–0.10 % [86, 87]. Arguably the most important putative advantage of MRgFUS is that the effect of treatment is immediate, rather than delayed, as is the case with GKRS [46, 85, 88]. It is not clear, for example, if GKRS for trigeminal neuralgia or ET has been successful until several weeks to months after treatment [88]. The effect with MRgFUS occurs in the machine [39, 46, 52]. The ability to visualize the target using real-time MRI, and also to monitor the temperature of the lesion using MR thermometry, are additional advances that heighten safety and provide confidence that the prescribed lesion and the actual lesion are the same.
The next decade will likely see rapid advances in both clinical and technological domains for MRgFUS. As results from preclinical models translate to clinical trials, clinicians treating the conditions listed in Table 1 may have an additional tool for managing their patients and, in particular, those patients who may not want, or cannot tolerate, an operation. Larger studies, with longer follow-up will help characterize the long-term clinical and radiological effects, allowing better comparisons with open neurosurgical procedures. Furthermore, in addition to ablation and BBB disruption, other emerging applications of MRgFUS include noninvasive modulation of brain function [89], as well as brain structure [90].
The most important advances for MRgFUS will be technical. Currently, the typical procedure length for Vim thalamotomy using MRgFUS is approximately 4 h. With evolving software, off-line analysis of skull and brain anatomy, and experience, this will be significantly shortened. MRgFUS treatments also currently require a complete head shave. Advances in the efficient transmission of acoustic energy through the skull may obviate this need in the future. In addition, current treatments are restricted to being at least 2.5 cm away from the inner table of the skull owing to excessive heating and poor contouring of targets near bony anatomy. Expanding the “treatment envelope” is a priority for researchers, and will facilitate treatment throughout the brain, including the skull base, orbits, and spinal cord.
Conclusion
The ability to focus ultrasound energy through the skull and onto brain targets a few millimeters in size represents a substantial technical achievement and, potentially, a major milestone in neurotherapeutics. Several phase I studies have demonstrated that transcranial MRgFUS is safe, and can achieve clinical and radiologic effects similar to those with open neurosurgical approaches. Larger studies with long-term follow-up are now required to better characterize the response and validate these early results. If borne out, the expanding list of indications for MRgFUS may yet grow, with the technology becoming an increasingly available, and desired, option for both patients and clinicians.
Electronic supplementary material
(PDF 1224 kb)
Acknowledgments
We thank Eyal Zadicario and Lynn Golumbic of InSightec for the images in shown in Figs 1 and 2. NL has nothing to declare. KH is an inventor of intellectual property related to trans-skull focused ultrasound treatments owned by Brigham and Women’s Hospital (Boston, MA, USA) and licensed to InSightec. AML, TGM and MLS are consultants to the Focused Ultrasound Foundation.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
References
- 1.Lynn JG, Zwemer RL, Chick AJ. The biological application of focused ultrasonic waves. Science. 1942;96:119–120. doi: 10.1126/science.96.2483.119. [DOI] [PubMed] [Google Scholar]
- 2.Fry FJ, Ades HW, Fry WJ. Production of reversible changes in the central nervous system by ultrasound. Science. 1958;127:83–84. doi: 10.1126/science.127.3289.83. [DOI] [PubMed] [Google Scholar]
- 3.Fry WJ. Use of intense ultrasound in neurological research. Am J Phys Med. 1958;37:143–147. [PubMed] [Google Scholar]
- 4.Fry WJ, Fry FJ. Fundamental neurological research and human neurosurgery using intense ultrasound. IRE Trans Med Electron. 1960;ME-7:166–181. doi: 10.1109/iret-me.1960.5008041. [DOI] [PubMed] [Google Scholar]
- 5.Ram Z, Cohen ZR, Harnof S, et al. Magnetic resonance imaging-guided, high-intensity focused ultrasound for brain tumor therapy. Neurosurgery. 2006;59:949–955. doi: 10.1227/01.NEU.0000254439.02736.D8. [DOI] [PubMed] [Google Scholar]
- 6.Cline HE, Hynynen K, Watkins RD, et al. Focused US system for MR imaging-guided tumor ablation. Radiology. 1995;194:731–737. doi: 10.1148/radiology.194.3.7862971. [DOI] [PubMed] [Google Scholar]
- 7.Cline HE, Schenck JF, Watkins RD, Hynynen K, Jolesz FA. Magnetic resonance-guided thermal surgery. Magn Reson Med. 1993;30:98–106. doi: 10.1002/mrm.1910300115. [DOI] [PubMed] [Google Scholar]
- 8.Cline HE, Schenck JF, Hynynen K, Watkins RD, Souza SP, Jolesz FA. MR-guided focused ultrasound surgery. J Comput Assist Tomogr. 1992;16:956–965. doi: 10.1097/00004728-199211000-00024. [DOI] [PubMed] [Google Scholar]
- 9.Hynynen K, Damianou C, Darkazanli A, Unger E, Schenck JF. The feasibility of using MRI to monitor and guide noninvasive ultrasound surgery. Ultrasound Med Biol. 1993;19:91–92. doi: 10.1016/0301-5629(93)90022-g. [DOI] [PubMed] [Google Scholar]
- 10.Hynynen K, Darkazanli A, Unger E, Schenck JF. MRI-guided noninvasive ultrasound surgery. Med Phys. 1993;20:107–115. doi: 10.1118/1.597093. [DOI] [PubMed] [Google Scholar]
- 11.Clement GT, Hynynen K. A non-invasive method for focusing ultrasound through the human skull. Phys Med Biol. 2002;47:1219–1236. doi: 10.1088/0031-9155/47/8/301. [DOI] [PubMed] [Google Scholar]
- 12.Hynynen K, McDannold N, Clement G, et al. Pre-clinical testing of a phased array ultrasound system for MRI-guided noninvasive surgery of the brain—a primate study. Eur J Radiol. 2006;59:149–156. doi: 10.1016/j.ejrad.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 13.McDannold N, Moss M, Killiany R, et al. MRI-guided focused ultrasound surgery in the brain: tests in a primate model. Magn Reson Med. 2003;49:1188–1191. doi: 10.1002/mrm.10453. [DOI] [PubMed] [Google Scholar]
- 14.McDannold NJ, Jolesz FA. Magnetic resonance image-guided thermal ablations. Top Magn Reson Imaging. 2000;11:191–202. doi: 10.1097/00002142-200006000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Hynynen K, McDannold N, Mulkern RV, Jolesz FA. Temperature monitoring in fat with MRI. Magn Reson Med. 2000;43:901–904. doi: 10.1002/1522-2594(200006)43:6<901::aid-mrm18>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 16.Ishihara Y, Calderon A, Watanabe H, et al. A precise and fast temperature mapping using water proton chemical shift. Magn Reson Med. 1995;34:814–823. doi: 10.1002/mrm.1910340606. [DOI] [PubMed] [Google Scholar]
- 17.Kuroda K, Chung AH, Hynynen K, Jolesz FA. Calibration of water proton chemical shift with temperature for noninvasive temperature imaging during focused ultrasound surgery. J Magn Reson Imaging. 1998;8:175–181. doi: 10.1002/jmri.1880080130. [DOI] [PubMed] [Google Scholar]
- 18.Hynynen K, Vykhodtseva NI, Chung AH, Sorrentino V, Colucci V, Jolesz FA. Thermal effects of focused ultrasound on the brain: determination with MR imaging. Radiology. 1997;204:247–253. doi: 10.1148/radiology.204.1.9205255. [DOI] [PubMed] [Google Scholar]
- 19.Vykhodtseva NI, Hynynen K, Damianou C. Pulse duration and peak intensity during focused ultrasound surgery: theoretical and experimental effects in rabbit brain in vivo. Ultrasound Med Biol. 1994;20:987–1000. doi: 10.1016/0301-5629(94)90058-2. [DOI] [PubMed] [Google Scholar]
- 20.Hynynen K, Clement GT, McDannold N, et al. 500-element ultrasound phased array system for noninvasive focal surgery of the brain: a preliminary rabbit study with ex vivo human skulls. Magn Reson Med. 2004;52:100–107. doi: 10.1002/mrm.20118. [DOI] [PubMed] [Google Scholar]
- 21.Hynynen K, McDannold N, Vykhodtseva N, Jolesz FA. Noninvasive MR imaging-guided focal opening of the blood-brain barrier in rabbits. Radiology. 2001;220:640–646. doi: 10.1148/radiol.2202001804. [DOI] [PubMed] [Google Scholar]
- 22.Jordao JF, Ayala-Grosso CA, Markham K, et al. Antibodies targeted to the brain with image-guided focused ultrasound reduces amyloid-beta plaque load in the TgCRND8 mouse model of Alzheimer's disease. PLoS One. 2010;5:e10549. doi: 10.1371/journal.pone.0010549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elias WJ, Khaled M, Hilliard JD, et al. A magnetic resonance imaging, histological, and dose modeling comparison of focused ultrasound, radiofrequency, and Gamma Knife radiosurgery lesions in swine thalamus. J Neurosurg. 2013;119:307–317. doi: 10.3171/2013.5.JNS122327. [DOI] [PubMed] [Google Scholar]
- 24.Mougenot C, Quesson B, de Senneville BD, et al. Three-dimensional spatial and temporal temperature control with MR thermometry-guided focused ultrasound (MRgHIFU) Magn Reson Med. 2009;61:603–614. doi: 10.1002/mrm.21887. [DOI] [PubMed] [Google Scholar]
- 25.Rieke V, Butts PK. MR thermometry. J Magn Reson Imaging. 2008;27:376–390. doi: 10.1002/jmri.21265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McDannold N, Vykhodtseva N, Jolesz FA, Hynynen K. MRI investigation of the threshold for thermally induced blood-brain barrier disruption and brain tissue damage in the rabbit brain. Magn Reson Med. 2004;51:913–923. doi: 10.1002/mrm.20060. [DOI] [PubMed] [Google Scholar]
- 27.Vykhodtseva N, Sorrentino V, Jolesz FA, Bronson RT, Hynynen K. MRI detection of the thermal effects of focused ultrasound on the brain. Ultrasound Med Biol. 2000;26:871–880. doi: 10.1016/s0301-5629(00)00216-7. [DOI] [PubMed] [Google Scholar]
- 28.Vykhodtseva N, McDannold N, Hynynen K. Progress and problems in the application of focused ultrasound for blood-brain barrier disruption. Ultrasonics. 2008;48:279–296. doi: 10.1016/j.ultras.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hynynen K, Clement G. Clinical applications of focused ultrasound-the brain. Int J Hyperth. 2007;23:193–202. doi: 10.1080/02656730701200094. [DOI] [PubMed] [Google Scholar]
- 30.Kimmel E. Cavitation bioeffects. Crit Rev Biomed Eng. 2006;34:105–161. doi: 10.1615/critrevbiomedeng.v34.i2.10. [DOI] [PubMed] [Google Scholar]
- 31.Hynynen K, Chung AH, Colucci V, Jolesz FA. Potential adverse effects of high-intensity focused ultrasound exposure on blood vessels in vivo. Ultrasound Med Biol. 1996;22:193–201. doi: 10.1016/0301-5629(95)02044-6. [DOI] [PubMed] [Google Scholar]
- 32.Xu Z, Ludomirsky A, Eun LY, et al. Controlled ultrasound tissue erosion. IEEE Trans Ultrason Ferroelectr Freq Control. 2004;51:726–736. doi: 10.1109/tuffc.2004.1308731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simon JC, Sapozhnikov OA, Khokhlova VA, Wang YN, Crum LA, Bailey MR. Ultrasonic atomization of tissue and its role in tissue fractionation by high intensity focused ultrasound. Phys Med Biol. 2012;57:8061–8078. doi: 10.1088/0031-9155/57/23/8061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith NB, Hynynen K. The feasibility of using focused ultrasound for transmyocardial revascularization. Ultrasound Med Biol. 1998;24:1045–1054. doi: 10.1016/s0301-5629(98)00086-6. [DOI] [PubMed] [Google Scholar]
- 35.Martin E, Jeanmonod D, Morel A, Zadicario E, Werner B. High-intensity focused ultrasound for noninvasive functional neurosurgery. Ann Neurol. 2009;66:858–861. doi: 10.1002/ana.21801. [DOI] [PubMed] [Google Scholar]
- 36.Louis ED, Ottman R, Hauser WA. How common is the most common adult movement disorder? estimates of the prevalence of essential tremor throughout the world. Mov Disord. 1998;13:5–10. doi: 10.1002/mds.870130105. [DOI] [PubMed] [Google Scholar]
- 37.Hubble JP, Busenbark KL, Wilkinson S, Penn RD, Lyons K, Koller WC. Deep brain stimulation for essential tremor. Neurology. 1996;46:1150–1153. doi: 10.1212/wnl.46.4.1150. [DOI] [PubMed] [Google Scholar]
- 38.Koller W, Pahwa R, Busenbark K, et al. High-frequency unilateral thalamic stimulation in the treatment of essential and parkinsonian tremor. Ann Neurol. 1997;42:292–299. doi: 10.1002/ana.410420304. [DOI] [PubMed] [Google Scholar]
- 39.Lipsman N, Schwartz ML, Huang Y, et al. MR-guided focused ultrasound thalamotomy for essential tremor: a proof-of-concept study. Lancet Neurol. 2013;12:462–468. doi: 10.1016/S1474-4422(13)70048-6. [DOI] [PubMed] [Google Scholar]
- 40.Lyons KE, Pahwa R. Deep brain stimulation and tremor. Neurotherapeutics. 2008;5:331–338. doi: 10.1016/j.nurt.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elias WJ, Shah BB. Tremor. JAMA. 2014;311:948–954. doi: 10.1001/jama.2014.1397. [DOI] [PubMed] [Google Scholar]
- 42.Koller WC, Vetere-Overfield B. Acute and chronic effects of propranolol and primidone in essential tremor. Neurology. 1989;39:1587–1588. doi: 10.1212/wnl.39.12.1587. [DOI] [PubMed] [Google Scholar]
- 43.Hua SE, Lenz FA, Zirh TA, Reich SG, Dougherty PM. Thalamic neuronal activity correlated with essential tremor. J Neurol Neurosurg Psychiatry. 1998;64:273–276. doi: 10.1136/jnnp.64.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Benabid AL, Pollak P, Seigneuret E, Hoffmann D, Gay E, Perret J. Chronic VIM thalamic stimulation in Parkinson's disease, essential tremor and extra-pyramidal dyskinesias. Acta Neurochir Suppl (Wien) 1993;58:39–44. doi: 10.1007/978-3-7091-9297-9_8. [DOI] [PubMed] [Google Scholar]
- 45.Kumar K, Kelly M, Toth C. Deep brain stimulation of the ventral intermediate nucleus of the thalamus for control of tremors in Parkinson's disease and essential tremor. Stereotact Funct Neurosurg. 1999;72:47–61. doi: 10.1159/000029671. [DOI] [PubMed] [Google Scholar]
- 46.Elias WJ, Huss D, Voss T, et al. A pilot study of focused ultrasound thalamotomy for essential tremor. N Engl J Med. 2013;369:640–648. doi: 10.1056/NEJMoa1300962. [DOI] [PubMed] [Google Scholar]
- 47.Chao Y, Gang L, Na ZL, Ming WY, Zhong WS, Mian WS. Surgical management of Parkinson's disease: update and review. Interv Neuroradiol. 2007;13:359–368. doi: 10.1177/159101990701300407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matsumoto K, Asano T, Baba T, Miyamoto T, Ohmoto T. Long-term follow-up results of bilateral thalamotomy for parkinsonism. Appl Neurophysiol. 1976;39:257–260. doi: 10.1159/000102501. [DOI] [PubMed] [Google Scholar]
- 49.Tasker RR. Deep brain stimulation is preferable to thalamotomy for tremor suppression. Surg Neurol. 1998;49:145–153. doi: 10.1016/s0090-3019(97)00459-x. [DOI] [PubMed] [Google Scholar]
- 50.Zirh A, Reich SG, Dougherty PM, Lenz FA. Stereotactic thalamotomy in the treatment of essential tremor of the upper extremity: reassessment including a blinded measure of outcome. J Neurol Neurosurg Psychiatry. 1999;66:772–775. doi: 10.1136/jnnp.66.6.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cetas JS, Saedi T, Burchiel KJ. Destructive procedures for the treatment of nonmalignant pain: a structured literature review. J Neurosurg. 2008;109:389–404. doi: 10.3171/JNS/2008/109/9/0389. [DOI] [PubMed] [Google Scholar]
- 52.Jeanmonod D, Werner B, Morel A, et al. Transcranial magnetic resonance imaging-guided focused ultrasound: noninvasive central lateral thalamotomy for chronic neuropathic pain. Neurosurg Focus. 2012;32:E1. doi: 10.3171/2011.10.FOCUS11248. [DOI] [PubMed] [Google Scholar]
- 53.Manzoni GC, Torelli P. Epidemiology of typical and atypical craniofacial neuralgias. Neurol Sci. 2005;26(Suppl. 2):s65–s67. doi: 10.1007/s10072-005-0410-0. [DOI] [PubMed] [Google Scholar]
- 54.Haines SJ, Jannetta PJ, Zorub DS. Microvascular relations of the trigeminal nerve. An anatomical study with clinical correlation. J Neurosurg. 1980;52:381–386. doi: 10.3171/jns.1980.52.3.0381. [DOI] [PubMed] [Google Scholar]
- 55.Alahmadi H, Zadeh G, Laperriere N, et al. Trigeminal nerve integrated dose and pain outcome after gamma knife radiosurgery for trigeminal neuralgia. J Radiosurg SBRT. 2012;1:295–301. [PMC free article] [PubMed] [Google Scholar]
- 56.Monteith SJ, Medel R, Kassell NF, et al. Transcranial magnetic resonance-guided focused ultrasound surgery for trigeminal neuralgia: a cadaveric and laboratory feasibility study. J Neurosurg. 2013;118:319–328. doi: 10.3171/2012.10.JNS12186. [DOI] [PubMed] [Google Scholar]
- 57.McDannold N, Clement GT, Black P, Jolesz F, Hynynen K. Transcranial magnetic resonance imaging- guided focused ultrasound surgery of brain tumors: initial findings in 3 patients. Neurosurgery. 2010;66:323–332. doi: 10.1227/01.NEU.0000360379.95800.2F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Colen RR, Jolesz F, editors. MR-guided focused ultrasound of the brain. Berlin: Springer-Verlag; 2012. [Google Scholar]
- 59.Kinoshita M. Targeted drug delivery to the brain using focused ultrasound. Top Magn Reson Imaging. 2006;17:209–215. doi: 10.1097/RMR.0b013e3180332e79. [DOI] [PubMed] [Google Scholar]
- 60.Kinoshita M, McDannold N, Jolesz FA, Hynynen K. Noninvasive localized delivery of Herceptin to the mouse brain by MRI-guided focused ultrasound-induced blood-brain barrier disruption. Proc Natl Acad Sci U S A. 2006;103:11719–11723. doi: 10.1073/pnas.0604318103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Burgess A, Huang Y, Waspe AC, Ganguly M, Goertz DE, Hynynen K. High-intensity focused ultrasound (HIFU) for dissolution of clots in a rabbit model of embolic stroke. PLoS One. 2012;7:e42311. doi: 10.1371/journal.pone.0042311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Alkins R, Burgess A, Ganguly M, et al. Focused ultrasound delivers targeted immune cells to metastatic brain tumors. Cancer Res. 2013;73:1892–1899. doi: 10.1158/0008-5472.CAN-12-2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cho EE, Drazic J, Ganguly M, Stefanovic B, Hynynen K. Two-photon fluorescence microscopy study of cerebrovascular dynamics in ultrasound-induced blood-brain barrier opening. J Cereb Blood Flow Metab. 2011;31:1852–1862. doi: 10.1038/jcbfm.2011.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Samiotaki G, Konofagou EE. Dependence of the reversibility of focused- ultrasound-induced blood-brain barrier opening on pressure and pulse length in vivo. IEEE Trans Ultrason Ferroelectr Freq Control. 2013;60:2257–2265. doi: 10.1109/TUFFC.2013.6644731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jolesz F, McDannold N, Clement G, Kinoshita M, Fennessy F, Tempany C, editors. MRI-guided FUS and its clinical applications. Berlin: Springer Science + Business Media; 2008. [Google Scholar]
- 66.Wei KC, Chu PC, Wang HY, et al. Focused ultrasound-induced blood-brain barrier opening to enhance temozolomide delivery for glioblastoma treatment: a preclinical study. PLoS One. 2013;8:e58995. doi: 10.1371/journal.pone.0058995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Treat LH, McDannold N, Zhang Y, Vykhodtseva N, Hynynen K. Improved anti-tumor effect of liposomal doxorubicin after targeted blood-brain barrier disruption by MRI-guided focused ultrasound in rat glioma. Ultrasound Med Biol. 2012;38:1716–1725. doi: 10.1016/j.ultrasmedbio.2012.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.de Lau LM, Breteler MM. Epidemiology of Parkinson's disease. Lancet Neurol. 2006;5:525–535. doi: 10.1016/S1474-4422(06)70471-9. [DOI] [PubMed] [Google Scholar]
- 69.Lozano AM, Lipsman N. Probing and regulating dysfunctional circuits using deep brain stimulation. Neuron. 2013;77:406–424. doi: 10.1016/j.neuron.2013.01.020. [DOI] [PubMed] [Google Scholar]
- 70.Kinoshita M, McDannold N, Jolesz FA, Hynynen K. Targeted delivery of antibodies through the blood-brain barrier by MRI-guided focused ultrasound. Biochem Biophys Res Commun. 2006;340:1085–1090. doi: 10.1016/j.bbrc.2005.12.112. [DOI] [PubMed] [Google Scholar]
- 71.Banks WA, Terrell B, Farr SA, Robinson SM, Nonaka N, Morley JE. Passage of amyloid beta protein antibody across the blood-brain barrier in a mouse model of Alzheimer's disease. Peptides. 2002;23:2223–2226. doi: 10.1016/s0196-9781(02)00261-9. [DOI] [PubMed] [Google Scholar]
- 72.Burgess A, Ayala-Grosso CA, Ganguly M, Jordao JF, Aubert I, Hynynen K. Targeted delivery of neural stem cells to the brain using MRI-guided focused ultrasound to disrupt the blood-brain barrier. PLoS One. 2011;6:e27877. doi: 10.1371/journal.pone.0027877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Thevenot E, Jordao JF, O'Reilly MA, et al. Targeted delivery of self-complementary adeno-associated virus serotype 9 to the brain, using magnetic resonance imaging-guided focused ultrasound. Hum Gene Ther. 2012;23:1144–1155. doi: 10.1089/hum.2012.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mendelow AD, Gregson BA, Rowan EN, Murray GD, Gholkar A, Mitchell PM. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial lobar intracerebral haematomas (STICH II): a randomised trial. Lancet. 2013;382:397–408. doi: 10.1016/S0140-6736(13)60986-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Monteith SJ, Kassell NF, Goren O, Harnof S. Transcranial MR-guided focused ultrasound sonothrombolysis in the treatment of intracerebral hemorrhage. Neurosurg Focus. 2013;34:E14. doi: 10.3171/2013.2.FOCUS1313. [DOI] [PubMed] [Google Scholar]
- 76.Holscher T, Ahadi G, Fisher D, Zadicario E, Voie A. MR-guided focused ultrasound for acute stroke: a rabbit model. Stroke. 2013;44(6 Suppl. 1):S58–S60. doi: 10.1161/STROKEAHA.111.000688. [DOI] [PubMed] [Google Scholar]
- 77.Wright C, Hynynen K, Goertz D. In vitro and in vivo high-intensity focused ultrasound thrombolysis. Investig Radiol. 2012;47:217–225. doi: 10.1097/RLI.0b013e31823cc75c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Freeman W, Watts JW. Prefrontal lobotomy: The surgical relief of mental pain. Bull NY Acad Med. 1942;18:794–812. [PMC free article] [PubMed] [Google Scholar]
- 79.Freeman W, Watts JW. Prefrontal lobotomy; survey of 331 cases. Am J Med Sci. 1946;211:1–8. doi: 10.1097/00000441-194601000-00001. [DOI] [PubMed] [Google Scholar]
- 80.Freeman W, Watts JW. Psychosurgery. Prog Neurol Psychiatry. 1946;1:649–661. [PubMed] [Google Scholar]
- 81.Lipsman N, Sankar T, Downar J, Kennedy SH, Lozano AM, Giacobbe P. Neuromodulation for treatment-refractory major depressive disorder. CMAJ. 2014;186:33–39. doi: 10.1503/cmaj.121317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dougherty DD, Baer L, Cosgrove GR, et al. Prospective long-term follow-up of 44 patients who received cingulotomy for treatment-refractory obsessive-compulsive disorder. Am J Psychiatry. 2002;159:269–275. doi: 10.1176/appi.ajp.159.2.269. [DOI] [PubMed] [Google Scholar]
- 83.Dougherty DD, Weiss AP, Cosgrove GR, et al. Cerebral metabolic correlates as potential predictors of response to anterior cingulotomy for treatment of major depression. J Neurosurg. 2003;99:1010–1017. doi: 10.3171/jns.2003.99.6.1010. [DOI] [PubMed] [Google Scholar]
- 84.Hurwitz TA, Honey CR, Allen J, et al. Bilateral anterior capsulotomy for intractable depression. J Neuropsychiatry Clin Neurosci. 2012;24:176–182. doi: 10.1176/appi.neuropsych.11080189. [DOI] [PubMed] [Google Scholar]
- 85.Kondziolka D, Ong JG, Lee JY, Moore RY, Flickinger JC, Lunsford LD. Gamma knife thalamotomy for essential tremor. J Neurosurg. 2008;108:111–117. doi: 10.3171/JNS/2008/108/01/0111. [DOI] [PubMed] [Google Scholar]
- 86.Noren G. Long-term complications following gamma knife radiosurgery of vestibular schwannomas. Stereotact Funct Neurosurg. 1998;70(Suppl. 1):65–73. doi: 10.1159/000056408. [DOI] [PubMed] [Google Scholar]
- 87.Patel SR, Sheth SA, Mian MK, et al. Single-neuron responses in the human nucleus accumbens during a financial decision-making task. J Neurosci. 2012;32:7311–7315. doi: 10.1523/JNEUROSCI.0027-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lim SY, Hodaie M, Fallis M, Poon YY, Mazzella F, Moro E. Gamma knife thalamotomy for disabling tremor: a blinded evaluation. Arch Neurol. 2010;67:584–588. doi: 10.1001/archneurol.2010.69. [DOI] [PubMed] [Google Scholar]
- 89.Legon W, Sato TF, Opitz A, et al. Transcranial focused ultrasound modulates the activity of primary somatosensory cortex in humans. Nat Neurosci. 2014;17:322–329. doi: 10.1038/nn.3620. [DOI] [PubMed] [Google Scholar]
- 90.Scarcelli T, Jordao JF, O'Reilly MA, Ellens N, Hynynen K, Aubert I. Stimulation of hippocampal neurogenesis by transcranial focused ultrasound and microbubbles in adult mice. Brain Stimul. 2014;7:304–307. doi: 10.1016/j.brs.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jordao JF, Thevenot E, Markham-Coultes K, et al. Amyloid-beta plaque reduction, endogenous antibody delivery and glial activation by brain-targeted, transcranial focused ultrasound. Exp Neurol. 2013;248:16–29. doi: 10.1016/j.expneurol.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Alkins R, Huang Y, Pajek D, Hynynen K. Cavitation-based third ventriculostomy using MRI-guided focused ultrasound. J Neurosurg. 2013;119:1520–1529. doi: 10.3171/2013.8.JNS13969. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 1224 kb)