Abstract
Microglia/macrophages (M) are major contributors to postinjury inflammation, but they may also promote brain repair in response to specific environmental signals that drive classic (M1) or alternative (M2) polarization. We investigated the activation and functional changes of M in mice with traumatic brain injuries and receiving intracerebroventricular human bone marrow mesenchymal stromal cells (MSCs) or saline infusion. MSCs upregulated Ym1 and Arginase-1 mRNA (p < 0.001), two M2 markers of protective M polarization, at 3 and 7 d postinjury, and increased the number of Ym1+ cells at 7 d postinjury (p < 0.05). MSCs reduced the presence of the lysosomal activity marker CD68 on the membrane surface of CD11b-positive M (p < 0.05), indicating reduced phagocytosis. MSC-mediated induction of the M2 phenotype in M was associated with early and persistent recovery of neurological functions evaluated up to 35 days postinjury (p < 0.01) and reparative changes of the lesioned microenvironment. In vitro, MSCs directly counteracted the proinflammatory response of primary murine microglia stimulated by tumor necrosis factor-α + interleukin 17 or by tumor necrosis factor-α + interferon-γ and induced M2 proregenerative traits, as indicated by the downregulation of inducible nitric oxide synthase and upregulation of Ym1 and CD206 mRNA (p < 0.01). In conclusion, we found evidence that MSCs can drive the M transcriptional environment and induce the acquisition of an early, persistent M2-beneficial phenotype both in vivo and in vitro. Increased Ym1 expression together with reduced in vivo phagocytosis suggests M selection by MSCs towards the M2a subpopulation, which is involved in growth stimulation and tissue repair.
Electronic supplementary material
The online version of this article (doi:10.1007/s13311-014-0277-y) contains supplementary material, which is available to authorized users.
Keywords: Traumatic brain injury, Microglia, Macrophages, Mesenchymal stromal cells, Immunomodulation
Introduction
Traumatic brain injury (TBI) is the leading cause of mortality and disability among young people in high-income countries [1]. Mechanical “primary” injury is responsible only for a part of the subsequent neurologic damage. In the following days/weeks, waves of toxic cascades convert at-risk tissue into dead brain tissue, with a major impact on the final outcome [2]. However, TBI also induces lasting neurorestorative processes [3, 4], which contribute to the spontaneous recovery. Thus, promotion of endogenous restorative mechanisms may represent an interesting therapeutic approach. A critical review of TBI trial failures has highlighted the need to focus on strategies that simultaneously affect multiple injury mechanisms and foster repair [5, 6]. In this context, mesenchymal stromal cells (MSCs) that interact with parenchymal brain cells in multiple ways are promising candidates [7–10].
Mononuclear phagocytes (microglial cells, perivascular macrophages, and blood-borne macrophages, all referred to here as “M”) have a prominent role in tissue surveillance and the response to changes in brain homeostasis. Activated M are consistently detected in the pericontusional tissue after TBI [11, 12], but their specific contribution to the progression of injury is far from being completely elucidated [13–15]. In response to TBI, M are capable of adopting diverse, complex activation states, allowing them to participate in the cytotoxic response, but also in immune regulation and injury resolution [13, 16, 17]. These states can be classified into four main phenotypes: classically activated M1 phenotype, with cytotoxic properties; alternative activated M2a phenotype, with proregenerative functions; M2b immunoregulatory phenotype; and M2c deactivated phenotype. The activation state of M is reflected by the expression of cell surface antigens with recognized functions [18, 19] that can be used to characterize changes of microglial phenotypes over time after acute brain injury and to describe, in vivo, the environmental changes related to a specific M state [20].
We recently showed long-term protection (on sensorimotor and cognitive functions and anatomical damage up to 5 weeks) of human MSCs transplanted 24 h after TBI in mice [21]. Infused MSCs are able to reprogram the local inflammatory microenvironment from detrimental to beneficial, favoring endogenous neurorestorative mechanisms [9, 21]. Using the same protocol of injury and MSC infusion in the same experimental model, we assessed, in vivo, M activation and functional polarization after human bone marrow MSC treatment. Similarly to our previous study, we chose intracerebroventricular (ICV) infusion of MSCs. Notably, in patients with severe TBI, ICV cannulation is recommended by authoritative guidelines for intracranial pressure monitoring. Thus, this approach would allow the focal injection of MSCs without exposing the patient to additional, potentially harmful, surgical procedures.
We investigated 1) the M phenotype following MSC transplantation to injured mice; and 2) the involvement of M and their phagocytic activity in the protection induced by MSCs. In addition we assessed the direct ability of MSCs to drive a phenotypic switch in primary microglial cultures under inflammatory conditions.
Materials and Methods
Isolation and Culture of Human MSCs
The local institutional review board approved the study, and informed consent was obtained from healthy donors. MSCs were isolated and expanded ex vivo from bone marrow aspirates, as previously described [22]. Briefly, total nucleated cells were isolated from the washouts of sealed bone marrow collection bags and filters. Cells were plated, without further separation, at 800 × 103 cells/cm2 in Dulbecco’s modified Eagle medium (Lonza, Basel, Switzerland) supplemented with 5 % freshly thawed platelet lysate (PL), 2 mM L-glutamine (LiStarFish, Milan, Italy), and 1 % penicillin/streptomycin (Sigma-Aldrich, St. Louis, MO, USA). After 24 h, nonadherent cells were removed. Adherent cells were trypsinized after reaching 70–80 % confluence and seeded at 100–200 cells/cm2. MSCs at passage 3 were screened by flow cytometry for the expression of CD34, CD45, CD73, CD90, CD105, human leukocyte antigen-ABC, and CD11b. MSCs were also tested for their capacity to differentiate into adipocytes and osteoblasts. Cells were used for the experiments between P 3 and 5, and preparations from individual donors (n = 2) were injected into individual sham-operated/TBI mice.
In vivo Studies
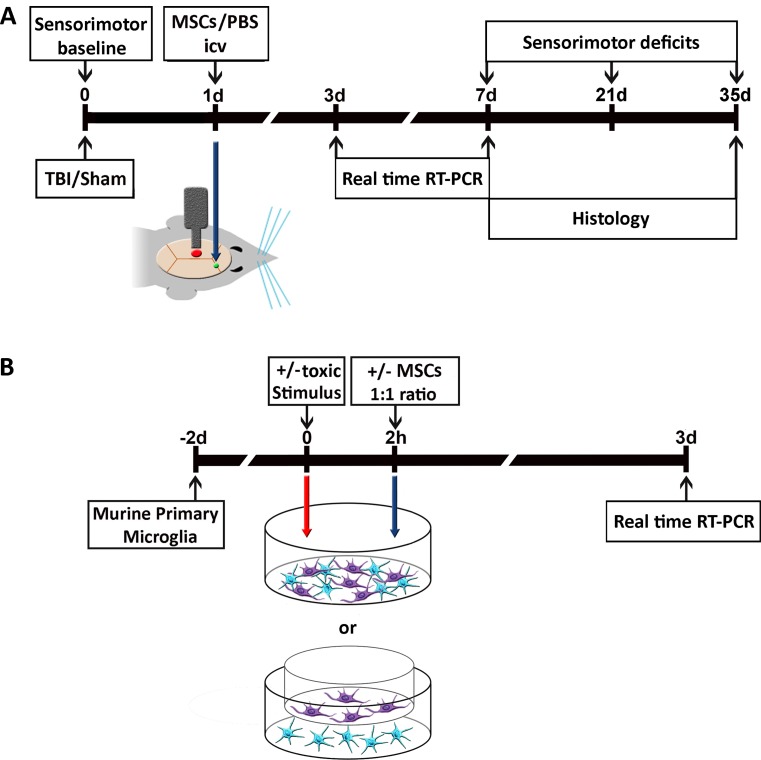
In vivo experiments were conducted according to the experimental design shown in Fig. 1A.
Fig. 1.
Experimental design of in vivo and in vitro experiments. (A) In vivo experiments: traumatic brain injury (TBI)/sham surgery was done 1 d before treatment. Mesenchymal stromal cells (MSCs) or phosphate buffered saline (PBS) were infused in the contralateral ventricle. Sensorimotor deficits were evaluated at 0, 7, 21, and 35 d. Animals were sacrificed at 3 or 7 d for real time reverse transcription (RT) polymerase chain reaction (PCR) or at 7 or 35 d for histological analysis. (B) In vitro experiments: primary murine microglial cells were cultured for 48 h then, at the timepoints indicated in the plan, were exposed to 1) proinflammatory stimuli [tumor necrosis factor (TNF)-α/interleukin (IL-17) or TNF-α/interferon (IFN)-γ] for M1 classical activation; 2) MSCs; 3) proinflammatory stimuli followed by either direct or indirect (transwell) MSC co-culture. Unexposed cultures served as controls. After 72 h, co-cultures were analyzed by real-time PCR or immunohistochemistry. icv = intracerebroventricular
Animals
Procedures involving animals and their care were conducted in conformity with the institutional guidelines at the IRCCS – Institute for Pharmacological Research “Mario Negri” in compliance with national (Decreto Legge nr 116/92, Gazzetta Ufficiale, supplement 40, February 18, 1992; Circolare nr 8, Gazzetta Ufficiale, July 14, 1994) and international laws and policies [EEC Council Directive 86/609, OJL 358, 1, Dec. 12, 1987; Guide for the Care and Use of Laboratory Animals, U.S. National Research Council, (Eighth Edition) 2011]. The protocol used and details of this study are also in accordance with Animal Research: Reporting In Vivo Experiments guidelines. Male C57Bl/6J mice (20–24 g; Harlan Laboratories, Milan, Italy) were housed in a specific pathogen-free vivarium at a constant temperature (21 ± 1 °C) with a 12-h light–dark cycle, and free access to food and water.
Experimental Brain Injury
Anesthetized mice (sodium pentobarbital, 65 mg/kg i.p.) were placed in a stereotaxic frame and subjected to craniectomy followed by induction of controlled cortical impact brain injury as previously described [9]. Briefly, a 3-mm rigid impactor driven by a pneumatic piston and rigidly mounted at an angle of 20° from the vertical plane was applied perpendicularly to the exposed dura mater over the left parieto-temporal cortex (antero-posteriority: –2.5 mm, laterality: –2.5 mm) at a velocity of 5 m/s and depth of 1 mm. The craniotomy was then covered with a cranioplasty and the scalp sutured. During all surgical procedures, the body temperature of the mice was maintained at the 37 °C. Sham-operated mice received identical anesthesia without brain injury.
MSC Preparation and Transplantation
MSCs were resuspended in phosphate-buffered saline (PBS), before transplantation. Cell number was evaluated by light microscopy. Viability of MSCs was evaluated by the Trypan blue exclusion test and cell concentration was adjusted to 150,000 cells/5 μl PBS. In a set of experiments, MSCs were labeled with PKH26 red fluorescence cell linker (Sigma-Aldrich), according to manufacturer’s instructions, in order to visualize cell localization and interaction with host tissue.
Twenty-four hours after surgery, a hole was drilled in the scalp of anesthetized mice, contralateral to the injured side at coordinates 0 mm caudal to bregma, 1 mm lateral to the midline, and 3 mm beneath the dura mater. MSCs were infused ICV over 5 min and the needle was left in place afterwards for another 5 min. Control mice were infused with PBS alone (5 μl) following the same procedures. No animals died after transplantation.
Sensorimotor Deficits
Sensorimotor deficits were evaluated by neuroscore and beam walk tests [9, 21, 23] before injury (day 0) and at 7, 21, and 35 days post-TBI. For neuroscore, animals were scored from 4 (normal) to 0 (severely impaired) for 1) forelimb function, 2) hind limb function, and 3) resistance to lateral pulsion, as previously described [9, 24]. The maximum score per animal is 12. The beam walk test measures the number of foot faults of a trained mouse walking twice on an elevated, narrow wooden beam (5 mm wide and 100 cm long). The best score is 0 [9, 23].
Real-Time Reverse Transcription Polymerase Chain Reaction
On day 3 or 7, mouse ipsilateral cortical areas (including all the tissue above the rhinal fissure [25]) were dissected out, rapidly frozen on dry ice, and stored at –80 °C until analysis. Total RNA was obtained from tissue specimen using Trizol reagent (Gibco BRL, Gaithersburg, MD, USA) [26]. Samples of total RNA (1.5 μg) were treated with DNAse (Applied Biosystems, Foster City, CA, USA) and reverse-transcribed with random hexamer primers using Multi-Scribe Reverse Transcriptase (TaqMan Reverse transcription reagents; Applied Biosystems). Real-time reverse transcription polymerase chain reaction (RT-PCR) was performed according to the manufacturer’s instructions. Expression of the following genes was analyzed: CD11b, TNFα, CD86, CD68, Ym1, Arginase-1, CD206, SOCS3, CCL2, IL-1β, IL-10, IGF1, VEGF, GFAP. β-Actin was used as the reference gene and relative gene expression levels were determined according to the manufacturer’s ΔΔCt method (Applied Biosystems). Primers were designed to match selectively mouse, but not human, sequences using Primer Express 2.0 software (Applied Biosystems) or Primer3 (http://bioinfo.ut.ee/primer3-0.4.0/) based on GenBank accession numbers (Table 1).
Table 1.
List of primers used for real-time reverse transcription polymerase chain reaction
| Gene | NCBI ref. sequence | Forward primer | Reverse primer |
|---|---|---|---|
| β-actin | NM_007393.3 | GCCCTGAGGCTCTTTTCCAG | TGCCACAGGATTCCATACCC |
| CD11b | AK089521 | GAGCAGCACTGAGATCCTGTTTAA | ATACGACTCCTGCCCTGGAA |
| TNF-α | NM_013693.2 | AGACCCTCACACTCAGATCATCTTC | TTGCTACGACGTGGGCTACA |
| CD86 | NM_019388.3 | GTTACTGTGGCCCTCCTCCTT | CTGATTCGGCTTCTTGTGACATA |
| CD68 | AK002264 | GGATTGGATTGAGGAAGGAACTG | GCCGCATGGCAGAGATG |
| Ym1 | NM_009892.2 | TCTGGTGAAGGAAATGCGTAAA | GCAGCCTTGGAATGTCTTTCTC |
| Arginase-1 | NM_007482.3 | CATGGGCAACCTGTGTCCTT | TCCTGGTACATCTGGGAACTTTC |
| CD206 | NM_008625.2 | CCCAAGGGCTCTTCTAAAGCA | CGCCGGCACCTATCACA |
| SOCS3 | NM_007707.3 | TTTCTTATCCGCGACAGCTC | GGATGCGTAGGTTCTTGGTC |
| CCL2 | NM_011333.3 | GCCTGCTGTTCACAGTTGC | ATTGGGATCATCTTGCTGGT |
| IL-1β | NM_008361.3 | AGTTGACGGACCCCAAAAGA | GGACAGCCCAGGTCAAAGG |
| IL-10 | NM_010548.2 | CGGCTGAGGCGCTGT | TGCCTTGCTCTTATTTTCACAGG |
| IGF1 | NM_001111274.1 | CACACTGACATGCCCAAGAC | CCTTTCCTTCTCCTTTGCAG |
| VEGF | NM_009505.4 | CCAGACCTCTCACCGGAAAG | CTGTCAACGGTGACGATGATG |
| GFAP | NM_001131020.1 | GAAAACCGCATCACCATTCC | TCGGATCTGGAGGTTGGAGA |
NCBI = National Center for Biotechnology Information.
Brain Transcardial Perfusion
Seven or 35 days after injury, mice were deeply anesthetized with Equitensin (120 μl/mouse i.p.) and transcardially perfused with 20 ml of PBS, 0.1 mol/l, pH 7.4, followed by 50 ml of chilled paraformaldehyde (4 %) in PBS. The brains were carefully removed from the skull, and transferred to 30 % sucrose in PBS at 4 °C overnight for cryoprotection. The brains were then rapidly frozen by immersion in isopentane at –45 °C for 3 min, sealed in vials, and stored at –70 °C until use.
Immunohistochemistry
Immunohistochemistry was done on 20-μm brain coronal sections using rat antimouse-CD11b (1:700; kindly provided by Dr. Doni), rat antimouse-CD68 (1:200; Serotec, Kidlington, UK), rat antimouse-CD45 (1:800; BD Pharmingen, Franklin Lakes, NJ, USA), rabbit antimouse-Ym1 (1:400; Stem Cell Technologies, Vancouver, Canada). Positive cells were stained by reaction with 3,3’-diaminobenzidine (Vector Laboratories, Burlingame, CA, USA) as previously described [20]. For negative control staining, the primary antibodies were omitted, and no staining was observed. Quantitative analysis was made in defined anatomic boundaries, acquiring the same focal plan throughout the samples [9, 27]. Unbiased, operator nondependent tissue sampling was done using a BX61 Olympus microscope equipped with a motorized stage and managed with AnalySIS software (Olympus, Tokyo, Japan). Quantification fields at 40× magnification were selected over 3 brain coronal sections per mouse, 0.4 mm posterior to bregma (12 fields), 1.6 mm posterior to bregma (12 fields), and 2.8 mm posterior to bregma (9 fields). The first row of fields was positioned at the contusion edge, spacing each field by 361.2 μm (distance between centres of the fields). A second and a third row of fields were positioned further from the lesion and aligned to the first row. The distance between each row was 722.4 μm. The immunostained area for each marker was measured using Fiji software [28], expressed as positive pixels/total assessed pixels, and reported as the percentage staining area for subsequent statistical analysis. Two independent investigators blinded to the identity of the samples did the immunohistochemical analysis of brain sections and quantification.
Immunofluorescence and Confocal Analysis
Immunofluorescence was done on 20-μm coronal sections as previously described [20, 29]. The primary antibodies used were antimouse CD11b (1:30000, kindly provided by Dr. Doni), antimouse Ym1 (1:400; Stem Cell Technologies), antimouse CD68 (1:200, Serotec) and antimouse growth associated protein 43 (GAP-43; 1:500 [30]). The fluoro-conjugated secondary antibodies used were Alexa 546 antirat, Alexa 594, or Alexa 647 antirabbit (all 1:500; Invitrogen, Carlsbad, CA, USA). Biotinylated antirat or antirabbit antibodies (1:200; Vector Laboratories) were also used, followed by fluorescent signal coupling with a streptavidin Tyramide Signal Amplification kit (cyanine 5; Perkin Elmer, Waltham, MA, USA). Appropriate negative controls were run without the primary antibodies. None of the immunofluorescence reactions gave an unspecific fluorescent signal in the negative controls. Immunofluorescence was acquired using a scanning sequential mode to avoid bleed-through effects by an IX81 microscope equipped with a motorized stage and a confocal scan unit FV500 with 3 laser lines [argon-kryton (488 nm), helium-neon red (646 nm), and helium-neon green (532 nm; Olympus)] and an ultraviolet diode. Co-localization was analyzed over 3-dimensional fields measuring 180 × 135 × 7 μm, obtained by stacking 31 confocal planes at a resolution of 800 × 600 resolution, distanced by a z-axis step of 0.23 μm. Three-dimensional fields were positioned over the same sections as for immunohistochemical analysis (0.4 mm, 1.6 mm, and 2.8 mm posterior to bregma) using the motorized stage under the control of xy Stage software (Olympus).
For each coronal section, 4 nonoverlapping fields, over a 2 × 2 matrix, for CD68/CD11b co-localization in the cortex, and 3 fields (1 × 3 matrix) for Ym1/CD68 co-localization in the hippocampus were aligned. Quantification of double-positive voxels (co-localization) was performed with Imaris (Bitplane, Bern, Switzerland) using the ImarisColoc algorithm [31]. Signal intensity over a volume with no positive staining (background) was calculated for green and red channels, and used as the lower signal threshold. Voxels that were over lower thresholds for both channels were co-localized. A co-localization channel (yellow) containing only co-localized voxels was generated and visualized by surface rendering (IsoSurface; Imaris) using the thresholds applied for co-localization analysis. Analysis was performed by an operator blinded to the study. Co-localization is expressed as percentage of double-positive voxels over total CD68 or Ym1-positive voxels.
Study Design and Blinding
C57Bl/6J male mice, divided into 4 equal experimental groups, were used: 1) SHAM PBS—sham-operated mice receiving PBS (5 μL, ICV) 24 h after surgery; 2) SHAM MSCs—sham-operated mice receiving MSCs (150,000/5 μL ICV) 24 h after surgery; 3) TBI PBS—TBI mice receiving PBS (5 μL ICV) 24 h after surgery; 4) TBI MSCs—TBI mice receiving MSCs (150,000/5 μL ICV) 24 h after surgery.
For real-time RT-PCR analysis, mice were euthanized 3 or 7 d (n = 8) postinjury. For immunohistochemistry analysis, mice (n = 6) were euthanized 7 or 35 d postinjury.
Mice were randomly allocated to surgery (sham-operated or TBI), taking care to distribute them equally across experimental days and batches to avoid systematic errors.
All surgeries and injuries were done by the same investigator, who was masked to the treatment of each mouse. At the end of the procedure, a second investigator assigned a masked code to each mouse (including the sham-operated group). MSCs or PBS treatment and all subsequent behavioral, biochemical, and histologic evaluations were done by investigators unaware of the injury or treatment status of the animals. Sham-operated mice were used as the contralateral hemiphere is not a proper control after TBI [9, 32].
In vitro Studies
In vitro experiments were done according to the design depicted in Figure 1B.
Microglial Cultures and In Vitro Polarization
Primary mouse microglial cells were isolated from mixed cultures of cortical and hippocampal astrocytes, established from P2 C57Bl/6J mouse pups and maintained in MEM supplemented with 20 % fetal calf serum and 5.5 g/l glucose (glial medium). To promote microglial proliferation, 1 week after plating, the culture medium was supplemented with granulocyte macrophage colony-stimulating factor produced from X-63 granulocyte macrophage colony-stimulating factor cells. After 5–7 d, microglia were harvested by shaking mixed glial cultures and seeded on polyornithine-coated glass coverslips (16 mm diameter, 1 × 105 cells) or 60-mm tissue Petri dishes (8 × 105 cells). To minimize activation, half of the medium in which microglia were kept after shaking from mixed cultures was replaced with fresh low serum (1 %) medium.
Two stimuli were used to drive M1 phenotypes. Microglia cells were exposed either to a combination of tumor necrosis factor (TNF)-α (200 U/ml) and interleukin (IL)-17 (500 U/ml), or to a combination of TNF-α (200 U/ml) and interferon (IFN)-γ (500 U/ml) [33]. Two hours after the toxic stimuli or in control conditions, microglia–MSC co-cultures were obtained by plating MSCs on purified microglia in a microglia:MSC ratio of 1:1, and were maintained in glial medium for 72 h. At the end of the treatment, microglia were washed and either harvested with TRIZOL (Invitrogen) for RT-PCR analysis or fixed with 4 % paraformaldehyde for immunocytochemistry. In a set of experiments, MSCs were co-cultured with microglia indirectly, using a 0.3-μm pore size transwell (Corning Life Sciences, Pittson, PA, USA).
Reverse Transcriptase-Coupled PCR
Total RNA was isolated from murine microglia or microglia–MSC co-cultures using Nucleo Spin miRNA kit (Macherey-Nagel GmbH & Co. Düren Germany) following the manufacturer’s protocol. To remove any contaminating genomic DNA, total RNA was digested with DNase. Complementary DNA (cDNA) synthesis was done using the SuperScriptIII RT-PCR system (Invitrogen) and random hexamers as the primer. The resulting cDNAs were amplified using a mouse-specific TaqMan Gene Expression Assay (Applied Biosystems). mRNA expression was normalized to the label of mouse-specific GAPDH mRNA. Mouse-specific TaqMan Assays were tested on MSC cDNA to confirm the specificity for murine sequences; their inability to detect human sequences and relative gene expression levels were determined according to the manufacturer’s ΔΔCt method (Applied Biosystems).
Cell Fluorescence Analysis
Pure microglial cultures and microglia–MSC co-cultures were fixed at room temperature for 25 min with 4 % paraformaldehyde in 0.1 M phosphate buffer containing 0.12 M sucrose. Fixed cells were permeabilized with detergent and labeled with anti-CD206 monoclonal Abs (1:100; AbD Serotec, Oxford, UK) and anti-YM1 polyclonal Abs (1:100; Stem Cell Technologies), followed by Alexa-488 antirat Abs (1:500) and Alexa-568 anti-rabbit Abs (1:200; all Invitrogen). In one set of experiments, MSCs co-cultured with microglia were identified by the mesenchymal cell marker CD105 (eBioscience, San Diego, CA, USA). The coverslips were mounted in 70 % glycerol in phosphate buffer containing 1 mg/ml phenylenediamine. Cells were observed with a Leica SP5 (Leica Microsystems, Wetzlar, Germany) confocal microscope.
Statistical Analysis
We used a standard software package for statistical analysis (GraphPad Prism version 6.0; GraphPad, San Diego, CA, USA). All data are presented as mean ± S.D. For sensorimotor deficits (neuroscore and beam walk tests), groups were compared using a two-way analysis of variance for repeated measures (RM), followed by Tukey’s test. For immunohistochemical analysis and in vitro studies of microglia in basal condition, groups were compared using the unpaired t test. For gene expression analysis groups were compared by a two-way ANOVA, followed by Tukey’s test. p-values < 0.05 were considered to be statistically significant. Assumptions of normality were checked using the Kolmogorov–Smirnov test.
Results
MSC Infusion Induces Protection on Sensorimotor Deficits 7 d After TBI
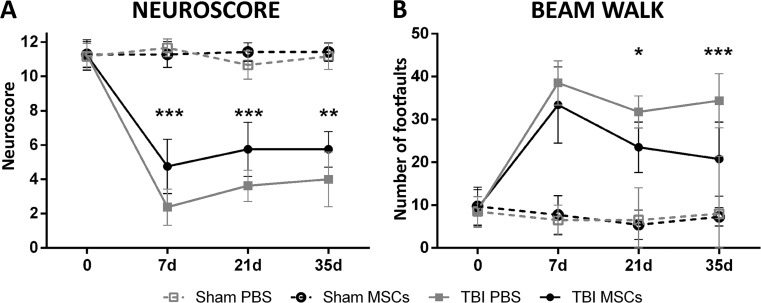
Using the same protocol of injury and MSC infusion, we have recently demonstrated that MSCs improve sensorimotor and cognitive dysfunctions induced by TBI and reduce the contusion volume at 5 weeks after surgery [21]. Here, we analyzed sensorimotor deficits at 7, 21, and 35 days after TBI, to evaluate early timepoints and to confirm efficacy later on. Significant protection after MSC infusion was observed with the neuroscore at every timepoint considered (7 d: TBI PBS: 2.37 ± 1.06, TBI MSCs: 4.75 ± 1.58, p < 0.001; 21 d: TBI PBS: 3.62 ± 0.91, TBI MSCs: 5.75 ± 1.58; 35 d: TBI PBS: 4.00 ± 1.60, TBI MSCs: 5.75 ± 1.03, p < 0.010; Fig. 2A) and with the beam walk test at 21 and 35 d (21 d: TBI PBS: 31.75 ± 3.73, TBI MSCs: 25.50 ± 5.88, p < 0.05; 35d: TBI PBS: 34.37 ± 6.30, TBI MSCs: 20.75 ± 8.65, p < 0.001; Fig. 2B).
Fig. 2.
Effects of infusion of mesenchymal stromal cells (MSCs) on sensorimotor deficits. Infusion of MSCs induced early and persistent improvement of sensorimotor deficits, as measured by (A) neuroscore or (B) beam walk tests. (A) Neuroscore test showed sensorimotor improvement in traumatic brain injury (TBI) MSCs mice from 7 d, while (B) the beam walk test showed a significant improvement of TBI MSCs from 21 d. Data are mean ± SD, n = 8, 2-way analysis of variance for RM followed by Tukey’s test. PBS = phosphate buffered saline
MSCs Drive the Upregulation of M2 Gene Expression at 3 and 7 d After TBI
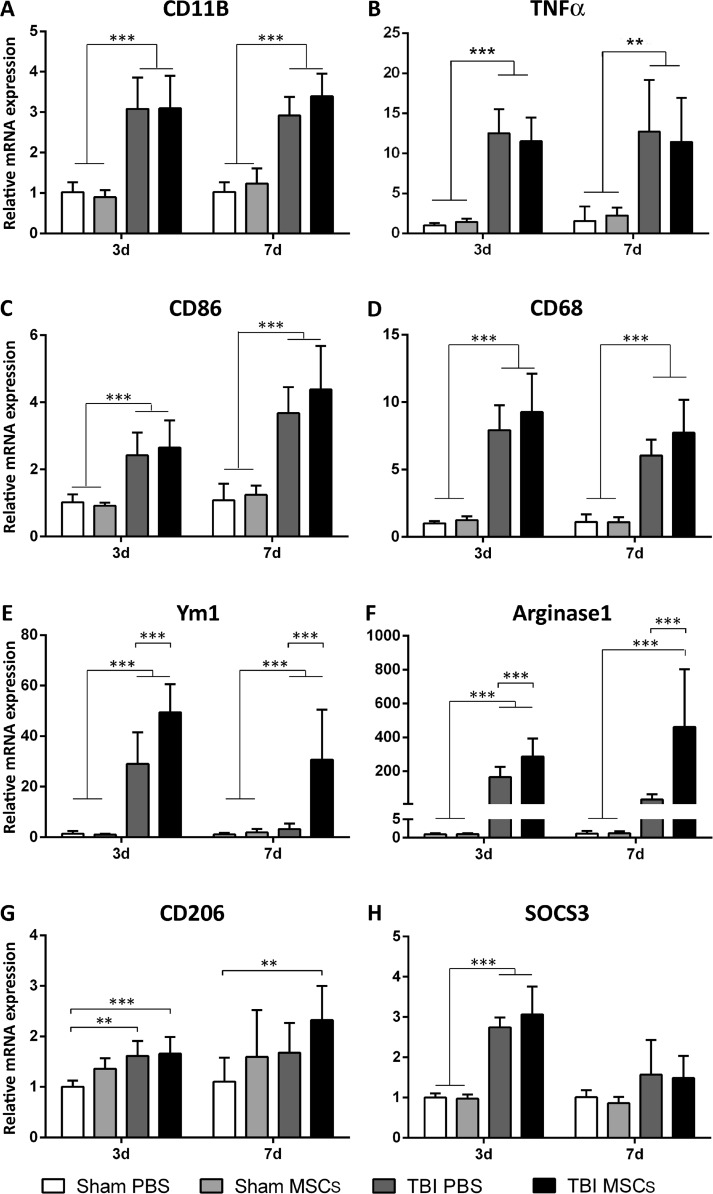
Three and 7 d after TBI, we measured the mRNA expression of CD11b, TNFα, and CD86 (markers of M activation), CD68 (a marker associated with the lysosomal activity of myeloid cells), and Ym1, Arginase-1, CD206, and SOCS3 (4 markers of M2a or 2c polarization). Compared with sham-operated mice, CD11b, TNFα, and CD86 showed induction at both timepoints after TBI (CD11b: 3.08 ± 0.77 at 3 d; 2.92 ± 0.46 at 7 d; TNFα: 12.51 ± 3.00 at 3 d; 12.73 ± 6.47 at 7 d; CD86: 2.42 ± 0.67 at 3 d; 3.67 ± 0.78 at 7d). No further increases were detected in TBI MSCs mice at both timepoints (CD11b: 3.10 ± 0.80 at 3 d; 3.39 ± 0.56 at 7 d; TNFα: 11.51 ± 2.96 at 3 d; 11.43 ± 5.50 at 7 d; CD86: 2.65 ± 0.81 at 3 d; 4.38 ± 1.30 at 7 d; Fig. 3A–C). Similar changes were observed for CD68 (7.92 ± 1.85 at 3 d, 6.05 ± 1.17 at 7 d), with no significant changes after MSC infusion (9.27 ± 2.85 at 3 d; 7.73 ± 2.45 at 7 d; Fig. 3D). Ym1, Arginase-1, and SOCS3 were significantly upregulated at 3 d (Ym1: 29.06 ± 12.50; Arginase-1: 165.1 ± 59.23; SOCS3: 2.74 ± 0.25), but not at 7 d (Ym1: 3.23 ± 2.22; Arginase-1: 32.45 ± 30.21; SOCS3: 1.57 ± 0.86), after TBI. Of note, TBI mice injected with MSCs showed a more marked and lasting increase in the Ym1 and Arginase-1, but not SOCS3 transcripts than TBI PBS mice (3 d: Ym1: 49.40 ± 11.18, +70 %; Arginase-1: 286.00 ± 106.70, +73 %; SOCS3: 3.06 ± 0.69, +11 %; 7 d: Ym1: 30.73 ± 19.75, +850 %; Arginase-1: 460.20 ± 342.10, +1300 %; SOCS3: 1.49 ± 0.55, –5 %; Fig. 3E, F, H). Compared with sham-operated mice, CD206 was transiently induced in TBI PBS at 3 d, but not at 7 d (3 d: 1.61 ± 0.30, 7 d: 1.68 ± 0.95), CD206 induction was longer lasting after infusion of MSCs (3 d: 1.66 ± 0.33, 7 d: 2.33 ± 0.67; Fig. 3G). MSCs in sham-operated mice did not experience a change in mRNA expression for any of the assessed genes.
Fig. 3.
mRNA expression of genes related to microglia activation and polarization in brain cortices, 3 and 7d after surgery. (A–D) CD11b, TNFα, CD86, and CD68 were significantly upregulated in traumatic brain injury (TBI) mice compared with sham-operated mice both at 3 and 7 d after surgery, with no difference between TBI phosphate-buffered saline (PBS) and TBI mesenchymal stromal cells (MSCs) mice. (E–H) Ym1, Arginase-1, SOCS3, and CD206 were significantly upregulated in TBI mice compared with sham-operated mice at 3 d, but not at 7 d, after surgery. Infusion of MSCs significantly increased the expression of Ym1 and Arginase-1 in TBI MSCs mice compared with TBI PBS mice at both timepoints considered. No difference in the mRNA expression of SOCS3 was found between TBI PBS mice and TBI MSCs mice. At 7 d, TBI MSCs mice showed increased expression of CD206 compared with sham PBS mice. Data are expressed as the fold induction compared with the sham PBS group. Data are mean ± SD, n = 8. **p < 0.01, ***p < 0.001 versus sham or TBI PBS mice. Two-way analysis of variance followed by Tukey’s test
MSC Infusion Induces Increased CD45high Cell Infiltration and Reduces M Lysosomal Activity in the Ipsilateral Cortex 7 d After TBI
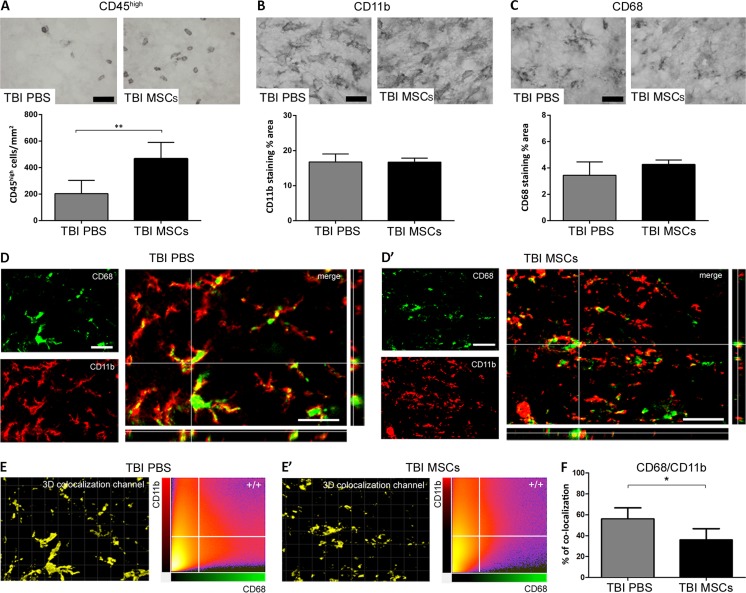
We measured the number of CD45high-positive cells 7 d after TBI by immunohistochemistry. CD45high cells were significantly increased in TBI MSCs mice compared with mice receiving PBS (Fig. 4A), indicating an increased infiltration of peripheral immune cells mediated by MSCs [34].
Fig. 4.
Immunohistochemical analysis of CD45, CD11b, and CD68, and quantification of their co-localization in the injured cortex. Representative micrographs of (A) CD45, (B) CD11b, and (C) CD68 immunostainings and their quantifications 7 d after traumatic brain injury (TBI). The number of CD45high cells was significantly increased in TBI mesenchymal stromal cells (MSCs) mice, whereas no differences were observed in the expression of either CD11b or CD68. Representative micrographs of CD68 (green) and CD11b (red) co-localization in (D) TBI phosphate-buffered saline (PBS) and (D’) TBI MSCs mice. (D, merge) In TBI PBS mice, CD68 often co-localized with the membrane marker CD11b, while in TBI MSCs mice (D’, merge) it remained mainly located in the cytoplasm, thus yielding less co-localization with CD11b. (E, E’) Quantification of co-localized voxels in the 3-dimensional confocal acquisitions showed a reduction of CD68/CD11b-positive voxels after infusion of MSCs, indicating a reduction of lysosomal activity in TBI MSCs mice compared with TBI PBS mice (F). Data are mean ± SD, n = 8 (A, B, E). *p < 0.05, unpaired t test. Bars = 20 μm
We measured the expression and co-localization of CD11b and CD68 7 d after TBI by immunohistological techniques. Neither marker quantitatively changed its protein expression after the administration of MSCs (Fig. 4B, C), in line with gene expression data (Fig. 3A–D). Analysis of 3-dimensional images revealed that CD68 was always expressed by CD11b + cells, as previously described [35]. As, during lysosomal activity, CD68 is localized at the plasma membrane [36, 37], we assessed the extent of CD68 co-localization with CD11b, the latter being a surface marker of myeloid cells. We found that the percentage of CD68 and CD11b double-positive voxels was 56.04 ± 10.56 in TBI PBS mice, and that it significantly decreased to 35.98 ± 10.71 following an infusion of MSCs (Figure 4D’, E’, F), thus suggesting a reduction in the phagocytic activity.
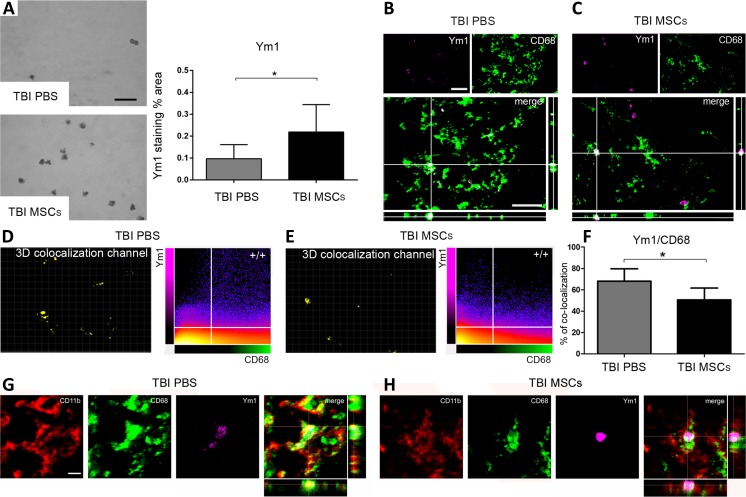
MSC Infusion Enhances the Presence of M2 Polarized Cells Within the Lesion Site 7 d After TBI
Ym1 protein expression in the ipsilateral cortex was significantly upregulated 7 d after TBI in mice infused with MSCs (staining percentage area in TBI PBS: 0.10 ± 0.06; TBI MSCs: 0.22 ± 0.13; Fig. 5A). We then measured Ym1 co-localization with CD68 by analyzing 3-dimensional immunofluorescence images (Fig. 5B, C). MSC infusion significantly reduced Ym1/CD68 co-localization (co-localized voxel percentage in TBI PBS: 68.14 ± 11.53; TBI MSCs: 50.57 ± 10.99; Fig. 5D–F). Furthermore triple immunofluorescence analysis revealed a different pattern of cellular CD68/CD11b co-localization in Ym1+ cells in TBI PBS versus TBI MSCs mice, with the latter showing a decrease in CD68 membrane localization (Fig. 5G, H).
Fig. 5.
Immunohistochemical analysis of Ym1 in the injured cortex and quantification of its co-localization with CD68 in the injured hippocampus. Representative micrographs of (A) Ym1 immunostaining 7 d after traumatic brain injury (TBI) in phosphate-buffered saline (PBS)- or mesenchymal stromal cells (MSCs) treated mice, and its related quantification, which shows an increase in the expression of Ym1 in TBI MSCs mice compared with TBI PBS mice. Co-localization of Ym1 (purple) and CD68 (green) in (B) TBI PBS and (C) TBI MSCs mice. Bar = 20 μm. (D, E) Quantification of co-localized voxels in the 3-dimensional (3D) confocal acquisitions showed a reduction of (F) Ym1/CD68-positive voxels after infusion of MSCs. Triple immunofluorescence for CD11b (red), CD68 (green), and Ym1 (purple) for (G) TBI PBS and (H) TBS MSCs mice. (G) In TBI PBS mice, co-localization between Ym1 and CD68 (white) is observed in cells with strong co-localization between CD68 and CD11b (yellow). Xyz-view and 3D renderings of co-localized pixels (centre and right panels) better illustrate this. (H) In TBI MSCs mice, cells with reduced Ym1/CD68 co-localization (white) show diminished CD68/CD11b co-localization. The Xyz-view and 3D renderings of co-localized pixels are shown in centre and right panels in (H). Bar = 5 μm. Data are mean ± SD, n = 8 (A, F). *p < 0.05, unpaired t test
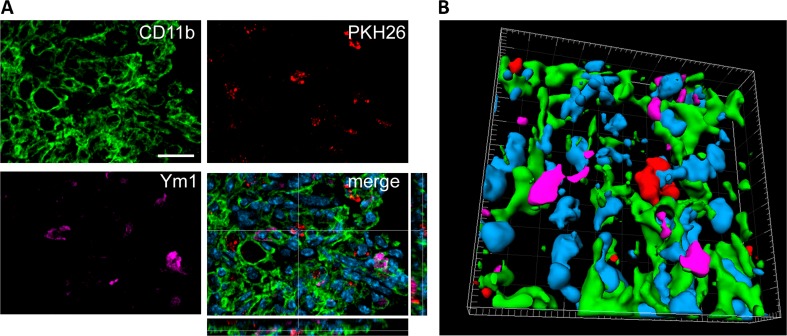
Physical contact between M2-polarized cells co-expressing CD11b and Ym1 and PKH26-labeled MSCs was observed in some, but not all, cases (Fig. 6), indicating that M2-induced polarization by MSCs does not require direct cell–cell contact.
Fig. 6.
Localization of CD11b/Ym1 double-positive cells with PKH26-labeled mesenchymal stromal cells (MSCs) in the injured cortex. (A) At 7 d after traumatic brain injury, in mice infused with MSCs, the cells positive for CD11b (green) and Ym1 (purple) showed direct contact with infused MSCs (PKH26, red, A), as better depicted in (B) the 3-dimensional rendering. Bar = 20 μm
Ym1 and CD68 protein expression in TBI mice infused with MSCs was dramatically decreased at 35d after TBI compared with 7 d (Ym1: –94 %, CD68: –80 %, data not shown), suggesting that these events played a role at early timepoints.
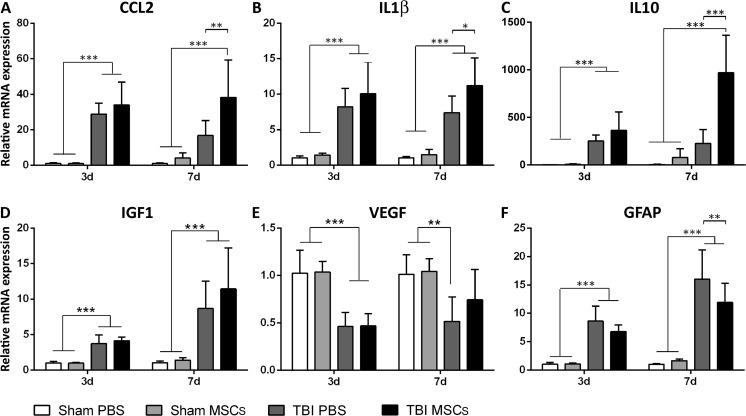
MSC Infusion Induces Early Microenvironmental Changes
We next assessed possible alterations of cytokine and growth factor gene expression after MSC infusion. At 3 d, TBI upregulated the cytokines CCL2 (28.88 ± 6.10), IL-1β (8.21 ± 2.62), and IL-10 (252.40 ± 61.82), and the growth factor IGF1 (3.73 ± 1.25), and also IL-1β (7.39 ± 2.36) and IGF1 (9.88 ± 4.95) at 7 d after injury compared with sham PBS mice (Fig. 7A–D). More importantly, TBI MSCs mice showed significant upregulation of CCL2 (38.21 ± 21.11; Fig. 7A), IL-1β (11.21 ± 3.89; Fig. 7B), and IL-10 (969.96 ± 392.72; Fig. 7C) on d 7 compared with the TBI PBS group. No significant differences were found in the gene expression of IGF1 at either times (Fig. 7D).
Fig. 7.
mRNA expression of cytokines and growth factors in brain cortices, 3 and 7 d after surgery. (A–D) CCL2, IL-1β, IL-10, and IGF1 were significantly upregulated in traumatic brain injury (TBI) compared with sham-operated mice at 3 d after surgery. (A–B–D) The upregulation of CCL2, IL-1β, and IGF1 in TBI mice persisted at 7 d after surgery. (A–D) There was no difference in the expression of CCL2, IL-1β, IL-10, and IGF1 between TBI phosphate buffered saline (PBS) and TBI MSCs mice at 3 d. (A–C) At 7 d, mesenchymal stromal cells (MSCs) infusion significantly increased the expression of CCL2, IL-1β, and IL-10 in TBI MSCs mice compared with TBI PBS mice, while (D) no difference was found in the expression of IGF1. (E) The expression of VEGF was downregulated in TBI PBS mice compared with sham-operated groups both at 3 d and 7 d. TBI MSCs mice showed a trend toward an increase in the expression of VEGF at 7d, restoring VEGF expression close to the levels observed in sham-operated animals. (F) The expression of GFAP was significantly upregulated in TBI mice compared with sham-operated mice at both 3 and 7 d after surgery. Infusion of MSCs induced a significant reduction in the expression of GFAP at 7 d after surgery. Data are shown as fold induction compared with sham PBS group and are mean ± SD, n = 8. **p < 0.01, ***p < 0.001 versus sham or TBI PBS, 2-way analysis of variance followed by Tukey’s test
We analyzed possible early changes in VEGF and GFAP gene expression in the cortical contusion core and bordering regions to clarify whether early acquisition of a beneficial M phenotype was associated with more general reprogramming of the microenviroment towards a proregenerative state, involving endothelial cells and astrocytes. As expected VEGF was lower in TBI PBS both 3 d (0.46 ± 0.15) and 7 d (0.52 ± 0.26) after surgery (Fig. 7E) compared with sham-operated mice. MSC infusion did not cause significant changes in TBI mice at d 3 (0.47 ± 0.13), but it increased the expression of VEGF, close to the levels observed in sham-operated animals at 7 d (0.74 ± 0.32; Fig. 7E), suggesting proangiogenic activity. In TBI mice, there was an 8.64 ± 2.62- and 16.01 ± 5.18-fold induction in GFAP at 3 and 7 d, respectively, compared with sham PBS mice, revealing a strong reaction of astrocytes to the mechanical insult. MSC infusion significantly reduced GFAP gene expression in TBI mice at both timepoints (3 d: 6.78 ± 1.17; 7 d: 11.92 ± 3.37; Fig. 7F).
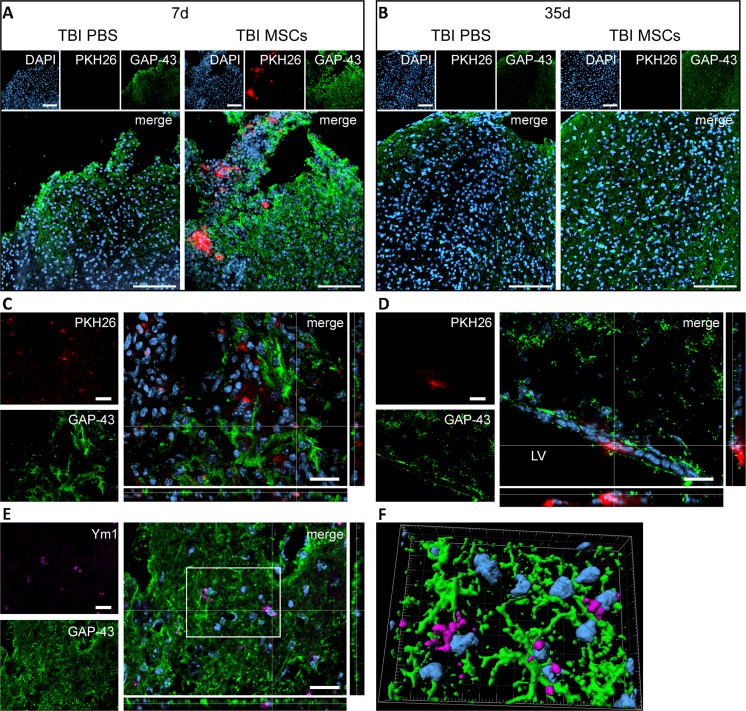
Interestingly, MSCs induced an increase in the immunofluorescence for GAP-43—a marker of axonal regeneration—in the contused cortex at both 7 and 35 d after TBI (qualitative observation based on 4 mice per condition; Fig. 8A, B). MSCs labeled with PKH26 prior to infusion were found only at 7 d. At this timepoint, PKH26-positive cells localized either far from GAP-43-positive cells or in close proximity to neurons undergoing axonal regeneration (Fig. 8C), with the latter localization being particularly evident in those cases in which MSCs reached the subventricular zone niche (Fig. 8D).
Fig. 8.
Localization of PKH26-labeled mesenchymal stromal cells (MSCs) and immunofluorescence for growth associated protein 43 (GAP-43) and Ym1 in the injured cortex. Representative micrographs at low magnification showing the presence of PKH26-labeled MSCs (red) in cortical areas positive for GAP-43 (green) at (A) 7 d or (B) 35 d after traumatic brain injury (TBI; nuclei are in blue, bar = 100 μm). PKH26 was visible only at 7 d in mice infused with MSCs, while no PKH26 positivity was detectable at 35 d in either phosphate-buffered saline (PBS)- or MSC-treated mice. GAP-43 appeared to be increased at 7 and 35 d in mice receiving MSCs. (C) At 7 d, in TBI MSCs mice, PKH26-positive cells were found either in association with GAP-43-positive cells or in areas negative for GAP-43. (D) When MSCs reached the neurogenic niche in the subventricular zone, they localized close to GAP-43-positive cells. (E) M2 polarized cells (Ym1-positive, purple) were located in areas positive for GAP-43 and showed strong association with GAP-43-positive cells [(F) 3-dimensional rendering]. Bars in (C–E) = 20 μm. DAPI = 4’6-diamidino-2-phenylindole; LV = lateral ventricle
M2-polarized cells (Ym1-positive) were present in areas showing intense GAP-43 staining and in contact with GAP-43-positive neurons (Fig. 8E, F).
Exposure to MSCs Switches Microglia from M1 to M2 Polarization in vitro
To establish a direct link between MSC infusion and changes in the activation state of M, we co-cultured primary murine microglial cells, under basal or inflammatory conditions, with MSCs.
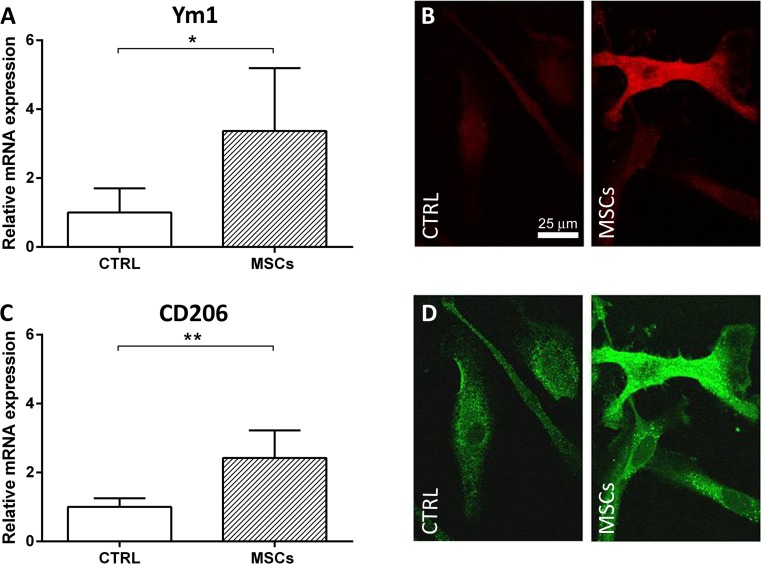
Exposure to MSCs significantly upregulated Ym1 (relative mRNA expression: 3.37 ± 1.82) and CD206 (relative mRNA expression: 2.42 ± 0.80) transcripts in microglia cells (Fig. 9A, C). The CD206 transcript was similarly upregulated when microglia were co-cultured with MSCs using the transwell system (relative mRNA expression: 2.75 ± 0.25). Accordingly, immunofluorescence analysis showed that 3 d after co-culturing with MSCs, a small proportion of microglial cells became positive for Ym1, while the vast majority had higher levels of CD206 protein expression than the pure microglial cultures maintained in basal conditions (Fig. 9B, D). No major changes in CD68 protein expression could be observed after co-culturing MSCs with microglia, which is characterized in vitro by a high basal expression of CD68.
Fig. 9.
In vitro analysis of microglia markers after exposure to mesenchymal stromal cells (MSCs). Microglia expression of (A, B) Ym1 and (C, D) CD206 in control conditions or in co-culture with MSCs for 72 h. (A, C) Quantification of mRNA expression indicates an upregulation of both M2 markers induced by MSC co-culture. Representative confocal images of pure microglial cultures and microglia co-cultured with MSCs stained for (B) Ym1 and (D) CD206. Data are mean ± SD from three independent experiments.*p < 0.05, **p < 0.01, unpaired t test. CTRL = control
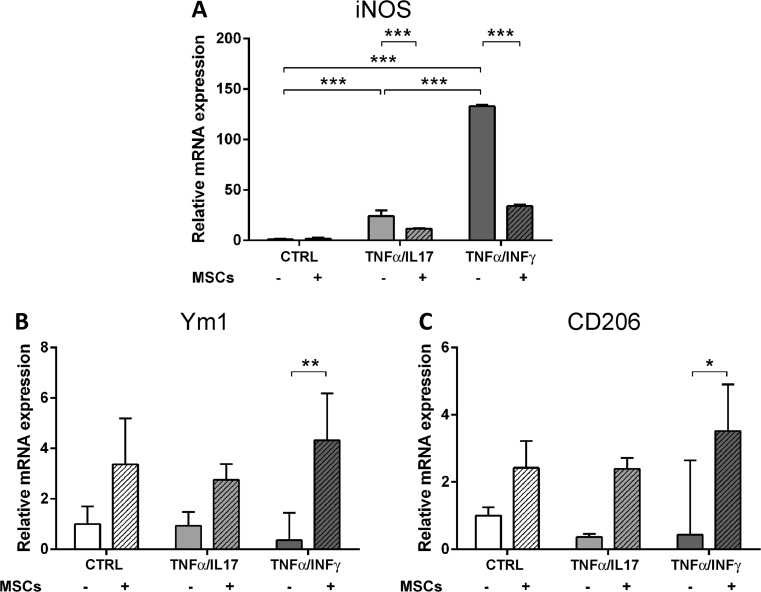
Next, we used 2 different proinflammatory stimuli (TNF-α/IL-17 or TNF-α/IFN-γ) to verify the ability of MSCs to revert directly the M1 phenotype acquired by microglia after an inflammatory challenge and to steer traits towards M2 polarization (see experimental plan, Fig. 1B). As expected, exposure to both proinflammatory stimuli upregulated transcription of inducible nitric oxide synthase (iNOS) (TNF-α/IL-17: 24.06 ± 5.68; TNF-α/IFN-γ: 133.00 ± 1.50; Fig. 10A) compared with controls. When MSCs were applied to microglia 2 h after the proinflammatory stimulus, they reverted the upregulation of iNOS (TNF-α/IL-17 + MSCs: 11.61 ± 0.39; TNF-α/IFN-γ + MSCs: 34.00 ± 1.47; Fig. 10A), and increased Ym1 and CD206 when considering the most effective proinflammatory challenge, namely TNF-α/INF-γ (Ym1: TNF-α/IFN-γ: 0.36 ± 1.09, TNF-α/IFN-γ + MSCs: 4.32 ± 1.86; CD206: TNF-α/IFN-γ: 0.44 ± 2.20, TNF-α/IFN-γ + MSCs: 3.52 ± 1.39; Fig. 10B, C). Similar results were obtained following indirect co-culture of microglia challenged with TNF-α/IL-17 with MSCs (iNOS expression: –60 % in direct co-cultures; –49 % in transwell system; CD206 expression: +300 % in direct co-cultures; +460 % in transwell system).
Fig. 10.
In vitro analysis of M1 and M2 polarization markers following exposure to proinflammatory stimuli and mesenchymal stromal cells (MSCs). mRNA expression for (A) iNOS, (B) Ym1, and (C) CD206 in control or activated [exposed to tumor necrosis factor (TNF)-α/interleukin (IL)-17 or TNF-α/interferon (IFN)-γ] microglia, maintained in vitro in isolation or co-cultured with MSCs. Data are mean ± SD from 3 independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001 2-way analysis of variance followed by Tukey’s test
Discussion
We found that ICV infusion of MSCs after TBI induces early and lasting acquisition of protective M2 traits by M. MSC-induced M2 polarization is indicated by 1) upregulation of the M2 expression markers Ym1, Arginase-1, and CD206 in mice 3 and 7 d after TBI, and 2) reduction of the lysosomal activity in M at the contusion core and bordering regions 7 d after TBI. This phenotypic profile suggests selection of M towards the M2a subpopulation, characterized by proregenerative activity and reduced phagocytosis [38]. Induction of an M beneficial phenotype by MSCs is associated with other proregenerative changes of the lesioned microenvironment, and with early and persistent recovery of neurologic functions. These results were obtained by infusing human MSCs, the most relevant cells from a translational perspective, into the mouse-injured brain [39]. Indeed, we recently reported the absence of innate inflammatory responses after human MSC infusion in immunocompetent mice [21]. Importantly, we have established a direct link between MSC activity and M phenotypical switch in an in vitro co-culture model, where MSCs were able to reverse the M1 proinflammatory phenotype acquired by microglia after 2 independent inflammatory challenges and to steer traits towards M2 polarization.
By interacting with other inflammatory cells, resident microglia and recruited monocytes retain a prominent role in post-injury damage, contributing to both pathogenic and protective mechanisms. M can directly protect neurons [40] and participate in hippocampal neurogenic processes after brain injury [41]. In addition, M eliminate invading neutrophils by phagocytosis [42], though they are a target for infiltrated T cells, which can orchestrate their state of polarization [43], allowing M to participate in the cytotoxic response, immune regulation, and injury resolution. The M2 phenotype is acquired early after acute brain injury, but vanishes very soon, tipping the balance toward the M1 phenotype, which contributes to excessive inflammatory and immune reactions exacerbating injury progression [19, 35]. Our results, in line with this evidence, show the transient upregulation of M2 markers (at 3 d, but not at 7 d post-TBI) in TBI PBS compared with sham-operated mice. More importantly, we showed that MSC infusion skews M at the injured site by enhancing the amount of “alternatively activated” M positive for M2 markers both 3 and 7 d after TBI.
One previous study reported that intravenous administration of multipotent adult progenitor cells (MAPCs) raised the percentage of T regulatory cells in the periphery (spleen and blood), and the ratio of M2:M1 macrophages in the brain. Remarkably, it also reported that direct contact between MAPCs and splenocytes is required for modulation of parenchymal microglia after the administration of MAPCs, thereby indicating a central role of the spleen in MAPC-mediated neuroprotection [44]. Conversely, our data provide evidence that MSCs can also act as a local “bioreactor” within the brain. In our model, ICV-injected MSCs can be detected both in the ventricles and at the lesion site in TBI mice, while they are confined in the ventricles in sham-operated mice [21], supporting a local action of MSCs on host M.
Phenotypic M changes are associated with increased recruitment of CD45high-positive infiltrating monocytes, with no major changes in overall CD11b immunoreactivity in TBI mice infused with MSCs. These data indicate a crucial role of infiltrating monocytes in switching the inflammatory environment towards a protective phenotype upon MSC administration.
In vitro experiments with MSCs and purified microglial cells show that MSCs directly upregulate the polarization of M2 and counteract the microglia reaction to an inflammatory challenge by switching microglia from a cytotoxic phenotype to a beneficial one. Moreover, data obtained in a transwell system, together with the analysis of the physical interaction between MSCs and the M2 cells in the injured brain, strongly suggest that MSCs control the polarization of M through the release of active molecules rather than through cell–cell contact [9, 44]. Our data extend previous in vitro evidence obtained using lipopolysaccharide-stimulated BV2 cells and MSCs [45, 46], showing reductions in proinflammatory mediators (e.g., iNOS, TNF-α, and IFN-γ) after exposure to MSCs.
To gain insight into the functional meaning of M polarization in vivo, we analyzed the expression of CD68, a lysosomal glycoprotein associated with phagocytic function [36, 47] and involved in lysosomal trafficking [48, 49]. CD68 was similarly expressed at both the mRNA and protein level in TBI mice infused or not with MSCs. However, the cellular distribution of the antigen differed after MSC infusion. Indeed, quantitative confocal analysis showed that the expression of CD68, which normally sits in the cytoplasm, was decreased at the membrane level after MSC transplantation. Notably, CD68 on the cell surface is associated with an active scavenging commitment, in line with surface functions, such as internalization of target ligands by elicited macrophages [37]. The decrease of CD68 on the cell membrane after the infusion of MSCs suggests a functional switch of M towards a less active phagocytic cell population. The role of phagocytosis in the lesion progression is complex and, unlike other M markers, phagocytic activity does not seem to be clearly linked to a specific M1 or M2 state of polarization. Cells belonging to the M2c class of protective polarization, as well as M1 toxic cells, all have phagocytic functions [38]. On the one hand, phagocytosis is needed to remove cell debris and dying cells, thus limiting the propagation of danger signals that can further exacerbate damage progression (“secondary phagocytosis”). On the other hand, under certain conditions such as inflammation, phagocytosis can target viable neurons, thus causing their death (“primary phagocytosis”). After injury, such detrimental phagocytic activity may result from exposure of eat-me signals on otherwise viable neurons as a result of subtoxic and reversible insult [50, 51]. The ability of MSCs to influence and limit “primary phagocytosis” might be a key pathway to confer protection after TBI. The complexity of in vivo phagocytosis accounts for the apparently divergent results obtained in vitro by Giunti et al. [45], who reported that MSCs enhance the phagocytic activity of LPS-activated microglia.
To further characterize the functional state of M after TBI and infusion of MSCs, we quantified Ym1 protein expression and Ym1/CD68 co-localization. MSCs significantly increased the number of Ym1+ cells, but it reduced their expression of CD68, suggesting that this M population may belong to the M2a subtype (characterized by the expression of Ym1, Arg1, and CD206), which is involved in growth stimulation and tissue repair [38], but not in phagocytic activity.
Gene expression in the pericontusional tissue showed that MSC administration was also associated with a more general reprogramming of the microenvironment likely involving other parenchymal cell populations. In fact, MSC infusion reduces GFAP and attenuates down-regulation of VEGF, and increases immunoreactivity of the proregenerative axonal regeneration marker GAP-43. These changes are consistent with data showing reduction of the gliotic scar and increase in vessel density described by our group [9, 21] and others [52–54]. Analysis of inflammatory cytokines and chemokines showed significant upregulation of IL-1β after infusion of MSCs at 7 d after TBI. A similar increase in IL-1β was reported in BV2 cells challenged with LPS after co-culturing of MSCs [45]. Furthermore, data from a model of spinal cord injury indicate that IL-1β may support the induction of alternative M activation [55], suggesting a role in injury resolution and brain repair, as well as being involved in the proinflammatory response. We also detected significant upregulation of CCL2 after infusion of MSCs in TBI mice at 7 d. CCL2 promotes chemotaxis of monocytes and hematopoietic progenitors to inflammation sites [56, 57] and may therefore indicate increased mobilization and infiltration of peripheral immune cells by MSCs. Consistently, we show here a significant increase in CD45high-positive infiltrating monocytes in the injured tissue. This could explain the modified inflammatory milieu that is potentially more prone to promote M2 polarization. Indeed, among infiltrating immune cells, as mentioned previously, lymphocytes are recognized orchestrators of M polarization. In particular regulatory T cells and T helper 2 cells, through the release of IL-4, are able to induce the M2 phenotype [43, 58, 59]. Finally, CXC chemokines are recognized as regulatory linkers between inflammation and angiogenesis, providing fine-tuning that leads to tissue repair [60]. Globally, these data indicate that MSCs, besides inducing beneficial traits in M, act on other brain cell populations, skewing the balance of the microenvironment towards protection.
In conclusion, MSCs induce an early, lasting acquisition of a protective M2 phenotype both in vivo and in vitro. Elevated M expression of Ym1 and a reduction of phagocytosis suggest polarization towards the M2a subpopulation, which is involved in growth stimulation and tissue repair. Protective/proregenerative changes of the lesioned microenvironment occur early after injury and may contribute to lasting protective and remodeling processes.
Electronic supplementary material
(PDF 1224 kb)
(PDF 1224 kb)
Acknowledgments
This work was supported by Progetto ricerca finalizzata FR-CCM-2008-1248388 and FISM2012/R/17 to C.V. We thank Professor Maria Pia Abbracchio and Dr. Davide Lecca, 20133 University of Milan, for useful discussions; and Ilaria Prada (IN CNR, Milan) for assistance with some of the experiments. We also thank the association “Esserci con Cate per i Bimbi”, which supported this work.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Contributor Information
Claudia Verderio, Email: c.verderio@in.cnr.it.
Maria-Grazia De Simoni, Email: desimoni@marionegri.it.
References
- 1.Lingsma HF, Roozenbeek B, Steyerberg EW, Murray GD, Maas AIR. Early prognosis in traumatic brain injury: from prophecies to predictions. Lancet Neurol. 2010;9:543–554. doi: 10.1016/S1474-4422(10)70065-X. [DOI] [PubMed] [Google Scholar]
- 2.Xiong Y, Mahmood A, Chopp M. Neurorestorative treatments for traumatic brain injury. Discov Med. 2010;10:434–442. [PMC free article] [PubMed] [Google Scholar]
- 3.Christie KJ, Turnley AM. Regulation of endogenous neural stem/progenitor cells for neural repair-factors that promote neurogenesis and gliogenesis in the normal and damaged brain. Front Cell Neurosci. 2012;6:70. doi: 10.3389/fnhum.2012.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xiong Y, Mahmood A, Meng Y, et al. Delayed administration of erythropoietin reducing hippocampal cell loss, enhancing angiogenesis and neurogenesis, and improving functional outcome following traumatic brain injury in rats: comparison of treatment with single and triple dose. J Neurosurg. 2010;113:598–608. doi: 10.3171/2009.9.JNS09844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loane DJ, Faden AI. Neuroprotection for traumatic brain injury: translational challenges and emerging therapeutic strategies. Trends Pharmacol Sci. 2010;31:596–604. doi: 10.1016/j.tips.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maas AIR, Stocchetti N, Bullock R. Moderate and severe traumatic brain injury in adults. Lancet Neurol. 2008;7:728–741. doi: 10.1016/S1474-4422(08)70164-9. [DOI] [PubMed] [Google Scholar]
- 7.Ohtaki H, Ylostalo JH, Foraker JE, et al. Stem/progenitor cells from bone marrow decrease neuronal death in global ischemia by modulation of inflammatory/immune responses. Proc Natl Acad Sci U S A. 2008;105:14638–14643. doi: 10.1073/pnas.0803670105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sarnowska A, Braun H, Sauerzweig S, Reymann KG. The neuroprotective effect of bone marrow stem cells is not dependent on direct cell contact with hypoxic injured tissue. Exp Neurol. 2009;215:317–327. doi: 10.1016/j.expneurol.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 9.Zanier ER, Montinaro M, Vigano M, et al. Human umbilical cord blood mesenchymal stem cells protect mice brain after trauma. Crit Care Med. 2011;39:2501–2510. doi: 10.1097/CCM.0b013e31822629ba. [DOI] [PubMed] [Google Scholar]
- 10.Nakajima H, Uchida K, Guerrero AR, et al. Transplantation of mesenchymal stem cells promotes an alternative pathway of macrophage activation and functional recovery after spinal cord injury. J Neurotrauma. 2012;29:1614–1625. doi: 10.1089/neu.2011.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar A, Loane DJ. Neuroinflammation after traumatic brain injury: Opportunities for therapeutic intervention. Brain Behav Immun. 2012;26:1191–1201. doi: 10.1016/j.bbi.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 12.Kumar A, Stoica BA, Sabirzhanov B, Burns MP, Faden AI, Loane DJ. Traumatic brain injury in aged animals increases lesion size and chronically alters microglial/macrophage classical and alternative activation states. Neurobiol Aging. 2013;34:1397–1411. doi: 10.1016/j.neurobiolaging.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shechter R, Schwartz M. Harnessing monocyte-derived macrophages to control central nervous system pathologies: no longer “if” but “how”. J Pathol. 2013;229:332–346. doi: 10.1002/path.4106. [DOI] [PubMed] [Google Scholar]
- 14.Lai AY, Todd KG. Differential regulation of trophic and proinflammatory microglial effectors is dependent on severity of neuronal injury. Glia. 2008;56:259–270. doi: 10.1002/glia.20610. [DOI] [PubMed] [Google Scholar]
- 15.Madinier A, Bertrand N, Mossiat C, et al. Microglial involvement in neuroplastic changes following focal brain ischemia in rats. PLoS ONE. 2009;4:e8101. doi: 10.1371/journal.pone.0008101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci. 2009;29:13435–13444. doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Longhi L, Perego C, Ortolano F, et al. Tumor necrosis factor in traumatic brain injury: effects of genetic deletion of p55 or p75 receptor. J Cereb Blood Flow Metab. 2013;33:1182–1189. doi: 10.1038/jcbfm.2013.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chhor V, Le Charpentier T, Lebon S, et al. Characterization of phenotype markers and neuronotoxic potential of polarised primary microglia in vitro. Brain Behav Immun. 2013;32:70–85. doi: 10.1016/j.bbi.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu X, Li P, Guo Y, et al. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke. 2012;43:3063–3070. doi: 10.1161/STROKEAHA.112.659656. [DOI] [PubMed] [Google Scholar]
- 20.Fumagalli S, Perego C, Ortolano F, De Simoni M-G. CX3CR1 deficiency induces an early protective inflammatory environment in ischemic mice. Glia. 2013;61:827–842. doi: 10.1002/glia.22474. [DOI] [PubMed] [Google Scholar]
- 21.Pischiutta F, D’Amico G, Dander E, et al. Immunosuppression does not affect human bone marrow mesenchymal stromal cell efficacy after transplantation in traumatized mice brain. Neuropharmacology. 2014;79:119–126. doi: 10.1016/j.neuropharm.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 22.Dander E, Lucchini G, Vinci P, et al. Mesenchymal stromal cells for the treatment of graft-versus-host disease: understanding the in vivo biological effect through patient immune monitoring. Leukemia. 2012;26:1681–1684. doi: 10.1038/leu.2011.384. [DOI] [PubMed] [Google Scholar]
- 23.Zanier ER, Pischiutta F, Villa P, et al. Six-month ischemic mice show sensorimotor and cognitive deficits associated with brain atrophy and axonal disorganization. CNS Neurosci Ther. 2013;19:695–704. doi: 10.1111/cns.12128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ortolano F, Colombo A, Zanier ER, et al. c-Jun N-terminal kinase pathway activation in human and experimental cerebral contusion. J Neuropathol Exp Neurol. 2009;68:964–971. doi: 10.1097/NEN.0b013e3181b20670. [DOI] [PubMed] [Google Scholar]
- 25.Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. Academic Press, San Diego, CA, USA, 2004.
- 26.Capone C, Frigerio S, Fumagalli S, et al. Neurosphere-derived cells exert a neuroprotective action by changing the ischemic microenvironment. PLoS ONE. 2007;2:e373. doi: 10.1371/journal.pone.0000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Donnelly DJ, Gensel JC, Ankeny DP, van Rooijen N, Popovich PG. An efficient and reproducible method for quantifying macrophages in different experimental models of central nervous system pathology. J Neurosci Methods. 2009;181:36–44. doi: 10.1016/j.jneumeth.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schindelin J, Arganda-Carreras I, Frise E, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gesuete R, Storini C, Fantin A, et al. Recombinant C1 inhibitor in brain ischemic injury. Ann Neurol. 2009;66:332–342. doi: 10.1002/ana.21740. [DOI] [PubMed] [Google Scholar]
- 30.Curtis R, Hardy R, Reynolds R, Spruce BA, Wilkin GP. Down-regulation of GAP-43 During Oligodendrocyte Development and Lack of Expression by Astrocytes In Vivo: Implications for Macroglial Differentiation. Eur J Neurosci. 1991;3:876–886. doi: 10.1111/j.1460-9568.1991.tb00099.x. [DOI] [PubMed] [Google Scholar]
- 31.Riglar DT, Rogers KL, Hanssen E, et al. Spatial association with PTEX complexes defines regions for effector export into Plasmodium falciparum-infected erythrocytes. Nat Commun. 2013;4:1415. doi: 10.1038/ncomms2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Longhi L, Perego C, Ortolano F, et al. C1-inhibitor attenuates neurobehavioral deficits and reduces contusion volume after controlled cortical impact brain injury in mice. Crit Care Med. 2009;37:659–665. doi: 10.1097/CCM.0b013e318195998a. [DOI] [PubMed] [Google Scholar]
- 33.Verderio C, Muzio L, Turola E, et al. Myeloid microvesicles are a marker and therapeutic target for neuroinflammation. Ann Neurol. 2012;72:610–624. doi: 10.1002/ana.23627. [DOI] [PubMed] [Google Scholar]
- 34.Stein VM, Baumgärtner W, Schröder S, Zurbriggen A, Vandevelde M, Tipold A. Differential expression of CD45 on canine microglial cells. J Vet Med A Physiol Pathol Clin Med. 2007;54:314–320. doi: 10.1111/j.1439-0442.2007.00926.x. [DOI] [PubMed] [Google Scholar]
- 35.Perego C, Fumagalli S, De Simoni M-G. Temporal pattern of expression and colocalization of microglia/macrophage phenotype markers following brain ischemic injury in mice. J Neuroinflammation. 2011;8:174. doi: 10.1186/1742-2094-8-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramprasad MP, Terpstra V, Kondratenko N, Quehenberger O, Steinberg D. Cell surface expression of mouse macrosialin and human CD68 and their role as macrophage receptors for oxidized low density lipoprotein. Proc Natl Acad Sci U S A. 1996;93:14833–14838. doi: 10.1073/pnas.93.25.14833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kurushima H, Ramprasad M, Kondratenko N, Foster DM, Quehenberger O, Steinberg D. Surface expression and rapid internalization of macrosialin (mouse CD68) on elicited mouse peritoneal macrophages. J Leukoc Biol. 2000;67:104–108. doi: 10.1002/jlb.67.1.104. [DOI] [PubMed] [Google Scholar]
- 38.David S, Kroner A. Repertoire of microglial and macrophage responses after spinal cord injury. Nat Rev Neurosci. 2011;12:388–399. doi: 10.1038/nrn3053. [DOI] [PubMed] [Google Scholar]
- 39.Franquesa M, Hoogduijn MJ, Reinders ME, et al. Mesenchymal Stem Cells in Solid Organ Transplantation (MiSOT) Fourth Meeting: lessons learned from first clinical trials. Transplantation. 2013;96:234–238. doi: 10.1097/TP.0b013e318298f9fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lambertsen KL, Clausen BH, Babcock AA, et al. Microglia protect neurons against ischemia by synthesis of tumor necrosis factor. J Neurosci. 2009;29:1319–1330. doi: 10.1523/JNEUROSCI.5505-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sierra A, Encinas JM, Deudero JJP, et al. Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell. 2010;7:483–495. doi: 10.1016/j.stem.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Denes A, Vidyasagar R, Feng J, et al. Proliferating resident microglia after focal cerebral ischaemia in mice. J Cereb Blood Flow Metab. 2007;27:1941–1953. doi: 10.1038/sj.jcbfm.9600495. [DOI] [PubMed] [Google Scholar]
- 43.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122:787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walker PA, Bedi SS, Shah SK, et al. Intravenous multipotent adult progenitor cell therapy after traumatic brain injury: modulation of the resident microglia population. J Neuroinflammation. 2012;9:228. doi: 10.1186/1742-2094-9-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Giunti D, Parodi B, Usai C, et al. Mesenchymal stem cells shape microglia effector functions through the release of CX3CL1. Stem Cells. 2012;30:2044–2053. doi: 10.1002/stem.1174. [DOI] [PubMed] [Google Scholar]
- 46.Kim Y-J, Park H-J, Lee G, et al. Neuroprotective effects of human mesenchymal stem cells on dopaminergic neurons through anti-inflammatory action. Glia. 2009;57:13–23. doi: 10.1002/glia.20731. [DOI] [PubMed] [Google Scholar]
- 47.Micklem K, Rigney E, Cordell J, et al. A human macrophage-associated antigen (CD68) detected by six different monoclonal antibodies. Br J Haematol. 1989;73:6–11. doi: 10.1111/j.1365-2141.1989.tb00210.x. [DOI] [PubMed] [Google Scholar]
- 48.Holness CL, Simmons DL. Molecular cloning of CD68, a human macrophage marker related to lysosomal glycoproteins. Blood. 1993;81:1607–1613. [PubMed] [Google Scholar]
- 49.Travaglione S, Falzano L, Fabbri A, Stringaro A, Fais S, Fiorentini C. Epithelial cells and expression of the phagocytic marker CD68: scavenging of apoptotic bodies following Rho activation. Toxicol In Vitro. 2002;16:405–411. doi: 10.1016/S0887-2333(02)00028-0. [DOI] [PubMed] [Google Scholar]
- 50.Neher JJ, Neniskyte U, Zhao J-W, Bal-Price A, Tolkovsky AM, Brown GC. Inhibition of microglial phagocytosis is sufficient to prevent inflammatory neuronal death. J Immunol. 2011;186:4973–4983. doi: 10.4049/jimmunol.1003600. [DOI] [PubMed] [Google Scholar]
- 51.Neher JJ, Neniskyte U, Brown GC. Primary phagocytosis of neurons by inflamed microglia: potential roles in neurodegeneration. Front Pharmacol. 2012;3:27. doi: 10.3389/fphar.2012.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bonilla C, Zurita M, Otero L, Aguayo C, Vaquero J. Delayed intralesional transplantation of bone marrow stromal cells increases endogenous neurogenesis and promotes functional recovery after severe traumatic brain injury. Brain Inj. 2009;23:760–769. doi: 10.1080/02699050903133970. [DOI] [PubMed] [Google Scholar]
- 53.Mahmood A, Lu D, Qu C, Goussev A, Chopp M. Treatment of traumatic brain injury with a combination therapy of marrow stromal cells and atorvastatin in rats. Neurosurgery. 2007;60:546–553. doi: 10.1227/01.NEU.0000255346.25959.99. [DOI] [PubMed] [Google Scholar]
- 54.Qu C, Mahmood A, Lu D, Goussev A, Xiong Y, Chopp M. Treatment of traumatic brain injury in mice with marrow stromal cells. Brain Res. 2008;1208:234–239. doi: 10.1016/j.brainres.2008.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sato A, Ohtaki H, Tsumuraya T, et al. Interleukin-1 participates in the classical and alternative activation of microglia/macrophages after spinal cord injury. J Neuroinflammation. 2012;9:65. doi: 10.1186/1742-2094-9-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Babcock AA, Kuziel WA, Rivest S, Owens T. Chemokine expression by glial cells directs leukocytes to sites of axonal injury in the CNS. J Neurosci. 2003;23:7922–7930. doi: 10.1523/JNEUROSCI.23-21-07922.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Si Y, Tsou C-L, Croft K, Charo IF. CCR2 mediates hematopoietic stem and progenitor cell trafficking to sites of inflammation in mice. J Clin Invest. 2010;120:1192–1203. doi: 10.1172/JCI40310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11:889–896. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- 59.Butovsky O, Bukshpan S, Kunis G, Jung S, Schwartz M. Microglia can be induced by IFN-gamma or IL-4 to express neural or dendritic-like markers. Mol Cell Neurosci. 2007;35:490–500. doi: 10.1016/j.mcn.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 60.Cho HH, Kim YJ, Kim JT, et al. The role of chemokines in proangiogenic action induced by human adipose tissue-derived mesenchymal stem cells in the murine model of hindlimb ischemia. Cell Physiol Biochem. 2009;24:511–518. doi: 10.1159/000257495. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 1224 kb)
(PDF 1224 kb)