Summary
Neurologists are often the first medical providers to evaluate patients with possible infectious meningitis. Knowledge of the clinical presentations and cerebrospinal fluid, microbiologic, and neuroimaging findings for different etiologies is essential to make a prompt diagnosis and initiate appropriate treatment. Tuberculosis is a common cause of meningitis in developing countries with a high prevalence of pulmonary tuberculosis. However, tuberculosis affects populations in every country and all neurologists need to be vigilant for possible cases of tuberculous meningitis presenting to their medical facilities. This article discusses the challenges of diagnosing and treating tuberculous meningitis and highlights recent advances in diagnostic technology.
Tuberculosis (TB) is a highly prevalent global human infection caused by Mycobacterium tuberculosis (MTB). One-third of the world's population is infected with latent TB. These individuals are not clinically affected but carry a lifetime risk of 10% for developing active disease.1 There were an estimated 8.6 million incident cases of TB globally in 2012, with 1.3 million deaths.2 A total of 22 high-burden countries accounted for 81% of all estimated incident cases. TB is the leading cause of death in people living with HIV, accounting for approximately 1 in 5 deaths.
The largest share of the global burden of TB is in the Southeast Asia, Western Pacific, and African regions.2 However, TB is a global epidemic, with cases reported annually in every country. In the United States in 2012, 9,951 new cases of TB were reported, with 7.7% of cases coinfected with HIV.3 The majority of cases (63%) were foreign-born persons. The top 5 countries of birth were Mexico, the Philippines, India, Vietnam, and China. In the United Kingdom in 2012,4 8,751 new cases of TB were reported, with 73% among people born in high-burden countries of South Asia and sub-Saharan Africa. In the United States and the United Kingdom, populations at higher risk for TB include racial and ethnic minorities, homeless persons, prisoners, and immunosuppressed individuals.3,4
Clinical presentation of tuberculous meningitis
Tuberculous meningitis (TBM) can occur as the sole manifestation of TB or concurrent with pulmonary or other extrapulmonary sites of infection.5–7 TBM carries a high mortality and morbidity, particularly among patients coinfected with HIV.8–12 Delays in seeking medical care, diagnosis, and initiation of treatment are contributing factors to the high mortality and morbidity, especially in resource-limited regions. When diagnosed promptly, TBM can be cured with supervised medication administration and supportive care.
Patients with TBM develop typical symptoms and signs of meningitis including headache, fever, and stiff neck, although meningeal signs may be absent in the early stages. The duration of symptoms before presentation ranges from several days to several months.9,10 Especially in resource-limited settings, TBM cases may present in advanced clinical stages, with Glasgow Coma Scale scores of 10 or less.9–12 Cranial nerve (CN) palsies, hemiparesis, paraparesis, and seizures are common and should raise the possibility of MTB as the etiology of meningitis. Patients often present with multiple CN palsies, most commonly involving CN III, VI, and VII. Chest X-ray is suggestive of active or previous pulmonary TB in approximately 50% of cases (figure 1).5
Figure 1. Chest X-ray of pulmonary tuberculosis.
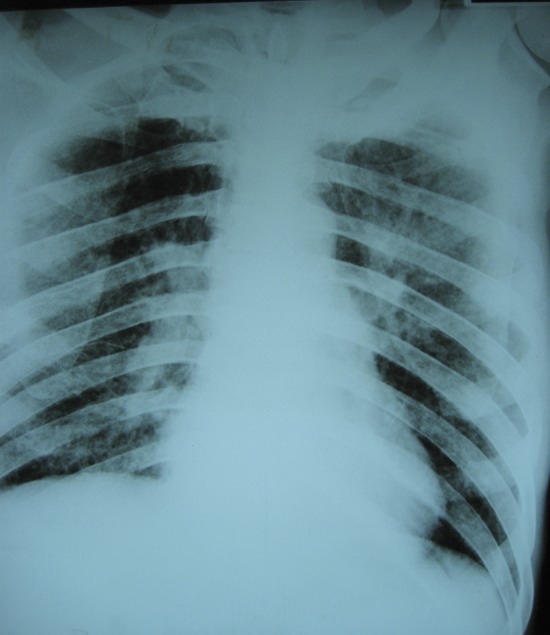
Bilateral infiltrates and hilar adenopathy suggestive of active tuberculosis.
CSF in TBM
If the clinical presentation is suggestive of TBM, cerebrospinal fluid (CSF) should be sent for routine analyses (cell counts and differential, protein level, glucose level) and microbiologic tests for bacteria, fungi, and MTB. Pleocytosis with lymphocytic predominance, high protein levels, and low glucose levels are the hallmark findings in the CSF of patients with TBM. In a consecutive series of 88 HIV-negative patients with definite TBM diagnosed by positive CSF culture,13 the following median values were determined from CSF analyses: cell count (136/µL), mononuclear cell percentage (63%), protein concentration (160 mg/dL), and CSF glucose/blood glucose ratio (0.13). Atypical CSF findings may be obtained in HIV coinfected individuals including normal cell counts (≤5 cells/µL), polymorphonuclear cell predominance, and normal glucose levels (>2.2 mmol/L).12
The CSF findings noted above are not specific for TBM and can be seen in other conditions including non-MTB bacterial meningitis, fungal meningitis, carcinomatous meningitis, and subarachnoid hemorrhage. For all patients with suspected TBM, CSF samples should be examined by Ziehl-Neelsen (ZN) staining for acid-fast bacilli, Gram staining for bacteria, India ink preparations for fungi, and antigen testing for Cryptococcus neoformans. In non-MTB bacterial meningitis, 60%–90% of CSF Gram stains are positive, allowing rapid identification of the causative organism for most cases.14 In contrast, ZN staining has a very low sensitivity in cases of TBM.9,13 For example, in a prospective observational study from Indonesia, only 11% of CSF samples taken from 207 suspected cases of TBM were positive by ZN staining.13 In the same study, a low percentage of CSF samples yielded growth of MTB by solid culture (36%) and by liquid culture (44%). Since MTB cultures can take several weeks or longer to detect growth, a presumptive diagnosis of TBM in cases with a negative CSF ZN stain needs to be made without waiting for the results of CSF MTB culture.
A recent diagnostic advance is Xpert MTB/RIF, an automated rapid nucleic acid amplification test for MTB endorsed by the WHO in December 2010.15 Xpert technology employs single-use sealed disposable cartridges that avoid contamination of testing equipment and requires minimal technical training to operate. The Xpert MTB/RIF assay employs 3 specific primers and 5 unique molecular probes to detect MTB and rifampicin resistance simultaneously in less than 2 hours. Molecular probes are included to detect DNA of sample processing control bacteria (figure 2). Rifampicin resistance is strongly indicative of multi-drug-resistant TB (MDR-TB), defined as TB caused by organisms resistant to isoniazid and rifampicin.15
Figure 2. Xpert MTB/RIF nucleic acid amplification test.
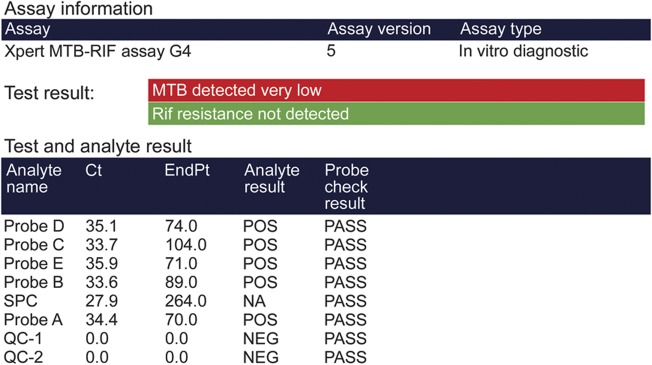
Xpert MTB/RIF results on the CSF of a 53-year-old patient with tuberculous meningitis and HIV coinfection. Ziehl-Neelsen stain of CSF for acid-fast bacilli was negative.
A 2013 Cochrane Review of Xpert MTB/RIF in pulmonary TB that included 18 unique studies reported a pooled sensitivity of the assay of 98% for smear-positive, culture-positive TB and 68% for smear-negative, culture-positive TB, and an assay specificity of 98%.15 A prospective study from South Africa reported a sensitivity of 67% and a specificity of 94% for Xpert MTB/RIF comparing 54 HIV-infected patients with definite TBM and 65 patients with “non-TBM” meningitis.16 A recent study of suspected TBM cases (20.8% HIV-infected) from Vietnam reported a sensitivity of 72% and a specificity of 99.5% for Xpert MTB/RIF comparing 151 definite cases of TBM with 197 cases of not TBM meningitis.17
Neuroimaging in TBM
Contrast-enhanced brain CT or MRI can help support a diagnosis of TBM because of the high frequency of abnormalities on initial presentation (figure 3). The most common findings in descending order are meningeal enhancement, hydrocephalus, basal exudates, infarcts, and tuberculomas.18 Infarcts occur as a result of vasculitis affecting the vessels of the Circle of Willis, the perforating branches of the middle cerebral artery, and the vertebrobasilar circulation.
Figure 3. Neuroimaging in tuberculous meningitis.
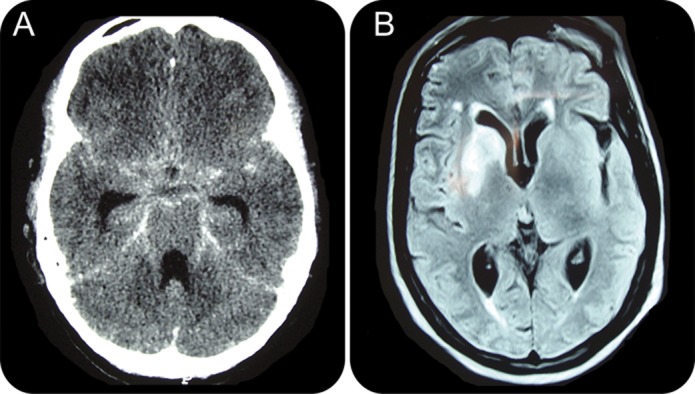
(A) Brain CT of a 19-year-old patient with tuberculous meningitis (TBM) shows hydrocephalus and basal meningeal enhancement. (B) Brain MRI of a 56-year-old patient with TBM shows basal ganglia infarcts.
Treatment of TBM
If the clinical presentation, CSF findings, and neuroimaging studies of a patient with meningitis are compatible with TBM and other etiologies (e.g., bacterial and fungal) have been excluded by initial CSF analysis, a presumptive diagnosis of TBM should be made and treatment should be initiated promptly. Parenteral empirical antibiotics should be administered to cover non-MTB bacterial etiologies until CSF and blood cultures for bacteria are negative for >48 hours. Guidelines for the treatment of TBM in adults and children have been published by the WHO in 201019,20 and the British Infection Society in 20095 and are based on standard regimens for pulmonary TB (table). Controlled trials to determine the optimal drug regimen and treatment duration for TBM have not been conducted.
Table.
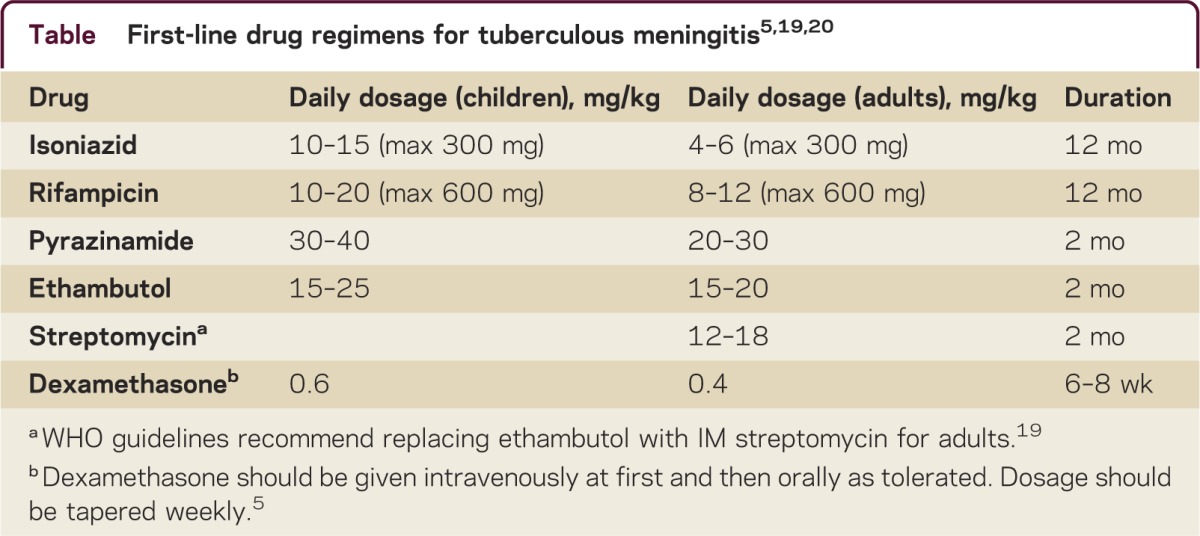
The British Infection Society guidelines recommend a first-line regimen of 2 months of isoniazid, rifampicin, pyrazinamide, and ethambutol followed by 10 months of isoniazid and rifampicin.5 The WHO guidelines recommend a first-line regimen of 2 months of isoniazid, rifampicin, pyrazinamide, and ethambutol (children) or streptomycin (adults) followed by 10 months of isoniazid and rifampicin.19,20 Both guidelines recommend adjunctive corticosteroids based on a Cochrane review that concluded that adjunctive corticosteroids reduce the risk of death or disabling residual neurologic deficit from TBM.8 Newer regimens combining fluoroquinolones with high-dose rifampicin show promise for improving outcomes in TBM.21–23 For patients with suspected or confirmed MDR-TB, recently published WHO guidelines should be followed24 and an expert in treating MDR-TB should be consulted. The Food and Drug Administration recently approved the use of bedaquiline fumarate as part of combination therapy for adults with a diagnosis of pulmonary MDR-TB. Although clinical trials of bedaquiline for the treatment of extrapulmonary MDR-TB are not available, published guidelines indicate that use on a case-by-case basis is reasonable given the high mortality rate and limited treatment options.25
HIV-infected patients receiving treatment for TBM are at risk for clinical deterioration after initiation of antiretroviral therapy (ART) due to immune reconstitution inflammatory syndrome (TBM-IRIS).26,27 The rapid restoration of immune function in the presence of mycobacterial antigens provokes an intensified inflammatory reaction resulting in worsening, recurrent, or new clinical signs and symptoms. Diagnosis of TBM-IRIS requires exclusion of other causes of clinical deterioration including drug resistance, poor adherence to treatment, misdiagnosis, and other opportunistic infections. The optimal time to initiate ART is uncertain. Some guidelines recommend deferring ART to 4–6 weeks after beginning anti-TB medication.26 Although corticosteroids do not appear to prevent TBM-IRIS,27 restarting or increasing the dose and duration of corticosteroid treatment is recommended for the management of TBM-IRIS.26,27
DISCUSSION
TB is one of the most challenging causes of meningitis to diagnose because of the difficulties in rapidly identifying MTB in CSF samples. TBM should be strongly considered in any patient presenting with symptoms and signs of meningitis in regions with a high burden of TB and in high-risk individuals in regions with lower burdens of TB. Clinical, microbiologic, and radiologic findings should be used conjunctively to support a diagnosis of TBM. Empirical treatment with anti-TB drugs is the standard of care in presumptive cases with negative ZN staining of CSF for acid-fast bacilli. The Xpert MTB/RIF assay provides the ability to rapidly diagnose infection with MTB, although additional studies are needed to define the sensitivity and specificity for the diagnosis of TBM.
STUDY FUNDING
No targeted funding reported.
DISCLOSURES
J.H. Chin receives research support from NIH Fogarty International Center, Global Health Fellows and Scholars Research Training, 1R25TW009338-01, Junior Faculty Fellow, 2013–2014. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
REFERENCES
- 1.Zumla A, Raviglione M, Hafner R, von Reyn CF. Current concepts: tuberculosis. N Engl J Med 2013;368:745–755 [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Global tuberculosis report 2013. Available at: http://www.who.int/tb/publications/global_report/en/index.html. Accessed January 10, 2014
- 3.Centers for Disease Control and Prevention. Trends in tuberculosis: United States 2012. MMWR Morb Mortal Wkly Rep 2013;62:201–205 [PMC free article] [PubMed] [Google Scholar]
- 4.Tuberculosis in the UK: Annual Report on Tuberculosis Surveillance in the UK, 2013. London: Public Health England; 2013. Available at: http://www.hpa.org.uk/Publications/InfectiousDiseases/Tuberculosis/1308TBintheUK2013report/. Accessed January 10, 2014 [Google Scholar]
- 5.Thwaites G, Fisher M, Hemingway C, Scott G, Solomon T, Innes J. British Infection Society guidelines for the diagnosis and treatment of tuberculosis of the central nervous system in adults and children. J Infect 2009;59:167–187 [DOI] [PubMed] [Google Scholar]
- 6.Cherian A, Thomas SV. Central nervous system tuberculosis. Afr Health Sci 2011;11:116–127 [PMC free article] [PubMed] [Google Scholar]
- 7.Nelson C, Zunt JR. Tuberculosis of the central nervous system in immunocompromised patients: HIV Infection and solid organ transplant recipients. Clin Infect Dis 2011;53:915–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prasad K, Singh M. Corticosteroids for managing tuberculous meningitis (review). Cochrane Database Syst Rev 2008;1:CD002244 [DOI] [PubMed]
- 9.Thwaites GE, Nguyen DB, Nguyen HD, et al. Dexamethasone for the treatment of tuberculous meningitis in adolescents and adults. N Engl J Med 2004;351:1741–1751 [DOI] [PubMed] [Google Scholar]
- 10.Katrak SM, Shembalkara PK, Bijwe SR, Bhandarkar LD. The clinical, radiological and pathological profile of tuberculous meningitis in patients with and without human immunodeficiency virus infection. J Neurol Sci 2000;181:118–126 [DOI] [PubMed] [Google Scholar]
- 11.Cecchinia D, Ambrosioni J, Brezzo C, et al. Tuberculous meningitis in HIV-infected patients: drug susceptibility and clinical outcome. AIDS 2007;21:373–374 [DOI] [PubMed] [Google Scholar]
- 12.Marais S, Pepper DJ, Schutz C, Wilkinson RJ, Meintjes G. Presentation and outcome of tuberculous meningitis in a high HIV prevalence setting. PLoS ONE 2011;6:e20077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chaidir L, Ganiem AR, vander Zanden A, et al. Comparison of real time IS6110-PCR, microscopy, and culture for diagnosis of tuberculous meningitis in a cohort of adult patients in Indonesia. PLoS ONE 2012;7:e52001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tunkel AR, Hartman BJ, Kaplan SL, et al. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis 2004;39:1267–1284 [DOI] [PubMed] [Google Scholar]
- 15.Steingart KR, Sohn H, Schiller I, et al. Xpert® MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults (review). Cochrane Database Syst Rev 2013;1:CD009593 [DOI] [PMC free article] [PubMed]
- 16.Patel VB, Theron G, Lenders L, et al. Diagnostic accuracy of quantitative PCR (Xpert MTB/RIF) for tuberculous meningitis in a high burden setting: a prospective study. PLoS Med 2013;10:e1001536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nguyen TQN, Heemskerk D, Do DAT, et al. Evaluation of Xpert MTB/RIF for the diagnosis of tuberculous meningitis. J Clin Microbiol 2014;52:226–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raut T, Garg RK, Jain A, et al. Hydrocephalus in tuberculous meningitis: incidence, its predictive factors and impact on the prognosis. J Infect 2013;66:330–337 [DOI] [PubMed] [Google Scholar]
- 19.WHO. Treatment of tuberculosis: guidelines: 4th ed. Available at: http://www.who.int/tb/publications/2010/9789241547833/en/index.html. Accessed January 10, 2014 [PubMed] [Google Scholar]
- 20.WHO. Rapid advice: treatment of tuberculosis in children. Available at: http://apps.who.int/iris/handle/10665/44444. Accessed January 10, 2014 [PubMed]
- 21.Heemskerk D, Day J, Tran THC, et al. Intensified treatment with high dose rifampicin and levofloxacin compared to standard treatment for adult patients with tuberculous meningitis (TBM-IT): protocol for a randomized controlled trial. Trials 2011;12:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruslami R, Ganiem AR, Dian S, et al. Intensified regimen containing rifampicin and moxifloxacin for tuberculous meningitis: an open-label, randomized controlled phase 2 trial. Lancet Infect Dis 2013;13:27–35 [DOI] [PubMed] [Google Scholar]
- 23.Thwaites GE, van Toorn R, Schoeman J. Tuberculous meningitis: more questions, still too few answers. Lancet Neurol 2013;12:999–1010 [DOI] [PubMed] [Google Scholar]
- 24.WHO. Guidelines for the Programmatic Management of Drug-Resistant Tuberculosis. 2011 Update. Available at: http://www.who.int/tb/challengesdr/programmatic_guidelines_for_mdrtb/en/index.html. Accessed January 10, 2014 [Google Scholar]
- 25.Centers for Disease Control and Prevention. Provisional CDC guidelines for the use and safety monitoring of bedaquiline fumarate (Sirturo) for the treatment of multidrug-resistant tuberculosis. MMWR Recomm Rep 2013;62:1–12 [PubMed] [Google Scholar]
- 26.Bahr N, Boulware DR, Marais S, et al. Central nervous system immune reconstitution inflammatory syndrome. Curr Infect Dis Rep 2013;15:583–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marais S, Meintjes G, Pepper DJ, et al. Frequency, severity, and prediction of tuberculous meningitis immune reconstitution inflammatory syndrome. Clin Infect Dis 2013;56:450–460 [DOI] [PMC free article] [PubMed] [Google Scholar]


