Summary
Distinguishing dementia due to multiple sclerosis (MS) from that of an accompanying neurodegenerative dementia coexisting with MS has been difficult. The recent introduction of Alzheimer disease (AD) biomarkers of amyloid-β and neuronal degeneration has improved diagnosis of AD premortem. We describe 3 patients with MS with coexisting AD, 1 diagnosed at autopsy before AD biomarkers were available and 2 diagnosed premortem by decreased CSF amyloid-β1-42/tau index, MRI, and 18F-flourodeoxyglucose-PET patterns. AD biomarkers may be of diagnostic value in selected patients with severe dementia and MS.
Cognitive dysfunction is common in multiple sclerosis (MS), affecting approximately 50% of individuals.1 The cognitive deficits tend to be mild with a “subcortical pattern” of deficits in speed of processing, attention, and immediate recall.1 Cortical demyelination in MS is increasingly recognized2 and rare patients with MS have severe progressive cortical dementia in relative isolation from other clinical impairment.3,4 CSF and imaging biomarkers for Alzheimer disease (AD) allow increased diagnostic confidence of AD premortem compared to the requirement of pathologic examination (usually at autopsy).5 The clinical utility of AD biomarkers remains to be established. We describe 3 cases of MS where dementia was complicated by coexisting AD and highlight how premortem clinical diagnosis can be aided by AD biomarkers.
Case 1
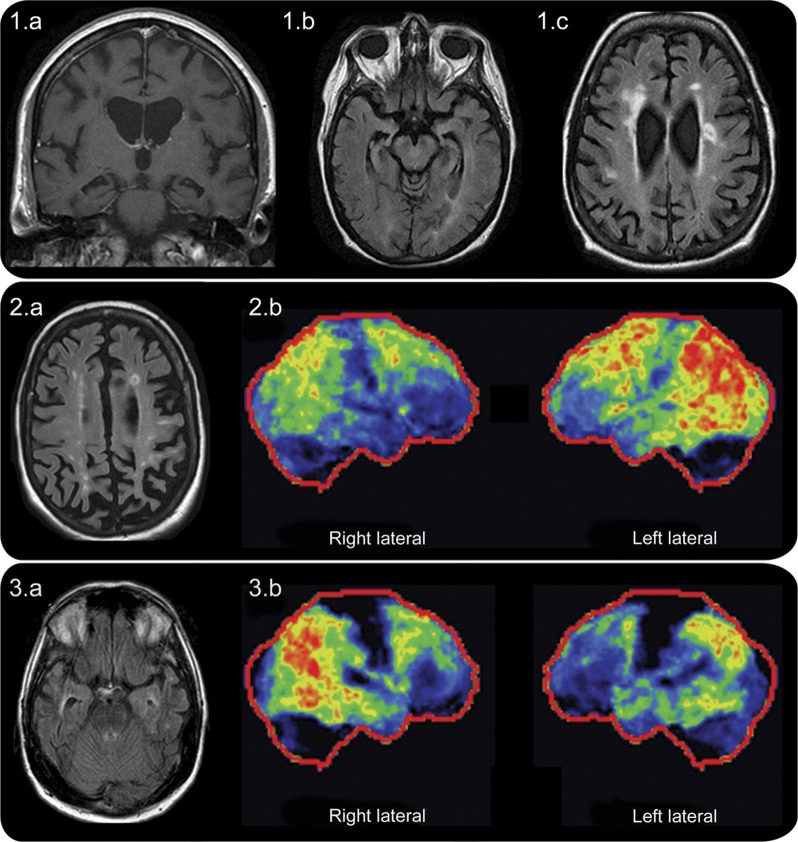
A 56-year-old woman developed short-term memory and calculation impairment followed by progressively severe dementia over 7 years. She had no history of typical MS attacks. Neurologic examination revealed severe cognitive difficulties, aphasia, and limb apraxia. Brain MRI revealed bilateral mesial temporal lobe atrophy with multiple nonenhancing periventricular MS lesions (figure 1, A–C). CSF showed elevated unique oligoclonal bands and immunoglobulin G index. Primary progressive MS with severe dementia was diagnosed. Autopsy following death revealed definite chronic MS pathology with focal white matter lesions with marked myelin loss and perivascular lymphocytic inflammation. In addition, severe AD pathology highlighted by neurofibrillary tangles, neuritic plaques, and amyloid angiopathy (Braak stage 6 [of 6]) was found.
Figure. MRI and 18F-fluorodeoxyglucose PET findings.
Case 1: MRI demonstrates prominent bilateral mesial temporal and hippocampal atrophy on coronal T1-weighted MRI (1a) and axial fluid-attenuated inversion recovery (FLAIR) images (1b) with classic periventricular lesions of multiple sclerosis (MS) also present on axial FLAIR images (1c). Case 2: MRI axial FLAIR image demonstrates a typical ovoid periventricular lesion of MS in the left frontal region and multiple periventricular T2-signal hyperintensities along with severe parietal lobe atrophy (2a); hypometabolism (brighter colors indicate increasing severity of hypometabolism with red reflecting the most severe hypometabolism and dark regions indicating normal metabolism) on 18F-fluorodeoxyglucose-PET (FDG-PET) is demonstrated to be moderate to severe in the temporo-parietal region (worse on the left) and milder in the frontal regions (2b). Case 3: MRI demonstrates right greater than left mesial temporal atrophy illustrated by an enlarged lateral ventricle on that side; typical periventricular lesions of MS next to the left and right lateral ventricles are also noted (3a); right greater than left temporo-parietal FDG-PET hypometabolism with less severe hypometabolism in the frontal region (3b).
Case 2
A 53-year-old woman developed positional vertigo 8 years prior to our evaluation. MRI head at that time revealed typical demyelinating brain lesions, but her symptoms were attributed to peripheral vertigo and no immunomodulatory treatments for MS were instituted. One year later, she developed paroxysmal episodes of right upper extremity numbness and imbalance with falls consistent with paroxysmal symptoms of demyelination. Coincident with the paroxysmal symptoms, she developed short-term memory impairment followed by progressive dementia. Neuropsychological testing 4 years after symptom onset revealed constructional apraxia, executive dysfunction (inability to complete Trails B test), dyscalculia, inattention, difficulty with working memory, and severe deficits in delayed recall. The pattern of abnormalities on neuropsychological testing was not believed to be specific for any one cognitive disorder. Neurologic examination 8 years after onset revealed aphasia, apraxia, and Kokmen short test of mental status score of 14/38 (29/38 or less consistent with dementia).6 Brain and spinal cord MRI showed typical MS lesions along with prominent parietal lobe atrophy (figure 2A). Serologic evaluations were negative for alternative causes of dementia. Brain 18F-fluorodeoxyglucose-PET (FDG-PET) revealed bilateral (left greater than right) fronto-temporal-parietal and posterior cingulate hypometabolism (figure 2B), highly suggestive of AD. CSF revealed elevated oligoclonal bands, a total tau of 1,027.9 pg/mL, and amyloid-β1-42 concentration of 315 pg/mL corresponding to a reduced amyloid-β1-42/tau index (0.22; normal, >1) (AD sensitivity 85%–94%; specificity 83%–89%). Donepezil and memantine were instituted with some mild subjective symptomatic improvement reported after telephone follow-up; however, objective evaluation on retesting was unavailable.
Case 3
A 53-year-old man developed bilateral upper extremity paresthesias that did not remit 11 years prior to presentation. He underwent an MRI at that time that was consistent with MS and he was commenced on interferon-β-1a IM once weekly. His neurologic symptoms were stable until he developed a progressive dementia over the 2 years prior to our evaluation. His mother had known AD. Kokmen short test of mental status score was 13/38. Brain and spine MRI showed multiple typical MS lesions and prominent right greater than left focal mesial temporal lobe atrophy (figure 3A). Serologic evaluations were negative for alternative causes of dementia. He continued to deteriorate despite donepezil. Brain FDG-PET showed bilateral temporo-parietal and posterior cingulate hypometabolism highly suggestive of AD (figure 3B). CSF revealed elevated oligoclonal bands, a total tau of 306.1 pg/mL, and an amyloid-β1-42 concentration of 238.2 pg/mL corresponding to a decreased amyloid-β1-42/tau index of 0.4 (normal >1) (AD sensitivity 85%–94%; specificity 83%–89%).
DISCUSSION
AD pathology may coexist with MS and may lead to severe dementia. We demonstrate that AD biomarkers may diagnose this complicated clinical picture premortem. The use of AD biomarkers may provide diagnostic clarity to patients and clinicians and assist with therapeutic decisions. Selection of medications such as acetylcholinesterase inhibitors or memantine, or both, which are effective in AD may be considered even as they have been ineffective in typical MS-related cognitive disorders.7
AD biomarkers are classified as markers of (1) amyloid-β (via PET amyloid imaging or CSF) or (2) neuronal injury or degeneration (CSF tau, FDG-PET, MRI).5 Cases 2 and 3 correspond to high biomarker probability of AD demonstrated by amyloid-β accumulation (on CSF biomarkers) and neuronal degeneration (typical FDG PET, MRI findings, and elevated CSF tau). The FDG-PET features in our cases with temporo-parietal and posterior cingulate hypometabolism were highly suggestive of AD and differ from the global FDG-PET hypometabolism reported in MS.8 Overall, our case suggests that AD biomarkers may be of value in selected patients with severe dementia and MS.
While we suspect AD was a relevant cause of the underlying dementia in all these cases, it is difficult to determine the contribution from each disorder to the cognitive decline and any possible additive effect of the 2 pathologies. The clinical course in these patients is difficult to clearly define but mostly suggests primary progressive MS. No clear inflammatory clinical attacks of MS with definite improvement were seen. Given the severity of the dementia seen and the uncertainty of the relative contributions of MS and AD to the severity of the dementia, we would not consider the patients to have the radiologically isolated syndrome, where neuroimaging findings highly characteristic of MS are seen essentially without symptoms and typically without signs of MS.9
Neuropsychological testing is an additional tool that may help distinguish dementia subtypes. Early executive dysfunction and deficits in verbal memory on neuropsychological testing are characteristic of AD.10 However, in case 2, neuropsychological testing was unable to suggest one particular dementia subtype, possibly related to the dual pathologies present. Formal neuropsychological testing was not performed in cases 1 and 3 due to the severity of dementia as testing in those with Kokmen scores less than 17/38 is of limited diagnostic utility.
Our report is limited by the lack of definitive pathologic confirmation in cases 2 and 3. Although AD biomarkers may be of value in selected patients with severe dementia and MS, the use of these biomarkers may be limited to academic institutions until their practical utility is formalized. Given the frequency of milder cognitive problems in MS, we do not recommend AD biomarker evaluation in those with minor cognitive dysfunction as a mildly positive or indeterminate result may be misleading. In selected patients with moderate to severe cortical dementia and MS (such as the cases we describe), evaluating AD biomarkers may be reasonable. Further studies are necessary to investigate the utility of AD biomarkers in this setting and their potential to guide therapy.
STUDY FUNDING
No targeted funding reported.
DISCLOSURES
E. Flanagan reports no disclosures. D. Knopman has served on Data Safety Monitoring Boards for Lilly Pharmaceuticals and the DIAN study; has served as a consultant to TauRx, McGill University Alzheimer Program, the Bluefield Project, Lundbeck Pharmaceuticals, and Janssen Alzheimer Immunotherapy program; has received speaker honoraria from University of Toronto, University of Pennsylvania, St John's Hospital, and the American Society of Neuroradiology; serves as Deputy Editor for Neurology®; has been an investigator in a clinical trial sponsored by Baxter Pharmaceuticals and Janssen Pharmaceuticals; and receives research support from the NIH. B.M. Keegan is compensated as a Chief Editor of eMedicine; has consulted for Bristol-Myers Squibb, Novartis, and Bionest; and receives research support from Terumo BCT. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
REFERENCES
- 1.Calabrese P, Penner IK. Cognitive dysfunctions in multiple sclerosis: a “multiple disconnection syndrome”? J Neurol 2007;254(suppl 2):II18–II21 [DOI] [PubMed] [Google Scholar]
- 2.Lucchinetti CF, Popescu BF, Bunyan RF, et al. Inflammatory cortical demyelination in early multiple sclerosis. N Engl J Med 2011;365:2188–2197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zarei M, Chandran S, Compston A, Hodges J. Cognitive presentation of multiple sclerosis: evidence for a cortical variant. J Neurol Neurosurg Psychiatry 2003;74:872–877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Staff NP, Lucchinetti CF, Keegan BM. Multiple sclerosis with predominant, severe cognitive impairment. Arch Neurol 2009;66:1139–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011;7:263–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kokmen E, Smith GE, Petersen RC, Tangalos E, Ivnik RC. The short test of mental status: correlations with standardized psychometric testing. Arch Neurol 1991;48:725–728 [DOI] [PubMed] [Google Scholar]
- 7.Krupp LB, Christodoulou C, Melville P, et al. Multicenter randomized clinical trial of donepezil for memory impairment in multiple sclerosis. Neurology 2011;76:1500–1507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blinkenberg M, Rune K, Jensen CV, et al. Cortical cerebral metabolism correlates with MRI lesion load and cognitive dysfunction in MS. Neurology 2000;54:558–564 [DOI] [PubMed] [Google Scholar]
- 9.Okuda DT, Mowry EM, Beheshtian A, et al. Incidental MRI anomalies suggestive of multiple sclerosis: the radiologically isolated syndrome. Neurology 2009;72:800–805 [DOI] [PubMed] [Google Scholar]
- 10.Mayeux R. Clinical practice: early Alzheimer's disease. N Engl J Med 2010;362:2194–2201 [DOI] [PubMed] [Google Scholar]