Abstract
Here, we demonstrate that biomolecule-directed metal clusters are applicable in the study of hard X-ray excited optical luminescence, promising a new direction in the development of novel X-ray-activated imaging probes.
X-rays are widely used in imaging applications such as diffraction imaging of crystals and medical imaging. In particular, X-ray computed tomography (CT) is a critical tool for clinical and disease diagnostics.1,2 The principle of conventional CT is based on X-ray attenuation caused by photoelectric absorption and scattering.3,4 In addition to conventional CT, a number of novel methodologies are presently under development, including state-of-the-art instrument technologies and chemical probes to fulfill diagnosis criteria.5 To advance these new approaches, X-ray fluorescence and X-ray excited optical luminescence (XEOL) are being proposed as potential photo-physical mechanisms to exploit for imaging.6 Among these novel methodologies, we have utilized hard X-ray-excited optical luminescence (hXEOL) as a new methodology called X-ray luminescence CT (XLCT).7 In previous papers by Anker, Sham, and us, a radio-luminescent probe was employed to specifically target a biological signature and produce an optical contrast when irradiated with hard X-rays (i.e. >20 keV).7–10 Despite excellent hXEOL properties of inorganic nanoparticles, there are still several drawbacks for medical and biological imaging, such as poor biocompatibility and stability in vivo.10 To overcome these issues, we have reported that iridium complex doped biocompatible polymer dots (P-dots) induce optical luminescence upon hard X-ray irradiation. In vivo study of this new class of probes is currently ongoing.11 In addition, the basis of hXEOL has not yet been reported in detail except in few studies.8,9 Therefore, to understand the nature of hXEOL, there is still a need to explore and discover compounds and materials that emit light upon hard X-ray irradiation.
Metal clusters that emit luminescence upon ultraviolet (UV) or visible light irradiation have recently been developed as imaging probes. In particular, biomolecule-directed metal clusters have emerged as promising candidates for applications such as in vitro and in vivo bio-sensing and fluorescence imaging.12–16 These biomolecule-directed metal clusters emit in the near infrared region, upon ultraviolet (UV) light or blue light irradiation.12,17 Furthermore, a few studies on protein-directed metal clusters suggested their possible use as the X-ray CT absorption contrast agents.18,19 Prompted by these studies, we hypothesized that biomolecule-directed metal clusters will produce luminescence upon excitation by hard X-rays. Herein, for the first time, we explore the possibility of hXEOL via biomolecule-directed metal clusters and propose it as a potential platform for new X-ray imaging. Fig. 1 shows the strategy of this study. We hypothesized that XEOL would be induced from biomolecule-directed metal clusters and that obtaining a distinctive luminescence image of these metal clusters upon X-ray irradiation is possible. In the present study, we choose previously described biomolecule-directed metal clusters, the single-stranded DNA (ssDNA) directed Ag clusters,13 lysozyme VI (Lyz)16 or bovine serum albumin (BSA)15 directed Au clusters as a proof of concept. These metal clusters have been demonstrated by several groups for in vitro as well as in vivo imaging studies using UV or visible light irradiation. In the present study, their X-ray activity is studied further to investigate whether these probes could function for XLCT. These metal clusters were synthesized as previously described by Dickson et al.,20 Ying et al.,15 and Tseng et al. using slightly modified methods.16 The detailed procedures are described in the experimental section.
Fig. 1.
Overview of the study. X-ray excited optical luminescence was characterized to investigate the hypothesis that hard X-ray-induced optical luminescence would be induced via ssDNA-, Lyz-, and BSA-directed metal clusters.
First, the steady state luminescence upon UV light (365 nm) excitation was investigated. Consistent with earlier studies,15,16,20 visible range luminescence from the biomolecule-directed metal clusters was clearly observed upon UV irradiation (Fig. S1a, ESI†). The ssDNA-directed Ag cluster exhibits a pink color upon UV excitation, although the ssDNA sequence used in this study has been reported to emit blue,21 yellow,20 and green22 in earlier studies. This is possibly because of different synthetic conditions, such as the variety of buffers and their concentrations. Protein-directed Au clusters synthesized in this study emit blue for Lyz and red for BSA upon UV light excitation, consistent with earlier studies.15,16 To further confirm the formation of luminescent metal clusters, the steady state excitation and luminescence spectra were recorded (Fig. S1b, ESI†). The luminescence maximum was at 607 nm for ssDNA-, 421 nm for Lyz- and 667 nm for BSA-directed metal clusters, respectively. For ssDNA clusters, the number of Ag atoms has been estimated to be about 5–11, according to the literature, and determining the number of silver atoms from steady state photo-luminescence measurement is not easy.23 Therefore, we denote the Ag cluster with ssDNA used in this study as Agx–DNA in the following. Consistent with earlier studies,15,16 it was also confirmed on the basis of the Spherical Jellium model that Lyz- or BSA-directed Au clusters were Au8 or Au25, respectively. Absolute quantum yields (QYs) for metal clusters were measured to quantify photo-physical properties upon UV or visible light irradiation (Table S1, ESI†). The measurement of photo-luminescence quantum yields upon UV or visible light irradiation showed that the Agx–DNA cluster is the brightest among the three samples. These results further confirm that metal cluster-directed biomolecules produce luminescence under UV and visible light irradiation.
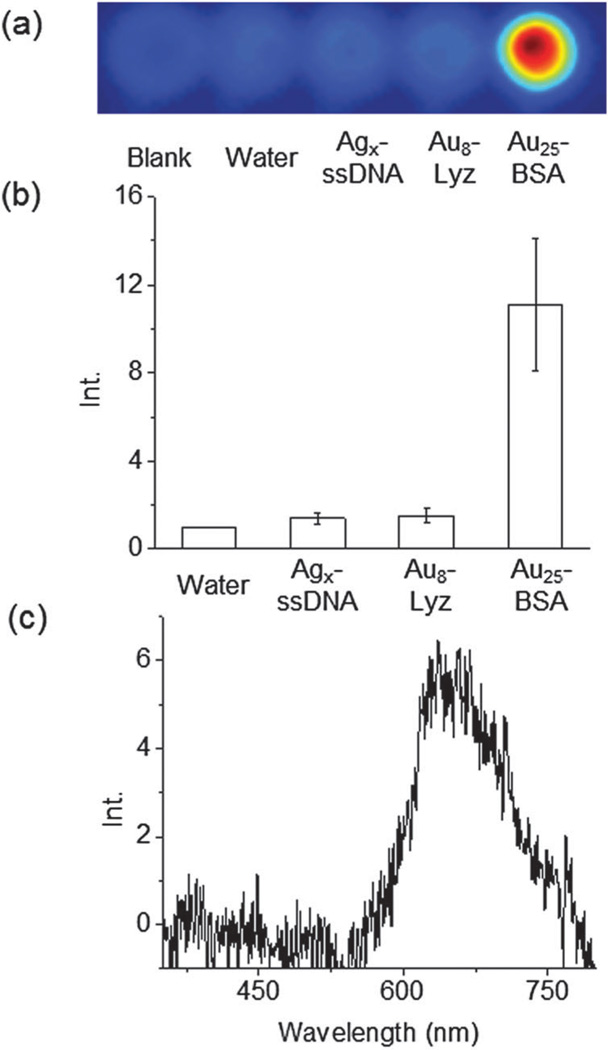
To test the hypothesis of hXEOL via biomolecule-directed metal clusters, hard X-ray-induced luminescence from these metal clusters was measured in aqueous solution (Fig. 2a). A hard X-ray source was employed for X-ray irradiation. The X-ray spectrum of the source used in this study is shown in the ESI† (Fig. S2). A maximum X-ray excitation was observed at around 0.05 nm (25 keV) when the X-ray source was operated at 50 kVp. For hXEOL imaging, a sample solution of the biomolecule-directed cluster was placed in a plastic tube and irradiated with hard X-rays to induce optical emission. The emission was measured using an EM-CCD camera to analyze the hXEOL. The detailed method is provided in the experimental section and in our previous work.11 Fig. 2a shows a typical image of optical luminescence upon hard X-ray irradiation. It is noteworthy that significant luminescence from Agx–DNA and Au8–Lyz clusters was not observed, despite clear luminescence and reasonable QYs upon UV or visible light irradiation. In contrast, distinctive luminescence was observed in the Au25–BSA cluster upon hard X-ray irradiation. It is worth noting that Au25–BSA has the lowest absolute QY upon visible light irradiation among the three samples investigated in this study (Table S1, ESI†). These results suggest that the steady state photophysical properties do not necessarily represent the hXEOL properties. The relative intensity of luminescence of Au25–BSA against water is greater by a factor of 11 (25 mg mL−1 of BSA for the synthesis), which is comparable to that of iridium P-dots described previously.11 Metal ions (gold and silver ions), ssDNA, and proteins themselves (Lyz and BSA) did not provide any distinctive luminescence signals upon hard X-ray irradiation (Fig. S3, ESI†). These results further suggest that the hard X-ray-induced luminescence from Au25–BSA is produced by the Au metal clusters in the BSA molecule. We also confirmed comparable luminescence from transferrin and human serum albumin directed gold clusters (data not shown). Furthermore, to confirm whether the luminescence originates from the metal clusters upon hard X-ray excitation, the XEOL spectrum of Au25–BSA was recorded and is shown in Fig. 2c. A luminescence spectrum similar to the steady state luminescence spectrum shown in Fig. S1 (ESI†) was observed. This result further supports the hypothesis that the hXEOL arises from Au25–BSA clusters.
Fig. 2.
hXEOL imaging and spectra in solution. (a) Images upon X-ray irradiation. From left; blank, water, Agx–ssDNA, Au8–Lyz and Au25–BSA clusters. (b) Relative luminescence intensity upon X-ray irradiation. (c) X-ray excited optical luminescence spectra of Au25–BSA clusters.
X-ray photons above the L3 absorption edge of Au (~12 keV) have sufficient energy to knock out inner-shell electrons (photoelectric absorption) or outer-shell electrons (incoherent Compton scattering) from Au atoms. Additionally, photoelectric absorption is followed by emissions of fluorescent X-rays and Auger electrons as the inner-shell holes get filled with electrons from lower energy states, leading to a cascade of events. Similar processes also occur in organic molecules that surround the gold nanocluster. Energetic electrons produced during these events travel through matter up to tens of micrometers (continuous slowing down approximation), losing their energy through electron–electron inelastic scattering (thermalization). This thermalization process produces excited electrons and holes within the gold nanocluster that can recombine to emit luminescence.24 Although not all of the metal clusters are applicable for the hXEOL and the specific mechanisms of the luminescence upon hard X-ray irradiation should be examined in future, these results clearly demonstrate that BSA-directed metal clusters produce optical luminescence upon hard X-ray irradiation. Further experiments such as the measurement of the XAFS spectrum via resulting photoluminescence yield (PLY) using a synchrotron X-ray source by other specialists of X-ray spectroscopy would provide more detailed mechanistic aspects after the publication of this communication.25,26 One big issue concerning XEOL imaging or X-ray imaging in general is radiation damage. Fortunately for hard X-rays, the absorption cross section is low for low Z elements. If a low brightness source (conventional X-ray) is used, it would further reduce the risk, but studies are necessary to establish the protocol.
In this study, the hard X-ray excited optical luminescence of biomolecule-directed metal clusters was investigated. It was found, for the first time, that luminescence is induced from BSA-directed metal clusters, whereas Lyz-directed metal clusters and ssDNA-directed silver clusters show less or no significant emission upon X-ray irradiation. To the best of our knowledge, this is the first demonstration of luminescence imaging using biomolecule-directed metal clusters upon hard X-ray irradiation. Although the specific mechanisms of XEOL in metal clusters are still unclear, these results are likely to provide a novel application of these metal clusters and expand the new field in radiation chemistry. Future work will be dedicated to detailed mechanistic studies such as metal cluster size dependency on XEOL and, more importantly, tumour imaging using biomolecule-directed metal clusters for the realization of multimodal imaging with CT and X-ray fluorescence imaging.27 Moreover, these systems may work as useful tools in crystallographic studies to determine the position of crystals during mounting of the samples on the stage.
Supplementary Material
Acknowledgments
This work was supported by the JST PRESTO program to YO, the World Premier International Research Center Initiative (WPI) and the Foundation Advanced Technology Institute.
Footnotes
Electronic supplementary information (ESI) available: Fig. S1–S3 and Table S1 and experimental information.
Notes and references
- 1.Rabin O, Manuel Perez J, Grimm J, Wojtkiewicz G, Weissleder R. Nat. Mater. 2006;5:118–122. doi: 10.1038/nmat1571. [DOI] [PubMed] [Google Scholar]
- 2.Ale A, Ermolayev V, Herzog E, Cohrs C, Hrabe de Angelis M, Ntziachristos V. Nat. Methods. 2012;9:615–620. doi: 10.1038/nmeth.2014. [DOI] [PubMed] [Google Scholar]
- 3.Yu S-B, Watson AD. Chem. Rev. 1999;99:2353–2377. doi: 10.1021/cr980441p. [DOI] [PubMed] [Google Scholar]
- 4.Oh MH, Lee N, Kim H, Park SP, Piao Y, Lee J, Jun SW, Moon WK, Choi SH, Hyeon T. J. Am. Chem. Soc. 2011;133:5508–5515. doi: 10.1021/ja200120k. [DOI] [PubMed] [Google Scholar]
- 5.Chen H, Rogalski MM, Anker JN. Phys. Chem. Chem. Phys. 2012;14:13469–13486. doi: 10.1039/c2cp41858d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen H-Y, Patrick AL, Yang Z-Q, Van Derveer DG, Anker JN. Anal. Chem. 2011;83:5045–5049. doi: 10.1021/ac200054v. [DOI] [PubMed] [Google Scholar]
- 7.Pratx G, Carpenter CM, Sun C, Rao RP, Xing L. Opt. Lett. 2010;35:3345–3347. doi: 10.1364/OL.35.003345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen H, Longfield DE, Varahagiri VS, Nguyen KVT, Patrick AL, Qian H, Van Derveer DG, Anker JN. Analyst. 2011;136:3438–3445. doi: 10.1039/c0an00931h. [DOI] [PubMed] [Google Scholar]
- 9.Armelao L, Heigl F, Kim P-SG, Rosenberg RA, Regier TZ, Sham T-K. J. Phys. Chem. C. 2012;116:14163–14169. [Google Scholar]
- 10.Sun C, Pratx G, Carpenter Colin M, Liu H, Cheng Z, Gambhir Sanjiv S, Xing L. Adv. Mater. 2011;23:H195–H199. doi: 10.1002/adma.201100919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Osakada Y, Pratx G, Hanson L, Solomon PE, Xing L, Cui B. Chem. Commun. 2013;49:4319–4321. doi: 10.1039/c2cc37169c. [DOI] [PubMed] [Google Scholar]
- 12.Choi S, Yu J, Patel SA, Tzeng Y-L, Dickson RM. Photochem. Photobiol. Sci. 2011;10:109–115. doi: 10.1039/c0pp00263a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng J, Nicovich PR, Dickson RM. Annu. Rev. Phys. Chem. 2007;58:409–431. doi: 10.1146/annurev.physchem.58.032806.104546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shang L, Dong S, Nienhaus GU. Nano Today. 2011;6:401–418. [Google Scholar]
- 15.Xie J, Zheng Y, Ying JY. J. Am. Chem. Soc. 2009;131:888–889. doi: 10.1021/ja806804u. [DOI] [PubMed] [Google Scholar]
- 16.Chen T-H, Tseng W-L. Small. 2012;8:1912–1919. doi: 10.1002/smll.201102741. [DOI] [PubMed] [Google Scholar]
- 17.Wu X, He X, Wang K, Xie C, Zhou B, Qing Z. Nanoscale. 2010;2:2244–2249. doi: 10.1039/c0nr00359j. [DOI] [PubMed] [Google Scholar]
- 18.Liu C-L, Wu H-T, Hsiao Y-H, Lai C-W, Shih C-W, Peng Y-K, Tang K-C, Chang H-W, Chien Y-C, Hsiao J-K, Cheng J-T, Chou P-T. Angew. Chem. Int. Ed. 2011;50:7056–7060. doi: 10.1002/anie.201100299. [DOI] [PubMed] [Google Scholar]
- 19.Zhang A, Tu Y, Qin S, Li Y, Zhou J, Chen N, Lu Q, Zhang B. J. Colloid Interface Sci. 2012;372:239–244. doi: 10.1016/j.jcis.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 20.Richards CI, Choi S, Hsiang J-C, Antoku Y, Vosch T, Bongiorno A, Tzeng Y-L, Dickson RM. J. Am. Chem. Soc. 2008;130:5038–5039. doi: 10.1021/ja8005644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kennedy TAC, MacLean JL, Liu J. Chem. Commun. 2012;48:6845–6847. doi: 10.1039/c2cc32841k. [DOI] [PubMed] [Google Scholar]
- 22.Chen W-Y, Lan G-Y, Chang H-T. Anal. Chem. 2011;83:9450–9455. doi: 10.1021/ac202162u. [DOI] [PubMed] [Google Scholar]
- 23.Petty JT, Story SP, Hsiang J-C, Dickson RM. J. Phys. Chem. Lett. 2013;4:1148–1155. doi: 10.1021/jz4000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sham T-K, Rosenberg RA. Chem Phys Chem. 2007;8:2557–2567. doi: 10.1002/cphc.200700226. [DOI] [PubMed] [Google Scholar]
- 25.Kim P-SG, Hu Y, Brandys M-C, Burchell TJ, Puddephatt RJ, Sham TK. Inorg. Chem. 2007;46:949–957. doi: 10.1021/ic0609352. [DOI] [PubMed] [Google Scholar]
- 26.Atak K, Engel N, Lange KM, Golnak R, Gotz M, Soldatov M, Rubensson J-E, Kosugi N, Aziz EF. Chem Phys Chem. 2012;13:3106–3111. doi: 10.1002/cphc.201200314. [DOI] [PubMed] [Google Scholar]
- 27.Sham TK, Gordon RA. AIP Conf. Proc. 2010;1234:133–136. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.