Abstract
Purpose
Although anatomic segmentectomy has been considered a compromised procedure by many surgeons, recent retrospective, single-institution series have demonstrated tumor recurrence and patient survival rates that approximate those achieved by lobectomy. The primary objective of this study was to use propensity score matching to compare outcomes after these anatomic resection approaches for stage I non–small-cell lung cancer.
Patients and Methods
A retrospective data set including 392 segmentectomy patients and 800 lobectomy patients was used to identify matched segmentectomy and lobectomy cohorts (n = 312 patients per group) using a propensity score matching algorithm that accounted for confounding effects of preoperative patient variables. Primary outcome variables included freedom from recurrence and overall survival. Factors affecting survival were assessed by Cox regression analysis and Kaplan-Meier estimates.
Results
Perioperative mortality was 1.2% in the segmentectomy group and 2.5% in the lobectomy group (P = .38). At a mean follow-up of 5.4 years, comparing segmentectomy with lobectomy, no differences were noted in locoregional (5.5% v 5.1%, respectively; P = 1.00), distant (14.8% v 11.6%, respectively; P = .29), or overall recurrence rates (20.2% v 16.7%, respectively; P = .30). Furthermore, when comparing segmentectomy with lobectomy, no significant differences were noted in 5-year freedom from recurrence (70% v 71%, respectively; P = .467) or 5-year survival (54% v 60%, respectively; P = .258). Segmentectomy was not found to be an independent predictor of recurrence (hazard ratio, 1.11; 95% CI, 0.87 to 1.40) or overall survival (hazard ratio, 1.17; 95% CI, 0.89 to 1.52).
Conclusion
In this large propensity-matched comparison, lobectomy was associated with modestly increased freedom from recurrence and overall survival, but the differences were not statistically significant. These results will need further validation by prospective, randomized trials (eg, Cancer and Leukemia Group B 140503 trial).
INTRODUCTION
Although surgical resection of clinical stage I non–small-cell lung cancer (NSCLC) remains the standard of care, controversy exists with regard to the extent of parenchymal resection required for local control of the tumor and disease-free survival.1–5 Intentional use of anatomic segmentectomy for the treatment of small peripheral lung cancers was first performed by Jensik et al.6 Lobectomy has been considered the standard of surgical care for early-stage NSCLC identified in the physiologically fit patient7; however, anatomic segmentectomy performed by open surgical techniques or through video-assisted thoracic surgical approaches has gained enthusiasm by many surgical groups over the years.8–10 Additionally, nonsurgical approaches using hypofractionated, stereotactic radiotherapy and image-guided probing of the tumor for delivery of radiofrequency or microwave energy to focally ablate small peripheral lung cancers are also challenging the concept of universal selection of anatomic lobectomy for clinical stage I NSCLC.11,12
We focused our present analysis on clinical stage I NSCLC, because describing the results of various surgical resection approaches related to pathologic stage I disease necessarily biases outcomes toward surgical interventions with greater objective intraoperative staging of the pulmonary hilum and mediastinum. This is an important point of distinction, in that it is appreciated that pathologic upstaging from the pretreatment clinical stage may be seen in up to 30% of patients, even with modern computed tomography (CT) and positron emission tomography staging techniques being used.13,14
To accomplish this analysis, we explored our clinical outcomes with anatomic segmentectomy and lobectomy for clinical stage I NSCLC using a propensity-matched risk model and multivariable parameter assessment,15,16 because results of randomized studies directly comparing these anatomic resection approaches are lacking.
PATIENTS AND METHODS
Patients
Approval for this study was provided by the Institutional Review Board of the University of Pittsburgh, and individual patient consent was waived. We performed a retrospective analysis of 1,192 patients who underwent anatomic segmentectomy (n = 392) or lobectomy (n = 800) for clinical stage I NSCLC derived from the Lung Cancer Database of the University of Pittsburgh. Patients were staged according to the seventh edition of the International Union Against Cancer/American Joint Committee on Cancer lung cancer staging system. Pathologic information was derived from the published case synoptic for each patient.
Propensity Score Matching
Propensity score matching is a method for creating similar case (segmentectomy) and control (lobectomy) sets from an existing data set for a retrospective analysis.17 The sets are similar in that the cases and controls are matched on a set of variables that would otherwise confound comparisons between the cases and controls; in this case, the matching variables would be those that the surgeon would consider in choosing the type of surgery and baseline clinical characteristics. The significant potential confounders are identified by logistic regression, and each patient is assigned a score based on those confounders. Cases and controls are then matched on those scores, rather than the individual confounders (which is usually infeasible). The outcomes (eg, overall survival) can then be compared between the case and control sets without adjusting for the confounders. Short of a prospective, randomized trial, this propensity-matching technique provides the fairest comparison of matched sets in the setting of a retrospective study.
Of the original 1,192 patients in the database, 42 were removed secondary to tumor size greater than 5 cm or because their propensity scores could not be calculated because of missing values, leaving 763 lobectomies and 387 segmentectomies on which the propensity score matching was performed. The variables for the propensity score matching were chosen by first performing a logistic regression of segmentectomy versus lobectomy on the following factors: age (years); sex; ever smoked (yes or no); forced expiratory volume in 1 second (FEV1); preoperative hypertension, chronic obstructive pulmonary disease (COPD), diabetes mellitus, gastroesophageal reflux disease, coronary artery disease, or prior cancer history (all yes or no); preoperative estimate of tumor size (cm); and date of surgery. The potential predictors that were not statistically significant (P > .05) were removed, and the propensity score was calculated from the logistic regression refit to the reduced variable group, which included age, FEV1, preoperative COPD, tumor size, and date of surgery. Segmentectomy and lobectomy patients were then matched 1:1 using a greedy five-digit matching algorithm. Out of the 387 segmentectomy patients and 763 lobectomy patients, 312 pairs were matched; the 1:1 matching algorithm could not find lobectomies for the remaining 75 segmentectomies. Patient demographics and tumor characteristics for the matched groups are listed in Table 1.
Table 1.
Patient Demographic and Clinical Characteristics
| Characteristic | Segmentectomy (n = 312) |
Lobectomy (n = 312) |
P | ||
|---|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | ||
| Age, years | |||||
| Mean | 68.5 | 68.4 | .88 | ||
| SD | 9.2 | 9.2 | |||
| Sex | .75 | ||||
| Male | 139 | 144 | |||
| Female | 173 | 168 | |||
| Comorbidities | |||||
| HTN | 165 | 52.9 | 152 | 48.7 | .34 |
| DM | 57 | 18.3 | 44 | 14.1 | .19 |
| COPD | 103 | 33.0 | 105 | 33.7 | .93 |
| Previous cancer | 77 | 24.7 | 71 | 22.8 | .64 |
| Smoking status | 1.00 | ||||
| Ever | 290 | 92.9 | 290 | 92.9 | |
| Never | 22 | 7.1 | 22 | 7.1 | |
| PFTs: FEV1, % | .68 | ||||
| Mean | 77.8 | 75.1 | |||
| SD | 22.5 | 19.7 | |||
| Histology | |||||
| Adenocarcinoma | 177 | 56.7 | 183 | 58.7 | .69 |
| Squamous | 89 | 28.5 | 97 | 31.1 | .54 |
| Tumor size, cm | .88 | ||||
| Mean | 2.2 | 2.2 | |||
| SD | 1.0 | 1.1 | |||
| Tumor stage | .44 | ||||
| IA | 248 | 239 | |||
| IB | 64 | 73 | |||
| Follow-up time, years | 5.3 | 5.4 | .86 | ||
Abbreviations: COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; FEV1, forced expiratory volume in 1 second; HTN, hypertension; PFTs, pulmonary function tests; SD, standard deviation.
Operative Technique
Surgeries performed included 312 lobectomies and 312 anatomic segmentectomies. A video-assisted thoracoscopic surgery approach was used in 54.6% of the patients in this study, with thoracotomy performed in 45.4% of patients. Anatomic segmentectomy was performed as described previously using the clinical and anatomic parameters for lesion selection.2,10 Criteria favoring the use of anatomic segmentectomy include T1 tumors (< 3 cm in diameter), tumors confined to discrete anatomic segmental boundaries, absence of proximal segmental bronchial involvement/lesion confined to the outer half of the lung parenchyma, absence of visceral pleural involvement, and absence of clinically positive hilar or mediastinal adenopathy.
Anatomic segmentectomy was accomplished by the removal of one or more pulmonary parenchymal segments with its corresponding bronchovascular and lymphatic supply. In contradistinction to wedge resection (which does not involve anatomic hilar dissection), anatomic segmentectomy is accomplished by individual isolation and division of the targeted segmental bronchial and vascular structures and complete excision of the segmental pedicle.18,19 The endostapler was then applied to encompass a parenchymal margin of resection on the adjacent segment of the lung (extended segmentectomy) ensuring a resection margin equivalent to the diameter of the tumor.10,20 Systematic hilar and mediastinal nodal sampling was then performed. Lobectomy was also accomplished using the individual isolation, ligation, and division of the corresponding bronchovascular contributions to the lobe, similar to the technique used for anatomic segmentectomy. The distribution of resected segments and lobes is provided in Table 2.
Table 2.
Distribution of Anatomic Segmentectomy and Lobectomy Procedures
| Anatomic Location | Lobectomy (n = 312) |
Segmentectomy (n = 312) |
||
|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | |
| Right upper lobe | ||||
| Apical segment | 24 | 7.7 | ||
| Anterior segment | 126 | 40.4 | 15 | 4.8 |
| Posterior segment | 37 | 11.9 | ||
| Apicoposterior segment | 11 | 3.5 | ||
| Right middle lobe | 28 | 9.0 | ||
| Medial segment | 2 | 0.6 | ||
| Lateral segment | 8 | 2.6 | ||
| Right lower lobe | 43 | 13.8 | ||
| Superior segment | 38 | 12.2 | ||
| Basilar segment | 32 | 10.3 | ||
| Left upper lobe | 85 | 27.2 | ||
| Upper division segment | 77 | 24.7 | ||
| Lingula segment | 21 | 6.7 | ||
| Left lower lobe | 30 | 9.6 | ||
| Superior segment | 25 | 8.0 | ||
| Basilar segment | 22 | 7.1 | ||
Follow-Up
Perioperative data were actively collected from the hospital chart, anesthesia, and operating room records as well as the electronic medical record and/or office charts for each patient. Complications were documented for each patient based on standard definitions established for the Society of Thoracic Surgeons General Thoracic Surgery Database.21 All patients were observed postoperatively with CT scans at 2 weeks and at 4- to 6-month intervals for the first 2 years, and then yearly thereafter. Perioperative mortality was defined as any death within the first 30 days after surgery or during the same hospitalization. Ninety-day mortality was also calculated. Locoregional recurrence was defined as evidence of tumor within the same lobe, the hilum, or the mediastinal lymph nodes. Distant recurrences were defined as evidence of tumor in another lobe, the pleural space, or elsewhere outside the hemithorax. Median follow-up time was 5.4 years for the entire cohort.
Statistical Analysis
Wilcoxon and t tests were used to compare the distributions of continuous data (age, tumor size, number of lymph nodes removed, operative time, and estimated blood loss), and the χ2 or Fisher's exact test was used to compare the frequencies of categorical measures (eg, sex, histology, stage) between lobectomies and segmentectomies. All comparisons were two-tailed. Postoperative complications were tabulated and categorized according to those used in the Society of Thoracic Surgeons National Database.21 Thirty- and 90-day mortality were also assessed.
Primary outcome variables included freedom from recurrence and overall survival. Locoregional recurrence was defined as any recurrence in the ipsilateral lobe, hilum, or mediastinum for segmentectomy patients and any hilar or mediastinal recurrence noted for lobectomy patients, without evidence of distant metastases for either resection mode used. Distant recurrence was defined as any recurrence in a distinctly different lobe of the ipsilateral lung, contralateral mediastinum, or hilum and extrathoracic metastatic disease. Freedom from recurrence was defined as the time from surgery to the first diagnosis of local, regional, or distant disease recurrence or until last follow-up; death was considered a censoring event for recurrence. Overall survival was defined as the time from surgery to death or last follow-up. Overall survival and freedom from recurrence functions were estimated using the Kaplan-Meier method. Survival functions were compared using the log-rank test.
Multiple clinical variables were evaluated for their association with time to recurrence in univariable analysis. Variables demonstrating a significant association with recurrence in univariable analysis (P < .05) were then analyzed in a forward proportional hazards (Cox) regression model. Corresponding hazard ratios, CIs, and P values for each variable were determined using the SAS software package (SAS Institute, Cary, NC).
RESULTS
Differences Between Matched and Unmatched Patients
The average age was 66.9 years in the unmatched patients (n = 526) and 68.4 years in the matched set (n = 624). Mean FEV1 values were 81.9% and 76.4%, respectively. Average tumor sizes were 2.6 cm in the unmatched set and 2.2 cm in the matched set. In the unmatched set, 19.3% of patients had preoperative COPD versus 33.4% of patients in the matched set. Patients in the matched set had surgery an average of 4.9 months later (in calendar time) than in the unmatched set.
Clinical Characteristics
Patient age, sex, comorbidities, pathologic cell type distribution, and tumor size were equivalent between the matched groups (Table 1). The mean patient age was 68.5 years. The female-to-male ratio was 283:341 for the entire cohort. The vast majority of patients were current or former smokers (92.9%), with a moderate degree of pulmonary impairment (FEV1, 76.4%). The mean tumor size was 2.2 cm. The clinical stage distribution was as follows: stage IA, 487 patients (78.0%); and stage IB, 137 patients (22.0%). The most common lobar and segmental resections performed were right upper lobectomy (n = 126, 40.4%) and left upper division segmentectomy (n = 77, 24.7%), respectively (Table 2). Lobectomy was associated with a significantly increased number of harvested lymph nodes compared with segmentectomy (median, 12 v six nodes, respectively; P < .001). There was no significant difference in the number of lymph node stations sampled with lobectomy versus segmentectomy (median, three v three stations, respectively; P = .45), suggesting that the difference in nodal sampling was related, at least in part, to the extent of parenchyma resected. Final pathologic stage distribution was as follows: stage IA, 345 patients (55.3%); stage IB, 202 patients (32.4%); and stage IIA to IIIB, 77 patients (12.3%). Overall agreement between clinical and pathologic stage was 63.7%. Pathologic upstaging occurred in 32.3% of patients; 4.0% of patients were downstaged.
Perioperative Outcomes
Overall morbidity was similar between the segmentectomy (36.9%) and lobectomy (32.7%) groups (P = .31). The 30-day mortality rate for segmentectomy was 1.2%, compared with 2.5% for lobectomy (P = .38). The 90-day mortality rates were 2.6% for segmentectomy versus 4.8% for lobectomy (P = .20; Table 3).
Table 3.
Perioperative Outcomes: Segmentectomy Versus Lobectomy
| Outcome | Segmentectomy (n = 312) |
Lobectomy (n = 312) |
P | ||
|---|---|---|---|---|---|
| Proportion | 95% CI | Proportion | 95% CI | ||
| Mortality | |||||
| 30 day | 0.012 | 0.004 to 0.32 | 0.025 | 0.011 to 0.050 | .38* |
| 90 day | 0.026 | 0.011 to 0.050 | 0.048 | 0.027 to 0.078 | .20* |
| Site of recurrence | |||||
| Locoregional | 0.057 | 0.034 to 0.089 | 0.057 | 0.034 to 0.089 | 1.00* |
| Distant | 0.153 | 0.115 to 0.198 | 0.127 | 0.093 to 0.169 | .42* |
| 5-year freedom from recurrence† | 0.70 | 0.63 to 0.78 | 0.71 | 0.64 to 0.78 | .47‡ |
| 5-year overall survival† | 0.54 | 0.47 to 0.61 | 0.60 | 0.54 to 0.67 | .26‡ |
Fisher's exact test.
Estimates from Kaplan-Meier survival function estimate and 95% CIs.
Log-rank test comparing survival functions.
Recurrence and Survival
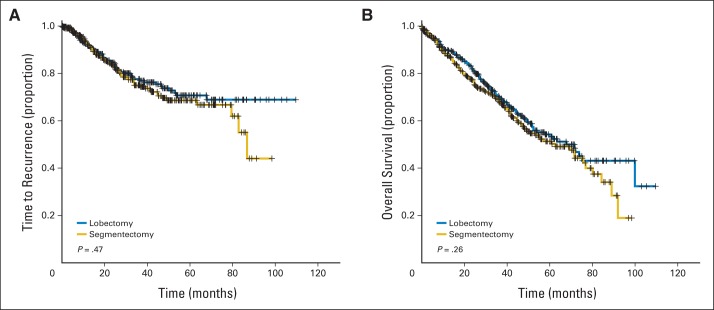
At a mean follow-up of 5.4 years, comparing segmentectomy with lobectomy, no differences were noted in either locoregional (5.5% v 5.1%, respectively; P = 1.00) or distant (14.8% v 11.6%, respectively; P = .29) recurrence rates. Kaplan-Meier estimates of freedom from recurrence (70% v 71%, respectively; P = .467) and overall survival (54% v 60%, respectively; P = .258) at 5 years similarly demonstrated no statistically significant difference between groups (Table 3, Fig 1). Importantly, no significant differences were seen in time to recurrence when comparing patients with T1a tumors (≤ 2 cm; P = .585), T1b tumors (2 to 3 cm; P = .395), or T2a tumors (3 to 5 cm; P = .432).
Fig 1.
Kaplan-Meier survival estimates for (A) time to recurrence and (B) overall survival between propensity score–matched patients undergoing segmentectomy or lobectomy.
Multivariable Analysis
On univariable analysis, none of the patient or operative variables analyzed demonstrated an association with recurrence (Table 4). Of importance, anatomic segmentectomy was not found to be a predictor of recurrence. When examining predictors of overall survival, age, male sex, preoperative COPD, preoperative coronary artery disease, and tumor size (all P < .05) were all associated with reduced survival in univariable and multivariable analyses (Tables 4 and 5). Of importance, anatomic segmentectomy was not found to be an independent predictor of recurrence (hazard ratio, 1.11; 95% CI, 0.87 to 1.40) or overall survival (hazard ratio, 1.17; 95% CI, 0.89 to 1.52).
Table 4.
Univariable Analysis of Recurrence and Overall Survival
| Factor | P |
|---|---|
| Recurrence | |
| Patient variable | |
| Age | .849 |
| Sex | .111 |
| Ever-smoker | .950 |
| Comorbidities | |
| COPD | .609 |
| Hypertension | .729 |
| Diabetes mellitus | .734 |
| CAD | .082 |
| GERD | .159 |
| Previous cancer history | .165 |
| Pulmonary function (FEV1) | .606 |
| Operative variables | |
| Surgery | .391 |
| Laterality | .584 |
| Lobe involved | .067 |
| Operative time | .818 |
| Lymph nodes examined | .140 |
| Tumor size | .491 |
| Tumor histology | .881 |
| Overall survival | |
| Patient variables | |
| Age | < .001* |
| Sex | < .001* |
| Ever-smoker | .717 |
| Comorbidities | |
| COPD | .022* |
| Hypertension | .729 |
| Diabetes mellitus | .273 |
| CAD | .004* |
| GERD | .106 |
| Previous cancer history | .165 |
| Pulmonary function (FEV1) | .089 |
| Operative variables | |
| Surgery | .154 |
| Laterality | .986 |
| Lobe involved | .706 |
| Operative time | .143 |
| Tumor size | .008* |
| Tumor histology | .523 |
Abbreviations: CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 second; GERD, gastroesophageal reflux disease.
Statistically significant.
Table 5.
Multivariable Analysis of Overall Survival
| Factor | Hazard Ratio | P |
|---|---|---|
| Age | 1.04 | < .001 |
| Sex | 1.53 | < .001 |
| Preoperative COPD | 1.35 | .018 |
| Preoperative CAD | 1.83 | .003 |
| Tumor size | 1.19 | .006 |
| Surgery | 1.23 | .126 |
Abbreviations: CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease.
DISCUSSION
How should we approach the peripheral, small, clinically node-negative NSCLC today? Pneumonectomy was the standard of care for resectable lung cancer for nearly two decades.22–26 However, by the late 1950s, lobectomy had surpassed it as the preferred mode of resection for peripheral lung cancers as a result of observed increases in long-term survival with lobectomy (19% v 12% for pneumonectomy) at 5 years, as well as a reduction in operative morbidity and mortality.27 These generally poor results by today's standards can be primarily attributed to the limitations of clinical staging during that period and to a near totally subjective evaluation of the associated medical conditions (eg, impaired functional lung reserve, cardiac disease) that affect early postsurgical and long-term survival.
The findings of large-population CT screening programs for early lung cancer identification,28–40 coupled with these clinical staging advances, have led to an increased number of clinically suspicious lung nodules suggestive of early-stage NSCLC in modern practice. The following question has thus arisen: Is lobectomy needed for the small peripheral symptomatic lung nodule serendipitously identified by CT scanning?41,42
Over the years, our group has been influenced by the insights of breast surgical oncologists considering lesser resections for small breast cancers43 and has explored the potential merits of parenchymal preservation for the patient with lung cancer in the setting of clinical stage I NSCLC.40–42 Despite a growing number of reports suggesting the utility of a selective application of sublobar resection, and specifically anatomic segmentectomy, for peripheral stage I NSCLC, resection of even the smallest peripheral lung cancer is still considered by many surgeons as a compromised therapy that should be only considered for the physiologically impaired patient who is not a candidate for anatomic lobectomy.1,4,41 This perception has been primarily established by the findings of the Lung Cancer Study Group, which represents the only prospective, multicenter, randomized trial comparing lobectomy with sublobar resection performed to date. In this study, a three-fold increase in the rate of locoregional recurrence was noted in the sublobar resection arm compared with the lobectomy arm (17.2% v 6.4%, respectively), which included patients undergoing either wedge resection or segmentectomy. Interestingly, we and others have previously found that anatomic segmentectomy may have a reduced risk of locoregional recurrence compared with nonanatomic wedge resection and that anatomic segmentectomy more closely approximates the oncologic results after lobectomy.42 The results of the current propensity-matched study suggest that anatomic segmentectomy can achieve perioperative (morbidity and mortality) and oncologic outcomes (recurrence and survival) that are not statistically significantly different compared with those achieved by lobectomy for clinical stage I disease, and thus anatomic segmentectomy should be considered as a valid alternative to lobectomy in properly selected patients.
In this study, we also identified that a similar hilar and mediastinal lymph node station examination was accomplished by segmentectomy and lobectomy. Although a greater number of nodes removed during mediastinal evaluation may lead to greater discovery of surprise node-positive disease, this occurred less than 4% of the time in the recently reported results of the American College of Surgeons Oncology Group Z0030 investigation of mediastinal node dissection versus systematic sampling after resection of stage I NSCLC. Additionally, no improvement in survival could be demonstrated in this trial with the standard use of mediastinal and hilar node dissection over systemic sampling.44 In the current study, although lobectomy yielded a greater absolute number of lymph nodes (median, 12 nodes with lobectomy v six nodes with segmentectomy; P < .001), when comparing segmentectomy with lobectomy, there was no significant difference in the number of lymph node stations assessed (median of three lymph node stations in both groups; P = .45) or degree of clinical to pathologic upstaging (36.5% v 29.5%, respectively; P = .073), and no corresponding statistically significant difference in recurrence or overall survival (Table 3, Fig 1).
Approaching the problem of small peripheral lung cancer from a different direction, we see the emergence of enthusiasm with nonsurgical image-guided ablative and focused radiotherapeutic approaches to the small peripheral lung nodule suspicious for lung cancer.11,12,45 The commentary of advocates of nonsurgical approaches to peripheral lung cancer relate to the avoidance of any morbidity related to surgery with the theoretical possibility of equivalent long-term survival. But as the saying goes, “pay me now or pay me later.” It seems that the risk of local lung injury leading to intermediate and late-term unintended negative consequences, such as progressive radiation pneumonitis, fibrosis, and loss of pulmonary function,46–48 and the incremental loss of opportunity to identify a significant minority of patients with more advanced cancers who may benefit from adjuvant therapy are concerns.
Today more than ever, we should not be satisfied with the concept of the simple local extirpation of the point of disease presentation manifested in the apparently locally confined NSCLC. Again, an emphasis on improving the overall outcome of clinical stage I NSCLC, which includes patients with occult/surprise lymph node positivity (identified in up to 30% of patients), mandates the pathologic assessment of the disease through surgical resection. Accordingly, tissue still remains the issue in the surgical management of early-stage NSCLC, particularly in this age of individualized therapy for the patient with lung cancer.49–53 Surgical resection of peripheral lung cancers represents the standard of care. Similar to the arguments with breast cancer surgery, the advantages of surgery are pathologic assessment of surgical margins, the establishment of pathologic regional nodal status, and, in this era of increasing enthusiasm for adjuvant systemic therapy, provision of tissue for pharmacogenomic assessment.
Limitations of this study include the potential introduction of surgical and selection bias associated with the retrospective assessment of prospectively collected data. We have attempted to directly address this fundamental limitation with the use of propensity matching and multivariable analysis. Another limitation of this study is that the presented data are derived from a single institution with expertise in minimally invasive and open lobectomy and segmentectomy techniques. The generalization of the observed outcomes to broad clinical practice must therefore be cautiously scrutinized. Multicenter, prospective, randomized studies currently under way (Cancer and Leukemia Group B 140503; Japan Clinical Oncology Group 0802/West Japan Oncology Group 4607L) will thus be necessary to confirm the results of this study.
In conclusion, the information obtained from this propensity-matched analysis supports this concept of anatomic segmental resection for the small peripheral lung cancer anatomically confined to segmental boundaries. However, confirmation of clear, generous margins of resection and the assurance of accurate intraoperative pathologic nodal staging of the lesion are important considerations that should lead us to favor lobectomy over segmentectomy when an issue. For the small peripheral lung cancer, however, anatomic segmentectomy appears to offer comparable local control and the opportunity for prolonged disease-free and overall survival that is not statistically different when compared with lobectomy.
Footnotes
Supported in part by National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute Grant No. 1K08 HL097078-1, jointly sponsored by the Thoracic Surgery Foundation for Research and Education. Also supported by NIH/National Cancer Institute Grant No. P50 CA090440-11 (D.P.N.).
Presented in part at the 48th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, June 1-5, 2012.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Neil A. Christie, Varian (C) Stock Ownership: None Honoraria: None Research Funding: James D. Luketich, Accuray Expert Testimony: None Patents, Royalties, and Licenses: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Rodney J. Landreneau, Matthew J. Schuchert
Collection and assembly of data: Joseph J. Wizorek, Matthew J. Schuchert
Data analysis and interpretation: Daniel P. Normolle, Neil A. Christie, Omar Awais, Ghulam Abbas, Arjun Pennathur, Manisha Shende, Benny Weksler, James D. Luketich, Matthew J. Schuchert
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Ginsberg RJ, Rubinstein LV Lung Cancer Study Group. Randomized trial of lobectomy versus limited resection for T1N0 non-small cell lung cancer. Ann Thorac Surg. 1995;60:615–622. doi: 10.1016/0003-4975(95)00537-u. [DOI] [PubMed] [Google Scholar]
- 2.Pettiford BL, Schuchert MJ, Santos R, et al. Role of sublobar resection (segmentectomy and wedge resection) in the surgical management of non-small cell lung cancer. Thorac Surg Clin. 2007;17:175–190. doi: 10.1016/j.thorsurg.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Lewis RJ. The role of video-assisted thoracic surgery for carcinoma of the lung: Wedge resection to lobectomy by simultaneous individual stapling. Ann Thorac Surg. 1993;56:762–768. doi: 10.1016/0003-4975(93)90975-n. [DOI] [PubMed] [Google Scholar]
- 4.Whitson BA, Groth SS, Andrade RS, et al. Survival after lobectomy versus segmentectomy for stage I non-small cell lung cancer: A population-based analysis. Ann Thorac Surg. 2011;92:1943–1950. doi: 10.1016/j.athoracsur.2011.05.091. [DOI] [PubMed] [Google Scholar]
- 5.Mery CM, Pappas AN, Bueno R, et al. Similar long-term survival of elderly patients with non-small cell lung cancer treated with lobectomy or wedge resection within the surveillance, epidemiology, and end results database. Chest. 2005;128:237–245. doi: 10.1378/chest.128.1.237. [DOI] [PubMed] [Google Scholar]
- 6.Jensik RJ, Faber LP, Milloy FJ, et al. Segmental resection for lung cancer: A fifteen year experience. J Thorac Cardiovasc Surg. 1973;66:563–572. [PubMed] [Google Scholar]
- 7.Warren WH, Faber LP. Segmentectomy versus lobectomy in patients with stage I pulmonary carcinoma: Five-year survival and patterns of intrathoracic recurrence. J Thorac Cardiovasc Surg. 1994;107:1087–1093. [PubMed] [Google Scholar]
- 8.Read RC, Yoder G, Schaeffer RC. Survival after conservative resection for T1 N0 M0 non-small cell lung cancer. Ann Thorac Surg. 1990;49:242–247. doi: 10.1016/0003-4975(90)90242-x. [DOI] [PubMed] [Google Scholar]
- 9.Okada M, Yoshikawa K, Hatta T, et al. Is segmentectomy with lymph node assessment an alternative to lobectomy for non-small cell lung cancer of 2 cm or smaller? Ann Thorac Surg. 2001;71:956–961. doi: 10.1016/s0003-4975(00)02223-2. [DOI] [PubMed] [Google Scholar]
- 10.Schuchert MJ, Pettiford BL, Keeley S, et al. Anatomic segmentectomy in the treatment of stage I non-small cell lung cancer. Ann Thorac Surg. 2007;84:926–932. doi: 10.1016/j.athoracsur.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 11.Onishi H, Shirato H, Nagata Y, et al. Hypofractionated stereotactic radiotherapy (HypoFXSRT) for stage I non-small cell lung cancer: Updated results of 257 patients in a Japanese multi-institutional study. J Thorac Oncol. 2007;2(suppl 3):S94–S100. doi: 10.1097/JTO.0b013e318074de34. [DOI] [PubMed] [Google Scholar]
- 12.Fakiris AJ, McGarry RC, Yiannoutsos CT, et al. Stereotactic body radiation therapy for early-stage non-small-cell lung carcinoma: Four-year results of a prospective phase II study. Int J Radiat Oncol Biol Phys. 2009;75:677–682. doi: 10.1016/j.ijrobp.2008.11.042. [DOI] [PubMed] [Google Scholar]
- 13.López-Encuentra A, García-Luján R, Rivas JJ, et al. Comparison between clinical and pathologic staging in 2,994 cases of lung cancer. Ann Thorac Surg. 2005;79:974–979. doi: 10.1016/j.athoracsur.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 14.Schuchert MJ, Abbas G, Pennathur A, et al. Anatomic lung resection for clinical stage I non-small cell lung cancer (NSCLC): Equivalent outcomes following anatomic segmentectomy and lobectomy. Presented at the American College of Chest Physicians Annual Meeting; November 4, 2010; Vancouver, British Columbia, Canada. [Google Scholar]
- 15.Baser O. Too much ado about propensity score models? Comparing methods of propensity score matching. Value Health. 2006;9:377–385. doi: 10.1111/j.1524-4733.2006.00130.x. [DOI] [PubMed] [Google Scholar]
- 16.Austin PC. A critical appraisal of propensity-score matching in the medical literature between 1996 and 2003. Stat Med. 2008;27:2037–2049. doi: 10.1002/sim.3150. [DOI] [PubMed] [Google Scholar]
- 17.Dehejia R, Wahba S. Propensity score-matching methods for nonexperimental causal studies. Rev Econ Statistics. 2002;84:151–161. [Google Scholar]
- 18.Szwerc MF, Landreneau RJ, Santos RS, et al. Minithoracotomy combined with mechanically stapled bronchial and vascular ligation for anatomical lung resection. Ann Thorac Surg. 2004;77:1904–1910. doi: 10.1016/j.athoracsur.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 19.Schuchert MJ, Abbas G, Pettiford BL, et al. Preliminary results of anatomic lung resection using energy-based tissue and vessel coagulative fusion technology. J Thorac Cardiovasc Surg. 2010;140:1168–1173. doi: 10.1016/j.jtcvs.2010.06.064. [DOI] [PubMed] [Google Scholar]
- 20.Sawabata N, Ohta M, Matsumura A, et al. Optimal distance of malignant negative margin in excision of nonsmall cell lung cancer: A multicenter prospective study. Ann Thorac Surg. 2004;77:415–420. doi: 10.1016/S0003-4975(03)01511-X. [DOI] [PubMed] [Google Scholar]
- 21.Clark RE. The Society of Thoracic Surgeons National Database status report. Ann Thorac Surg. 1994;57:20–26. doi: 10.1016/0003-4975(94)90359-x. [DOI] [PubMed] [Google Scholar]
- 22.Baue AE. Landmark perspective: Evarts A. Graham and the first pneumonectomy. JAMA. 1984;251:261–264. doi: 10.1001/jama.251.2.260. [DOI] [PubMed] [Google Scholar]
- 23.Ellis H. The first pneumonectomies for lung cancer. J Perioper Pract. 2008;18:130–131. doi: 10.1177/175045890801800306. [DOI] [PubMed] [Google Scholar]
- 24.Archibald E. The technic of total unilateral pneumonectomy. Ann Surg. 1934;100:796–811. doi: 10.1097/00000658-193410000-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.MacLean LD, Entin MA. Norman Bethune and Edward Archibald: Sung and unsung heroes. Ann Thorac Surg. 2000;70:1746–1752. doi: 10.1016/s0003-4975(00)02043-9. [DOI] [PubMed] [Google Scholar]
- 26.Ochsner A, DeBakey M. Surgical considerations of primary carcinoma of the lung: Review of the literature and report of 19 cases. Surgery. 1940;8:992–1023. [Google Scholar]
- 27.Churchill ED, Sweet RH, Soutter L, et al. The surgical management of carcinoma of the lung: A study of the cases treated at the Massachusetts General Hospital from 1930 to 1950. J Thorac Surg. 1950;20:349–365. [PubMed] [Google Scholar]
- 28.Sone S, Takashima S, Li F, et al. Mass screening for lung cancer with mobile spiral computed tomography scanner. Lancet. 1998;351:1242–1245. doi: 10.1016/S0140-6736(97)08229-9. [DOI] [PubMed] [Google Scholar]
- 29.International Early Lung Cancer Action Program Investigators. Henschke CI, Yankelevitz DF, et al. Survival of patients with stage I lung cancer detected on CT screening. N Engl J Med. 2006;355:1763–1771. doi: 10.1056/NEJMoa060476. [DOI] [PubMed] [Google Scholar]
- 30.National Lung Screening Trial Research Team. Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Donington JS. Point: Are limited resections appropriate in non-small cell lung cancer? Yes. Chest. 2012;141:588–590. doi: 10.1378/chest.11-3108. [DOI] [PubMed] [Google Scholar]
- 32.Detterbeck FC. Counterpoint: Are limited resections appropriate in non-small cell lung cancer? No: Don't overdo it, and don't get confused. Chest. 2012;141:590–592. doi: 10.1378/chest.11-3110. [DOI] [PubMed] [Google Scholar]
- 33.Keenan RJ, Landreneau RJ, Maley RH, Jr, et al. Segmental resection spares pulmonary function in patients with stage I lung cancer. Ann Thorac Surg. 2004;78:228–233. doi: 10.1016/j.athoracsur.2004.01.024. [DOI] [PubMed] [Google Scholar]
- 34.El-Sherif A, Gooding WE, Santos R, et al. Outcomes of sublobar resection versus lobectomy for stage I non-small cell lung cancer: A 13-year analysis. Ann Thorac Surg. 2006;82:408–415. doi: 10.1016/j.athoracsur.2006.02.029. [DOI] [PubMed] [Google Scholar]
- 35.Macke RA, Schuchert MJ, McCormack KN, et al. Long-term impact of anatomic lung resection on pulmonary function. Presented at the 25th Annual Meeting of the General Thoracic Surgery Club, Poster Session; March 8, 2012; San Diego, CA. [Google Scholar]
- 36.Errett LE, Wilson J, Chiu RC, et al. Wedge resection as an alternative procedure for peripheral bronchogenic carcinoma in poor-risk patients. J Thorac Cardiovasc Surg. 1985;90:656–661. [PubMed] [Google Scholar]
- 37.Pastorino U, Valente M, Bedini V, et al. Limited resection for stage I lung cancer. Eur J Surg Oncol. 1991;17:42–46. [PubMed] [Google Scholar]
- 38.Okada M, Koike T, Higashiyama M, et al. Radical sublobar resection for small-sized non-small cell lung cancer: A multicenter study. J Thorac Cardiovasc Surg. 2006;132:769–775. doi: 10.1016/j.jtcvs.2006.02.063. [DOI] [PubMed] [Google Scholar]
- 39.Donahue JM, Morse CR, Wigle DA, et al. Oncologic efficacy of anatomic segmentectomy in stage IA lung cancer patients with T1a tumors. Ann Thorac Surg. 2012;93:381–387. doi: 10.1016/j.athoracsur.2011.10.079. [DOI] [PubMed] [Google Scholar]
- 40.Schuchert MJ, Abbas G, Awais O, et al. Anatomic segmentectomy for the solitary pulmonary nodule and early-stage lung cancer. Ann Thorac Surg. 2012;93:1780–1787. doi: 10.1016/j.athoracsur.2011.11.074. [DOI] [PubMed] [Google Scholar]
- 41.Landreneau RJ, Sugarbaker DJ, Mack MJ, et al. Wedge resection versus lobectomy for stage I (T1 N0 M0) non-small-cell lung cancer. J Thorac Cardiovasc Surg. 1997;113:691–698. doi: 10.1016/S0022-5223(97)70226-5. [DOI] [PubMed] [Google Scholar]
- 42.El-Sherif A, Fernando HC, Santos R, et al. Margin and local recurrence after sublobar resection of non-small cell lung cancer. Ann Surg Oncol. 2007;14:2400–2405. doi: 10.1245/s10434-007-9421-9. [DOI] [PubMed] [Google Scholar]
- 43.Litière S, Werutsky G, Fentiman IS, et al. Breast conserving therapy versus mastectomy for stage I-II breast cancer: 20 year follow-up of the EORTC 10801 phase 3 randomised trial. Lancet Oncol. 2012;13:412–419. doi: 10.1016/S1470-2045(12)70042-6. [DOI] [PubMed] [Google Scholar]
- 44.Darling GE, Allen MS, Decker PA, et al. Randomized trial of mediastinal lymph node sampling versus complete lymphadenectomy during pulmonary resection in the patient with N0 or N1 (less than hilar) non-small cell carcinoma: Results of the American College of Surgery Oncology Group Z0030 Trial. J Thorac Cardiovasc Surg. 2011;141:662–670. doi: 10.1016/j.jtcvs.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dupuy DE. Image-guided thermal ablation of lung malignancies. Radiology. 2011;260:633–655. doi: 10.1148/radiol.11091126. [DOI] [PubMed] [Google Scholar]
- 46.Timmerman R, McGarry R, Yiannoutsos C, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol. 2006;24:4833–4839. doi: 10.1200/JCO.2006.07.5937. [DOI] [PubMed] [Google Scholar]
- 47.Ong CL, Palma D, Verbakel WF, et al. Treatment of large stage I-II lung tumors using stereotactic body radiotherapy (SBRT): Planning considerations and early toxicity. Radiother Oncol. 2010;97:431–436. doi: 10.1016/j.radonc.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 48.Lopez Guerra JL, Gomez D, Zhuang Y, et al. Change in diffusing capacity after radiation as an objective measure for grading radiation pneumonitis in patients treated for non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2012;83:1573–1579. doi: 10.1016/j.ijrobp.2011.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rocco G. The surgeon's role in molecular biology. J Thorac Cardiovasc Surg. 2012;144(suppl):S18–S22. doi: 10.1016/j.jtcvs.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 50.Kim DN, Nam TK, Choe KS, et al. Personalized combined modality therapy for locally advanced non-small cell lung cancer. Cancer Res Treat. 2012;44:74–84. doi: 10.4143/crt.2012.44.2.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Langer CJ. Individualized therapy for patients with non-small cell lung cancer: Emerging trends and challenges. Crit Rev Oncol Hematol. 2012;83:130–144. doi: 10.1016/j.critrevonc.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 52.Salgia R, Hensing T, Campbell N, et al. Personalized treatment of lung cancer. Semin Oncol. 2011;38:274–283. doi: 10.1053/j.seminoncol.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 53.Paik PK, Johnson ML, D'Angelo SP, et al. Driver mutations determine survival in smokers and never-smokers with stage IIIB/IV lung adenocarcinomas. Cancer. 2012;118:5840–5847. doi: 10.1002/cncr.27637. [DOI] [PMC free article] [PubMed] [Google Scholar]