Abstract
Purpose
A major concern with sublobar resection (SR) for non–small-cell lung cancer (NSCLC) is high local recurrence (LR). Adjuvant brachytherapy may reduce LR This multicenter randomized trial compares SR to SR with brachytherapy (SRB).
Patients and Methods
High-risk operable patients with NSCLC ≤ 3 cm were randomly assigned to SR or SRB. The primary end point was time to LR, where LR included recurrence at the staple line (local progression), in the primary tumor lobe away from the staple line, and in ipsilateral hilar nodes. The trial was designed to have a 90% power to detect a hazard ratio (HR) of 0.315 in favor of SRB, using a one-sided type I error rate of 0.05 with a sample size of 100 eligible patients in each arm.
Results
Two hundred twenty-four patients were randomly assigned; 222 patients were evaluable for intent-to-treat analysis. Median age was 71 years (range, 49 to 87 years). No differences were found in baseline characteristics. Median follow-up time was 4.38 years (range, 0.04 to 5.59 years). There was no difference in time to LR (HR, 1.01; 95% CI, 0.51 to 1.98; log-rank P = .98) or in the types of LR. Local progression occurred in only 17 (7.7%) of 222 patients. In patients with potentially compromised margins (margin < 1 cm, margin-to-tumor ratio < 1, positive staple line cytology, wedge resection, nodule size > 2.0 cm), SRB did not reduce LR, although trends favored the SRB arm. This was most marked in 14 patients with positive staple line cytology (HR, 0.22; P = .24). Three-year overall survival rates were similar for patients in the SR (71%) and SRB (71%) arms (P = .97).
Conclusion
Brachytherapy did not reduce LR after SR. This finding may have been related to closer attention to parenchymal margins by surgeons participating in this study.
INTRODUCTION
Sublobar resection (SR) for non–small-cell lung cancer (NSCLC) has been associated with higher locoregional recurrence rates compared with lobectomy.1 For this reason, SR is generally reserved for high-risk operable patients who, although able to undergo general anesthesia, are considered too high risk for lobectomy. One approach that may reduce increased local recurrence (LR) after SR is the addition of adjuvant radiation therapy.2 However external-beam radiation can be challenging to implement in high-risk patients. Another approach to improve local control is adjuvant intraoperative brachytherapy. This has the advantages of 100% patient compliance and minimizes radiation injury to non–tumor-bearing areas of the lung.3–6 The American College of Surgeons Oncology Group (ACOSOG) Z4032 trial is a prospective randomized clinical trial that compared SR plus adjuvant intraoperative brachytherapy (SRB) with SR alone. This study began enrollment in January 2006 and completed accrual in January 2010. The primary objective of the study was to determine whether patients treated with SRB had a longer time to LR compared with patients treated by SR alone. Here, we report the primary end point results from this study.
PATIENTS AND METHODS
Eligible patients were required to have biopsy-proven stage I lung cancers 3 cm or less in maximum diameter (ie, stage IA or the subset of stage IB with visceral pleural involvement). Patients were defined as high risk for lobectomy if they met at least one major criterion or two minor criteria listed in Table 1. Evaluation by an ACOSOG-approved thoracic surgeon was required to determine that the patient was either not a candidate for lobectomy (standard-risk operable patient) or not a candidate for any form of pulmonary resection (medically inoperable patient). Any suspicious lymph nodes seen on positron emission tomography (PET) or computed tomography (CT) scan required biopsy by mediastinoscopy, endobronchial ultrasound, or lymph node sampling at the time of resection. Patients were preregistered and randomly assigned. The preregistration process allowed sites to order brachytherapy seeds for patients randomly assigned to the brachytherapy arm and also allowed histologic confirmation of NSCLC at the time of surgery. Once confirmation of stage I NSCLC was obtained, patients were fully registered. Wedge or segmental resection was allowed and could be performed by video-assisted thoracic surgery or thoracotomy. Two methods of brachytherapy were allowed.2,3 In the first technique, polyglactin sutures containing iodine-125 (125I) seeds (Oncura, Princeton, NJ) were placed parallel to and 5 mm away from the staple line on each side of the resection margin. The suture strands were fixed to the lung surface with several 3.0 silk or polyglactin sutures placed 1 to 2 cm apart. With the second technique, a polyglycolic mesh implant was created. The same 125I suture strands were woven into a piece of vicryl mesh. The strands were placed at 1-cm intervals. The mesh was then sutured over the staple line. The dosimetry goal of the brachytherapy was to deliver 100 Gy at 5 to 7 mm along the central axis of the resection margin.
Table 1.
Major and Minor Eligibility Criteria for Z4032 Trial
| Criterion | SR* (n = 114) |
SRB* (n = 108) |
||
|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | |
| Major criteria | ||||
| FEV1 ≤ 50% predicted | 67 | 58.8 | 49 | 45.4 |
| DLCO ≤ 50% predicted | 72 | 63.2 | 74 | 68.5 |
| Minor criteria | ||||
| Age ≥ 75 years | 43 | 37.7 | 42 | 38.9 |
| FEV1 51% to 60% predicted | 18 | 15.8 | 25 | 23.1 |
| DLCO 51% to 60% predicted | 19 | 16.7 | 19 | 17.6 |
| Pulmonary hypertension (defined as a pulmonary artery systolic pressure > 40 mmHg) as estimated by echocardiography or right heart catheterization | 4 | 3.5 | 1 | 0.9 |
| Poor left ventricular function (defined as an ejection fraction of ≤ 40%) | 9 | 7.9 | 3 | 2.8 |
| Resting or exercise arterial Po2 ≤ 55 mmHg or Spo2 ≤ 88% | 5 | 4.4 | 6 | 5.6 |
| Pco2 > 45 mmHg | 3 | 2.6 | 3 | 2.8 |
| Modified Medical Research Council Dyspnea Scale ≥ 3 | 31 | 27.2 | 17 | 15.7 |
NOTE. Eligible patients must have met either one major or two minor criteria.
Abbreviations: DLCO, diffusing capacity of the lung for carbon monoxide; FEV1, forced expiratory volume in 1 second; Pco2, partial pressure of carbon dioxide; Po2, partial pressure of oxygen; Spo2, saturation of peripheral oxygen; SR, sublobar resection; SRB, sublobar resection with brachytherapy.
Patients may have multiple criteria.
A touch-prep of the surgical specimen was performed to assess the adequacy of the surgical margin using the method described by Sawabata et al.7 After removal of the specimen, a glass slide was run at least three times across the staple line margin before the pathologist cut the specimen. The slide was then fixed for cytologic examination, with a positive staple line defined as the presences of at least three malignant cells or clustered malignant cells. We did not mandate that the results of the staple line cytology be obtained at the time of surgery. Additionally, we did not require that a specific margin size be obtained because this was high-risk operable group.
Adverse events were recorded using the Common Terminology Criteria for Adverse Events Version 3.0.8 A report of 30- and 90-day adverse events has previously been published from this study.9 There were no significant differences in grade 3 or higher adverse events between the study arms.
The primary end point was the time to LR. LR was defined as recurrence within the primary tumor lobe at the staple line (local progression), recurrence within the primary tumor lobe away from the staple line (involved lobe failure), or recurrence within hilar lymph nodes. Regional recurrence was defined as recurrence within another lobe on the same side as the resection or within ipsilateral mediastinal or subcarinal lymph nodes. Distant recurrence was defined as recurrence within contralateral, mediastinal, or hilar lymph nodes or distant metastatic disease. Follow-up imaging included serial CT scans obtained at months 3, 6, 12, 18, 24, and 36 after resection. In the SRB group, an additional scan was obtained at 1 month for implant dosimetry. If LR was suspected, tissue diagnosis was strongly recommended. If this was felt not to be feasible, then PET scans were obtained. Evidence of growth on serial CT, with increased uptake on PET, was considered diagnostic of recurrent cancer in the absence of a tissue diagnosis.
All patients provided written informed consent. At each participating site, institutional review board approval was obtained in accord with an assurance filed with and approved by the US Department of Health and Human Services.
Statistical Analysis
This randomized phase III trial was designed to assess whether the time to LR is significantly longer for patients randomly assigned to SRB compared with patients assigned to SR. From the literature,1,10 an estimate of the proportion of patients free from LR at 2 years who were treated by SR only is between 80% and 85%. Assuming 85% as a conservative estimate of the proportion of patients free from LR at 2 years in the SR arm, the trial sample size was determined to detect a hazard ratio (HR) of Δ = 0.315 (10% higher proportion of patients free from LR at year 2 in SRB arm). Assuming at least 90% power, a one-sided type I error rate of 0.05, constant accrual rate, and a minimum follow-up time of 3 years on all alive patients for LR, a total of 32 LRs were required to be observed (0.081 × 100 × 3 + 0.026 × 100 × 3) using a log-rank test. Thus, the accrual goal, including a 12% nonevaluable rate, was 226 patients (or 200 eligible patients). An interim futility analysis based on the O'Brien-Fleming boundary was planned after 15 LRs were observed. If the observed HR was ≥ 0.97 (P ≥ .48), the recommendation would be to stop further accrual (if the trial was still accruing) to the trial and conclude futility.
All recurrences were centrally reviewed by review of pathology and imaging reports. In questionable cases, central review of images was also performed. The central assessment was used in the final analysis.
The time to LR was analyzed using the log-rank test as a primary analysis. Time to LR was censored at the time of a distant/regional recurrence, at death, or at 5 years of follow-up. However, as a secondary analysis, because distant or regional recurrence or death without LR is truly a competing risk (because treatment for the distant/regional recurrence may influence the time to LR), the cumulative incidence function was used to estimate the probability of LR and was compared using the approach of Pepe and Mori11 and Gray.12 Time to LR or death (LRD) and time to any recurrence (AnyR) were also analyzed similarly. Overall survival (OS) was analyzed using Kaplan-Meier curves and log-rank tests. χ2 and Fisher's exact tests were used to compare treatment groups with respect to patterns of LR, as well as the OS, LR, LRD, and AnyR rates. Subgroup analyses using a logistic regression model for 3-year end points and Cox proportional hazards model for time-to-event end points were performed to identify potentially vulnerable subgroups considered to be at higher risk for LR (margin size < 1.0 cm, margin-to-tumor ratio < 1, positive staple line cytology, wedge resection, and clinical nodule size > 2.0 cm). Two-sided P ≤ .05 was considered statistically significant. This study was monitored by the ACOSOG Data Safety and Monitoring Committee on a biannual basis.
RESULTS
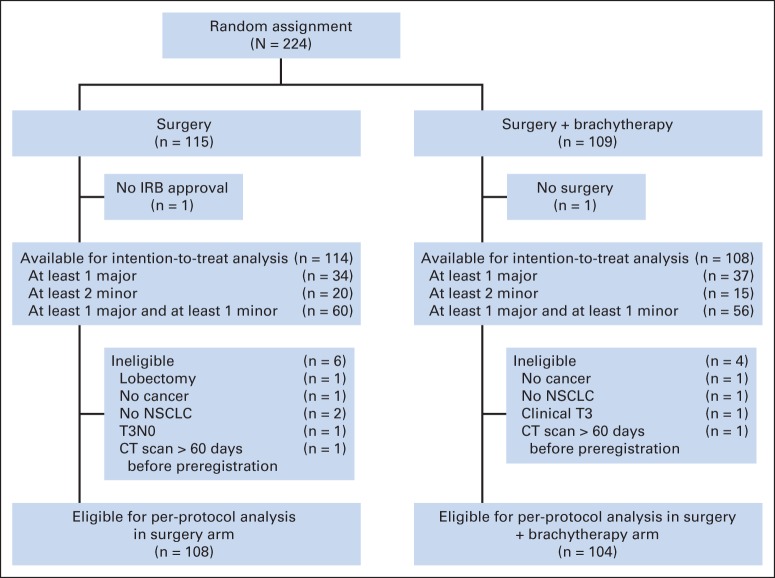
Data were frozen for this analysis on July 15, 2013. A total of 224 patients were registered. One patient from the SR arm had the intervention at a hospital that was not institutional review board approved and was deemed not evaluable. One patient randomly assigned to the SRB arm did not have surgery and was also not evaluable. A total of 222 patients were included in the intent-to-treat (ITT) cohort. An additional 10 registered patients (six in the SR arm and four in the SRB arm) were found to be ineligible (Fig 1). Thus, 212 patients (108 in the SR arm and 104 in the SRB arm) were included in the patients treated per protocol cohort.
Fig 1.
CONSORT diagram. CT, computed tomography; IRB, institutional review board; NSCLC, non–small-cell lung cancer.
Table 2 lists the baseline patient characteristics for the two cohorts. There were no significant differences between the arms in baseline characteristics, except for American Society of Anesthesiology class. A higher percentage of patients in the SR arm than in the SRB arm were Society of Anesthesiology class III or higher (ITT cohort: 91.2% v 78.7%, respectively; P = .02; per-protocol cohort: 90.7% v 79.8%, respectively; P = .05). Appendix Table A1 (online only) provides the follow-up information for the ITT and per-protocol cohorts.
Table 2.
Baseline Patient Demographic and Clinical Characteristics
| Characteristic | Intention-to-Treat Cohort (n = 222) |
Per-Protocol Cohort (n = 212) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SR (n = 114) |
SRB (n = 108) |
P* | SR (n = 108) |
SRB (n = 104) |
P* | |||||
| No. of Patients | % | No. of Patients | % | No. of Patients | % | No. of Patients | % | |||
| Age, years | .37† | .47† | ||||||||
| Median | 70 | 72 | 70 | 71 | ||||||
| Range | 49-85 | 50-87 | 49-85 | 50-87 | ||||||
| Sex | .79 | .89 | ||||||||
| Female | 65 | 57.0 | 59 | 54.6 | 61 | 56.5 | 57 | 54.8 | ||
| Male | 49 | 43.0 | 49 | 45.4 | 47 | 43.5 | 47 | 45.2 | ||
| PS | .55 | .72 | ||||||||
| 0 | 20 | 17.5 | 25 | 23.1 | 19 | 17.6 | 23 | 22.1 | ||
| 1 | 66 | 57.9 | 60 | 55.6 | 63 | 58.3 | 58 | 55.8 | ||
| 2 | 28 | 24.6 | 23 | 21.3 | 26 | 24.1 | 23 | 22.1 | ||
| Clinical nodule size, cm | .78 | .78 | ||||||||
| ≤ 2 | 73 | 64.0 | 67 | 62.0 | 70 | 64.8 | 65 | 62.5 | ||
| > 2 | 41 | 36.0 | 41 | 38.0 | 38 | 35.2 | 39 | 37.5 | ||
| Tumor stage | .054 | .12 | ||||||||
| T1 | 114 | 100 | 104 | 96.3 | 108 | 100 | 101 | 97.1 | ||
| T2 | 0 | 0 | 3 | 2.8 | 0 | 0 | 3 | 2.9 | ||
| T3 | 0 | 0 | 1 | 0.9 | 0 | 0 | 0 | 0 | ||
| Metastasis stage M0 | 114 | 100 | 108 | 100 | NA | 108 | 100 | 104 | 100 | NA |
| Nodal stage N0 | 114 | 100 | 108 | 100 | NA | 108 | 100 | 104 | 100 | NA |
| ASA class on surgery day‡ | .02 | .05 | ||||||||
| I/II | 10 | 8.8 | 21 | 19.4 | 10 | 9.3 | 20 | 19.2 | ||
| III/IV | 104 | 91.2 | 85 | 78.7 | 98 | 90.7 | 83 | 79.8 | ||
| Baseline FEV1, %‡ | .25† | .31† | ||||||||
| Median | 48 | 53 | 48 | 53 | ||||||
| Range | 22-117 | 25-110 | 22-117 | 25-110 | ||||||
| Baseline DLCO, %§ | .36† | .25† | ||||||||
| Median | 47 | 45 | 46 | 44 | ||||||
| Range | 18-97 | 8-137 | 18-97 | 8-83 | ||||||
Abbreviations: ASA, American Society of Anesthesiology; DLCO, diffusing capacity of the lung for carbon monoxide; FEV1, forced expiratory volume in 1 second; NA, not applicable; PS, performance status; SR, sublobar resection; SRB, sublobar resection with brachytherapy.
Fisher's exact test.
Wilcoxon rank sum test.
One patient in the SRB arm was missing data.
Three patients in the SR arm and two patients in the SRB arm were missing data.
Primary End Point Results
ITT cohort.
A total of 34 LRs, 22 regional recurrences, and 26 distant recurrences were reported as of July 15, 2013 (Table 3). Fourteen LRs were confirmed by biopsy (five in the SR arm and nine in the SRB arm; P = .46), and 33 LRs had documentation by CT (16 in the SR arm and 17 in the SRB arm; P = .34).
Table 3.
Comparison of SR Versus SRB in All Reported Recurrence Events and 2- and 3-Year Recurrence End Points
| Event | Intention-to-Treat Cohort (n = 222) |
Per-Protocol Cohort (n = 212) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SR (n = 114) |
SRB (n = 108) |
P* | SR (n = 108) |
SRB (n = 104) |
P* | |||||
| No. of Patients | % | No. of Patients | % | No. of Patients | % | No. of Patients | % | |||
| All reported events | ||||||||||
| LR | 16 | 14.0 | 18 | 16.7 | .59 | 16 | 14.8 | 18 | 17.3 | .62 |
| LRD | 49 | 43.0 | 53 | 49.1 | .36 | 47 | 43.5 | 50 | 48.1 | .51 |
| RR | 10 | 8.8 | 12 | 11.1 | .56 | 9 | 8.3 | 12 | 11.5 | .43 |
| DR | 13 | 11.4 | 13 | 12.0 | .88 | 13 | 12.0 | 13 | 12.5 | .92 |
| Any recurrence (local, regional, distant) | 30 | 26.3 | 29 | 26.9 | .93 | 29 | 26.9 | 29 | 27.9 | .87 |
| OS | 70 | 61.4 | 60 | 55.6 | .38 | 66 | 61.1 | 59 | 56.7 | .52 |
| Recurrence end point | ||||||||||
| LR at 2 years | 14 | 12.3 | 10 | 9.3 | .47 | 14 | 13.0 | 10 | 9.6 | .44 |
| LR at 3 years | 14 | 12.3 | 13 | 12.0 | .96 | 14 | 13.0 | 13 | 12.5 | .92 |
| LRD at 3 years | 37 | 32.5 | 33 | 30.6 | .76 | 36 | 33.3 | 32 | 30.8 | .69 |
| RR at 3 years | 8 | 7.0 | 9 | 8.3 | .71 | 7 | 6.5 | 9 | 8.7 | .55 |
| DR at 3 years | 10 | 8.8 | 12 | 11.1 | .56 | 10 | 9.3 | 12 | 11.5 | .59 |
| Any recurrence (local, regional, distant) at 3 years | 24 | 21.1 | 22 | 20.4 | .90 | 23 | 21.3 | 22 | 21.2 | .98 |
| OS at 3 years | 81 | 71.1 | 77 | 71.3 | .97 | 76 | 70.4 | 74 | 71.2 | .90 |
Abbreviations: DR, distant recurrence; LR, local recurrence; LRD, local recurrence/death; OS, overall survival; RR, regional recurrence; SR, sublobar resection; SRB, sublobar resection with brachytherapy.
χ2 test.
LR rates at 5 years of follow-up were 14.0% and 16.7% in the SR and SRB arms, respectively (P = .59). There was no difference in time to LR (HR, 1.01; 95% CI, 0.51 to 1.98; log-rank P = .98; competing risk P = .91; competing events: 35.1% in SR arm and 37% in SRB arm). The pattern of LR, in particular local progression, was not significantly different between the arms (Fig 2). Notably, local progression occurred in only 17 (7.7%) of 222 patients (95% CI, 4.5% to 12.0%) after surgery. LR rates at 2 and 3 years were 12.3% and 12.3%, respectively, in the SR arm and 9.3% and 12.0%, respectively, in the SRB arm and were not significantly different (P = .47 at 2 years, P = .96 at 3 years).
Fig 2.
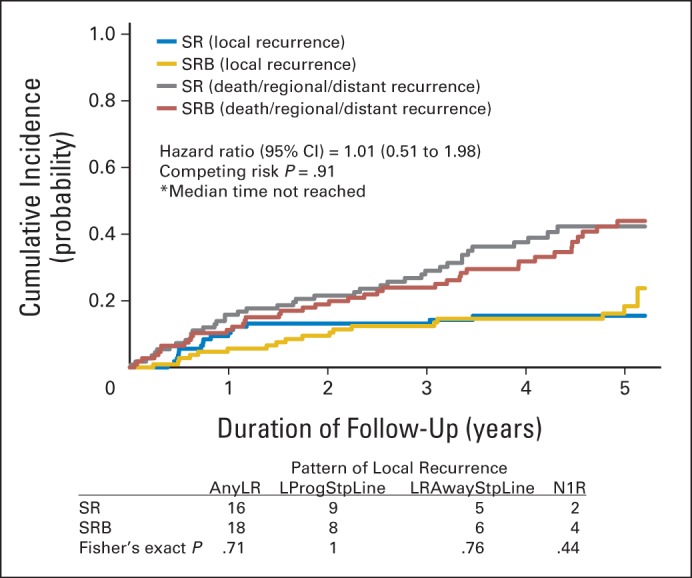
Pattern of local recurrence and cumulative incidence graph by arm for local recurrence with death or regional or distant recurrence as competing events in the intent-to-treat cohort. AnyLR, any local recurrence; LProgStpLine, local progression at staple line; LRAwayStpLine, lobar recurrence away from the staple line; N1R, nodal (N1) recurrence; SR, sublobar resection; SRB, sublobar resection with brachytherapy.
LRD rates at 5 years of follow-up were 43.0% and 49.1% in the SR and SRB arms, respectively (P = .36). Time to LRD was not significantly different between the arms (HR, 0.99; 95% CI, 0.67 to 1.47; log-rank P = .98; competing risk P = .86; competing events: 12.3% in the SR arm and 10.2% in the SRB arm; Appendix Fig A1, online only).
Per-protocol cohort.
A total of 34 LRs, 21 regional recurrences, and 26 distant recurrences were reported as of July 15, 2013 (Table 3). LR rates at 5 years of follow-up were 14.8% and 17.3% in the SR and SRB arms, respectively (P = .62). There was no difference in time to LR (HR, 1.00; 95% CI, 0.51 to 1.96; log-rank P = .99; competing risk P = .93; competing events: 34.3% in the SR arm and 35.6% in the SRB arm). The pattern of LR was similar to that in the ITT cohort (Appendix Fig A2, online only). LR rates at 2 and 3 years were 13.0% and 13.0%, respectively, in the SR arm and 9.6% and 12.5%, respectively, in the SRB arm, with no significant difference (P = .44 at 2 years; P = .92 at 3 years).
LRD rates at 5 years of follow-up were 43.5% and 48.1% in the SR and SRB arms, respectively (P = .51). Time to LRD was not significantly different between the arms (HR, 0.98; 95% CI, 0.66 to 1.46; log-rank P = .92; competing risk P = .94; competing events: 12.0% in the SR arm and 10.6% in the SRB arm; Appendix Fig A3, online only).
Secondary End Point Results
When considering all types of recurrence (local, regional, or distant; AnyR), no statistically significant differences were found in time to AnyR in the ITT cohort (HR, 0.87; 95% CI, 0.52 to 1.44; log-rank P = .58; competing risk P = .61; competing events: 22.8% in the SR arm and 26.9% in the SRB arm) and in the per-protocol cohort (HR, 0.89; 95% CI, 0.53 to 1.48; log-rank P = .65; competing risk P = .69; competing events: 22.2% in the SR arm and 25.0% in the SRB arm). Recurrences of any type were reported in 26.3% and 26.9% of patients in the SR and SRB arms, respectively, in the ITT cohort and in 26.9% and 27.9% of patients, respectively, in the per-protocol cohort (Table 3).
OS rates at 5 years of follow-up were 61.4% and 55.6% in the SR and SRB arms, respectively, in the ITT cohort (P = .38). In the ITT cohort, 92 patients died as a result of cancer (40.2%), other disease (51.1%), or unknown causes (8.7%). In the per-protocol cohort, OS rates at 5 years of follow-up were 61.1% and 56.7% in the SR and SRB arms, respectively (P = .52). In the per-protocol cohort, 87 patients died as a result of cancer (41.3%), other disease (50.6%), or unknown causes (8.0%). Figure 3 and Appendix Figure A4 (online only) depict the Kaplan-Meier curves for OS in the ITT and per-protocol cohort, respectively.
Fig 3.
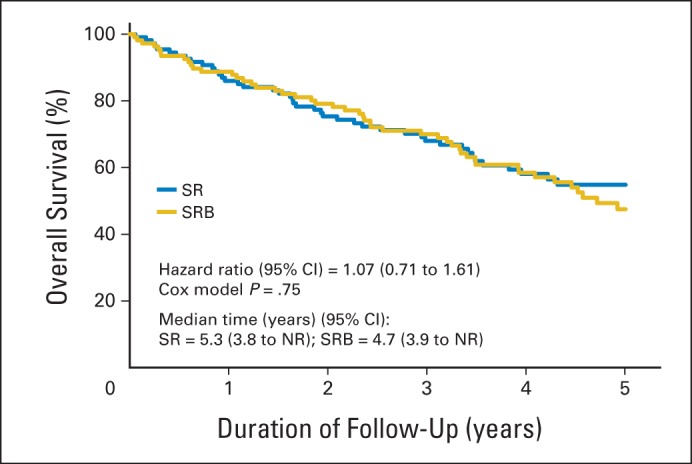
Kaplan-Meier curves for overall survival in the intention-to-treat cohort. NR, not reached; SR, sublobar resection; SRB, sublobar resection with brachytherapy.
Subset Analyses
Patients deemed to be at higher risk for LR included those with a margin less than 1 cm, margin-to-tumor ratio less than 1, positive staple line cytology, or clinical nodule size greater than 2 cm and those who underwent wedge rather than segmental resection. There was a trend favoring SRB among 14 patients who had positive staple line cytology (HR, 0.22); however, no statistically significant differences were found (Table 4 and Appendix Table 2, online only).
Table 4.
Comparison in Subgroups at High Risk of Recurrence: 3-Year End Points on Per-Protocol Cohort
| Subgroup | Total No. of Patients |
Local Recurrence–Free Rate |
Local Recurrence–Free Survival Rate |
Overall Survival Rate |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SR |
SRB |
P* | SR |
SRB |
P* | SR |
SRB |
P* | |||||||||
| SR | SRB | No. of Patients | % | No. of Patients | % | No. of Patients | % | No. of Patients | % | No. of Patients | % | No. of Patients | % | ||||
| Tumor margin < 1.0 cm | 42 | 46 | 34 | 81 | 37 | 80 | .95 | 29 | 69 | 30 | 65 | .70 | 30 | 71 | 32 | 70 | .85 |
| Margin-to-tumor ratio < 1.0 | 70 | 67 | 60 | 86 | 57 | 85 | .92 | 48 | 69 | 42 | 63 | .47 | 50 | 71 | 44 | 66 | .47 |
| Staple line cytology positive | 4 | 10 | 3 | 75 | 9 | 90 | .47 | 2 | 50 | 6 | 60 | .73 | 2 | 50 | 7 | 70 | .48 |
| Wedge resection | 74 | 81 | 63 | 85 | 69 | 85 | .99 | 48 | 65 | 55 | 68 | .69 | 50 | 68 | 57 | 70 | .71 |
| Clinical nodule size > 2.0 cm | 38 | 39 | 33 | 87 | 34 | 87 | .96 | 25 | 66 | 26 | 67 | .94 | 26 | 68 | 28 | 72 | .75 |
Abbreviations: SR, sublobar resection; SRB, sublobar resection with brachytherapy.
χ2 test.
DISCUSSION
SR has traditionally been used for high-risk operable patients with NSCLC. The major concern with SR has been the higher rate of LR compared with lobectomy.1 One method to decrease LR in the high-risk operable patient is to perform segmental rather than a wedge resection.13 However, segmental resection is more challenging, particularly if a minimally invasive technique is used. Some patients may also have difficulty tolerating the relatively longer periods of single-lung ventilation required to perform anatomic dissection of a segmental bronchus, artery, and vein, and so wedge resection is often performed. Another factor cited to improve oncologic outcome is to optimize the surgical margin to either 1 or 2 cm or to achieve a margin-to-tumor ratio of ≥ 1.14
Adjuvant intraoperative brachytherapy has previously been reported to decrease LR to rates normally associated with lobectomy.3,4 Our phase III study was performed based on these earlier studies. Despite initial enthusiasm for adjuvant brachytherapy, we found no difference in outcome between patients treated with or without brachytherapy. One main limitation of this trial was that it was powered to detect a large HR (0.315) and thus underpowered to detect perhaps a lower but clinically meaningful difference. In this study, in the per-protocol cohort, the overall LR rate at 3 years was 12.7%. This LR rate is lower than prior studies of SR, such as the 17% rate reported in the Lung Cancer Study Group randomized trial.1 Factors that may have contributed to this lower rate include improved instrumentation and a more conscientious attention to achieving a negative margin by emphasizing margin size. These factors may have contributed to the equivalent outcomes of SRB compared with SR alone in our study. The theoretical benefit of brachytherapy is presumably provided through extending the therapeutic margin after resection. Therefore, the benefit of brachytherapy may only be in those few patients with compromised margins. It is noteworthy that only 14 patients (6.6% of the per-protocol cohort) in our study had positive staple line cytology, and this group had the strongest trend favoring the brachytherapy arm in reducing LR.
The prospective nature of this study, with protocol-directed serial imaging with CT scans, is another factor that may have contributed to the equivalent outcomes of SRB. Previous retrospective studies likely had inconsistent follow-up and may not have involved serial CT scan imaging. Patients lost to follow-up or who died without routine detailed imaging would have been censored to the outcome of LR, despite possibly harboring occult recurrent disease. This effect would be amplified in high-risk patients, with patients dying from other causes before LR is diagnosed. In our high-risk population with small cancers, 51% of patients died from unrelated causes in the per-protocol cohort. It remains unclear whether brachytherapy would have any benefit in standard-risk patients (although these patients would likely be treated with lobectomy) or in larger tumors where SR is selected.
We attempted to standardize reproducible brachytherapy techniques across many centers by credentialing of surgeons and sites before site activation. Surgeons participated in workshops where brachytherapy techniques were taught and practiced. Sites were required to submit copies of the preprocedure CT scan, dose parameters (which included activity per seed, total seeds per strand, total activity, and measured strand separation), and three-dimensional calculated color isodose distributions for central review. This information regarding brachytherapy is currently being analyzed and will be reported as part of a planned secondary analysis.
Over the last decade, a number of new approaches including radiofrequency ablation and stereotactic body radiation therapy (SBRT) have been used to treat medically inoperable patients with lung cancer15,16 and are being suggested as alternatives for operable patients with early-stage NSCLC.17 By report, local control achieved with SBRT has demonstrated results approaching that of lobectomy15; however, the definitions of LR need to be specifically defined. Our results presented here and the results of the Radiation Therapy Oncology Group 0236 phase II study of SBRT in medically inoperable patients suggest similar local control18; however, the definitions of LR are different between the two studies. In the Radiation Therapy Oncology Group 0236 study, local failure was defined as progression or recurrence within the treated tumor and within 1 cm of the planning treatment volume. Outside of this area, the recurrence would be labeled as regional or distant recurrence. In contrast, our LR definition included local progression as a subtype of LR. Local progression was defined as recurrence at the staple line (similar to SBRT definitions of local failure). In our study, local progression occurred in only 8.0% of patients overall on the per-protocol cohort. This low incidence of local progression should be considered as the current benchmark for local progression in high-risk operable patients undergoing SR.
Previously in this study population, we demonstrated that resection can be undertaken with low 30- and 90-day mortality.9 The 3-year OS rate (per-protocol cohort) in our study was 70.8% and serves as a benchmark for comparing resection to alternative local control therapies for high-risk NSCLC.
In conclusion, this randomized prospective study demonstrates no advantage in local control when using adjuvant intraoperative brachytherapy in high-risk operable patients with stage I NSCLC of ≤ 3 cm. This negative finding may have been related to closer attention to parenchymal margins by surgeons participating in this study.
Supplementary Material
Acknowledgment
We thank the American College of Surgeons Oncology Group and Alliance staff, in particular the leadership of Heidi Nelson and David Ota, for assistance in the completion of this project. We also thank all of the investigators and their site research teams who enrolled patients onto this study (see Appendix, online only). Finally, we wish to thank the brave patients with non–small-cell lung cancer and their caregivers who participated in this study.
Appendix
The following investigators and their site research teams enrolled patients onto this study: University of Pittsburgh (Pittsburgh, PA), Mayo Clinic (Rochester, MN), Washington University (St Louis, MO), University of Virginia (Charlottesville, VA), Benedictine Hospital (Kingston, NY), University of Cincinnati (Cincinnati, OH), Jameson Hospital (New Castle, PA), University of Michigan (Ann Arbor, MI), Latter Day Saints Hospital (Salt Lake City, UT), Memorial Medical Center (Springfield, IL), Rhode Island Hospital (Providence, RI), Valley Hospital (Ridgewood, NJ), William Beaumont Hospital (Royal Oak, MI), Northwestern University (Chicago, IL), Medical City Dallas (Dallas, TX), Allegheny Cancer Center Network (Pittsburgh, PA), Boston Medical Center (Boston, MA), City of Hope Medical Center (Duarte, CA), Portland Veterans' Administration Medical Center (Portland, OR), University of Philadelphia (Philadelphia, PA), Virginia Mason Medical Center (Seattle, WA), Medical University of South Carolina (Charleston, SC), Memorial Hospital (Chattanooga, TN), South Nassau Community Hospital (Oceanside, NY), Southern Illinois University School of Medicine (Springfield, IL), Swedish Hospital (Seattle, OR), University of Tennessee (Knoxville, TN), Dartmouth Hitchcock Medical Center (Lebanon, NH), Emory University (Atlanta, GA), Fox Chase Cancer Center (Philadelphia, PA), Oregon Health Sciences University (Portland, OR), Vanderbilt University Medical Venter (Nashville, TN), Intermountain Medical Center (Murray, UT), London Health Sciences Centre (London, Ontario, Canada), Methodist Hospital (Houston, TX), Miami Valley Hospital (Dayton, OH), Monmouth Medical Center (Long Branch, NJ), Northshore University Health System (Evanston, IL), Providence Medical Center (Portland, OR), Roswell Park Memorial Institute (Buffalo, NY), and Thomas Jefferson University Hospital (Philadelphia, PA).
Table A1.
Follow-Up Summary
| Data Status | Intention-to-Treat Cohort (n = 222) |
Per-Protocol Cohort (n = 212) |
||||||
|---|---|---|---|---|---|---|---|---|
| SR (n = 114) |
SRB (n = 108) |
SR (n = 108) |
SRB (n = 104) |
|||||
| No. of Patients | % | No. of Patients | % | No. of Patients | % | No. of Patients | % | |
| Completed 5-year follow-up | 34 | 29.8 | 33 | 30.6 | 33 | 30.6 | 32 | 30.8 |
| Early termination (before 5 years) | 56 | 49.1 | 54 | 50.0 | 53 | 49.1 | 51 | 49.0 |
| Patient withdrew consent | 6 | 4 | 6 | 4 | ||||
| Lost contact | 2 | 1 | 2 | 1 | ||||
| Other (noncompliance/other complicating disease/unknown) | 4 | 1 | 3 | 1 | ||||
| Dead | 44 | 48 | 42 | 45 | ||||
| Still in follow-up | 24 | 21.1 | 21 | 19.4 | 22 | 20.4 | 21 | 20.2 |
| Follow-up status | ||||||||
| Alive | 70 | 61.4 | 60 | 55.6 | 66 | 61.1 | 59 | 56.7 |
| Dead | 44 | 38.6 | 48 | 44.4 | 42 | 38.9 | 45 | 43.3 |
| Follow-up time of those alive, years | ||||||||
| Median | 4.08 | 4.69 | 4.25 | 4.56 | ||||
| Range | 0.04-5.59 | 0.12-5.57 | 0.04-5.59 | 0.12-5.57 | ||||
Abbreviations: SR, sublobar resection; SRB, sublobar resection with brachytherapy.
Table A2.
Cox Proportional Hazards Models for Subgroups at High Risk of Recurrence: Univariable Subgroup Analysis on Per-Protocol Cohort
| Subgroup | Total No. of Patients |
Local Recurrence |
Local Recurrence or Death |
Death |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SR | SRB | Hazard Ratio (SRB v SR) | 95% CI | P* | Hazard Ratio (SRB v SR) | 95% CI | P* | Hazard Ratio (SRB v SR) | 95% CI | P* | |
| Tumor margin < 1.0 cm | 42 | 46 | 0.85 | 0.33 to 2.21 | .74 | 0.95 | 0.46 to 1.98 | .89 | 0.96 | 0.44 to 2.07 | .91 |
| Margin-to-tumor ratio < 1.0 | 70 | 67 | 0.99 | 0.41 to 2.38 | .98 | 1.12 | 0.63 to 1.99 | .69 | 1.20 | 0.66 to 2.19 | .54 |
| Staple line cytology positive | 4 | 10 | 0.22 | 0.01 to 3.58 | .24 | 0.50 | 0.09 to 2.81 | .43 | 0.46 | 0.08 to 2.76 | .38 |
| Wedge resection | 74 | 81 | 0.85 | 0.38 to 1.94 | .71 | 0.80 | 0.46 to 1.37 | .41 | 0.82 | 0.47 to 1.45 | .50 |
| Clinical nodule size > 2.0 cm | 38 | 39 | 0.84 | 0.24 to 2.92 | .79 | 0.83 | 0.38 to 1.79 | .63 | 0.78 | 0.34 to 1.76 | .54 |
Abbreviations: SR, sublobar resection; SRB, sublobar resection with brachytherapy.
Log-rank test.
Fig A1.
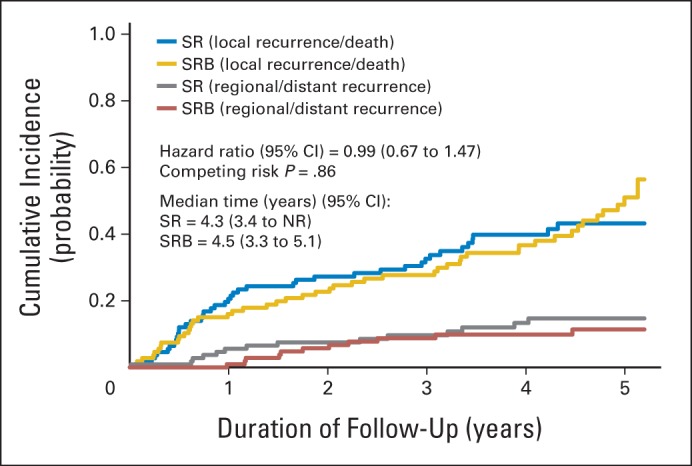
Cumulative incidence graph by arm for local recurrence or death with regional or distant recurrence as competing events in the intention-to-treat cohort. NR, not reached; SR, sublobar resection; SRB, sublobar resection with brachytherapy.
Fig A2.
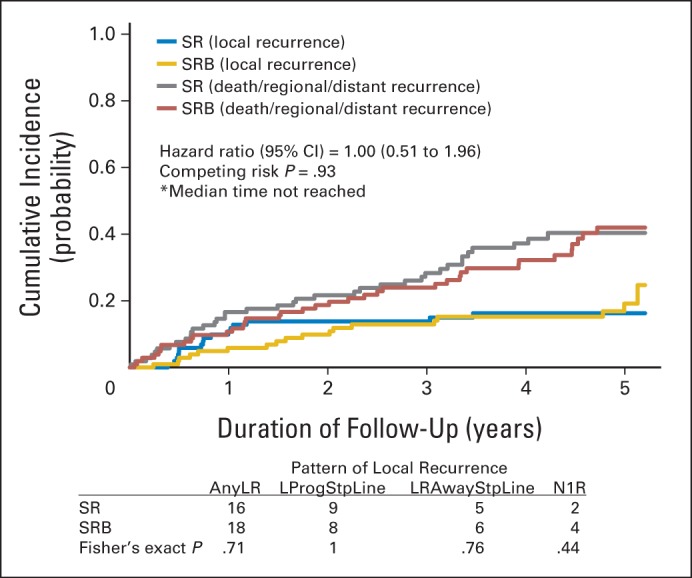
Pattern of local recurrence and cumulative incidence graph by arm for local recurrence with death or regional or distant recurrence as competing events in the per-protocol cohort. AnyLR, any local recurrence; LProgStpLine, local progression at staple line; LRAwayStpLine, lobar recurrence away from the staple line; N1R, nodal (N1) recurrence; SR, sublobar resection; SRB, sublobar resection with brachytherapy.
Fig A3.
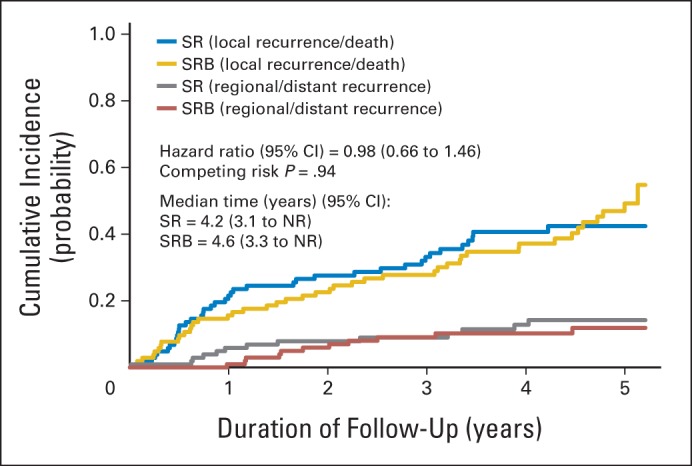
Cumulative incidence graph by arm for local recurrence or death with regional or distant recurrence as competing events in the per-protocol cohort. NR, not reached; SR, sublobar resection; SRB, sublobar resection with brachytherapy.
Fig A4.
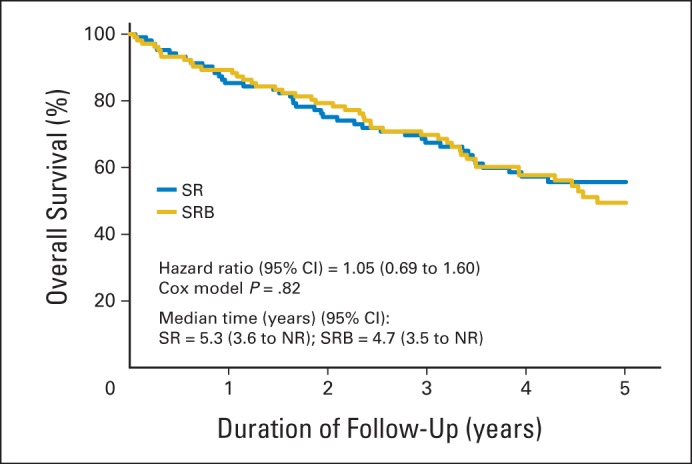
Kaplan-Meier curves for overall survival in the per-protocol cohort. NR, not reached; SR, sublobar resection; SRB, sublobar resection with brachytherapy.
Footnotes
Written on behalf of the Alliance for Clinical Trials in Oncology.
Supported by National Cancer Institute (NCI) U10 Grant No. CA076001 and by an additional grant from Oncura, Princeton, NJ. Also supported in part by NCI Grants No. CA31946 to the Alliance for Clinical Trials in Oncology and CA33601 to the Alliance Statistics and Data Center.
Presented at the 49th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, May 31-June 4, 2013.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information: NCT00107172.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Thomas A. DiPetrillo, SPEC (C) Stock Ownership: None Honoraria: None Research Funding: None Expert Testimony: None Patents, Royalties, and Licenses: Thomas A. DiPetrillo, Small Business Innovation Research grant (SPEC) to develop patent Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Hiran C. Fernando, Rodney J. Landreneau, Sumithra J. Mandrekar, Francis C. Nichols, Shauna L. Hillman, Dwight E. Heron, Bryan F. Meyers, Thomas A. DiPetrillo, Benedict D.T. Daly, Joe B. Putnam Jr
Provision of study materials or patients: Hiran C. Fernando, Rodney J. Landreneau, Francis C. Nichols, Dwight E. Heron, Bryan F. Meyers, Thomas A. DiPetrillo, David R. Jones, Sandra L. Starnes, Benedict D.T. Daly, Joe B. Putnam Jr
Collection and assembly of data: Sumithra J. Mandrekar, Shauna L. Hillman, Angelina D. Tan
Data analysis and interpretation: Hiran C. Fernando, Rodney J. Landreneau, Sumithra J. Mandrekar, Francis C. Nichols, Shauna L. Hillman, Dwight E. Heron, Bryan F. Meyers, Thomas A. DiPetrillo, David R. Jones, Sandra L. Starnes, Angelina D. Tan
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Ginsberg RJ, Rubenstein LV. Randomized trial of lobectomy versus limited resection for T1NO non-small cell lung cancer. Ann Thorac Surg. 1995;60:615–623. doi: 10.1016/0003-4975(95)00537-u. [DOI] [PubMed] [Google Scholar]
- 2.Shennib H, Bogart J, Herndon JE, et al. Video-assisted wedge resection and local radiotherapy for peripheral lung cancer in high-risk patients: The Cancer and Leukemia Group B (CALGB) 9335, a phase II, multi-institutional cooperative group study. J Thorac Cardiovasc Surg. 2005;129:813–818. doi: 10.1016/j.jtcvs.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 3.d'Amato TA, Galloway M, Szydlowski G, et al. Intraoperative brachytherapy following thoracoscopic wedge resection of stage I lung cancer. Chest. 1998;114:1112–1115. doi: 10.1378/chest.114.4.1112. [DOI] [PubMed] [Google Scholar]
- 4.Lee W, Daly BD, DiPetrillo TA, et al. Limited resection for non-small cell lung cancer: Observed local control with implantation of I-125 brachytherapy seeds. Ann Thorac Surg. 2003;75:237–243. doi: 10.1016/s0003-4975(02)04098-5. [DOI] [PubMed] [Google Scholar]
- 5.Fernando HC, Santos RS, Benfield JR, et al. Lobar and sublobar resection with and without brachytherapy for small stage IA non-small cell lung cancer. J Thorac Cardiovasc Surg. 2005;129:261–267. doi: 10.1016/j.jtcvs.2004.09.025. [DOI] [PubMed] [Google Scholar]
- 6.Voynov G, Heron DE, Lin CJ, et al. Intraopeative (125)I Vicryl mesh brachytherapy after sublobar resection for high-risk stage I non-small cell lung cancer. Brachytherapy. 2005;4:278–285. doi: 10.1016/j.brachy.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 7.Sawabata N, Matsumura A, Ohota M, et al. Cytologically malignant margins of wedge resected stage I non-small cell lung cancer. Ann Thorac Surg. 2002;74:1953–1957. doi: 10.1016/s0003-4975(02)03993-0. [DOI] [PubMed] [Google Scholar]
- 8.National Institutes of Health, National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) Version 3. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf.
- 9.Fernando HC, Landreneau RJ, Mandrekar SJ, et al. Thirty and ninety-day outcomes after sublobar resection with and without brachytherapy for non-small cell lung cancer: Results from a multicenter phase III study. J Thorac Cardiovasc Surg. 2011;142:1143–1151. doi: 10.1016/j.jtcvs.2011.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Warren WH, Faber LP. Segmentectomy versus lobectomy in patients with stage I pulmonary carcinoma: Five-year survival and patterns of recurrence. J Thorac Cardiovasc Surg. 1994;107:1087–1093. [PubMed] [Google Scholar]
- 11.Pepe MS, Mori M. Kaplan-Meier, marginal or conditional probability curves in summarizing competing risks failure time data? Stat Med. 1993;12:737–751. doi: 10.1002/sim.4780120803. [DOI] [PubMed] [Google Scholar]
- 12.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 13.El-Sherif A, Fernando HC, Santos R, et al. Margin and local recurrence after sublobar resection of non-small cell lung cancer. Ann Surg Oncol. 2007;14:2400–2405. doi: 10.1245/s10434-007-9421-9. [DOI] [PubMed] [Google Scholar]
- 14.Schuchert MJ, Pettiford BL, Keeley S, et al. Anatomic segmentectomy in the treatment of stage I non-small cell lung cancer. Ann Thorac Surg. 2007;84:926–933. doi: 10.1016/j.athoracsur.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 15.Timmerman RD, Park C, Kavanagh BD. The North American experience with stereotactic body radiation therapy in non-small cell lung cancer. J Thorac Oncol. 2007;2(suppl 3):S101–S112. doi: 10.1097/JTO.0b013e318074e4fa. [DOI] [PubMed] [Google Scholar]
- 16.Dupuy DE, DiPetrillo T, Gandhi S, et al. Radiofrequency ablation followed by conventional radiotherapy for medically inoperable stage I non-small cell lung cancer. Chest. 2006;129:738–745. doi: 10.1378/chest.129.3.738. [DOI] [PubMed] [Google Scholar]
- 17.Grillis IS, Mangona VS, Welsh R, et al. Outcomes after stereotactic lung radiotherapy or wedge resection for stage I non–small-cell lung cancer. J Clin Oncol. 2010;28:928–935. doi: 10.1200/JCO.2009.25.0928. [DOI] [PubMed] [Google Scholar]
- 18.Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010;303:1070–1076. doi: 10.1001/jama.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.