Abstract
Purpose
To investigate the efficacy and safety of aerobic training (AT) in patients with cancer with medically stable heart failure (HF).
Patients and Methods
A retrospective analysis of 90 patients with cancer who have HF and who were randomly assigned to AT (n = 47) or guideline-based usual care (UC; n = 43) was performed. AT consisted of three supervised sessions per week at 20 to 45 minutes per session at 60% to 70% of heart rate reserve for 12 weeks followed by home-based sessions for 4 to 12 months. The primary end point was all-cause mortality and hospitalization. Secondary end points were other clinical events, safety, and change in exercise capacity (VO2peak) and health-related quality of life (HRQOL).
Results
Median follow-up was 35 months. In intention-to-treat (ITT) analyses, all-cause mortality or hospitalization at 2 years was 74% in the AT group compared with 67% in the UC group (adjusted hazard ratio [HR], 1.11; 95% CI, 0.69 to 1.77; P = .676). The incidence of cardiovascular mortality or cardiovascular hospitalization was significantly higher in the AT group compared with the UC group (41% v 67%; adjusted HR, 1.94; 95% CI, 1.12 to 3.16; P = .017). There were no differences in any VO2peak or HRQOL end points. In post hoc analyses based on adherence to AT, all-cause mortality and hospitalization was 66% in adherent patients (≥ 90 minutes per week) compared with 84% in nonadherent patients (< 90 minutes per week).
Conclusion
In ITT analyses, AT did not improve clinical outcomes in patients with cancer who had HF. Post hoc analyses suggested that patients not capable of adhering to the planned AT prescription may be at increased risk of clinical events.
INTRODUCTION
The incidence of therapy-induced progressive declines in left ventricular function leading to overt heart failure (HF; ie, cardiac toxicity) is a major but under-recognized cause of competing mortality in long-term survivors of cancer.1–3 The importance of this problem is likely to increase with continual improvements in cancer-specific outcomes together with the aggressive use of newer cytotoxic agents (taxane-based regimens) and the introduction of molecularly targeted therapies, all of which have different cardiovascular safety profiles from historical regimens. Modern adjuvant therapy is also generally administered for longer durations, which increases the period of exposure and possibly the risk of cardiac toxicity.4
Management of patients with cancer who have signs or symptoms of overt cardiac toxicity represents a significant challenge. Effective management has, however, been significantly hampered by the lack of oncology-specific standard-of-care guidelines for cardiovascular toxicities.5 A major barrier to the development of such guidelines is that patients with a prior history of cancer are generally excluded from cardiovascular trials, and patients with HF (or other cardiovascular diseases) are typically ineligible for oncology trials. Treatment recommendations for general HF populations (ie, angiotensin-converting enzyme inhibitors and beta blockers6) are supported by evidence in patients with cancer who have HF,7,8 although data are limited.5,9
In patients with general HF, randomized trials demonstrate that aerobic training (AT) improves exercise capacity (VO2peak) with concomitant improvements in health-related quality of life (HRQOL), left ventricular ejection fraction, and possibly overall mortality.10–13 Similarly, promising data in the oncology setting indicates that AT is safe and is associated with significant improvements in VO2peak and HRQOL.14,15 However, the vast majority of studies have been conducted in patients with early-stage disease without significant underlying comorbid disease.15 Furthermore, the monitoring and reporting of adverse events (AEs) in trials to date has been less than optimal.16 Finally, whether AT improves clinical events in patients with cancer has not been investigated.16 Thus, the safety and efficacy of AT in high-risk (or complex) patients with cancer remains uncertain.
We conducted an ancillary analysis of the Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training (HF-ACTION)17,18 study to investigate the safety and efficacy of AT in patients with cancer with stable HF. We hypothesized that AT would be safe and would confer significant reductions in clinical events with concomitant improvements in VO2peak and HRQOL compared with usual care (UC).
PATIENTS AND METHODS
Eligibility and Trial Overview
Full details regarding the study sample, recruitment, and procedures have been reported previously.17,18 In brief, HF-ACTION was a multicenter, randomized controlled trial involving 2,331 patients with stable HF (ie, left ventricular ejection fraction ≤ 35% and New York Heart Association class II to IV symptoms) enrolled between April 2003 and February 2007 at 82 clinical medical centers. There was no specific exclusion criterion for patients with cancer or cardiomyopathy as a result of anticancer therapy. At baseline, history of cancer in the last 5 years, excluding minor skin cancer, was collected for each participant. Cancer type, stage, and anticancer therapy were not collected. Etiology of HF was categorized as either ischemic or nonischemic, with no further delineation of cause within the nonischemic category. The protocol was approved by the respective institutional review boards or ethics committees for each of the clinical sites and the coordinating center.
AT Intervention
The initial prescription consisted of three group-based, supervised AT (treadmill or stationary cycle ergometer) sessions per week lasting 20 to 45 minutes per session at 60% to 70% of heart rate reserve (HRR) for 12 weeks. AT was initiated at 15 to 30 minutes per session at a heart rate corresponding to 60% of measured HRR obtained during the baseline cardiopulmonary exercise test (CPET). After six sessions, AT duration and intensity were increased to 30 to 35 minutes and 70% of HRR, respectively. Patients were encouraged to begin home-based AT after 18 supervised sessions with full transition after 36 supervised AT sessions. Participants were provided with home AT equipment (cycle or treadmill [ICON, Logan, UT]) and heart rate monitors (Polar USA, New York, NY). The home-based AT prescription goal was 5 days per week for 40 minutes at a heart rate of 60% to 70% of HRR. Adherence was evaluated by measuring attendance at supervised AT sessions and by activity logs, telephone and clinic follow-up, and heart rate monitoring data for home-based sessions. Adherence was defined a priori as ≥ 90 minutes per week of supervised AT and ≥ 120 minutes per week of home-based AT from month 4 onward.17
UC
UC participants were instructed to maintain their usual exercise levels and not to initiate structured AT during the study period. All patients, regardless of treatment group, received standardized UC materials and physical activity recommendations as described previously.17
Follow-Up
All patients were asked to return for clinic visits every 3 months for the first 2 years of participation and yearly thereafter for up to 4 years. Cardiopulmonary exercise testing and 6-minute walk testing were performed, and health status questionnaires were completed at the 3- and 12-month follow-up visits. Follow-up was completed on March 15, 2008. Searches of the Social Security Death Index and the National Death Index were performed for patients lost to follow-up.
Primary and Secondary Clinical End Points
The primary end point was a composite of all-cause mortality and hospitalization. Secondary clinical end points and/or events were all-cause mortality, the composite of cardiovascular mortality and cardiovascular hospitalization, and the composite of cardiovascular mortality and HF hospitalization. It was not possible to blind patients or site investigators to AT; however, all deaths and other clinical end points were adjudicated by a clinical end points committee. Cardiovascular AEs included worsening HF, myocardial infarction, unstable angina, serious adverse arrhythmia, stroke, and transient ischemic attack.
Cardiopulmonary Exercise Testing
VO2peak was assessed by using a maximal CPET with expired gas analysis. CPET data were forwarded to a core laboratory for analysis. VO2peak was defined as the highest VO2 for a given 15-second to 20-second interval within the last 90 seconds of exercise or the first 30 seconds of recovery.19 CPET measures were VO2peak reported in mL · kg–1 · min−1 (relative) and L · min−1 (absolute), VO2peak at ventilatory threshold, peak heart rate, and respiratory exchange ratio. During exercise, heart rate and rhythm were monitored continuously by using a 12-lead electrocardiogram. All tests were reviewed by investigators to identify significant arrhythmias or ischemia that would preclude AT and to establish appropriate AT heart rate zones. The 6-minute walk test was conducted according to established guidelines.20
HRQOL
HRQOL was assessed by using the Kansas City Cardiomyopathy Questionnaire, a 23-item self-administered disease-specific questionnaire.21 The Kansas City Cardiomyopathy Questionnaire is scored from 0 to 100, with higher scores representing better HRQOL. In addition to an overall summary score, subscores for physical limitations, symptoms, quality of life, and social limitations are also reported.
Statistical Analysis
All statistical analyses were conducted by the coordinating center (Duke Clinical Research Institute, Durham, NC) by using SAS software version 9.2 (SAS Institute, Cary NC). Differences in baseline characteristics between groups were assessed by using χ2 tests for categorical variables and t tests for continuous variables. AEs were compared by using Fisher's exact tests. Statistical comparisons of the study groups with respect to the primary and secondary outcomes were performed according to the intention-to-treat (ITT) principle. Cumulative event rates were calculated by using the Kaplan-Meier method and reported at 2 years of follow-up. The Cox proportional hazard regression model was used to compare groups by using all available follow-up data. Data on clinical end points was collected until the time of final contact with the patient, including patients who withdrew consent or were lost to follow-up, at which point follow-up was censored. Relative risks were expressed as hazard ratios (HRs) with 95% CIs. HRs were adjusted for age, sex, race, and use of beta blockers.
Linear mixed effects models were used to examine change in VO2peak and HRQOL. Patients missing VO2peak or HRQOL data at month 3 or month 12 contributed to the analysis with the available information. In post hoc analysis, we also explored the effect of AT as a function of protocol-specified adherence for the supervised AT portion of the trial (ie, < 90 minutes per week v ≥ 90 minutes per week). All linear mixed effects models were unadjusted. As with ITT analyses, cumulative event rates were calculated by using the Kaplan-Meier method and were reported at 2 years of follow-up. The Cox proportional hazards regression model (without adjustment) was used to compare groups by using all available follow-up data. Relative risks were expressed as HRs with 95% CIs. All statistical tests were two-tailed with a significance level α = .05.
RESULTS
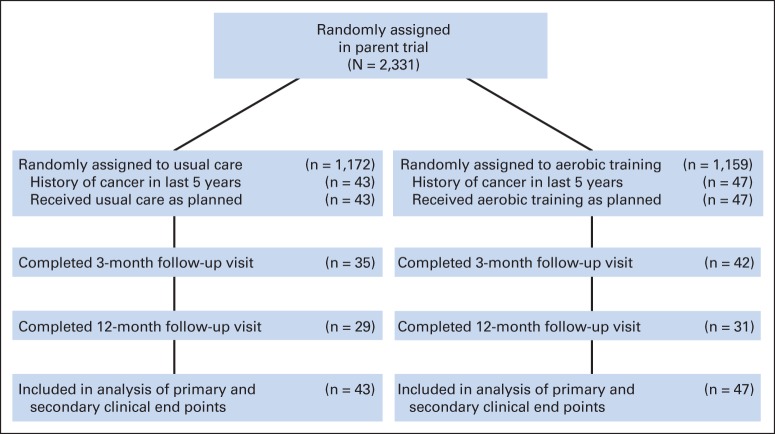
Of the 2,331 patients randomly assigned in the parent trial, 90 (3.8%) had a medical history of cancer in the last 5 years. Participants' characteristics were balanced at baseline (Table 1), except a higher proportion of patients in the UC group were receiving beta blockers. In terms of HF etiology, 58% presented with ischemic etiology and 42% presented with nonischemic etiology. The study flow is presented in Figure 1. Of the 90 randomly assigned patients (AT, n = 47; UC, n = 43), 77 (85%) and 60 (66%) completed VO2peak HRQOL assessments at 3 months and 12 months, respectively. Median adherence to AT was 91 minutes per week in months 0 to 3 and 116 minutes per week in months 10 to 12. The median duration of follow-up for the primary end point was 35 months.
Table 1.
Demographic and Medical Characteristics of Participants
| Characteristic | All Patients |
UC Group |
AT Group |
P | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | Mean | SD | No. | % | Mean | SD | No. | % | Mean | SD | ||
| No. of patients | 90 | 100 | 43 | 48 | 47 | 52 | |||||||
| Age, years | 66 | 11 | 66 | 11 | 66 | 10 | .861 | ||||||
| Female sex | 23 | 26 | 10 | 23 | 13 | 28 | .632 | ||||||
| Weight, kg | 85.1 | 19.8 | 86.6 | 21.7 | 83.8 | 18.2 | .507 | ||||||
| BMI, kg/m2 | 28 | 6 | 29 | 8 | 28 | 5 | .385 | ||||||
| Heart rate, beats · min–1 | 71 | 11 | 72 | 11 | 70 | 11 | .269 | ||||||
| Systolic blood pressure, mmHg | 116 | 19 | 117 | 20 | 115 | 18 | .634 | ||||||
| Diastolic blood pressure, mmHg | 70 | 10 | 69 | 9 | 70 | 11 | .422 | ||||||
| NYHA class | .344 | ||||||||||||
| II | 57 | 63 | 29 | 67 | 28 | 60 | |||||||
| III | 31 | 34 | 14 | 33 | 17 | 36 | |||||||
| IV | 2 | 2 | 0 | 0 | 2 | 4 | |||||||
| Race/ethnicity | .810 | ||||||||||||
| White | 59 | 66 | 28 | 65 | 31 | 67 | |||||||
| African American | 27 | 30 | 14 | 33 | 13 | 28 | |||||||
| Other | 3 | 3 | 1 | 2 | 2 | 4 | |||||||
| Left ventricular ejection fraction, % | 26 | 8 | 27 | 8 | 24 | 8 | .188 | ||||||
| Comorbid conditions | |||||||||||||
| Diabetes | 34 | 38 | 19 | 44 | 15 | 32 | .230 | ||||||
| Previous MI | 44 | 49 | 18 | 42 | 26 | 55 | .202 | ||||||
| Hypertension | 52 | 58 | 28 | 65 | 24 | 51 | .177 | ||||||
| Atrial fibrillation or atrial flutter | 18 | 20 | 7 | 16 | 11 | 23 | .398 | ||||||
| Sodium | 139.5 | 2.7 | 139.5 | 2.9 | 139.4 | 2.4 | .941 | ||||||
| Serum creatinine | 1.5 | 1.3 | 1.4 | 0.5 | 1.6 | 1.8 | .353 | ||||||
| Medications and devices | |||||||||||||
| ACE inhibitor or ARB | 85 | 94 | 40 | 93 | 45 | 96 | .573 | ||||||
| Beta blocker | 79 | 88 | 41 | 95 | 38 | 81 | .036 | ||||||
| Aldosterone | 34 | 38 | 13 | 30 | 21 | 45 | .157 | ||||||
| Loop diuretic | 65 | 72 | 33 | 77 | 32 | 68 | .359 | ||||||
| Digoxin | 44 | 49 | 19 | 44 | 25 | 53 | .393 | ||||||
| Implantable cardioverter-defibrillator | 35 | 39 | 15 | 35 | 20 | 43 | .456 | ||||||
| Biventricular pacemaker | 16 | 18 | 8 | 19 | 8 | 17 | .844 | ||||||
NOTE. Continuous variables are reported as mean and standard deviation (SD); categorical variables are reported as No. and %.
Abbreviations: ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blocker; AT, aerobic training; BMI, body mass index; MI, myocardial infarction; NYHA, New York Heart Association; UC, usual care.
Fig 1.
CONSORT diagram.
Primary and Secondary Clinical End Points
For the primary end point, there was no difference in all-cause mortality or hospitalization or hospitalization between the AT and UC groups. The cumulative incidence of all-cause mortality or hospitalization was 74% in the AT group compared with 67% in the UC group (adjusted HR, 1.11; 95% CI, 0.69 to 1.77; P = .676; Table 2). The incidence of the combined end point of cardiovascular mortality and cardiovascular hospitalization was higher in the AT group compared with the UC group (67% v 41%; adjusted HR, 1.94; 95% CI, 1.12 to 3.16; P = .017; Table 2). Similarly, there was no difference in the combined end point of cardiovascular mortality and HF hospitalization in the AT group compared with the UC group (32% v 20%; adjusted HR, 1.77; 95% CI, 0.85 to 3.70; P = .128).
Table 2.
Differences in Clinical Events by Treatment Group (intention to treat)
| Event | UC (n = 43) |
AT (n = 47) |
HR | 95% CI | P* | ||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | ||||
| All-cause mortality or all-cause hospitalization | 27 | 67 | 33 | 74 | 1.11 | 0.69 to 1.77 | .676 |
| Cardiovascular mortality and cardiovascular hospitalization | 16 | 41 | 29 | 67 | 1.94 | 1.12 to 3.16 | .017 |
| Cardiovascular mortality and HF hospitalization | 8 | 20 | 14 | 32 | 1.77 | 0.85 to 3.70 | .128 |
| All-cause mortality, all-cause hospitalization, emergency department visit, or urgent clinic visit for HF exacerbation | 35 | 95 | 37 | 95 | 0.97 | 0.62 to 1.54 | .914 |
| All-cause mortality | 4 | 11 | 5 | 11 | 1.03 | 0.38 to 2.85 | .948 |
| Cardiovascular-related mortality | 1 | 3 | 3 | 7 | 1.36 | 0.38 to 4.81 | .636 |
NOTE. Cumulative event rates were calculated by using the Kaplan-Meier method and were reported at 2 years of follow-up. The Cox proportional hazards regression model was used to compare groups by using all available follow-up data. Data are No. of clinical events and Kaplan Meier estimates at 2 years (%).
Abbreviations: AT, aerobic training; HF, heart failure; HR, hazard ratio; UC, usual care.
Adjusted for age, sex, race, and use of beta blockers.
AEs (Safety)
The incidence of the composite end point of any AEs was significantly higher in the AT group compared with the UC group (45% v 23%; P = .046); this difference was driven by a higher incidence of worsening HF (19% v 5%; P = .052) and serious arrhythmia (17% v 2%; P = .032), respectively (Table 3).
Table 3.
Differences in AEs (Safety) by Treatment Group (intention to treat)
| Event | UC (n = 43) |
AT (n = 47) |
P* | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Worsening HF | 2 | 5 | 9 | 19 | .052 |
| Unstable angina | 7 | 16 | 6 | 13 | .767 |
| Serious arrhythmia | 1 | 2 | 8 | 17 | .032 |
| Stroke | 1 | 2 | 1 | 2 | 1.000 |
| Transient ischemic attack | 2 | 5 | 0 | 0 | .226 |
| Any of the above events | 10 | 23 | 21 | 45 | .046 |
| Hospitalization after exercise | 1 | 2 | 2 | 4 | 1.000 |
Abbreviations: AE, adverse event; AT, aerobic training; HF, heart failure; UC, usual care.
Fisher's exact test.
Effects on VO2peak, 6-Minute Walk Distance, and HRQOL
VO2peak increased by +0.6 mL · kg–1 · min−1 from baseline to month 3 and by +0.5 mL · kg–1 · min–1 from month 3 to 12 in the AT group compared with +0.8 mL · kg–1 · min–1 and +0.5 mL · kg–1 · min–1 for the corresponding time points in the UC group (P = .710; Table 4). No significant group-by-time interactions were observed for any CPET outcomes or 6-minute walk distance. In general, HRQOL outcomes improved over time in the AT group but no significant group-by-time interactions were observed.
Table 4.
Changes in Exercise Capacity and 6-Minute Walk Distance (intention to treat)
| Variable | UC |
AT |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline |
Month 3 |
Month 12 |
P* | Baseline |
Month 3 |
Month 12 |
P* | P† | |||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||||
| Heart rate, beats · min–1 | 116 | 23 | 118 | 23 | 118 | 23 | .797 | 117 | 22 | 117 | 19 | 115 | 18 | .539 | .558 |
| VO2peak, mL · kg–1 · min–1 | 14.3 | 4.4 | 15.1 | 4.1 | 15.6 | 4.1 | .622 | 14.5 | 4.8 | 15.1 | 5.1 | 15.6 | 5.3 | .602 | .710 |
| VO2peak, L · min–1 | 1.2 | 0.4 | 1.3 | 0.4 | 1.3 | 0.4 | .361 | 1.2 | 0.5 | 1.3 | 0.5 | 1.3 | 0.5 | .560 | .528 |
| VO2peak at VT, mL · kg–1 · min–1 | 10.3 | 2.0 | 10.7 | 2.4 | 11.3 | 2.3 | .213 | 11.1 | 2.4 | 10.9 | 2.5 | 11.6 | 2.8 | .546 | .675 |
| RER | 1.09 | 0.12 | 1.11 | 0.13 | 1.12 | 0.11 | .620 | 1.09 | 0.10 | 1.13 | 0.11 | 1.11 | 0.14 | .039 | .383 |
| 6-Minute walk distance, m | 356 | 109 | 391 | 97 | 383 | 79 | .259 | 358 | 119 | 390 | 105 | 382 | 116 | .040 | .541 |
NOTE. Sample size for the usual care (UC) group: baseline, n = 43; month 3, n = 35; month 12, n = 29; for the aerobic training (AT) group: baseline, n = 46; month 3, n = 42; month 12, n = 31.
Abbreviations: RER, respiratory exchange ratio; SD, standard deviation; VO2peak, peak oxygen consumption; VT, ventilatory threshold.
P value comparing the three time points (derived from an unadjusted model). Significant P values indicate that at least one time point is different.
P value for the interaction between time and treatment.
Post Hoc Analyses
During the supervised phase of AT, 53% of the patients (n = 25) were adherent (ie, ≥ 90 minutes per week). For the primary clinical end point, the rate of all-cause mortality or hospitalization was lower in adherent patients (66.1%) compared with nonadherent patients (ie, < 90 minutes per week; 83.9%; Table 5); the corresponding rate for all-cause mortality was 4.0% versus 19.3%, respectively. Similar patterns were observed for the composite end points of cardiovascular mortality and cardiovascular hospitalization and cardiovascular mortality and HF hospitalizations (Table 5). There were no significant changes in VO2peak or 6-minute maximum walking distance in the adherent and nonadherent groups, although significant improvements in several HRQOL domains were observed in adherent patients.
Table 5.
Clinical Events As a Function of Adherence in the AT Group (post hoc analysis)
| Event | UC |
< 90 minutes/week |
≥ 90 minutes/week |
|||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| All-cause mortality and all-cause hospitalization | 27 | 67 | 17 | 84 | 16 | 66 |
| Cardiovascular mortality and cardiovascular hospitalization | 16 | 41 | 16 | 83 | 13 | 54 |
| Cardiovascular mortality and HF hospitalization | 8 | 20 | 10 | 52 | 4 | 16 |
| All-cause mortality, all-cause hospitalization, emergency department visit, or urgent clinic visit for HF exacerbation | 35 | 95 | 18 | 100 | 19 | 91 |
| All-cause mortality | 4 | 11 | 4 | 19 | 1 | 4 |
| Cardiovascular-related mortality | 1 | 3 | 2 | 11 | 1 | 4 |
NOTE. Sample size for the usual care (UC) group: n = 43; for the aerobic training (AT) group: < 90 minutes/week, n = 22; ≥ 90 minutes/week, n = 25. Cumulative event rates were calculated by using the Kaplan-Meier method and were reported at 2 years of follow-up. The Cox proportional hazards regression model was used to compare groups by using all available follow-up data. Data are No. of clinical events and Kaplan-Meier estimates at 2 years (%).
Abbreviation: HF, heart failure.
DISCUSSION
Contrary to our primary hypothesis, ITT analysis indicated that AT was not associated with improvements in the primary clinical end point of all-cause mortality or hospitalization, or any other clinical end points in comparison to UC. In contrast to the parent trial,17 in this trial, AT was associated with a nonsignificant 11% higher incidence of all-cause mortality or hospitalization compared with a 7% to 11% reduction in the parent trial.17 Similar trends were observed across virtually all other clinical end points under the principle of ITT. The higher incidence of clinical events with AT appear to be primarily driven by worsening HF and serious arrhythmia that, together, contribute to a significantly higher overall AE rate in the AT group (45% v 34%). Under ITT, AT was also not associated with improvements in VO2peak or HRQOL, which is contrary to the consistent favorable effects observed in general HF13,22,23 and the oncology setting.14,15
Intriguingly, post hoc analyses as a function of protocol-specified AT adherence indicated that higher AT dose (ie, ≥ 90 minutes per week) was associated with an improvement in VO2peak and several domains of HRQOL from baseline to month 3. Hence, supervised AT may be a safe and efficacious strategy in patients with cancer who have HF and are capable of adhering to the prescribed AT regimen and is associated with a magnitude of benefit similar to that in the general HF population.17 These findings may be important, given the likely etiologic mechanistic differences between anticancer therapy-induced HF and general HF,24,25 and the rapidly growing number patients with cancer who are at risk for or who present with cardiac toxicity.1–3 To this end, approximately 40% of patients with HF in this substudy had HF with a nonischemic etiology, which may have been related to prior anticancer therapy, although it remains unknown whether the response to AT differs as a function of HF etiology (ischemic v nonischemic) in patients with cancer.
It is important to highlight that caution must be used when interpreting our findings, given the highly exploratory nature of this study. This was an unplanned retrospective analysis of prospectively collected data not designed or powered to investigate the question of interest. In addition, data pertaining to cancer diagnosis and specific treatment-related characteristics, including type and length and/or dose of cancer therapy were not available. Similarly, it is not known whether study groups were balanced on cancer-specific characteristics, although groups were balanced on all other demographic and medical characteristics including HF etiology. Although this information has an impact on the generalizability of our findings, we contend that it does not significantly impact the results or interpretation of our findings because although patients with cancer previously treated with certain anticancer therapies are at higher risk of HF, those not treated with these agents are also at high risk, especially because the vast majority of those diagnosed are age 65 and older.26 HF is a major cause of mortality in this age group.27 Thus, this study and its findings are relevant to all patients diagnosed with cancer regardless of type and treatment, and the current definition and management of HF is the same regardless of prior therapy with cardiotoxic anticancer agents.5 In other words, treatment of HF in patients with the cancer who were included in this substudy would not have been altered on the basis of their specific cancer diagnosis or prior or concurrent therapy.
An unexpected finding was that the incidence of clinical events in the low (nonadherent) AT dose group (ie, < 90 minutes per week) was markedly higher compared with that in adherent patients and that reported in the parent trial.17 There was also no change in VO2peak or HRQOL from baseline to month 3 in this subgroup. Interestingly, a similar volume of AT was associated with clinical benefit in the parent trial.28 Collectively, these data provide initial evidence to suggest that a small, but significant subgroup of patients with HF and cancer either are too ill (at presentation) to adhere to standard AT guidelines or may even experience an adverse response to AT. The clinical characteristics of the low AT dose group were similar to those of the high AT dose group and those of the overall sample in the parent trial, suggesting that this subgroup of patients was not atypical. Alternatively, recent work suggests that a subpopulation of individuals may exhibit an adverse cardiovascular and/or metabolic response to AT29 that is distinct from classic acute exercise (stress) –induced events (eg, sudden cardiac death). Such a subpopulation may also exist in patients with cancer who have HF, although the underlying mechanisms are not known. Interestingly, exposure of juvenile animals to anthracycline-containing chemotherapy causes long-term impairments that render the myocardium more susceptible to pathologic (ie, myocardial infarction) as well as physiologic (ie, exercise) stress-induced HF.30 Whether this mechanism contributes to our observations cannot be determined. Nevertheless, our initial findings highlight the critical need for development of individualized pre-exercise screening and exercise prescriptions not only to identify those patients at risk for adverse response patterns but also to optimize the efficacy of exercise therapy in clinical populations, including patients with cancer.
In summary, in this unplanned retrospective study, AT did not improve clinical outcomes or VO2peak and HRQOL in patients with cancer who have HF compared with UC. Furthermore, post hoc analyses suggested that patients not capable of adhering to AT may be at increased risk of clinical events. Adequately powered prospective trials investigating the safety and efficacy (as well as response modifiers) of exercise training, as well as other therapeutic strategies, in patients with cancer with or at risk of anticancer therapy-induced HF are warranted.
Supplementary Material
Footnotes
Supported by research Grants No. CA138624, CA142566, CA133895, CA164751, and CA179992 from the National Cancer Institute (L.W.J.). Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training (HF-ACTION) was supported by Grants No. 5U01-HL063747 (Duke Clinical Research Institute Coordinating Center), 5U01HL066461 (Economic and Quality of Life), HL068973 (grant enrolling centers, Boston Medical Center), HL066501 (Case Western Reserve University), HL066482 (Emory University), HL064250 (Henry Ford Hospital), HL066494 (Ohio State University), HL064257 (Oregon Health & Science University), HL066497 (University of Alabama), HL068980 (University of California, Los Angeles), HL064265 (University of Colorado), HL066491 (Wake Forest University), and HL064264 (Washington University, St Louis, MO) from the National Heart, Lung and Blood Institute.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information: NCT00047437.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Lee W. Jones, William E. Kraus, David J. Whellan, Christopher M. O'Connor
Financial support: David J. Whellan
Administrative support: David J. Whellan
Provision of study materials or patients: David J. Whellan
Collection and assembly of data: David J. Whellan
Data analysis and interpretation: Lee W. Jones, Pamela S. Douglas, Michel G. Khouri, John R. Mackey, Daniel Wojdyla, Christopher M. O'Connor
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Mackey JR, Martin M, Pienkowski T, et al. Adjuvant docetaxel, doxorubicin, and cyclophosphamide in node-positive breast cancer: 10-year follow-up of the phase 3 randomised BCIRG 001 trial. Lancet Oncol. 2013;14:72–80. doi: 10.1016/S1470-2045(12)70525-9. [DOI] [PubMed] [Google Scholar]
- 2.Darby SC, Ewertz M, Hall P. Ischemic heart disease after breast cancer radiotherapy. N Engl J Med. 2013;368:2527. doi: 10.1056/NEJMc1304601. [DOI] [PubMed] [Google Scholar]
- 3.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572–1582. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 4.Koelwyn GJ, Khouri M, Mackey JR, et al. Running on empty: Cardiovascular reserve capacity and late effects of therapy in cancer survivorship. J Clin Oncol. 2012;30:4458–4461. doi: 10.1200/JCO.2012.44.0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khouri MG, Douglas PS, Mackey JR, et al. Cancer therapy-induced cardiac toxicity in early breast cancer: Addressing the unresolved issues. Circulation. 2012;126:2749–2763. doi: 10.1161/CIRCULATIONAHA.112.100560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 7.Jensen BV, Skovsgaard T, Nielsen SL. Functional monitoring of anthracycline cardiotoxicity: A prospective, blinded, long-term observational study of outcome in 120 patients. Ann Oncol. 2002;13:699–709. doi: 10.1093/annonc/mdf132. [DOI] [PubMed] [Google Scholar]
- 8.Cardinale D, Colombo A, Lamantia G, et al. Anthracycline-induced cardiomyopathy: Clinical relevance and response to pharmacologic therapy. J Am Coll Cardiol. 2010;55:213–220. doi: 10.1016/j.jacc.2009.03.095. [DOI] [PubMed] [Google Scholar]
- 9.Mann DL, Krone RJ. Cardiac disease in cancer patients: An overview. Prog Cardiovasc Dis. 2010;53:80–87. doi: 10.1016/j.pcad.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 10.Belardinelli R, Georgiou D, Cianci G, et al. Randomized, controlled trial of long-term moderate exercise training in chronic heart failure: Effects on functional capacity, quality of life, and clinical outcome. Circulation. 1999;99:1173–1182. doi: 10.1161/01.cir.99.9.1173. [DOI] [PubMed] [Google Scholar]
- 11.Giannuzzi P, Temporelli PL, Corrà U, et al. Antiremodeling effect of long-term exercise training in patients with stable chronic heart failure: Results of the Exercise in Left Ventricular Dysfunction and Chronic Heart Failure (ELVD-CHF) Trial. Circulation. 2003;108:554–559. doi: 10.1161/01.CIR.0000081780.38477.FA. [DOI] [PubMed] [Google Scholar]
- 12.Hambrecht R, Gielen S, Linke A, et al. Effects of exercise training on left ventricular function and peripheral resistance in patients with chronic heart failure: A randomized trial. JAMA. 2000;283:3095–3101. doi: 10.1001/jama.283.23.3095. [DOI] [PubMed] [Google Scholar]
- 13.Wisløff U, Støylen A, Loennechen JP, et al. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: A randomized study. Circulation. 2007;115:3086–3094. doi: 10.1161/CIRCULATIONAHA.106.675041. [DOI] [PubMed] [Google Scholar]
- 14.Jones LW, Liang Y, Pituskin EN, et al. Effect of exercise training on peak oxygen consumption in patients with cancer: A meta-analysis. Oncologist. 2011;16:112–120. doi: 10.1634/theoncologist.2010-0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmitz KH, Courneya KS, Matthews C, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42:1409–1426. doi: 10.1249/MSS.0b013e3181e0c112. [DOI] [PubMed] [Google Scholar]
- 16.Jones LW, Alfano CM. Exercise-oncology research: Past, present, and future. Acta Oncol. 2013;52:195–215. doi: 10.3109/0284186X.2012.742564. [DOI] [PubMed] [Google Scholar]
- 17.O'Connor CM, Whellan DJ, Lee KL, et al. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301:1439–1450. doi: 10.1001/jama.2009.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whellan DJ, O'Connor CM, Lee KL, et al. Heart failure and a controlled trial investigating outcomes of exercise training (HF-ACTION): Design and rationale. Am Heart J. 2007;153:201–211. doi: 10.1016/j.ahj.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 19.Bensimhon DR, Leifer ES, Ellis SJ, et al. Reproducibility of peak oxygen uptake and other cardiopulmonary exercise testing parameters in patients with heart failure (from the Heart Failure and A Controlled Trial Investigating Outcomes of exercise traiNing) Am J Cardiol. 2008;102:712–717. doi: 10.1016/j.amjcard.2008.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brooks D, Solway S, Gibbons WJ. ATS statement on six-minute walk test. Am J Respir Crit Care Med. 2003;167:1287. doi: 10.1164/ajrccm.167.9.950. [DOI] [PubMed] [Google Scholar]
- 21.Flynn KE, Piña IL, Whellan DJ, et al. Effects of exercise training on health status in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301:1451–1459. doi: 10.1001/jama.2009.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haykowsky MJ, Brubaker PH, Stewart KP, et al. Effect of endurance training on the determinants of peak exercise oxygen consumption in elderly patients with stable compensated heart failure and preserved ejection fraction. J Am Coll Cardiol. 2012;60:120–128. doi: 10.1016/j.jacc.2012.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edelmann F, Gelbrich G, Düngen HD, et al. Exercise training improves exercise capacity and diastolic function in patients with heart failure with preserved ejection fraction: Results of the Ex-DHF (Exercise training in Diastolic Heart Failure) pilot study. J Am Coll Cardiol. 2011;58:1780–1791. doi: 10.1016/j.jacc.2011.06.054. [DOI] [PubMed] [Google Scholar]
- 24.Force T, Kolaja KL. Cardiotoxicity of kinase inhibitors: The prediction and translation of preclinical models to clinical outcomes. Nat Rev Drug Discov. 2011;10:111–126. doi: 10.1038/nrd3252. [DOI] [PubMed] [Google Scholar]
- 25.Zhang S, Liu X, Bawa-Khalfe T, et al. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat Med. 2012;18:1639–1642. doi: 10.1038/nm.2919. [DOI] [PubMed] [Google Scholar]
- 26.Pinder MC, Duan Z, Goodwin JS, et al. Congestive heart failure in older women treated with adjuvant anthracycline chemotherapy for breast cancer. J Clin Oncol. 2007;25:3808–3815. doi: 10.1200/JCO.2006.10.4976. [DOI] [PubMed] [Google Scholar]
- 27.Writing Committee Members. Yancy CW, Jessup M, et al. 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240–e327. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- 28.Keteyian SJ, Leifer ES, Houston-Miller N, et al. Relation between volume of exercise and clinical outcomes in patients with heart failure. J Am Coll Cardiol. 2012;60:1899–1905. doi: 10.1016/j.jacc.2012.08.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bouchard C, Blair SN, Church TS, et al. Adverse metabolic response to regular exercise: Is it a rare or common occurrence? PloS One. 2012;7:e37887. doi: 10.1371/journal.pone.0037887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang C, Zhang X, Ramil JM, et al. Juvenile exposure to anthracyclines impairs cardiac progenitor cell function and vascularization resulting in greater susceptibility to stress-induced myocardial injury in adult mice. Circulation. 2010;121:675–683. doi: 10.1161/CIRCULATIONAHA.109.902221. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.