Abstract
Background
Members of the Semaphorin 3 family (Sema3s) influence the development of the central nervous system, and some are implicated in regulating aspects of visual system development. However, we lack information about the timing of expression of the Sema3s with respect to different developmental epochs in the mammalian visual system. In this time-course study in the rat, we document for the first time changes in the expression of RNAs for the majority of Class 3 Semaphorins (Sema3s) and their receptor components during the development of the rat retina and superior colliculus (SC).
Results
During retinal development, transcript levels changed for all of the Sema3s examined, as well as Nrp2, Plxna2, Plxna3, and Plxna4a. In the SC there were also changes in transcript levels for all Sema3s tested, as well as Nrp1, Nrp2, Plxna1, Plxna2, Plxna3, and Plxna4a. These changes correlate with well-established epochs, and our data suggest that the Sema3s could influence retinal ganglion cell (RGC) apoptosis, patterning and connectivity in the maturing retina and SC, and perhaps guidance of RGC and cortical axons in the SC. Functionally we found that SEMA3A, SEMA3C, SEMA3E, and SEMA3F proteins collapsed purified postnatal day 1 RGC growth cones in vitro. Significantly this is a developmental stage when RGCs are growing into and within the SC and are exposed to Sema3 ligands.
Conclusion
These new data describing the overall temporal regulation of Sema3 expression in the rat retina and SC provide a platform for further work characterising the functional impact of these proteins on the development and maturation of mammalian visual pathways.
Keywords: Retinal ganglion cells, Collapse assay, qPCR, Neuropilins, Plexins, Cell adhesion molecules
Background
The mammalian visual system is complex, and the developmental events that give rise to this highly organised system are similarly complex and exquisitely ordered. The timing of the major developmental events of the mammalian visual system has been well characterised, and many of the molecular cues controlling these critical events have been elucidated (for example Ephs/Ephrins, Slits/Robo, Netrin/DCC, and various neurotrophic factors [1]–[7]). However despite our increased knowledge about these molecular cues, they are not sufficient to explain completely the complexity and timing of the development of visual pathways in mammals. It is likely then that other molecules are also involved during the development and maturation of the mammalian visual system, and in this context recent attention has turned to the Semaphorins.
Semaphorins (Semas) have been implicated in neural and vascular aspects of visual system development in various species including frog [8],[9], zebrafish [10]–[14], goldfish [15], and chicken [16]–[21]. In mouse the membrane bound Class 5 and Class 6 Semaphorins are involved in lamination of the retina [22]–[24], and guidance of retinal ganglion cell (RGC) axons [25] via contact-mediated interactions. However many previously discovered molecular cues in the mammalian visual system are diffusible, and the only vertebrate secreted members of the Semaphorins are the Class 3 Semaphorins (Sema3s) [26] which are known to be expressed in the developing rat retina [27]. It is possible then that the Sema3s also influence the complexity and timing of the developing mammalian visual system.
The Sema3s consist of Sema3a through Sema3g[16],[26],[28]–[36] and their main receptors are the Neuropilins which form multimeric receptor complexes with Plexins and cell adhesion molecules [37]. Sema3s were initially discovered as axon guidance molecules [30],[38], but are now known to also mediate apoptosis, cell migration, immune response, organogenesis, tumour suppression and promotion, and vasculature development [39]–[44]. Sema3s affect at least some aspects of the development of the visual system of rodents [45],[46], from where much of our knowledge of molecular and activity-driven influences on mammalian visual system development has come [6],[47].
In summary, while we understand much about the timing of developmental events in the mammalian visual system, investigation of a potential role for the Sema3s in these events would benefit from knowledge about the timing of expression of Sema3s and their receptors during visual system maturation. We sought to address this gap in our knowledge by building on a previous study from our laboratory [27], focusing on Sema3s known to be expressed in RGCs and extending the work to include a greater number of receptors as well as analysis of a major central target for those RGCs, the superior colliculus (SC). To that end, we quantified transcript expression in the rat retina and SC over a range of embryonic (E) and postnatal (P) developmental time points (E16, E19, birth – P0, P7, P14, P21 and adult) which were chosen to correspond to previously established developmental epochs such as the timing of cell birth, migration, naturally occurring cell death, axonal and dendritic growth, and synaptogenesis (Figure 1). We also investigated the hypothesis that Sema3 ligands in the SC can influence RGC axon guidance using an in vitro growth cone collapse assay on isolated RGCs from a developmental stage when they would be growing into and within the SC in vivo.
Figure 1.
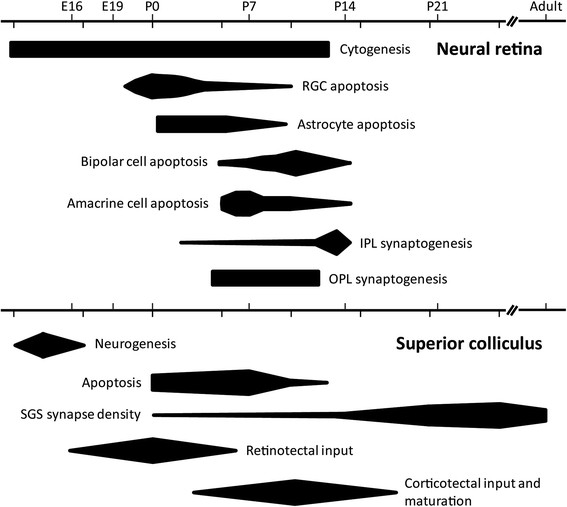
Developmental epochs in the rat neural retina and superior colliculus. Cytogenesis in the rat retina occurs from E9 through to P12, with distinct and overlapping waves of cell birth and differentiation [48]–[51]. Death of differentiated retinal ganglion cells (RGCs) is predominantly perinatal; starting at E20, peaking between birth and P3, falling sharply at P5 and continuing on until around P10 [52]–[57]. Astrocyte apoptosis is observed from P0, peaking between then and P5, and is associated with vasculature development [51],[58]. Bipolar and amacrine cell apoptosis occurs concurrently with different peaks, from P5 until P13/P14 [54]. Synapse generation in the inner plexiform layer (IPL) starts at P2 with amacrine cells [59],[60], and from P12 in bipolar cells [59], both peaking around the time of eye opening [54],[59]. Outer plexiform layer (OPL) synaptogenesis begins at P4 [59]–[62] and, while its time-course in the rat is not certain, in the mouse continues until about P12 [63]. Neurogenesis in the rat superior colliculus (SC) occurs from E12 to E17 [64],[65]. Apoptosis in the visual layers of the rat SC (stratum griseum superficiale; SGS) occurs from birth, peaking at between P4 and P7, and lasting until P13 [66],[67]. Circuit maturation in the SGS, as judged by synapse density and composition, is mostly postnatal [68],[69] in three stages: P0-P12/P14 (eye opening), P12-P24 increasing number of synapses, P21-P30/P40 increasing ratio of inhibitory to excitatory terminals [70]. Retinal axons first enter the SC at E16/E17 [71], and continue to arrive until P5/P6 [52],[72]. Cortico-tectal innervation starts at P3, with marked ingrowth between P6 and P12, and adult-like innervation observable from P18 [73]. Scale: downwards ticks mark 5 days, upwards ticks mark time points in this study; IPL: inner plexiform layer; OPL: outer plexiform layer; RGC: retinal ganglion cell; SGS: stratum griseum superficiale.
Results
Developmental expression profiles for the Sema3s and their receptors are presented in Figures 2, 3, 4 and 5, with those showing statistically significant changes in expression explored further. Statistical comparisons illustrated in Figures 2, 3, 4 and 5 are summaries of statistically significant peaks in expression; the entirety of statistical comparisons can be found in Additional file 1: Table S1. Plxna3 in situ hybridisations at P1 and P7 are presented in Figure 6 showing co-expression in βIII-tubulin positive cells (presumptive RGCs). The effects of exogenous recombinant Sema3s on RGC growth cone collapse are presented in Figure 7, and data detailing expression and detection of those recombinant Sema3s are presented in Additional file 2: Figure S1.
Figure 2.

RNA expression levels of the Class 3 Semaphorins in the rat retina during development. Sema3s: Sema3f is the most predominant transcript, and Sema3a the least. All transcripts increased expression through development and maintained relatively high levels into adulthood. Sema3a: Expression rises slightly to a peak at P14, dropping at P21 before rising again at adulthood. Sema3b: Expression is lowest in the embryonic retina, around half that found from P14 onwards. Sema3c: Transcription is stable up to P0 then rises twofold by P21 and into adulthood. Sema3e: Expression levels rise steadily after birth, reaching fourfold higher levels at their peak in the adult. Sema3f: There are two periods of increased expression, a doubling at birth before falling again by P7 and then rising back by P21 and into adulthood. Error bars are SEM; * p < 0.05, ** p < 0.01; n = 4–5.
Figure 3.
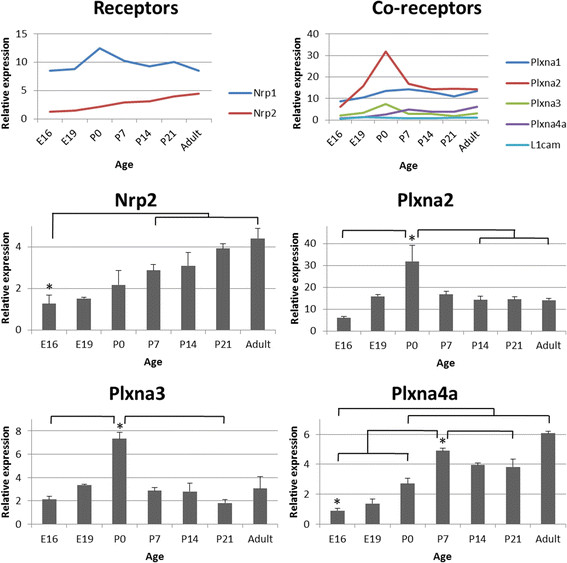
RNA expression levels of the Class 3 Semaphorin receptors in the rat retina during development. Receptors: Nrp1 transcript levels are around twofold higher than the peak level of Nrp2. Co-receptors: Plxna2 had the highest peak expression levels, and L1cam the lowest. Plxna2 and Plxna3 expression levels are very similar, with Plxna2 having around fourfold higher peak levels. Nrp2: RNA levels are lowest in the embryo, rising from P7 onwards and peaking at around fourfold higher in the adult. Plxna2: Expression peaks at birth at fivefold higher than at E16, drops again by P14 and is maintained at that level into the adult. Plxna3: RNA levels increase fourfold from E16 to their peak at birth before falling back to embryonic levels by P21. Plxna4a: Expression triples from E16 to P0, doubles again at P7, falls back one third by P21 before increasing again to peak in the adult. Error bars are SEM;* p < 0.05; n = 4–5.
Figure 4.
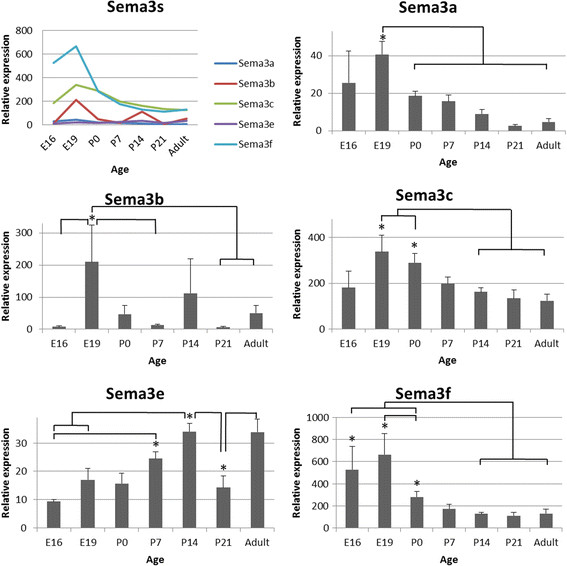
RNA expression levels of the Class 3 Semaphorins in the rat superior colliculus during development. Sema3s: All Sema3 RNA expression levels, except for Sema3e, peak at E19 and are lowest by P21 and into the adult. Sema3b, Sema3c and Sema3f are the predominant transcripts with around 100 times higher levels than Sema3a and Sema3e. Sema3a: There is a high variability in expression at E16, peak expression at E19, falling twofold by birth and eightfold in the adult. Sema3b: Expression changes markedly throughout development, with high variability levels at both E19 and P14. Expression rises twenty-eightfold from E16 to E19 before falling back 17 fold by P7 and back to E16 levels at P21. Transcript levels then rise eightfold from P21 to adult. Sema3c: RNA levels peak at E19 and P0, falling twofold by P14 and maintaining that level into the adult. Sema3e: Transcript levels double and triple from the embryo to P7 and P14 respectively, dropping back at P21 and increasing again into the adult. Sema3f: RNA expression levels are highest in the embryo, dropping fivefold after birth and into the adult. Error bars are SEM; * p < 0.05; n = 4–5.
Figure 5.
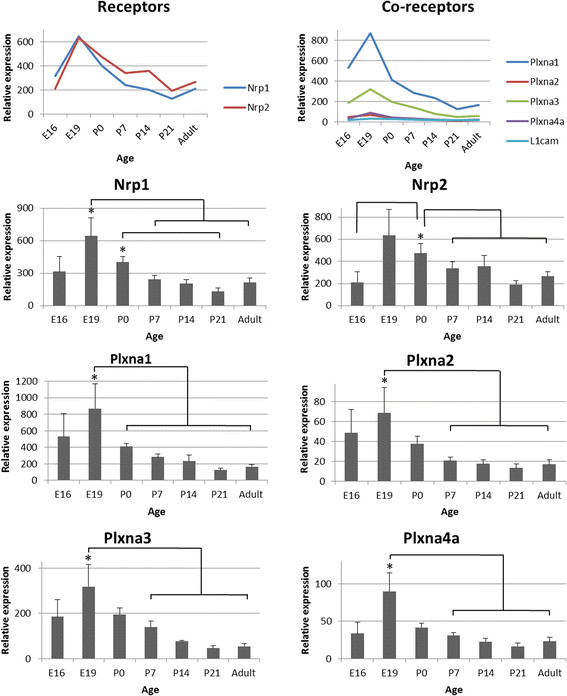
RNA expression levels of the Class 3 Semaphorin receptors in the rat superior colliculus during development. Receptors: Nrp1 and Nrp2 share similar expression profiles, with peaks around E19 and birth and then falling away into the adult. Co-receptors: All co-receptors bar L1cam follow the same expression profile, peaking at E19 before falling after birth and into the adult. Plxna1 and Plxna3 are the predominant transcripts. Nrp1: Peak RNA expression is at E19, falling threefold by P7 and maintained into the adult. Nrp2: Transcript levels double from E16 to P0, with high variability at E19. Expression falls from P0 to P7 and is maintained at around half peak levels in the adult. Plxna1: RNA levels are highly variable at E16 and E19 where they peak. From birth expression levels are halved, continue to decline up to P21 where they are sevenfold lower than at peak, and this level is maintained into the adult. Plxna2: RNA levels are highly variable at E16 and E19 where they peak. Expression drops threefold by P7 and is maintained into the adult. Plxna3: RNA levels are highly variable at E16 and E19 where they peak. Expression falls to half peak at P7, one quarter peak at P14 and is sevenfold lower than peak by P21 and in the adult. Plxna4a: RNA levels are highly variable at E16 and E19 where they peak before falling fourfold by P7 and into the adult. Error bars are SEM; * p < 0.05; n = 4–5.
Figure 6.

Location of Plxna3 transcript in the neonate rat retina. Double in situ hybridisation (ISH) and immunofluorescence staining in P1 (A-C) and P7 retina (D-F). Expression was restricted mainly to the ganglion cell layer (GCL), with overlap of Plxna3 ISH and βIII-Tubulin positive putative retinal ganglion cells (C,F). GCL and dark arrows: ganglion cell layer; scale bars: 50 μm.
Figure 7.

Collapse assay of immunopurified P1/P2 rat retinal ganglion cells exposed to exogenous recombinant Sema3 proteins. P1 retinal ganglion cells (RGCs) were immunopurified and seeded in BD Culture Slides coated with PDL and laminin, grown overnight. The following day (effectively P2), RGCs were exposed to recombinant Sema3s for 30 minutes before fixation and immunostaining. Growth cones were considered collapsed if the F-actin did not extend past the end of the βIII-tubulin positive neurite with an arc of less approximately 60°. Only terminal growth cones were counted, because lateral extensions can occur in response to the collapse of the leading growth cone. Additionally, growth cones were only scored if their neurites were at least two soma widths long. All cultures treated with conditioned media containing recombinant Sema3 proteins showed statistically significantly higher numbers of collapsed growth cones when compared to cultures treated with control conditioned media (A). Also shown here are examples of neurons from each culture condition with growth cones scored as collapsed (hollow arrows) and uncollapsed (solid arrows). Solid arrows: uncollapsed growth cones; hollow arrows: collapsed growth cones; *** p < 0.001; scale bar: 20 μM.
Changes in Class 3 Semaphorins and their receptor components during rat retinal development
Expression levels in the retina could be separated into three qualitative groups: relatively high expression of Sema3f and Plxna2; moderate expression of Nrp1 and Plxna1; and relatively low expression of the rest (Figures 2, and 3). There were statistically significant changes in the level of expression of all Sema3 RNAs in the retina, while of the receptors only Nrp2, Plxna2, Plxna3, and Plxna4a showed statistically significant changes (Figures 2, and 3; Additional file 1: Table S1).
Relative to other time points, Sema3a transcript expression levels were significantly increased at P14 and in the adult. Similar to Sema3a, Sema3b transcript expression was relatively stable during retinal development until P14, at which time there was increased expression that was maintained into adulthood. Sema3c RNA expression was also relatively steady through to P0, increasing significantly through to P21, and remaining at that level into the adult. Sema3e RNA levels appeared to increase gradually with retinal maturation and were significantly higher than E16-P7 levels at P21 and in adult rats. Sema3f transcription was temporarily greater at P0 and then increased again at P21 and beyond. Nrp2 RNA expression gradually increased throughout retina maturation, levels at P7 and beyond being significantly greater than in embryos. Plxna2 and Plxna3 showed nearly identical patterns of altered transcript expression during development, both peaking significantly at the time of birth (P0). Plxna4a RNA levels peaked later at P7, but also again in the adult.
Many of the significant peaks in transcript expression occurred after the main developmental epochs. However, the changes that were quantified in the retina before P21 occurred during periods of RGC apoptosis, and synapse generation and maturation.
Changes in Class 3 Semaphorins and their receptor components during development of the rat superior colliculus
There were four qualitative groups of transcript expression levels in the SC: Plxna1 having the highest; followed by Sema3f, Nrp1, and Nrp2; then Sema3b, Sema3c and Plxna3; and lower amounts for the remainder (Figures 4, and 5). With the exception of L1cam, all genes of interest changed expression significantly during SC maturation.
Sema3a RNA levels were highly variable at E16 (149% coefficient of variance; CV), peaked at E19, and a decline with increasing postnatal age. The expression profile of Sema3b differed from other transcripts; there was large biological variation at many time points, with CVs greater than 100%, and four apparent peaks in expression, only one of which reached significance (E19). Sema3c transcript expression peaked at E19 and P0, before falling to adult levels by around P14. In contrast to the other Sema3s, Sema3e transcript levels increased steadily from E16 to P14, before a decrease at P21, then again increased in the adult. Sema3f expression was highest at E16 and E19, with RNA levels lower at birth and remaining at those reduced levels during postnatal development. There was relatively high biological variation in expression levels of Nrp1, Nrp2 and all PlexinAs at E16 and E19 (CV > 100%) compared to other time-points. Nrp1 RNA expression peaked at E19 then decreased steadily to P7 at levels that were maintained into the adult. Nrp2 transcript levels peaked at E19 before falling steadily to E16 levels at P7 and beyond. Plxna1 RNA expression also peaked at E19, a pattern that was repeated for the remaining three PlexinA RNAs, all of which had significantly decreased RNA expression by P0 and P7 that remained at low levels into adulthood.
The patterns of expression of Sema3s in the developing SC were substantially different from those seen in the retina, with peak expression occurring around or before birth. Temporally, these changes in transcript expression occurred during the period of SC neurogenesis, synapse generation and maturation, and innervation of the SC by extrinsic afferents including those from the retina and visual cortex.
Expression of Plxna3 in the neonatal retina
Our qPCR data showed that peak Plxna3 RNA expression in the retina occurred during the period of peak RGC apoptosis (Figure 3). While rat retinal cells expressing the Sema3 receptor components Nrp1, Nrp2, Plxna1 and Plxna2 have previously been identified using ISH [74] there has been no such characterisation for Plxna3. We used ISH to examine the cellular location of Plxna3 transcripts in P1 and P7 rat retinas (Figure 6) and found that Plxna3 RNA was clearly expressed in the ganglion cell layer at both these ages, with transcript expression overlapping βIII-tubulin (RGCs) immunostaining. This is in agreement with previously published data in the mouse retina [22]. Qualitatively, changes in the level of Plxna3 expression in our ISH data are consistent with the reduction in expression between P0 and P7 in our qPCR material.
Growth cone collapse assay for purified neonatal RGCS in vitro
It has been suggested that Sema3s in the SC can assist in the guidance of ingrowing RGC axons [45], and our data revealed increased expression of these ligands in the SC during this period (Figure 4). To further investigate the capability of Sema3s to influence RGC axons as they grow into and within the SC we used a growth cone collapse assay to assess the capacity of developmentally appropriate RGCs to respond to Sema3 proteins. Recombinant Sema3 proteins (SEMA3A-GFP, SEMA3C-FLAG, SEMA3E-FLAG, and SEMA3F-AP) in conditioned media were used to challenge immunopurified P1/P2 RGCs in vitro. These proteins were detected in the conditioned media by western blot against their artificial epitopes (Additional file 2: Figure S1A). Detected bands for SEMA3A-GFP correspond to the approximately 130 kDa and 90 kDa bands reported previously [75]. SEMA3C-FLAG was detected at around 80 kDa as expected, and also in a presumably processed form at around 70 kDa. Both SEMA3C-FLAG and SEMA3E-FLAG were detected in media at approximately 100 kDa, which might represent glycosylated forms of the proteins. SEMA3F-AP was detected at 150 kDa, in line with expectations. Transfected cells were analysed in culture by epifluorescence and immunocytochemistry and were positive for recombinant proteins while appropriate controls were negative (Additional file 2: Figure S1B-G).
Growth cones were visible as F-actin positive extensions from the ends of βIII-tubulin positive neurites. Typically uncollapsed growth cones displayed the common ‘hand like’ morphology, and collapsed growth cones appeared as thin stumps. Growth cones were counted only at the end of neurites, even when significant growth cone-like processes were observed along the shaft of a neurite, as these lateral extensions can occur in response to the collapse of the leading growth cone [76]. Examples of collapsed versus uncollapsed growth cones are illustrated in Figure 7. Cultures treated with conditioned media containing any of the Sema3s had significantly higher percentages of collapsed growth cones, with the average percentage of the two replicates and statistical significance level presented in Figure 7A. Our data demonstrate that neonatal rat RGCs are competent to respond to SEMA3A, SEMA3C, SEMA3E, and SEMA3F proteins at a developmental time point during which their axons are entering into and growing within the SC in vivo[52],[71],[72].
Discussion
We found statistically significant changes in gene expression of Sema3s and their co-receptors during maturation of the rat retina and SC. These changes differed in degree and time-course between the retina and SC so were not due to generalised or non-specific systemic changes. It is likely that the observed tissue-specific changes in expression reflect the biological roles of these molecules and are in some way related to the development and maturation of each structure [20],[21],[45]. While our data cannot localise these changes in expression to distinct cell populations, previous work in the retina suggests that the transcripts studied are heavily expressed in rat RGCs [27]. Importantly we also showed that neonatal rat RGCs are competent to respond to Sema3s at a developmental time point when their axons are encountering the Sema3s in the SC. We are wary of over-analysing temporal association data, but to help place our results in context we briefly discuss our data below with respect to known developmental processes (Figure 1).
Class 3 Semaphorins and morphogenesis of the retina and superior colliculus
Sema5s and Sema6s are known to mediate lamination of the mouse retina, signalling through Plxna2, Plxna3 and Plxna4[22]–[24]. In addition, Sema3s guide cells as they differentiate and migrate within CNS tissues such as the cortex, cerebellum, and hippocampus [39],[77],[78], and presumably they are able diffuse through tissues like other guidance cues in the mammalian visual system such as the Ephs and Netrin. Is the expression of Sema3s also involved in morphogenesis of the rat retina and SC? Retinal neurogenesis occurs from E10/E11 through to the second postnatal week [48], and while there is expression of all Sema3s and their receptors and co-receptors during much of this period, expression levels are for the most part relatively low. Rod photoreceptor generation peaks at P0, as does transcript expression of Sema3f, Plxna2 and Plxna3 RNAs. However there appears to be very little Sema3f transcript expression in the outer nuclear layer (ONL) at this age [27], despite evidence of relatively high expression of Sema3f RNA in the ONL of adult mice [79].
On the other hand, rat SC neurogenesis occurs from E12 to E17 [64],[65] and the SC has an adult like laminar structure at P7 [80]. Sema3c and Nrp2 RNA expression displayed the same upwards then downwards sweep as neurogenesis in the SC: elevated at E19 and P0, falling by P7. Similarly, Sema3a, Sema3b, and Sema3f as well as Nrp1 and all PlexinA co-receptors showed peak expressions at E19 that declined thereafter, coincidental with early cytoarchitectural patterning of the SC. These data therefore suggest that the Sema3s might play a role in patterning the SC, but not the retina.
Class 3 Semaphorins and retinal ganglion cell apoptosis
Sema3s, specifically Sema3a and Sema3f, have been implicated in apoptosis of neurons including RGCs [81]–[86]. During retinal development, RGC apoptosis is predominantly perinatal [52]–[57] and we observed three genes that had clear peaks in retinal RNA expression at P0: Sema3f, Plxna2, and Plxna3. Interestingly, Plxna2 and Plxna3 are co-receptors for Sema3f and Sema3a[37], and Plxna3 has been shown to be directly involved in Sema3a mediated cell death [84]. Sema3a, Sema3f, and Plxna2 are expressed in neonatal rat RGCs [27], and we here show that in the rat Plxna3 RNA is predominantly expressed in the retinal ganglion cell layer (presumptive RGCs) at P1 (Figure 7). Importantly we also showed that P1/P2 rat RGCs were responsive to both Sema3a and Sema3f (Figure 7). Our data provide further suggestive evidence that the Sema3s may influence RGC apoptosis.
Class 3 Semaphorin expression during the period of synapse generation and maturation in retina and superior colliculus
Sema3s are also involved in terminal axon branching, synaptogenesis, pruning of terminal arbors, regulation of synaptic plasticity, and maturation of dendrites [42],[87]–[92]. Synaptogenesis in the rat retina starts around P2 and is ongoing at P7 [54],[59]–[62], when transcripts for Sema3c and Sema3e both become more abundant. Conversely, Sema3b and Sema3f RNAs increased after completion of retinal synaptogenesis (P14) [63]. The maintained relatively high transcript levels in the adult rat retina of Sema3b, Sema3c, Sema3e, and Sema3f might indicate a role in regulating synaptic plasticity in the mature retina. It has been reported that Plxna2, Plxna3, and Plxna4 are important for proper lamination of the inner plexiform layer (IPL) of the mouse retina, with mRNA expressed in amacrine cells and protein heavily expressed in the IPL [22]–[24]. We see no clear signal supporting this finding in the rat retina, although this may be a consequence of analysing global rather than cellular expression. In the SC, the majority of synaptogenesis is postnatal [68]–[70], and Sema3e RNA expression in the SC peaks during the period when many synapses are being formed (P14) suggesting a role for this molecule in SC synaptogenesis.
Effect of Sema3s on guiding RGC and other axons into and within the superior colliculus
RGC axons enter the rat SC between E16 and P5/P6 [52],[71],[72], and previous results suggest that Sema3f may contribute in helping to guide these axons to their correct retinotopic targets [45]. Furthermore, the Sema3 co-receptor L1cam is required for correct mapping of retinal axons in the mouse [93],[94]. Previous data reveal that during development of the mouse SC at least some of the retino-tectal afferent fibres are positive for Nrp1, Nrp2, and L1cam[93]–[95], and P1 mouse RGC axons express the receptors Nrp2 and Plxna1[45]. While previous studies have shown responsiveness of some rodent RGCs to some Sema3s [45], these were not conducted at a developmental time point where the RGC axons are growing into and within their Sema3 expressing targets [52],[71],[72]. Here we show for the first time that rat P1/P2 RGCs are capable of responding to exogenous Sema3 proteins at an age when many of these neurons are encountering these Sema3s as they grow axons into and within the SC. [52],[71],[72]. Our data show that almost all these Sema3s, bar Sema3e, had peak expression levels in the SC during this period of retinal afferent ingrowth, but that Sema3c and Sema3f had around tenfold higher expression compared to Sema3a and Sema3e. This difference in RNA expression levels might indicate that if these molecules are having an effect then the more likely candidates are Sema3c and Sema3f. However, some caution must be used when extrapolating levels of RNA expression to physiological significance; L1cam, Plxna3 and Plxna4a all had relatively low expression in the retina despite their known effects in patterning the retina [22],[23],[93],[94]. Nevertheless, our data do support the suggestion that Sema3f may play a role in the formation of the retinotopic map in the mammalian SC [45].
Sema3s have also previously been shown to influence the pattern of long-distance cortico-tectal projections [96]. Rat visual cortical axons reach the SC at about P3, with marked ingrowth between P6 and P12, and then final maturation between P12-P18 [73]. Our data show elevated Sema3e expression during this period of ingrowth and maturation of cortical fibers in the SC, suggesting that Sema3e might influence the development of corticotectal projections.
Conclusions
In conclusion, the analysis of the Sema3s and their co-receptors in the developing rat visual system revealed a number of new and potentially important findings. In the retina, mRNA expression levels changed for all Sema3s examined and there were age-specific changes in Nrp2, Plxna2, Plxna3, and Plxna4a mRNA expression. In the SC there were also maturational changes in transcript levels for all Sema3s, Neuropilins, and PlexinAs, although the time-course of these changes differed markedly from those seen in the retina. These developmental changes were associated with periods of RGC apoptosis; neurogenesis in the SC; synapse generation, maturation, and plasticity in the retina and SC; and innervation by retino- and cortico-tectal axons in the SC. Importantly, and consistent with a broad role for the Sema3 family in the maturing visual system, purified P1/P2 RGCs were sensitive to Sema3a, Sema3c, Sema3e, and Sema3f mediated growth cone collapse in vitro. These new data, providing a framework for future studies aimed at elucidating such roles of the Sema3s. These new data describing the overall temporal regulation of Sema3 expression in the rat retina and SC highlight maturational events that might be influenced by Sema3s, providing a platform for further work characterising the functional impact of these proteins on mammalian visual system development.
Methods
Experiments conformed to the Australian National Health and Medical Research Council (NHMRC) guidelines and were approved by the Animal Ethics Committee of The University of Western Australia. Animals were sourced from the Animal Resources Centre (Western Australia). All statistical tests were performed using SPSS v19 (IBM, USA). E0 was the day vaginal plug detected, P0 was date of birth.
Tissue collection
Tissue collection for qPCR
Embryonic Wistar rats aged E16 or E19 were dissected after caesarean section from mothers under halothane anaesthesia (5% (v/v) halothane (Rhone Merieux, Australia) in 80:20 N2O:O2) and immediately placed in ice-cold F-10 media (Gibco, Australia). Dams were sacrificed by intraperitoneal (IP) overdose (50 mg/100 g body weight) of pentobarbitone sodium (Lethabarb; Virbac, Australia). Postnatal and adult (8 to 10 week old) rats were anaesthetised by overdose of Lethabarb (IP, 32.5 mg/100 g body weight). Eyes and SCs were quickly dissected out and placed into fresh ice-cold F-10 media. Retinas were dissected free of the surrounding tunica and vitreous, left and right retinas pooled and placed immediately into RNAlater (Ambion, USA). Similarly, SCs were dissected free of surrounding meninges and placed in RNAlater. All tissues stored in RNAlater were cooled to 4°C within one hour for overnight storage, before transfer into −20°C for longer term storage. Four to five animals were used per group.
Tissue collection for in situ hybridisation
P1 and P7 Wistar rats were anaesthetised by overdose injection of Lethabarb before transcardial perfusion with 0.05% (w/v) heparin (David Bull Laboratories, Australia) in phosphate buffered saline (PBS), followed by 4% paraformaldehyde (w/v; Sigma-Aldrich, Australia) in 0.1 M Sorenson’s Buffer (4% PFA; pH 7.4). Eyes and whole brains were dissected out and postfixed in 4% PFA for 30 minutes before cryoprotection in diethylpyrocarbonate (DEPC; Sigma-Aldrich, Australia) treated PBS containing 30% sucrose (w/v; Sigma-Aldrich, Australia) overnight at 4°C. PBS/sucrose solution was gradually replaced with Jung tissue freezing medium (Leica Microsystems, Australia) over several days. After infusion tissues were snap frozen in isopropanol (2-propanol; Sigma-Aldrich, Australia). 20 μM sections were cut on a cryostat, placed on SuperFrost Plus slides (Menzel-Gläser, Germany), and stored at −80°C until processing by in situ hybridisation (ISH).
qPCR
Methodology for qPCR was as previously detailed [97], and briefly described here. Total RNA was extracted with Tri Reagent (Molecular Research Center, USA) and treated with recombinant DNase I (rDNase I; DNA-free; Ambion, USA). First strand cDNA was synthesised using Omniscript (Qiagen, Australia) and random hexamers (Promega, Australia). Previously validated primer pairs [97] were used to quantify RNA transcript expressions of Sema3a, Sema3b, Sema3c, Sema3e, Sema3f, Plxna1, Plxna2, Plxna3, Plxna4a, Nrp1, Nrp2, L1cam, and internal reference genes Ppia, Rnr1, and Rpl19. qPCR runs were performed on either a Rotor-Gene 3000 or 6000 (Qiagen, USA), using Bio-Rad iQ SYBR 2x Mastermix (Bio-Rad, Australia) in 10 μL reactions containing 500 nM of each primer, and Cq values obtained from using the inbuilt second derivative maximum (SDM) equation. qPCR efficiencies are averages of efficiencies calculated from individual reactions using LinRegPCR [98],[99]. Initial fluorescence levels (N0) were calculated from Cq and mean efficiency values [100], and then normalised to the geometric mean of appropriate internal reference genes [101]. BestKeeper analysis found all internal reference genes were appropriate internal controls, as judged by the standard deviation in Cq values. However, Rpl19 was not used as an internal control because its RNA expression changed significantly between developmental groups (Kruskal-Wallis ANOVA, p < 0.05 and p < 0.01 in the retina and SC respectively), and was relatively poorly correlated to the BestKeeper index SC (Spearman’s R = 0.444). Thus expression data were normalised to the geometric mean of Rnr1 and Ppia expression [101].
Normalised data were analysed for changes between developmental groups by Kruskal-Wallis ANOVA (analysis of variance). When Kruskal Wallis ANOVA resulted in p < 0.05 pairwise tests of statistical significance between time points were performed by Mann–Whitney U tests.
In situ hybridisation
Plasmid for Plxna3 riboprobe was kindly provided by Professor Joost Verhaagen (Netherlands Institute for Neuroscience, Amsterdam). Sense and anti-sense probes were produced by restriction endonuclease (RE; New England Biolabs, USA) digest followed by in vitro RNA transcription (IVT) with digoxigenin labelled RNA (Roche, Australia) and RNA polymerase (Roche, Australia). RE used were: sense, Kpn I; anti-sense, Spe I. Riboprobes were hydrolysed for one hour at 60°C, precipitated with LiCl and 100% ethanol, resuspended in DEPC treated double deionised water (DDW), and stored at −80°C before use.
P1 and P7 retinal sections were processed for ISH with the above riboprobe using methodology previously described [27], with α-dig-AP fragments (Roche, Australia) and NBT/BCIP (Roche, Australia). Sections were differentiated in 70% ethanol if necessary, and stored in PBS before proceeding to immunohistochemistry (IHC). Sense riboprobes produced limited, diffuse staining, and all experimental runs showed positive anti-sense riboprobe staining in control tissue (adult rat cerebellum; data not shown).
Immunohistochemistry
After ISH processing, sections were processed for immunofluorescence using standard methods. Antibody diluent was 0.1% (w/v) Triton-X100, 10% (v/v) normal goat serum (NGS; Chemicon, USA), in PBS. Primary antibody (α-βIII-tubulin (TUJ1); mouse monoclonal, 1:2,000 dilution; Covance, USA) incubation was 4°C overnight in a humidified chamber, followed by secondary antibody (α-mouse FITC, goat raised; 1:400 dilution; ICN Cappel, USA) incubation for 2 hours in a dark humidified chamber at room temperature. Slides were coverslipped using fluorescent mounting media (DAKO, Australia).
Cell cultures were stained for βIII-tubulin and F-actin. βIII-tubulin was labelled by IHC according to standard practice, using the same antibody diluent, incubation times, and antibodies as described above. Following immunostaining for βIII-tubulin, cultures were incubated with phalloidin-FITC (Sigma-Aldrich, Australia) diluted 1:1,000 in PBS for 2 hours at room temperature to stain F-actin, then were washed 3 times with PBS, before being coverslipped using DAKO fluorescent mounting media.
Recombinant Class 3 Semaphorin conditioned media
Expression plasmids (Sema3a-GFP, Sema3c-FLAG, Sema3e-FLAG, Sema3f-AP) were also a gift from Professor Joost Verhaagen. The Sema3a-GFP and Sema3f-AP plasmids have previously been characterised [75],[102], containing rat and mouse cDNA respectively. Sema3c-FLAG contains mouse Sema3c cDNA, and Sema3e-FLAG contains human Sema3e cDNA. Plasmid constructs were verified by sequencing.
HEK-293 T cultures were grown in DMEM: FBS (Dulbecco’s Modified Eagle Medium:fetal bovine serum; Gibco, Australia) 9:1 fortified with gentamycin (50 μg/mL; Invitrogen, Australia), and then grown without the presence of antibiotics overnight. Cells were transfected with plasmids using Lipofectamine 2000 (Invitrogen, Australia), using Opti-MEM (Invitrogen, Australia) as per manufacturer’s instructions. Media was changed to original growth media with antibiotics 6 hours after transfection, and conditioned media collected 30 hours later. Control conditioned medium was obtained by the same methods, using sham (no plasmid) transfected cultures.
Immunoprecipitation and detection of recombinant proteins
SEMA3A-GFP was purified from conditioned media using magnetic GFP-Trap beads (Chromotek, Germany). FLAG tagged proteins (SEMA3C-FLAG and SEMA3E-FLAG), and Myc tagged proteins (SEMA3F-AP) were immunoprecipitated from conditioned media using Dynabeads Protein A/G (Invitrogen, Australia) and 5 μg α-FLAG antibody (Sigma-Aldrich, Australia), or 5 μg α-Myc antibody (CellSignaling, USA). Proteins were eluted in 2X Laemmili Buffer (250 mM Tris, 10% (v/v) glycerol, 4% (w/v) SDS, 2% (v/v) β-mercaptoethanol, 0.005% (w/v) bromophenol blue, in DDW pH 6.8), separated by SDS-PAGE (Mini-PROTEAN TGX Stain-Free Precast Gels; Bio-Rad, Australia) and transferred to nitrocellulose membrane (Trans-Blot Turbo Mini Nitrocellulose Transfer Pack; Bio-Rad, Australia). Recombinant proteins were detected by western blot using 5% (w/v) skim milk powder in Tris buffered saline containing Tween (TBS-T; 100 mM Tris, 154 mM NaCl, 0.1% (v/v) Tween 20, in DDW pH 7.5) blocking buffer, α-GFP antibody (mouse monoclonal clones 7.1 and 13.1, 0.4 μg/mL; Roche, Australia), α-FLAG antibody (mouse monoclonal, 10 μg/mL; Sigma-Aldrich, Australia), α-Myc antibody (mouse monoclonal, 1:1,000 dilution; Cell Signaling, USA), and α-mouse-HRP secondary antibody (1:10,000 dilution; Peirce Scientific, Australia). Proteins were visualised by chemiluminescence (Immun-Star; Bio-Rad, USA) using the Chemi-Doc system (Bio-Rad, USA).
Immunopurified P1/P2 retinal ganglion cell cultures
P1 Wistar pups were euthanised by IP Lethabarb overdose and retinas dissected free in PBS. Retinas were dissociated with MiltenyiBiotec Tissue Dissociation Kit – Postnatal Neurons (MiltenyBiotec, Australia), and RGCs purified using the MACS RGC Isolation Kit (MiltenyiBiotec, Australia) according to manufacturer’s instructions. Purified RGCs were resuspended in growth media (Neurobasal media (Invitrogen, Australia), 1X B27 supplement (Invitrogen, Australia), 1.25 μM transferrin (Sigma-Aldrich, Australia), 0.2 μM progesterone (Sigma-Aldrich, Australia), 100 μM putrescine (Sigma-Aldrich, Australia), 230 nM sodium selenite (Sigma-Aldrich), 1.5 μM bovine serum albumin (Sigma-Aldrich, Australia), 872 nM bovine pancreas insulin (Sigma-Aldrich, Australia), 1 mM L-glutamine (Invitrogen, Australia), 30.6 μM N-acetyl-cysteine (Sigma-Aldrich, Australia), 6 nM triiodithyronine (T3; Sigma-Aldrich, Australia), 1 mM sodium pyruvate (Sigma-Aldrich, Australia)) fortified with growth factors (5 μM insulin (Sigma-Aldrich, Australia), 5 μM forskolin (Sigma-Aldrich, Australia), 440 pM rat ciliary neurotrophic factor (CNTF; PeproTech, USA), 1.85 nM human brain-derived neurotrophic factor (BDNF; PeproTech, USA) to increase survival as previously described [103]. Cells were seeded onto PDL (100 μg/mL; Sigma-Aldrich, USA) and mouse laminin (10 μg/mL; Invitrogen, Australia) coated BD CultureSlides (BD, Australia) at approximately 6,000 cells per mm2.
Purified retinal ganglion cell growth cone collapse assay
Purified RGC cultures were grown overnight at 37°C and 5% pCO2. The following day (effectively P2) 30 μL of conditioned media was added to the 300 μL of growth media in each well and incubated at 37°C and 5% pCO2 for 30 minutes. Cultures were then fixed with 4% PFA and stained for βIII-tubulin and F-actin. Epifluorescent photomicrographs were taken of all neurons in all cultures, and encoded such that analysis was completed blind to treatment group. Growth cones were scored as either collapsed or uncollapsed [104]: uncollapsed if the neurite itself was at least two cell body widths in length, and the growth cone spread with an arc of at least approximately 60°; other growth cones on neurites at least two cell body widths in length were classified as collapsed. The number of collapsed and uncollapsed growth cones was normalised to totals in the control group, and compared using a Chi-square test. The minimum number of growth cones counted per group was 50, and the assay was repeated to confirm results.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
AS carried out qPCR, in situ hybridisations, immunohistochemistry, data analyses, project coordination, manuscript preparation and revision. CL performed immunopreciptiation and western blotting. GWP participated in experimental design and manuscript revision. ARH conceived experimental design, aided in project management, and contributed heavily to manuscript revision. All authors read and approved the final manuscript.
Additional files
Supplementary Material
Pairwise statistical comparisons of expression data. Normalised data were analysed for changes between developmental groups by Kruskal-Wallis ANOVA (analysis of variance). When Kruskal Wallis ANOVA resulted in p < 0.05 pairwise tests of statistical significance between time points were performed by Mann–Whitney U tests. e: p = 0.05; * p < 0.05; ** p < 0.01.
Recombinant Sema3 protein expression in conditioned media. A: Presented are three different blots, lined up against the same ladder. Controls were from cultures that underwent a sham transfection (no plasmid). Boxes indicated bands of expected molecular weights. Detected bands for SEMA3A-GFP correspond to the approximately 130 kDa and 90 kDa bands reported previously [75]. SEMA3C-FLAG was detected at around 80 kDa as expected, and also in a presumably processed form at around 70 kDa. Both SEMA3C-FLAG and SEMA3E-FLAG were detected in media at approximately 100 kDa, which might represent glycosylated forms of the proteins. SEMA3F-AP was detected at 150 kDa, in line with expectations. B-G: recombinant Class 3 Semaphorin expression in HEK-293 T cells transfected with expression plasmids for Sema3a-GFP, Sema3c-FLAG, Sema3e-FLAG, and Sema3f-AP, as well as a no plasmid control. SEMA3A-GFP expression was visualised directly by epifluorescence (B), SEMA3B-FLAG (C) and SEMA3E-FLAG (D) was detected following fluorescence immunocytochemistry, and SEMA3F-AP was detected by the presence of colour reaction product from NBT/BCIP (F). Scale bar: 50 μM.
Contributor Information
Anil Sharma, Email: anil@propriocept.com.
Chrisna J LeVaillant, Email: chrisna.levaillant@uwa.edu.au.
Giles W Plant, Email: gplant@stanford.edu.
Alan R Harvey, Email: alan.harvey@uwa.edu.au.
Acknowledgements
This study was supported by grants from the Western Australia Neurotrauma Research Program. Manuscript preparation was partly funded by a PhD Completion Scholarship from The University of Western Australia. We are indebted to Professor Joost Verhaagen for his advice, and for the generous gift of some of the reagents used in this study.
References
- Scicolone G, Ortalli AL, Carri NG. Key roles of Ephs and ephrins in retinotectal topographic map formation. Brain Res Bull. 2009;79:227–247. doi: 10.1016/j.brainresbull.2009.03.008. [DOI] [PubMed] [Google Scholar]
- Erskine L, Herrera E. The retinal ganglion cell axon’s journey: insights into molecular mechanisms of axon guidance. Dev Biol. 2007;308:1–14. doi: 10.1016/j.ydbio.2007.05.013. [DOI] [PubMed] [Google Scholar]
- Isenmann S, Kretz A, Cellerino A. Molecular determinants of retinal ganglion cell development, survival, and regeneration. Prog Retin Eye Res. 2003;22:483–543. doi: 10.1016/s1350-9462(03)00027-2. [DOI] [PubMed] [Google Scholar]
- Feldheim DA, O’Leary DDM. Visual map development: bidirectional signaling, bifunctional guidance molecules, and competition. Cold Spring Harb Perspect Biol. 2010;2:a001768. doi: 10.1101/cshperspect.a001768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin T, Hindges R, O’Leary DD. Regulation of axial patterning of the retina and its topographic mapping in the brain. Curr Opin Neurobiol. 2003;13:57–69. doi: 10.1016/s0959-4388(03)00014-x. [DOI] [PubMed] [Google Scholar]
- Reese BE. Development of the retina and optic pathway. Vis Res. 2011;51:613–632. doi: 10.1016/j.visres.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada T, Harada C, Parada LF. Molecular regulation of visual system development: more than meets the eye. Genes Dev. 2007;21:367–378. doi: 10.1101/gad.1504307. [DOI] [PubMed] [Google Scholar]
- Campbell DS, Regan AG, Lopez JS, Tannahill D, Harris WA, Holt CE. Semaphorin 3A elicits stage-dependent collapse, turning, and branching in Xenopus retinal growth cones. J Neurosci. 2001;21:8538–8547. doi: 10.1523/JNEUROSCI.21-21-08538.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita EM, Bertolesi GE, Hehr CL, Johnston J, McFarlane S. Neuropilin-1 biases dendrite polarization in the retina. Development. 2013;140:2933–2941. doi: 10.1242/dev.088286. [DOI] [PubMed] [Google Scholar]
- Halloran MC, Severance SM, Yee CS, Gemza DL, Raper JA, Kuwada JY. Analysis of a Zebrafish semaphorin reveals potential functions in vivo. Dev Dyn. 1999;214:13–25. doi: 10.1002/(SICI)1097-0177(199901)214:1<13::AID-DVDY2>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Liu Y, Berndt J, Su F, Tawarayama H, Shoji W, Kuwada JY, Halloran MC. Semaphorin3D guides retinal axons along the dorsoventral axis of the tectum. J Neurosci. 2004;24:310–318. doi: 10.1523/JNEUROSCI.4287-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai JA, Halloran MC. Semaphorin 3d guides laterality of retinal ganglion cell projections in zebrafish. Development. 2006;133:1035–1044. doi: 10.1242/dev.02272. [DOI] [PubMed] [Google Scholar]
- Callander DC, Lamont RE, Childs SJ, McFarlane S. Expression of multiple class three semaphorins in the retina and along the path of zebrafish retinal axons. Dev Dyn. 2007;236:2918–2924. doi: 10.1002/dvdy.21315. [DOI] [PubMed] [Google Scholar]
- Dell AL, Fried-Cassorla E, Xu H, Raper JA. cAMP-induced expression of neuropilin1 promotes retinal axon crossing in the zebrafish optic chiasm. J Neurosci. 2013;33:11076–11088. doi: 10.1523/JNEUROSCI.0197-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenzweig S, Raz-Prag D, Nitzan A, Galron R, Paz M, Jeserich G, Neufeld G, Barzilai A, Solomon AS. Sema-3A indirectly disrupts the regeneration process of goldfish optic nerve after controlled injury. Graefes Arch Clin Exp Ophthalmol. 2010;248:1423–1435. doi: 10.1007/s00417-010-1377-y. [DOI] [PubMed] [Google Scholar]
- Luo Y, Shepherd I, Li J, Renzi MJ, Chang S, Raper JA. A family of molecules related to collapsin in the embryonic chick nervous system. Neuron. 1995;14:1131. doi: 10.1016/0896-6273(95)90261-9. [DOI] [PubMed] [Google Scholar]
- Shepherd I, Luo Y, Raper JA, Chang S. The distribution of collapsin-1 mRNA in the developing chick nervous system. Dev Biol. 1996;173:185–199. doi: 10.1006/dbio.1996.0016. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Nakamura F, Jin Z, Kalb RG, Strittmatter SM. Semaphorins A and E act as antagonists of neuropilin-1 and agonists of neuropilin-2 receptors. Nat Neurosci. 1998;1:487–493. doi: 10.1038/2203. [DOI] [PubMed] [Google Scholar]
- Henke-Fahle S, Beck KW, Püschel AW. Differential responsiveness to the chemorepellent Semaphorin 3A distinguishes Ipsi- and contralaterally projecting axons in the chick midbrain. Dev Biol. 2001;237:381–397. doi: 10.1006/dbio.2001.0376. [DOI] [PubMed] [Google Scholar]
- Steinbach K, Volkmer H, Schlosshauer B. Semaphorin 3E/collapsin-5 inhibits growing retinal axons. Exp Cell Res. 2002;279:52–61. doi: 10.1006/excr.2002.5595. [DOI] [PubMed] [Google Scholar]
- Steffensky M, Steinbach K, Schwarz U, Schlosshauer B. Differential impact of semaphorin 3E and 3A on CNS axons. Int J Dev Neurosci. 2006;24:65–72. doi: 10.1016/j.ijdevneu.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Matsuoka RL, Chivatakarn O, Badea TC, Samuels IS, Cahill H, Katayama K, Kumar SR, Suto F, Chedotal A, Peachey NS, Nathans J, Yoshida Y, Giger RJ, Kolodkin AL. Class 5 transmembrane semaphorins control selective Mammalian retinal lamination and function. Neuron. 2011;71:460–473. doi: 10.1016/j.neuron.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka RL, Nguyen-Ba-Charvet KT, Parray A, Badea TC, Chedotal A, Kolodkin AL. Transmembrane semaphorin signalling controls laminar stratification in the mammalian retina. Nature. 2011;470:259–263. doi: 10.1038/nature09675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun LO, Jiang Z, Rivlin-Etzion M, Hand R, Brady CM, Matsuoka RL, Yau KW, Feller MB, Kolodkin AL. On and off retinal circuit assembly by divergent molecular mechanisms. Science. 2013;342:1241974. doi: 10.1126/science.1241974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwajima T, Yoshida Y, Takegahara N, Petros TJ, Kumanogoh A, Jessell TM, Sakurai T, Mason C. Optic chiasm presentation of Semaphorin6D in the context of Plexin-A1 and Nr-CAM promotes retinal axon midline crossing. Neuron. 2012;74:676–690. doi: 10.1016/j.neuron.2012.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unified Nomenclature for the Semaphorins/Collapsins. Cell. 1999;97:551–552. doi: 10.1016/s0092-8674(00)80766-7. [DOI] [PubMed] [Google Scholar]
- de Winter F, Cui Q, Symons N, Verhaagen J, Harvey AR. Expression of class-3 semaphorins and their receptors in the neonatal and adult rat retina. Invest Ophthalmol Vis Sci. 2004;45:4554–4562. doi: 10.1167/iovs.04-0173. [DOI] [PubMed] [Google Scholar]
- Roche J, Boldog F, Robinson M, Robinson L, Varella-Garcia M, Swanton M, Waggoner B, Fishel R, Franklin W, Gemmill R, Drabkin H. Distinct 3p21.3 deletions in lung cancer and identification of a new human semaphorin. Oncogene. 1996;12:1289–1297. [PubMed] [Google Scholar]
- Kolodkin AL, Matthes DJ, Goodman CS. The semaphorin genes encode a family of transmembrane and secreted growth cone guidance molecules. Cell. 1993;75:1389. doi: 10.1016/0092-8674(93)90625-z. [DOI] [PubMed] [Google Scholar]
- Luo Y, Raible D, Raper JA. Collapsin: a protein in brain that induces the collapse and paralysis of neuronal growth cones. Cell. 1993;75:217. doi: 10.1016/0092-8674(93)80064-l. [DOI] [PubMed] [Google Scholar]
- Feiner L, Koppel AM, Kobayashi H, Raper JA. Secreted chick semaphorins bind recombinant neuropilin with similar affinities but bind different subsets of neurons in situ. Neuron. 1997;19:539–545. doi: 10.1016/s0896-6273(00)80370-0. [DOI] [PubMed] [Google Scholar]
- Püschel AW, Adams RH, Betz H. Murine semaphorin D/collapsin is a member of a diverse gene family and creates domains inhibitory for axonal extension. Neuron. 1995;14:941. doi: 10.1016/0896-6273(95)90332-1. [DOI] [PubMed] [Google Scholar]
- Sekido Y, Bader S, Latif F, Chen J-Y, Duh F-M, Wei M-H, Albanesi JP, Lee C-C, Lerman MI, Minna JD. Human semaphorins A (V) and IV reside in the 3p21.3 small cell lung cancer deletion region and demonstrate distinct expression patterns. Proc Natl Acad Sci U S A. 1996;93:4120–4125. doi: 10.1073/pnas.93.9.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang R. Isolation of the human semaphorin III/F gene (SEMA3F) at chromosome 3p21, a region deleted in lung cancer. Genomics. 1996;32:39. doi: 10.1006/geno.1996.0074. [DOI] [PubMed] [Google Scholar]
- Taniguchi M, Masuda T, Fukaya M, Kataoka H, Mishina M, Yaginuma H, Watanabe M, Shimizu T. Identification and characterization of a novel member of murine semaphorin family. Genes Cells. 2005;10:785–792. doi: 10.1111/j.1365-2443.2005.00877.x. [DOI] [PubMed] [Google Scholar]
- Stevens CB, Halloran MC. Developmental expression of sema3G, a novel zebrafish semaphorin. Gene Expr Patterns. 2005;5:647–653. doi: 10.1016/j.modgep.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Sharma A, Verhaagen J, Harvey AR. Receptor complexes for each of the Class 3 Semaphorins. Front Cell Neurosci. 2012;6:28. doi: 10.3389/fncel.2012.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodkin AL, Matthes DJ, O’Connor TP, Patel NH, Admon A, Bentley D, Goodman CS. Fasciclin IV: sequence, expression, and function during growth cone guidance in the grasshopper embryo. Neuron. 1992;9:831–845. doi: 10.1016/0896-6273(92)90237-8. [DOI] [PubMed] [Google Scholar]
- Roth L, Koncina E, Satkauskas S, Cremel G, Aunis D, Bagnard D. The many faces of semaphorins: from development to pathology. Cell Mol Life Sci. 2009;66:649–666. doi: 10.1007/s00018-008-8518-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazdani U, Terman JR. The semaphorins. Genome Biol. 2006;7:211. doi: 10.1186/gb-2006-7-3-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizui M, Kumanogoh A, Kikutani H. Immune semaphorins: novel features of neural guidance molecules. J Clin Immunol. 2009;29:1–11. doi: 10.1007/s10875-008-9263-7. [DOI] [PubMed] [Google Scholar]
- Pasterkamp RJ, Giger RJ. Semaphorin function in neural plasticity and disease. Curr Opin Neurobiol. 2009;19:263–274. doi: 10.1016/j.conb.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Kumanogoh A, Kikutani H. Semaphorins and their receptors in immune cell interactions. Nat Immunol. 2008;9:17–23. doi: 10.1038/ni1553. [DOI] [PubMed] [Google Scholar]
- Neufeld G, Kessler O. The semaphorins: versatile regulators of tumour progression and tumour angiogenesis. Nat Rev Cancer. 2008;8:632–645. doi: 10.1038/nrc2404. [DOI] [PubMed] [Google Scholar]
- Claudepierre T, Koncina E, Pfrieger FW, Bagnard D, Aunis D, Reber M. Implication of neuropilin 2/semaphorin 3F in retinocollicular map formation. Dev Dyn. 2008;237:3394–3403. doi: 10.1002/dvdy.21759. [DOI] [PubMed] [Google Scholar]
- Kim J, Oh WJ, Gaiano N, Yoshida Y, Gu C. Semaphorin 3E-Plexin-D1 signaling regulates VEGF function in developmental angiogenesis via a feedback mechanism. Genes Dev. 2011;25:1399–1411. doi: 10.1101/gad.2042011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefton AJ, Dreher B, Harvey A. In: The Rat Nervous System. 3. Paxinos G, editor. Elsevier Academic Press, San Diego; 2004. Visual system; pp. 1083–1165. [Google Scholar]
- Rapaport DH, Wong LL, Wood ED, Yasumura D, LaVail MM. Timing and topography of cell genesis in the rat retina. J Comp Neurol. 2004;474:304–324. doi: 10.1002/cne.20134. [DOI] [PubMed] [Google Scholar]
- Agathocleous M, Harris WA. In: Retinal Development. Sernagor E, Eglen S, Harris B, Wong R, editor. Cambridge University Press, Cambridge; 2006. Cell determination; pp. 75–98. [Google Scholar]
- Rapaport DH. In: Retinal Development. Sernagor E, Eglen S, Harris B, Wong R, editor. Cambridge University Press, Cambridge; 2006. Retinal Neurogenesis; pp. 30–58. [Google Scholar]
- Chan-Ling T, Chu Y, Baxter L, Weible Ii M, Hughes S. In vivo characterization of astrocyte precursor cells (APCs) and astrocytes in developing rat retinae: differentiation, proliferation, and apoptosis. Glia. 2009;57:39–53. doi: 10.1002/glia.20733. [DOI] [PubMed] [Google Scholar]
- Dallimore EJ, Park KK, Pollett MA, Taylor JSH, Harvey AR. The life, death and regenerative ability of immature and mature rat retinal ganglion cells are influenced by their birthdate. Exp Neurol. 2010;225:353–365. doi: 10.1016/j.expneurol.2010.07.007. [DOI] [PubMed] [Google Scholar]
- Dreher B, Potts RA, Bennett MR. Evidence that the early postnatal reduction in the number of rat retinal ganglion cells is due to a wave of ganglion cell death. Neurosci Lett. 1983;36:255–260. doi: 10.1016/0304-3940(83)90009-5. [DOI] [PubMed] [Google Scholar]
- Horsburgh GM, Sefton AJ. Cellular degeneration and synaptogenesis in the developing retina of the rat. J Comp Neurol. 1987;263:553–566. doi: 10.1002/cne.902630407. [DOI] [PubMed] [Google Scholar]
- Potts RA, Dreher B, Bennett MR. The loss of ganglion cells in the developing retina of the rat. Dev Brain Res. 1982;3:481–486. doi: 10.1016/0165-3806(82)90013-x. [DOI] [PubMed] [Google Scholar]
- Crespo D, O’Leary DD, Cowan WM. Changes in the numbers of optic nerve fibers during late prenatal and postnatal development in the albino rat. Brain Res. 1985;351:129–134. doi: 10.1016/0165-3806(85)90238-x. [DOI] [PubMed] [Google Scholar]
- Perry VH, Henderson Z, Linden R. Postnatal changes in retinal ganglion cell and optic axon populations in the pigmented rat. J Comp Neurol. 1983;219:356–368. doi: 10.1002/cne.902190309. [DOI] [PubMed] [Google Scholar]
- Fruttiger M. Development of the retinal vasculature. Angiogenesis. 2007;10:77–88. doi: 10.1007/s10456-007-9065-1. [DOI] [PubMed] [Google Scholar]
- Dhingra NK, Ramamohan Y, Raju TR. Developmental expression of synaptophysin, synapsin I and syntaxin in the rat retina. Brain Res Dev Brain Res. 1997;102:267–273. doi: 10.1016/s0165-3806(97)00085-0. [DOI] [PubMed] [Google Scholar]
- Sarthy PV, Bacon W. Developmental expression of a synaptic vesicle-specific protein in the rat retina. Dev Biol. 1985;112:284–291. doi: 10.1016/0012-1606(85)90399-9. [DOI] [PubMed] [Google Scholar]
- Kapfhammer JP, Christ F, Schwab ME. The expression of GAP-43 and synaptophysin in the developing rat retina. Brain Res Dev Brain Res. 1994;80:251–260. doi: 10.1016/0165-3806(94)90110-4. [DOI] [PubMed] [Google Scholar]
- Weidman TA, Kuwabara T. Postnatal development of the rat retina. An electron microscopic study. Arch Ophthalmol. 1968;79:470–484. doi: 10.1001/archopht.1968.03850040472015. [DOI] [PubMed] [Google Scholar]
- Olney JW. An electron microscopic study of synapse formation, receptor outer segment development, and other aspects of developing mouse retina. Invest Ophthalmol. 1968;7:250–268. [PubMed] [Google Scholar]
- Altman J, Bayer SA. Time of origin of neurons of the rat superior colliculus in relation to other components of the visual and visuomotor pathways. Exp Brain Res. 1981;42:424–434. doi: 10.1007/BF00237507. [DOI] [PubMed] [Google Scholar]
- Mustari MJ, Lund RD, Graubard K. Histogenesis of the superior colliculus of the albino rat: a tritiated thymidine study. Brain Res. 1979;164:39–52. doi: 10.1016/0006-8993(79)90004-0. [DOI] [PubMed] [Google Scholar]
- Giordano DL, Murray M, Cunningham TJ. Naturally occurring neuron death in the optic layers of superior colliculus of the postnatal rat. J Neurocytol. 1980;9:603–614. doi: 10.1007/BF01205028. [DOI] [PubMed] [Google Scholar]
- Giordano DL, Cunningham TJ. Optic afferents, neuron maturation, and neuron survival in the rat superior colliculus. Brain Res. 1982;256:365–368. doi: 10.1016/0165-3806(82)90150-x. [DOI] [PubMed] [Google Scholar]
- Warton SS, Jones DG. Postnatal development of the superficial layers in the rat superior colliculus: a study with Golgi-Cox and Kluver-Barrera techniques. Exp Brain Res. 1985;58:490–502. doi: 10.1007/BF00235865. [DOI] [PubMed] [Google Scholar]
- Warton SS, McCart R. Synaptogenesis in the stratum griseum superficiale of the rat superior colliculus. Synapse. 1989;3:136–148. doi: 10.1002/syn.890030205. [DOI] [PubMed] [Google Scholar]
- Lund RD, Lund JS. Development of synaptic patterns in the superior colliculus of the rat. Brain Res. 1972;42:1–20. doi: 10.1016/0006-8993(72)90038-8. [DOI] [PubMed] [Google Scholar]
- Bunt SM, Lund RD, Land PW. Prenatal development of the optic projection in albino and hooded rats. Brain Res. 1983;282:149–168. doi: 10.1016/0165-3806(83)90093-7. [DOI] [PubMed] [Google Scholar]
- Dallimore EJ, Cui Q, Beazley LD, Harvey AR. Postnatal innervation of the rat superior colliculus by axons of late-born retinal ganglion cells. Eur J Neurosci. 2002;16:1295–1304. doi: 10.1046/j.1460-9568.2002.02178.x. [DOI] [PubMed] [Google Scholar]
- Thong IG, Dreher B. The development of the corticotectal pathway in the albino rat: transient projections from the visual and motor cortices. Neurosci Lett. 1987;80:275–282. doi: 10.1016/0304-3940(87)90467-8. [DOI] [PubMed] [Google Scholar]
- de Winter F, Oudega M, Lankhorst AJ, Hamers FP, Blits B, Ruitenberg MJ, Pasterkamp RJ, Gispen WH, Verhaagen J. Injury-induced class 3 semaphorin expression in the rat spinal cord. Exp Neurol. 2002;175:61–75. doi: 10.1006/exnr.2002.7884. [DOI] [PubMed] [Google Scholar]
- de Wit J, de Winter F, Klooster J, Verhaagen J. Semaphorin 3A displays a punctate distribution on the surface of neuronal cells and interacts with proteoglycans in the extracellular matrix. Mol Cell Neurosci. 2005;29:40–55. doi: 10.1016/j.mcn.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Davenport RW, Thies E, Cohen ML. Neuronal growth cone collapse triggers lateral extensions along trailing axons. Nat Neurosci. 1999;2:254–259. doi: 10.1038/6360. [DOI] [PubMed] [Google Scholar]
- Tran TS, Kolodkin AL, Bharadwaj R. Semaphorin regulation of cellular morphology. Annu Rev Cell Dev Biol. 2007;23:263–292. doi: 10.1146/annurev.cellbio.22.010605.093554. [DOI] [PubMed] [Google Scholar]
- Torre ER, Gutekunst C-A, Gross RE. Expression by midbrain dopamine neurons of Sema3A and 3F receptors is associated with chemorepulsion in vitro but a mild in vivo phenotype. Mol Cell Neurosci. 2010;44:135–153. doi: 10.1016/j.mcn.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buehler A, Sitaras N, Favret S, Bucher F, Berger S, Pielen A, Joyal JS, Juan AM, Martin G, Schlunck G, Agostini HT, Klagsbrun M, Smith LE, Sapieha P, Stahl A. Semaphorin 3F forms an anti-angiogenic barrier in outer retina. FEBS Lett. 2013;587:1650–1655. doi: 10.1016/j.febslet.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards MA, Caviness VS Jr, Schneider GE. Development of cell and fiber lamination in the mouse superior colliculus. J Comp Neurol. 1986;248:395–409. doi: 10.1002/cne.902480308. [DOI] [PubMed] [Google Scholar]
- Shirvan A, Kimron M, Holdengreber V, Ziv I, Ben-Shaul Y, Melamed S, Melamed E, Barzilai A, Solomon AS. Anti-semaphorin 3A antibodies rescue retinal ganglion cells from cell death following optic nerve axotomy. J Biol Chem. 2002;277:49799–49807. doi: 10.1074/jbc.M204793200. [DOI] [PubMed] [Google Scholar]
- Shirvan A, Ziv I, Fleminger G, Shina R, He Z, Brudo I, Melamed E, Barzilai A. Semaphorins as mediators of neuronal apoptosis. J Neurochem. 1999;73:961–971. doi: 10.1046/j.1471-4159.1999.0730961.x. [DOI] [PubMed] [Google Scholar]
- Gagliardini V, Fankhauser C. Semaphorin III can induce death in sensory neurons. Mol Cell Neurosci. 1999;14:301–316. doi: 10.1006/mcne.1999.0787. [DOI] [PubMed] [Google Scholar]
- Ben-Zvi A, Manor O, Schachner M, Yaron A, Tessier-Lavigne M, Behar O. The Semaphorin receptor PlexinA3 mediates neuronal apoptosis during dorsal root ganglia development. J Neurosci. 2008;28:12427–12432. doi: 10.1523/JNEUROSCI.3573-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagnard D, Vaillant C, Khuth ST, Dufay N, Lohrum M, Püschel AW, Belin MF, Bolz J, Thomasset N. Semaphorin 3A-vascular endothelial growth factor-165 balance mediates migration and apoptosis of neural progenitor cells by the recruitment of shared receptor. J Neurosci. 2001;21:3332–3341. doi: 10.1523/JNEUROSCI.21-10-03332.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagnard D, Sainturet N, Meyronet D, Perraut M, Miehe M, Roussel G, Aunis D, Belin MF, Thomasset N. Differential MAP kinases activation during semaphorin3A-induced repulsion or apoptosis of neural progenitor cells. Mol Cell Neurosci. 2004;25:722–731. doi: 10.1016/j.mcn.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Schlomann U, Schwamborn JC, Muller M, Fassler R, Püschel AW. The stimulation of dendrite growth by Sema3A requires integrin engagement and focal adhesion kinase. J Cell Sci. 2009;122:2034–2042. doi: 10.1242/jcs.038232. [DOI] [PubMed] [Google Scholar]
- Polleux F, Morrow T, Ghosh A. Semaphorin 3A is a chemoattractant for cortical apical dendrites. Nature. 2000;404:567–573. doi: 10.1038/35007001. [DOI] [PubMed] [Google Scholar]
- Morita A, Yamashita N, Sasaki Y, Uchida Y, Nakajima O, Nakamura F, Yagi T, Taniguchi M, Usui H, Katoh-Semba R, Takei K, Goshima Y. Regulation of dendritic branching and spine maturation by semaphorin3A-Fyn signaling. J Neurosci. 2006;26:2971–2980. doi: 10.1523/JNEUROSCI.5453-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vo T, Carulli D, Ehlert EME, Kwok JCF, Dick G, Mecollari V, Moloney EB, Neufeld G, de Winter F, Fawcett JW, Verhaagen J. The chemorepulsive axon guidance protein semaphorin3A is a constituent of perineuronal nets in the adult rodent brain. Mol Cell Neurosci. 2013;56:186–200. doi: 10.1016/j.mcn.2013.04.009. [DOI] [PubMed] [Google Scholar]
- Cioni JM, Telley L, Saywell V, Cadilhac C, Jourdan C, Huber AB, Huang JZ, Jahannault-Talignani C, Ango F. SEMA3A signaling controls layer-specific interneuron branching in the cerebellum. Curr Biol. 2013;23:850–861. doi: 10.1016/j.cub.2013.04.007. [DOI] [PubMed] [Google Scholar]
- Uesaka N, Uchigashima M, Mikuni T, Nakazawa T, Nakao H, Hirai H, Aiba A, Watanabe M, Kano M. Retrograde semaphorin signaling regulates synapse elimination in the developing mouse brain. Science. 2014;344:1020–1023. doi: 10.1126/science.1252514. [DOI] [PubMed] [Google Scholar]
- Demyanenko GP, Maness PF. The L1 cell adhesion molecule is essential for topographic mapping of retinal axons. J Neurosci. 2003;23:530–538. doi: 10.1523/JNEUROSCI.23-02-00530.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhusi M, Schlatter MC, Demyanenko GP, Thresher R, Maness PF. L1 interaction with ankyrin regulates mediolateral topography in the retinocollicular projection. J Neurosci. 2008;28:177–188. doi: 10.1523/JNEUROSCI.3573-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong S, Zheng C, Doughty ML, Losos K, Didkovsky N, Schambra UB, Nowak NJ, Joyner A, Leblanc G, Hatten ME, Heintz N. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature. 2003;425:917–925. doi: 10.1038/nature02033. [DOI] [PubMed] [Google Scholar]
- Low LK, Liu XB, Faulkner RL, Coble J, Cheng HJ. Plexin signaling selectively regulates the stereotyped pruning of corticospinal axons from visual cortex. Proc Natl Acad Sci U S A. 2008;105:8136–8141. doi: 10.1073/pnas.0803849105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Pollett MA, Plant GW, Harvey AR. Changes in mRNA expression of class 3 semaphorins and their receptors in the adult rat retino-collicular system after unilateral optic nerve injury. Invest Ophthalmol Vis Sci. 2012;53:8367–8377. doi: 10.1167/iovs.12-10799. [DOI] [PubMed] [Google Scholar]
- Ramakers C, Ruijter JM, Deprez RH, Moorman AF. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci Lett. 2003;339:62–66. doi: 10.1016/s0304-3940(02)01423-4. [DOI] [PubMed] [Google Scholar]
- Ruijter JM, Ramakers C, Hoogaars WM, Karlen Y, Bakker O, van den Hoff MJ, Moorman AF. Amplification efficiency: linking baseline and bias in the analysis of quantitative PCR data. Nucleic Acids Res. 2009;37:e45. doi: 10.1093/nar/gkp045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schefe JH, Lehmann KE, Buschmann IR, Unger T, Funke-Kaiser H. Quantitative real-time RT-PCR data analysis: current concepts and the novel “gene expression’s CT difference” formula. J Mol Med (Berl) 2006;84:901–910. doi: 10.1007/s00109-006-0097-6. [DOI] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:Research0034. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Chedotal A, He Z, Goodman CS, Tessier-Lavigne M. Neuropilin-2, a novel member of the neuropilin family, is a high affinity receptor for the semaphorins Sema E and Sema IV but not Sema III. Neuron. 1997;19:547–559. doi: 10.1016/s0896-6273(00)80371-2. [DOI] [PubMed] [Google Scholar]
- Meyer-Franke A, Kaplan MR, Pfieger FW, Barres BA. Characterization of the signaling interactions that promote the survival and growth of developing retinal ganglion cells in culture. Neuron. 1995;15:805–819. doi: 10.1016/0896-6273(95)90172-8. [DOI] [PubMed] [Google Scholar]
- Kapfhammer JP, Xu H, Raper JA. The detection and quantification of growth cone collapsing activities. Nat Protoc. 2007;2:2005–2011. doi: 10.1038/nprot.2007.295. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Pairwise statistical comparisons of expression data. Normalised data were analysed for changes between developmental groups by Kruskal-Wallis ANOVA (analysis of variance). When Kruskal Wallis ANOVA resulted in p < 0.05 pairwise tests of statistical significance between time points were performed by Mann–Whitney U tests. e: p = 0.05; * p < 0.05; ** p < 0.01.
Recombinant Sema3 protein expression in conditioned media. A: Presented are three different blots, lined up against the same ladder. Controls were from cultures that underwent a sham transfection (no plasmid). Boxes indicated bands of expected molecular weights. Detected bands for SEMA3A-GFP correspond to the approximately 130 kDa and 90 kDa bands reported previously [75]. SEMA3C-FLAG was detected at around 80 kDa as expected, and also in a presumably processed form at around 70 kDa. Both SEMA3C-FLAG and SEMA3E-FLAG were detected in media at approximately 100 kDa, which might represent glycosylated forms of the proteins. SEMA3F-AP was detected at 150 kDa, in line with expectations. B-G: recombinant Class 3 Semaphorin expression in HEK-293 T cells transfected with expression plasmids for Sema3a-GFP, Sema3c-FLAG, Sema3e-FLAG, and Sema3f-AP, as well as a no plasmid control. SEMA3A-GFP expression was visualised directly by epifluorescence (B), SEMA3B-FLAG (C) and SEMA3E-FLAG (D) was detected following fluorescence immunocytochemistry, and SEMA3F-AP was detected by the presence of colour reaction product from NBT/BCIP (F). Scale bar: 50 μM.


