Abstract
Noninvasive biomarkers with diagnostic value and prognostic applications have long been desired to replace muscle biopsy for Duchenne muscular dystrophy (DMD) patients. Growing evidence indicates that circulating microRNAs are biomarkers to assess pathophysiological status. Here, we show that the serum levels of six muscle-specific miRNAs (miR-1/206/133/499/208a/208b, also known as myomiRs) were all elevated in DMD patients (P < 0.01). The receiver operating characteristic curves of circulating miR-206, miR-499, miR-208b, and miR-133 levels reflected strong separation between Becker's muscular dystrophy (BMD) and DMD patients (P < 0.05). miR-206, miR-499, and miR-208b levels were positively correlated with both age and type IIc muscle fiber content in DMD patients (2–6 years), indicating that they might represent the stage of disease as well as the process of regeneration. miR-499 and miR-208b levels were correlated with slow and fast fiber content and might reflect the ratio of slow to fast fibers in DMD patient (>6 years). Fibroblast growth factor, transforming growth factor-β, and tumor necrosis factor-α could affect the secretion of myomiRs, suggesting that circulating myomiRs might reflect the effects of cytokines and growth factors on degenerating and regenerating muscles. Collectively, our data indicated that circulating myomiRs could serve as promising biomarkers for DMD diagnosis and disease progression.
Keywords: biomarkers, Duchenne muscular dystrophy, secretion, serum miRNAs
Introduction
Duchenne muscular dystrophy (DMD) is a lethal X-linked recessive neuromuscular disease affecting 1 in 3,500 newborn males. DMD is caused by mutations in dystrophin, a cytoskeletal protein that connects the cytoskeleton of a muscle fiber to the surrounding extracellular matrix through the cell membrane. Mutations leading to complete absence of dystrophin protein cause myofiber necrosis, accompanied by a complex series of events including phagocytosis, infiltration of inflammatory cells and decrease of muscle fibers with subsequent fibrosis and fat replacement leading to subsequent loss of ambulation and premature death by respiratory or heart failure.1,2 Loss of dystrophin renders myofibers susceptible to exercise induced injury, which triggers continuous degeneration and regeneration.3 In contrast, mutations leading to the production of less defective yet still partially functional dystrophin protein result in a much milder dystrophic phenotype in affected patients. These patients were known as Becker's muscular dystrophy (BMD) patients.4
As for patients with suspected DMD, the preliminary diagnostic criterion is the serum creatine kinase (CK) level, which is markedly increased in early stages of the disease. However, it is not always reliable since CK levels increase in normal individuals due to exercise or muscle trauma. Moreover, it is not suitable as a screening test for those who have already been using wheelchairs, because CK levels may fall when disease progresses, probably due to the loss of muscle tissue.5,6 In addition, the CK level in BMD patients is also highly elevated, which makes CK level a poor biomarker to distinguish DMD from BMD. The precise diagnosis for DMD should contain a combination of genetic analysis, muscle biopsy, and clinical observation of muscle strength and function. Nonetheless, muscle biopsy is invasive and provides only local information, which may not reflect the state of all muscles, while muscle strength assessment is subject to extensive intra- and inter-patient variability.7 Therefore, more reliable biomarkers with diagnostic value and prognostic applications have long been desired. Although there is no cure for DMD, several strategies including gene therapy, cell therapy, and pharmacotherapy have been recently tested in animal models of DMD8,9 and some of them have already entered clinical trials. Considering the various clinical treatments and trials currently ongoing for DMD, there is an urgent need to develop better noninvasive biomarkers that could be used to monitor the progression of DMD pathology, guide therapeutic decisions, and evaluate the efficacy of potential therapies.
MicroRNAs (miRNAs) are important regulators for numerous physiological and pathological processes.10,11,12 Growing evidence revealed that miRNAs also exist in various body fluids such as serum, plasma, saliva, urine, and milk.13,14,15 Altered profiles of circulating miRNAs have been shown to associate with various diseases, including tissue injury, cancers, and diabetes. Consequently, circulating miRNAs become promising biomarkers to assess the pathophysiological status and monitor the effectiveness of clinical treatment. It has been shown that serum levels of several myomiRs (miR-1, miR-133a, and miR-206) are increased not only in animal models, such as mdx mice and CXMDJ dogs, but also in DMD patients.16,17,18 In the human study, the authors showed that miR-1, miR-133, and miR-206 were able to discriminate DMD patients from healthy controls and BMD patients. Most importantly, the increased levels of these miRNAs but not the CK was correlates with the severity of muscle damage show a very clear inverse correlation with North Star Ambulatory Assessment (NSAA) scores. However, only 26 DMD and 5 BMD patients were recruited for serum miRNA level study and only data from 10 DMD patients between 3 and 6 years of age were used for evaluating the correlation between serum miRNA levels and clinical assessments. Therefore, more comprehensive investigation is required to confirm these findings in cohort with more patients across broader age groups.
The major goal of this study was to identify potential circulating miRNA biomarker for DMD diagnosis and prognosis. MyomiRs are suggested to have a role either in myoblast proliferation, differentiation, and muscle regeneration, which are key events in the pathogenesis of DMD. Besides three classical myomiRs (miR-1, miR-133, and miR-206), we also investigated the serum levels of another two myomiRs, miR-499 and miR-208, which have been shown also play critical roles in muscle biology and exercise adaptation.19 miR-499 is expressed mainly in the slow skeletal muscle and cardiac muscle; miR-208a is expressed mainly in the cardiac muscle and miR-208b in the slow skeletal muscle.20,21 We hypothesize that these myomiR are indicative for the disease pathophysiology and have potential as prognostic biomarkers. We examined serum levels of myomiRs in mdx mice, DMD and BMD patients and compared the serum levels of miRNAs with clinical assessment including age, CK value, and muscle fiber composition. Based on our results, we proposed that specific serum circulating miRNAs are not only novel biomarkers for DMD diagnosis but also may be useful to monitor the pathological progression.
Results
Serum levels of myomiRs in dystrophic subjects
To test the value of serum miRNAs as potential biomarkers for DMD diagnosis, we quantified the levels of myomiRs (miR-1, miR-133, miR-206, miR-208a, miR-208b, and miR-499) in the serum of dystrophin-deficient mice (mdx mice) by real-time PCR (Supplementary Figure S1). Consistent with an earlier report,16 the levels of miR-1, miR-133, and miR-206 were all significantly elevated. We also observed a significant increase in the levels of miR-499 and miR-208b. In contrast, the serum level of miR-208a did not change in mdx mice.
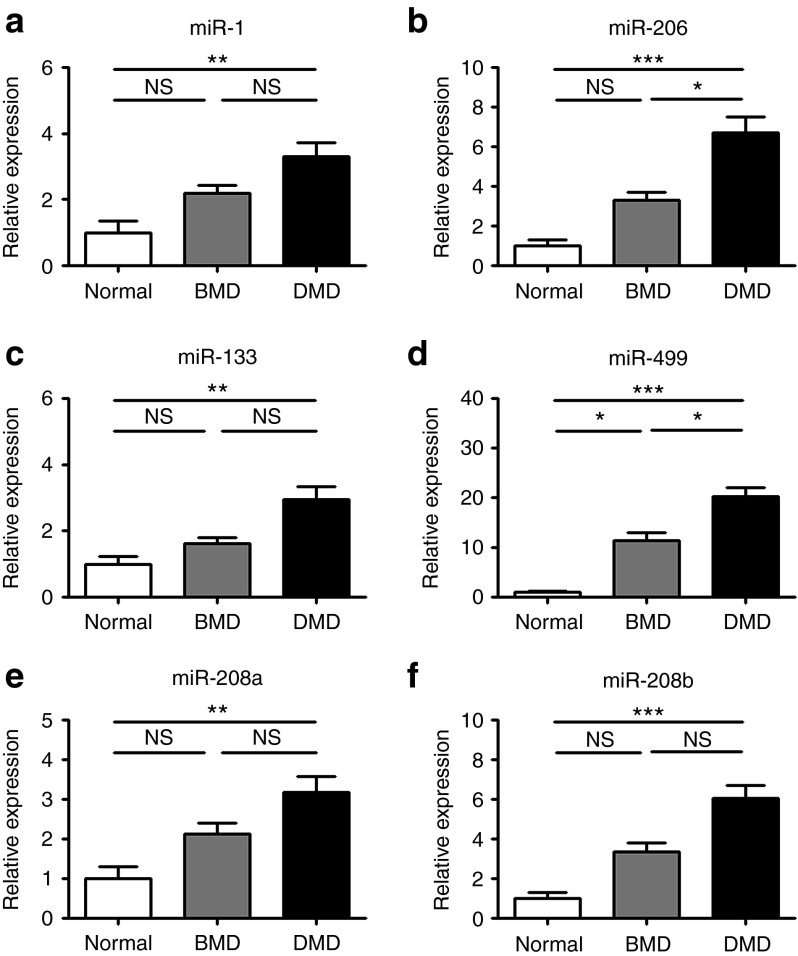
Subsequently, we determined the levels of these myomiRs in serum samples from healthy (n = 23), Becker (n = 15), and Duchenne (n = 52) children, aged from 1 to 14 years old (Figure 1a–f). These patients were not on glucocorticoid treatment and were still ambulant except one patient (Supplementary Table S1). As expected, the levels of miR-1, miR-133, miR-206, miR-208b, and miR-499 were significantly elevated in DMD patients compared to healthy control subjects. To be noted, the level of miR-208a was also significantly increased in DMD subjects. In addition, the levels of these six myomiRs were also elevated in BMD patients, although to a less extent and not statistically significant except for miR-499. Importantly, the levels of miR-206 and miR-499 were significantly higher in DMD than those in BMD patients. The levels of miR-1 miR-133, miR-208a, and miR-208b showed a tendency to be higher in DMD than those in BMD; however, the differences did not reach statistical significance. We also compared the CK value in these BMD and DMD patients. We observed that the CK values were slightly higher in DMD patients than those in BMD but not significantly (Supplementary Figure S2).
Figure 1.
Elevation of myomiRs in dystrophic children serum. (a–f) Serum levels of miRNAs (miR-1, miR-206, miR-133, miR-499, miR-208a, and miR-208b) in dystrophic children and normal controls (normal, n = 23; BMD, n = 15; DMD, n = 52) were determined by real-time PCR. ANOVA was used for statistical analysis. SEs are shown as error bars. *P < 0.05, **P < 0.01, ***P < 0.001. BMD, Becker's muscular dystrophy; DMD, Duchenne muscular dystrophy; NS, not significant; SE, standard error.
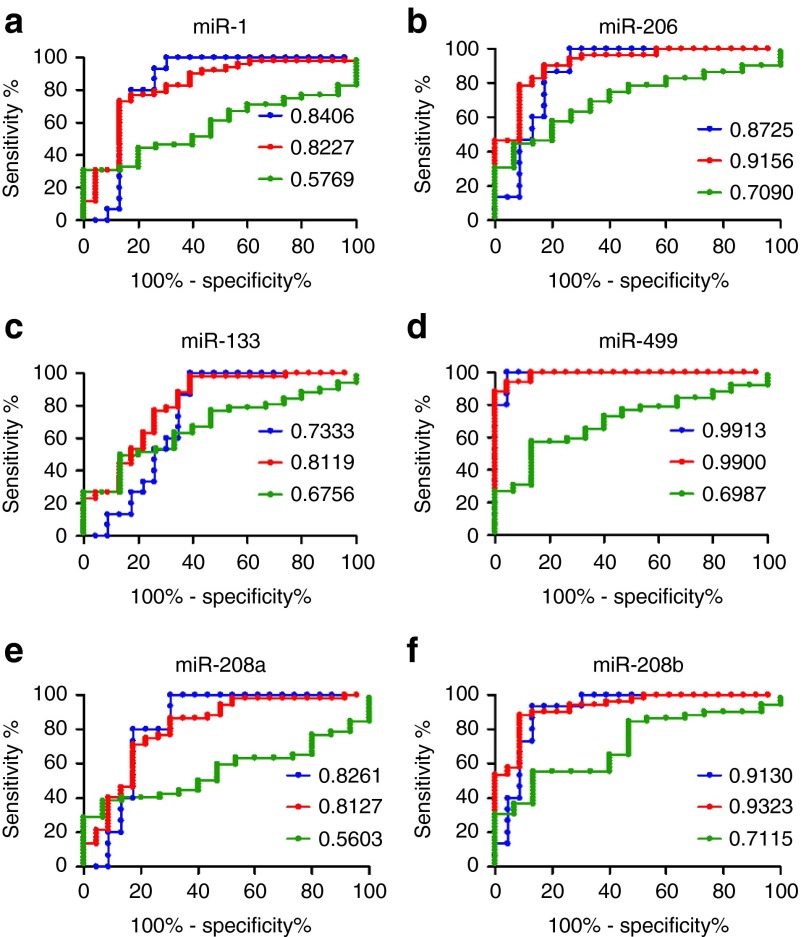
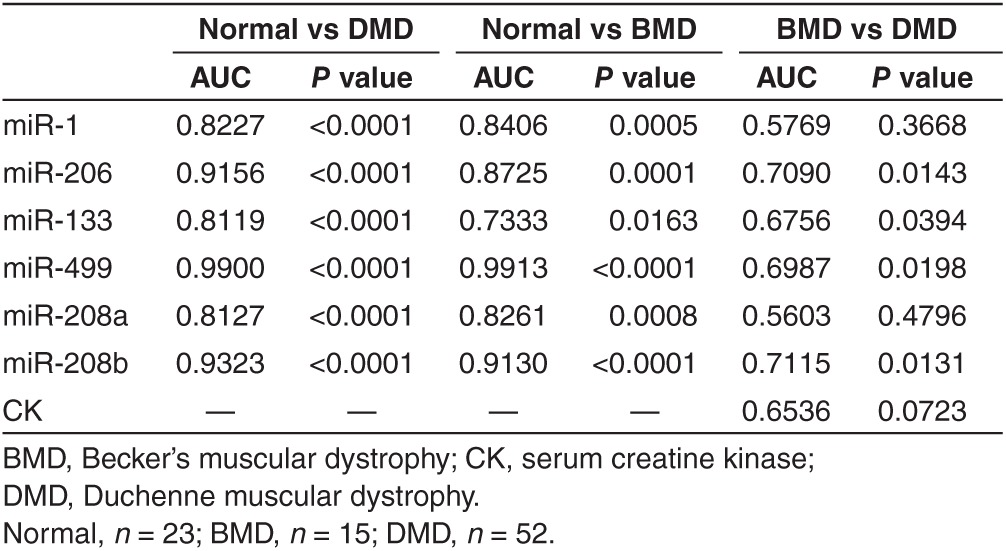
The statistical significance of these measurements and diagnostic power of serum miRNAs were evaluated by receiver operating characteristics (ROC) analysis (Figure 2a–f and Table 1). Our results demonstrated that miR-206, miR-499, and miR-208b displayed better sensitivity and specificity to discriminate DMD from normal subjects (AUC: 0.9156, 0.99, and 0.9323, respectively, P < 0.0001; Figure 2b,d,f and Table 1). Also miR-1, miR-133, and miR-208a displayed a very good statistical scores (AUC: 0.8227, 0.8119, and 0.8127 respectively, P < 0.0001; Figure 2a,c,e and Table 1). Similarly, miR-206, miR-499, and miR-208b had higher sensitivity and specificity to discriminate BMD from controls (AUC: 0.8725, 0.9913, and 0.913, respectively, P ≤ 0.0001), while miR-1, miR-133, and miR-208a were less potent (AUC: 0.8406, 0.7333, and 0.8261, respectively, P = 0.0005, P = 0.0163, and P = 0.0008, respectively; Figure 2a–f and Table 1). Most importantly, ROC curve analysis displayed the diagnostic power of serum miR-208b, miR-206, miR-499, and miR-133 in discriminating DMD from BMD patients (AUC: 0.7115, 0.7090, 0.6987, and 0.6756, listed from high to low, P = 0.0131, P = 0.0143, P = 0.0198, and P = 0.0394, respectively; Figure 2f,b,d,c and Table 1). We also performed ROC analysis to evaluate the diagnostic power of CK value in discriminating DMD from BMD. As shown in Table 1, CK did not have diagnostic power in discriminating DMD from BMD patients (AUC: 0.6536, P = 0.0723). These results indicated that circulating miRNA signatures have diagnostic value in patients with DMD.
Figure 2.
ROC analysis of serum levels of myomiRs. (a–f) ROC curves were plotted to evaluate the diagnostic power of myomiRs (miR-1, miR-206, miR-133, miR-499, miR-208a, and miR-208b). Red line, DMD versus normal; blue line, BMD versus normal; green line, BMD versus DMD. The area under the ROC curve (AUC) was calculated for the measurement of discrimination accuracy (normal, n = 23; BMD, n = 15; DMD, n = 52). BMD, Becker's muscular dystrophy; DMD, Duchenne muscular dystrophy; ROC, receiver–operator characteristic.
Table 1. Receiver–operator characteristic (ROC) curve analysis of miRNAs and CK for diagnosis of dystrophic children.
The correlation between serum level of myomiR and age
The absence of dystrophin results in progressive degeneration of skeletal and cardiac muscle. We speculated that the circulating miRNA levels might change during aging and might correlate with the extent of muscle degeneration or the stage of the disease. To test our hypothesis, we first performed a case-by-case correlation analysis between age and serum miRNA/CK levels of dystrophic children of all ages. As expected, we did not observe any statistical significance of correlations (Supplementary Table S2). Subsequently, we analyzed the correlation of serum miRNA or CK levels with age in different age groups (≤2, 2–6, and >6 years old; Supplementary Table S2). Interestingly, serum miR-206, miR-499, and miR-208b levels were positively correlated with age in DMD patients aged 2–6 years (Figure 3a–c and Supplementary Table S2). No correlation between CK value and age was observed in DMD or BMD patients in any age groups (Supplementary Table S2). These results indicated that these age-associated serum miRNA signatures might represent the extent of muscle degeneration or the stage of the disease.
Figure 3.
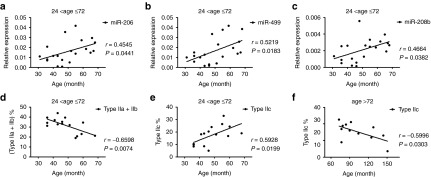
Correlation between serum miRNA level or muscle fiber type composition and age. (a–c) Case-by- case comparison of miR-206 (a), miR-499 (b), and miR-208b (c) levels with age in 20 DMD children between 2 and 6 years of age. Regression lines are displayed. (d,e) The percentage of fast-twitch (type IIa and type IIb) muscle fibers (d) shows an inverse correlation, while the percentage of type IIc (e) shows a positive correlation with age in DMD children from 2 to 6 years old (n = 15). (f) In DMD patients older than 6 years, the percentage of type IIc shows a negative correlation with age. DMD, Duchenne muscular dystrophy; r, correlation coefficient.
The correlation between serum miRNA level and muscle fiber type composition
Since myomiRs play regulatory roles in muscle growth, differentiation, fiber determination, and metabolism, we hypothesized that the serum levels of these miRNA might reflect the muscle structure and function and correlate with the pathogenesis of DMD or BMD. In order to test our hypothesis, we first determined the fiber type composition in the muscle-biopsy specimens from these DMD and BMD subjects. We were not able to observe any correlation of fiber type composition with age in dystrophic subjects of all ages (Supplementary Table S3). In agreement with the concept that fast muscle fibers are preferentially affected,22 we found that the percentage of fast-twitch (type IIa and type IIb) muscle fibers decreased in DMD patients aged 2–6 years (Figure 3d and Supplementary Table S3). In contrast, in the same age group (2–6 years), the percentage of type IIc muscle fibers increased gradually (Figure 3e and Supplementary Table S3), while in patients older than 6 years the percentage of type IIc muscle fiber decreased (Figure 3f). Because many of type IIc muscle fibers have characteristics of regenerating fibers and reflect an active regenerating process,23,24,25,26 our result suggested that the regeneration becomes increasingly active in patients between the ages of 2 and 6 years due to continuous degeneration, while in older patients (>6 years) after degeneration and regeneration cycle depletes the satellite cell pool, these patients fail to regenerate and recover muscle mass. We did not observe any correlation between type IIc fibers and age in all age groups of BMD, in turn, suggesting that muscle regeneration in BMD patients remains active (Supplementary Table S3).
Then, we evaluated the correlation between serum miRNA levels and muscle fiber composition. As shown in Table 2, serum level of miR-499 was negatively correlated with fast-twitch fiber composition, while serum level of miR-133 was positively correlated with fast-twitch fiber composition and negatively correlated with slow-twitch fiber composition (Table 2). Since both serum miRNA levels and muscle fiber composition might be correlated with age at different stages of disease, in order to obtain exact correlations, we analyzed the correlation between them in different age groups. We found that the serum levels of miR-206, miR-499, and miR-208b were positively correlated with the percentage of type IIc muscle fibers in DMD patients between 2 and 6 years old (Figure 4a–c and Table 2), suggesting that the serum miR-206, miR-499, and miR-208b might serve as real-time biomarkers to monitor the activity of regeneration in DMD patients between 2 and 6 years old. In addition, we observed that serum level of miR-499 and miR-208b were positively correlated with the percentage of slow-twitch (type I) muscle fibers and negatively correlated with fast-twitch (type IIa and IIb) muscle fiber composition in DMD patient older than 6 years (Table 2). Similar correlations were observed for miR-206 although one of them tended to be significant (P = 0.0575; Table 2). This observation suggested that the serum levels of miR-206, miR-499, and miR-208b might reflect the ratio of slow to fast muscle fibers in DMD patients older than 6 years (Figure 4d–f). Interestingly, these three miRNAs were mainly expressed in solues muscle (slow-twitch muscle) of mice (Supplementary Figure S3). In contrast to these three miRNAs, miR-1, and miR-133 were enriched in tibialis anterior muscle (TA, fast-twitch muscle) of mice (data not shown). These observed correlations between serum miRNA levels and muscle fiber composition might be due to the differential expression pattern of miRNA in muscles comprising of different fiber types. As we expected, no correlation between CK value and muscle fiber composition was observed in all age groups (Table 2).
Table 2. Correlation between muscle fiber type composition and serum miRNAs/CK level of DMD children.
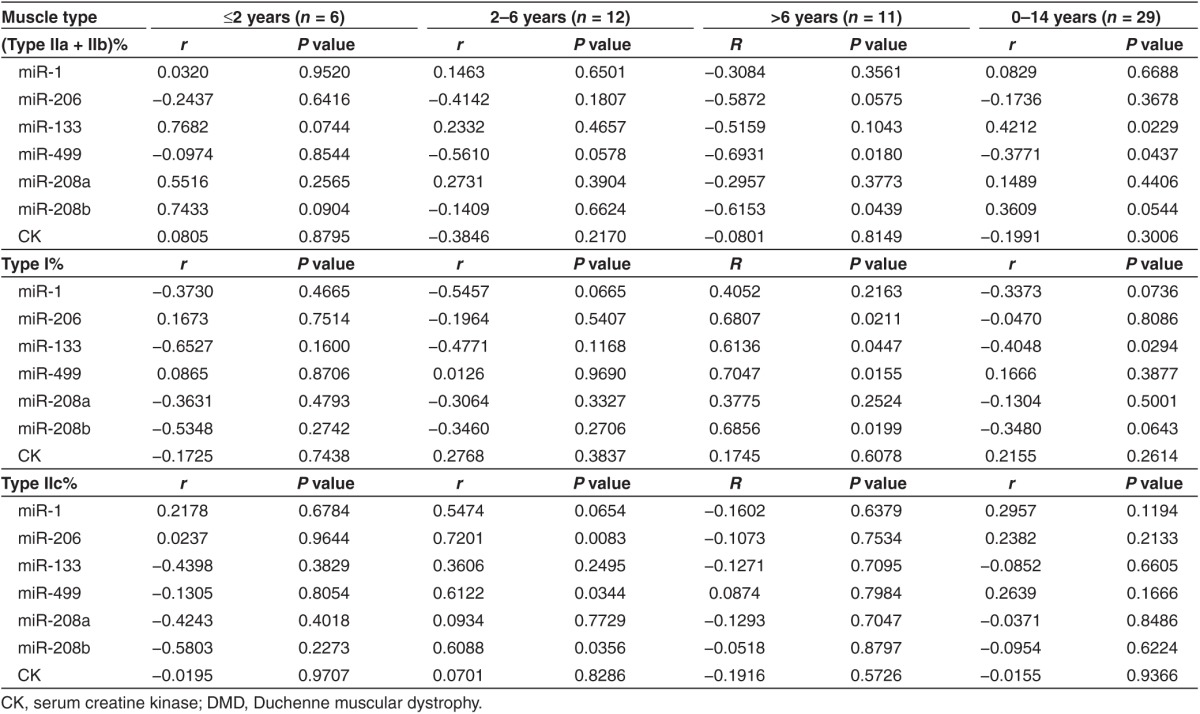
Figure 4.
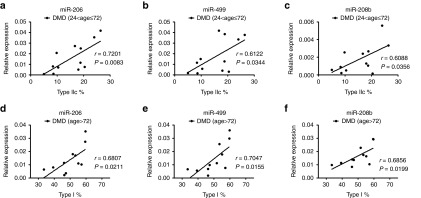
Correlation between serum miRNA level and type IIc or type I muscle fiber composition. (a–c) Serum levels of miR-206 (a), miR-499 (b) and miR-208b (c) show positive correlation with type IIc muscle fiber composition in DMD children between 2 and 6 years old (24< age ≤72; n = 12). (d–f) Serum levels of miR-206 (d), miR-499 (e) and miR-208b (f) show positive correlation with type I muscle fiber composition in DMD children older than 6 years old (age > 72; n = 11). DMD, Duchenne muscular dystrophy; r, correlation coefficient.
The regulation of miRNA secretion by cytokines and growth factors
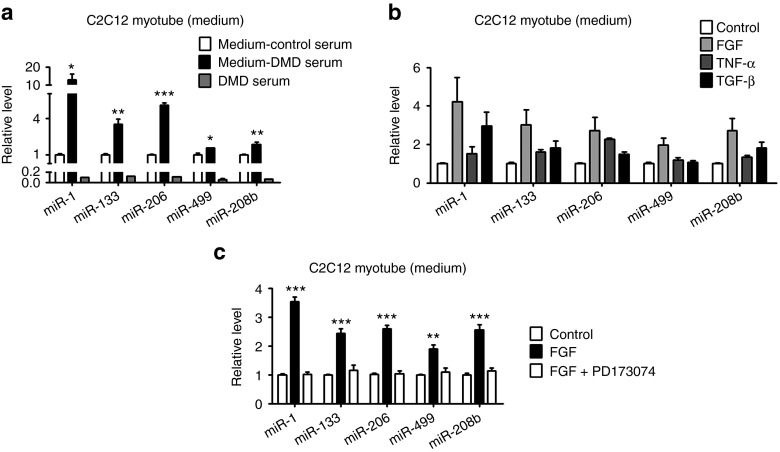
The exploration of circulating miRNAs brings about an important advance for biomarker discovery. Circulating miRNAs have been profiled in many diseases. However, the source of these circulating miRNAs and the underlying mechanism are not fully elucidated. We first tested whether the serum from DMD patient could affect the secretion of myomiRs. Interestingly, we found that DMD serum increased the miR-1, miR-133, miR-206, miR-499, and miR-208b levels in the medium of differentiated C2C12 cells (Figure 5a). We also directly measure the miRNA levels with the same amount of the DMD serum in the same amount of medium and detected very low levels of these miRNAs, suggesting that the human miRNA contamination from added DMD serum is limited (Figure 5a).
Figure 5.
The effect of DMD serum, cytokine, and growth factors on the level of myomiRs in culture medium of C2C12 myotubes. The levels of myomiR (miR-1, miR-133, miR-206, miR499, and miR-208b) in the culture medium of C2C12 myotubes were examined by using real-time PCR. (a) C2C12 myotubes were treated with 20 μl serum from DMD patients or control subjects. The culture medium from these two groups were collected for measurement and designated as medium-DMD serum and medium-Control serum, respectively, as indicated. Since the primers could not distinguish human and mouse miRNAs, a third group was set to measure the miRNA levels in 20 μl DMD serum and designated as DMD serum as indicated. Student t test was used to analyze the data from DMD serum treatment and control serum treatment. (b) C2C12 myotubes were incubated with FGF, TNF-α, or TGF-β. (c) Differentiated C2C12 myocytes were preincubated with FGFR1 inhibitor (PD173074) before FGF was added to the culture medium. Error bars represent the SEs. Student's t test was used. *P < 0.05, **P < 0.01, ***P < 0.001 versus control group. DMD, Duchenne muscular dystrophy; SE, standard error.
It is known that cytokines and growth factors are enriched in regenerating muscles and play important roles in inflammation and regeneration.27,28 To figure out which factors or molecules in the DMD serum might contribute to miRNA secretion, we investigated the effect of tumor necrosis factor-α (TNF-α), fibroblast growth factor (FGF), and transforming growth factor-β (TGF-β). As shown in Figure 5b, these factors have differential impact on the level of myomiRs in the culture medium of C2C12 myotubes. Moreover, consistent with the finding that FGF could increase the level of these myomiRs in the culture medium, inhibition of FGF signaling by PD173074, a specific inhibitor for fibroblast growth factor receptor 1 (FGFR1), resulted in a reduction of myomiR levels in the culture medium (Supplementary Figure S4). Furthermore, the effect of FGF could be blocked by pre-incubating C2C12 myotubes with PD173074 (Figure 5c). In addition, TGF-β affected the level of miR-1, miR-133, miR-206, and miR-208b but had little effect on miR-499 level, while TNF-α increased the level of miR-133, miR-206, and miR-208b, but not miR-1 and miR-499 (Figure 5b). To rule out the possibility that treatment of TNF-α, FGF, and TGF-β could disturb the membrane integrity, leading to the leak of some miRNA out of the cells, we checked the effect of these growth factors and cytokines on the cell viability. As shown in Supplementary Figure S5a–d, the treatment did not significantly affect the cell viability of C2C12 myotubes.
Based on these findings, we proposed that the different circulating miRNA profiles might also reflect the action of different cytokines and growth factors on degenerating and regenerating muscles. On the other hand, the differential effects of cytokine and growth factors on the secretion of different miRNAs suggested that the secretion might be regulated by separated mechanisms, which requires further investigation.
Discussion
Comprehensive approaches including gene therapy, cell therapy, and pharmacotherapy have been proposed for an ultimate cure for DMD. Considering the various clinical treatments and trials currently ongoing for DMD, the identification of serum biomarkers can be valuable to diagnose, prognosis of diseases, and guide therapeutic decisions. Although CK is commonly used as a biomarker of muscular diseases to evaluate the level of muscle damage and necrosis,29 but it is not always reliable since CK level varies considerably upon several independent stress conditions, such as exercise. Growing evidence has indicated that serum miRNAs are promising biomarkers for diseases, like tissue injury, cancers, and cardiovascular disease.15,30,31 The complexity of the regulation and function of miRNA provides us more opportunities to identify disease-associated biomarkers. Here our study suggested that specific circulating miRNAs in serum could serve as potential biomarker for DMD diagnosis. We focused on myomiRs since these functional miRNAs have been studied extensively in muscles. The elevation of serum levels of myomiRs (miR-1, miR-133, miR-206, miR-208a, miR-208b, and miR-499) were observed in DMD patients. Based on our result, among these miRNAs, miR-499 is the best biomarker to discriminate DMD from healthy control subjects (AUC = 0.99). Although miR-208b is not the best biomarker to discriminate DMD from healthy controls, it displayed the greatest power to distinguish DMD and BMD (AUC = 0.7115). Our study here suggested that serum miRNA profiles might be considered as biomarkers for DMD diagnosis.
An early report suggested that the levels of serum miRNAs might be associated with pathogenesis of DMD.16 Different fiber compositions contribute to muscle functions and features such as contraction and metabolism. During degeneration and regeneration, muscle undergoes composition changes in muscle fiber types. We hypothesized that varied fiber type compositions might reflect the structure and function of muscle and might be used as a signature of disease progression. In this study, we compared the muscle fiber composition with age and explored the correlation between serum miRNA levels and muscle fiber composition. We uncovered that serum levels of miR-206, miR-499, and miR-208b are positively correlated with age and type IIc muscle fibers from 2 to 6 years in DMD patients (Figures 3 and 4). Therefore, we suggested that these miRNAs might be useful in monitoring the activity of regeneration in DMD patients between 2 and 6 years old. Moreover, these biomarkers are not replaceable since muscle biopsy is invasive, after first diagnosis.
We also showed that miR-206, miR-499, and miR-208b are positively correlated with slow-twitch muscle fibers in DMD patients more than 6 years old. It is possible that the serum miRNA levels represent the residual muscle mass. Hence these results allowed us to propose that the serum miRNAs could be valuable prognostic biomarkers to monitor DMD diseases progression. This result is consistent with the concept that miR-206, miR-208b, and miR-499 are enriched in slow-twitch muscle fiber and fast-twitch muscle fibers are the first to degenerate. In other words, the less affected slow muscle fibers might contribute to the higher level of serum miRNA at late stage of DMD.
To elucidate the mechanisms underlying circulating miRNA profile variation, we incubated the cultured muscle cells with DMD serum to mimic the microenvironment in vivo and found that DMD serum is capable of increasing the level of myomiR in the culture medium. This finding suggested that the myomiRs in serum might be secreted from muscle tissues. Also, DMD serum might contain regulatory molecules that could regulate miRNA secretion. A previous report showed that the expression of cytokines including TNF-α was not only upregulated in DMD muscles but also increased with age.32 It is intriguing that we found TNF-α as well as other growth factors (FGF and TGF-β) could increase the levels of muscle specific miRNAs in C2C12 cells under differentiation culture condition. We speculated that the increase of these miRNAs in the serum is secreted from differentiating muscle cells through the exact mechanisms remain to be explored. It has been shown that initial myofiber damage is exacerbated by the endogenous inflammatory response.33,34,35 We, therefore proposed that the serum levels of these miRNAs may partly reflect the degeneration-induced inflammatory response in patients between 2 and 6 years old. This would be consistent with the fact that there is no significant change in serum revels of some abundant miRNAs or housekeeping miRNAs, such as miR-16, in DMD patients. Although it is likely that the serum myomiRs could also be directly leaked from damaged muscle cells, we propose that serum miRNA might be mainly derived from selective secretion. Further work is required to validate our hypothesis in the future.
A recent study from Dr. Muntoni's group suggested that miR-1, miR-133a, and miR-133b showed a similar age pattern: higher and gradually decreasing level in patients between 4 to 10 years old and considerably lower levels of miRNAs in older patients (from 13 to 17 years of age).18 Based on the age profile of miR-1, miR-133a, and miR-133b, the authors speculated that the three dystromirs can be utilized as biomarkers for the remaining muscle mass in DMD. Our results are in general in agreement with this study that the increases in miRNA is only significant in young DMD patients (<10 years old), whereas the levels of the same miRNA start to drop in the older patients. Since the DMD patients recruited in our study are much younger than those in their study and only one patient had lost ambulation, we have more chance to detect possible correlation between circulating miRNAs with disease progression in early stage. Actually, we found the serum levels of miR-206, miR-499, and miR-208b were positively correlated with age in young patient (from 2 to 6 years of age). The more elaborated parts of our study are the observation that serum levels of miRNA is related to muscle fiber type and growth factors might influence the secretion/leakage of miRNA from myocytes. Together, the data from these two studies provided us more information about the change of the amount of miRNAs over time.
Taken together, we identified serum miRNA signatures for DMD prognosis. The signatures are associated with fiber type composition and disease progression. Thus analysis of the pattern of myomiRs could be useful for assessing disease severity and effectiveness of therapeutic interventions.
Materials and methods
Mouse and human studies. Animals were maintained and experiments were performed according to the protocol approved by the Animal Care and Use Committees of Institute for Nutritional Sciences (permit number: 2011-AN-14). Mice were housed in a temperature controlled room (22 °C) with a 12-hour light/dark cycle and had free access to food and water. Mdx mice were from Dr.Xiang Gao (Nanjing University, Nanjing, China). Eight weeks old male mice were used in this study. Mouse muscle pads were harvested and frozen in liquid nitrogen and stored at −80 °C. Blood samples were centrifuged to prepare serum and stored at −80 °C.
Human study was reviewed and approved by Ethics Committee of Children's Hospital of Fudan University. All human participants gave written informed consent. The serum samples were collected at Children's Hospital of Fudan University. Patient inclusion criteria were: genetically and muscle biopsy proven DMD or BMD diagnosis, patient still ambulant without any help (only for children younger than 6-years-old), no severe or moderate learning difficulties or behavioral problems. The bicep (upper arm muscle) which is about 5 mm in diameter and 1 cm in length was selected for muscle biopsy analysis and myosin ATPase Staining.
Reagents, cell culture and differentiation. Mouse C2C12 myoblasts (gift from Dr. Jia Li, SIBS, CAS, China) were maintained in a humidified incubator at 37 °C and 5% CO2 in Dulbecco's modified Eagle medium containing 10% fetal bovine serum, and 1% penicillin and streptomycin (Gibco, Grand Island, NY). Myoblast differentiation was induced as described before.36,37 On day 4 after induction of differentiation, cells were treated with pooled DMD serum (20 μl), or incubated with 5 ng/ml TGF-β (Santa Cruz, Dallas, TX), 10 ng/ml FGF (Santa Cruz), or 50 ng/ml TNF-α (gift from Dr.Yingying Le SIBS, CAS, China) for 12 hours before culture mediums (2 ml) were harvested. FGFR1 inhibitor PD173074 was from Han-Xiang Chemical (final concentration 10 nmol/l).
RNA isolation and qRT-PCR analysis. The miRNAs were extracted from serum and cell culture medium using miRcute miRNA isolation kit (Tiangen, Beijing, China). Small RNA was reverse-transcribed by using stem-loop RT which was performed according to the protocols described before38 Real-time PCR was performed as described before39 on an ABI 7900 Real-Time PCR System (Applied Biosystems, Grand Island, NY). The primers for miR-133 detected both miR-133a and miR-133b. We chose miR-223 as an internal control for real-time PCR analysis of serum miRNA. Primers used in this study are listed in Supplementary Table S5.
Myosin ATPase staining. Muscle fibers were counted, measured, and classified by ATPase reaction which was performed as described before with minor modification.40 Briefly, fiber types were determined by the differential staining resulting from pre-incubation at either pH 10.5, 4.6, or 4.2 for human fibers. The first method distinguishes type I (light staining) fibers from type II (dark staining) fibers, the second distinguishes type I (dark staining) from IIa (light staining) and IIb or IIc(medium staining) fibers, and the third distinguishes IIc (medium staining) from IIa or IIb (bright staining)fibers. The three preincubation reagents were (i) 20 mmol/l sodium barbital, 18 mmol/l CaCl2, pH 10.5, (ii) 50 mmol/l sodium acetate, 30 mmol/l sodium barbital brought to pH on 4.6 with HCl, and the same as (ii) but adjusted to pH 4.2. The preincubation times were 15 minutes at pH 10.5 and 5 minutes at the acid pH's. After preincubation, the sections were incubated for 30 mm in 20 mmol/l sodium barbital, pH 10.5, containing 9 mmol/l CaCl2 and 2.7 mmol/l ATP; rinsed in three changes of 1% CaCI2 (3 minutes each); immersed for 3 minutes in 2% CoCl2; and rinsed in three changes of tap water. After staining for 1 minutes in 1% (NH4)2S, the sections were washed with several changes of tap water, dehydrated with ethanol, cleared in xylene, and mounted in DPX.
Statistical analysis. Data are presented as means ± standard error unless otherwise indicated. ANOVA and student's t-test were performed for statistical analysis as indicated. P values of < 0.05 were considered to be significant. ROC curves were plotted to evaluate the diagnostic effects of the profiles. The area under the ROC curve (AUC) was calculated for the measurement of discrimination accuracy. GraphPad software was used for all the statistical analysis.
SUPPLEMENTARY MATERIAL Figure S1. Serum levels of myomiRs in dystrophic mouse model. Figure S2. The serum level of CK in BMD and DMD patients. Figure S3. The expression levels of miR-499, miR-208a, miR-208b, miR-206 and miR-21 in fast-twitch (tibialis anterior, TA) and slow-twitch (soleus, SOL) muscles of C57/B6 mice. Figure S4. The impact of FGFR1 inhibitor PD173074 on myomiR levels in the medium of C2C12 myotubes. Figure S5. The effect of FGF, TNF-α, or TGF-β on cell viability of C2C12 myotubes. Table S1. Patient information. Table S2. Correlation between age and serum miRNA/CK level of dystrophic children. Table S3. Correlation between age and muscle fiber type composition of dystrophic children. Table S4. Primer information.
Acknowledgments
This work was supported by grants from the Ministry of Science and Technology of China (973 Program 2010CB912500 and 2012BAK01B00), the National Natural Science Foundation (31371189, 31070679, 31100550, and 81172009) , and Shanghai Institutes for Biological Sciences, CAS (SIBS 2012004). We also would like to thank Shanghai Charity Foundation (The Special Fund for DMD). The authors declare that they have no conflict of interest.
Supplementary Material
Serum levels of myomiRs in dystrophic mouse model.
The serum level of CK in BMD and DMD patients.
The expression levels of miR-499, miR-208a, miR-208b, miR-206 and miR-21 in fast-twitch (tibialis anterior, TA) and slow-twitch (soleus, SOL) muscles of C57/B6 mice.
The impact of FGFR1 inhibitor PD173074 on myomiR levels in the medium of C2C12 myotubes.
The effect of FGF, TNF-α, or TGF-β on cell viability of C2C12 myotubes.
Patient information.
Correlation between age and serum miRNA/CK level of dystrophic children.
Correlation between age and muscle fiber type composition of dystrophic children.
Primer information.
References
- Emery AE. The muscular dystrophies. Lancet. 2002;359:687–695. doi: 10.1016/S0140-6736(02)07815-7. [DOI] [PubMed] [Google Scholar]
- Haslett JN, Sanoudou D, Kho AT, Bennett RR, Greenberg SA, Kohane IS, et al. Gene expression comparison of biopsies from Duchenne muscular dystrophy (DMD) and normal skeletal muscle. Proc Natl Acad Sci USA. 2002;99:15000–15005. doi: 10.1073/pnas.192571199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace GQ, McNally EM. Mechanisms of muscle degeneration, regeneration, and repair in the muscular dystrophies. Annu Rev Physiol. 2009;71:37–57. doi: 10.1146/annurev.physiol.010908.163216. [DOI] [PubMed] [Google Scholar]
- Bieber FR, Hoffman EP. Duchenne and Becker muscular dystrophies: genetics, prenatal diagnosis, and future prospects. Clin Perinatol. 1990;17:845–865. [PubMed] [Google Scholar]
- Nicholson GA, Morgan GJ, Meerkin M, Strauss ER, McLeod JG. The effect of aerobic exercise on serum creatine kinase activities. Muscle Nerve. 1986;9:820–824. doi: 10.1002/mus.880090905. [DOI] [PubMed] [Google Scholar]
- Florence JM, Fox PT, Planer GJ, Brooke MH. Activity, creatine kinase, and myoglobin in Duchenne muscular dystrophy: a clue to etiology. Neurology. 1985;35:758–761. doi: 10.1212/wnl.35.5.758. [DOI] [PubMed] [Google Scholar]
- Mazzone ES, Messina S, Vasco G, Main M, Eagle M, D'Amico A, et al. Reliability of the North Star Ambulatory Assessment in a multicentric setting. Neuromuscul Disord. 2009;19:458–461. doi: 10.1016/j.nmd.2009.06.368. [DOI] [PubMed] [Google Scholar]
- Aartsma-Rus A, van Ommen GJ. Progress in therapeutic antisense applications for neuromuscular disorders. Eur J Hum Genet. 2010;18:146–153. doi: 10.1038/ejhg.2009.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossu G, Sampaolesi M. New therapies for Duchenne muscular dystrophy: challenges, prospects and clinical trials. Trends Mol Med. 2007;13:520–526. doi: 10.1016/j.molmed.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Filipowicz W, Bhattacharyya SN, Sonenberg N. Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight. Nat Rev Genet. 2008;9:102–114. doi: 10.1038/nrg2290. [DOI] [PubMed] [Google Scholar]
- Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- Creemers EE, Tijsen AJ, Pinto YM. Circulating microRNAs: novel biomarkers and extracellular communicators in cardiovascular disease. Circ Res. 2012;110:483–495. doi: 10.1161/CIRCRESAHA.111.247452. [DOI] [PubMed] [Google Scholar]
- Chen X, Gao C, Li H, Huang L, Sun Q, Dong Y, et al. Identification and characterization of microRNAs in raw milk during different periods of lactation, commercial fluid, and powdered milk products. Cell Res. 2010;20:1128–1137. doi: 10.1038/cr.2010.80. [DOI] [PubMed] [Google Scholar]
- Michael A, Bajracharya SD, Yuen PS, Zhou H, Star RA, Illei GG, et al. Exosomes from human saliva as a source of microRNA biomarkers. Oral Dis. 2010;16:34–38. doi: 10.1111/j.1601-0825.2009.01604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zen K, Zhang CY. Circulating microRNAs: a novel class of biomarkers to diagnose and monitor human cancers. Med Res Rev. 2012;32:326–348. doi: 10.1002/med.20215. [DOI] [PubMed] [Google Scholar]
- Mizuno H, Nakamura A, Aoki Y, Ito N, Kishi S, Yamamoto K, et al. Identification of muscle-specific microRNAs in serum of muscular dystrophy animal models: promising novel blood-based markers for muscular dystrophy. PLoS One. 2011;6:e18388. doi: 10.1371/journal.pone.0018388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacchiarelli D, Legnini I, Martone J, Cazzella V, D'Amico A, Bertini E, et al. miRNAs as serum biomarkers for Duchenne muscular dystrophy. EMBO Mol Med. 2011;3:258–265. doi: 10.1002/emmm.201100133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaharieva IT, Calissano M, Scoto M, Preston M, Cirak S, Feng L, et al. Dystromirs as serum biomarkers for monitoring the disease severity in Duchenne muscular Dystrophy. PLoS One. 2013;8:e80263. doi: 10.1371/journal.pone.0080263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby TJ, McCarthy JJ. MicroRNAs in skeletal muscle biology and exercise adaptation. Free Radic Biol Med. 2013;64:95–105. doi: 10.1016/j.freeradbiomed.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond MJ, Glynn EL, Fry CS, Dhanani S, Volpi E, Rasmussen BB. Essential amino acids increase microRNA-499, -208b, and -23a and downregulate myostatin and myocyte enhancer factor 2C mRNA expression in human skeletal muscle. J Nutr. 2009;139:2279–2284. doi: 10.3945/jn.109.112797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rooij E, Quiat D, Johnson BA, Sutherland LB, Qi X, Richardson JA, et al. A family of microRNAs encoded by myosin genes governs myosin expression and muscle performance. Dev Cell. 2009;17:662–673. doi: 10.1016/j.devcel.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster C, Silberstein L, Hays AP, Blau HM. Fast muscle fibers are preferentially affected in Duchenne muscular dystrophy. Cell. 1988;52:503–513. doi: 10.1016/0092-8674(88)90463-1. [DOI] [PubMed] [Google Scholar]
- Nonaka I, Takagi A, Sugita H. The significance of type 2C muscle fibers in Duchenne muscular dystrophy. Muscle Nerve. 1981;4:326–333. doi: 10.1002/mus.880040409. [DOI] [PubMed] [Google Scholar]
- Taniguchi M, Kurahashi H, Noguchi S, Fukudome T, Okinaga T, Tsukahara T, et al. Aberrant neuromuscular junctions and delayed terminal muscle fiber maturation in alpha-dystroglycanopathies. Hum Mol Genet. 2006;15:1279–1289. doi: 10.1093/hmg/ddl045. [DOI] [PubMed] [Google Scholar]
- Anderson JE, Bressler BH, Ovalle WK. Functional regeneration in the hindlimb skeletal muscle of the mdx mouse. J Muscle Res Cell Motil. 1988;9:499–515. doi: 10.1007/BF01738755. [DOI] [PubMed] [Google Scholar]
- Nonaka I, Sunohara N, Satoyoshi E, Terasawa K, Yonemoto K. Autosomal recessive distal muscular dystrophy: a comparative study with distal myopathy with rimmed vacuole formation. Ann Neurol. 1985;17:51–59. doi: 10.1002/ana.410170113. [DOI] [PubMed] [Google Scholar]
- Husmann I, Soulet L, Gautron J, Martelly I, Barritault D. Growth factors in skeletal muscle regeneration. Cytokine Growth Factor Rev. 1996;7:249–258. doi: 10.1016/s1359-6101(96)00029-9. [DOI] [PubMed] [Google Scholar]
- Kuru S, Inukai A, Kato T, Liang Y, Kimura S, Sobue G. Expression of tumor necrosis factor-alpha in regenerating muscle fibers in inflammatory and non-inflammatory myopathies. Acta Neuropathol. 2003;105:217–224. doi: 10.1007/s00401-002-0635-4. [DOI] [PubMed] [Google Scholar]
- Vassella F, Richterich R, Rossi E. The diagnostic value of serum creatine kinase in neuromuscular and muscular disease. Pediatrics. 1965;35:322–330. [PubMed] [Google Scholar]
- Wang K, Zhang S, Marzolf B, Troisch P, Brightman A, Hu Z, et al. Circulating microRNAs, potential biomarkers for drug-induced liver injury. Proc Natl Acad Sci USA. 2009;106:4402–4407. doi: 10.1073/pnas.0813371106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Zhao J, Evan G, Xiao C, Cheng Y, Xiao J. Circulating microRNAs: novel biomarkers for cardiovascular diseases. J Mol Med (Berl) 2012;90:865–875. doi: 10.1007/s00109-011-0840-5. [DOI] [PubMed] [Google Scholar]
- Messina S, Vita GL, Aguennouz M, Sframeli M, Romeo S, Rodolico C, et al. Activation of NF-kappaB pathway in Duchenne muscular dystrophy: relation to age. Acta Myol. 2011;30:16–23. [PMC free article] [PubMed] [Google Scholar]
- Tidball JG. Inflammatory processes in muscle injury and repair. Am J Physiol Regul Integr Comp Physiol. 2005;288:R345–R353. doi: 10.1152/ajpregu.00454.2004. [DOI] [PubMed] [Google Scholar]
- Grounds MD, Torrisi J. Anti-TNFalpha (Remicade) therapy protects dystrophic skeletal muscle from necrosis. FASEB J. 2004;18:676–682. doi: 10.1096/fj.03-1024com. [DOI] [PubMed] [Google Scholar]
- Evans NP, Misyak SA, Robertson JL, Bassaganya-Riera J, Grange RW. Immune-mediated mechanisms potentially regulate the disease time-course of duchenne muscular dystrophy and provide targets for therapeutic intervention. PM R. 2009;1:755–768. doi: 10.1016/j.pmrj.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Li X, Chen C, Li Y, Zhao L, Jing Y, et al. Attenuation of p38-mediated miR-1/133 expression facilitates myoblast proliferation during the early stage of muscle regeneration. PLoS One. 2012;7:e41478. doi: 10.1371/journal.pone.0041478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YC, Li Y, Wang XY, Zhang D, Zhang H, Wu Q, et al. Circulating miR-130b mediates metabolic crosstalk between fat and muscle in overweight/obesity. Diabetologia. 2013;56:2275–2285. doi: 10.1007/s00125-013-2996-8. [DOI] [PubMed] [Google Scholar]
- Chen C, Ridzon DA, Broomer AJ, Zhou Z, Lee DH, Nguyen JT, et al. Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic Acids Res. 2005;33:e179. doi: 10.1093/nar/gni178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Shan S, Zhang Y, Liu W, Ding W, Ren W, et al. Ligand-dependent corepressor acts as a novel corepressor of thyroid hormone receptor and represses hepatic lipogenesis in mice. J Hepatol. 2012;56:248–254. doi: 10.1016/j.jhep.2011.07.014. [DOI] [PubMed] [Google Scholar]
- Hintz CS, Coyle EF, Kaiser KK, Chi MM, Lowry OH. Comparison of muscle fiber typing by quantitative enzyme assays and by myosin ATPase staining. J Histochem Cytochem. 1984;32:655–660. doi: 10.1177/32.6.6202737. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Serum levels of myomiRs in dystrophic mouse model.
The serum level of CK in BMD and DMD patients.
The expression levels of miR-499, miR-208a, miR-208b, miR-206 and miR-21 in fast-twitch (tibialis anterior, TA) and slow-twitch (soleus, SOL) muscles of C57/B6 mice.
The impact of FGFR1 inhibitor PD173074 on myomiR levels in the medium of C2C12 myotubes.
The effect of FGF, TNF-α, or TGF-β on cell viability of C2C12 myotubes.
Patient information.
Correlation between age and serum miRNA/CK level of dystrophic children.
Correlation between age and muscle fiber type composition of dystrophic children.
Primer information.