Abstract
We investigated respiratory pathogens in a prospective cohort study of young children living in the Peruvian Andes. In the study we assessed viral respiratory infections among young children, and explored interactions of viruses with common respiratory bacteria, especially Streptococcus pneumoniae. Through weekly household visits, data were collected on the signs and symptoms of acute respiratory illness (ARI), nasal samples were collected to test for viruses during episodes of ARI, and nasopharyngeal samples were collected on a monthly basis to monitor bacterial colonisation. We also collected data on vaccination coverage, patterns of social mixing, geographic information, and environmental and socio-demographic variables. Understanding the interaction of respiratory viruses with bacteria and its impact on the burden and severity of ARIs in rural areas of developing countries is critical to designing strategies for preventing such infections. Investigators interested in more details about this study or in accessing these resources should contact Dr. Carlos G. Grijalva at Vanderbilt University (carlos.grijalva@vanderbilt.edu).
Keywords: Epidemiology, acute respiratory illness, children, pneumococcus, influenza
Why was the cohort set up?
Every year, children aged <5 years, regardless of their country of residence or socio-economic status, have from 3–6 episodes of acute respiratory illness (ARI).1 Pneumonia is the most serious ARI, accounting for approximately 18% of all mortality in childhood3–5 with 95% of the deaths from this disease occurring in the developing world.3,4
Although the precise reasons for the increased severity of ARI in developing countries are unclear, exposure to indoor air pollution is considered an important factor. In rural settings, cooking and heating with solid fuels on open fires or stoves without chimneys are common.6 According to the World Health Organization (WHO), more than half of the world’s population relies on dung, wood, crop waste, or coal to meet basic energy needs.7 Indoor exposure to smoke is thought to be responsible for approximately 50% of all childhood pneumonia deaths worldwide.7
Most severe pneumonias are caused by bacteria, particularly Streptococcus pneumoniae,5 which often colonizes the nasopharynx of children and is commonly transmitted from person to person.8 Although most nasopharyngeal colonization with S. pneumoniae does not result in disease, the acquisition of a new serotype is often associated with illness.8,9
Recent studies have focused on the potential interaction between influenza virus and S. pneumoniae. Colonization with pneumococci has seasonal patterns similar to those of influenza,9 the incidence of pneumococcal pneumonia increases during epidemics of influenza,10,11 and influenza has been identified in persons with pneumococcal pneumonia.12,13 A secondary analysis of data from an efficacy trial of a 9-valent pneumococcal conjugate vaccine in South African children examined pneumonia without wheezing as an outcome. The incidence of influenza-associated pneumonia was reduced by 45% in subjects vaccinated with the pneumococcal conjugate as compared with that in controls.14 This decline was observed only among children infected with human immunodeficiency virus (HIV), and reductions were also observed for pneumonia associated with non-influenza viruses.14,15 However, a similar randomized trial of an 11-valent pneumococcal-conjugate vaccine in the Philippines (mainly in non-HIV-infected children), which included all pneumonias, failed to replicate these observations.16 Experimental infection of animals with pneumococcus after influenza infection results in more severe disease than that caused by either pathogen alone.17–20 It is not clear whether factors common to both pathogens (i.e. neuraminidase)18,19 and/or other immunological host factors (e.g. interferon-gamma)21 mediate this interaction. It has also been found that infection of ferrets with influenza A virus increased the transmission of certain pneumococcal serotypes.22 Although studies have failed to demonstrate that influenza increases pneumococcal colonization in adult humans,23 similar studies have not been conducted in children.
Understanding pneumococcal colonization patterns in rural communities of developing countries is crucial for implementing effective strategies for preventing pneumococcal pneumonia. Early pneumococcal colonization has resulted in a lower vaccine-associated antibody response to colonizing serotypes.24-26 Nevertheless, the clinical relevance of these findings in settings with high pneumococcal colonization remains unknown.
The present study will provide information about the distribution of colonizing bacteria, especially S. pneumoniae, the role of respiratory viruses in pneumococcal colonization, and the impact of other environmental factors, such as indoor smoke exposure, on bacterial colonization and the incidence and severity of ARIs among young Andean children.
How is it funded?
The study is supported in part by a Clinical and Translational Science Awards (CTSA) grant to Vanderbilt University from the US National Institutes of Health (NIH), an investigator-initiated research grant from Pfizer, and a grant from the Thrasher Research Fund. The study protocol was approved by the Vanderbilt Institutional Review Board (Nashville, TN, USA), and by the Ethics Committee of the Instituto de Investigacion Nutricional, IIN (Lima, Peru). The study was implemented in communities that participated in a community-cluster randomized trial, that evaluated an integrated home-based intervention package described below. It was conducted by the IIN and the Swiss Tropical and Public Health Institute, in Basel, Switzerland and supported by the UBS Optimus Foundation.27
Who is in the cohort?
The study was conducted in the Province of San Marcos, Department of Cajamarca, located in the northern highlands of Peru. Cajamarca has a population of about 1.5 million, is 2700 m above sea level, and has a dry and sunny equatorial climate. San Marcos, in southeastern Cajamarca, includes areas ranging from approximately 1500–4000 m above sea level. Nearly all inhabits of San Marcos are descended from the same ethnic group of Spanish people who mixed with the local Quechua population. The population is primarily rural, with low income, low educational level, and limited access to health-care services; 74% of the population is poor. San Marcos has a United Nations Development Program (UNDP) Human Development Index of 0.553, which ranks it in the 20th percentile of Peru. In 2007, the average life expectancy in San Marcos was 70 years, and 76% of the inhabitants were able to read and write. The median per-capita income per month in 2007 was US$71, ranking San Marcos in the 36th percentile for Peru.28
Investigators obtained approvals from each local community in the study area. Field activities began with a local census to enumerate all families and to identify potential participants. Trained field workers visited all houses and interviewed family members, using a standardized census form. These activities provided basic demographic information about the population in the study area, composition of the households, and number of children eligible for enrollment in the study.
To assure a representative sample of the communities in the study area, broad selection criteria were used for enrollment, consisting of: (i) families with children aged <3 years (including newborns); and (ii) intention to remain in the study area for the next year.
Identified households with potentially eligible children were visited and, after informed consent was obtained, children were enrolled and follow-up was begun. At enrollment, field workers obtained demographic and socio-economic information and data on health-services utilization and risk factors for ARI, including vaccination history. Consent was obtained to access participants’ medical and vaccination records for the duration of their participation.
During 2008–2009, before the study began, a community-cluster randomized trial conducted in the study area assessed the effects of an integrated home-based intervention package on clinical outcomes (without assessments of disease etiology).27 In that trial, communities were randomly allocated to either psychomotor stimulation of their children (control arm) or provision of an improved stove (to reduce indoor smoke) and sink, solar disinfection of water, and information about kitchen hygiene (intervention arm). A subset of eligible households that participated in the randomized trial was invited to participate in our prospective cohort study to assess the effects of reduced indoor exposure on pneumococcal colonization and the incidence of viral ARIs.
From May 2009 through September 2011, 892 children were enrolled in the study with the aim of maintaining a dynamic cohort with approximately 500 children under observation at any one time. Of 403 children enrolled in the randomized trial who were invited to participate in our observational study, 272 (67.5%) agreed to participate and were enrolled in this study, accounting for 30% of the total cohort (Figure 1).
Figure 1.
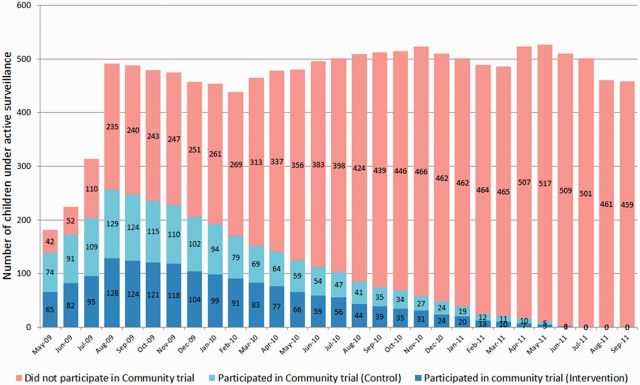
Composition of study cohort
The median age of enrolled children was 4.6 months, and 52% of the children were male. The median number of people living in study households was 5, and the materials of which the houses were predominantly made were typical of rural Andean settings, including dirt floors, tile roofs, and mud brick walls. Most houses used open fires or traditional stoves and wood for cooking. Most of the children (91%) in the study received health care though the public health insurance system of Peru (Table 1).
Table 1.
Selected baseline characteristics of enrolled children (N = 892)
| Demographics | |
| Age in months, median (IQR) | 4.6 (0.5, 17.1) |
| Male | 52% |
| Socioeconomic characteristics | |
| Number of people living in household, median (IQR) | 5 (4, 6) |
| Number of children <5 years old in household, median (IQR) | 1 (1, 2) |
| Number of bedrooms in house, median (IQR) | 1 (1, 2) |
| Number of rooms (excluding bedrooms) in house, median (IQR) | 2 (1, 3) |
| At least one family member attending school | 54% |
| Family owns house | 68% |
| House floor: dirt | 91% |
| House roof: tile | 93% |
| House walls: mud bricks | 93% |
| Water supply: pipeline | 82% |
| Sewage: connected to public system | 9% |
| House energy: electricity | 40% |
| House kitchen: open fire/traditional stove | 63% |
| Energy for stoves: wood | 93% |
| Smoking in house | 8% |
| Children taken to weekend public markets | 65% |
| Father: works in agriculture | 64% |
| Mother: elementary education or less | 65% |
| Health care related characteristics | |
| Children enrolled in public health insurance system | 91% |
| Children had vaccination card available | 93% |
IQR, interquartile range
During follow-up, 126 children (14%) were withdrawn from the study by their parents (Table 2). As compared with children who were not withdrawn, those who were withdrawn from the study were older at enrollment (median age 9 months vs. 3 months, respectively, P < 0.001), less likely to use traditional stoves or open fires for cooking (52% vs. 63% respectively, P < 0.001), and less likely to have vaccination cards available (90% vs. 94%, respectively, P = 0.011). However, there were no differences in sex, maternal education level, number of people living in the household, or enrollment in the public health insurance system between children who were and were not withdrawn.
Table 2.
Reasons for ending follow-up
| End of study (September 30 2011) | 459 (51.4%) |
| Reached 3 years of age | 246 (27.6%) |
| Consent withdrawn | 126 (14.1%) |
| Moved outside area/unable to locate | 56 (6.3%) |
| Death | 5 (0.6%) |
How often have they been followed up?
Trained field workers visited each household weekly to collect information on ARI through the use of a standardized questionnaire. Field workers were trained to recognize respiratory signs and symptoms by using educational material prepared by the Pan American (World) Health Organization in the Integrated Management of Childhood Illnesses (IMCI)–WHO.29-31 Weekly household visits allowed timely sampling of information about ARI and maximized the probability of detection of respiratory viruses while minimizing recall bias.32
Follow-up for each child continued from enrollment through the first of: (i) attainment of the age of 3 years; (ii) withdrawal of consent; (iii) loss to follow-up (travel outside the area or inability to locate); (iv) death; or (v) the end of the study (September 30, 2011). We maintained a relatively stable cohort size by enrolling additional newborns throughout the study. The reasons for loss to follow-up are listed in Table 2.
A physician was available for children with complicated ARIs, defined by the presence of WHO–IMCI danger signs (e.g. tachypnea, chest retractions, etc.).29–31 Cell phones allowed prompt communication between field workers and study headquarters for immediate feedback and to summon the physician to evaluate children with complicated ARIs.
What has been measured?
Acute respiratory infections
At each weekly visit, field workers recorded children’s respiratory signs and symptoms for the preceding 7 days, enabling day-by-day reconstruction of episodes of ARI (Figure 2). If a child had cough or fever at the time of the visit or the previous day, WHO-IMCI danger signs were assessed (Figure 3). Surveillance forms similar to those used for this purpose had been used in other limited-resource settings and were shown to be valid tools for identifying pneumonia.32 Field workers were trained in the use of the forms, a comprehensive field-procedures manual including support algorithms for the coding of forms was developed, and regular group meetings were held for the review of forms.
Figure 2.
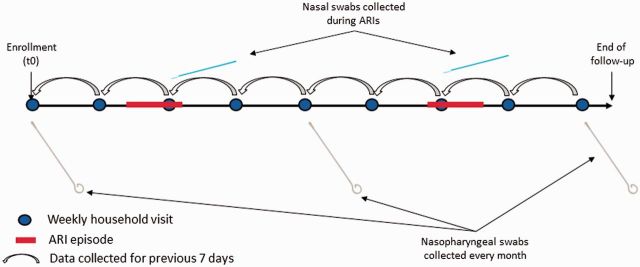
Household visits and respiratory sample collection strategy
Figure 3.
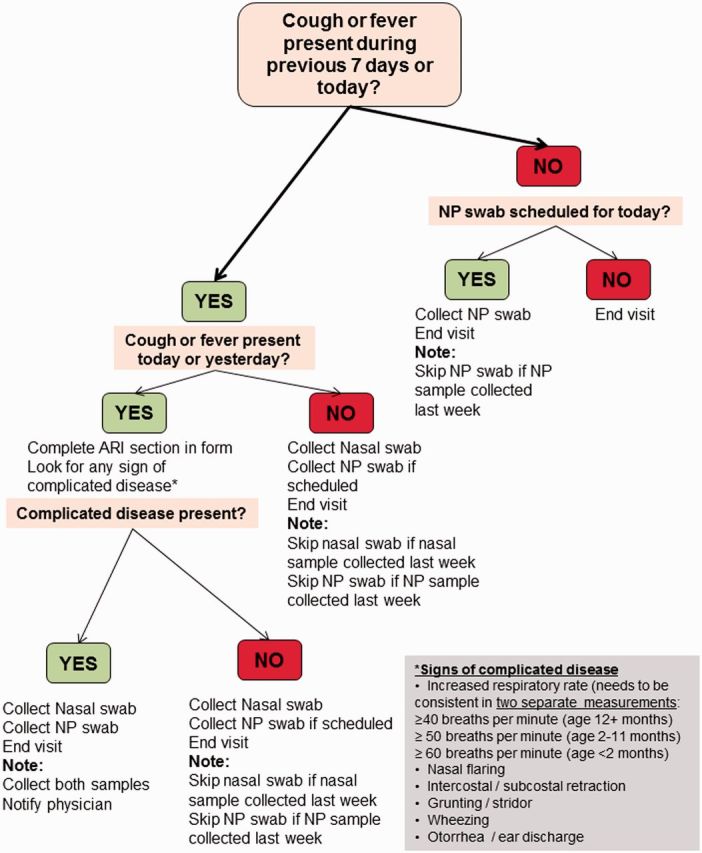
Algorithm for ARI assessment and sample collection. NP, nasopharyngeal
Field supervisors prepared visit schedules; reviewed study forms at the end of each day for data completeness; and assured prompt delivery of samples to the laboratory. Incomplete forms or inconsistent information necessitated return of the field worker or supervisor to the household. Furthermore, supervisors performed audits of a random subset of households to confirm the accuracy of the data collected. At the computer centre established in San Marcos, data from the study forms and collected samples were double entered by trained personnel. Weekly checks were done for data completeness and consistency.
Respiratory samples
Field workers were trained in standard procedures for collecting and transporting samples. The collection of nasal swabs followed procedures previously reported.33,34 In brief, one swab was placed into each nostril sequentially and rotated beneath the turbinates to collect epithelial cells and absorb secretions. The swab was then inserted into a tube with Remel M4RT viral transport medium and transported in envelopes with cold packs to the local research laboratory in San Marcos within 8 hours of sample collection. At the local research laboratory, two 800-µl aliquots were preserved as original samples and three 200-µl aliquots were preserved in lysis cryovials with 300-µl lysis buffer (MagNA Pure LC, Roche, Indianapolis, IN and MagMAX, Ambion, Grand Island, NY). All vials were labeled and stored at –70°C in the local research laboratory. Samples were sent to Vanderbilt University for identification of influenza viruses, respiratory syncytial virus, human metapneumovirus, rhinovirus, adenovirus, and parainfluenza viruses through real-time reverse transcription–polymerase chain reaction (RT–PCR) as previously described.34–37
Nasopharyngeal samples, collected monthly whether or not respiratory symptoms were present, and during complicated ARIs, were obtained according to WHO recommendations for identifying pneumococcal colonization.38 In brief, samples were collected with a deep nasopharyngeal Rayon polyester swab, and were immediately placed in a tube with 1 ml of transport medium (skim milk–tryptone–glucose–glycerine (STGG). The specimens were transported in envelopes with cold packs to the local laboratory within 8 hours of collection. Specimens were preserved with the swab in the medium at –70°C. Samples were sent to Emory University for identification of colonizing bacteria including S. pneumoniae, H. influenzae, and S. aureus. Laboratory studies included bacterial culture, phenotypic identification, molecular detection and quantification of bacterial load through quantitative PCR (qPCR), and pneumococcal serotyping through multiplex PCR as previously reported.39–41
Nasal swabs were collected for each new episode of ARI, defined as cough or fever present within the previous 7 days, including the visit date. Nasal swabs were not collected in consecutive weeks unless new manifestations of a complicated ARI developed. Thus, our approach considered a minimum 7-day window period to separate episodes of ARI. Detailed procedure manuals were provided to field workers. The flow of activities and the corresponding sample collection algorithm for field workers are shown in Figures 2 and 3.
Social contacts
Contact patterns are considered important determinants of the transmission of ARI.42 During designated visits by field workers, members of selected households were invited to record all contacts experienced from 6 am of the previous day through 6 am of the day of the recording. Contact information for children was provided by their parents. Field workers used a structured questionnaire previously used for assessments of social contacts in Europe42 and adapted after field-testing. For this assessment, a contact was classified as physical contact [skin-to-skin contact (i.e. a kiss or handshake)] or non-physical contact (conversation with another person who is physically present but with whom there is no skin-to-skin contact). Participants also provided information about the sex and age of the contact person(s). Each contact was further characterized by location, duration, and the estimated frequency of similar contacts.42 Collection of contact assessment data was completed between August and October 2011. A total of 114 families were included in this assessment, and contact questionnaires were completed for 96% of 588 eligible household members.
What has been found? Key findings and publications
From May 2009–September 2011, a total of 55 661 household visits were scheduled, 89% were executed (i.e. field workers reached their target households), and 79% were successfully completed (i.e. information was collected during the visit). A total of 4655 nasal and 10 722 nasopharyngeal swabs were collected. Collection of nasopharyngeal swabs was completed in 88% of scheduled collections.
During follow-up, there were 319 476 child-days of observation and an estimated incidence of ARI of 5 episodes per child-year. The median duration of episodes of ARI was 5 days, and approximately 1 in 12 ARIs present at the time of the household visit were complicated by the presence of WHO–IMCI danger signs. During episodes of ARI, cough (76%), fever (70%), and rhinorrhea (74%) were commonly present. Approximately 25% of episodes of ARI resulted in health care encounters and 1% resulted in hospitalization. Overall, 33% of children enrolled in the study received at least one dose of influenza vaccine and 62% received at least one dose of pneumococcal conjugate vaccine (Table 3).
Table 3.
Follow-up measurements
| ARI incidence | |
| Total child-days of observation | 319 476 |
| Number of ARIs | 4635 |
| Incidence of ARI per child-year | 5 |
| ARI symptoms | |
| Cough | 76% |
| Fever | 70% |
| Rhinorrhea | 74% |
| Complicated ARI | |
| Household visits with ARIs present at the visit or previous day | 2535 |
| IMCI–WHO danger signs present | 208 |
| Percent with complicated ARI | 8% |
| Health care encounters | |
| Health care encounters with ARI | 25% |
| Hospitalizations with ARI | 1% |
| Vaccination | |
| At least one dose of influenza vaccine | 33% |
| At least one dose of pneumococcal conjugate vaccine | 62% |
We also assessed the performance of qPCR relative to traditional bacterial culture for detecting colonizing S. pneumoniae, H. influenzae, and S. aureus in 446 nasopharyngeal samples collected from 360 healthy children during 2009. Concurrent bacterial colonization involving these 3 bacteria was also studied. Quantitative PCR detected more bacteria than did culture. A comparison of the results of culture and qPCR showed that 60.1% and 77.4%, respectively, of the swabs with the two techniques were positive for S. pneumoniae, 23.6% and 37.3% were positive for H. influenzae, and 11.9% and 40.8% were positive for S. aureus. There was a positive association between colonization with S. pneumoniae and H. influenzae both with culture (odds ratio [OR] 3.11) and qPCR (OR 1.95). The densities of S. pneumoniae and H. influenzae, as measured with qPCR, were positively correlated with one another (r = 0.32). A negative association was found between the presence of S. pneumoniae and that of S. aureus with both culture (OR 0.45) and qPCR (OR 0.61). Quantitative PCR improved the yield of nasopharyngeal samples from young children, especially at a density of <104 colony forming units (CFU)/ml, where culture was poorly sensitive. The effect of bacterial density on detection by culture, and the observed density-related interactions, support the use of qPCR in subsequent studies of ARI.43
What are the main strengths and weaknesses?
Strengths
Populations living in rural high-altitude communities other than those in San Marcos have characteristics similar to those of our study population, and the results of our study would be informative for those settings. Even though vaccination with influenza and pneumococcal conjugate vaccines is routine in the developed world, the long-term sustainability of routine vaccination with either or both of these vaccines is unclear in many developing countries. Our study will provide information on the preventive potential of these vaccines and will allow quantification of the effects of pneumococcal conjugate vaccines in preventing colonization in these settings.
In developing countries, access to health care is often limited by poverty or cultural barriers. In these settings, traditional studies based at health care centres might select relatively sicker or wealthier children and may not accurately assess the true disease burden in a particular area. Our study avoids these issues by using weekly household visits to assure a systematic and comprehensive assessment of all episodes of ARI.
Weaknesses
A limitation of our study is the lack of radiologic equipment in the study communities for identifying pneumonia. Such equipment is costly to acquire and maintain. Nevertheless, our study is designed to reflect the reality of numerous rural populations in which access to health care and radiologic examination is limited. We therefore followed WHO recommendations (i.e. IMCI–WHO protocols) for recognizing major causes of childhood disease in these settings.29–31 Although our field workers were trained in the assessment of respiratory signs and symptoms based on these protocols, some measurements may be subject to misclassification.
Another limitation of the study is the potential recall bias introduced during data collection. Although we performed intensive surveillance through weekly household visits, some studies have used more frequent home visits (e.g. twice per week or daily) in attempts to minimize recall issues. We considered using those alternatives, but they required a substantial increase in resources, making them prohibitive. Alternative surveillance visits made every 2 weeks may be affected by recall issues.32,44
Although influenza and pneumococcal conjugate vaccines were incorporated into the Peruvian infant vaccination schedule in 2009, routine vaccination has not been uniformly implemented in Peru. Vaccination with pneumococcal conjugate vaccine will have an effect on colonization with vaccine serotypes, and as vaccination coverage increases, our ability to assess the interaction between respiratory viruses and specific pneumococcal vaccine serotypes may be limited. Nevertheless, our study will allow us to assess the progressive use and timing of vaccination with influenza and pneumococcal conjugate vaccines, and the effects of the vaccination programs on pneumococcal colonization in this rural population.
Despite intensive weekly surveillance, our study may have missed some episodes of ARI. Parents sometimes took their sick children directly to the health care centre before the scheduled study visit, and did so without notifying the study personnel. Accordingly, we reviewed medical records of these enrollees to capture the relevant episodes of ARI, but were unable to collect specimens for microbial testing.
Although our project focused on the major respiratory pathogens, the relevance of other micro-organisms to public health is increasingly recognized. Additional resources will be needed to study other pathogens. Sample aliquots collected in our study will be preserved for future evaluations.
Can I get hold of the data? Where can I find out more?
We welcome initiatives to further explore the data made available by our study and/or to combine our data with other resources for additional studies. Investigators interested in more details about this study, or in accessing these resources, should contact Dr Carlos G. Grijalva at Vanderbilt University (carlos.grijalva@vanderbilt.edu).
Funding
The present study is supported by the Vanderbilt University CTSA grant UL1 RR024975-01 from the US National Institutes of Health, an investigator-initiated research grant IIR WS1898786(0887X1-4492) from Pfizer, and a grant from the Thrasher Research Fund (grant 02832-9).
Acknowledgements
We are indebted to the communities of San Marcos, Cajamarca, Peru for their participation in this study. We also appreciate the approval and continuous support of the Cajamarca Health Region authorities. Additionally, we are indebted to the field workers and field supervisors whose efforts in difficult geographical areas and harsh weather conditions allowed this study to be conducted. Dr. Carlos Grijalva reviewed the references in this paper for accuracy and completeness. He has full access to the study data and takes responsibility for the integrity of the data and the accuracy of this report.
Conflict of interest: C.G.G. received research support for the study from Pfizer and has served as consultant for Glaxo–Smith-Kline. J.V.W. serves on the Scientific Advisory Board of Quidel. K.P.K. has received research support from Pfizer and has provided consultancy to Pfizer and Glaxo–Smith-Kline.
KEY MESSAGES.
Young Andean children had an average of 5 ARI episodes per year. This incidence is similar to the 3-6 ARIs per year usually described for children aged <5 years without regard to country of residency or socioeconomic status.
Approximately 1 in 12 children with an ARI present at the time of the household visit had ‘danger signs’ of severe disease [according to definitions from the World Health Organization-Integrated Management of Childhood Illnesses (WHO-IMCI)].
Over 75% of children carried Streptococcus pneumoniae in their nasopharynx.
References
- 1.Williams BG, Gouws E, Boschi-Pinto C, Bryce J, Dye C. Estimates of worldwide distribution of child deaths from acute respiratory infections. Lancet Infect Dis. 2002;2:25–32. doi: 10.1016/s1473-3099(01)00170-0. [DOI] [PubMed] [Google Scholar]
- 2. Jamison DT, Breman JG, Measham AR. Disease Control Priorities in Developing Countries, 2nd edn. Washington DC: Oxford University Press and The World Bank. Available from http://www.dcp2.org/pubs/DCP (5 November 2012, date last accessed)
- 3.Liu L, Johnson HL, Cousens S, et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379:2151–61. doi: 10.1016/S0140-6736(12)60560-1. [DOI] [PubMed] [Google Scholar]
- 4.Mulholland K. Childhood pneumonia mortality—a permanent global emergency. Lancet. 2007;370:285–89. doi: 10.1016/S0140-6736(07)61130-1. [DOI] [PubMed] [Google Scholar]
- 5.Pneumococcal conjugate vaccine for childhood immunization—WHO position paper. Wkly Epidemiol Rec. 2007;82:93–104. [PubMed] [Google Scholar]
- 6.Smith KR, McCracken JP, Weber MW, et al. Effect of reduction in household air pollution on childhood pneumonia in Guatemala (RESPIRE): a randomised controlled trial. Lancet. 2011;378:1717–26. doi: 10.1016/S0140-6736(11)60921-5. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization. Indoor air pollution and health. Available from http://www.who.int/mediacentre/factsheets/fs292/en/index.html (12 November 2012, date last accessed) [Google Scholar]
- 8.O'Brien KL, Dagan R, Makela PH. Nasopharyngeal carriage. In: Siber GR, Klugman KP, Makela PH, editors. Pneumococcal Vaccines. Washington, DC: ASM Press; 2008. pp. 279–300. [Google Scholar]
- 9.Gray BM, Converse GM, III, Dillon HC., Jr Epidemiologic studies of Streptococcus pneumoniae in infants: acquisition, carriage, and infection during the first 24 months of life. J Infect Dis. 1980;142:923–33. doi: 10.1093/infdis/142.6.923. [DOI] [PubMed] [Google Scholar]
- 10.Deaths related to 2009 pandemic influenza A (H1N1) among American Indian/Alaska Natives—12 states, 2009. MMWR Morb Mortal Wkly Rep. 2009;58:1341–44. [PubMed] [Google Scholar]
- 11.Weinberger DM, Simonsen L, Jordan R, et al. Impact of the 2009 influenza pandemic on pneumococcal pneumonia hospitalizations in the United States. J Infect Dis. 2012;205:458–65. doi: 10.1093/infdis/jir749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Michelow IC, Olsen K, Lozano J, et al. Epidemiology and clinical characteristics of community-acquired pneumonia in hospitalized children. Pediatrics. 2004;113:701–07. doi: 10.1542/peds.113.4.701. [DOI] [PubMed] [Google Scholar]
- 13.Juven T, Mertsola J, Waris M, et al. Etiology of community-acquired pneumonia in 254 hospitalized children. Pediatr Infect Dis J. 2000;19:293–98. doi: 10.1097/00006454-200004000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Madhi SA, Klugman KP. A role for Streptococcus pneumoniae in virus-associated pneumonia. NatMed. 2004;10:811–13. doi: 10.1038/nm1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Madhi SA, Ludewick H, Kuwanda L, et al. Pneumococcal coinfection with human metapneumovirus. J Infect Dis. 2006;193:1236–43. doi: 10.1086/503053. [DOI] [PubMed] [Google Scholar]
- 16.Simoes EAF, Nohynek H, Lucero M, et al. International Symposium on Pneumococci and Pneumococcal Diseases (ISPPD) Efficacy of an 11-valent pneumococcal conjugate vaccine (11-PCV) against severe and non-severe viral lower respiratory infection (LRI), in Filipino infants. Abstract book, p. 71 (Shift I, poster # 80). Israel: Tel Aviv, 14–18 March 2010. [Google Scholar]
- 17.Berendt RF, Long GG, Walker JS. Influenza alone and in sequence with pneumonia due to Streptococcus pneumoniae in the squirrel monkey. J Infect Dis. 1975;132:689–93. doi: 10.1093/infdis/132.6.689. [DOI] [PubMed] [Google Scholar]
- 18.Peltola VT, McCullers JA. Respiratory viruses predisposing to bacterial infections: role of neuraminidase. Pediatr Infect Dis J. 2004;23:S87–97. doi: 10.1097/01.inf.0000108197.81270.35. [DOI] [PubMed] [Google Scholar]
- 19.McCullers JA. Insights into the interaction between influenza virus and pneumococcus. Clin Microbiol Rev. 2006;19:571–82. doi: 10.1128/CMR.00058-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gerone PJ, Ward TG, Chappell WA. Combined infections in mice with influenza virus and Diplococcus pneumoniae. Am J Hyg. 1957;66:331–41. doi: 10.1093/oxfordjournals.aje.a119906. [DOI] [PubMed] [Google Scholar]
- 21.Sun K, Metzger DW. Inhibition of pulmonary antibacterial defense by interferon-gamma during recovery from influenza infection. Nat Med. 2008;14:558–64. doi: 10.1038/nm1765. [DOI] [PubMed] [Google Scholar]
- 22.McCullers JA, McAuley JL, Browall S, Iverson AR, Boyd KL, Henriques Normark B. Influenza enhances susceptibility to natural acquisition of and disease due to Streptococcus pneumoniae in ferrets. J Infect Dis. 2010;202:1287–95. doi: 10.1086/656333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wadowsky RM, Mietzner SM, Skoner DP, Doyle WJ, Fireman P. Effect of experimental influenza A virus infection on isolation of Streptococcus pneumoniae and other aerobic bacteria from the oropharynges of allergic and nonallergic adult subjects. Infect Immun. 1995;63:1153–57. doi: 10.1128/iai.63.4.1153-1157.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Madhi SA, Violari A, Klugman KP, et al. Inferior quantitative and qualitative immune responses to pneumococcal conjugate vaccine in infants with nasopharyngeal colonization by Streptococcus pneumoniae during the primary series of immunization. Vaccine. 2011;29:6994–7001. doi: 10.1016/j.vaccine.2011.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vakevainen M, Soininen A, Lucero M, et al. Serotype-specific hyporesponsiveness to pneumococcal conjugate vaccine in infants carrying pneumococcus at the time of vaccination. J Pediatr. 2010;157:778–83.e1. doi: 10.1016/j.jpeds.2010.04.071. [DOI] [PubMed] [Google Scholar]
- 26.Dagan R, Givon-Lavi N, Greenberg D, Fritzell B, Siegrist CA. Nasopharyngeal carriage of Streptococcus pneumoniae shortly before vaccination with a pneumococcal conjugate vaccine causes serotype-specific hyporesponsiveness in early infancy. J Infect Dis. 2010;201:1570–79. doi: 10.1086/652006. [DOI] [PubMed] [Google Scholar]
- 27.Hartinger SM, Lanata CF, Hattendorf J, et al. A community randomised controlled trial evaluating a home-based environmental intervention package of improved stoves, solar water disinfection and kitchen sinks in rural Peru: rationale, trial design and baseline findings. Contemp Clin Trials. 2011;32:864–73. doi: 10.1016/j.cct.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 28. Instituto Nacional de Estadística e Informática INEI. www.inei.gob.pe (22 April 2011, date last accessed)
- 29.Integrated management of childhood illness: conclusions. WHO Division of Child Health and Development. Bull World Health Organ. 1997;75:119–28. [PMC free article] [PubMed] [Google Scholar]
- 30.Gove S. Integrated management of childhood illness by outpatient health workers: technical basis and overview. The WHO Working Group on Guidelines for Integrated Management of the Sick Child. Bull World Health Organ. 1997;75:7–24. [PMC free article] [PubMed] [Google Scholar]
- 31.Lambrechts T, Bryce J, Orinda V. Integrated management of childhood illness: a summary of first experiences. Bull World Health Organ. 1999;77:582–94. [PMC free article] [PubMed] [Google Scholar]
- 32.Lanata CF, Quintanilla N, Verastegui HA. Validity of a respiratory questionnaire to identify pneumonia in children in Lima, Peru. Int J Epidemiol. 1994;23:827–34. doi: 10.1093/ije/23.4.827. [DOI] [PubMed] [Google Scholar]
- 33.Griffin MR, Walker FJ, Iwane MK, Weinberg GA, Staat MA, Erdman DD. Epidemiology of respiratory infections in young children: insights from the new vaccine surveillance network. Pediatr Infect Dis J. 2004;23:S188–92. doi: 10.1097/01.inf.0000144660.53024.64. [DOI] [PubMed] [Google Scholar]
- 34.Poehling KA, Edwards KM, Weinberg GA, et al. The underrecognized burden of influenza in young children. N Engl J Med. 2006;355:31–40. doi: 10.1056/NEJMoa054869. [DOI] [PubMed] [Google Scholar]
- 35.Lu X, Holloway B, Dare RK, et al. Real-time reverse transcription-PCR assay for comprehensive detection of human rhinoviruses. J Clin Microbiol. 2008;46:533–39. doi: 10.1128/JCM.01739-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kodani M, Yang G, Conklin LM, et al. Application of TaqMan low-density arrays for simultaneous detection of multiple respiratory pathogens. J Clin Microbiol. 2011;49:2175–82. doi: 10.1128/JCM.02270-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klemenc J, Asad Ali S, Johnson M, et al. Real-time reverse transcriptase PCR assay for improved detection of human metapneumovirus. J Clin Virol. 2012;54:371–75. doi: 10.1016/j.jcv.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O'Brien KL, Nohynek H. Report from a WHO working group: standard method for detecting upper respiratory carriage of Streptococcus pneumoniae. Pediatr Infect Dis J. 2003;22:133–40. doi: 10.1097/01.inf.0000048676.93549.d1. [DOI] [PubMed] [Google Scholar]
- 39.Albrich WC, Madhi SA, Adrian PV, et al. Use of a rapid test of pneumococcal colonization density to diagnose pneumococcal pneumonia. Clin Infect Dis. 2012;54:601–09. doi: 10.1093/cid/cir859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vernet G, Saha S, Satzke C, et al. Laboratory-based diagnosis of pneumococcal pneumonia: state of the art and unmet needs. Clin Microbiol Infect. 2011;17:1–13. doi: 10.1111/j.1469-0691.2011.03496.x. [DOI] [PubMed] [Google Scholar]
- 41.Klugman KP, Madhi SA, Albrich WC. Novel approaches to the identification of Streptococcus pneumoniae as the cause of community-acquired pneumonia. Clin Infect Dis. 2008;47:S202–06. doi: 10.1086/591405. [DOI] [PubMed] [Google Scholar]
- 42.Mossong J, Hens N, Jit M, et al. Social contacts and mixing patterns relevant to the spread of infectious diseases. PLoS Med. 2008;5:e74. doi: 10.1371/journal.pmed.0050074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chien YW, Vidal JE, Grijalva CG, et al. Density interactions between Streptococcus pneumoniae, Haemophilus influenzae and Staphylococcus aureus in the nasopharynx of young Peruvian children. Pediatr Infect Dis J. 2013;32:72–77. doi: 10.1097/INF.0b013e318270d850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Feikin DR, Audi A, Olack B, et al. Evaluation of the optimal recall period for disease symptoms in home-based morbidity surveillance in rural and urban Kenya. Int J Epidemiol. 2010;39:450–58. doi: 10.1093/ije/dyp374. [DOI] [PMC free article] [PubMed] [Google Scholar]


