Abstract
Background: Chinese women’s reproductive patterns have changed significantly over the past several decades. However, relatively little is known about the pace and characteristics of these changes either overall or by region and socioeconomic status.
Methods: We examined the cross-sectional data from the China Kadoorie Biobank cohort study that recruited 300 000 women born between 1930 and 1974 (mean age: 51 years) from 10 socially diverse urban and rural regions of China. Temporal trends in several self-reported reproductive characteristics, and effect modification of these trends by area and education (as a surrogate for socioeconomic status), were examined.
Results: The overall mean age at menarche was 15.4 (standard deviation 1.9) years, but decreased steadily over the 45 birth cohorts from 16.1 to 14.3 years, except for an anomalous increase of ∼1 year for women exposed to the 1958-61 famine in early adolescence. Similarly large changes were seen for other characteristics: mean parity fell (urban: 4.9 to 1.1; rural: 5.9 to 1.4); mean age at first birth increased (urban: 19.0 to 25.9 years; rural: 18.3 to 23.8 years); and birth spacing increased after 1980 to over 5 years. Breastfeeding declined after 1950 in urban and, after 1980, in rural women; and 68% of urban and 48% of rural women experienced a terminated pregnancy. Mean age at menopause increased from 47.9 to 49.3 years.
Conclusions There have been striking changes in reproductive factors over time and between areas among these Chinese women. Their effects on major chronic diseases should be investigated.
Keywords: Reproductive, temporal trends, China, cohort study
Key Messages.
The reproductive patterns of Chinese women have changed significantly over the past several decades, partly or wholly due to the major social and economic changes that have occurred in China.
Mean age at menarche decreased by approximately 2 years between women born in 1930 and those born in 1974.
Women who experienced the 1958-61 famine around puberty had, on average, a 1-year delay in menarche, with somewhat greater effect among the rural and the least educated women. In contrast, there was no interruption in the downward trend for women who experienced famine around the time of birth (either in utero or in the first few months of life).
Parity fell steadily from 1950 (as in other surrounding countries), with no obvious inflection after the introduction of the one-child policy, suggesting that fertility rates may have eventually fallen to below replacement level without strict fertility control, particularly in some urban areas.
The main effect of the one-child policy seems to have been to increase the mean age at first birth, especially in urban areas, and there was a sudden increase in the spacing between first and second births after its introduction.
Introduction
The reproductive patterns of Chinese women have changed significantly over the past several decades, partly or wholly due to the major social and economic changes that have occurred in China.1,2 These changes include: the introduction of a strict family planning policy (the so-called ‘one-child’ policy); recent rapid urbanization and economic development; and periodic social upheavals (e.g. wars, famines). However, the pace and characteristics of these changes—either overall or by region and socioeconomic status—have not been properly investigated, nor have the potential long-term consequences of these changes on major chronic diseases.
In China, a strict family planning policy was introduced during the late 1970s, which not only restricts the number of children a couple can have (usually one in urban and up to two in rural areas) but also encourages late marriage and childbirth. The seemingly large and profound impact on reproductive patterns of Chinese women has not always been considered in the context of other rapid social and demographic changes seen since the 1950s.1
Studies from predominantly Western populations have shown that reproductive characteristics change as countries become more affluent and better educated.3 As a result of improved nutrition, the mean age at menarche falls and at menopause rises,4 and as women become more educated, the fertility rate declines5 as well as the likelihood and duration of breast feeding.6
There is evidence from the West that exposure to famine can cause a sharp, but temporary, increase in mean age at menarche,7 modestly decreased reproductive function8 and a small decrease in age at natural menopause.9 However, there are only limited Chinese data on the effects of famine on reproductive factors.10
Although there have been reports of temporal changes in reproductive characteristics among Chinese women,1,2 they have been small, from limited geographical areas or were conducted many years ago, and have been unable to assess reliably whether the pace of change was affected by urbanization and socioeconomic status. We report data on 300 000 women from the China Kadoorie Biobank study (CKB),11 who were recruited during 2004-08 from 10 socially diverse areas of China. These women were born between 1925 and 1978 and so experienced the major social and economic changes described during their reproductive lives (1950s to 1990s). We aim to examine the temporal trend of reproductive characteristics both overall and by urbanization, and to assess the impact of major socioeconomical factors on any such trends.
Methods
The CKB study is a blood-based prospective cohort study of 0.5 million Chinese adults. Detailed information about the study design and procedures has been reported previously.11,12 Briefly, the baseline survey took place from 2004 to 2008 in 10 geographically defined areas of China (eFigure 1, available as Supplementary data at IJE online). At the baseline survey, socio-demographic, lifestyle and medical data were collected. Women were asked about their reproductive history, including age at menarche and menopause, gravidity and parity, duration of breastfeeding for each live birth, use of oral contraceptive pills and history of hysterectomy, ovarian or breast surgery. All participants are being followed up for cause-specific morbidity and mortality but only the baseline data have been used for this report to perform a retrospective analysis of the women’s reproductive histories.
Ethical approval was obtained from the ethical review committee of the Chinese Center for Disease Control and Prevention, Beijing, China and the Oxford Tropical Research Ethics Committee, University of Oxford, UK. All study participants provided written informed consent.
Statistical methods
Only women with known age at birth, known educational history and the relevant reproductive history data were included in these analyses. Analyses of childbearing characteristics were further restricted to women aged 35 years or over, of whom more than 95% had finished child bearing. Only women with at least one live birth were included in analyses of age at first birth and breastfeeding, and only women with at least two live births were included in analyses of time between first and second births. Analyses of menopause included only women aged 57 years or over (of whom 99% reported being post-menopausal) and who had experienced natural menopause. For analyses of breastfeeding, the average duration of breastfeeding per baby was calculated for each woman. For analyses of abortion (termination of pregnancy or miscarriage), the rate is the percentage of women reporting at least one abortion. The abortion rates differed greatly in Gansu (a rural area in central China: eFigure 1, available as Supplementary data at IJE online) from the other regions (see Results), and so have been presented separately.
To describe the population, crude distributions of age, education and the reproductive factors were calculated separately for women in urban and rural areas, and overall. To assess temporal trends, area-adjusted means of each reproductive factor were calculated by year-of-birth of the woman or by year-of-birth of the first child, whichever seemed most appropriate, and plotted as a 3-year moving average. Years with fewer than 100 women were not plotted because the results were too unreliable.
Women with inconsistent data on any reproductive factor (n = 166) were removed from the dataset and, to avoid extreme values (which may or may not have been data entry errors) skewing the results, women in the top and bottom 0.1% of values for any reproductive variable within a 5-year age band were also excluded (n = 1917). No attempt was made to impute values for this 0.7% of excluded data. All analyses were performed using SAS version 9.2.
Results
Of 302 669 women recruited into the study, 300 586 had all the data required to include them in the analyses, and the main demographic and reproductive characteristics of these women are given in Table 1 (and, for each of the 10 areas, in eTable 1 available as Supplementary data at IJE online). The mean [standard deviation (SD)] baseline age was 51 (11) years and the mean year of birth was 1955. Urban and younger women tended to be better educated: one-third of all urban women completed high school, whereas only 6% of all rural women did so (one-third had no formal education at all). Over 99% of the women had ever been married, with 9% widowed and 2% divorced at the time of the baseline survey. 88% of the women reported experiencing menarche between ages 13 to 18, with mean (SD) of 15.4 (1.9) years. Almost all the women (99%) had experienced at least one pregnancy and >99% of those had at least one live birth. Urban women had fewer children on average and the mean age at first birth was older than for rural women (Table 1; both P <0.0001). Most women had breastfed at least one of their babies (97%), with 93% of them breastfeeding each baby for more than 6 months, on average. Among women who had breastfed, rural women breastfed for an average 4 months longer than urban women (16.4 vs 12.2 months, P <0.0001). Excluding Gansu, 58% of women had experienced terminated pregnancy (68% urban, 48% rural), whereas 8% of women had experienced a miscarriage (6% urban, 10% rural). The pattern in Gansu had very different rates: only 3% of women had experienced termination of pregnancy whereas 16% had miscarried. The mean (SD) age at menopause was 48.2 (4.3) years among the 83 791 women who were naturally post-menopausal at the time of interview, and was slightly higher in urban than rural women (48.5 vs 48.0 years, P <0.0001). These crude analyses, however, disguise temporal trends in all reproductive factors.
Table 1.
Demographic and reproductive characteristics
| Characteristics | Urban | Rural | Overall |
|---|---|---|---|
| Number of women | 134 173 | 166 413 | 300 586 |
| Age (years) | |||
| Mean (SD) | 52.1 (10.7) | 50.0 (10.2) | 50.9 (10.5) |
| Birth cohort (%) | |||
| <1930 | <1 | <1 | <1 |
| 1930–1939 | 13 | 8 | 10 |
| 1940–1949 | 21 | 20 | 21 |
| 1950–1959 | 32 | 32 | 32 |
| 1960–1969 | 28 | 34 | 31 |
| >= 1970 | 5 | 6 | 6 |
| Mean (SD) | 1954 (10.7) | 1956 (10.2) | 1955 (10.5) |
| Highest education (%) | |||
| No formal school | 17 | 32 | 25 |
| Primary school | 20 | 40 | 31 |
| Middle school | 30 | 22 | 25 |
| High school | 23 | 6 | 14 |
| University/College | 9 | <1 | 4 |
| Age at menarche (%) | |||
| <13 | 6 | 5 | 5 |
| 13–14 | 30 | 26 | 28 |
| 15–16 | 36 | 38 | 37 |
| 17–18 | 22 | 25 | 23 |
| > = 19 | 5 | 6 | 6 |
| Mean (SD) | 15.3 (2.0) | 15.5 (1.9) | 15.4 (1.9) |
| Number with at least one pregnancy | 132 456 | 165 333 | 297 789 |
| (excluding Gansu) | – | 135 188 | 267 644 |
| Number of live births (%) | |||
| 0 | <1 | <1 | <1 |
| 1 | 52 | 21 | 35 |
| 2 | 24 | 39 | 32 |
| 3–4 | 20 | 31 | 26 |
| > = 5 | 4 | 9 | 7 |
| Mean (SD) | 1.9 (1.2) | 2.5 (1.4) | 2.2 (1.3) |
| Median (Q1, Q3) | 1 (1, 2) | 2 (2, 3) | 2 (1, 3) |
| History of abortiona | |||
| No history of abortion | 29 | 45 | 37 |
| History of induced abortionb | 68 | 48 | 58 |
| History of spontaneous abortionb | 6 | 10 | 8 |
| Number with at least one live birth | 131 613 | 165 006 | 296 619 |
| Age at first birth (%) | |||
| <20 | 4 | 13 | 9 |
| 20–21 | 11 | 27 | 20 |
| 22–23 | 19 | 29 | 24 |
| 24–25 | 28 | 21 | 24 |
| > = 26 | 37 | 10 | 22 |
| Mean (SD) | 24.7 (3.3) | 22.3 (2.7) | 23.4 (3.2) |
| Average duration of breastfeeding (%) | |||
| Never breastfed | 5 | 1 | 3 |
| 1 – 6 months | 9 | 4 | 7 |
| 7 – 12 months | 59 | 37 | 47 |
| 13 – 18 months | 17 | 26 | 22 |
| 19 – 24 months | 7 | 21 | 15 |
| > 24 months | 3 | 10 | 7 |
| Mean (SD) | 12.2 (6.4) | 16.4 (7.9) | 14.5 (7.6) |
| Median (Q1, Q3) | 12 (10, 13) | 15 (12, 21) | 12 (11, 18) |
| Age at menopause (years)c | |||
| Number of women | 41 190 | 42 601 | 83 791 |
| <43 | 8 | 11 | 9 |
| 43–47 | 22 | 24 | 23 |
| 48–53 | 58 | 54 | 56 |
| >= 54 | 12 | 11 | 12 |
| Mean (SD) | 48.5 (4.3) | 48.0 (4.4) | 48.2 (4.3) |
aExcludes Gansu because of very different rates (81% no abortion; 3% termination of pregnancy; 16% miscarriage).
bSome women will have experienced both termination of pregnancy and miscarriage.
cExcludes women <57 years old or who had surgical menopause (hysterectomy, oophorectomy) or who had a daiagnosis of cancer.
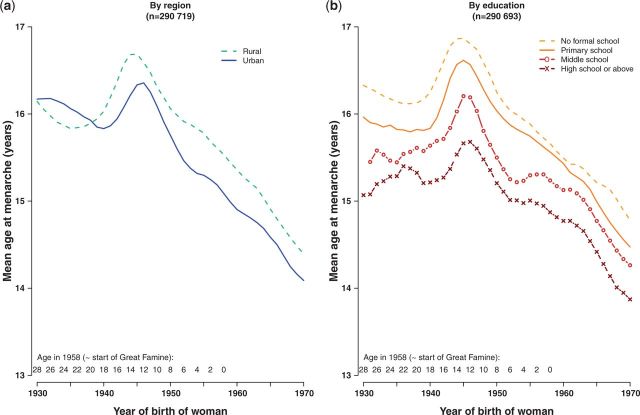
Figure 1 shows the temporal trend for mean age at menarche for women born 1930–70, by area and by highest level of education. Overall, mean age at menarche decreased from 16.1 to 14.3 years over 40 years. Although the downward trend was predominantly linear, there was an anomalous increase among women born around 1940–50, who were aged 8–18 years at the approximate start of the famines in 1958–61. The deviation in the temporal trend began earlier and lasted for longer in rural compared with urban women (Figure 1a). Education was a strong determinant of age at menarche, with the mean age at menarche decreasing with increasing level of education in every birth cohort (Figure 1b). Moreover, among the more educated women the anomalous increase due to famine was later and less extreme. This effect of education was more marked among urban women than among rural women (eFigure 2, available as Supplementary data at IJE online).
Figure 1.
Time trends in mean age at menarche (a) by region and (b) by highest level of education
Temporal trends in characteristics associated with childbearing are shown in Figure 2. In both urban and rural women, parity fell dramatically from 1950 onwards. These decreases were continuous with no sudden falls or plateaus until, among urban women, parity reached almost one (Figure 2a). In urban areas, higher education was associated with lower parity: in 1950, urban women with no formal education had two more children, on average, than women educated to high school or above; but this effect of education was greatly reduced by 1980 (eFigure 3, available as Supplementary data at IJE online). By contrast, there was no difference by education among rural women. Mean age at first birth increased over time, at least up to 1980, after which the mean age at first birth decreased again (Figure 2b). For urban women, this increase was steady and continuous over the 30-year period (from 19.0 to 25.9 years), but there was some fluctuation among rural women, with a small fall for first births in 1962–66, just after the end of the famine period. From 1980-85, there was a significant drop of about 1 year in the mean age at first birth among both urban and rural women, before it started to rise again. The peak occurred earlier and the fall was less extreme among the most highly educated urban women, whereas in rural women, education had less effect on the trend (eFigure 4, available as Supplementary data at IJE online).
Figure 2.
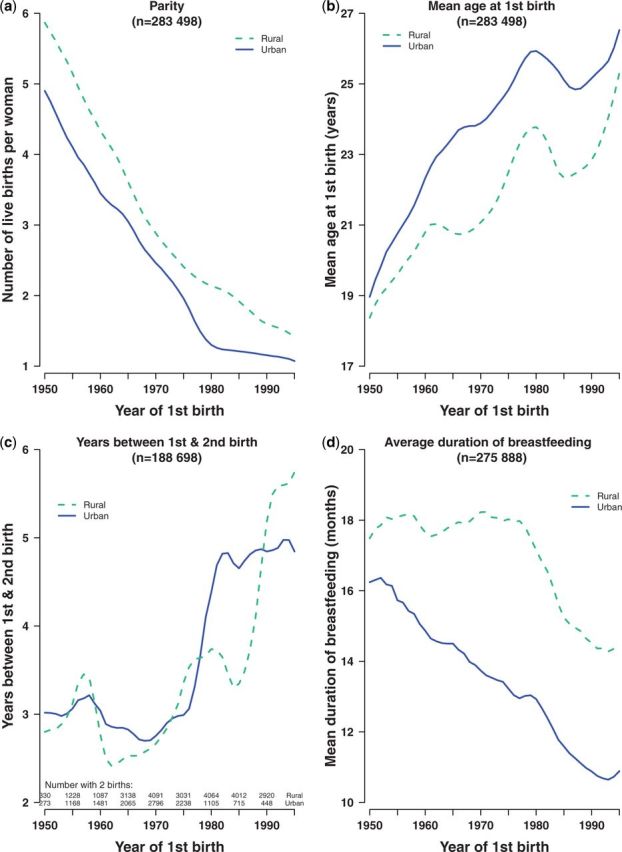
Time trends in reproductive characteristics, by region. (a) Parity, (b) mean age at first birth, (c) years between first and second birth and (d) mean duration of breastfeeding [All among women aged 35 years or over. (a) and (b): among women with at least one live birth; (c) among women with at least two live births; (d) among women who had ever breastfed]
Birth spacing showed a more unusual pattern (Figure 2c). Among urban mothers whose first birth was before 1975, there were just under 3 years, on average, between first and second babies, although there was a small temporary increase during the late 1950s. After 1975, the gap leapt rapidly to almost 5 years for women having their first child in 1982. This increase was greatest among the most highly educated urban women (to over 6 years between births: eFigure 5, available as Supplementary data at IJE online). The trend was more erratic among rural women: birth spacing eventually increased to a mean of 5.5 years regardless of education (eFigure 5, available as Supplementary data at IJE online).
Breastfeeding was more common and lasted longer in rural than in urban areas (Figure 2d). For urban women, the mean duration of breastfeeding fell steadily from 16 to 11 months. In rural areas, the mean lactation period was 18 months up to 1980, then dropped rapidly to 14 months. In both urban and rural areas, women who had completed high school were less likely to breastfeed than those with no formal schooling (94% vs 99%: data not shown) and, among those who did breastfeed, there was an inverse association with level of education, which diminished with time (eFigure 6, available as Supplementary data at IJE online).
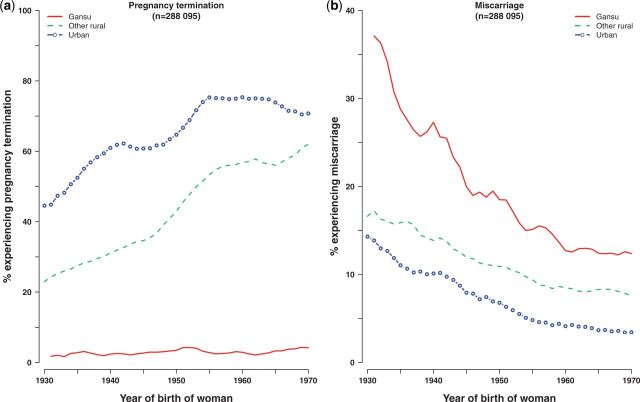
Figure 3 shows termination and miscarriage rates for all urban, Gansu and all other rural areas. Termination of pregnancy was far higher in urban than in rural areas for every year of birth, and the rate increased with year of birth from 45% to 71% among urban and from 23% to 62% among rural women (Figure 3a). By contrast, miscarriage fell from 14% to 3% among urban and 17% to 8% among rural women. In Gansu, termination rates were consistently very low (<5%) whereas miscarriage rates fell from 37% to 12%.
Figure 3.
Time trends in termination of pregnancy and miscarriage, by region. Percentage experiencing event = number of women with at least one such event/total number of women
Age at menopause rose steadily in both urban and rural women, increasing by 11 [standard error (SE) 0.3] months for every 10-years-later year of the woman’s birth (i.e. from 47.9 to 49.3 years) but, across all birth cohorts, urban women experienced menopause on average 7 months later than rural women (Figure 4a). There was some association between education and age at menopause, with the most highly educated women experiencing menopause on average 1 year later than those with no education (Figure 4b).
Figure 4.
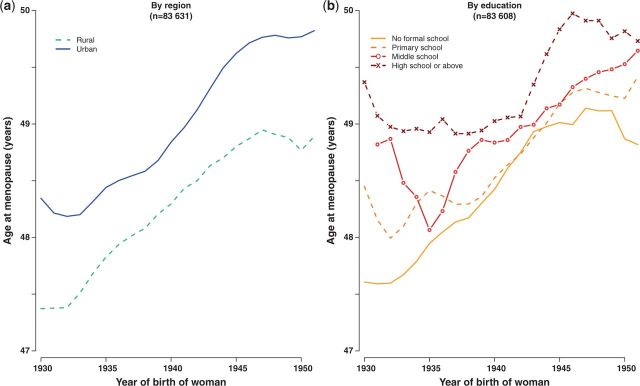
Time trends in mean age at menopause (a) by region and (b) by highest level of education
Discussion
In this study of 300 000 Chinese women born between 1930 and 1974 in 10 diverse regions of China, we observed some intriguing patterns over time and between social groups in all of the reproductive factors studied. On average, a woman in this study experienced menarche at 15 years of age, had two children (with the first live birth at 23 years) who were each breastfed for a mean of 14 months, and completed menopause at 48 years, giving her 33 years of reproductive life. However, this summary disguises significant differences between urban and rural women, by education and, more strikingly, changes over time.
Mean age at menarche decreased by approximately 2 years over the 45-year birth cohorts. This downward trend has been observed in many countries during the 20th century, but the pace and degree of the changes observed in these Chinese women are greater than in the UK or USA13 although comparable to Japan.14 Most striking, however, was the 1-year delay in menarche among the women who experienced famine around puberty. Although this peripubertal effect of famine on menarche has been observed in other studies,7,14–16 the women were drawn from small geographical areas and so it was not possible to investigate the effect of famine under different social and economic circumstances. In our study, the effect of famine on menarche was greater among the rural and the least educated women: the famine hit the urban areas later and was less severe, so it is perhaps not surprising that the delay in menarche occurred later and was less severe there. The dampening effect of education (as a proxy for social class) suggests that the more highly educated women were somewhat protected from the effects of famine. There was no interruption in the downward trend for women who experienced famine around the time of birth (either in utero or in the first few months of life). This is concordant with the Dutch famine birth cohort17 which also found no apparent association between perinatal famine exposure and age at menarche. It is not possible in a Chinese population to disentangle the long-term effects of pre-menarcheal famine (either around menarche or in utero) on reproductive function from the changes in other social conditions that occurred after the famine. Women who underwent puberty during the famine were having their first babies in the late 1960s, and there is no good evidence from these data to suggest that they suffered any long-term reproductive consequences of prepubescent famine. Women who were born during the famine were having their first babies during the 1980s, and so trying to disentangle any potential effects of in utero or neonatal famine from those of the fertility control measures imposed during the 1980s1 is virtually impossible.
Though not nationally representative, our findings suggest that the reproductive behaviour of these Chinese women was already changing well before the introduction of the one-child policy, probably due to China’s economic development and improved education.12 Parity (which is approximately equivalent to the fertility rate among these women, since 99% of them had at least one live birth) fell steadily from 1950, with no obvious inflection after the one-child policy was introduced, suggesting that fertility rates may have eventually fallen to below replacement level without strict fertility control, particularly in some urban areas. Indeed, during the latter half of the 20th century, fertility rates also fell in the neighbouring countries, so that by the end of the 20th century their total fertility rates were lower than in China: official data from the United Nations show that China’s total fertility rate was just over 1.8, compared with 1.1 in Hong Kong, 1.6 in Singapore and South Korea and 1.4 in Taiwan.18
As in high-income countries,19 these data show that mean age at first birth has increased significantly in China. The greater increase in mean age at first birth among urban women compared with rural women reflects the greater economic and social changes that have occurred as well as stricter implementation of the family planning policy in urban China. However, there was an anomalous fall in age for both urban and rural women having their first babies in the 1980s (that is, around the introduction of the one-child policy). This fall in mean age at first birth is intriguing and has not been reported elsewhere. Birth spacing shows the most striking pattern and is the one aspect of childbearing that appears to have been affected by both the famine and the fertility control policies. In both urban and rural women, there was a temporary rise and fall in the spacing between first and second births in the late 1950s, but more striking is the sudden increase among urban women at the end of the 1970s; the increase among rural women was less extreme and occurred about a decade later. This is in keeping with the greater enforcement of the family planning policies in urban areas.20 Finally, both the incidence and the duration of breastfeeding fell with time, in common with other studies observing this pattern as women become more economically active and better educated.6
In our study, the younger and more educated women experienced menopause later. There is no previous information from large-scale population-based studies in China, but limited data from developed countries suggest that the mean age at menopause seems to have either remained stable or increased slightly over the 20th century.21,22
The quality of the data collected in CKB is excellent, with very little missing information and good reproducibility.23 Moreover, any misreporting is likely to be random,24 which would attenuate any trends.25
The striking variations and rapid changes in Chinese women’s reproductive characteristics demonstrated by this large study may well have long-term consequences on major chronic diseases. Several smaller studies have shown associations between reproductive factors—such as age at menarche, age at first birth, breastfeeding and age at menopause—and risk of breast cancer.14,26,27 More controversially, gender differences in cardiovascular disease rates2 have prompted hypotheses that endogenous estrogen may play a role.28,29 However, few of these studies have been large enough on their own to quantify reliably the relevance of the major reproductive characteristics described here to risk, and there is extremely limited prospective evidence from China. With over 300 000 women and established linkages with mortality registries and coded hospitalization episodes,12 the CKB should soon be able to provide large-scale prospective evidence about the effects of reproductive characteristics on the risk of major chronic diseases in Chinese women.
Supplementary Data
Supplementary data are available at IJE online.
Funding
The baseline survey and the first re-survey were supported by a research grant from the Kadoorie Charitable Foundation in Hong Kong. The long-term continuation of the project during 2009–14 is supported by programme grants from the Wellcome Trust in the UK (088158/Z/09/Z] and the Chinese Ministry of Science and Technology (2011BAI09B01). The UK Medical Research Council, the British Heart Foundation (BHF) and Cancer Research UK also provide core funding to the Clinical Trial Service Unit and Epidemiological Studies Unit at Oxford University for the project.
Supplementary Material
Acknowledgements
We thank Judith Mackay in Hong Kong; Yu Wang, Gonghuan Yang, Zhengfu Qiang, Lin Feng, Maigen Zhou, Wenhua Zhao and Yan Zhang in China CDC; Lingzhi Kong, Xiucheng Yu and Kun Li in the Ministry of Health of China; and Yiping Chen, Sarah Clark, Martin Radley, Hongchao Pan and Jill Boreham in the Clinical Trial Service Unit (CTSU), Oxford, for assisting with the design, planning, organization and conduct of the study and data analysis. The most important acknowledgement is to the participants in the study and the members of the survey teams in each of the 10 regional centres, as well as to the project development and management teams based at Beijing, Oxford and the 10 regional centres. CTSU acknowledges support from the BHF Centre of Research Excellence, Oxford.
Members of the China Kadoorie Biobank collaborative group are listed as follows.
(i) International steering committee
Liming Li, Junshi Chen, Fan Wu (ex-member), Rory Collins, Richard Peto, Zhengming Chen.
(ii) Study coordinating centres
International Coordinating Centre, Oxford: Zhengming Chen, Garry Lancaster, Xiaoming Yang, Alex Williams,Margaret Smith, Ling Yang, Yumei Chang, Iona Millwood, Yiping Chen, Qiuli Zhang, Sarah Lewington, Gary Whitlock.
National Coordinating Centre, Beijing: Yu Guo, Guoqing Zhao, Zheng Bian, Can Hou,Yunlong Tan.
(iii) Regional Coordinating Centres
Qingdao Centre for Disease Control: Zengchang Pang, Shanpeng Li, Shaojie Wang.
Licang Centre for Disease Control: Silu lv.
Heilongjiang Provincial Centre for Disease Control: Zhonghou Zhao, Shumei Liu, Zhigang Pang.
Nangang Centre for Disease Control: Liqiu Yang, Hui He, Bo Yu.
Hainan Provincial Centre for Disease Control: Shanqing Wang, Hongmei Wang.
Meilan Centre for Disease Control: Chunxing Chen, Xiangyang Zheng.
Jiangsu Provincial Centre for Disease Control: Xiaoshu Hu, Minghao Zhou, Ming Wu, Ran Tao.
Suzhou Centre for Disease Control: Yeyuan Wang, Yihe Hu, Liangcai Ma.
Wuzhong Centre for Disease Control: Renxian Zhou.
Guanxi Provincial Centre for Disease Control: Zhenzhu Tang, Naying Chen, Ying Huang.
Liuzhou Centre for Disease Control: Mingqiang Li, Zhigao Gan, Jinhuai Meng, Jingxin Qin.
Sichuan Provincial Centre for Disease Control: Xianping Wu, Ningmei Zhang.
Pengzhou Centre for Disease Control: Guojin Luo, Xiangsan Que, Xiaofang Chen.
Gansu Provincial Centre for Disease Control: Pengfei Ge, Xiaolan Ren,Caixia Dong.
Maiji Centre for Disease Control: Hui Zhang, Enke Mao, Zhongxiao Li.
Henan Provincial Centre for Disease Control: Gang Zhou, Shixian Feng.
Huixian Centre for Disease Control: Yulian Gao, Tianyou He, Li Jiang, Huarong Sun.
Zhejiang Provincial Centre for Disease Control: MinYu, Danting Su, Feng Lu.
Tongxiang Centre for Disease Control: Yijian Qian, Kunxiang Shi, Yabin Han, Lingli Chen.
Hunan Provincial Centre for Disease Control: Guangchun Li, Huilin Liu, LI Yin.
Liuyang Centre for Disease Control: Youping Xiong, Zhongwen Tan,Weifang Jia.
Conflict of interest: None declared.
References
- 1.Ding QJ, Hesketh T. Family size, fertility preferences, and sex ratio in China in the era of the one child family policy: results from national family planning and reproductive health survey. BMJ 2006;333:371–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen J, Peto R, Pan W, Liu B, Boreham J. Geographic Study of Mortality, Biochemistry, Diet and Lifestyle in Rural China . Oxford, UK: Oxford University Press, 2006. [Google Scholar]
- 3.dos Santos Silva I, Beral V. Socioeconomic differences in reproductive behaviour. IARC Sci Publ 1997;138:285–308. [PubMed] [Google Scholar]
- 4.Dorjgochoo TMDP, Kallianpur AMDMPH, Gao Y-TMD, et al. Dietary and lifestyle predictors of age at natural menopause and reproductive span in the Shanghai Women's Health Study. Menopause 2008;15:924–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lavy V, Zablotsky A. Mother's Schooling, Fertility, and Children's Education: Evidence from a Natural Experiment. Cambridge, MA: National Bureau of Economic Research, 2011. [Google Scholar]
- 6.Thulier D, Mercer J. Variables associated with breastfeeding duration. J Obstet Gynecol Neonatal Nurs 2009;38:259–68. [DOI] [PubMed] [Google Scholar]
- 7.van Noord PAH, Kaaks R. The effect of wartime conditions and the 1944–45 ‘Dutch Famine’ on recalled menarcheal age in participants of the DOM breast cancer screening project. Ann Hum Biol 1991;18:57–70. [DOI] [PubMed] [Google Scholar]
- 8.Elias SG, van Noord PAH, Peeters PHM, den Tonkelaar I, Grobbee DE. Childhood exposure to the 1944–1945 Dutch famine and subsequent female reproductive function. Hum Reprod 2005;20:2483–88. [DOI] [PubMed] [Google Scholar]
- 9.Elias SG, van Noord PAH, Peeters PHM, den Tonkelaar I, Grobbee DE. Caloric restriction reduces age at menopause: the effect of the 1944-1945 Dutch famine. Menopause 2003;10:399–405. [DOI] [PubMed] [Google Scholar]
- 10.Cai Y, Feng W. Famine, social disruption, and involuntary fetal loss: Evidence from Chinese survey data. Demography 2005;42:301–22. [DOI] [PubMed] [Google Scholar]
- 11.Chen Z, Lee L, Chen J, et al. Cohort profile: the Kadoorie Study of Chronic Disease in China (KSCDC). Int J Epidemiol 2005;34:1243–49. [DOI] [PubMed] [Google Scholar]
- 12.Chen Z, Chen J, Collins R, et al. China Kadoorie Biobank of 0.5 million people: survey methods, baseline characteristics and long-term follow-up. Int J Epidemiol 2011;40:1652–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wyshak G, Frisch R. Evidence for a secular trend in age at menarche. N Engl J Med 1982;306:1033–35. [DOI] [PubMed] [Google Scholar]
- 14.Hoel DG, Wakabayashit T, Pike MC. Secular trends in the distributions of the breast cancer risk factors — menarche, first birth, menopause, and weight—in Hiroshima and Nagasaki, Japan. Am J Epidemiol 1983;118:78–89. [DOI] [PubMed] [Google Scholar]
- 15.Onland-Moret NC, Peeters PHM, van Gils CH, et al. Age at menarche in relation to adult height: The EPIC Study. Am J Epidemiol 2005;162:623–32. [DOI] [PubMed] [Google Scholar]
- 16.Kalichman L, Malkin I, Livshits G, Kobyliansky E. Age at menarche in a Chuvashian rural population. Ann Hum Biol 2006;33:390–97. [DOI] [PubMed] [Google Scholar]
- 17.Lumey LH, Stein AD. In utero exposure to famine and subsequent fertility: The Dutch Famine Birth Cohort Study. Am J Public Health 1997;87:1962–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. United Nations, Department of Economic and Social Affairs, Population Division, World Population Prospects: The 2012 Revision, New York, 2013. http://data.un.org/Data.aspx?d=PopDiv&f=variableID%3A54 (21 January 2013, date last accessed).
- 19. Social Policy Division - Directorate of Employment, Labour and Social Affairs. http://www.oecd.org/els/soc/SF2.3%20Mean%20age%20of%20mother%20at%20first%20childbirth%20-%20updated%20240212.pdf (7 February 2013, date last accessed).
- 20.Attané I. China's family planning policy: an overview of its past and future. Stud Fam Plann 2002;33:103–13. [DOI] [PubMed] [Google Scholar]
- 21.Nichols HB, Trentham-Dietz A, Hampton JM, et al. From menarche to menopause: trends among US women born from 1912 to 1969. Am J Epidemiol 2006;164:1003–11. [DOI] [PubMed] [Google Scholar]
- 22.Dratva J, Gómez Real F, Schindler C, et al. Is age at menopause increasing across Europe? Results on age at menopause and determinants from two population-based studies. Menopause 2009;16:385–94 10. [DOI] [PubMed] [Google Scholar]
- 23.Murugasen S. Age at menarche and menopause: their correlates and association with selected cardiovascular disease risk factors among 300 000 Chinese women in the China Kadoorie Biobank . MSc Thesis. Oxford University, Clinical Trial Service Unit and Epidemiological Studies Unit (CTSU) 2011. [Google Scholar]
- 24.Cooper R, Blell M, Hardy R, et al. Validity of age at menarche self-reported in adulthood. [Erratum appears in J Epidemiol Community Health 2007;61:175.] J Epidemiol Community Health 2006;60:993–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clarke R, Shipley M, Lewington S, et al. Underestimation of risk associations due to regression dilution in long-term follow-up of prospective studies. Am J Epidemiol 1999;150:341–53. [DOI] [PubMed] [Google Scholar]
- 26.Kelsey JL, Gammon MD, John EM. Reproductive factors and breast cancer. Epidemiol Rev 1993;15:36–47. [DOI] [PubMed] [Google Scholar]
- 27.McPherson K, Steel CM, Dixon JM. Breast cancer—epidemiology, risk factors, and genetics. BMJ 2000;321:624–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huxley VH. Sex and the cardiovascular system: the intriguing tale of how women and men regulate cardiovascular function differently. Adv Physiol Educ 2007;31:17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang X, Shu X-O, Gao Y-T, Yang G, Li H, Zheng W. Pregnancy, childrearing, and risk of stroke in Chinese women. Stroke 2009;40:2680–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.