Abstract
The Seek and Treat for Optimal Prevention of HIV/AIDS (STOP HIV/AIDS) cohort is a census of all identified HIV-positive individuals in the province of British Columbia. It was formed through the linkage of nine provincial treatment, surveillance and administrative databases. This open cohort allows for bidirectional analyses from 1996 onward and is refreshed annually. Extensive data collection for cohort members includes demographic information, detailed clinical and laboratory data, complete prescription drug use including antiretroviral agents, and information on health service utilization encompassing inpatient and outpatient care, addictions treatment and palliative care. This cohort provides an unprecedented opportunity to evaluate, over an extended time period, patterns and determinants of key outcomes including engagement in the cascade of HIV care from diagnosis to treatment to viral suppression as well as monitoring trends in medical costs, health outcomes and other key healthcare delivery indicators at a population level with wide-ranging, high-quality data. The overall purpose of these activities is to enable the development and implementation of strategically targeted interventions to improve access to testing, care and treatment for all HIV-positive individuals living in British Columbia. As a programme of the Ministry of Health, the STOP HIV/AIDS Evaluation Evaluation Team welcomes input from all stakeholders and possible partners via contact with the Director of Operations for the British Columbia Centre for excellence in HIV AIDS, Ms. Irene Day (iday@cfenet.ubc.ca).
Key Messages.
This open bidirectional cohort includes comprehensive demographic, clinical, laboratory, healthcare utilization and treatment information for all persons in British Columbia, Canada, with HIV infection.
The cohort construction exercise validated the utility of using case-finding algorithms and subsequent hypothesis testing to defining a population-level cohort using administrative databases.
Application to the cascade of care provides a framework to monitor changes in cascade components which, thus far, indicate improvement in each stage, from the proportion diagnosed to the percentage suppressed over time.
This cohort will allow for prospective monitoring of the cascade of HIV diagnosis and care thus enabling identification of cascade leakage points and targets for intervention and evaluation of HIV-related programming.
Background: the context of cohort development and cohort purpose
At the end of 2010, an estimated 11 700 (range 9400 to 14 000) individuals in the province of British Columbia (BC) were living with HIV.1 As a result of comprehensive support from the provincial government, which provides medical care (HIV and non-HIV related), HIV testing, antiretroviral treatment (ART) and laboratory monitoring free of charge to all HIV-positive individuals,2 great strides have been made in controlling the HIV epidemic. Moreover, innovative prevention efforts, including the first medically supervised safer injecting facility in North America3 and other harm reduction strategies as well as early adoption of emerging treatment options, have helped to curb the spread of HIV. BC continues to be a leader in HIV care and treatment and the only jurisdiction in Canada recording diminishing rates of new HIV diagnoses: 6.3 (289 cases) per 100 000 population in 2011from 6.6 (300 cases) per 100 000 population in 2010.1,4
Despite these advances, HIV in BC remains a health crisis. An estimated quarter of HIV-positive individuals in Canada are unaware of their sero-status,5 and 40% have died of HIV-related causes without ever accessing treatment.6 Fewer than half of all HIV-positive individuals in the province, who are medically eligible under defined 2010 IAS-USA guidelines,7–9 are consistently receiving highly active antiretroviral treatment (HAART).10 These numbers make clear that enhanced efforts are needed to effectively diagnose individuals with HIV and engage and retain HIV-positive individuals in care. A possible solution advocates the expansion in uptake of HAART for the purpose not only of maintaining optimum immune reconstitution for HIV-positive individuals but also as a means to reduce the incidence of HIV10–13 known as Treatment as Prevention or TasP.
Driven by current trends HIV epidemiology and treatment and catalyzed by the emerging construct of TasP, in 2009 the BC Ministry of Health (BC-MoH), the BC Centre for Disease Control (BCCDC) and the BC Centre for Excellence in HIV/AIDS (BC-CfE) launched a 5-year, 48 million Canadian dollar initiative to address existing gaps in HIV diagnosis, care and treatment, resulting in the Seek and Treat for Optimal Prevention (STOP) HIV/AIDS pilot programme. This pilot was implemented in two regions with a high burden of HIV and increased HIV-related mortality: Vancouver and Northern Interior Health Service Delivery Areas (HSDAs), while retaining the other HSDAs (Fraser, Vancouver Island and Interior HSDAs) as ‘controls’. Subsequently, driven by an expanding body of research confirming the utility, safety and cost-effectiveness of TasP, a decision was made in December 2012 to implement TasP province wide and remove its status as a ‘pilot’, making it the new operating standard.
In order to meet provincial mandates of programme evaluation including monitoring and interpreting key outcomes, a cohort of all persons identified as having HIV and living in BC was established. Complete monitoring and evaluation requires comprehensive access to health administrative databases, treatment and testing databases and ongoing linkages between these datasets. The resulting database will allow unprecedented ability to provide detailed quantification of the cascade of care14 and the effect on its components of programmatic TasP innovations. Key long-term outcomes for evaluation include trends in mortality and HIV incidence as well as the burden of disease at both individual and population levels.
STOP HIV/AIDS cohort construction and membership
Cohort formation and data linkages
The cohort includes all HIV-positive persons identified as being HIV-positive between 1 January 1996 and 31 March 2010. In BC, HIV testing and surveillance are conducted through the BCCDC which retains a record of all positive HIV tests performed in the province. Many more people have HIV than are identified through this standard surveillance system; for instance, individuals may be tested outside the province and move to BC, whereas others are simply never formally diagnosed even though they may be hospitalized for HIV-related illness or die of HIV-related causes. Such individuals are never captured in BCCDC surveillance statistics. Moreover, other individuals test anonymously, making linkage to subsequent care and treatment activities impossible. Clearly, reliance on testing data alone cannot provide an accurate number or form a complete cohort of HIV-positive persons. Therefore, we developed, tested and applied an algorithm using a previously validated methodological approach15 to a number of data sources to construct a ‘best estimate’ cohort.
A detailed description of this procedure has recently been published.16 In brief, we identified potential HIV-positive individuals from a review of nine databases. As outlined in Table 1, these data sources represent the vast majority of health administrative data in BC, incorporating many facets of care including in- and outpatient services, HIV-related clinical and laboratory data, home care and supportive services, mental health services and prescription drug utilization. In the context of BC’s universal healthcare system, every person who has any contact with any government-funded healthcare service is captured in these data through their personal provincial health number (PHN) that is provided to all province residents at birth or through immigration.
Table 1.
Data sources and key variables used to construct the cohort and populate the STOP HIV cohort database
| Database | Data steward | Contents | Key data |
|---|---|---|---|
| Drug Treatment and Laboratory Database | BCCfE | Clinical and laboratory tests and HIV-related treatment and treatment outcomes | Antiretroviral therapy (ARV) history, CD4, pVL, occurrence of AIDS-dDefining Illnesses, results of drug resistance testing |
| Provincial HIV/AIDS Surveillance Database | BCCDC | All HIV testing events in BC | Positive HIV tests, date of first positive test and occurrence of AIDS-defining illnesses |
| Medical Services Plan | BCMoH | Outpatient care billing for all medical care services provided as fee-for-service | Diagnostic and laboratory procedures, encounter claims and all fee-for-service dates, diagnoses and costs billed |
| Discharge Abstract Database | BCMoH | Inpatient care | Procedures, chronic and rehabilitation services, transfers and deaths for all inpatient and day surgery patientsa |
| Home and Community Care | BCMoH | Community-based support services | Range of services including hospice and home nursing care, adult day services, assisted living, respite care, residential and convalescent care |
| Mental Health Services | BCMoH | Mental health services | Mental health services used including fee-for-service institutional care, community clinics and acute care. |
| Addictions Information Management Systems | BCMoH | Referral to treatment for alcohol, drug or gambling addictions | History of illicit drug use and related service utilization patterns |
| PharmaNet | BCMoH | Prescription drug dispensation | Drug type and name, date and length of prescription, dosage, prescriber code and drug costs |
| Vital Statistics | Statistics Canada | Mortality statistics | Deaths including date and probable causes |
BCCfE, British Columbia Centre for Excellence in HIV/AIDS; BCCDC, British Columbia Centre for Disease Control; BC MoH, British Columbia Ministry of Health.
aExcludes abortion procedures and outpatient care such as emergency room utilization and imaging.
In terms of cohort membership, individuals with a documented positive HIV test in the BCCDC testing database, as well as those who have one or more positive HIV plasma viral load (pVL) tests and/or who have received antiretroviral medications, are considered HIV positive with certainty. Individuals identified only within the additional health administrative datasets as having received care for an HIV- or AIDS-related medical condition in BC are also considered for inclusion in the cohort. This latter group of individuals is identified through administrative data sources using international classification of disease (ICD) diagnostic codes (ICD-9 and ICD-10) associated with HIV/AIDS. In our algorithm, ‘unconfirmed’ potential HIV-positive cases were considered likely cases and included in the cohort if they had three medical services plan (MSP) claims for an HIV-related condition or one or more record of HIV-related hospitalization.
To summarize, individuals enter the cohort when any of the following HIV-defining events is detected in the data: (i) a positive HIV test; (ii) report of an AIDS-defining illness; (iii) a detectable HIV pVL; (iv) initiation of HIV ART; or (v) an HIV-related hospitalization or three HIV-related Medical Service Plan (MSP) claims.16
From date of cohort entry forward as well as retrospectively to 1996, all hospital visits, physician visits, laboratory tests, drug dispensations and other instances of health service utilization are tracked through the data collected in these databases for each cohort member. Annual data refreshes ensure that the cohort remains open with newly identified HIV-positive individuals entering the cohort and that all participants continue to be included in non-nominal prospective monitoring.
Database linkage was executed by data stewards in each collaborating agency and coordinated by the Vancouver Coastal Health Authority. Clients were matched to the Health Registry by PHN and then supplemental manual and probabilistic matching. The final de-identified datasets were provided to the analytical team at the BC-CfE. A privacy impact assessment was completed for this study. The study received ethical approval through the UBC / Providence Health Care Research Ethics Board.
Cohort composition
Using the algorithm described above, a total of 12 349 unique individuals were included in the final baseline STOP HIV/AIDS cohort, capturing prevalent cases from 1 January 1996 to 31 March 2010. The majority of individuals were identified through membership in two or more source databases. Among the five health authorities (HAs) in the province, the Vancouver Coastal HA was home to more than half of the cohort, followed by the Fraser and Vancouver Island HAs, which is consistent with the nature of the epidemic in BC. Some 8733 individuals have ever accessed ART and, of the total, 9597 were alive as of 31 March 2010, of whom 69% have ever accessed ART.
Table 2 summarizes selected characteristics of cohort members, comparing those who have ever vs never accessed ART. Among those accessing treatment, 83% were male vs 74% in the untreated group. Median age at cohort entry was similar for both treated and untreated groups at 44 (interquartile range (IQR): 37–51) and 47 (IQR: 4153), respectively, as was age at diagnosis. Individuals ever vs never accessing ART, however, had a longer median follow-up (10 years vs 7 years), and were more likely to be alive as of 31 March 2010 (69% vs 31%). Those receiving ART were, as would be expected, more likely to have had pVL (15% vs 72%) and have a CD4 cell count (76% vs 21%) or pVL (72% vs 15%) recorded in the year prior to administrative censorship of the cohort (1 April 2009 to 31 March 2010). The wide disparities in IDU status and hepatitis C infection likely reflect the fact that IDU status is unknown for a large proportion of those never treated, as these factors are usually assessed at treatment initiation if not captured at time of HIV testing.
Table 2.
Selected characteristics of STOP HIV/AIDS cohort participants in BC stratified by antiretroviral treatment utilization
| Characteristic | n | Ever treated n (%) | Never treated n (%) |
|---|---|---|---|
| Gender | 12349 | ||
| Male | 7259 (82.74) | 2658 (74.33) | |
| Female | 1514 (17.26) | 2252 (25.67) | |
| Age at first known HIV (years, median, IQR) | 12349 | 37 (30–45) | 37 (31–43) |
| IDU status (ever) | 12349 | ||
| Yes | 3070 (34.99) | 157 (4.39) | |
| No | 3988 (45.46) | 192 (5.37) | |
| Unknown | 1715 (19.55) | 3227 (90.24) | |
| Hepatitis C co-infection (ever) | 12349 | ||
| Yes | 3086 (35.18) | 156 (4.36) | |
| No | 4003 (45.63) | 265 (7.41) | |
| Unknown | 1684 (19.20) | 3155 (88.23) | |
| AIDS-defining illness at treatment initiation | 12349 | ||
| Yes | 1370 (15.62) | N/A | |
| No | 7403 (84.38) | ||
| Unknown | |||
| Positive HIV test recorded | 5343 | 3843 (43.8) | 1500 (41.95) |
| Years on HIV treatment (median, IQR) | 8773 | 7 (3–12) | N/A |
| Alive as of 31 March 2010 | 9597 | 6647 (69.26) | 2950 (30.74) |
| Age at censoring (years, median, IQR) | 9597 | 44 (37–51) | 47 (41–53) |
| Last plasma viral load in past yeara | 9597 | ||
| No plasma viral load | 983 (14.79) | 2113 (71.63) | |
| <400 | 5015 (75.45) | 127 (4.31) | |
| 400-10 000 | 284 (4.27) | 301 (10.20) | |
| 10 001-20 000 | 63 (0.95) | 116 (3.93) | |
| 20 001-30 000 | 40 (0.60) | 72 (2.44) | |
| 30 000+ | 262 (3.94) | 221 (7.49) | |
| Last CD4 cell count in past yeara | 9597 | ||
| No CD4 cell count | 1374 (20.67) | 2245 (76.10) | |
| <50 cells/ml | 61 (0.92) | 5 (0.17) | |
| 51-200 | 461 (6.94) | 27 (0.92) | |
| 201-300 | 572 (8.61) | 66 (2.24) | |
| 301-500 | 1772 (26.66) | 218 (7.39) | |
| 500+ | 2407 (36.21) | 389 (13.19) | |
| MSP services #/year (median, IQR) | 11786 | 70 (44–105) | 22 (8–49) |
| Days of inpatient care | 8170 | 5 (2–10) | 5 (2–11) |
IQR, interquartile range.
aOn-year period from 1 April, 2009 to 31 March 2010.
Patient follow-up
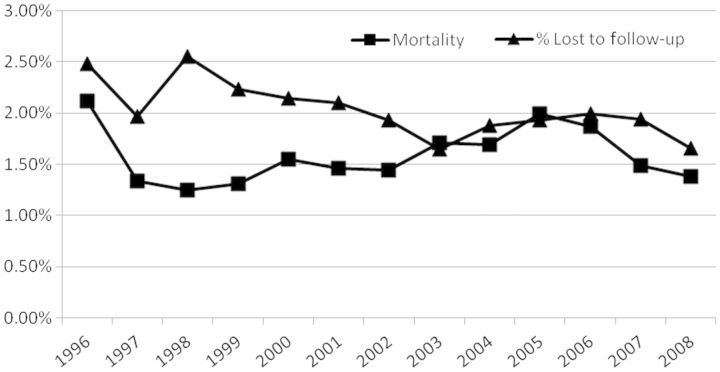
Median duration of follow-up in the cohort is 6.6 years. An estimated 22% of cohort members have died since 1996 and approximately 15% were lost to follow-up between 1996 and 2008 (Figure 1). Individuals lost to follow-up were defined as those without a CD4 or pVL test, dispensed ART, hospitalization, medical services plan (MSP) claim or PharmaNet claim in the past 18 months (reflecting administrative censoring in 2008 to allow for 18 months of follow-up to occur). Overall, 63% of the cohort participants were administratively censored (i.e. not removed from the cohort due to loss to follow-up or death), 14.8% of the cohort participants were lost and 22.3% deceased. Those never on HAART were more likely to have been lost to follow-up (31.7% vs 7.9% among those treated with HAART).
Figure 1.
Loss to follow-up and mortality among STOP HIV/AIDS cohort participants in British Columbia from 1996 to 2008 (N = 12 349), defined as those without health service utilization recorded in any of the nine databases used to create the STOP cohort.
Overall, loss to follow-up has remained less than 3% per annum and has declined over time. These low rates minimize the chance of bias due to informative censoring and ensure that estimations from cohort analyses are based on as close to a totality of HIV-positive individuals on treatment in the province as possible. Annual mortality rates have also remained below 3%, ranging between 1% and 2.5%. The imminent data re-linkage will update the cohort to 31 March 2012 and annual updates thereafter will maintain the open cohort approach. An exceptional feature of the data is that all records identify the specific date(s) of service delivery/prescription fill, allowing for temporal relationships between interactions with services and health outcomes to be established.
We hypothesize that the majority of individuals who have been lost to follow-up have moved out of the province of BC and are seeking care elsewhere. It is unlikely that they have died, given our comprehensive and real-time linkage with Vital Statistics. Moreover, monitoring of non-HIV related claims indicates that lost subjects have effectively ceased to engage in any facet of the medical system, including hospital use, physician visits or HIV monitoring and treatment. Figure 2 illustrates the cohort membership including entry and loss over the follow-up period. Over time the proportion of the cohort consisting of previously linked individuals increases, capturing a greater proportion of all existing cases. Correspondingly, the proportion of the cohort that is new (defined as diagnosed in the year of interest) is declining and, in each year, the proportion of new cases that remains unlinked is diminishing.
Figure 2.
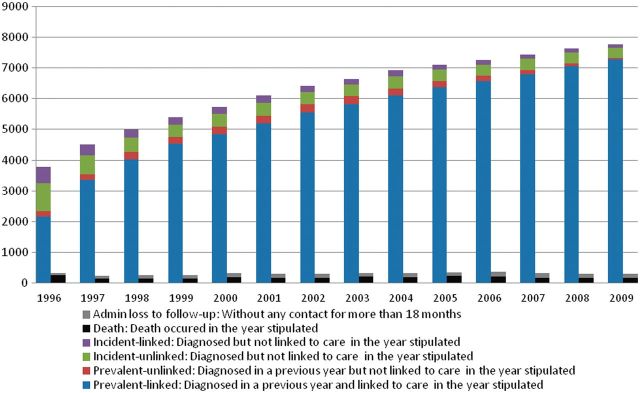
Cohort membership: 1996 to 2009. HIV-positive: based on annual HIV prevalence estimates reported by the Public Health Agency of Canada. Diagnosed: defined as the first instance of a confirmed HIV-positive test, detectable plasma viral load (pVL), HIV-related MSP billing or hospitalization, reported AIDS-defining illness or antiretroviral treatment. Linked: among diagnosed cases, defined as the first instance of HIV-related service following HIV diagnosis among those with confirmed HIV test and the first instance of HIV-related service ≥30 days following HIV diagnosis among those with no confirmed HIV test. Retained: among individuals linked to HIV care, defined as three HIV-related services provided on distinct dates, within the calendar year or access to antiretroviral treatment. Need antiretroviral therapy (ART): among individuals with any record of CD4 and/or pVL, individuals qualify if they have reached 2009 IAS-USA initiation criteria, CD4 ≤350 or AIDS-defining illness. Accessing ART: among those linked to HIV care, defined as receiving an antiretroviral drug dispensation at least once in the calendar year. Adherent: among individuals on antiretroviral therapy, defined as having at least 80% adherence in 2009, or from the point of antiretroviral initiation that year. Suppressed: no detectable pVL in two consecutive measurements during 2009.
Findings to date
A large number of evaluations are being conducted or planned. Thus far three articles have been published or accepted for publication and eight abstracts have been presented or accepted for presentation at national and international conferences based on these publications regarding the STOP programme or cohort. An ongoing updated list of publications may be accessed at http://www.cfenet.ubc.ca under the ‘publications’ banner.
The first important output of this cohort is the methodology that allowed us to identify individuals eligible for cohort inclusion and the construction of the cohort itself which is described in the ‘cohort construction’ portion of this document.16 In essence we demonstrated the ability to identify HIV-infected subjects in the HAART era using an existing algorithm, and validated this algorithm with a series of tests of a priori hypothesis.
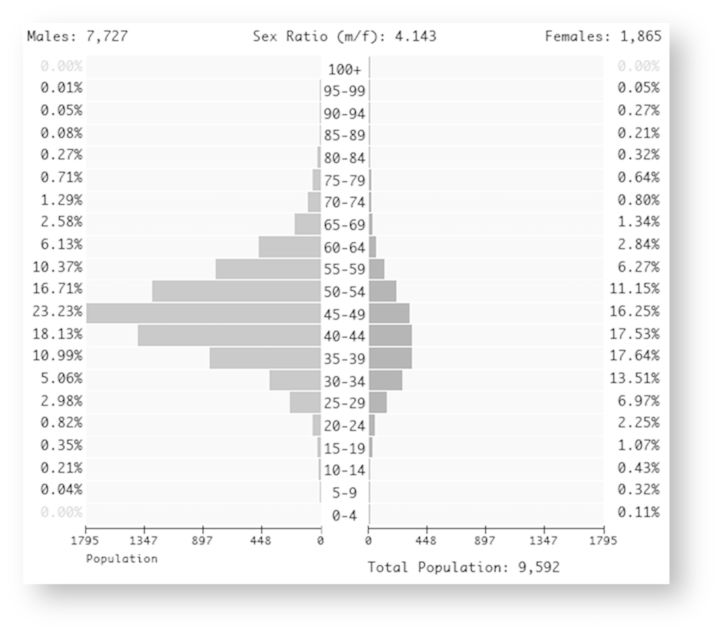
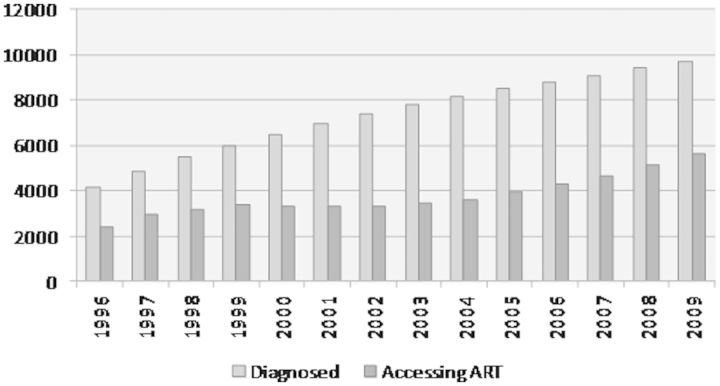
In terms of the cohort characteristics, almost 80% of HIV-positive individuals alive as of 2009 and living in BC are between the ages of 35 and 60 years (Figure 3). In addition, there are four times as many HIV-infected men as women in the cohort. Our findings indicate that the number of diagnosed HIV-positive individuals in the province of BC increased from 4178 in 1996 to 9700 by 2009 (Figure 4). Over the same period, the proportion of diagnosed individuals on ART has remained relatively constant, ranging from 57.1% in 1996 to 57.7% in 2009.
Figure 3.
Age pyramid stratified by sex for STOP HIV/AIDS cohort participants alive in 2009.
Figure 4.
Identified HIV-positive individuals and number on ART in British Columbia from 1996-2009, i.e. positive HIV test on record or with healthcare resource utilization patterns consistent with HIV.
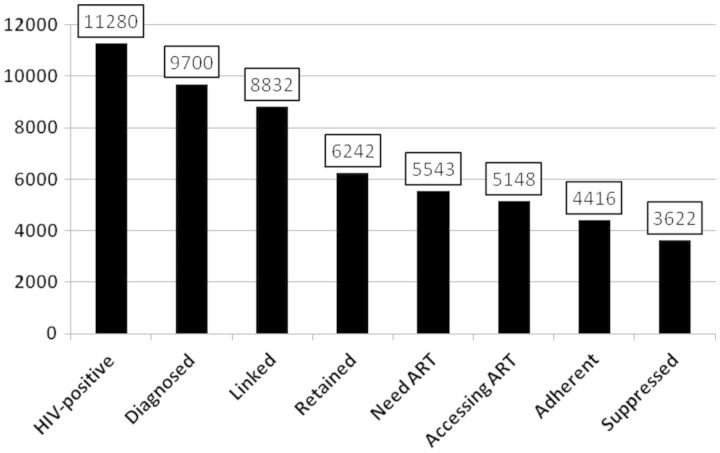
We also assessed the spectrum of engagement in care for patients in our cohort, illustrated cross-sectionally in Figure 5 for the year 2009. The cascade of HIV care, conceptualized as seven stages along the HIV care continuum, describes the engagement of HIV-positive individuals in care, from initial diagnosis to viral suppression.14 Expanding Gardner’s seventh stage of adherence into two final stages (adherent and suppressed), our eight stages are as follows: HIV-positive; diagnosed with HIV; linked to HIV care; retained in HIV care; ART needed; accessing ART; adherent to ART; and fully suppressed. In a recently published article we present the retrospective annualized cascades for all British Columbians living with HIV between 1996 and 2011.17 We discovered simultaneous trends of decrease in the estimated proportion of HIV-positive people remaining undiagnosed and improvement in the proportion of individuals entering each stage of the cascade. Notably, in the final step of the cascade, in 1996 only an estimated 1% of HIV-infected individuals were virally suppressed compared with approximately 35% in 2011.
Figure 5.
Spectrum of engagement in care for STOP HIV/AIDS cohort participants in British Columbia in 2009.
HIV prevalence in the province, the first stage of the cascade, is estimated based on data obtained from the Public Health Agency of Canada (PHAC). With PHAC estimates of prevalence in 2005, 2008 and 2011, we interpolated prevalence for 2009, estimating that there were some 11 280 people with HIV in BC.18,19 Of these, 9700were diagnosed as such; 91% of individuals who were diagnosed were subsequently linked to care but only 64% were retained in care. Approximately 53% of those with HIV were accessing ART. Overall, 46% were adherent to ART and 37% of HIV-positive individuals achieved pVL suppression. As illustrated here, it is clear that although more precise estimates of true prevalence are needed, expanded testing to identify HIV-positives is a priority. Of note, the cohort includes 3576 individuals who have never accessed ART, thus representing a critical target population requiring further study to inform efforts regarding engagement into HIV care.
Partner cohort studies
The HAART Observational Medical Evaluation and Research (HOMER) cohort is a partner study coordinated through the BC-CfE. The HOMER cohort primarily uses the robust clinical data within the BC-CfE Registry to evaluate mortality, prognostic factors and treatment responses specifically among individuals accessing HAART in BC, with the aim of informing treatment priorities and therapeutic guidelines and evaluating the population-level health effects of HAART. HOMER restricts inclusion to Drug Treatment Program (DTP) participants who were ART-naïve in BC prior to enrolment, aged ≥19 years of age at ART initiation, whose first ART regimen included three or more antiretroviral agents initiated on/after 1 August 1996. As previously described, The STOP HIV/AIDS cohort includes all HIV-positive individuals resident in BC, aged over 18 months, who were diagnosed with HIV between 1 January 1996 and 31 March 2010. Given that cohort enrolment is not limited to HIV-positive individuals who have initiated treatment, the STOP HIV/AIDS cohort is more expansive in scope than the HOMER cohort. Ultimately, the STOP HIV/AIDS seeks to critically evaluate the cascade of care and the expansion of HAART provision in BC under the umbrella of Treatment as Prevention.
Strengths and weaknesses of the study
Such a comprehensive combination of administrative, surveillance and treatment databases is unique. With this population-based, provincial cohort, policy makers and evaluation teams alike have access to comprehensive longitudinal information on patterns of health resources use and their impact on trends in HIV-related morbidity and mortality. The comprehensive capture and low rate of attrition ensures long-term viability of the cohort. The cohort allows unprecedented characterization of the HIV epidemic in BC, illuminating successes and identifying gaps in care. The formation of the cohort has also given investigators the ability to populate the cascade of care, monitoring important long-term outcomes such as viral suppression and effects on incidence: the ultimate goals of TasP. Annual data refreshes will allow investigations to keep pace with the dynamics of the epidemic, treatment patterns and outcomes and provide a timely overview of the success or otherwise of interventions.
As to study challenges, out-migration may inflate the number of individuals included in the cohort, whereas in-migration, resulting in an unobserved censorship, may result in under-estimates of health resource utilization. Although the cohort represents all those who have ever been diagnosed or sought care for HIV in BC, we are unable to estimate total prevalence for the province since those not testing or identified as HIV-positive through our care algorithm would not be captured or included. The cohort currently does not include complete information on HIV-positive individuals who are incarcerated; however, discussions to establish linkages with Corrections Canada are under way.
Data access and additional information
Requests and suggestions for analyses must have approval of all stakeholders and pertain to stated STOP HIV/AIDS objectives. Requests will be approved based on the BC-MoH’s discretion. A website with more information can be found at: http://www.cfenet.ubc.ca/our-work/programs/stop-hiv-aids. Enquiries may be sent by e-mail to: info@cfenet.ubc.ca.
Funding
This study was funded by the British Columbia Ministry of Health through the STOP HIV/AIDS pilot programme. The programme has recently been instituted as an ongoing healthcare programme and expanded to include all of BC.
Acknowledgements
We acknowledge all BCMoH and Vancouver Coastal Health Decision support staff involved in data access and procurement, including: Monika Lindegger, Clinical Prevention Services, BCCDC; Elsie Wong, PHAC; Al Cassidy, BC MoH; and Joleen Wright and Karen Luers, Vancouver Coastal Health Decision Support. We also acknowledge the assistance of David Milan and Suzanne Humphreys in early efforts towards this manuscript.
B.N is a Michael Smith Foundation for Health Research Scholar. J.M. has received grants from Abbott, Biolytical, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck and ViiV Healthcare. He is also is supported: by the Ministry of Health Services and the Ministry of Healthy Living and Sport, from the Province of British Columbia; through a Knowledge Translation Award from the Canadian Institutes of Health Research (CIHR); and through an Avant-Garde Award (No. 1DP1DA026182) from the National Institute of Drug Abuse, at the US National Institutes of Health. He has also received support from the International AIDS Society, United Nations AIDS Program, World Health Organization, National Institute on Drug Abuse, National Institutes of Health Research-Office of AIDS Research, National Institute of Allergy & Infectious Diseases, the United States President’s Emergency Plan for AIDS Relief (PEPfAR), Bill & Melinda Gates Foundation, French National Agency for Research on AIDS & Viral Hepatitis (ANRS), Public Health Agency of Canada.
The STOP HIV/AIDS Study Group includes Julio Montaner, Bohdan Nosyk, Viviane Lima, Rolando Barrios, Robert S Hogg, Patty Daly, Mark Gilbert, Reka Gustafson, Perry RW Kendall, Ciro Panessa, Nancy South and Gina McGowan.
Conflict of interest: None declared.
References
- 1.BC Centre for Disease Control. HIV in British Columbia: Annual Surveillance Report 2011. 2012. http://www.bccdc.ca/util/about/annreport/default.htm (12 December 2013, date last accessed). [Google Scholar]
- 2.Lima VD, Hogg RS, Montaner JSG. Expanding HAART treatment to all currently eligible individuals under the 2008 IAS-USA guidelines in British Columbia, Canada. PLoS One 2010;5:10991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marshall BDL, Milloy MJ, Wood E, Montaner JSG, Kerr T. Reduction in overdose mortality after the opening of North America’s first medically supervised safer injecting facility: A retrospective population-based study. Lancet 2011;377:1429–37. [DOI] [PubMed] [Google Scholar]
- 4.Hogg RS, Heath K, Lima VD, et al. Disparities in the burden of HIV/AIDS in Canada. PLoS One 2012;7:e47260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang Q, Boulos D, Yan P, et al. Estimates of the number of prevalent and incident human immunodeficiency virus (HIV) infections in Canada, 2008. Can J Public Health 2010;101:486–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joy R, Druyts EF, Brandson EK, et al. Impact of neighborhood-level socioeconomic status on HIV disease progression in a universal health care setting. J Acquir Immune Defic Syndr 2008;47:500. [DOI] [PubMed] [Google Scholar]
- 7.Czarnogorski M, Brown J, Lee V, et al. The prevalence of undiagnosed HIV infection in those who decline HIV screening in an urban emergency department. AIDS Res Treat 2011;2011:879065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown J, Shesser R, Simon G, et al. Routine HIV screening in the emergency department using the new US Centers for Disease Control and prevention guidelines. J Acquir Immune Defic Syndr 2007;46:395–401. [DOI] [PubMed] [Google Scholar]
- 9.Thompson MA, Aberg JA, et al. Antiretroviral treatment of adult HIV infection: 2010 recommendations of the International AIDS Society-USA panel. JAMA 2010;304:321–33. [DOI] [PubMed] [Google Scholar]
- 10.Montaner JS, Hogg R, Wood E, et al. The case for expanding access to highly active antiretroviral therapy to curb the growth of the HIV epidemic. Lancet 2006;368:531–36. [DOI] [PubMed] [Google Scholar]
- 11.Wood E, Kerr T, Marshall BDL, et al. Longitudinal community plasma HIV-1-RNA concentrations and incidence of HIV-1 among injecting drug users: a prospective cohort study. BMJ 2009;338:b1649:1191–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Montaner JSG, Lima VD, Barrios R, et al. Association of highly active antiretroviral therapy coverage, population viral load, and yearly new HIV diagnoses in British Columbia, Canada: a population-based study. Lancet 2010;376:532–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011;365:493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gardner EM, McLees MP, Steiner JF, del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis 2011;52:793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Antoniou T, Zagorski B, Loutfy MR, Strike C, Glazier RH. Validation of case-finding algorithms derived from administrative data for identifying adults living with human immunodeficiency virus infection. PLos One 2011;6:e21748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nosyk B, Colley G, Yip B, et al. Application and validation of case-finding algorithms for identifying individuals with human immunodeficiency virus from administrative data in British Columbia, Canada. PLoS One 2013;8:e54416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nosyk B, Montaner JSG, Colley G, et al. for the STOP HIV/AIDS Study Group. The cascade of HIV care in British Columbia, Canada, 1996-2011: a population-based retrospective cohort study. Lancet Infect Dis 2013; 27 September. doi: 10.1016/S1473-3099(13)70254-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Public Health Agency of Canada. HIV and AIDS in Canada – Surveillance Report to December 31, 2009 . 2009. http://www.phac-aspc.gc.ca/aids-sida/publication/survreport/2009/dec/index-eng.php (8 June 2011, date last accessed). [Google Scholar]
- 19.Public Health Agency of Canada. HIV/AIDS Epi Updates, July 2010. 2010. http://www.phac-aspc.gc.ca/aids-sida/publication/epi/2010/index-eng.php (9 June 2011, date last accessed). [Google Scholar]