Abstract
Autosomal recessive polycystic kidney disease, an inherited disorder characterized by the formation of cysts in renal collecting ducts and biliary dysgenesis, is caused by mutations of the polycystic kidney and hepatic disease 1 (PKHD1) gene. Expression of PKHD1 is tissue specific and developmentally regulated. Here, we show that a 2.0-kb genomic fragment containing the proximal promoter of mouse Pkhd1 directs tissue-specific expression of a lacZ reporter gene in transgenic mice. LacZ is expressed in renal collecting ducts beginning during embryonic development but is not expressed in extrarenal tissues. The Pkhd1 promoter contains a binding site for the transcription factor hepatocyte nuclear factor (HNF)-1β, which is required for activity in transfected cells. Mutation of the HNF-1β-binding site abolishes the expression of the lacZ reporter gene in renal collecting ducts. Transgenes containing the 2.0-kb promoter and 2.7 kb of additional genomic sequence extending downstream to the second exon are expressed in the kidney, intrahepatic bile ducts, and male reproductive tract. This pattern overlaps with the endogenous expression of Pkhd1 and coincides with sites of expression of HNF-1β. We conclude that the proximal 2.0-kb promoter is sufficient for tissue-specific expression of Pkhd1 in renal collecting ducts in vivo and that HNF-1β is required for Pkhd1 promoter activity in collecting ducts. Additional genomic sequences located from exons 1-2 or elsewhere in the gene locus are required for expression in extrarenal tissues.
Keywords: polycystic kidney disease, gene transcription, transcription factor, transgenic mice, fibrocystin, autosomal recessive polycystic kidney disease, polycystic kidney and hepatic disease 1
autosomal recessive polycystic kidney disease (ARPKD) affects 1:20,000 live births and represents one of the most common inherited causes of kidney failure in infants and children (41). ARPKD is characterized by the formation of cysts in renal collecting ducts and abnormal development of intrahepatic bile ducts. Neonates with the severe form of ARPKD present with bilateral nephromegaly, intrauterine kidney failure, and Potter sequence (1, 11). Older affected individuals may present with portal hypertension due to biliary dysgenesis and hepatic fibrosis. ARPKD is caused by mutations in the polycystic kidney and hepatic disease 1 (PKHD1) gene located on chromosome 6p12.2 (29, 36, 40). PKHD1 is a large gene consisting of 67 coding exons extending over ∼500 kb of genomic DNA. The orthologous mouse gene, Pkhd1, consists of 68 exons and encodes a protein comprising 4,059 amino acids (26). PKHD1 has been reported to undergo extensive alternative splicing, although a recent study (2) has suggested that much of the observed transcript diversity may be due to alternative polyadenylation sites. Pkhd1 knockout mice develop phenotypes that are similar to those seen in humans with ARPKD, including dilated and cystic renal tubules, biliary dysgenesis, liver cysts, periportal fibrosis, and pancreatic cysts (8, 9, 25, 38, 39).
The protein product of PKHD1, called fibrocystin or polyductin, is a large membrane protein (>450 kDa) that is located in the apical plasma membrane, primary cilium, basal body, and mitotic spindle (24, 34, 37, 42). The function of fibrocystin is uncertain, but it shares structural similarity with the hepatocyte growth factor receptor and contains a G8 domain that is frequently found in ligand-binding membrane proteins. Fibrocystin undergoes regulated proteolysis, releasing a large ectodomain that is shed into urinary exosomes and a COOH-terminal domain that translocates to the nucleus (14, 16, 19). The mechanism of cyst formation in ARPKD may involve alterations in oriented cell division, apoptosis, cell proliferation, actin cytoskeleton, cell-matrix interactions, and intra- and intercellular signaling (2, 23, 27, 35).
Expression of PKHD1 is tissue specific and developmentally regulated. The 16-kb human PKHD1 mRNA transcript is primarily expressed in the kidney, liver, lung, and pancreas (29, 36, 40). In adult and fetal human tissues, fibrocystin has been localized in renal collecting ducts, thick ascending limbs of loops of Henle, bile ducts, pancreatic ducts, epididymis, and testis (24, 37). The longest mouse Pkhd1 transcript is 13 kb and most abundantly expressed in the kidney (26). In mouse embryos, Pkhd1 is expressed in the mesonephric tubules, mesonephric (Wolffian) ducts, and metanephros (24, 26, 42). Within the metanephros, Pkhd1 is expressed in the branching ureteric bud but not in comma- and S-shaped bodies or metanephric mesenchyme. Postnatally, Pkhd1 is highly expressed in collecting ducts with lower levels in loops of Henle and proximal tubules and no expression in glomeruli. In the liver, Pkhd1 is expressed in developing bile ducts, and expression persists in intrahepatic and extrahepatic bile ducts in the adult. In situ hybridization using probes from specific exons has identified expression in the large blood vessels, testis, ganglia, pancreas, adrenal gland, and trachea, suggesting that alternative splicing may produce transcripts with distinct expression patterns (26). Analysis of Pkhd1lacZ/+ knockin mice has shown expression in renal collecting ducts, proximal tubules, Bowman's capsules, intrahepatic bile ducts, and pancreatic ducts (38).
The molecular mechanisms that control Pkhd1 gene expression remain poorly understood. Previous studies have shown that genomic fragments containing the proximal promoter and 5′ noncoding sequence of Pkhd1 are transcriptionally active in transfected renal epithelial cells (13). The proximal promoter contains a binding site for hepatocyte nuclear factor (HNF)-1β, a transcription factor that is expressed in epithelial cells in the kidney, liver, and other organs (17). HNF-1β binds to the Pkhd1 promoter in vitro and activates transcription by recruiting coactivators that have histone acetylase activity (15). The resulting chromatin remodeling may stimulate Pkhd1 gene transcription. Consistent with this model, expression of dominant negative mutant HNF-1β (DN-HNF-1β) or kidney-specific knockout of HNF-1β in transgenic mice reduces the levels of Pkhd1 mRNA transcripts in the kidney (10, 13, 15). In the present study, we explored the mechanisms underlying these findings using reporter gene assays in transgenic mice.
MATERIALS AND METHODS
Luciferase reporter plasmids.
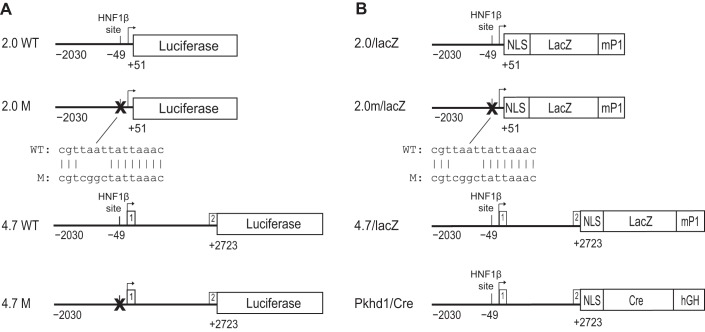
A plasmid containing the noncoding region of mouse Pkhd1 extending from exon 2 to 6.7 kb upstream to the transcription initiation site has been previously described (13). The plasmid was digested with BlpI and SmaI, and the 4.7-kb fragment was cloned into pGL3-Basic (Promega). The resulting plasmid (4.7WT/luc) contains 2,030 bp of the promoter region, exon 1 (noncoding, 156 bp), intron 1 (2,537 bp), and 30 bp of exon 2 inserted upstream to a Photinus luciferase reporter gene (Fig. 1A). 4.7WT/luc plasmid DNA was amplified using long-range PCR with Herculase (Stratagene, La Jolla, CA) and primers (RVPrimer3, 5′-CTAGCAAAATAGGCTGTCCC-3′ and 5′-GCAACCCGGGCAGGTAGCTCACTTCCTGTT-3′). The 2.0-kb product containing 2,030 bp of the promoter region and 51 bp of exon 1 was digested with SmaI and KpnI and cloned into pGL3-Basic to produce the plasmid 2.0WT/luc. Luciferase reporter plasmids (2.0m/luc and 4.7m/luc) containing a mutation of the HNF-1β-binding site located at nucleotide position1 −49 were produced by site-directed mutagenesis using QuickChange kits (Stratagene). The sequences of the wild-type and mutant plasmids were verified by DNA sequencing.
Fig. 1.
Schematic diagram of polycystic kidney and hepatic disease 1 (Pkhd1) reporter genes. A: maps of the constructs used for luciferase reporter gene assays in transfected cells. Wild-type (WT) and mutated (M) sequences are shown. Top: 2.0WT is a genomic fragment (horizontal line) containing 2.0 kb of the proximal 5′ flanking sequence, transcription initiation site (arrow), and 51 bp of exon 1 of mouse Pkhd1 linked to a Photinus luciferase reporter gene. A consensus hepatocyte nuclear factor (HNF)-1β-binding site is located 49 bp upstream from the transcription initiation site. In 2.0M, site-directed mutagenesis was used to mutate the HNF-1β-binding site in the Pkhd1 promoter. Bottom: the alignment of the wild-type and mutated sequences. 4.7WT is a 4.7-kb genomic fragment containing 2.0 kb of the proximal 5′ flanking sequence, exon 1, intron 1, and 30 bp of exon 2 of mouse Pkhd1 linked to a Photinus luciferase reporter gene. 4.7M contains a mutation of the consensus HNF-1β-binding site in the 4.7-kb genomic fragment (X). B: maps of the constructs used to generate transgenic mice. 2.0/lacZ is the genomic fragment containing 2.0 kb of the proximal 5′ flanking sequence and 51 bp of exon 1 of mouse Pkhd1 linked to an Esherichia coli lacZ reporter gene containing a nuclear localization signal (NLS) and an intron and polyadenylation signal from the mouse protamine-1 (mP1) gene. For 2.0m/lacZ, site-directed mutagenesis was used to mutate the HNF-1β-binding site in the Pkhd1 promoter. 4.7/lacZ is a 4.7-kb genomic fragment containing 2.0 kb of the proximal 5′ flanking sequence, exon 1, intron 1, and 30 bp of exon 2 of mouse Pkhd1 linked to the lacZ reporter gene. In Pkhd1/Cre, the 4.7-kb genomic fragment is linked to Cre recombinase containing NLS and a human growth hormone minigene (hGH). Reporter genes are not drawn to scale.
A 2,682-bp genomic fragment containing 115 bp of exon 1, intron 1, and 30 bp of exon 2 was amplified from the 4.7WT/luc plasmid using Platinum Taq DNA Polymerase High Fidelity (Invitrogen) and the following primers: 5′-TAGAATACGCGTGAGCTACCTGCATCTTAGCTAGC-3′ and 5′-TAGAATCTCGAGGGGTTGATTGGCGCCATGTAG-3′. The PCR product was digested with MluI and XhoI and cloned into the corresponding restriction sites in pGL3-Promoter (Promega). The resulting plasmid, Pkhd1(2.7 kb), contained the 2,682-bp region of Pkhd1 ligated upstream to the Simian virus 40 (SV40) basal promoter and Photinus luciferase reporter gene.
Cell culture and reporter gene assays.
mIMCD3 cells (mouse inner medullary collecting duct cells) were grown in DMEM supplemented with 10% heat-inactivated FBS. Normal mouse cholangiocytes (NMCs) were a generous gift from Dr. Yoshiyuki Ueno (Tohoku University School of Medicine) and were grown in MEM supplemented with heat-inactivated FBS. 53A cells expressing DN-HNF-1β in response to treatment with mifepristone have been previously described (22). To perform reporter gene assays, cells were plated in six-well plastic dishes at a density of 1.5 × 105 cells/dish. After 24 h, when cells had reached 50% confluence, cells were transfected with equimolar amounts of plasmid DNA using Effectene (Qiagen). Forty-eight hours after transfection, cells were lysed and assayed for luciferase activity as previously described (13). To control for differences in transfection efficiency, cells were cotransfected with 0.02 ng of phRL-CMV encoding Renilla luciferase (Promega), and relative luciferase activity was calculated as the ratio of Photinus and Renilla luciferase.
LacZ reporter plasmids.
The lacZ reporter plasmids generated in this study were derived from pnLacF (32), which encodes Esherichia coli β-galactosidase fused to a nuclear localization signal from SV40. The 2.0-kb Pkhd1 PCR fragment extending from nucleotides −2030 to +51 was amplified as previously described, digested with SmaI and KpnI, and purified with a nucleotide removal kit (Qiagen). The plasmid pnLacF was digested with XbaI and blunt ended with Klenow fragment. The linearized plasmid was gel purified, digested with KpnI, and ligated to the 2.0-kb Pkhd1 fragment to generate the 2.0/lacZ reporter plasmid (Fig. 1B). Plasmid 2.0m/lacZ was generated by introducing a mutation of the HNF-1β-binding site at position −49 using site-directed mutagenesis as previously described (13). Plasmid 4.7/lacZ was generated by digesting the 4.7WT/luc plasmid with Kpn1 and Nco1 and cloning the 4.7-kb Pkhd1 restriction fragment into the KpnI and NcoI sites in pnLacF (Fig. 1B).
Generation of transgenic mice.
Transgenic mice carrying the Pkhd1 promoter linked to a lacZ reporter gene were generated as follows: plasmids 2.0/lacZ and 2.0m/lacZ were digested with KpnI and BglII, and plasmid 4.7/lacZ was digested with KpnI and SphI. Transgene DNA was purified by agarose gel electrophoresis, anion-exchange chromatography (EluTip-D, Schleicher & Schuell, Keene, NH), and ethanol precipitation. Transgenic mice were produced by the UT Southwestern Transgenic Core Facility, as previously described (32). Transgene DNA was microinjected into the pronuclei of fertilized C57/BL6J oocytes, transferred to pseudopregnant foster mothers, and permitted to develop to term. Progeny were genotyped by PCR analysis of tail biopsies and confirmed by Southern blot analysis. The primers used for PCR were as follows: 5′-ATCCTCTGCATGGTCAGGTC-3′ and 5′-CGTGGCCTGATTCATTCC-3′ for 4.7/lacZ mice, 5′-TCTAAGTGGCTCGCGTTAAT-3′ and 5′-CAGCTGGCGAAAGGGGGATG-3′ for 2.0/lacZ mice, and 5′-TCTAAGTGGCTCGCGTCGGC-3′ and 5′-CAGCTGGCGAAAGGGGGATG-3′ for 2.0m/lacZ mice. Expression of the transgenes was analyzed in founders and in F1 progeny derived from crosses with C57/BL6J and B6D2/F1J hybrid mice. Embryos were obtained from timed-pregnant mice in which the morning of detection of the copulatory plug was designated as embryonic day 0.5 (E0.5). Nontransgenic, age-matched littermates were used as controls. Pkhd1/Cre transgenic mice were generated by cloning the 4.7-kb Pkhd1 fragment into the plasmid pNukCre upstream to a Cre recombinase gene containing a nuclear localization signal (Fig. 1B) (33). Transgene DNA was purified and microinjected into fertilized oocytes as described above. Transgenic founders were bred to create permanent lines, and expression of the Cre transgene was detected by crossing with R26R or RYFP reporter mice. Pkhd1lacZ/+ knockin mice containing a lacZ gene replacing exons 1–3 of Pkhd1 have been previously described (38). All experiments involving animals were performed under the auspices of the UT Southwestern Institutional Animal Care and Research Advisory Committee. Unless otherwise indicated in the figures, experiments were performed in adult mice at 8–12 wk of age.
X-Gal staining.
Tissues were perfused with ice-cold PBS followed by PBS containing 2% paraformaldehyde. Kidneys were bisected, and tissues were immersed in 2% paraformaldehyde for 1 h. After fixation, samples were rinsed with PBS and incubated overnight at 4°C in PBS containing 30% sucrose. Cryoprotected samples were embedded in OCT medium, frozen in liquid nitrogen, sectioned at 5–10 μm, and mounted on Vectabond-coated slides. Specimens were immersed in PBS containing 20 mM Tris·Cl (pH 7.4), 2.0 mM spermidine, 2 mM MgCl2, 0.02% Nonidet P-40, 0.01% sodium deoxycholate, 5 mM potassium ferrocyanide, 5 mM potassium ferricyanide, and 1 mg/ml 5-bromo-4-chloro-3-indoyl-β-d-galactosidase (X-Gal, Stratagene). Staining with X-Gal was performed at 37°C in the dark with continuous agitation. Stained specimens were rinsed with PBS, counterstained with eosin, and photographed under bright-field illumination using a Zeiss Axioplan 2 microscope. Whole mount X-Gal staining was performed as previously described (18). Stained tissues were photographed using a Zeiss Stemi 2000-C microscope.
Antibody and lectin staining.
Paraformaldehyde-fixed cryosections were stained with antibodies against β-galactosidase (Promega), green fluorescent protein (GFP; Aves), HNF-1β (Santa Cruz Biotechnology), or Na+-K+-2Cl− cotransporter 2 (NKCC2; Alpha Diagnostic). Secondary antibodies were conjugated to Alexa fluor 488 or 594 (Molecular Probes, Eugene, OR). Lectin staining was performed using FITC-coupled Lotus tetragonolobus agglutinin (LTA) and Dolichos biflorus agglutinin (DBA; Vector Laboratories, Burlingame, CA). Some sections were stained with biotinylated DBA or LTA followed by detection with rhodamine avidin D (Vector Labs).
Chromatin immunoprecipitation.
Chromatin immunoprecipitation (ChIP) was performed using the ChIP-IT Kit (Active Motif) with modifications. Briefly, mIMCD3 cells or NMCs were cross-linked with 1% formaldehyde for 15 min at room temperature. Mouse kidney tissues were cut into small pieces and cross-linked in 1% formaldehyde with rocking at room temperature. Cross-linked tissues were homogenized into a single cell suspension and resuspended in ChIP buffer. DNA was sheared by sonication to produce an average fragment size of 500–1,000 bp. Immunoprecipitation was performed with 5 μg rabbit anti-HNF-1β (sc-22840-X, Santa Cruz Biotechnology) or 5 μg rabbit IgG (sc-2027) as a negative control. Precipitated complexes were captured on protein G agarose beads. Purified genomic DNA was amplified using promoter-specific primers and quantified using semiquantitative PCR; 1% of the input DNA was also amplified to normalize for enrichment, and DNA products were resolved on a 1% agarose gel.
Statistical analysis.
Statistical analysis was performed using Student's t-test for pairwise comparisons or ANOVA and Tukey's test for multiple comparisons. Contingency tables were analyzed using Fisher's exact test. P < 0.05 was considered significant.
RESULTS
The HNF-1β-binding site is required for Pkhd1 promoter activity in transfected renal epithelial cells and cholangiocytes.
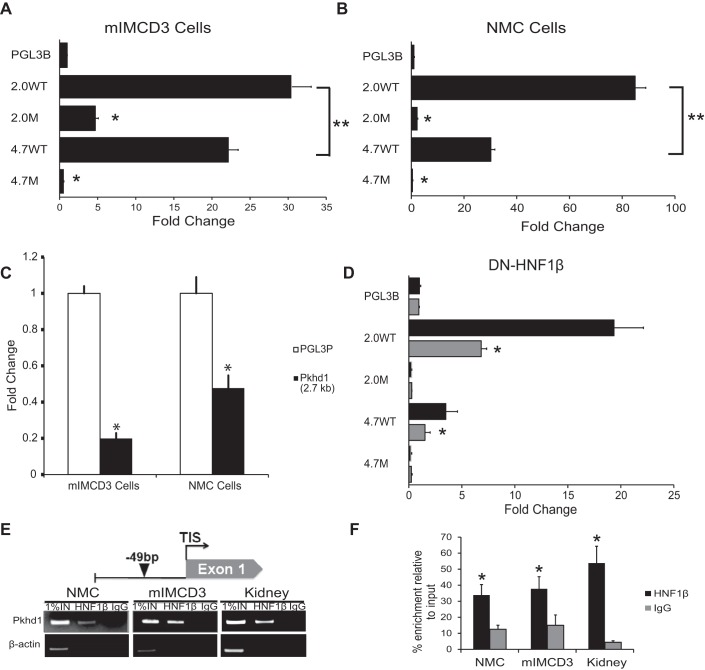
A previous study (13) has shown that a 4.5-kb genomic fragment containing 1.8 kb of the promoter region, exon 1, intron, 1, and exon 2 of mouse Pkhd1 can direct expression of a luciferase reporter gene in transfected mIMCD3 renal epithelial cells. To study the activity of the promoter itself, a DNA fragment containing 2,030 bp of the proximal 5′ flanking sequence, the transcription initiation site, and 51 bp of the first exon of Pkhd1 was cloned into the plasmid pGL3-Basic (Fig. 1A). When the resulting reporter plasmid (2.0WT/luc) was transfected into mIMCD3 cells, luciferase activity was stimulated 30-fold compared with empty pGL3-Basic (Fig. 2A). Transfection of mIMCD3 cells with a reporter plasmid (4.7WT/luc) containing the 2.0-kb promoter and 2.7 kb of additional genomic sequence extending downstream to the second exon stimulated luciferase activity 22-fold compared with pGL3-Basic (Fig. 2A). In addition to renal epithelial cells, Pkhd1 is endogenously expressed in bile ducts. Therefore, we tested the transcriptional activity of Pkhd1 genomic fragments in NMCs. Figure 2B shows that transfection of NMCs with reporter plasmids containing the 2.0-kb promoter produced an 85-fold stimulation of luciferase activity and that the 4.7-kb genomic fragment produced a 30-fold stimulation compared with empty pGL3-Basic. These results indicate that both 2.0- and 4.7-kb promoter fragments are transcriptionally active in cultured renal collecting duct cells and cholangiocytes. In both cell types, the transcriptional activity of the 2.0-kb fragment was significantly greater than the 4.7-kb fragment (double asterisks in Fig. 2, A and B). The additional 2.7-kb sequence that is present in the 4.7-kb fragment was cloned into pGL3-Promoter, a luciferase reporter plasmid containing a basal SV40 promoter. Transfection of the resulting plasmid, Pkhd1(2.7 kb), into mIMCD3 cells and NMCs inhibited luciferase activity compared with empty pGL3-Promoter (Fig. 2C). These results suggest that the 2.7-kb region contains regulatory elements that inhibit promoter activity, which likely explains the lower activity of the 4.7-kb fragment compared with the 2.0-kb fragment.
Fig. 2.
Pkhd1 promoter activity in transfected cells. A and B: mIMCD3 renal epithelial cells (A) and normal mouse cholangiocytes (NMCs; B) were transfected with equimolar amounts of the indicated luciferase reporter plasmids. Luciferase activity was measured after 48 h and normalized to empty pGL3-Basic (pGL3B). Data are means ± SE of six independent experiments. Both 2.0- and 4.7-kb genomic fragments drove transcription in mIMCD3 cells and NMCs. The 2.0-kb fragment had significantly greater activity than the 4.7-kb fragment (**P < 0.05). Mutation of the HNF-1β-binding site inhibited Pkhd1 promoter activity in both cell types. *P < 0.001 compared with wild type. C: mIMCD3 cells or NMCs were transfected with the plasmid Pkhd1(2.7 kb) or empty pGL3-Promoter (pGL3P), and luciferase activity was measured after 48 h. The 2.7-kb fragment inhibited promoter activity in both cell types. *P < 0.001 compared with pGL3-Promoter. D: 53A cells were transfected with the indicated reporter plasmids, and expression of dominant negative mutant HNF-1β (DN-HNF-1β) was induced with 10 nM mifepristone (Mif; shaded bars). Uninduced cells received vehicle [ethanol (EtOH); solid bars]. Luciferase activity was measured after 48 h and normalized to empty pGL3-Basic (pGL3B). *P < 0.05 compared with uninduced cells. E: schematic diagram of the mouse Pkhd1 promoter (top) showing the HNF-1β-binding site at −49 bp identified by chromatin immunoprecipitation (ChIP)-seq (arrowhead). ChIP (bottom) showed occupancy of the sites by endogenous HNF-1β in chromatin from NMCs, mIMCD3 cells, and the mouse kidney on postnatal day 28 (P28). Immunoprecipitation with IgG was used as a negative control, and the first lanes contained 1% of the input DNA. F: histogram showing densitometric analysis of three independent experiments. *P < 0.05 compared with control IgG.
We (13) have previously identified an evolutionarily conserved consensus binding site for the transcription factor HNF-1β located 49 bp upstream from the transcription initiation site in the proximal Pkhd1 promoter. A mutation that alters four conserved nucleotides within the HNF-1β-binding site located at position −49 bp was introduced into the 4.7-kb fragment and 2.0-kb promoter, and the effects on transcriptional activity were tested in mIMCD3 cells and NMCs. Figure 2, A and B, shows that mutation of the −49 bp site in the 2.0-kb promoter reduced luciferase activity 10-fold in transfected mIMCD3 cells and abolished promoter activity in transfected NMCs. Mutation of the −49 bp site in the 4.7-kb fragment also inhibited promoter activity in transfected mIMCD3 cells and NMCs. These results indicate that the HNF-1β-binding site at −49 bp is required for full transcriptional activity of the 2.0- and 4.7-kb fragments in both mIMCD3 cells and NMCs. To confirm these findings, we performed reporter gene assays in renal epithelial cells expressing DN-HNF-1β, which lacks the COOH-terminal activation domain and functions as a dominant negative mutant (22). Expression of DN-HNF-1β inhibited the activity of wild-type 2.0- and 4.7-kb fragments but did not affect fragments containing mutations of the HNF-1β-binding site (Fig. 2D). To verify that HNF-1β binds to the −49 bp site in vivo, ChIP assays were performed. Figure 2, E and F, shows that HNF-1β binds to the −49 bp site in chromatin extracted from NMCs, mIMCD3 cells, and the kidney. Taken together, these results indicate that HNF-1β directly binds to the −49 bp site and activates the Pkhd1 promoter.
Mutation of the HNF-1β-binding site inhibits Pkhd1 promoter activity in vivo.
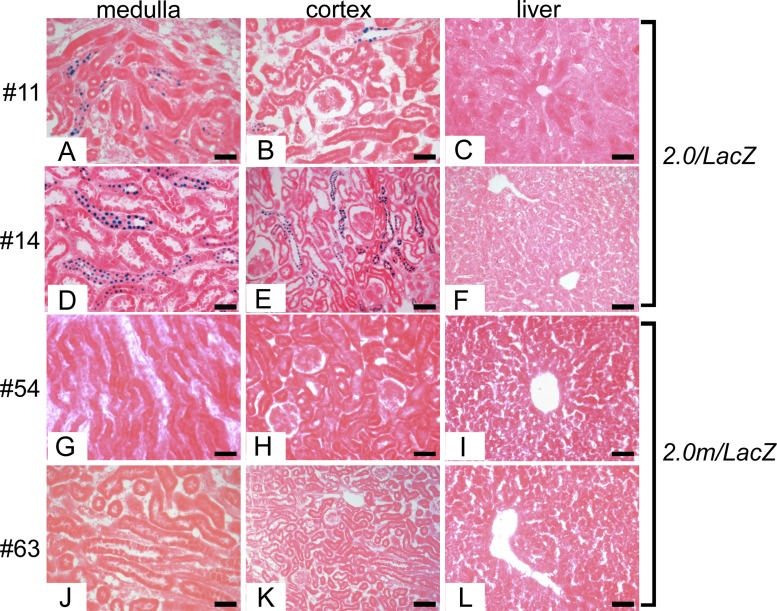
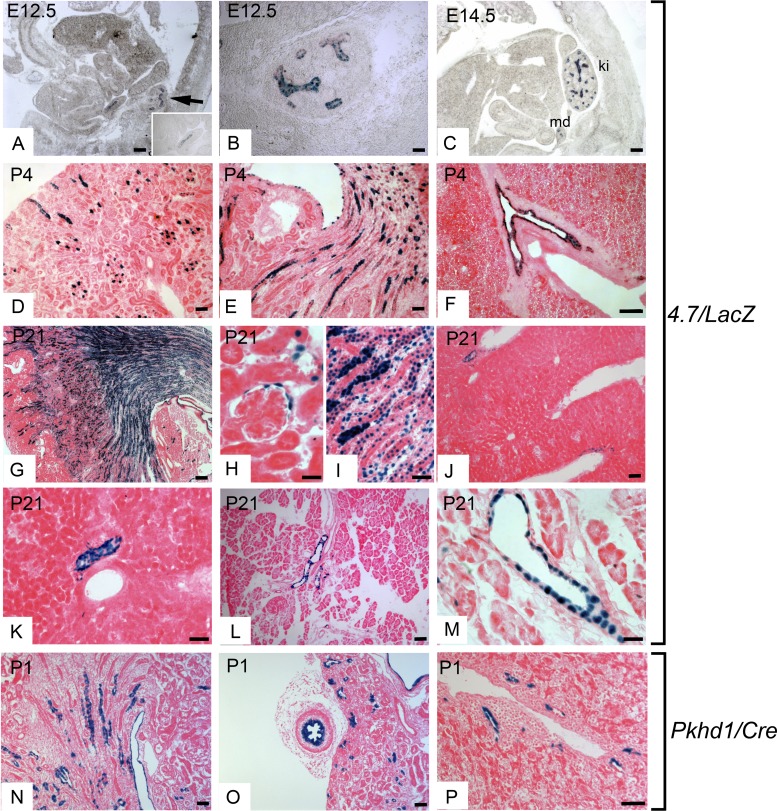
To determine whether the 2.0-kb Pkhd1 promoter is functional in vivo, we performed reporter gene assays in transgenic mice. We generated 2.0/lacZ transgenic mice containing 2,030 bp of the proximal 5′ flanking region, transcription initiation site, and 51 bp of the first exon of Pkhd1 linked to a reporter gene encoding E. coli β-galactosidase fused to a nuclear localization signal (Fig. 1B). The presence of the nuclear localization signal allows expression of the protein encoded by the transgene (nuclear) to be unequivocally distinguished from endogenous lysosomal β-galactosidase-like activity (cytoplasmic) (18). Eight independent founder mice carrying the 2.0/lacZ transgene were analyzed for transgene expression. Figure 3, A, B, D, and E, shows X-Gal staining of kidney sections from two independent founders (founders 11 and 14). The blue reaction product was present in the nuclei of a subset of tubular epithelial cells in the renal cortex and medulla, indicating that the 2.0-kb fragment contains a functional promoter that is active in the kidney. To identify the sites of expression in the kidney, sections were costained with antibodies against β-galactosidase and markers of specific nephron segments. Antibody staining confirmed that E. coli β-galactosidase encoded by the transgene was located in the nuclei of renal tubules (Fig. 4A). Costaining with nephron-specific markers revealed that the transgene was expressed in DBA-positive collecting ducts but was not expressed in LTA-positive proximal tubules or NKCC2-positive thick ascending limbs of loops of Henle (Fig. 4, A–I). Costaining with X-Gal and anti-NKCC2 antibody confirmed the lack of expression in thick ascending limbs. Taken together, these results indicate that the 2.0/lacZ transgene is expressed in cortical and medullary collecting ducts. No transgene expression was observed in glomeruli, blood vessels, or interstitial cells. Identical results were seen in five independent founders (Table 1), indicating that the observed expression was not solely due to position effects. Although the 2.0-kb promoter was active in cultured cholangiocytes, X-Gal staining of the liver did not show any blue reaction product in intrahepatic bile ducts (Fig. 3, C and F). The expression of lacZ in renal collecting ducts and absence in bile ducts suggests that regulatory elements located outside the 2.0-kb promoter are required for expression in cholangiocytes in vivo.
Fig. 3.
Expression of wild-type and mutant 2.0/lacZ transgenes in the kidney. X-Gal staining of adult kidney and liver sections from two independent 2.0/lacZ founders (founders 11 and 14) and two independent 2.0m/lacZ founders (founders 54 and 63) is shown. A, B, D, and E: blue nuclei indicate transgene expression in renal collecting ducts in mice carrying the wild-type 2.0-kb promoter. C, F, I, and L: neither the wild-type nor mutant transgene was expressed in the liver. G, H, J, and K: no transgene expression was observed in kidneys from mice carrying the 2.0-kb promoter with a mutation of the HNF-1β-binding site located at position −49 bp. Scale bars = 50 μm.
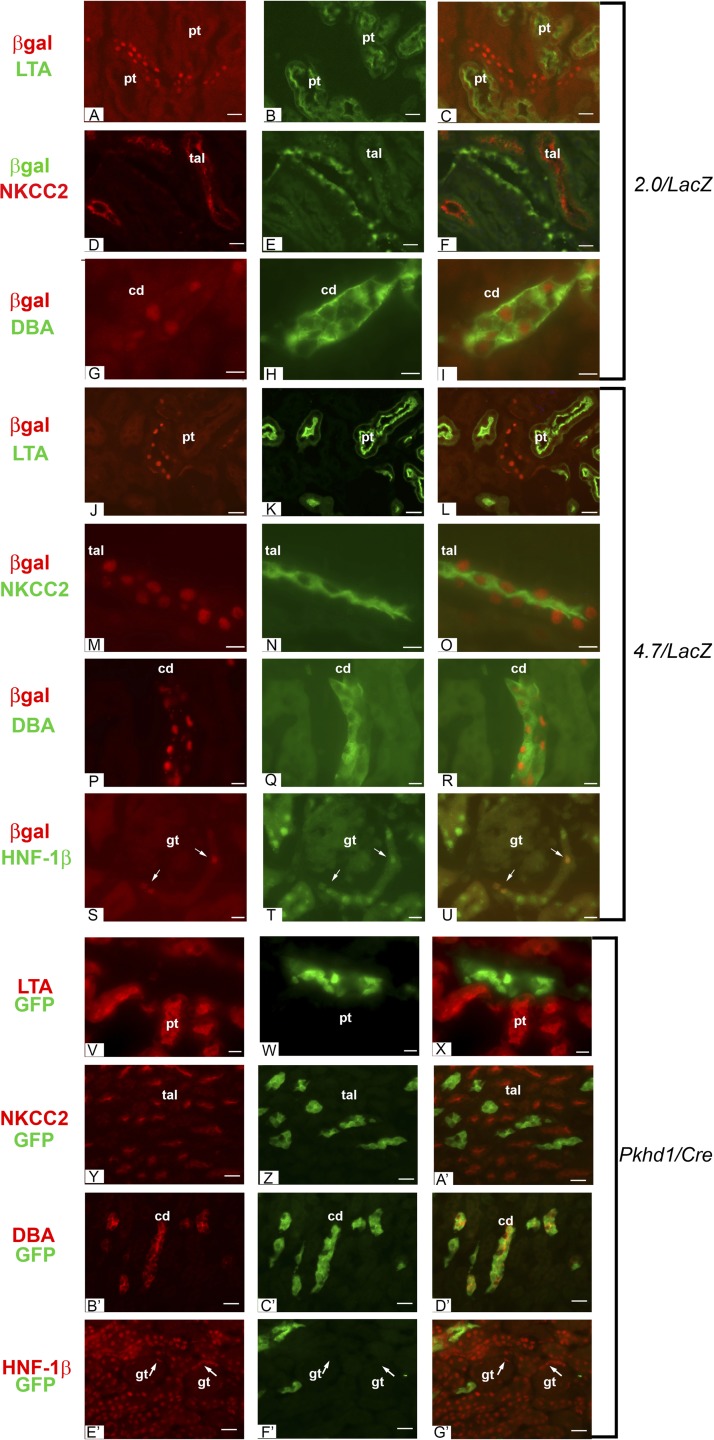
Fig. 4.
Expression of 2.0- and 4.7-kb transgenes in the kidney. A: antibody staining of kidney sections from adult 2.0/lacZ mice showed localization of E. coli β-galactosidase (red) in nuclei. B: costaining with FITC-conjugated Lotus tetragonolobus agglutinin (LTA; green) labeled proximal tubules (pt). C: merged image showing that the 2.0/lacZ transgene was not expressed in proximal tubules. D–F: costaining with antibodies against β-galactosidase (green) and Na+-K+-2Cl− cotransporter (NKCC2; red) showed no expression of the 2.0/lacZ transgene in thick ascending limbs of loops of Henle (tal). G–I: costaining with anti-β-galactosidase (red) and FITC-conjugated Dolichos biflorus agglutinin (DBA; green) showed expression of the 2.0/lacZ transgene in collecting ducts (cd). J–L: staining of kidney sections from adult 4.7/lacZ mice with an anti-β-galactosidase antibody (red) and FITC-conjugated LTA (green) showed that the transgene was not expressed in proximal tubules. M–O: costaining with antibodies against β-galactosidase (red) and NKCC2 (green) showed expression of the 4.7/lacZ transgene in thick ascending limbs of loops of Henle. P–R: costaining with anti-β-galactosidase antibody (red) and FITC-conjugated DBA (green) showed expression of the 4.7/lacZ transgene in collecting ducts. S–U: staining of the renal cortex from 4.7/lacZ mice with anti-β-galactosidase antibody (red) showed nuclear staining in parietal epithelia cells of Bowman's capsule (arrows), which also expressed HNF-1β (green). No transgene expression was observed in the glomerular tufts (gt). V–X: staining of kidney sections from adult Pkhd1/Cre;RYFP mice with an anti-green fluorescent protein (GFP) antibody (green) and biotinylated LTA followed by detection with rhodamine avidin D (red) showed that the transgene was not expressed in proximal tubules. Y–A′: costaining with antibodies against GFP (green) and NKCC2 (red) showed that the Pkhd1/Cre transgene was not expressed in thick ascending limbs of loops of Henle. B′–D′: costaining with anti-GFP antibody (green) and biotinylated DBA (red) showed expression of the Pkhd1/Cre transgene in collecting ducts. E′–G′: staining of the renal cortex from Pkhd1/Cre;RYFP mice with antibodies against GFP (green) and HNF-1β (red) showed no GFP expression in Bowman's capsule (arrows). Scale bars = 10 μm.
Table 1.
Expression of Pkhd1 transgenes in the kidney and extrarenal tissues
| Transgene | Founders Analyzed | Kidney Expression | Extrarenal Expression |
|---|---|---|---|
| 2.0/lacZ | 8 | 5 | 0 |
| 2.0m/lacZ | 8 | 0 | 0 |
| 4.7/lacZ | 11 | 4 | 1 |
| Pkhd1/Cre | 7 | 3 | 2 |
The first column shows the names of the transgenes. The second column shows the numbers of independent founders that were generated for each transgene. The third and fourth columns show the numbers of founders that expressed the transgene in the kidney and extrarenal tissues, respectively. Sixty-two percent of the founders carrying the 2.0/lacZ transgene showed expression in the kidney. In contrast, no founders carrying the same transgene with a mutated hepatocyte nuclear factor-1β-binding site (2.0m/lacZ) showed expression. This difference was statistically significant (P = 0.026 by Fisher's exact test). Only mice carrying the 4.7-kb polycystic kidney and hepatic disease 1 (Pkhd1) fragment (4.7/lacZ and Pkhd1/Cre) showed expression of the transgene in the liver and other extrarenal tissues.
To determine whether the HNF-1β-binding site located at position −49 bp in the proximal Pkhd1 promoter is necessary for promoter activity in the kidney, we generated 2.0m/lacZ transgenic mice containing a mutation that disrupts HNF-1β binding (Fig. 1B). Eight independent founders carrying the mutant 2.0m/lacZ transgene were analyzed. X-Gal staining of the kidneys showed no blue nuclei in the medulla or cortex (Fig. 3, G, H, J, and K). Similar to the wild-type promoter, no activity of the mutant promoter was observed in the liver (Fig. 3, I and L). Identical results were seen in multiple founders, indicating that the lack of expression of the mutant transgene was not due to position effects. A total of five of eight (62%) of the founders carrying the wild-type promoter showed expression in renal tubules, whereas zero of eight (0%) of the founders carrying the mutant promoter showed expression in the kidney. This difference was statistically significant (P = 0.026 by Fisher's exact test). These results indicate that the HNF-1β-binding site located at position −49 bp is required for Pkhd1 promoter activity in renal collecting ducts.
The 4.7-kb region containing the promoter and 5′ noncoding sequence directs expression in collecting ducts, loops of Henle, and Bowman's capsule.
The 2.0-kb Pkhd1 promoter was sufficient to direct reporter gene expression in renal collecting ducts; however, expression was not detected in proximal tubules and loops of Henle, which endogenously express Pkhd1. The regulatory elements that control gene transcription may be located in noncoding regions that are often evolutionarily conserved. The 5′ end of Pkhd1 extending from the first exon to the second exon is noncoding and is evolutionarily conserved in mice, humans, and other mammalian species. Therefore, we investigated whether the addition of this region can activate transgene expression in the other nephron segments where Pkhd1 is expressed. We produced transgenic mice carrying a 4.7-kb transgene including the 2.0-kb promoter, exon 1, intron 1, and 30 bp of exon 2 upstream to a lacZ reporter gene (Fig. 1B). Kidney sections were costained with antibodies against β-galactosidase and markers of specific nephron segments. Figure 4, J–L, shows that the transgene was not expressed in LTA-positive proximal tubules. Expression of β-galactosidase was identified in NKCC2-positive thick ascending limbs of loops of Henle (Fig. 4, M–O) and DBA-positive collecting ducts (Fig. 4, P–R). In addition, anti-β-galactosidase staining was observed in nuclei in glomerular parietal epithelial cells of Bowman's capsule, where it colocalized with HNF-1β (Fig. 4, S–U). These results indicate that a 4.7-kb transgene containing the Pkhd1 promoter and genomic sequence extending downstream to exon 2 is transcriptionally active in Bowman's capsules, loops of Henle, and collecting ducts, which are sites of endogenous Pkhd1 expression (26, 38, 42). No expression was detected in proximal tubules, possibly due to the low level of Pkhd1 expression in this nephron segment. Four independent 4.7/lacZ founders showed identical expression patterns in the kidney (Table 1).
Pkhd1 transgene expression in the developing kidney and extrarenal tissues.
To verify the tissue-specificity of the 4.7/lacZ transgene, we analyzed its expression in sagittal sections of whole embryos. Embryos were obtained from three independent 4.7/lacZ lines at E12.5 and E14.5, sectioned, and stained with X-Gal. Figure 5, A–C, shows that blue reaction product was only detected in the developing kidney and genitourinary tract, verifying the tissue specificity of the promoter. Transgene expression was detected in the Wolffian (mesonephric) duct, which is the anlage of the male reproductive tract, and in the branching ureteric bud, which gives rise to the renal collecting system (Fig. 5, A and B). In the E14.5 kidney (metanephros), the transgene was expressed exclusively in the branching ureteric bud (Fig. 5C). No expression was detected in the metanephric mesenchyme or S-shaped and comma-shaped bodies.
Fig. 5.
Developmental expression of 4.7/lacZ and Pkhd1/Cre transgenes. A: transgenic 4.7/lacZ embryos were harvested at embryonic day 12. 5 (E12.5), sectioned, and stained with X-Gal. The blue nuclei indicate Pkhd1 transgene expression in the metanephros (arrow) and Wolffian duct (inset). B: higher-magnification image of the metanephros at E12.5 showing transgene expression in the branching ureteric bud. C: sagittal section of an E14.5 embryo showing positive X-Gal staining (blue nuclei) in the kidney (ki) and mesonephric (Wolffian) duct (md). D and E: kidney section from a 4.7/lacZ mouse at P4 showing transgene expression in cortical and medullary collecting ducts. F: liver from a 4.7/lacZ mouse at P4 showing blue nuclei in cholangiocytes in intrahepatic bile ducts. G: kidney section from a 4.7/lacZ mouse at P21 showing blue nuclei in the renal cortex and medulla. H and I: higher-magnification images showing Pkhd1 transgene expression in Bowman's capsule and collecting ducts. J and K: liver sections from a 4.7/lacZ mouse at P21 showing positive X-Gal staining in intrahepatic bile ducts. L and M: images of X-Gal staining of the pancreas from a 4.7/lacZ mouse at P21 showing blue nuclei in intralobular exocrine pancreatic ducts. N: kidney cryosection from a Pkhd1/Cre;R26R bitransgenic mouse at P1 stained with X-Gal. Blue staining shows Cre/loxP recombination in collecting ducts and renal sinus. O: X-Gal staining of the ureter (left) and kidney (right) from a P1 Pkhd1/Cre;R26R mouse. Blue staining was observed in the luminal epithelium of the ureter, epithelium of the renal sinus, and collecting ducts in the kidney. P: X-Gal staining of a liver section from a Pkhd1/Cre;R26R mouse at P1 showing Cre/loxP recombination in periportal intrahepatic bile ducts. Scale bars = 50 μm.
X-Gal staining of kidneys from neonatal mice [postnatal day 4 (P4)] showed high expression in cortical and medullary collecting ducts (Fig. 5, D and E). In addition, lower levels of expression were detected in the epithelium of the renal pelvis, which is derived from the ureteric bud, and in developing loops of Henle (Fig. 5E). In the mature kidney (P21), X-Gal staining was present in nuclei of collecting ducts, thick ascending limbs of loops of Henle, and Bowman's capsules (Fig. 5, G–I). In addition to the kidney, one 4.7/lacZ transgenic line showed expression in extrarenal tissues. Staining of the liver at P4 and P21 showed expression in intrahepatic bile ducts (Fig. 5, F, J, and K). Expression in the liver was restricted to cholangiocytes; there was no expression in hepatocytes, endothelial cells, or interstitial cells. Expression of the transgene was also detected in pancreatic ducts at P21 (Fig. 5, L–M).
To confirm the expression of the 4.7-kb transgene in bile ducts, we generated Pkhd1/Cre transgenic mice carrying the identical 4.7-kb fragment inserted upstream to the Cre recombinase gene (Fig. 1). To detect transgene expression, Pkhd1/Cre mice were crossed with RYFP or R26R reporter mice carrying an enhanced yellow fluorescent protein (EYFP) or lacZ reporter gene that is activated by Cre/loxP recombination. Staining of kidneys from adult Pkhd1/Cre;RYFP mice with antibodies against GFP and nephron segment markers showed expression in renal collecting ducts but no expression in Bowman's capsules, proximal tubules, or loops of Henle (Fig. 4, V–G′). X-Gal staining of tissue sections from newborn Pkhd1/Cre:R26R mice showed expression in renal collecting ducts, epithelia of the renal sinus and ureter, and intrahepatic bile ducts (Fig. 5, N–P). Three independent Pkhd1/Cre founders showed expression in the kidney, and two lines showed expression in bile ducts. These results demonstrate that Pkhd1/Cre mice mediate Cre/loxP recombination in renal collecting ducts, the ureter, and intrahepatic bile ducts, which confirms the activity of the 4.7-kb transgene in these tissues. Table 1 shows the expression of the 4.7-kb transgenes in the kidney and extrarenal tissues.
Expression of Pkhd1 in the male reproductive tract.
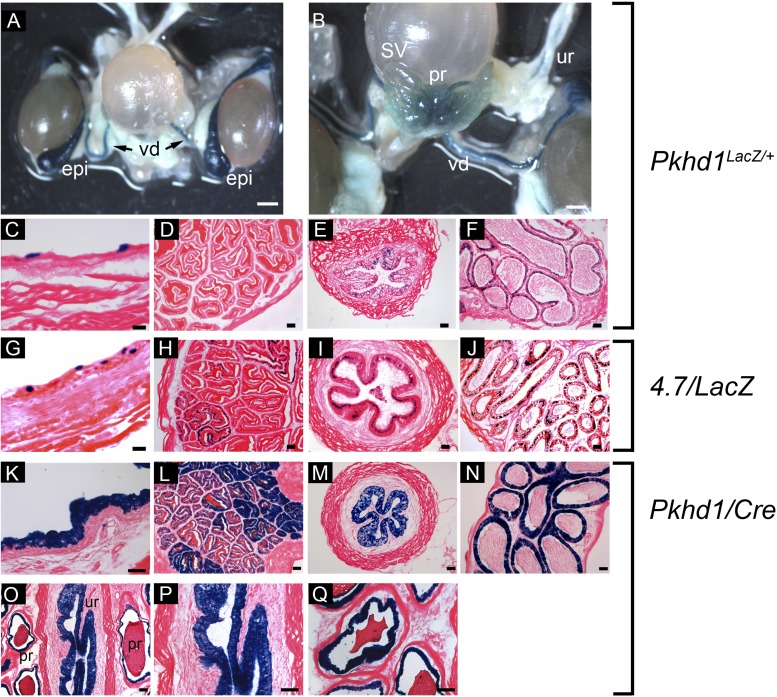
X-Gal staining of 4.7/lacZ embryos showed transgene expression in the Wolffian (mesonephric) duct, which is the embryonic anlage of the male reproductive tract. Therefore, we examined whether the transgene was also expressed in the adult derivatives, viz., the epididymis, vas deferens, and seminal vesicles. Since endogenous Pkhd1 gene expression has not previously been reported in these tissues in mice, we first analyzed Pkhd1lacZ/+ knockin mice carrying a lacZ reporter gene inserted by homologous recombination into the Pkhd1 locus. A previous study (38) has shown that expression of lacZ replicates the endogenous expression of Pkhd1 in the kidney, liver, and pancreas. Whole mount X-Gal staining of the genitourinary tract from an adult male Pkhd1lacZ/+ mouse showed strong staining in the vas deferens and epididymis and weak staining in seminal vesicles (Fig. 6, A and B). In addition, blue reaction product was detected in the ureter, prostate, and bladder. To identify the cellular sites of expression, tissues were sectioned and stained with X-Gal. Figure 6, C–F, shows heterocellular expression of lacZ in epithelial cells in the bladder, vas deferens, and epididymis. No blue reaction product was detected in the seminal vesicles, possibly reflecting lower sensitivity compared with whole mount staining. Having established that endogenous Pkhd1 is expressed in the male genitourinary tract, we next tested whether the 4.7-kb genomic fragment can direct transgene expression in these tissues. Figure 6 shows that the 4.7-kb fragment directs expression of lacZ and Cre recombinase in bladder (G and K), seminal vesicles (H and L), vas deferens (I and M), and epididymis (J and N). Blue reaction product was also detected in the prostate and urethra (Fig. 6, O–Q). Taken together, these data indicate that the 4.7-kb genomic fragment is sufficient to drive expression of Pkhd1 in the male genitourinary tract. Female mice showed expression in the kidney and liver but no expression in Müllerian duct derivatives (not shown).
Fig. 6.
Correlation between endogenous Pkhd1 gene expression and transcriptional activity of the 4.7-kb genomic fragment in the male genitourinary tract. A and B: whole mount X-Gal staining of the genitourinary tract from an adult male Pkhd1lacZ/+ knockin mouse showing sites of endogenous Pkhd1 gene expression. Strong staining was observed in the vas deferens (vd), epididymis (epi), prostate (pr), and ureter (ur); weak staining was seen in seminal vesicles (SV). C–F: X-Gal stained cryosections from an adult Pkhd1lacZ/+ mouse showing expression in the epithelia of the bladder (C), vas deferens (E), and epididymis (F). No expression was detected in sections of seminal vesicles (D). G–J: X-Gal-stained sections from an adult 4.7/lacZ mouse showing expression of the Pkhd1 transgene in the bladder (G), seminal vesicles (H), vas deferens (I), and epididymis (J). K–N: X-Gal-stained sections from a Pkhd1/Cre;R26R mouse showing positive staining in the bladder (K), seminal vesicles (L), vas deferens (M), and epididymis (N). O–Q: X-Gal staining of the prostate from an adult male Pkhd1/Cre;R26R mouse showing expression in the prostatic epithelium and urethra. Scale bars = 200 μm in A and B and 50 μm in C–Q.
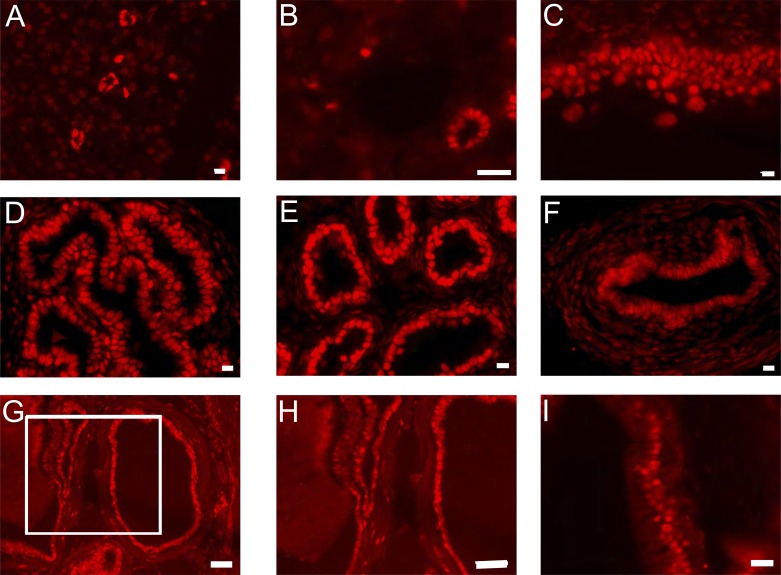
To determine whether the extrarenal expression of the 4.7-kb transgene correlates with sites of expression of HNF-1β, we stained tissue sections from adult wild-type mice with an anti-HNF-1β antibody. Consistent with previous studies of HNF-1β/lacZ knockin mice (5, 30), HNF-1β protein was present in nuclei of epithelial cells in pancreatic ducts, intrahepatic bile ducts, bladder, seminal vesicles, epididymis, vas deferens, prostate, and urethra (Fig. 7). These findings indicate that expression of Pkhd1 corresponds to sites of expression of HNF-1β in the kidney and extrarenal tissues.
Fig. 7.
Expression of HNF-1β in extrarenal tissues. Anti-HNF-1β antibody staining of adult wild-type mouse tissues showed expression of HNF-1β in the nuclei of epithelial cells in the pancreatic ducts (A), intrahepatic bile ducts (B), bladder (C), seminal vesicles (D), epididymis (E), vas deferens (F), prostate (G and H), and urethra (I). Scale bars = 10 μm.
DISCUSSION
In this study, we used transgenic and knockin mice to better understand the regulation of Pkhd1 gene expression in vivo. We show that a 2.0-kb region extending upstream from the first exon of mouse Pkhd1 can direct expression of a lacZ reporter gene in renal collecting ducts. Similar to endogenous Pkhd1 (26), expression of the transgene was restricted to epithelial cells in the kidney, indicating that promoter activity was cell specific. To our knowledge, this study is the first to demonstrate activity of the Pkhd1 promoter in vivo. The 2.0-kb region is located upstream from the longest Pkhd1 coding sequence in the GenBank database (Accession No. AY130764). Pkhd1 is large and subject to complex alternative splicing and potentially alternative promoters (26, 36). The present study suggests that the transcript encoding the longest open reading frame is expressed in renal collecting ducts under the control of the 2.0-kb promoter. None of the promoter fragments analyzed in this study showed expression in the lung, adrenal gland, testis, or blood vessels, which have also been reported to be sites of Pkhd1 expression (26, 40). It is possible that expression in these other tissues may be under the control of promoters located elsewhere in the gene locus.
The 2.0-kb Pkhd1 promoter contains an evolutionarily conserved binding site for the transcription factor HNF-1β located 49 bp upstream from the transcription initiation site. ChIP assays verified that HNF-1β binds to these sites in chromatin from kidney and cultured renal epithelial cells and cholangiocytes. Mutation of the −49 bp site strongly inhibited promoter activity in transfected mIMCD3 cells and NMCs, indicating that binding of HNF-1β to the site at −49 bp is required for full promoter activity. To test whether the HNF-1β-binding site at −49 bp is required for promoter activity in vivo, we generated transgenic mice carrying a mutant 2.0-kb promoter linked to a lacZ reporter gene. Sixty-two percent of the founders carrying the wild-type promoter showed expression in renal collecting ducts compared with none of the founders carrying the mutant promoter. This difference was statistically significant and indicates that HNF-1β binds to the −49 bp site and directly regulates Pkhd1 promoter activity in renal collecting ducts in vivo.
HNF-1β is a sequence-specific DNA-binding transcription factor that is expressed in epithelial cells of the kidney, liver, lung, gut, and pancreas (17). HNF-1β binds to DNA as a homodimer or heterodimer with HNF-1α and is important in organogenesis. Mutations of HNF-1β cause maturity-onset diabetes of the young 5, characterized by renal cystic disease, diabetes, cholestasis, pancreatic exocrine dysfunction, and genitourinary abnormalities (4, 7). Kidney-specific inactivation of HNF-1β and expression of DN-HNF-1β produces cystic kidney disease associated with downregulation of Pkhd1 (10, 13, 15). Liver-specific inactivation of HNF-1β produces ductal plate malformations and liver cysts similar to the phenotype of Pkhd1 knockout mice (6). Moreover, the phenotypes seen in children with ARPKD and HNF-1β mutations overlap. Taken together, PKHD1 and HNF-1β may participate in a common pathway of cystogenesis and biliary dysgenesis. The HNF-1β-binding site is conserved in the human PKHD1 promoter, which raises the possibility that mutations of the binding site might also inhibit PKHD1 transcription in humans. Further studies will be needed to determine whether mutations of the PKHD1 promoter or the HNF-1β-binding site are found in patients with ARPKD in whom mutations of the coding sequence have not been identified.
The activity of the 2.0-kb Pkhd1 promoter was restricted to renal collecting ducts, whereas previous studies have indicated that Pkhd1 and its protein product, fibrocystin/polyductin, are also expressed in loops of Henle and/or proximal tubules (8, 26, 37, 42). Transgenic 4.7/lacZ mice containing the longer 4.7-kb Pkhd1 genomic fragment showed expression in collecting ducts and thick ascending limbs of loops of Henle. No expression was detected in proximal tubules, possibly reflecting the low level of Pkhd1 expression in this nephron segment or a requirement for regulatory elements outside the 4.7-kb region. The 4.7/lacZ transgene was also expressed in glomerular parietal epithelial cells, which may explain the glomerular cysts seen in some Pkhd1 knockout mice (38) and mice expressing DN-HNF-1β (13). Although glomerular cysts have been observed in human ARPKD (20), they are not a typical feature of the disease and are not observed in all Pkhd1 mutant models. Pkhd1/Cre transgenic mice containing the 4.7-kb fragment linked to Cre recombinase showed expression in renal collecting ducts but not in loops of Henle or Bowman's capsules. The explanation for this variability is not known but may relate to different integration sites of the transgenes.
In the embryonic kidney, the 4.7-kb transgene was expressed in the branching ureteric bud, which gives rise to the renal collecting ducts. No transgene expression was detected in the metanephric mesenchyme or comma- or S-shaped bodies. This pattern is identical to the expression of Pkhd1 mRNA previously reported using in situ hybridization (26), which indicates that the 4.7-kb transgene recapitulates the developmental expression of Pkhd1 in the kidney. The expression of the 4.7-kb transgene overlaps with the expression of HNF-1β in the developing kidney (3, 21), suggesting that HNF-1β also regulates the activity of the Pkhd1 promoter during development.
Although the 2.0-kb promoter was active in cultured cholangiocytes, none of the transgenic mice carrying the 2.0/lacZ transgene showed expression in intrahepatic bile ducts in vivo. Reporter gene assays in transfected cells and transgenic mice can produce different results, which has been attributed to differences in chromatin structure between transiently transfected plasmids and chromosomally integrated DNA (31). The absence of bile duct expression in multiple independent founders that showed expression in the kidney suggests that the results cannot be explained by transgene integration into transcriptionally inactive heterochromatin (position effects). Instead, these findings suggest that additional elements located outside the 2.0-kb promoter are necessary for extrarenal Pkhd1 gene expression. To explore this possibility further, we generated transgenic mice carrying the 4.7-kb promoter fragment linked to a β-galactosidase reporter gene (4.7/lacZ) or a Cre recombinase gene (Pkhd1/Cre). Both 4.7/lacZ mice and Pkhd1/Cre mice showed expression in adult intrahepatic bile ducts. This pattern was observed in three independent founders, indicating that expression in bile ducts is not simply a consequence of the transgene insertion site (position effects). Although both 4.7/lacZ mice and Pkhd1/Cre mice showed expression in the postnatal liver, neither transgene was expressed in the embryonic liver. Pkhd1 is endogenously expressed in the developing liver, and mutations of PKHD1 cause ductal plate malformations in ARPKD patients and knockout mice. Taken together, these findings suggest that the 4.7-kb transgene contains elements that can direct expression in the postnatal liver but lacks elements required for expression in the developing liver.
Transgenes containing the 2.0-kb Pkhd1 promoter are expressed in renal collecting ducts but are not expressed in bile ducts. The addition of downstream genomic sequence contained from exons 1-2 produces expression in bile ducts. However, this region is deleted in Pkhd1lacZ/+ knockin mice, which also show expression in bile ducts. We conclude that the 2.0-kb promoter is sufficient for expression in renal collecting ducts, but additional genomic sequences located from exons 1-2 (4.7-kb transgene) or elsewhere in the gene locus (Pkhd1lacZ/+ mice) are needed for significant expression in extrarenal tissues. Similar to other genes, such as α-fetoprotein, tissue-specific expression of Pkhd1 may be controlled by multiple, redundant enhancers (12).
Both 4.7/lacZ mice and Pkhd1/Cre mice showed expression in the Wolffian duct and its adult derivatives, including the epididymis, vas deferens, and seminal vesicles. Expression was also observed in the epithelia of the prostate, bladder, and urethra. Analysis of Pkhd1lacZ/+ knockin mice confirmed that Pkhd1 is endogenously expressed in the epithelia of the bladder, prostate, epididymis, and vas deferens, and antibody staining showed that HNF-1β is also expressed in these tissues. The significance of Pkhd1 expression in the male reproductive tract is not known. However, epididymal cysts are seen in males with germline mutations of HNF-1β and have also been described in some patients with ARPKD, although they are not a typical feature (28).
In conclusion, the proximal 2.0 kb of the Pkhd1 promoter is sufficient for tissue-specific expression in renal collecting ducts in vivo, and the transcription factor HNF-1β is required for Pkhd1 promoter activity in collecting ducts. Additional genomic sequences located from exons 1-2 or elsewhere in the gene locus are required for expression in intrahepatic bile ducts and other extrarenal tissues. Pkhd1 is expressed in epithelial cells in the male reproductive tract, where it is coexpressed with HNF-1β. Finally, the Pkhd1/Cre transgenic mice generated in this study may be useful for tissue-specific gene targeting in renal collecting ducts, the male reproductive tract, and intrahepatic bile ducts.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grant R37 DK 042921 and the UT Southwestern O'Brien Kidney Research Core Center (NIDDK Grant P30 DK 079328). S. S. Williams and K. Aboudehen were supported by a NIDDK Training Grant T32 DK 007257. V. Patel is the recipient of National Institutes of Health Mentored Clinical-Scientist Development Award (NIDDK Grant K08 DK 084311).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.S.W., P.C.-S., S.H., X.S., V.P., and P.I. conception and design of research; S.S.W., P.C.-S., S.H., K.A., and X.S. performed experiments; S.S.W., P.C.-S., S.H., K.A., X.S., J.A.R., V.P., and P.I. analyzed data; S.S.W., P.C.-S., S.H., K.A., X.S., J.A.R., V.P., and P.I. interpreted results of experiments; S.S.W., P.C.-S., S.H., K.A., and P.I. prepared figures; S.S.W. and P.I. drafted manuscript; S.S.W., P.C.-S., S.H., K.A., X.S., J.A.R., V.P., and P.I. approved final version of manuscript; P.C.-S., S.H., J.A.R., V.P., and P.I. edited and revised manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. Yoshiyuki Ueno (Tohoku University School of Medicine) for providing NMCs. The Pkhd1/Cre mice described in this study are available from the UT Southwestern O'Brien Kidney Research Core Center (www.utsouthwestern.edu/nephrology/obrien).
Present address of S. S. Williams: Pediatric Nephrology, Our Lady of the Lake Children's Hospital, Baton Rouge, LA 70808.
Footnotes
REFERENCES
- 1.Adeva M, El-Youssef M, Rossetti S, Kamath PS, Kubly V, Consugar MB, Milliner DM, King BF, Torres VE, Harris PC. Clinical and molecular characterization defines a broadened spectrum of autosomal recessive polycystic kidney disease (ARPKD). Medicine (Baltimore) 85: 1–21, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Bakeberg JL, Tammachote R, Woollard JR, Hogan MC, Tuan HF, Li M, van Deursen JM, Wu Y, Huang BQ, Torres VE, Harris PC, Ward CJ. Epitope-tagged Pkhd1 tracks the processing, secretion, and localization of fibrocystin. J Am Soc Nephrol 22: 2266–2277, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barbacci E, Reber M, Ott MO, Breillat C, Huetz F, Cereghini S. Variant hepatocyte nuclear factor 1 is required for visceral endoderm specification. Development 126: 4795–4805, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Bellanne-Chantelot C, Chauveau D, Gautier JF, Dubois-Laforgue D, Clauin S, Beaufils S, Wilhelm JM, Boitard C, Noel LH, Velho G, Timsit J. Clinical spectrum associated with hepatocyte nuclear factor-1beta mutations. Ann Intern Med 140: 510–517, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Coffinier C, Barra J, Babinet C, Yaniv M. Expression of the vHNF1/HNF1b homeoprotein gene during mouse organogenesis. Mech Dev 89: 211–213, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Coffinier C, Gresh L, Fiette L, Tronche F, Schutz G, Babinet C, Pontoglio M, Yaniv M, Barra J. Bile system morphogenesis defects and liver dysfunction upon targeted deletion of HNF1β. Development 129: 1829–1838, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Edghill EL, Bingham C, Ellard S, Hattersley AT. Mutations in hepatocyte nuclear factor-1beta and their related phenotypes. J Med Genet 43: 84–90, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gallagher AR, Esquivel EL, Briere TS, Tian X, Mitobe M, Menezes LF, Markowitz GS, Jain D, Onuchic LF, Somlo S. Biliary and pancreatic dysgenesis in mice harboring a mutation in Pkhd1. Am J Pathol 172: 417–429, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garcia-Gonzalez MA, Menezes LF, Piontek KB, Kaimori J, Huso DL, Watnick T, Onuchic LF, Guay-Woodford LM, Germino GG. Genetic interaction studies link autosomal dominant and recessive polycystic kidney disease in a common pathway. Hum Mol Genet 16: 1940–1950, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gresh L, Fischer E, Reimann A, Tanguy M, Garbay S, Shao X, Hiesberger T, Fiette L, Igarashi P, Yaniv M, Pontoglio M. A transcriptional network in polycystic kidney disease. EMBO J 23: 1657–1668, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guay-Woodford LM, Desmond RA. Autosomal recessive polycystic kidney disease; the clinical experience in North America. Pediatrics 111: 1072–1080, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Hammer RE, Krumlauf R, Camper SA, Brinster RL, Tilghman SM. Diversity of α-fetoprotein gene expression in mice is generated by a combination of separate enhancer elements. Science 235: 53–58, 1987 [DOI] [PubMed] [Google Scholar]
- 13.Hiesberger T, Bai Y, Shao X, McNally BT, Sinclair AM, Tian X, Somlo S, Igarashi P. Mutation of hepatocyte nuclear factor-1β inhibits Pkhd1 gene expression and produces renal cysts in mice. J Clin Invest 113: 814–825, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hiesberger T, Gourley E, Erickson A, Koulen P, Ward CJ, Masyuk TV, Larusso NF, Harris PC, Igarashi P. Proteolytic cleavage and nuclear translocation of fibrocystin is regulated by intracellular Ca2+ and activation of protein kinase C. J Biol Chem 281: 34357–34364, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Hiesberger T, Shao X, Gourley E, Reimann A, Pontoglio M, Igarashi P. Role of the hepatocyte nuclear factor-1β (HNF-1β) C-terminal domain in Pkhd1 (ARPKD) gene transcription and renal cystogenesis. J Biol Chem 280: 10578–10586, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Hogan MC, Manganelli L, Woollard JR, Masyuk AI, Masyuk TV, Tammachote R, Huang BQ, Leontovich AA, Beito TG, Madden BJ, Charlesworth MC, Torres VE, LaRusso NF, Harris PC, Ward CJ. Characterization of PKD protein-positive exosome-like vesicles. J Am Soc Nephrol 20: 278–288, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Igarashi P, Shao X, McNally BT, Hiesberger T. Roles of HNF-1β in kidney development and congenital cystic diseases. Kidney Int 68: 1944–1947, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Igarashi P, Shashikant CS, Thomson RB, White DA, Liu-Chen S, Ruddle FH, Aronson PS. Ksp-cadherin gene promoter. II. Kidney-specific activity in transgenic mice. Am J Physiol Renal Physiol 277: F599–F610, 1999 [DOI] [PubMed] [Google Scholar]
- 19.Kaimori JY, Nagasawa Y, Menezes LF, Garcia-Gonzalez MA, Deng J, Imai E, Onuchic LF, Guay-Woodford LM, Germino GG. Polyductin undergoes notch-like processing and regulated release from primary cilia. Hum Mol Genet 16: 942–956, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lennerz JK, Spence DC, Iskandar SS, Dehner LP, Liapis H. Glomerulocystic kidney: one hundred-year perspective. Arch Pathol Lab Med 134: 583–605, 2010 [DOI] [PubMed] [Google Scholar]
- 21.Lokmane L, Heliot C, Garcia-Villalba P, Fabre M, Cereghini S. vHNF1 functions in distinct regulatory circuits to control ureteric bud branching and early nephrogenesis. Development 137: 347–357, 2010 [DOI] [PubMed] [Google Scholar]
- 22.Ma Z, Gong Y, Patel V, Karner CM, Fischer E, Hiesberger T, Carroll TJ, Pontoglio M, Igarashi P. Mutations of HNF-1β inhibit epithelial morphogenesis through dysregulation of SOCS-3. Proc Natl Acad Sci USA 104: 20386–20391, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mai W, Chen D, Ding T, Kim I, Park S, Cho SY, Chu JS, Liang D, Wang N, Wu D, Li S, Zhao P, Zent R, Wu G. Inhibition of Pkhd1 impairs tubulomorphogenesis of cultured IMCD cells. Mol Biol Cell 16: 4398–4409, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Menezes LF, Cai Y, Nagasawa Y, Silva AM, Watkins ML, Da Silva AM, Somlo S, Guay-Woodford LM, Germino GG, Onuchic LF. Polyductin, the PKHD1 gene product, comprises isoforms expressed in plasma membrane, primary cilium, and cytoplasm. Kidney Int 66: 1345–1355, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Moser M, Matthiesen S, Kirfel J, Schorle H, Bergmann C, Senderek J, Rudnik-Schoneborn S, Zerres K, Buettner R. A mouse model for cystic biliary dysgenesis in autosomal recessive polycystic kidney disease (ARPKD). Hepatology 41: 1113–1121, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Nagasawa Y, Matthiesen S, Onuchic LF, Hou X, Bergmann C, Esquivel E, Senderek J, Ren Z, Zeltner R, Furu L, Avner E, Moser M, Somlo S, Guay-Woodford L, Buttner R, Zerres K, Germino GG. Identification and characterization of Pkhd1, the mouse orthologue of the human ARPKD gene. J Am Soc Nephrol 13: 2246–2258, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Nishio S, Tian X, Gallagher AR, Yu Z, Patel V, Igarashi P, Somlo S. Loss of oriented cell division does not initiate cyst formation. J Am Soc Nephrol 21: 295–302, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nistal M, Gonzalez-Peramato P, Sousa G, Garcia-Cabezas MA, Rodriguez JI, Cajaiba MM. Cystic dysplasia of the epididymis: a disorder of mesonephric differentiation associated with renal maldevelopment. Virchows Arch 456: 695–702, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Onuchic LF, Furu L, Nagasawa Y, Hou X, Eggermann T, Ren Z, Bergmann C, Senderek J, Esquivel E, Zeltner R, Rudnik-Schoneborn S, Mrug M, Sweeney W, Avner ED, Zerres K, Guay-Woodford LM, Somlo S, Germino GG. PKHD1, the polycystic kidney and hepatic disease 1 gene, encodes a novel large protein containing multiple immunoglobulin-like plexin-transcription-factor domains and parallel β-helix 1 repeats. Am J Hum Genet 70: 1305–1317, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reber M, Cereghini S. Variant hepatocyte nuclear factor 1 expression in the mouse genital tract. Mech Dev 100: 75–78, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Recillas-Targa F. Multiple strategies for gene transfer, expression, knockdown, and chromatin influence in mammalian cell lines and transgenic animals. Mol Biotechnol 34: 337–354, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Shao X, Johnson JE, Richardson JA, Hiesberger T, Igarashi P. A minimal Ksp-cadherin promoter linked to a green fluorescent protein reporter gene exhibits tissue-specific expression in the developing kidney and genitourinary tract. J Am Soc Nephrol 13: 1824–1836, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Shao X, Somlo S, Igarashi P. Epithelial-specific Cre/lox recombination in the developing kidney and genitourinary tract. J Am Soc Nephrol 13: 1837–1846, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Wang S, Luo Y, Witman GB, Zhou J. The autosomal recessive polycystic kidney disease protein is localized to primary cilia with concentration in the basal body area. J Am Soc Nephrol 15: 592–602, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Wang S, Zhang J, Nauli SM, Li X, Starremans PG, Luo Y, Roberts KA, Zhou J. Fibrocystin/polyductin, found in the same protein complex with polycystin-2, regulates calcium responses in kidney epithelia. Mol Cell Biol 27: 3241–3252, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ward CJ, Hogan MC, Rossetti S, Walker D, Sneddon T, Wang X, Kubly V, Cunningham JM, Bacallao R, Ishibashi M, Milliner DS, Torres VE, Harris PC. The gene mutated in autosomal recessive polycystic kidney disease encodes a large, receptor-like protein. Nat Genet 30: 259–269, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Ward CJ, Yuan D, Masyuk TV, Wang X, Punyashthiti R, Whelan S, Bacallao R, Torra R, LaRusso NF, Torres VE, Harris PC. Cellular and subcellular localization of the ARPKD protein; fibrocystin is expressed on primary cilia. Hum Mol Genet 12: 2703–2710, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Williams SS, Cobo-Stark P, James LR, Somlo S, Igarashi P. Kidney cysts, pancreatic cysts, and biliary disease in a mouse model of autosomal recessive polycystic kidney disease. Pediatr Nephrol 23: 733–741, 2008 [DOI] [PubMed] [Google Scholar]
- 39.Woollard JR, Punyashtiti R, Richardson S, Masyuk TV, Whelan S, Huang BQ, Lager DJ, vanDeursen J, Torres VE, Gattone VH, LaRusso NF, Harris PC, Ward CJ. A mouse model of autosomal recessive polycystic kidney disease with biliary duct and proximal tubule dilatation. Kidney Int 72: 328–336, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Xiong H, Chen Y, Yi Y, Tsuchiya K, Moeckel G, Cheung J, Liang D, Tham K, Xu X, Chen XZ, Pei Y, Zhao ZJ, Wu G. A novel gene encoding a TIG multiple domain protein is a positional candidate for autosomal recessive polycystic kidney disease. Genomics 80: 96–104, 2002 [DOI] [PubMed] [Google Scholar]
- 41.Zerres K, Rudnik-Schoneborn S, Steinkamm C, Becker J, Mucher G. Autosomal recessive polycystic kidney disease. J Mol Med 76: 303–309, 1998 [DOI] [PubMed] [Google Scholar]
- 42.Zhang MZ, Mai W, Li C, Cho Sy Hao C, Moeckle G, Zhao R, Kim I, Wang J, Xiong H, Wange H, Sato Y, Wu Y, Nakanuma Y, Lilova M, Pei Y, Harris RC, Li S, Coffey RJ, Sun L, Wu D, Chen XZ, Breyer MD, Zhao ZJ, McKanna JA, Wu G. PKHD1 protein encoded by the gene for autosomal recessive polycystic kidney disease associates with basal bodies and primary cilia in renal epithelial cells. Proc Natl Acad Sci USA 101: 2311–2316, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]