Abstract
Rationale
Intestinal anaphylaxis (manifested by acute diarrhea) is dependent on IgE and mast cells.
Objective
We aimed to define the respective roles of IL-4 and IL-13 and their receptors in disease pathogenesis.
Methods
Wild-type mice and mice deficient in IL-4, IL-13, and IL-13Rα1 (part of the type 2 IL-4R) were sensitized with ovalbumin (OVA)/alum and subsequently given repeated intragastric OVA exposures. IL-4Rα chain was targeted with anti-IL-4Rα mAb prior to or after intragastric OVA exposures.
Results
IL-4−/− (and IL-4/13−/−) mice produced almost no IgE and were highly resistant to OVA-induced diarrhea, whereas allergic diarrhea was only partially impaired in IL-13−/− and IL-13Rα1−/− mice. IL-13Rα1-deficient mice developed decreased IgE despite having normal baseline IL-4 levels. Intestinal mast cell accumulation and activation also depended mainly on IL-4 and to a lesser extent on IL-13. Prophylactic anti-IL-4Rα mAb treatment, which blocks all IL-4 and IL-13 signaling, suppressed development of allergic diarrhea. However, treatment with anti-IL-4Rα mAb for 7 days only partially suppressed IgE and did not prevent intestinal diarrhea.
Conclusion
Endogenously-produced IL-13 supplements the ability of IL-4 to induce allergic diarrhea by promoting oral allergen sensitization rather than the effector phase of intestinal anaphylaxis.
Keywords: allergy, anaphylaxis, IL-4, IL-13, IL-13Ralpha1, intestine, mast cell
INTRODUCTION
Currently 2–6% of the population in the U.S. suffers from food allergy;1 a disease characterized by elevated total and Ag-specific IgE eosinophilia, mastocytosis and gastrointestinal dysfunction (e.g. vomiting, diarrhea and failure-to-thrive). The development of experimental models of gastrointestinal hypersensitivity has provided important insight into the immunological mechanisms responsible for this disease.1, 2
Allergen-induced acute diarrhea, which develops in mice sensitized intraperitoneally (i.p.) with OVA/alum followed by repeated intragastric (i.g.) OVA administration, is dependent on IgE, mast cells and mast cell-generated vasoactive mediators.3 The mild systemic features observed in this model led us to use the term “intestinal anaphylaxis” to describe the IgE-mediated mast cell degranulation that occurs in the small intestine and leads to increased intestinal permeability and acute diarrhea without shock.3, 4
Although increased quantities of both IL-4 and IL-13 are produced in the small and large intestine in this model, the roles of these cytokines and their receptors in the pathogenesis of intestinal anaphylaxis have not been explored.3, 5, 6 IL-4 and IL-13 both signal through receptors that contain IL-4Rα chain and activate STAT6, but only IL-4 signals through the type 1 receptor, whereas both cytokines signal through the type 2 receptor (composed of the IL-4Rα and IL-13Rα1 polypeptides). The relative roles of these two receptors can be distinguished by genetic deletion of the IL-13Rα1 chain, since such genetically engineered mice have an intact type 1 IL-4R, but lack the type 2 IL-4R.7, 8 T cell responses should not be directly affected by IL-13Rα1 deletion, because T cells lack the type 2 receptor.9 Most murine B cells also express little or no type 2 IL-4R;9 however, IL-4 and IL-13 signaling through this receptor might potentially influence the sensitization phase of allergic diarrhea by affecting the function of macrophages and dendritic cells.4, 10 Based on their role in expulsion of nematode parasites,4 IL-4 and IL-13 might also be involved in the effector phase of allergic diarrhea. Indeed, IL-4Rα positive non-bone marrow-derived cells have been implicated in parasite expulsion.11 Subsequent work by Shea-Donohue and colleagues has demonstrated parasite-induced STAT6 dependent alterations in both intestinal epithelial cell function and smooth muscle contractility.12, 13 Collectively, these studies suggest a role for IL4 and IL13 in the effector phase of the disease by increasing the sensitivity of intestinal tissues smooth muscle, epithelium, and vasculature to mediators released by mast cells.12–14
Defining the specific involvement of IL-4 and IL-13 is particularly important since therapeutic agents that block these cytokines or their common receptor (IL-4Rα) are being actively developed.15, 16 These approaches are particularly timely since safety concerns have been raised by an anti-IgE clinical trial for peanut allergy. 17
Using mice genetically deficient in IL-4, IL-13 or their receptors, we now demonstrate a central role for IL-4 in antigen-triggered intestinal mastocytosis and allergic diarrhea. Importantly, IL-13 and IL-13Rα1 are also shown to have a significant role.
MATERIALS AND METHODS
Animals
IL-4-deficient mice (BALB/c background) were obtained from Jackson Laboratory (Bar Harbor, ME). IL-13-deficient and IL-4/IL-13 double-deficient BALB/c background mice were originally obtained from Andrew McKenzie (Medical Research Council Laboratory of Molecular Biology, Cambridge, UK).18 IL-13Rα1-deficient mice were generated at Regeneron by Velocogene Technology as recently reported,7 and backcrossed into the BALB/c background for at least 6 generations. Animals involved in these studies were housed under specific pathogen-free conditions and treated in a humane manner according to institutional guidelines.
Intestinal Anaphylaxis Model
Mice were primed i.p. with OVA/alum and challenged repeatedly with OVA by oral gavage as previously described.3
In vivo cytokine capture assay
The in vivo cytokine capture assay (IVCCA) was used to monitor in vivo production of IL-4 as previously described.19
Intestinal mast cell quantification
Jejunum sections were stained for mast cells with chloroacetate esterase and numbers of mast cells/mm3 of jejunum were determined as previously described.3
ELISA
Mouse mast cell protease 1 (MMCP-1) and total IgE plasma levels were measured according to manufacturers' instructions (respectively, Moredun Scientific, Midlothian, United Kingdom and BD Biosciences-Pharmingen, San Diego, California, USA).
Antibody treatment
IL-4Rα was blocked with 2 mg of an antibody (4-3, anti-IL-4 receptor α chain hybrid IgG1 mAb) given either i.p. or i.v. respectively 24 h or 3 h prior to OVA exposure.
Statistical analysis
Data are expressed as mean ± SD. Statistical significance comparing different sets of mice was determined by Student's unpaired t-test or the non-parametric Mann Whitney U-test.
RESULTS
Allergen-induced diarrhea is mediated by IL-4 and potentially IL-13
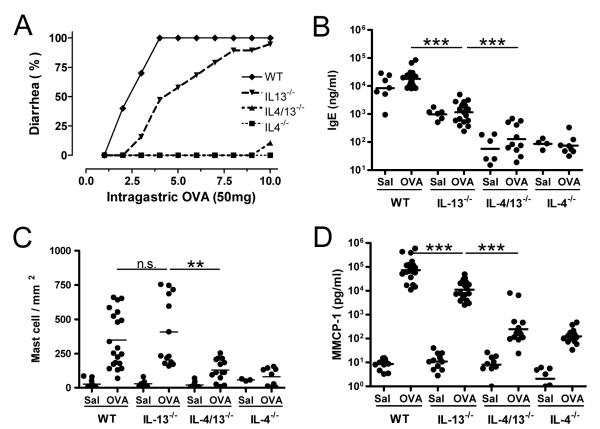
In order to determine the respective importance of IL-4 and IL-13 in intestinal anaphylaxis, we sensitized mice deficient in IL-4, IL-13 or both cytokines i.p. with OVA/alum and challenged them repeatedly i.g. with OVA. IL-4 deficiency almost completely protected against allergic diarrhea; two IL-4/IL-13-double-deficient mice developed diarrhea on the 10th OVA exposure (Figure 1A). Induction of allergic diarrhea required a significantly greater number of allergen challenges in IL-13-deficient mice than in wild type mice (Figure 1A).
Figure 1. Ablated intestinal anaphylaxis in the absence of IL-4 and IL-13.
(A) Occurrence of diarrhea was assessed in OVA/alum-primed wild type, IL-13−/−, IL-4/13−/− and IL-4−/− mice (n=9–14 mice/group) up to 60 minutes after 1–10 i.g. inoculations with 50 mg of OVA and (B) Jejunum mast cells numbers were assessed by morphometric analysis of chloroacetate esterase-stained cells. (C) IgE and (D) MMCP1 levels were measured by ELISA in blood drawn 60–90 minutes after the last saline or OVA inoculation. Serum IgE and MMCP1 levels were 490±204ng/ml and 9.7±7.8pg/ml respectively in naïve wild type mice, which had 35±25 mast cells/mm3 of jejunum. (** p < 0.01; *** p<0.001).
IgE and mast cell responses depend mainly upon IL-4
Because IgE and mast cells are essential for the development of allergic diarrhea,3 we compared serum IgE levels, jejunal mast cell numbers, and serum levels of MMCP1 (an enzyme released by degranulating mast cells) in wild-type, IL-4-, IL-13- and IL-4/IL-13-deficient mice that had been primed and challenged with OVA. Serum IgE levels were ~2 logs lower in IL-4- and IL-4/IL-13-deficient than in wild-type mice and ~1 log lower in IL-13-deficient than in wild-type mice (Figure 1B). Jejunal mast cell numbers following OVA immunization were decreased 2-3-fold in IL-4-, and IL-4/IL-13-deficient mice (Figure 1C). Most importantly, MMCP1 responses in OVA-immunized IL-4- and IL-4/IL-13-deficient mice were 2-3-logs lower than MMCP1 responses in similarly treated wild-type mice (Figure 1D), although OVA immunization stimulated an ~10-fold increase in MMCP1 levels even in the absence of IL-4. Despite non-significantly altered intestinal mast cell levels, MMCP1 levels were significantly lower in IL-13-deficient than in wild-type mice (Figure 1D).
IL-13Rα1−/− mice demonstrate a role for IL-13 in IgE and mast cell mediated allergic diarrhea
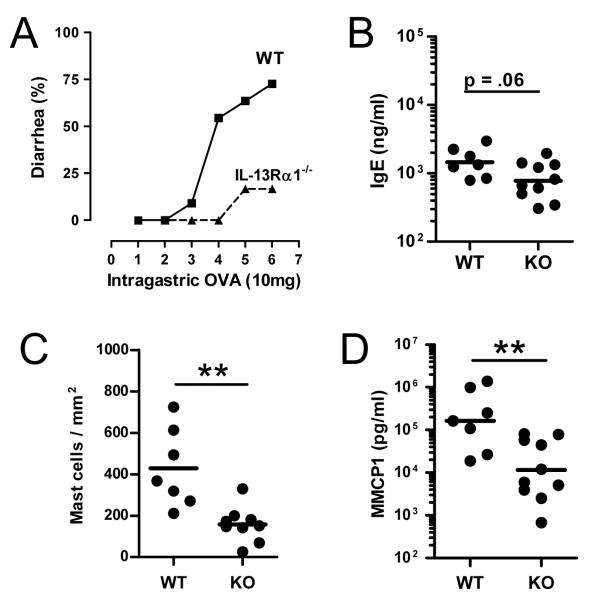
Because IL-13-deficient mice produce subnormal amounts of IL-4 (a consequence of deletion of an IL-4 promoter sequence in the IL-13 gene),20 the delayed development of intestinal anaphylaxis in IL-13−/− mice might result from diminished IL-4 production rather than the absence of IL-13. Indeed, we found that IL-4 production was reduced in naïve IL-13−/− mice compared to wild type mice as determined by in vivo cytokine capture assay (IVCCA) (66.1±23.1 vs. 115.1±39.3 pg/ml; p<0.01). This difference was not a direct effect of the absence of IL-13 signaling, because mice with defective IL-13 signaling (IL-13Rα1−/−) had comparable levels of IL-4 (187.9±31.4 vs. 170.7±22.0 pg/ml for −/− and +/+, respectively). Consequently, we evaluated the ability of IL-13Rα1-deficient mice to develop allergic diarrhea and found that it was also significantly impaired (Figure 2A).
Figure 2. IL-13 contributes to IgE and mast cell allergic responses.
(A) Diarrhea occurrences in OVA-alum primed wild-type and IL-13Rα1−/− mice after 1–6 i.g. inoculations with 10 mg of OVA. (B) Serum IgE levels, (C) jejunum mast cell numbers, and (D) serum MMCP1 levels were compared in wild-type and IL-13Rα1-deficient mice (7–18/group; ** p < 0.01; *** p<0.001).
The decrease in serum IgE level in IL-13Rα1-deficient mice was ~2-fold and did not quite reach statistical significance (Figure 2B). These data suggest that IL-13 has a modest stimulatory effect on IgE production in OVA-immunized mice, resulting in impaired mast cell stimulation in the absence of IL-13 signaling, as shown by MMCP1 levels that were ~1 log lower in IL-13Rα1-deficient mice than in wild-type mice.
Prophylactic targeting of IL-4rα alleviates allergic diarrhea
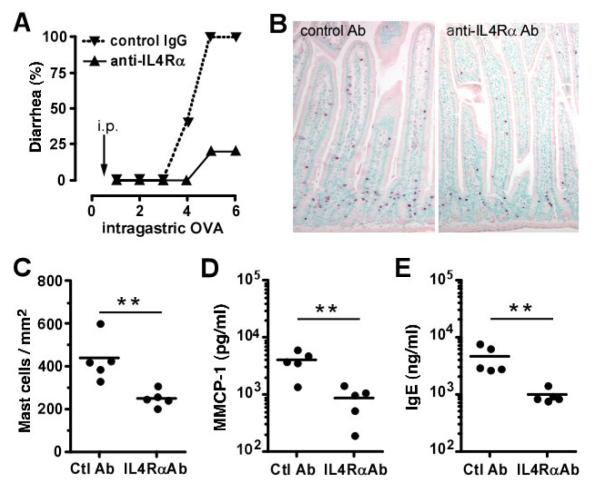
In order to inhibit the effects of IL-4 and IL-13 in experimental intestinal anaphylaxis, 4-3, a mAb to IL-4Rα that blocks both IL-4 and IL-13 effects in vitro and in an in vivo mouse model of allergic airway disease (unpublished data) was used. Initial experiments tested whether a single dose of 4-3, injected 1 day prior to the initiation of i.g. OVA administration, could inhibit the development of allergic diarrhea. This single dose strongly inhibited the development of allergic diarrhea after 4–6 i.g. doses of OVA (Figure 3A). This delay in the development of allergic diarrhea was associated with impaired intestinal mast cell accumulation (Figure 3B–C), decreased MMCP-1 plasma levels (Figure 3D), and lower plasma IgE (Figure 3E).
Figure 3. Prophylactic effects of anti-IL-4Rα mAb.
OVA/alum-primed BALB/c mice (5/group) were injected i.p. with either 2 mg of 4–3 anti-IL-4Rα mAb or a control IgG1 mAb 1 day before the first i.g. inoculation with OVA. (A) Development of diarrhea was assessed during the 60 minutes after 1–6 i.g. OVA inoculations. (B) Representative jejunum section of antibody treated mice stained with chloroacetate esterase. (C) Intestinal mast cell numbers were assessed by morphometric analysis. (D) Blood MMCP-1 and (E) total IgE levels were assessed 1-2 hours following the last allergen exposure (* p<0.05; ** p < 0.01).
IL-4 and IL-13 are not required for the effector phase of allergic diarrhea
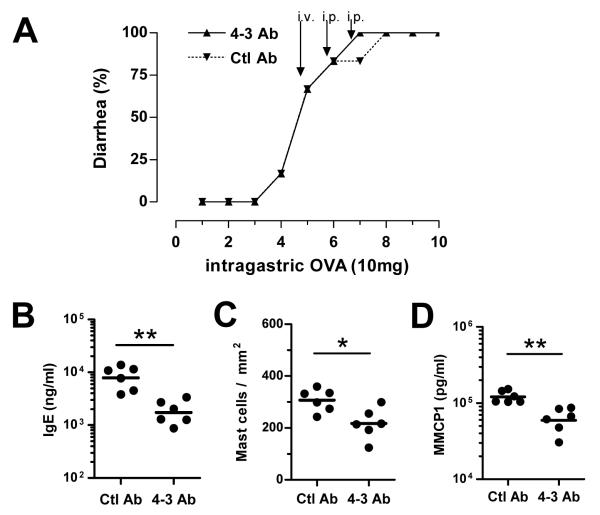
The ability of IL-4 and IL-13 to enhance smooth muscle contractility and epithelial permeability and secretion and the ability of these cytokines to increase sensitivity to mediators released by activated mast cells suggested that IL-4 and IL-13 might be contributing to the effector as well as the sensitization phase of intestinal anaphylaxis.12–14 However, administration of up to 3 doses of anti-IL-4Rα mAb over a 7 day period, starting after OVA-immunized mice had already developed allergic diarrhea, failed to decrease the incidence of diarrhea following high dose OVA challenge (Figure 4A), although it decreased total IgE levels (Figure 4B), intestinal mast cell numbers (Figure 4C) and MMCP1 blood levels (Figure 4D).
Figure 4. IL-4 and IL-13 are not required during the effector phase of allergic diarrhea.
(A) Once diarrhea had developed (after 4 i.g. challenges with OVA), BALB/c mice (6/group) were injected with 2 mg of 4–3 or control IgG1 mAb before the 5th, 6th and 7th OVA inoculations (n=6 mice/group). (B) One week and three OVA inoculations later, mice were sacrificed and plasma IgE levels were determined. (C) Jejunum mast cell numbers and (D) MMCP-1 blood levels were assessed 1–2 hours after the last OVA inoculation (* p<0.05; ** p < 0.01).
DISCUSSION
Taken together, our observations demonstrate that not only IL-4 but also IL-13 has a significant role in intestinal anaphylaxis. Our finding that IL-4-deficient mice are protected from OVA-induced diarrhea is supported by an earlier study using a different model, which shows that mice pre-treated with an anti-IL-4 antibody prior to sensitization failed to develop diarrhea.6 Confirmation of the delayed development of allergic diarrhea observed in IL-13-deficient mice with studies in IL-13Rα1-deficient mice was important because it could result from their decreased production of IL-4,20 which would be expected to decrease IgE and mast cell responses.21 In contrast, IL-4 production appears to be normal in IL-13Rα1-deficient mice. Although it could be argued that the delayed development of diarrhea in these mice might reflect the lack of IL-4, rather than IL-13, signaling through the type 2 IL-4 receptor, this seems unlikely, given the greater production of IL-13 than IL-45 and the more potent signaling of IL-13 than IL-4 through the type 2 receptor.22 In addition, this explanation cannot account for the accelerated development of allergic diarrhea in IL-13Rα2-deficient mice (data not shown).
Surprisingly, in contrast to observations made in allergic airway disease models,23 IL-13 is important in the induction, rather than the effector phase of intestinal anaphylaxis. This point is of importance, since the only available data so far in the gastrointestinal tract suggested that IL-13 and IL-4 had a significant impact on effector functions (e.g.; parasite expulsion).4 Blocking both IL-4 and IL-13 signaling with a high dose of a potent anti-IL-4Rα mAb for as long as 7 days had little effect on allergic diarrhea induced by a high dose of allergen. It is unlikely that this negative result reflected inadequate IL-4Rα blockade, because even a single dose of the 4-3 mAb suppressed allergic diarrhea for > 2 weeks in the prophylactic model.
These observations raise the question of how IL-13 contributes to the induction phase of intestinal anaphylaxis, inasmuch as T cells lack the type 2 IL-4R and IL-4 is much more potent than IL-13 at activating mouse mast cells and inducing isotype switching by mouse B cells.10, 24 One possibility is that IL-13 directly stimulates isotype switching by a small, but important B cell subset.25 This is consistent with the recent observation that baseline IgE levels are significantly lower in IL-13Rα1-deficient mice than in wild-type mice7 and earlier observations of increased IgE levels in IL-13Rα2-deficient mice and in IL-4-deficient mice that overproduce IL-13.26, 27 Alternatively, IL-13 effects on antigen presenting cells may indirectly promote IgE production and mastocytosis by contributing to Th2 cytokine production. In this regard, it is noteworthy that while both IL-4 and IL-13 can activate dendritic cells, only IL-4 stimulates their production of IL-12, which can inhibit Th2 cytokine production and B cell isotype switching to IgE by stimulating IFN-γ production.28
The observation that 10% of IL-4/IL-13-double-deficient mice developed diarrhea by the 10th allergen exposure indicates that the IL-4 requirement for induction of allergic diarrhea is not absolute. Detectable IgE serum levels have been observed in naïve IL-4Rα and IL-4/IL-13 deficient mice.21 Furthermore, OVA-specific IgE levels were observed in IL-4 and IL-4Rα deficient mice following OVA sensitization either through the i.p. route or through repeated intranasal instillations.29 This would support our findings that IgE is present in IL-4 and IL-4/IL-13-deficient mice following sensitization, albeit at barely detectable levels. Although we did not measure a significant induction of total plasma IgE levels following repeated intestinal allergen exposures, mast cell-bound OVA-specific IgE may have increased without a detectable increase in circulating levels of IgE in multiply-immunized IL-4- and IL-4/IL-13-deficient mice. We observed a 2-fold increase in mast cell accumulation associated with a log increase in MMCP-1 plasma levels in these mice, indicating that repeated immunization could induce mast cell activation through an IL-4/IL-13-independent pathway. Similar results were observed with STAT6-deficient mice (data not shown). Interestingly, intestinal mastocytosis is actually increased more in STAT6-deficient than in wild-type mice following infection with some nematodes.30
Finally, one would expect that blocking IL-4Rα should 1) normalize smooth muscle contractions and epithelial cell functions 2) reduce Th2-related antibody responses (IgE, IgG1), 3) decrease intestinal inflammation; and 4) impair mast cell degranulation with release of MMCP1, serotonin and PAF in allergen-immunized mice. Indeed, our findings demonstrate that a prophylactic approach blocks diarrhea development. Although treatment with anti-IL-4Rα for 7 days failed to block the diarrheal response to allergen challenge, it significantly decreased IgE, mast cell and MMCP1 responses despite continuing allergen administration. This suggests that a longer period of treatment with this mAb might suppress allergic diarrhea. The ability of this mAb to decrease IgE production may make it particularly effective as a treatment for food allergy when paired with a second biological agent, such as a non-activating anti-IgE mAb.
Key Messages.
Using mice genetically deficient in IL-4 and IL-13 signaling, we demonstrate a central role for IL-4 in antigen-triggered intestinal mastocytosis and allergic diarrhea; however, IL-4 and IL-13 are not absolutely required for the development of intestinal anaphylaxis. IL-13 contributes to allergic diarrhea and does so by promoting oral allergen sensitization rather than the effector phase of intestinal anaphylaxis.
Short-term treatment of established allergic diarrhea with an inhibitor of both IL-4 and IL-13 is not effective.
Acknowledgement
The authors would like to thank Matthew Doepker and Lynn Hassman for technical assistance. We are grateful to Amgen Corporation for their gift of anti-IL-4Rα mAb and to Regeneron for their gift of IL-13Rα1-deficient mice.
This research was partially supported by NIH grant AI45898-05, the Campaign Urging Research for Eosinophilic Diseases (CURED), the Food Allergy Project, and the U.S. Department of Veterans Affairs.
Abbreviations used
- Alum
aluminum potassium sulfate
- i.p.
intraperitoneal
- i.g.
intragastric
- IVCCA
in vivo cytokine capture assay
- mAb
monoclonal antibody
- MMCP1
mouse mast cell protease 1
- OVA
ovalbumin
- PAF
platelet activating factor
REFERENCES
- 1.Sampson HA. Update on food allergy. J Allergy Clin Immunol. 2004;113:805–19. doi: 10.1016/j.jaci.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 2.Knippels LM, van Wijk F, Penninks AH. Food allergy: what do we learn from animal models? Curr Opin Allergy Clin Immunol. 2004;4:205–9. doi: 10.1097/00130832-200406000-00012. [DOI] [PubMed] [Google Scholar]
- 3.Brandt EB, Strait RT, Hershko D, Wang Q, Muntel EE, Scribner TA, et al. Mast cells are required for experimental oral allergen-induced diarrhea. J Clin Invest. 2003;112:1666–77. doi: 10.1172/JCI19785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finkelman FD, Rothenberg ME, Brandt EB, Morris SC, Strait RT. Molecular mechanisms of anaphylaxis: lessons from studies with murine models. J Allergy Clin Immunol. 2005;115:449–57. doi: 10.1016/j.jaci.2004.12.1125. [DOI] [PubMed] [Google Scholar]
- 5.Knight AK, Blazquez AB, Zhang S, Mayer L, Sampson HA, Berin MC. CD4 T cells activated in the mesenteric lymph node mediate gastrointestinal food allergy in mice. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1234–43. doi: 10.1152/ajpgi.00323.2007. [DOI] [PubMed] [Google Scholar]
- 6.Kweon MN, Yamamoto M, Kajiki M, Takahashi I, Kiyono H. Systemically derived large intestinal CD4+ Th2 cells play a central role in STAT6-mediated allergic diarrhea. J Clin Invest. 2000;106:199–206. doi: 10.1172/JCI8490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Munitz A, Brandt EB, Mingler M, Finkelman FD, Rothenberg ME. Distinct roles for IL-13 and IL-4 via IL-13 receptor α1 and the type II IL-4 receptor in asthma pathogenesis. Proc Natl Acad Sci U S A. 2008;105:7240–5. doi: 10.1073/pnas.0802465105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramalingam TR, Pesce JT, Sheikh F, Cheever AW, Mentink-Kane MM, Wilson MS, et al. Unique functions of the type II interleukin 4 receptor identified in mice lacking the interleukin 13 receptor α1 chain. Nat Immunol. 2008;9:25–33. doi: 10.1038/ni1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zurawski G, de Vries JE. Interleukin 13, an interleukin 4-like cytokine that acts on monocytes and B cells, but not on T cells. Immunol Today. 1994;15:19–26. doi: 10.1016/0167-5699(94)90021-3. [DOI] [PubMed] [Google Scholar]
- 10.Wynn TA. IL-13 effector functions. Annu Rev Immunol. 2003;21:425–56. doi: 10.1146/annurev.immunol.21.120601.141142. [DOI] [PubMed] [Google Scholar]
- 11.Urban JF, Jr., Noben-Trauth N, Schopf L, Madden KB, Finkelman FD. Cutting edge: IL-4 receptor expression by non-bone marrow-derived cells is required to expel gastrointestinal nematode parasites. J Immunol. 2001;167:6078–81. doi: 10.4049/jimmunol.167.11.6078. [DOI] [PubMed] [Google Scholar]
- 12.Madden KB, Whitman L, Sullivan C, Gause WC, Urban JF, Jr., Katona IM, et al. Role of STAT6 and mast cells in IL-4- and IL-13-induced alterations in murine intestinal epithelial cell function. J Immunol. 2002;169:4417–22. doi: 10.4049/jimmunol.169.8.4417. [DOI] [PubMed] [Google Scholar]
- 13.Zhao A, McDermott J, Urban JF, Jr., Gause W, Madden KB, Yeung KA, et al. Dependence of IL-4, IL-13, and nematode-induced alterations in murine small intestinal smooth muscle contractility on Stat6 and enteric nerves. J Immunol. 2003;171:948–54. doi: 10.4049/jimmunol.171.2.948. [DOI] [PubMed] [Google Scholar]
- 14.Akiho H, Lovato P, Deng Y, Ceponis PJ, Blennerhassett P, Collins SM. Interleukin-4- and -13-induced hypercontractility of human intestinal muscle cells-implication for motility changes in Crohn's disease. Am J Physiol Gastrointest Liver Physiol. 2005;288:G609–15. doi: 10.1152/ajpgi.00273.2004. [DOI] [PubMed] [Google Scholar]
- 15.Wenzel S, Wilbraham D, Fuller R, Getz EB, Longphre M. Effect of an interleukin-4 variant on late phase asthmatic response to allergen challenge in asthmatic patients: results of two phase 2a studies. Lancet. 2007;370:1422–31. doi: 10.1016/S0140-6736(07)61600-6. [DOI] [PubMed] [Google Scholar]
- 16.Knackmuss S, Krause S, Engel K, Reusch U, Virchow JC, Mueller T, et al. Specific inhibition of interleukin-13 activity by a recombinant human single-chain immunoglobulin domain directed against the IL-13 receptor α1 chain. Biol Chem. 2007;388:325–30. doi: 10.1515/BC.2007.036. [DOI] [PubMed] [Google Scholar]
- 17.Chehade M. IgE and non-IgE-mediated food allergy: treatment in 2007. Curr Opin Allergy Clin Immunol. 2007;7:264–8. doi: 10.1097/ACI.0b013e32814a5607. [DOI] [PubMed] [Google Scholar]
- 18.McKenzie GJ, Fallon PG, Emson CL, Grencis RK, McKenzie AN. Simultaneous disruption of interleukin (IL)-4 and IL-13 defines individual roles in T helper cell type 2-mediated responses. J Exp Med. 1999;189:1565–72. doi: 10.1084/jem.189.10.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Finkelman F, Morris S, Orekhova T, Sehy D. The in vivo cytokine capture assay for measurement of cytokine production in the mouse. Curr Protoc Immunol. 2003;Chapter 6(Unit 6):28. doi: 10.1002/0471142735.im0628s54. [DOI] [PubMed] [Google Scholar]
- 20.Guo L, Hu-Li J, Zhu J, Pannetier C, Watson C, McKenzie GJ, et al. Disrupting IL-13 impairs production of IL-4 specified by the linked allele. Nat Immunol. 2001;2:461–6. doi: 10.1038/87778. [DOI] [PubMed] [Google Scholar]
- 21.Fish SC, Donaldson DD, Goldman SJ, Williams CM, Kasaian MT. IgE generation and mast cell effector function in mice deficient in IL-4 and IL-13. J Immunol. 2005;174:7716–24. doi: 10.4049/jimmunol.174.12.7716. [DOI] [PubMed] [Google Scholar]
- 22.Zdanov A, Wlodawer A. A new look at cytokine signaling. Cell. 2008;132:179–81. doi: 10.1016/j.cell.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 23.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, et al. Interleukin-13: central mediator of allergic asthma. Science. 1998;282:2258–61. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 24.Lai YH, Mosmann TR. Mouse IL-13 enhances antibody production in vivo and acts directly on B cells in vitro to increase survival and hence antibody production. J Immunol. 1999;162:78–87. [PubMed] [Google Scholar]
- 25.McCoy KD, Harris NL, Diener P, Hatak S, Odermatt B, Hangartner L, et al. Natural IgE production in the absence of MHC Class II cognate help. Immunity. 2006;24:329–39. doi: 10.1016/j.immuni.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 26.Wood N, Whitters MJ, Jacobson BA, Witek J, Sypek JP, Kasaian M, et al. Enhanced interleukin (IL)-13 responses in mice lacking IL-13 receptor α2. J Exp Med. 2003;197:703–9. doi: 10.1084/jem.20020906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Emson CL, Bell SE, Jones A, Wisden W, McKenzie AN. Interleukin (IL)-4-independent induction of immunoglobulin (Ig)E, and perturbation of T cell development in transgenic mice expressing IL-13. J Exp Med. 1998;188:399–404. doi: 10.1084/jem.188.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lutz MB, Schnare M, Menges M, Rossner S, Rollinghoff M, Schuler G, et al. Differential functions of IL-4 receptor types I and II for dendritic cell maturation and IL-12 production and their dependency on GM-CSF. J Immunol. 2002;169:3574–80. doi: 10.4049/jimmunol.169.7.3574. [DOI] [PubMed] [Google Scholar]
- 29.Grunewald SM, Teufel M, Erb K, Nelde A, Mohrs M, Brombacher F, et al. Upon prolonged allergen exposure IL-4 and IL-4Ralpha knockout mice produce specific IgE leading to anaphylaxis. Int Arch Allergy Immunol. 2001;125:322–8. doi: 10.1159/000053833. [DOI] [PubMed] [Google Scholar]
- 30.Finkelman FD, Shea-Donohue T, Morris SC, Gildea L, Strait R, Madden KB, et al. Interleukin-4- and interleukin-13-mediated host protection against intestinal nematode parasites. Immunol Rev. 2004;201:139–55. doi: 10.1111/j.0105-2896.2004.00192.x. [DOI] [PubMed] [Google Scholar]