SYNOPSIS
Diffusion weighted MRI (DWI) is a technique that assesses the cellularity, tortuosity of the extracellular/extravascular space and cell membrane density based upon differences in water proton mobility in tissues. The strength of the diffusion weighting is reflected by the b-value. DWI using several b-values enables quantification of the apparent diffusion coefficient (ADC). DWI is increasingly employed in liver imaging for multiple reasons: it can add useful qualitative and quantitative information to conventional imaging sequences, it is acquired relatively quickly, it is easily incorporated into existing clinical protocols, and it is a non-contrast technique. DWI is useful for focal liver lesion detection and characterization, for the assessment of post-treatment tumor response and for evaluation of diffuse liver disease. ADC quantification can be used to characterize lesions as cystic/necrotic or solid and for predicting tumor response to therapy. Advanced diffusion methods such as IVIM (intravoxel incoherent motion) may have potential for detection, staging and evaluation of the progression of liver fibrosis and for liver lesion characterization. The lack of standardization of DWI technique including choice of b-values and sequence parameters has somewhat limited its widespread adoption.
Keywords: Diffusion, liver MRI, apparent diffusion coefficient, liver lesion detection, liver lesion characterization, IVIM, cirrhosis, echo planar imaging
INTRODUCTION
Diffusion weighted imaging (DWI) is a magnetic resonance imaging (MRI) technique that reports on the physical process of microscopic thermal motion of water molecules in biologic tissues1,2. The differences in the mobility of water protons create image contrast, which is influenced by the interaction of water molecules with cellular membranes, macromolecules, degree of cellular density and the size of the extracellular extravascular space3. DWI is increasingly being employed in liver MRI given recent technologic advances and improvements in image quality, including the introduction of echo planar imaging (EPI), parallel imaging, multichannel coils and high amplitude gradients.
Accurate lesion detection and characterization is essential for treatment planning for patients with primary or secondary liver tumors, especially in selecting patients who may undergo liver resection, loco-regional or systemic therapies4,5. DWI can be employed for focal liver lesion detection and characterization, for the assessment of tumor response, and for evaluation of diffuse liver disease3. This sequence is easily incorporated into routine clinical protocols, especially given that DWI is a non-contrast technique and may be performed either before or after contrast administration. DWI can be acquired rapidly within a breath hold, and it provides both qualitative and quantitative information as an adjunct to conventional sequences. Quantification of apparent diffusion coefficient (ADC) has enabled differentiation of solid cellular lesions from cystic or necrotic lesions6. ADC quantification has also demonstrated promising results for predicting tumor response to therapy7,8. The purpose of this chapter is to review the basic principles of DWI, discuss protocol optimization, summarize the performance of DWI compared to conventional sequences, discuss the role of DWI in evaluation of treatment response and examine the potential role of DWI in the assessment of liver fibrosis.
DWI TECHNIQUE
1. Concepts
1.1. Principles of molecular diffusion
Diffusion describes random molecular motion occurring in tissues as a result of thermally activated translations of atoms and molecules. The resulting motion can be described as a stochastic process, with a Gaussian probability distribution given as:
| Eq. 1 |
where D is the diffusion coefficient and r is the distance traveled by the diffusing molecule during time t. D depends on the species undergoing diffusion and on the medium in which diffusion occurs. For water self-diffusion at 37° C, D = 3.0 mm2/s 9. Larger molecules tend to undergo slower diffusion resulting in lower D, as is also the case of diffusion in fluids of higher viscosity. The Gaussian distribution in Eq. 1 describes free diffusion of molecules in an infinite medium. An important variation of this concept is restricted diffusion where molecular motion is restrained within hard boundaries. Restricted diffusion, which is common in tissues, generally leads to lower D and non-Gaussian distributions.
1.2. DWI physics
Nuclear magnetic resonance offers an novel way to measure diffusion, via the application of magnetic field gradients. If such gradients are deployed in pulses of opposed polarity (using a gradient or spin echo sequence), moving spins undergo dephasing while static spins show a null phase at the echo time. By considering the effect of a stochastic diffusion process on the magnetic signal, one can derive the signal attenuation resulting from the application of pulsed gradients in a spin echo experiment10:
with S0 the signal in the absence of gradient and b a function of the applied gradient:
with γ the spin gyromagnetic ratio, G and δ the gradient strength and length, and Δ the time separating the gradient pair. The factor b, called b-value, determines the strength of the diffusion weighing. The diffusion gradients can be inserted in an imaging experiment as a preparation module, in order to provide additional contrast to the MRI signal and to estimate molecular diffusion in different organs and tissues.
1.3. Quantification of diffusion properties in tissues
In its simplest form, the diffusion experiment involves acquiring two sets of images, one at low or zero b-value, another at high b-value, to derive a voxel-wise diffusion coefficient. Since multiple tissues and compartments may be present in a single voxel, the derived coefficient is referred to as an ADC that reflects a sum of exponential decays rather than a single, pure diffusion constant. In addition to the ADC, which assumes monoexponential decay of diffusion signal, there are models that account for the more complex properties of tissues:
Flowing blood contributes to diffusion signal and leads to measureable effects on the diffusion decay. The intravoxel incoherent motion (IVIM) approach11 integrates these effects in a biexponential model where a faster decaying exponential, reflecting perfusion effects at low b-values, can be separated from slower exponential decay reflecting true water diffusion. This approach is especially appropriate in highly perfused organs such as the liver12–18.
Because of restricted diffusion in tissues, the Gaussian model of Eq. 1 is not valid anymore, and diffusion weighting has a less trivial form. A successful approach has been diffusion kurtosis imaging (DKI) that evaluates restricted diffusion by analyzing the non-Gaussian diffusion distribution, using an additional constant derived from acquisitions at higher b-value19. Recently, DKI has been investigated in liver explant studies, where results have been correlated with hepatocellular carcinoma (HCC) tumor cellularity20.
Biological tissues can be anisotropic, and therefore so can diffusion decay. The ADC measured using different diffusion gradient directions may differ in an anisotropic sample, therefore diffusion tensor imaging21 (DTI) has been proposed in order to extract direction-specific information using at least 6 different diffusion gradient directions. In the liver, acquisition protocols typically involve acquiring the diffusion trace, which is an average of all 3 principal diffusion directions. DTI has been investigated in abdominal organs22–25, but there is limited data on anisotropic properties in the liver 26–28.
1.4. Quality control
There are sources of variability for diffusion quantification across different platforms or centers. First, hardware variations exist between different platforms, leading to different estimates for ADC. Sasaki et al29 reported up to 9% variability in brain ADC in a multicenter study in 12 healthy subjects. Chenevert et al30 measured the ADC of a temperature-controlled ice-water sample in several platforms and reported 5% variability across 3 vendors at 1.5T and 3T. Another source for variability is the choice of b-values used to derive the ADC. Previous studies in liver DWI have used a b-value range of 0–1000 s/mm2. Because of confounding effects of perfusion, computing the ADC using a lower range of b-values (e.g. 0–400) may lead to higher values than using an extended range of b-values (e.g. 0–1000), and to different accuracy of the technique for disease detection31. According to Xing et al32, the ADC can be reliably estimated using 2 b-values chosen outside of the perfusion range, with magnitude such that bhigh−blow~1.1/D. In order to measure perfusion effects using the IVIM model, more b-values are needed and the b-values distributions must be appropriately chosen to sample the fast pseudo-diffusion (b<100) and slower true diffusion (100<b<1000) decays33,34.
1.5. Reproducibility and repeatability
Reproducibility and repeatability are important concepts for quantitative diffusion evaluation, particularly for longitudinal and multicentre studies. In the liver, Braithwaite et al35 reported mean coefficient of variation (CV=standard deviation divided by mean) of 14%. In hepatic tumors36, similar results were reported for short term reproducibility (95% limits of agreement: 30%), and ADC reproducibility was found to depend on lesion location (lower reproducibility in the left lobe) and size (lower reproducibility in smaller lesions). The reproducibility of liver IVIM was also reported13,14,17, showing higher reproducibility for D and ADC (CV <20%), compared to perfusion parameters PF (or f) and D* (or Dt) (CV >20%). IVIM reproducibility was further investigated in lesions in a recent study showing lower reproducibility of IVIM parameters in liver metastases13 and HCC37 compared to liver parenchyma. These findings may be due to higher variability of physiological processes in HCC, or to limitations of DWI for measurement of finite size lesions (lesions DWI signal is more sensitive to motion artifacts and to limited resolution).
2. Diffusion acquisition
2.1. Imaging strategy
The most widely used strategy for DWI is echo planar imaging (EPI), which allows acquisition of a full slice in a single shot. A typical protocol involves using fat-saturated single shot diffusion weighted EPI played in an interleaved multislice fashion to allow volume coverage. However, the EPI readout is also subject to ghosting and susceptibility artifacts38. Some alternative techniques to EPI include:
Segmented EPI39, which is performed by distributing the EPI acquisition over repeated cycles. This way, susceptibility artifacts are reduced by decreasing the EPI readout length. Compared to single shot EPI, segmented EPI is more sensitive to motion occurring between consecutive shots, leading to signal inconsistency and artifacts.
Turbo spin echo40 (TSE) and steady state free precession41 (SSFP), which may offer reduced B0 and susceptibility artifacts compared to EPI readouts. However this usually leads to increased echo trains, and to higher power deposition of the radiofrequency pulse train.
PROPELLER42, a modified segmented EPI technique where successive segments are acquired in a radial fashion. Compared to segmented EPI, PROPELLER may be more immune to motion artifacts, with options for robust motion correction43.
2.2. Control of physiologic motion
Physiologic motion is inherent to any liver imaging protocol, with breathing and cardiac motion resulting in subject and acquisition-dependent imaging artifacts. Single shot EPI is robust to motion because its acquisition time is faster than physiological processes. However, in a typical DWI acquisition with multiple b-values and signal averaging, residual fluctuations exist between successively acquired EPI images. Either breath hold (BH), free breathing (FB) or respiratory triggering (RT) technique may be employed. BH results in the shortest image acquisition time, with a limitation in the number of b-values that can be used. FB protocols typically result in blurred images. RT acquisition (using navigator echoes or bellows) may improve DWI data quality14,36,44,45, at the cost of increased imaging time. Cardiac motion artifacts usually appear in the left liver lobe as signal loss at high b-value, as a result of strong dephasing of coherently moving spins under the influence of diffusion gradients. This can be overcome by performing DWI acquisitions at diastole using an ECG or pulse trigger46, but this approach results in significantly increased scan time. Another promising approach to mitigate signal loss due to cardiac motion is to use motion-compensated diffusion gradients47,48 to cancel the dephasing of coherently moving tissues while maintaining diffusion-weighting.
2.3. Parallel imaging
Using multiple receiver radiofrequency coils, k-space acquisition can be accelerated by skipping lines and reconstructing the image using well established algorithms49–51. Applied to single shot EPI, parallel imaging allows for shorter EPI train, hence lower susceptibility artifacts and achievable higher resolution52. In order to avoid residual artifacts and severe signal loss, acceleration factors are typically limited to 3, depending on the number of coil elements. Another more recent application of parallel imaging is multiband imaging that allows for simultaneous excitation and acquisition of multiple slices53,54. Multiband imaging leads to increased slice coverage compared to regular DWI acquisition, with minimal signal degradation.
2.4. Effect of magnetic field strength: 1.5 vs. 3T
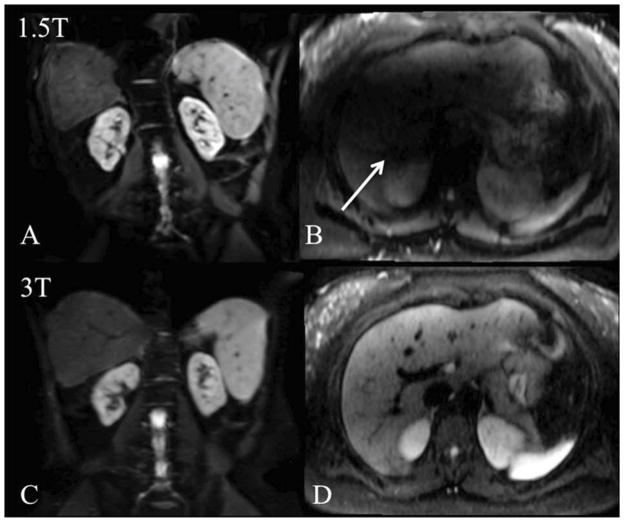
Because of the strong signal attenuation imposed by diffusion gradients, DWI suffers from low signal levels, as quantified by the signal to noise ratio (SNR). Low SNR, especially at high b-values, can be overcome by signal averaging or by increased static magnetic field. In this regard, a magnetic field strength of 3T might result in up to 2-fold SNR increase compared to 1.5T (4-fold reduction in signal averaging). However, Rozenkrantz et al55 reported worse image quality for abdominal DWI at 3T when compared to 1.5T. This study was using a first generation 3T system. Physical limitations at 3T may explain the moderate advantages of 3T DWI. First, higher EPI distortions may arise from stronger B0 and susceptibility variations. Second, the radiofrequency wavelength at 3T, of the same order as the human body, results in inhomogeneity of the excitation field B1. This in turn may lead to large signal variations across the liver and ghosting artifacts due to incomplete fat saturation. In addition to image quality degradation, previous reports have shown significant differences in ADC of abdominal organs at 1.5T and 3T56. Fig. 1 illustrates DWI artifacts at 3T compared to 1.5T.
Fig. 1.
Example of increased SS EPI diffusion artifacts at 3T (A–B) compared to 1.5T (C–D) in the same patients imaged with both systems (b400 images are shown). Image distortion on coronal SS EPI image due to higher B0 inhomogeneity (A) is observed in a 62 y male patient with HCV and fibrosis. Signal drop due to B1 field inhomogeneity at 3T (arrow on B) is observed in a 24 y female healthy subject, compared to 1.5T (D).
LIVER APPLICATIONS
1. Lesion Detection
Detection of malignant liver lesions is predicated on the belief that tumors have increased cellularity compared to background liver parenchyma and, consequentially, higher signal intensity on DWI. DWI is increasingly employed in oncologic imaging for detection of liver metastases, HCC and cholangiocarcinoma, the application of which will be discussed in detail below. Low b-value diffusion images using SS EPI (i.e. b <100) are most valuable for lesion detection given the suppression of vascular flow, producing “black blood” images, resulting in improved conspicuity for lesions located near vessels57,58. The suppression of intrahepatic vascular flow also avoids confusing a small lesion with a vessel on the T2WI sequence57. Compared to high b-value images (i.e. b1000), low b-value images have relatively higher contrast-to-noise and signal-to-noise ratios58–61. Low b-value images are also less affected by signal loss due to cardiac motion and geometric distortions induced by eddy currents62. Prior work has compared DWI lesion detection rates with conventional sequences, including T2 weighted images and post-contrast T1 weighted imaging (CE T1WI) using extracellular gadolinium based contrast agents (GBCAs), liver specific GBCAs and non-GBCAs liver specific agents, as discussed below. Studies comparing DWI to superparamagnetic iron oxide (SPIO) or mangafodipir trisodium (MnDPDP) will not be discussed in this review as these agents are either no longer available or not widely used.
1.1. Liver metastases
The accurate diagnosis of liver metastases is critical for treatment planning and for identifying patients suitable for liver resection, locoregional therapy or systemic chemotherapy63–65. MRI provides valuable and accurate information regarding lesion size, segmental location and distribution of liver metastases4,66. DWI has outperformed T2WI in several studies for liver lesion detection (including metastases), with DWI detection rates as high as 71–87.7% versus 55.6–70.1% for T2WI57,67–71. A current challenge remains the ability to detect small lesions, especially metastases <2 cm in size. In a prospective study of 129 lesions including 40 metastases, Coenegrachts et al demonstrated that 100% of metastases < 1 cm (40/40) were detected on low b-value DWI (b=20). Similarly, Parikh et al showed excellent metastatic lesion detection rate of 78.5% (57/72) for DWI compared to 45.8% (33/72) for T2WI for lesions 1–2 cm in size. In a retrospective series of 118 hepatic metastases, Bruegel et al reported DWI detection rate of 88–91% versus 45–62% for T2WI, a result that was also impressive for small lesions <1 cm, with DWI detection rate of 85% versus 26–44% for T2WI sequences68. The superior detection rates of optimized RT DWI may be explained by improved image quality compared to T2WI with a reduction in eddy current induced artifacts and image blurring6. Furthermore, diffusion images also have improved background fat saturation, suppression of intrahepatic vascular flow and liver to lesion contrast compared to T2WI58,67.
Routine liver MRI protocols typically include post-contrast T1WI for the detection of liver metastases. Both extracellular and combined extracellular-hepatocyte specific GBCAs such as gadoxetic acid (Primovist/Eovist, Bayer Healthcare) are widely used. DWI is not significantly affected by gadoxetic acid injection, and in order to minimize table time, DWI may be obtained between the dynamic and hepatobiliary (HBP) phases72. Results comparing DWI with dynamic CE T1WI and/or HBP for the detection of liver metastases have been mixed with most studies evaluating hypovascular metastases. Overall, DWI performs reasonably well compared to CE-MRI, with equivocal or slightly lower detection rates reported in most series. A recent meta-analysis by Wu et al examined the overall diagnostic value of DWI in detecting liver metastases and to compared its performance with CE-MRI62. The authors examined 11 studies (9 retrospective, 2 prospective) with 537 patients using either a variety of GBCAs or liver specific agents for comparison with DWI. These results show no major difference in the performance of DWI compared to either extra-cellular GBCAs or combined GBCAs68,70,73–81. DWI used in combination with CE-MRI or HBP imaging demonstrates higher sensitivity compared to DWI alone, with pooled sensitivities across multiple studies of 97% for the combined set versus 86% for DWI alone62. The sensitivity of DWI was statistically equivalent when compared to CE-MRI in several recent studies (66.3–84.4% vs. 76.0–79.7%, respectively)70,75,79. Subgroup analysis in several of these studies also showed that DWI is highly accurate in detecting small lesions. For example, Lowenthal et al demonstrated 92% sensitivity of DWI for detecting lesions <1 cm80. In a recent retrospective study of 144 pathologically proven metastases, the authors noted that assessment of DWI was very important in identifying small lesions, which may be missed or misinterpreted as peripheral vessels or imaging artifacts on HBP82. Interestingly, per-lesion analysis in the study by Hardie et al75 revealed a trend towards improved DWI detection rate for the less experienced observer for both lesions <1 cm and >1 cm, without reaching statistical significance. This finding was thought to be due to the simplicity of interpretation of DWI (Fig. 2, 3). Recent publications have evaluated DWI for detection of hypervascular liver metastases. DWI outperformed CE-MRI in a retrospective series of 41 patients with 162 hypervascular neuroendocrine liver metastases, with sensitivities of 71.6%–71.0% versus 47.5%–48.1% (P= 0.001)69. Further work is necessary to compare of the role of DWI for hypervascular and hypovascular liver metastases.
Summary: DWI is highly sensitive for detection of liver metastases, especially for small lesions < 2 cm. Combined DWI and CE T1WI yields the best performance for lesion detection compared to each sequence alone. In addition, DWI is a reasonable alternative to CE MRI in patients who cannot receive GBCAs.
Fig. 2.
51-year-old male with pancreatic cancer and liver metastases. Axial RT SS EPI DWI at 3T with b values of 50 and 800 s/mm2 (A, B) and ADC map (C), FSE T2WI (E) and axial late venous phase GRE T1-WI after injection of extracellular contrast medium (E). Both liver metastases (arrows) and the primary pancreatic tumor with secondary pancreatic duct distention (dashed arrow) are identified on b50 images (A), and remain hyperintense on high b value image (B). ADC value of the largest confluent metastasis was 0.97 × 10−3 mm2/s.
Fig. 3.
80 year old male with diffuse metastatic melanoma. Axial T1 in-phase (A) and opposed phase images (B), axial RT SS EPI DWI with b value of 1000 s/mm2 (C) and ADC map (D). Innumerable non-fat containing T1 hyperintense lesions are present throughout the liver, the largest confluent lesion measuring 5.2 cm in the caudate lobe (arrow). Lesions demonstrate diffusion restriction with low signal on ADC map (ADC value 0.87 × 10−3 mm2/s).
1.2. Hepatocellular carcinoma (HCC)
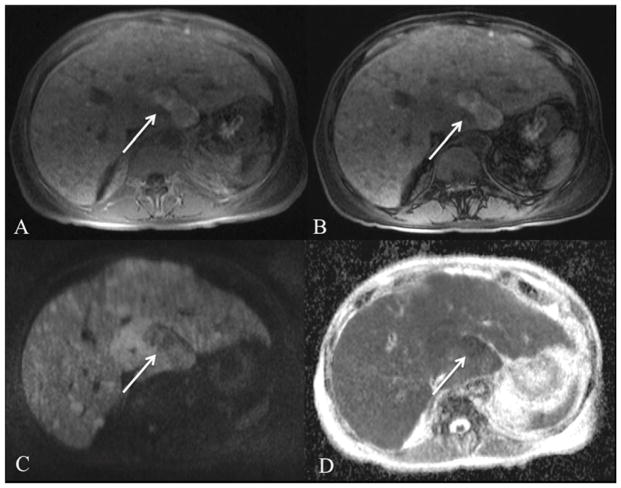
HCC is the most common primary liver cancer and the third most common cause of cancer related death in the United States83. Early diagnosis is essential given improved prognosis of early stage HCC and advances in surgical and locoregional therapies (LRT)84. The diagnosis of HCC is made at CE-CT or CE-MRI for lesions > 1 cm that demonstrate arterial hyperenhancement and portal venous/late venous phase washout5. Of note, the sensitivity for detecting small HCC <2 cm is considerably lower using CE-CT or CE-MRI85,86. Several authors have investigated DWI as a tool to improve noninvasive HCC detection. DWI is moderately sensitive for the detection of HCC with detection rates ranging from 45–91.2%71,87–89. The wide range of reported sensitivities likely reflects differences in study technique and selection bias, and thus, comparisons of reported results are somewhat challenging. Studies with very high reported sensitivities included either larger HCCs or cases with a high pre-test probability of HCC. Overall, DWI was better for HCC detection using low b value DWI (b0, b50) compared to conventional breath hold T2WI sequences (80.5% vs. 54%, p <0.001) and especially for small HCCs < 2 cm (79% vs. 46%, p<0.001)71. Excellent detection rate of HCCs < 2 cm of 91.2% was shown in a series of 58 HCCs using high b value DWI compared to post-contrast and T2WI88. Overall, DWI has shown mixed results compared to CE-MRI. In the study by Piana et al, there was better sensitivity for HCC detection using DWI compared to CE T1WI in 91 patients with 109 HCCs greater than 1 cm in size (72.5–81.7% vs. 59.6–59.6%)85. In contrast, CE-MRI was superior for HCC detection in 52 patients with 72 HCCs in the only published liver explant DWI correlation study (87.9% vs. 75.8%, p=0.02)90. In another study, DWI and gadoxetic acid images showed equivalent pooled sensitivity for HCC detection (87.1% vs. 91.1%, p > 0.05) in 130 patients with 179 surgically confirmed HCCs in a separate series91. Fig. 4 demonstrates the classic imaging characteristics of HCC with DWI and CE-MRI. Finally, published data has shown that the combination of DWI, CE-MRI and/or HBP images yields the best sensitivity for HCC detection compared to either sequence alone90,91. Sensitivities as high as 97.9% may be achieved using this the combination of sequences92. The added benefit of the combination of sequences is that the DWI and CE MRI provide complimentary information: DWI is particularly useful in identifying lesions near vessels and CE MRI is useful for identifying subcapsular lesions or lesions in the left lobe. DWI may also be very helpful in detecting infiltrative HCC, which often does not demonstrate typical arterial hypervascularity and delayed wash-out appearance (Fig. 5)93.
Fig. 4.
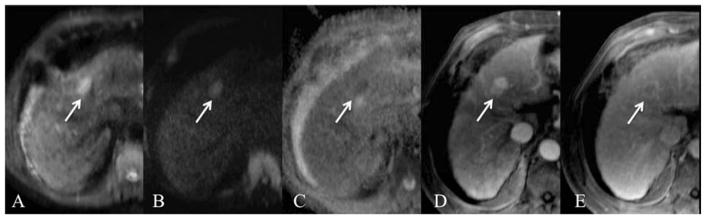
66-year-old male with HCC. Axial RT SS EPI DWI at 1.5T with b values of 50 and 1000 s/mm2 (A,B) and ADC map (C), contrast-enhanced (using extracellular contrast medium) T1WI on arterial phase (D) and late venous phase (E). There is a left hepatic lobe HCC (arrow) which is hyperintense on b50 (A) and b1000 (B) images with low ADC (1.31 × 10−3 mm2/s). The lesion demonstrates typical hypervascular hyperenhancement (D) with delayed phase washout and pseudocapsular enhancement (E).
Fig. 5.
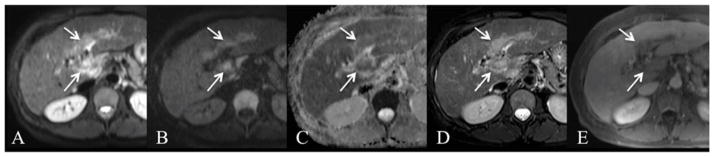
59-year-old female with infiltrative HCC invading the portal vein. Axial free breathing SS EPI DWI with b values of 50 and 1000 s/mm2 (A, B) and ADC map (C), fat suppressed FSE T2WI (D) and contrast-enhanced (using extracellular contrast medium) T1WI at portal venous phase (E). Tumor thrombus (arrows) demonstrates T2 hyperintensity and restricted diffusion on both b50 (A) and b1000 (B) images with low corresponding ADC value 1.45 × 10−3 mm2/s. The extent of tumor is better delineated on the DWI and T2 images (arrows, A–D) compared to post-contrast image (E).
There are several possible explanations for the lower DWI detection rate for HCC. A recognized limitation of DWI sequences for HCC detection is that not all HCCs are hyperintense. Approximately 8.8–38.9% of HCC are either isointense or hypointense on DWI sequences, and smaller lesions (<2 cm) are frequently inconspicuous (Fig. 6) 85,94,95. Well-differentiated HCC may be more frequently isointense than HCCs with higher tumor grade, however visual analysis of DW images alone is not reliable for this distinction94,96. The conspicuity and signal intensity of an HCC in a cirrhotic liver ultimately depend on a complex interaction between biologic and technical factors. HCCs are also more difficult to identify in the background of cirrhosis as the distorted liver parenchyma demonstrates diffusion restriction in relation with background fibrosis97,98. A retrospective study by Kim et al showed a trend towards decreased sensitivity for HCC detection with increasing severity of liver cirrhosis for two observers (Child-Pugh A 93.9–95.6%, Child-Pugh B 77.1–83.0%, Child-Pugh C 60.0–60.6%)99. Based on the data presented and the established benefits and limitations of each individual sequences, the optimal MRI protocol for detecting HCC in the cirrhotic liver should include the combination of DWI and CE/HBP MRI, thus yielding a maximized diagnostic accuracy.
Summary: DWI demonstrates moderate sensitivity for HCC detection. DWI should be used in combination with conventional MRI sequences, including post-contrast sequences, for HCC detection. Currently, DWI in isolation is insufficient for detecting HCC in cirrhosis.
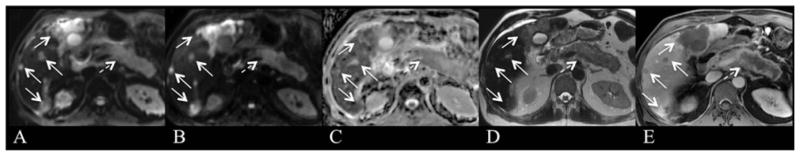
Fig. 6.
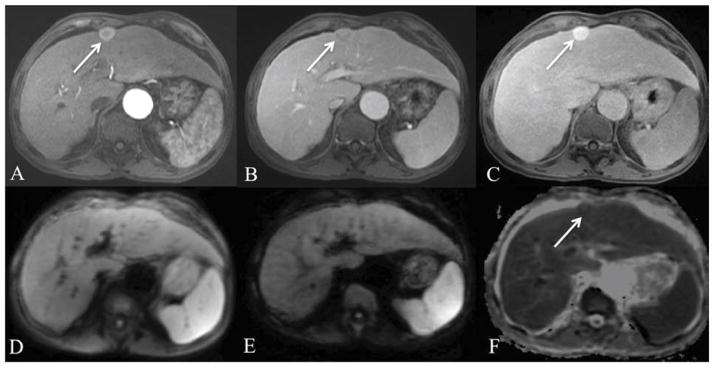
63-year-old male with hepatitis C cirrhosis and HCC. Axial arterial phase (A), portal venous phase (B), delayed hepatobiliary phase (C), axial free breathing SS EPI DWI with b values of 50 (D) and 500 s/mm2 (E) and ADC map (F) after injection of gadoxetic acid. 2.4 cm left hepatic lobe lesion (arrow) is hypervascular with portal venous phase washout/delayed pseudocapsule enhancement and demonstrates hyperintensity on delayed hepatobiliary phase images. The lesion is isointense on both low and high b-value DWI images and ADC map, with ADC value of 1.49 × 10−3 mm2/s. A well-differentiated HCC was found at histopathology.
1.3. Cholangiocarcinoma
Intrahepatic cholangiocarcinoma (ICC) is the second most common primary malignant tumor of the liver and is increasing in incidence100. Similar to HCC, ICC may arise in the context of hepatitis C and chronic liver disease101. There are three types of ICC classified based on morphology and growth pattern, including mass forming, periductal infiltrating type, and intraductal type102. The mass forming is the most common type and must be differentiated from HCC given the differences in treatment and prognosis. Recent reports have shown that small ICCs (< 3 cm) may have similar imaging appearance and enhancement pattern as HCC103,104. There is a paucity of data evaluating DWI imaging features and detection rates for ICC. Very few ICCs were included the previous work comparing DWI to T2 and CE-MRI. A recent study by Park et al describes a “target appearance” of ICC on DWI: peripheral hyperintense rim with a central area of hypointensity on high b-value images105. The central areas of hypointensity corresponded to fibrosis on histopathology. This finding was noted in 75% of ICCs (24/32) versus 3.1% of HCC (1/32) and may be a reliable imaging feature to distinguish small ICC from HCC. Further work is necessary to evaluate the role of DWI in the detection of ICC given its growing incidence and relatively poor prognosis.
Summary: There is limited data regarding the sensitivity of DWI for ICC detection. DWI may be of value in detecting ICC and shows a characteristic morphologic “target” appearance which should be verified in further studies.
2. Lesion characterization
2.1. Qualitative assessment
The noninvasive characterization of liver lesions without using GBCAs is increasingly attractive in the era of nephrogenic systemic fibrosis (NSF)106. Visual assessment of liver lesion morphology, signal intensity and changes in signal intensity at increasing b-values on DW images is useful for lesion characterization6. The changes in signal intensity observed on DW images over a range of b-values depend on true lesion water diffusivity, vascular microperfusion and T2 relaxation time. It is essential to separate true diffusion restriction from “T2 shine through”. “T2 shine through” is the phenomenon present in lesions with long T2 relaxation times, such as cysts or gallbladder content, causing persistent hyperintensity on high b-value images and corresponding high ADC values; thus, the high signal observed is due to the long T2 of the lesion contents (fluid)3. The observed behavior of a lesion on qualitative images must therefore be interpreted along with the corresponding ADC map, which is the graphical representation of the ratio of the diffusion-weighted signal intensities107. Mean ADC values (mm2/s) are obtained directly from the ADC map by placing a region of interest (ROI). ADC maps are calculated with a linear regression analysis of the function. The ROI used must be large enough to for sufficient SNR and to avoid the effects of volume averaging.
Previous work suggested that it is possible to characterize liver lesions as benign or malignant using DWI sequences71,106,108–111. Liver lesions are considered benign when demonstrating hyperintensity on low b-value DW images, attenuation of signal on higher b-value DWI images (b >500), and a corresponding high ADC value. Lesions are considered malignant when remaining hyperintense on higher b-value DW with a low corresponding ADC value71,108 (Fig. 7). Performing visual assessment of lesion characterization has been shown to be highly accurate71,108,112. In a study of 185 focal liver lesions including 76 metastases and 11 HCCs, Holzapfel et al. demonstrated accuracy of 93% for characterizing small lesions <1 cm113. However, the majority of the benign lesions were cysts or hemangiomas. Of note, limitations of these reports include the relatively small sample sizes and limited types of liver lesions evaluated106. DWI may provide added benefit to conventional sequences in the characterization of indeterminate lesions in the cirrhotic liver. For example, distinguishing well-differentiated HCC from benign cirrhotic nodules remains challenging in many cases. In a recent study with histopathologic correlation, high b-value DWI showed higher accuracy (79%) than did hypointensity on HBP for distinguishing well-differentiated HCCs from benign cirrhotic nodules114. DWI was also useful in distinguishing hypervascular HCC from hypervascular pseudolesions, as no pseudolesions were hyperintense at DWI in a series by Motosugi et al115. In addition, indeterminate hypovascular lesions with diffusion restriction often go on to develop hypervascularity and overt imaging features of HCC; thus DWI may be a strong predictor for progression to hypervascular HCC116.
Summary: Assessment of qualitative DW images allows for characterization of liver lesions as benign or malignant with a high degree of accuracy. DWI may play an added role to conventional sequences in characterizing indeterminate lesions in the cirrhotic liver.
Fig. 7.
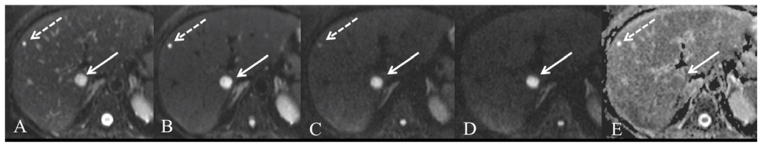
58 year old female with metastatic colon cancer and hepatic cyst. Axial respiratory-triggered SS EPI DWI at 1.5T with b values of 0, 50, 500 and 800 s/mm2 (A–D) and ADC map (E). Both lesions are easily detected on b50 images (B) with excellent suppression of intrahepatic vascular flow. The liver metastasis (solid arrow) remains hyperintense on high b value with low ADC (1.1 × 10−3 mm2/s). The cyst (dashed arrow) shows strong signal drop on intermediate b-value images and is not seen on high b value with high ADC (3.2 × 10−3 mm2/s).
2.2. Quantitative assessment
The ADC has been investigated as a tool to characterize liver lesions. As described previously in this text, the ADC is calculated from the diffusion sequence using multiple (at least 2) b-values. ADC quantification requires minimum acceptable SNR at higher b values and a minimum lesion size of 1.5–2 times the in-plane resolution in order to avoid partial volume averaging6. ADC images must be interpreted in conjunction with qualitative DW images and other sequences. Extensive prior work has shown that benign lesions generally have a significantly higher ADC value compared to malignant lesions71,108,110,111,117–119. Cysts and hemangiomas have statistically higher mean ADC values compared to all solid liver lesions (benign and malignant)106. In a large study of 542 lesions in 382 patients, the mean ADC values of specific liver lesions were as follows (measured as ×10−3 mm2/s): cysts (3.40 ± 0.48), hemangiomas (2.26 ± 0.70), FNHs (1.79 ± 0.39), hepatocellular adenomas (1.49 ± 0.39), metastases (1.50 ± 0.65), and HCC (1.54 ± 0.44)106. ADC cutoff values between 1.4–1.6 × 10−3 mm2/s have been reported in the literature for diagnosing malignant liver lesions, with reported sensitivities of 57.1–100% and specificities of 77–100% (Fig. 8) 71,106,108,110,118. Of note, these ADC cutoff values vary depending on the patient population studied and DWI acquisition parameters. There is significant overlap in ADC measurement between solid benign and malignant lesions, thus making this distinction with DWI challenging. There is no statistically significant difference in reported ADC values for FNH, hepatocellular adenomas, metastases and HCC106,120,121. There is also a paucity of literature examining differences in ADC value for HCC, dysplastic (DN) and regenerative nodules (RN). A report by Xu et al did find that the ADC values of HCC were significantly lower than DN (1.28 × 10−3 mm2/s vs. 1.53 × 10−3 mm2/s, respectively, p=0.0082) in a series of 54 patients with 40 HCCs and 19 DNs, with histopathologic confirmation122.
Summary: ADC quantification can be helpful in separating liver cysts and hemangiomas from malignant liver lesions. However, DWI with ADC quantification cannot reliably make the distinction between solid benign and malignant lesions, and between different malignant lesions.
Fig. 8.
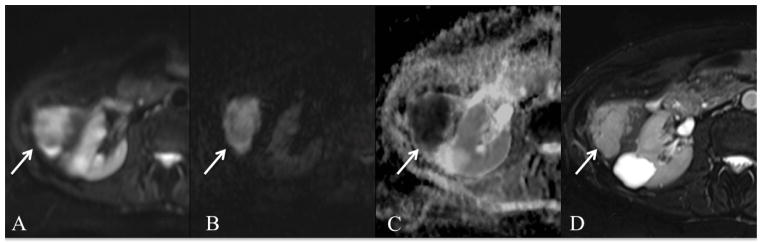
79-year-old female with subcapsular ovarian cancer metastasis. Axial respiratory triggered SS EPI DWI at 1.5T with b values of 50 and 1000 s/mm2 (A, B) and ADC map (C), and fat suppressed FSE T2WI (D). There is a metastatic lesion that is T2 hyperintense with diffusion restriction. ADC value was 1.0 × 10−3 mm2/s.
2.3. Common pitfalls in using DWI for lesion characterization
There are several important limitations to DWI characterization of focal liver lesions. As stated, DWI and ADC characterization cannot distinguish between benign and malignant solid liver lesions given overlap in signal intensity and ADC values (Fig. 9). Furthermore, malignant lesions may be heterogeneous and may contain cystic, mucinous or necrotic components (Fig. 10). The T2 prolongation of these complex components results in elevated ADC values, allowing for mischaracterization of a malignant lesion as benign (Fig. 11)3,6. In addition, the ADC of liver abscesses can demonstrate significant overlap with both hemangiomas and solid lesions (Fig. 12)106. Thus, it is essential to rely on clinical history in addition to the imaging features on conventional sequences in order to correctly diagnose inhomogeneous metastasis and hepatic abscess.
Fig. 9.
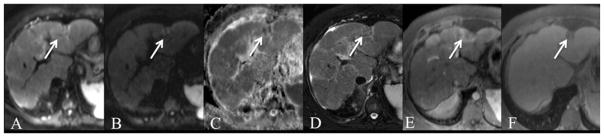
29-year-old male with biopsy-proven necrotizing granuloma. Axial RT SS EPI DWI with b values of 50 and 1000 s/mm2 (A, B) and ADC map (C), fat suppressed FSE T2WI (D) and gadobutrol enhanced GRE T1WI on arterial and equilibrium phases (E, F). There is a T2-weighted hypointense, enhancing lesion in segment 2 with foci of restricted diffusion and adjacent capsular retraction (arrow). ADC value was 1.19 × 10−3 mm2/s.
Fig. 10.
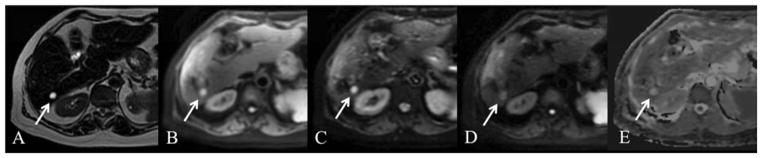
61 year old female with cystic metastasis (arrow). Axial T2 SSFSE (A), breath hold axial SS EPI DWI with b 50, 100, 500 (B, C, D) and ADC map (E). There is a 1.2 cm cystic lesion in segment 6 which is markedly hyperintense on T2 SSFSE. The lesion is hyperintense on low b value image with attenuation of signal on high b value images and high ADC signal (ADC value 3.4 × 10−3 mm2/s) (arrow). Imaging characteristics are consistent with a benign cyst, however this lesion represented a cystic neuroendocrine metastasis.
Fig. 11.
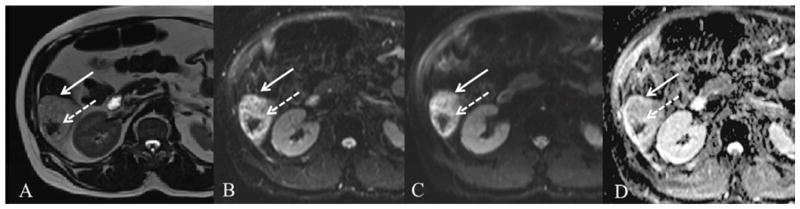
57 year old male with indeterminate liver lesion. There is a 6.9 cm segment 6 lesion (arrow) which is hyperintense on axial HASTE (A), FB SS EPI DWI b50 (B) and b500 (C) with corresponding high signal on ADC map (D). ADC value measured as 1.6 × 10−3 mm2/s. The central hypointense area represents susceptibility artifact from intra-lesional hemorrhage (dashed arrow) with corresponding “T2 black out” on DWI images. Given atypical findings on post-contrast T1WI (not shown), lesion was considered indeterminate and biopsy revealed epitheloid angiosarcoma.
Fig. 12.
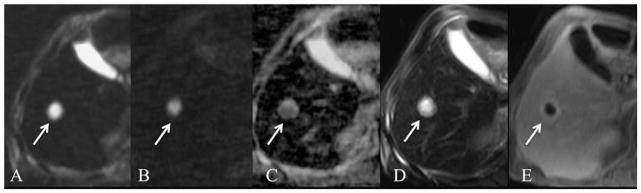
67-year-old male status post chemotherapy for diffuse large B-cell lymphoma. Axial respiratory triggered SS EPI DWI with b values of 50 and 800 s/mm2 (A, B) and ADC map (C), fat suppressed FSE T2WI (D) and gadobutrol enhanced GRE T1WI on late venous phase (E). A thick-walled T2 hyperintense lesion is present in the right hepatic lobe (arrow) demonstrating peripheral rim enhancement (E) and centrally restricted diffusion on both b 50 (A) and b750 (B). FNA guided biopsy of the lesion revealed pus, compatible with hepatic abscess.
Issues related to image quality may also affect interpretation of DWI and ADC. Patients with chronic liver disease and cirrhosis may have concomitant hepatic iron deposition, which may cause a significant T2 shortening effect in the liver that may result in decreased SNR on DW images, most pronounced on higher b values images, with falsely decreased ADC123–125. For example, ADC values were noted to be significantly lower in patients with hepatic siderosis in a series of 52 patients with cirrhosis124. Finally, interpretation of absolute ADC quantification has yet to be clarified as multiple factors influence ADC value (i.e. instrumental, sequencing, biologic). DW images are often of limited quality with relatively poor SNR, spatial resolution, and EPI related artifacts. DWI is still an imaging technique requiring image optimization to ensure consistent high quality performance126.
Summary: Qualitative and quantitative information provided by DWI sequence is best used for characterizing cystic/necrotic lesions and highly cellular solid lesions. One must be aware of potential pitfalls and limitations when interpreting DW images and ADC maps.
3. Tumor treatment response
DWI has been investigated as a tool to evaluate tumor response to therapy and potentially predict which lesions will respond to treatment, by providing information regarding tumor viability, cellularity and vascularity127. Changes in DWI signal intensity and ADC following treatment can precede changes in lesion size or enhancement and reflect tumor necrosis128. The ability to detect early tumor treatment response or lack of response has significant impact in clinical management and treatment planning. Making this distinction would enable repeat treatment or alternative therapy if necessary. Furthermore, given the significant impact and adverse effects of current treatments, including chemotherapy and LRT, knowledge of how likely a lesion is to respond to therapy will also facilitate clinical decision-making in terms of how aggressively to pursue treatment8.
Metastatic lesions to the liver have shown a significant increase in ADC values following systemic chemotherapy that precedes changes in lesion size129,130. Interestingly, a recent study by Marugami et al showed significant overlap in ADC values between responding and non-responding colorectal metastatic lesions following intra-arterial chemotherapy. Using a threshold change in ADC value of 3.5%, ROC analysis showed higher sensitivity and specificity values for % min ADC (100% and 92.6%, respectively) than for % mean ADC (66.7% and 74.1%, respectively) to identify responding lesions131. The % min ADC indicated the most diffusion restricting voxel within the ROI. Furthermore, liver metastases with low pre-treatment ADC values have responded better to systemic chemotherapy compared to lesions with high pre-treatment ADC values in several series. High pre-treatment ADC value of liver metastases is therefore a potential predictor of poor response to chemotherapy8,129,132. Metastatic lesions with higher ADC values may in fact have diminished blood supply and consequentially, more baseline cellular hypoxia and necrosis, which may limit the effectiveness of therapy. Another hypothesis to explain poor treatment response in lesions with high ADC value is the variation in local immune response: local host immune response results in increased tissue cellularity and cellular swelling, resulting in a lower ADC8. Fig. 13 demonstrates tumor recurrence in a patient with colon cancer who underwent wedge resection of the liver.
Fig. 13.
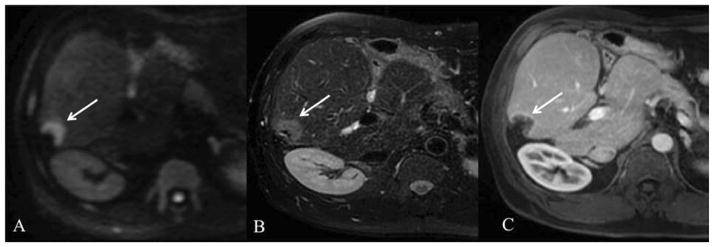
51 year old male status post partial right wedge resection for metastatic colon cancer with tumor recurrence. Axial respiratory-triggered SS EPI DWI with b value of 500 s/mm2 (A), axial breath-hold fat suppressed T2WI (B), and axial fat-suppressed GRE T1WI after gadoxetic acid injection at portal venous phase (C) show a hypovascular metastatic lesion at the resection margin (arrow), which is hyperintense on DWI and T2WI.
The development of LRT for HCC, including thermal radiofrequency ablation (RFA), trans-arterial chemoembolization (TACE), trans-arterial radioembolization (TARE), and external beam radiation, has revolutionized HCC treatment in non-operative patients or patients with unresectable disease. Lesions treated with RFA or TACE typically undergo coagulative hemorrhagic necrosis with heterogeneous hyperintensity on unenhanced T1WI, making evaluation of CE-MRI challenging133. Several authors have investigated DWI to evaluate HCC response to TACE and TARE7,128,134–138. LRT causes disruption of cellular membranes, cell death and tumor necrosis with the resultant increase in water diffusivity. An increase in ADC values has been reported following TACE in the early post-treatment period (few days up to 2 weeks) with measurable differences before and after treatment (Fig. 14)7,128,135. ADC values also increase post-TARE according to several preliminary series, but treatment effect has been noted at 1–3 months post-treatment 137,139,140. ADC has demonstrated a significant correlation with tumor necrosis, especially complete tumor necrosis assessed with histopathology141.
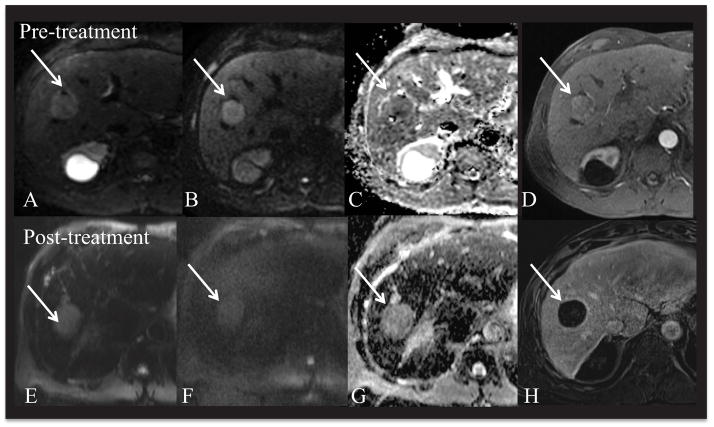
Fig. 14.
66 year old male with hepatitis C related cirrhosis and HCC. 3.2 cm HCC in segment 8 (arrow). Pre-treatment MRI (top) and post-treatment MRI (bottom row). Respiratory-triggered SS EPI DWI at b50, 1000 (A, B) and ADC (C) and arterial phase CE T1WI (gadobutrol) (D) demonstrate HCC in the right lobe with restricted diffusion (ADC value of 1.0 × 10−3 mm2/s). Patient underwent right hepatic lobe Y-90 radio-embolization. Follow-up MRI using free breathing SS EPI DWI at b50, b1000 (E, F) and ADC (G) demonstrate a significant increase in ADC value of 1.7 × 10−3 mm2/s. Post-contrast subtracted (H) image demonstrates complete interval necrosis.
Furthermore, studies have demonstrated differences in ADC values between viable and necrotic portions of HCCs7,141. In theory, the viable portion of the tumor restricts diffusion (hyperintense with low ADC value), whereas the necrotic portions will show relatively un-impeded water diffusion (hyperintense with high ADC value)142. Fig. 15 demonstrates an example of a patient who underwent TACE and RFA to an index HCC with partial necrosis, in additional to additional tumors. Recent publications have also investigated the role of pre-treatment ADC value in predicting response to TACE, with discordant results. Mannelli et al showed that HCCs with low pre-TACE ADC tended to be less responsive to TACE than those with higher ADC, whereas Yuan et al showed that HCCs with higher pre-TACE ADC were less responsive than those with lower ADC to TACE138,143. Thus, DWI and ADC quantification may play a very important role in the non-invasive, non-contrast assessment of post-treatment response and guiding clinical treatment planning in a select population of patients with HCC144. Pre-LRT ADC may be useful in predicting response to therapy; however large prospective studies are needed.
Summary: DWI is a potential useful non-invasive tool in evaluating response to therapy for liver metastases and HCC. Changes in ADC value precede changes in lesion size or enhancement, and correspond with tumor necrosis. Pre-treatment ADC values may also be of value in predicting response to treatment. Therefore, DWI and ADC quantification may provide early information valuable for treatment planning and clinical decision-making. However, large prospective studies are needed.
Fig. 15.
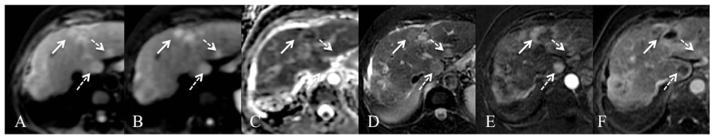
79-year-old female status post TACE/RFA of left hepatic lobe HCC. Axial free breathing SS EPI DWI with b values of 50 and 500 s/mm2 (A,B) and ADC map (C), fat suppressed FSE T2WI (D) and gadoxetate enhanced GRE T1WI on arterial phase (E) and late phase subtraction images (F). A left lobe approximately 50% necrotic treated HCC and adjacent arterial enhancing nodule (E) with venous wash-out (F) are present (arrow). The viable nodule shows T2-weighted hyperintensity (D) and restricted diffusion (A, B) with low ADC value of 0.70 × 10−3 mm2/s. There are additional HCCs in the left and caudate lobes (dashed arrows).
4. Liver fibrosis and cirrhosis
Liver fibrosis and cirrhosis affect parenchymal structure and function due to excessive extracellular matrix deposition. These microscopic changes may lead to reduced diffusion because of a larger proportion of macromolecules such as collagen (inducing restricted motion) and may also lead to reduced perfusion. Previous studies16,17,98,145,146 have reported correlations between liver ADC and fibrosis stage assessed by histopathology. In most studies, a decrease in ADC was observed with increasing fibrosis stage and in cirrhosis. Lewin et al145 reported AUCs of 0.79 and 0.92, for detection of stages F2–F4 and F3–F4, using ADC cutoff values of 1.24 and 1.21 × 10−3 mm2/s, respectively. Taouli et al98 observed AUCs of 0.896 and 0.896 for detection of stages F2–F4 and F3–F4, using cutoff values of 1.54 and 1.53 × 10−3 mm2/s. Wang et al146, in a study comparing DWI and MR elastography (MRE) for liver fibrosis detection, reported AUCs of 0.78–0.88 using ADC for detection of any stage of fibrosis, vs. AUC of 0.92–0.99 for MRE. Beyond ADC measurements, the IVIM technique has been tested recently for liver fibrosis and cirrhosis detection14,16,17 with variable differences observed in fibrotic liver for both diffusion and perfusion metrics (Fig. 16). Fibrosis is, however not the only source of altered diffusion properties in the non-cirrhotic and cirrhotic liver. The influence of inflammatory response on ADC has been studied in lesions147,148 and in the liver26 showing restricted diffusion possibly because of increased viscosity. Increased fat content may also lead to reduced ADC149.
Fig. 16.
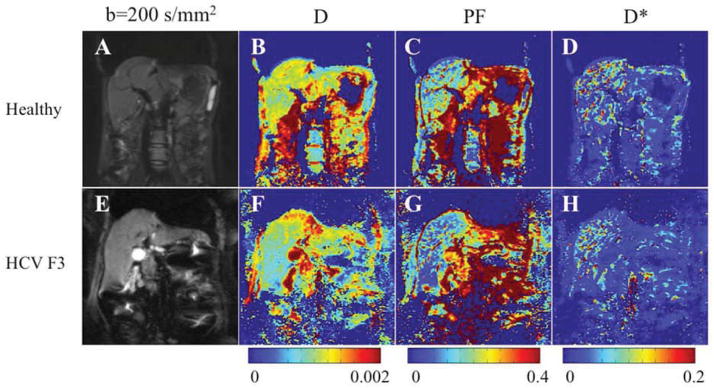
Parametric IVIM diffusion maps obtained with respiratory-triggered SS EPI sequence and 16 b-values (0–800) in a 26-year-old healthy woman and a 55-year-old woman with HCV and stage F3 fibrosis. A, E, Coronal single-shot echo-planar image DW image (b200). B, F: true diffusion coefficient (D) map. C, G: perfusion fraction (PF) map. D, H, D* (pseudodiffusion coefficient) map. D and PF maps show lower values in patient with HCV than in healthy subject. There is no visible difference in D* maps. Corresponding parameter values were: for D, 0.81 10−3 mm2/s and 1.06 10−3 mm2/s for the patient with HCV and the healthy volunteer, respectively; for PF, 5.1% and 9.8%, respectively; and for D*, 50.5 × 10−5 mm2/s and 49.4 × 10−5 mm2/s, respectively.
From: Dyvorne HA, Galea N, Nevers T, et al. Diffusion-weighted imaging of the liver with multiple b values: effect of diffusion gradient polarity and breathing acquisition on image quality and intravoxel incoherent motion parameters--a pilot study. Radiology 2013;266: 920–929; with permission.
LIMITATIONS
DWI sequence has several important limitations in terms of SS EPI image quality and ADC reproducibility. The SS EPI DWI sequence is limited given its relatively low spatial resolution and reduced SNR6. EPI artifacts including ghosting and distortion can result in image degradation, which is worse at higher field strength and in the left hepatic lobe. Despite gains in SNR, 3T SS EPI images may be further impaired by susceptibility artifacts caused by B1 inhomogeneities and dielectric/conductive artifacts55,58. Technical improvements including the application of strong gradients, multi-channel coils, high magnetic fields and advanced software platforms are being used to optimize DWI6,126.
Significant variability in ADC values has been reported, likely as a result of a combination of hardware and biologic factors6. The CV of ADC values measured in liver parenchyma ranges from 3.0–16.2% in several reports17,35,55,56. Several authors have investigated ADC reproducibility for malignant liver lesions. The DWI acquisition technique (BH, RT or FB), ADC calculation method, and selection of b-values all potentially influence the ADC measurement and the reproducibility for malignant liver lesions150. ADCs calculated from breath-hold DWI were more reproducible than from RT DWI in a recent study by Kim et al150. An additional recent study reports 95% limits of agreement of repeated ADC measurements of malignant liver lesions of 28.7–31.3% of the mean36. Greater reproducibility of ADC measurement for larger lesions and for lesions in the right hepatic lobe was also reported in this study36, as well as in a recent study using IVIM37. Further improvements and modifications to the DWI sequence and ADC calculation are needed to optimize ADC measurement reproducibility and accuracy, thus enabling reliable comparison of research studies and evaluation post-treatment tumor response. Standardization of imaging technique and ADC calculation methods across imaging platforms may serve to address these issues.
Summary: DWI sequences are limited due to relative poor SNR and EPI related artifacts including ghosting and distortion. ADC quantification is limited by poor measurement reproducibility for both liver parenchyma and lesions. Future DWI sequence improvements and modifications are needed to address these limitations.
FUTURE DIRECTIONS
5.1. IVIM in the clinic
Emerging research has investigated the role of intravoxel incoherent motion (IVIM) for the following potential clinical applications:
5.1.1. Non-invasive detection of liver fibrosis
Given the experience with DWI and monoexponential ADC calculation, preliminary work has shown that IVIM can distinguish the individual contributions of cellular restricted diffusion and tissue perfusion, as defined by true diffusion coefficient (D), perfusion related pseudodiffusion (PF), and pseudodiffusion coefficient (D*)16,17,35,151–153. In a study comparing RT and FB IVIM using 9 b-values, there was a significant decrease in liver true diffusion coefficient (D), perfusion fraction (PF), pseudodiffusion coefficient (D*) and ADC in cirrhotic livers compared to healthy livers17. Similarly, a recent study using tri-exponential IVIM analysis also demonstrated a significant reduction in D*, D, and PF in cirrhosis152. In a prospective pilot study by Dyvorne et al evaluating the effect of diffusion gradient polarity and breathing acquisition on image quality, PF and D were reduced in patients with HCV related liver fibrosis153. Overall, these findings reflect the physiologic and microscopic cellular changes to the liver with fibrosis and cirrhosis: namely, the decrease in hepatic blood flow (reduction in portal flow) and the increased deposition of proteins and macromolecules (collagen). A current challenge in the role of IVIM for liver fibrosis quantification remains the optimization of image quality and standardization of the technique and inter-platform reproducibility, for which further research is necessary44.
5.1.2. Liver lesion characterization
IVIM is being investigated for liver lesion characterization in hopes of addressing the existing challenges for ADC characterization, including the significant overlap in ADC values for benign and malignant lesions and the variability in ADC measurement across imaging platforms. The ability to discriminate and measure diffusion and perfusion contributions may be of value for highly cellular and hypervascular lesions151. A recent study by Yoon et al retrospectively examined 142 patients with 169 focal liver lesions (120 malignant lesions), and found that the diagnostic performance of true diffusion (D) was better than that of ADCtotal in the diagnosis of malignancy (AUC 0.971 using a cut off value 1.278 × 10−3 mm2/s)154. In addition, the authors found that the perfusion fraction (PF) and pseudodiffusion coefficient (D*) were significantly higher in hypervascular lesions compared to hypovascular lesions. Microvascular flow in hypervascular lesions thus contaminated the ADCtotal value, and therefore, the removal of perfusion contamination may be useful in revealing the increased cellularity in malignant hypervascular lesions thus allowing improved characterization154. The exciting new application of IVIM is limited by measurement reproducibility, for which further investigation is warranted37,155.
5.2. Combination and comparison with PET/MRI
Recent technologic advancements have led to the integration of DWI with FDG-PET, a well-established functional modality for various oncologic and non-oncologic applications, allowing for simultaneous data acquisition. PET-MRI has the potential to combine excellent soft tissue contrast resolution, anatomic resolution and functional information with reduction in overall radiation exposure. DWI and PET are predicated upon entirely different fundamental principles and mechanisms, DWI assessing random motion of water and PET assessing cell glucose metabolism within biologic tissues. Maximum standardized uptake value (SUVmax) is a quantitative measure of cellular glucose metabolism and has been down shown to correlate with histopathologic tumor grade and the presence of necrosis156,157. These modalities can provide complementary information. DWI may be of value for lesions that are associated with low 18F-FDG accumulation and for the assessment of organs with normal high levels of background 18F-FDG accumulation. PET may be of value for lesions close to the heart or spleen158.
Both DWI and PET can be acquired using FB technique with the ability to perform whole body imaging, multi-planar and 3-dimensional reformats. DWI and PET images are visually similar in appearance. Diagnostic performance of PET-MRI can be improved with acquisition of anatomic MR imaging sequences159. PET-MRI T1-weighted gradient-echo images and T2-weighted images have more accurate spatial registration than PET/CT images, although the DWI sequence can result in significant image misregistration from EPI-related geometric distortions, which is a potential weakness of the DWI-PET integration160.
Studies on correlation between PET and DWI are limited, and results of preliminary work have shown an inverse relationship between ADC and SUVmax161–163. A recent prospective study was the first to examine correlation between SUV and ADC in neoplastic lesions using a simultaneous hybrid PET/MRI system. In this study, there was a significant negative correlation for between lesion SUV ratio (SUVmax/mean liver SUV) and ADCmin (R=−0.46, p<0.04) for non-osseous soft tissue lesions164. Well-designed prospective studies are needed to provide more insight and experience into the PET-DWI imaging integration, especially in terms of patient tolerance, safety, diagnostic and prognostic capability and cost effectiveness158. Currently, there are no published clinical studies investigating the role of DWI-PET in liver malignancy.
5.3. New DWI sequences
The SS EPI sequence is the standard pulse sequence for liver MRI given its rapid acquisition time and relative insensitivity to patient motion165. Given enhancements in gradient systems and the emergence of parallel imaging, additional pulse sequences have been investigated for use in abdominal imaging, including spin-echo, stimulated-echo, fast-spin-echo, and steady-state free-precession sequences166. The most important alternative sequences used in the abdomen include the single-shot and PROPELLER fast-spin-echo and steady-state free-precession sequences166. Modifications of imaging parameters including selection of b-values is necessary when using these pulse sequence in order to obtain sufficient image quality and reliable quantification of diffusion parameters166.
Diffusion tensor imaging (DTI), which employs additional sensitizing gradients to plot the relative degree of diffusion in multiple directions, has been studied in anatomy where diffusion is preferentially restricted in one direction21. DTI has been investigated in the central nervous system for evaluation of non-isotropic tissue such as white matter and spinal cord diseases. Limited data is available for DTI in the liver 25,52. The use of multiple diffusion gradient directions with DTI results in a stronger applied gradient and shorter TE values52. Since more dimensions are plotted in DTI compared to conventional DWI, there is a theoretical advantage of more precise ADC calculation and information regarding tissue anisotropy. Preliminary work has investigated DTI in both healthy and fibrotic livers, with mixed results regarding image quality of DTI compared to conventional DWI26. Taouli et al showed no compromise in hepatic ADC values using DTI52. Further work is necessary to determine the role of DTI, if any, in the assessment of diffuse liver disease or focal liver lesion.
Summary: Emerging applications of DWI technology including IVIM, DWI/PET integration and new DWI sequences may further expand the role and ability of DWI to accurately detect and characterize diffuse and focal liver disease.
Conclusion
In this review, we have described our current understanding, applications and potential of DWI for the evaluation of focal and diffuse liver disease. DWI has many potential evolving applications, including focal liver lesion detection and characterization, the assessment of tumor response and the evaluation of diffuse liver disease. In our opinion, DWI should be incorporated into routine liver MRI protocols. DWI is best employed in combination with conventional sequences, especially contrast-enhanced sequences, for detection and characterization of malignant liver lesions. DWI currently has a limited role as an isolated technique for lesion characterization. The reader must be cognizant of the potential pitfalls and limitations of this technique. For patients with impaired renal function or GBCA allergy, DWI is a reasonable non-invasive alternative to CE MRI. Further sequence improvements and widespread standardization of technique, b-values and imaging parameters are necessary for DWI to reach its full potential. Future applications including IVIM and DWI/PET MRI show promise and warrant further investigation.
KEY POINTS.
DWI (diffusion-weighted imaging) is based upon differences in mobility of water protons in tissues. Single-shot echo planar (SS EPI) DWI sequences are most commonly used in liver imaging.
DWI is generally more sensitive than fast spin echo fat suppressed T2-weighted imaging (T2WI) for liver lesion detection. The combination of DWI and CE-T1 weighted imaging (contrast-enhanced T1-WI) is most sensitive for detection of malignant liver lesions.
ADC (apparent diffusion coefficient) quantification can be used to characterize liver lesions as cystic/necrotic or solid. However, ADC alone is insufficient for lesion characterization.
ADC has potential value in the evaluation of tumor treatment response, with changes in ADC preceding changes in lesion size.
ADC quantification and IVIM (intra voxel incoherent motion) DWI have diagnostic value in the non-invasive detection of liver fibrosis and cirrhosis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Le Bihan D. Molecular diffusion nuclear magnetic resonance imaging. Magnetic resonance quarterly. 1991 Jan;7(1):1–30. [PubMed] [Google Scholar]
- 2.Bammer R. Basic principles of diffusion-weighted imaging. European journal of radiology. 2003 Mar;45(3):169–184. doi: 10.1016/s0720-048x(02)00303-0. [DOI] [PubMed] [Google Scholar]
- 3.Taouli B, Koh DM. Diffusion-weighted MR imaging of the liver. Radiology. 2010 Jan;254(1):47–66. doi: 10.1148/radiol.09090021. [DOI] [PubMed] [Google Scholar]
- 4.Charnsangavej C, Clary B, Fong Y, Grothey A, Pawlik TM, Choti MA. Selection of patients for resection of hepatic colorectal metastases: expert consensus statement. Ann Surg Oncol. 2006 Oct;13(10):1261–1268. doi: 10.1245/s10434-006-9023-y. [DOI] [PubMed] [Google Scholar]
- 5.Bruix J, Sherman M, Llovet JM, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. Journal of hepatology. 2001 Sep;35(3):421–430. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 6.Galea N, Cantisani V, Taouli B. Liver lesion detection and characterization: Role of diffusion-weighted imaging. Journal of magnetic resonance imaging: JMRI. 2013 Jun;37(6):1260–1276. doi: 10.1002/jmri.23947. [DOI] [PubMed] [Google Scholar]
- 7.Kamel IR, Bluemke DA, Eng J, et al. The role of functional MR imaging in the assessment of tumor response after chemoembolization in patients with hepatocellular carcinoma. J Vasc Interv Radiol. 2006 Mar;17(3):505–512. doi: 10.1097/01.RVI.0000200052.02183.92. [DOI] [PubMed] [Google Scholar]
- 8.Tam HH, Collins DJ, Brown G, et al. The role of pre-treatment diffusion-weighted MRI in predicting long-term outcome of colorectal liver metastasis. The British journal of radiology. 2013 Oct;86(1030):20130281. doi: 10.1259/bjr.20130281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holz M, Heil SR, Sacco A. Temperature-dependent self-diffusion coefficients of water and six selected molecular liquids for calibration in accurate 1H NMR PFG measurements. Physical Chemistry Chemical Physics. 2000;2(20):4740–4742. [Google Scholar]
- 10.Stejskal EO, Tanner JE. Spin diffusion measurements: spin echoes in the presence of a time-dependent field gradient. J Chem Phys. 1965;42:288–292. [Google Scholar]
- 11.Le Bihan D, Breton E, Lallemand D, Aubin ML, Vignaud J, Laval-Jeantet M. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology. 1988;168(2):497–505. doi: 10.1148/radiology.168.2.3393671. [DOI] [PubMed] [Google Scholar]
- 12.Koh DM, Collins DJ, Orton MR. Intravoxel incoherent motion in body diffusion-weighted MRI: reality and challenges. AJR Am J Roentgenol. 2011 Jun;196(6):1351–1361. doi: 10.2214/AJR.10.5515. [DOI] [PubMed] [Google Scholar]
- 13.Andreou A, Koh DM, Collins DJ, et al. Measurement reproducibility of perfusion fraction and pseudodiffusion coefficient derived by intravoxel incoherent motion diffusion-weighted MR imaging in normal liver and metastases. European radiology. 2012 Oct 6; doi: 10.1007/s00330-012-2604-1. [DOI] [PubMed] [Google Scholar]
- 14.Dyvorne HA, Galea N, Nevers T, et al. Diffusion-weighted Imaging of the Liver with Multiple b Values: Effect of Diffusion Gradient Polarity and Breathing Acquisition on Image Quality and Intravoxel Incoherent Motion Parameters--A Pilot Study. Radiology. 2012 Dec 6; doi: 10.1148/radiol.12120686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kang KM, Lee JM, Yoon JH, Kiefer B, Han JK, Choi BI. Intravoxel Incoherent Motion Diffusion-weighted MR Imaging for Characterization of Focal Pancreatic Lesions. Radiology. 2013 Oct 14; doi: 10.1148/radiol.13122712. [DOI] [PubMed] [Google Scholar]
- 16.Luciani A, Vignaud A, Cavet M, et al. Liver cirrhosis: intravoxel incoherent motion MR imaging--pilot study. Radiology. 2008 Dec;249(3):891–899. doi: 10.1148/radiol.2493080080. [DOI] [PubMed] [Google Scholar]
- 17.Patel J, Sigmund EE, Rusinek H, Oei M, Babb JS, Taouli B. Diagnosis of cirrhosis with intravoxel incoherent motion diffusion MRI and dynamic contrast-enhanced MRI alone and in combination: preliminary experience. Journal of magnetic resonance imaging: JMRI. 2010 Mar;31(3):589–600. doi: 10.1002/jmri.22081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamada I, Aung W, Himeno Y, Nakagawa T, Shibuya H. Diffusion coefficients in abdominal organs and hepatic lesions: evaluation with intravoxel incoherent motion echo-planar MR imaging. Radiology. 1999 Mar;210(3):617–623. doi: 10.1148/radiology.210.3.r99fe17617. [DOI] [PubMed] [Google Scholar]
- 19.Jensen JH, Helpern JA, Ramani A, Lu H, Kaczynski K. Diffusional kurtosis imaging: the quantification of non-gaussian water diffusion by means of magnetic resonance imaging. Magn Reson Med. 2005 Jun;53(6):1432–1440. doi: 10.1002/mrm.20508. [DOI] [PubMed] [Google Scholar]
- 20.Rosenkrantz AB, Sigmund EE, Winnick A, et al. Assessment of hepatocellular carcinoma using apparent diffusion coefficient and diffusion kurtosis indices: preliminary experience in fresh liver explants. Magnetic resonance imaging. 2012 Dec;30(10):1534–1540. doi: 10.1016/j.mri.2012.04.020. [DOI] [PubMed] [Google Scholar]
- 21.Le Bihan D, Mangin JF, Poupon C, et al. Diffusion tensor imaging: concepts and applications. Journal of magnetic resonance imaging: JMRI. 2001 Apr;13(4):534–546. doi: 10.1002/jmri.1076. [DOI] [PubMed] [Google Scholar]
- 22.Gurses B, Kabakci N, Kovanlikaya A, et al. Diffusion tensor imaging of the normal prostate at 3 Tesla. European radiology. 2008 Apr;18(4):716–721. doi: 10.1007/s00330-007-0795-7. [DOI] [PubMed] [Google Scholar]
- 23.Kataoka M, Kido A, Yamamoto A, et al. Diffusion tensor imaging of kidneys with respiratory triggering: optimization of parameters to demonstrate anisotropic structures on fraction anisotropy maps. Journal of magnetic resonance imaging: JMRI. 2009 Mar;29(3):736–744. doi: 10.1002/jmri.21669. [DOI] [PubMed] [Google Scholar]
- 24.Manenti G, Carlani M, Mancino S, et al. Diffusion tensor magnetic resonance imaging of prostate cancer. Invest Radiol. 2007 Jun;42(6):412–419. doi: 10.1097/01.rli.0000264059.46444.bf. [DOI] [PubMed] [Google Scholar]
- 25.Sigmund EE, Vivier PH, Sui D, et al. Intravoxel incoherent motion and diffusion-tensor imaging in renal tissue under hydration and furosemide flow challenges. Radiology. 2012 Jun;263(3):758–769. doi: 10.1148/radiol.12111327. [DOI] [PubMed] [Google Scholar]
- 26.Taouli B, Chouli M, Martin AJ, Qayyum A, Coakley FV, Vilgrain V. Chronic hepatitis: role of diffusion-weighted imaging and diffusion tensor imaging for the diagnosis of liver fibrosis and inflammation. Journal of magnetic resonance imaging: JMRI. 2008 Jul;28(1):89–95. doi: 10.1002/jmri.21227. [DOI] [PubMed] [Google Scholar]
- 27.Cheung JS, Fan SJ, Gao DS, Chow AM, Man K, Wu EX. Diffusion tensor imaging of liver fibrosis in an experimental model. Journal of magnetic resonance imaging: JMRI. 2010 Nov;32(5):1141–1148. doi: 10.1002/jmri.22367. [DOI] [PubMed] [Google Scholar]
- 28.Taouli B, Vilgrain V, Dumont E, Daire JL, Fan B, Menu Y. Evaluation of liver diffusion isotropy and characterization of focal hepatic lesions with two single-shot echo-planar MR imaging sequences: prospective study in 66 patients. Radiology. 2003 Jan;226(1):71–78. doi: 10.1148/radiol.2261011904. [DOI] [PubMed] [Google Scholar]
- 29.Sasaki M, Yamada K, Watanabe Y, et al. Variability in absolute apparent diffusion coefficient values across different platforms may be substantial: a multivendor, multi-institutional comparison study. Radiology. 2008 Nov;249(2):624–630. doi: 10.1148/radiol.2492071681. [DOI] [PubMed] [Google Scholar]
- 30.Chenevert TL, Galban CJ, Ivancevic MK, et al. Diffusion coefficient measurement using a temperature-controlled fluid for quality control in multicenter studies. Journal of magnetic resonance imaging: JMRI. 2011 Oct;34(4):983–987. doi: 10.1002/jmri.22363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Girometti R, Furlan A, Esposito G, et al. Relevance of b-values in evaluating liver fibrosis: a study in healthy and cirrhotic subjects using two single-shot spin-echo echo-planar diffusion-weighted sequences. Journal of magnetic resonance imaging: JMRI. 2008 Aug;28(2):411–419. doi: 10.1002/jmri.21461. [DOI] [PubMed] [Google Scholar]
- 32.Xing D, Papadakis NG, Huang CL, Lee VM, Carpenter TA, Hall LD. Optimised diffusion-weighting for measurement of apparent diffusion coefficient (ADC) in human brain. Magnetic resonance imaging. 1997;15(7):771–784. doi: 10.1016/s0730-725x(97)00037-4. [DOI] [PubMed] [Google Scholar]
- 33.Lemke A, Stieltjes B, Schad LR, Laun FB. Toward an optimal distribution of b values for intravoxel incoherent motion imaging. Magnetic resonance imaging. 2011 Jul;29(6):766–776. doi: 10.1016/j.mri.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 34.Zhang JL, Sigmund EE, Rusinek H, et al. Optimization of b-value sampling for diffusion-weighted imaging of the kidney. Magn Reson Med. 2012 Jan;67(1):89–97. doi: 10.1002/mrm.22982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Braithwaite AC, Dale BM, Boll DT, Merkle EM. Short- and midterm reproducibility of apparent diffusion coefficient measurements at 3.0-T diffusion-weighted imaging of the abdomen. Radiology. 2009 Feb;250(2):459–465. doi: 10.1148/radiol.2502080849. [DOI] [PubMed] [Google Scholar]
- 36.Kim SY, Lee SS, Byun JH, et al. Malignant hepatic tumors: short-term reproducibility of apparent diffusion coefficients with breath-hold and respiratory-triggered diffusion-weighted MR imaging. Radiology. 2010 Jun;255(3):815–823. doi: 10.1148/radiol.10091706. [DOI] [PubMed] [Google Scholar]
- 37.Kakite S, Dyvorne HA, Besa C, et al. Hepatocellular carcinoma: Short-term reproducibility of ADC and IVIM parameters at 3.0T. J Magn Reson Imag. doi: 10.1002/jmri.24538. (in press) [DOI] [PubMed] [Google Scholar]
- 38.Le Bihan D, Poupon C, Amadon A, Lethimonnier F. Artifacts and pitfalls in diffusion MRI. Journal of magnetic resonance imaging: JMRI. 2006 Sep;24(3):478–488. doi: 10.1002/jmri.20683. [DOI] [PubMed] [Google Scholar]
- 39.Porter DA, Heidemann RM. High resolution diffusion-weighted imaging using readout-segmented echo-planar imaging, parallel imaging and a two-dimensional navigator-based reacquisition. Magn Reson Med. 2009 Aug;62(2):468–475. doi: 10.1002/mrm.22024. [DOI] [PubMed] [Google Scholar]
- 40.Lovblad KO, Jakob PM, Chen Q, et al. Turbo spin-echo diffusion-weighted MR of ischemic stroke. AJNR. American journal of neuroradiology. 1998 Feb;19(2):201–208. discussion 209. [PMC free article] [PubMed] [Google Scholar]
- 41.Lu L, Erokwu B, Lee G, et al. Diffusion-prepared fast imaging with steady-state free precession (DP-FISP): a rapid diffusion MRI technique at 7 T. Magn Reson Med. 2012 Sep;68(3):868–873. doi: 10.1002/mrm.23287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deng J, Miller FH, Salem R, Omary RA, Larson AC. Multishot diffusion-weighted PROPELLER magnetic resonance imaging of the abdomen. Invest Radiol. 2006 Oct;41(10):769–775. doi: 10.1097/01.rli.0000236808.84746.95. [DOI] [PubMed] [Google Scholar]
- 43.Pipe JG. Motion correction with PROPELLER MRI: application to head motion and free-breathing cardiac imaging. Magn Reson Med. 1999 Nov;42(5):963–969. doi: 10.1002/(sici)1522-2594(199911)42:5<963::aid-mrm17>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 44.Kwee TC, Takahara T, Koh DM, Nievelstein RA, Luijten PR. Comparison and reproducibility of ADC measurements in breathhold, respiratory triggered, and free-breathing diffusion-weighted MR imaging of the liver. Journal of magnetic resonance imaging: JMRI. 2008 Nov;28(5):1141–1148. doi: 10.1002/jmri.21569. [DOI] [PubMed] [Google Scholar]
- 45.Taouli B, Sandberg A, Stemmer A, et al. Diffusion-weighted imaging of the liver: comparison of navigator triggered and breathhold acquisitions. Journal of magnetic resonance imaging: JMRI. 2009 Sep;30(3):561–568. doi: 10.1002/jmri.21876. [DOI] [PubMed] [Google Scholar]
- 46.Murtz P, Flacke S, Traber F, van den Brink JS, Gieseke J, Schild HH. Abdomen: diffusion-weighted MR imaging with pulse-triggered single-shot sequences. Radiology. 2002 Jul;224(1):258–264. doi: 10.1148/radiol.2241011117. [DOI] [PubMed] [Google Scholar]
- 47.Johnson GA, Maki JH. In vivo measurement of proton diffusion in the presence of coherent motion. Invest Radiol. 1991 Jun;26(6):540–545. doi: 10.1097/00004424-199106000-00006. [DOI] [PubMed] [Google Scholar]
- 48.Ozaki M, Inoue Y, Miyati T, et al. Motion artifact reduction of diffusion-weighted MRI of the liver: use of velocity-compensated diffusion gradients combined with tetrahedral gradients. Journal of magnetic resonance imaging: JMRI. 2013 Jan;37(1):172–178. doi: 10.1002/jmri.23796. [DOI] [PubMed] [Google Scholar]
- 49.Griswold MA, Jakob PM, Heidemann RM, et al. Generalized autocalibrating partially parallel acquisitions (GRAPPA) Magn Reson Med. 2002;47(6):1202–1210. doi: 10.1002/mrm.10171. [DOI] [PubMed] [Google Scholar]
- 50.Pruessmann KP, Weiger M, Scheidegger MB, Boesiger P. SENSE: sensitivity encoding for fast MRI. Magn Reson Med. 1999 Nov;42(5):952–962. [PubMed] [Google Scholar]
- 51.Sodickson DK, Manning WJ. Simultaneous acquisition of spatial harmonics (SMASH): fast imaging with radiofrequency coil arrays. Magn Reson Med. 1997 Oct;38(4):591–603. doi: 10.1002/mrm.1910380414. [DOI] [PubMed] [Google Scholar]
- 52.Taouli B, Martin AJ, Qayyum A, et al. Parallel imaging and diffusion tensor imaging for diffusion-weighted MRI of the liver: preliminary experience in healthy volunteers. AJR Am J Roentgenol. 2004 Sep;183(3):677–680. doi: 10.2214/ajr.183.3.1830677. [DOI] [PubMed] [Google Scholar]
- 53.Setsompop K, Gagoski BA, Polimeni JR, Witzel T, Wedeen VJ, Wald LL. Blipped-controlled aliasing in parallel imaging for simultaneous multislice echo planar imaging with reduced g-factor penalty. Magn Reson Med. 2012 May;67(5):1210–1224. doi: 10.1002/mrm.23097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Setsompop K, Cohen-Adad J, Gagoski BA, et al. Improving diffusion MRI using simultaneous multi-slice echo planar imaging. NeuroImage. 2012 Oct 15;63(1):569–580. doi: 10.1016/j.neuroimage.2012.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rosenkrantz AB, Oei M, Babb JS, Niver BE, Taouli B. Diffusion-weighted imaging of the abdomen at 3.0 Tesla: image quality and apparent diffusion coefficient reproducibility compared with 1.5 Tesla. Journal of magnetic resonance imaging: JMRI. 2011 Jan;33(1):128–135. doi: 10.1002/jmri.22395. [DOI] [PubMed] [Google Scholar]
- 56.Dale BM, Braithwaite AC, Boll DT, Merkle EM. Field strength and diffusion encoding technique affect the apparent diffusion coefficient measurements in diffusion-weighted imaging of the abdomen. Invest Radiol. 2010 Feb;45(2):104–108. doi: 10.1097/RLI.0b013e3181c8ceac. [DOI] [PubMed] [Google Scholar]
- 57.Coenegrachts K, Delanote J, Ter Beek L, et al. Improved focal liver lesion detection: comparison of single-shot diffusion-weighted echoplanar and single-shot T2 weighted turbo spin echo techniques. The British journal of radiology. 2007 Jul;80(955):524–531. doi: 10.1259/bjr/33156643. [DOI] [PubMed] [Google Scholar]
- 58.van den Bos IC, Hussain SM, Krestin GP, Wielopolski PA. Liver imaging at 3.0 T: diffusion-induced black-blood echo-planar imaging with large anatomic volumetric coverage as an alternative for specific absorption rate-intensive echo-train spin-echo sequences: feasibility study. Radiology. 2008 Jul;248(1):264–271. doi: 10.1148/radiol.2481070034. [DOI] [PubMed] [Google Scholar]
- 59.Okada Y, Ohtomo K, Kiryu S, Sasaki Y. Breath-hold T2-weighted MRI of hepatic tumors: value of echo planar imaging with diffusion-sensitizing gradient. Journal of computer assisted tomography. 1998 May-Jun;22(3):364–371. doi: 10.1097/00004728-199805000-00005. [DOI] [PubMed] [Google Scholar]
- 60.Hussain SM, De Becker J, Hop WC, Dwarkasing S, Wielopolski PA. Can a single-shot black-blood T2-weighted spin-echo echo-planar imaging sequence with sensitivity encoding replace the respiratory-triggered turbo spin-echo sequence for the liver? An optimization and feasibility study. Journal of magnetic resonance imaging: JMRI. 2005 Mar;21(3):219–229. doi: 10.1002/jmri.20269. [DOI] [PubMed] [Google Scholar]
- 61.Moteki T, Horikoshi H. Evaluation of hepatic lesions and hepatic parenchyma using diffusion-weighted echo-planar MR with three values of gradient b-factor. Journal of magnetic resonance imaging: JMRI. 2006 Sep;24(3):637–645. doi: 10.1002/jmri.20682. [DOI] [PubMed] [Google Scholar]
- 62.Wu LM, Hu J, Gu HY, Hua J, Xu JR. Can diffusion-weighted magnetic resonance imaging (DW-MRI) alone be used as a reliable sequence for the preoperative detection and characterisation of hepatic metastases? A meta-analysis. Eur J Cancer. 2013 Feb;49(3):572–584. doi: 10.1016/j.ejca.2012.08.021. [DOI] [PubMed] [Google Scholar]
- 63.Cummings LC, Payes JD, Cooper GS. Survival after hepatic resection in metastatic colorectal cancer: a population-based study. Cancer. 2007 Feb 15;109(4):718–726. doi: 10.1002/cncr.22448. [DOI] [PubMed] [Google Scholar]
- 64.Benoist S, Nordlinger B. The role of preoperative chemotherapy in patients with resectable colorectal liver metastases. Ann Surg Oncol. 2009 Sep;16(9):2385–2390. doi: 10.1245/s10434-009-0492-7. [DOI] [PubMed] [Google Scholar]
- 65.Gillams AR, Lees WR. Five-year survival in 309 patients with colorectal liver metastases treated with radiofrequency ablation. European radiology. 2009 May;19(5):1206–1213. doi: 10.1007/s00330-008-1258-5. [DOI] [PubMed] [Google Scholar]
- 66.Koh DM, Collins DJ, Wallace T, Chau I, Riddell AM. Combining diffusion-weighted MRI with Gd-EOB-DTPA-enhanced MRI improves the detection of colorectal liver metastases. The British journal of radiology. 2012 Jul;85(1015):980–989. doi: 10.1259/bjr/91771639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zech CJ, Herrmann KA, Dietrich O, Horger W, Reiser MF, Schoenberg SO. Black-blood diffusion-weighted EPI acquisition of the liver with parallel imaging: comparison with a standard T2-weighted sequence for detection of focal liver lesions. Invest Radiol. 2008 Apr;43(4):261–266. doi: 10.1097/RLI.0b013e31816200b5. [DOI] [PubMed] [Google Scholar]
- 68.Bruegel M, Gaa J, Waldt S, et al. Diagnosis of hepatic metastasis: comparison of respiration-triggered diffusion-weighted echo-planar MRI and five t2-weighted turbo spin-echo sequences. AJR Am J Roentgenol. 2008 Nov;191(5):1421–1429. doi: 10.2214/AJR.07.3279. [DOI] [PubMed] [Google Scholar]
- 69.d’Assignies G, Fina P, Bruno O, et al. High sensitivity of diffusion-weighted MR imaging for the detection of liver metastases from neuroendocrine tumors: comparison with T2-weighted and dynamic gadolinium-enhanced MR imaging. Radiology. 2013 Aug;268(2):390–399. doi: 10.1148/radiol.13121628. [DOI] [PubMed] [Google Scholar]
- 70.Soyer P, Boudiaf M, Place V, et al. Preoperative detection of hepatic metastases: comparison of diffusion-weighted, T2-weighted fast spin echo and gadolinium-enhanced MR imaging using surgical and histopathologic findings as standard of reference. European journal of radiology. 2011 Nov;80(2):245–252. doi: 10.1016/j.ejrad.2010.06.027. [DOI] [PubMed] [Google Scholar]
- 71.Parikh T, Drew SJ, Lee VS, et al. Focal liver lesion detection and characterization with diffusion-weighted MR imaging: comparison with standard breath-hold T2-weighted imaging. Radiology. 2008 Mar;246(3):812–822. doi: 10.1148/radiol.2463070432. [DOI] [PubMed] [Google Scholar]
- 72.Choi JS, Kim MJ, Choi JY, Park MS, Lim JS, Kim KW. Diffusion-weighted MR imaging of liver on 3.0-Tesla system: effect of intravenous administration of gadoxetic acid disodium. European radiology. 2010 May;20(5):1052–1060. doi: 10.1007/s00330-009-1651-8. [DOI] [PubMed] [Google Scholar]
- 73.Nasu K, Kuroki Y, Nawano S, et al. Hepatic Metastases: Diffusion-weighted Sensitivity-encoding versus SPIO-enhanced MR Imaging. Radiology. 2006 Apr;239(1):122–130. doi: 10.1148/radiol.2383041384. [DOI] [PubMed] [Google Scholar]
- 74.Koh DM, Brown G, Riddell AM, et al. Detection of colorectal hepatic metastases using MnDPDP MR imaging and diffusion-weighted imaging (DWI) alone and in combination. European radiology. 2008 May;18(5):903–910. doi: 10.1007/s00330-007-0847-z. [DOI] [PubMed] [Google Scholar]
- 75.Hardie AD, Naik M, Hecht EM, et al. Diagnosis of liver metastases: value of diffusion-weighted MRI compared with gadolinium-enhanced MRI. European radiology. 2010 Jun;20(6):1431–1441. doi: 10.1007/s00330-009-1695-9. [DOI] [PubMed] [Google Scholar]
- 76.Shimada K, Isoda H, Hirokawa Y, Arizono S, Shibata T, Togashi K. Comparison of gadolinium-EOB-DTPA-enhanced and diffusion-weighted liver MRI for detection of small hepatic metastases. European radiology. 2010 Nov;20(11):2690–2698. doi: 10.1007/s00330-010-1842-3. [DOI] [PubMed] [Google Scholar]
- 77.Chung WS, Kim MJ, Chung YE, et al. Comparison of gadoxetic acid-enhanced dynamic imaging and diffusion-weighted imaging for the preoperative evaluation of colorectal liver metastases. Journal of magnetic resonance imaging: JMRI. 2011 Aug;34(2):345–353. doi: 10.1002/jmri.22671. [DOI] [PubMed] [Google Scholar]
- 78.Holzapfel K, Reiser-Erkan C, Fingerle AA, et al. Comparison of diffusion-weighted MR imaging and multidetector-row CT in the detection of liver metastases in patients operated for pancreatic cancer. Abdominal imaging. 2011 Apr;36(2):179–184. doi: 10.1007/s00261-010-9633-5. [DOI] [PubMed] [Google Scholar]
- 79.Kenis C, Deckers F, De Foer B, Van Mieghem F, Van Laere S, Pouillon M. Diagnosis of liver metastases: can diffusion-weighted imaging (DWI) be used as a stand alone sequence? European journal of radiology. 2012 May;81(5):1016–1023. doi: 10.1016/j.ejrad.2011.02.019. [DOI] [PubMed] [Google Scholar]
- 80.Lowenthal D, Zeile M, Lim WY, et al. Detection and characterisation of focal liver lesions in colorectal carcinoma patients: comparison of diffusion-weighted and Gd-EOB-DTPA enhanced MR imaging. European radiology. 2011 Apr;21(4):832–840. doi: 10.1007/s00330-010-1977-2. [DOI] [PubMed] [Google Scholar]
- 81.Kim YK, Lee MW, Lee WJ, et al. Diagnostic accuracy and sensitivity of diffusion-weighted and of gadoxetic acid-enhanced 3-T MR imaging alone or in combination in the detection of small liver metastasis (</= 1.5 cm in diameter) Invest Radiol. 2012 Mar;47(3):159–166. doi: 10.1097/RLI.0b013e31823a1495. [DOI] [PubMed] [Google Scholar]
- 82.Macera A, Lario C, Petracchini M, et al. Staging of colorectal liver metastases after preoperative chemotherapy. Diffusion-weighted imaging in combination with Gd-EOB-DTPA MRI sequences increases sensitivity and diagnostic accuracy. European radiology. 2013 Mar;23(3):739–747. doi: 10.1007/s00330-012-2658-0. [DOI] [PubMed] [Google Scholar]
- 83.Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. International journal of cancer. Journal international du cancer. 2001 Oct 15;94(2):153–156. doi: 10.1002/ijc.1440. [DOI] [PubMed] [Google Scholar]
- 84.Bruix J, Llovet JM. Major achievements in hepatocellular carcinoma. Lancet. 2009 Feb 21;373(9664):614–616. doi: 10.1016/S0140-6736(09)60381-0. [DOI] [PubMed] [Google Scholar]
- 85.Piana G, Trinquart L, Meskine N, Barrau V, Beers BV, Vilgrain V. New MR imaging criteria with a diffusion-weighted sequence for the diagnosis of hepatocellular carcinoma in chronic liver diseases. Journal of hepatology. 2011 Jul;55(1):126–132. doi: 10.1016/j.jhep.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 86.Forner A, Vilana R, Ayuso C, et al. Diagnosis of hepatic nodules 20 mm or smaller in cirrhosis: Prospective validation of the noninvasive diagnostic criteria for hepatocellular carcinoma. Hepatology. 2008 Jan;47(1):97–104. doi: 10.1002/hep.21966. [DOI] [PubMed] [Google Scholar]
- 87.Kim JE, Kim SH, Lee SJ, Rhim H. Hypervascular hepatocellular carcinoma 1 cm or smaller in patients with chronic liver disease: characterization with gadoxetic acid-enhanced MRI that includes diffusion-weighted imaging. AJR Am J Roentgenol. 2011 Jun;196(6):W758–765. doi: 10.2214/AJR.10.4394. [DOI] [PubMed] [Google Scholar]
- 88.Vandecaveye V, De Keyzer F, Verslype C, et al. Diffusion-weighted MRI provides additional value to conventional dynamic contrast-enhanced MRI for detection of hepatocellular carcinoma. European radiology. 2009 Oct;19(10):2456–2466. doi: 10.1007/s00330-009-1431-5. [DOI] [PubMed] [Google Scholar]
- 89.Hardie AD, Kizziah MK, Boulter DJ. Diagnostic accuracy of diffusion-weighted MRI for identifying hepatocellular carcinoma with liver explant correlation. Journal of medical imaging and radiation oncology. 2011 Aug;55(4):362–367. doi: 10.1111/j.1754-9485.2011.02286.x. [DOI] [PubMed] [Google Scholar]
- 90.Park MS, Kim S, Patel J, et al. Hepatocellular carcinoma: detection with diffusion-weighted versus contrast-enhanced magnetic resonance imaging in pretransplant patients. Hepatology. 2012 Jul;56(1):140–148. doi: 10.1002/hep.25681. [DOI] [PubMed] [Google Scholar]
- 91.Park MJ, Kim YK, Lee MW, et al. Small hepatocellular carcinomas: improved sensitivity by combining gadoxetic acid-enhanced and diffusion-weighted MR imaging patterns. Radiology. 2012 Sep;264(3):761–770. doi: 10.1148/radiol.12112517. [DOI] [PubMed] [Google Scholar]
- 92.Xu PJ, Yan FH, Wang JH, Lin J, Ji Y. Added value of breathhold diffusion-weighted MRI in detection of small hepatocellular carcinoma lesions compared with dynamic contrast-enhanced MRI alone using receiver operating characteristic curve analysis. Journal of magnetic resonance imaging: JMRI. 2009 Feb;29(2):341–349. doi: 10.1002/jmri.21650. [DOI] [PubMed] [Google Scholar]
- 93.Rosenkrantz AB, Lee L, Matza BW, Kim S. Infiltrative hepatocellular carcinoma: comparison of MRI sequences for lesion conspicuity. Clinical radiology. 2012 Dec;67(12):e105–111. doi: 10.1016/j.crad.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 94.Nasu K, Kuroki Y, Tsukamoto T, Nakajima H, Mori K, Minami M. Diffusion-weighted imaging of surgically resected hepatocellular carcinoma: imaging characteristics and relationship among signal intensity, apparent diffusion coefficient, and histopathologic grade. AJR Am J Roentgenol. 2009 Aug;193(2):438–444. doi: 10.2214/AJR.08.1424. [DOI] [PubMed] [Google Scholar]
- 95.Kim YK, Kim CS, Han YM, Lee YH. Detection of liver malignancy with gadoxetic acid-enhanced MRI: is addition of diffusion-weighted MRI beneficial? Clinical radiology. 2011 Jun;66(6):489–496. doi: 10.1016/j.crad.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 96.Sandrasegaran K, Tahir B, Patel A, et al. The usefulness of diffusion-weighted imaging in the characterization of liver lesions in patients with cirrhosis. Clinical radiology. 2013 Jul;68(7):708–715. doi: 10.1016/j.crad.2012.10.023. [DOI] [PubMed] [Google Scholar]
- 97.Sandrasegaran K, Akisik FM, Lin C, et al. Value of diffusion-weighted MRI for assessing liver fibrosis and cirrhosis. AJR Am J Roentgenol. 2009 Dec;193(6):1556–1560. doi: 10.2214/AJR.09.2436. [DOI] [PubMed] [Google Scholar]
- 98.Taouli B, Tolia AJ, Losada M, et al. Diffusion-weighted MRI for quantification of liver fibrosis: preliminary experience. AJR Am J Roentgenol. 2007 Oct;189(4):799–806. doi: 10.2214/AJR.07.2086. [DOI] [PubMed] [Google Scholar]
- 99.Kim AY, Kim YK, Lee MW, et al. Detection of hepatocellular carcinoma in gadoxetic acid-enhanced MRI and diffusion-weighted MRI with respect to the severity of liver cirrhosis. Acta Radiol. 2012 Oct 1;53(8):830–838. doi: 10.1258/ar.2012.120099. [DOI] [PubMed] [Google Scholar]
- 100.Patel T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology. 2001 Jun;33(6):1353–1357. doi: 10.1053/jhep.2001.25087. [DOI] [PubMed] [Google Scholar]
- 101.Kim SJ, Lee JM, Han JK, Kim KH, Lee JY, Choi BI. Peripheral mass-forming cholangiocarcinoma in cirrhotic liver. AJR Am J Roentgenol. 2007 Dec;189(6):1428–1434. doi: 10.2214/AJR.07.2484. [DOI] [PubMed] [Google Scholar]
- 102.Lim JH. Cholangiocarcinoma: morphologic classification according to growth pattern and imaging findings. AJR Am J Roentgenol. 2003 Sep;181(3):819–827. doi: 10.2214/ajr.181.3.1810819. [DOI] [PubMed] [Google Scholar]
- 103.Vilana R, Forner A, Bianchi L, et al. Intrahepatic peripheral cholangiocarcinoma in cirrhosis patients may display a vascular pattern similar to hepatocellular carcinoma on contrast-enhanced ultrasound. Hepatology. 2010 Jun;51(6):2020–2029. doi: 10.1002/hep.23600. [DOI] [PubMed] [Google Scholar]
- 104.Kim SA, Lee JM, Lee KB, et al. Intrahepatic mass-forming cholangiocarcinomas: enhancement patterns at multiphasic CT, with special emphasis on arterial enhancement pattern--correlation with clinicopathologic findings. Radiology. 2011 Jul;260(1):148–157. doi: 10.1148/radiol.11101777. [DOI] [PubMed] [Google Scholar]
- 105.Park HJ, Kim YK, Park MJ, Lee WJ. Small intrahepatic mass-forming cholangiocarcinoma: target sign on diffusion-weighted imaging for differentiation from hepatocellular carcinoma. Abdominal imaging. 2013 Aug;38(4):793–801. doi: 10.1007/s00261-012-9943-x. [DOI] [PubMed] [Google Scholar]
- 106.Miller FH, Hammond N, Siddiqi AJ, et al. Utility of diffusion-weighted MRI in distinguishing benign and malignant hepatic lesions. Journal of magnetic resonance imaging: JMRI. 2010 Jul;32(1):138–147. doi: 10.1002/jmri.22235. [DOI] [PubMed] [Google Scholar]
- 107.Koh DM, Collins DJ. Diffusion-weighted MRI in the body: applications and challenges in oncology. AJR Am J Roentgenol. 2007 Jun;188(6):1622–1635. doi: 10.2214/AJR.06.1403. [DOI] [PubMed] [Google Scholar]
- 108.Mohr A, Priebe M, Taouli B, Grimm J, Heller M, Brossmann J. Selective water excitation for faster MR imaging of articular cartilage defects: initial clinical results. European radiology. 2003 Apr;13(4):686–689. doi: 10.1007/s00330-002-1735-1. [DOI] [PubMed] [Google Scholar]
- 109.Demir OI, Obuz F, Sagol O, Dicle O. Contribution of diffusion-weighted MRI to the differential diagnosis of hepatic masses. Diagn Interv Radiol. 2007 Jun;13(2):81–86. [PubMed] [Google Scholar]
- 110.Ichikawa T, Haradome H, Hachiya J, Nitatori T, Araki T. Characterization of hepatic lesions by perfusion-weighted MR imaging with an echoplanar sequence. AJR Am J Roentgenol. 1998 Apr;170(4):1029–1034. doi: 10.2214/ajr.170.4.9530054. [DOI] [PubMed] [Google Scholar]
- 111.Namimoto T, Yamashita Y, Sumi S, Tang Y, Takahashi M. Focal liver masses: characterization with diffusion-weighted echo-planar MR imaging. Radiology. 1997 Sep;204(3):739–744. doi: 10.1148/radiology.204.3.9280252. [DOI] [PubMed] [Google Scholar]
- 112.Ichikawa T, Haradome H, Hachiya J, Nitatori T, Araki T. Diffusion-weighted MR imaging with a single-shot echoplanar sequence: detection and characterization of focal hepatic lesions. AJR Am J Roentgenol. 1998 Feb;170(2):397–402. doi: 10.2214/ajr.170.2.9456953. [DOI] [PubMed] [Google Scholar]
- 113.Holzapfel K, Bruegel M, Eiber M, et al. Characterization of small (</=10 mm) focal liver lesions: value of respiratory-triggered echo-planar diffusion-weighted MR imaging. European journal of radiology. 2010 Oct;76(1):89–95. doi: 10.1016/j.ejrad.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 114.Lee MH, Kim SH, Park MJ, Park CK, Rhim H. Gadoxetic acid-enhanced hepatobiliary phase MRI and high-b-value diffusion-weighted imaging to distinguish well-differentiated hepatocellular carcinomas from benign nodules in patients with chronic liver disease. AJR Am J Roentgenol. 2011 Nov;197(5):W868–875. doi: 10.2214/AJR.10.6237. [DOI] [PubMed] [Google Scholar]
- 115.Motosugi U, Ichikawa T, Sou H, et al. Distinguishing hypervascular pseudolesions of the liver from hypervascular hepatocellular carcinomas with gadoxetic acid-enhanced MR imaging. Radiology. 2010 Jul;256(1):151–158. doi: 10.1148/radiol.10091885. [DOI] [PubMed] [Google Scholar]
- 116.Kim YK, Lee WJ, Park MJ, Kim SH, Rhim H, Choi D. Hypovascular hypointense nodules on hepatobiliary phase gadoxetic acid-enhanced MR images in patients with cirrhosis: potential of DW imaging in predicting progression to hypervascular HCC. Radiology. 2012 Oct;265(1):104–114. doi: 10.1148/radiol.12112649. [DOI] [PubMed] [Google Scholar]
- 117.Papanikolaou N, Gourtsoyianni S, Yarmenitis S, Maris T, Gourtsoyiannis N. Comparison between two-point and four-point methods for quantification of apparent diffusion coefficient of normal liver parenchyma and focal lesions. Value of normalization with spleen. European journal of radiology. 2010 Feb;73(2):305–309. doi: 10.1016/j.ejrad.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 118.Bruegel M, Holzapfel K, Gaa J, et al. Characterization of focal liver lesions by ADC measurements using a respiratory triggered diffusion-weighted single-shot echo-planar MR imaging technique. European radiology. 2008 Mar;18(3):477–485. doi: 10.1007/s00330-007-0785-9. [DOI] [PubMed] [Google Scholar]
- 119.Gourtsoyianni S, Papanikolaou N, Yarmenitis S, Maris T, Karantanas A, Gourtsoyiannis N. Respiratory gated diffusion-weighted imaging of the liver: value of apparent diffusion coefficient measurements in the differentiation between most commonly encountered benign and malignant focal liver lesions. European radiology. 2008 Mar;18(3):486–492. doi: 10.1007/s00330-007-0798-4. [DOI] [PubMed] [Google Scholar]
- 120.Sandrasegaran K, Akisik FM, Lin C, Tahir B, Rajan J, Aisen AM. The value of diffusion-weighted imaging in characterizing focal liver masses. Acad Radiol. 2009 Oct;16(10):1208–1214. doi: 10.1016/j.acra.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 121.Agnello F, Ronot M, Valla DC, Sinkus R, Van Beers BE, Vilgrain V. High-b-value diffusion-weighted MR imaging of benign hepatocellular lesions: quantitative and qualitative analysis. Radiology. 2012 Feb;262(2):511–519. doi: 10.1148/radiol.11110922. [DOI] [PubMed] [Google Scholar]
- 122.Xu PJ, Yan FH, Wang JH, Shan Y, Ji Y, Chen CZ. Contribution of diffusion-weighted magnetic resonance imaging in the characterization of hepatocellular carcinomas and dysplastic nodules in cirrhotic liver. Journal of computer assisted tomography. 2010 Jul;34(4):506–512. doi: 10.1097/RCT.0b013e3181da3671. [DOI] [PubMed] [Google Scholar]
- 123.Sener RN. Echo-planar and gradient-echo diffusion MRI of normal brain iron in the globus pallidus. Clinical imaging. 2002 Nov-Dec;26(6):371–374. doi: 10.1016/s0899-7071(02)00487-4. [DOI] [PubMed] [Google Scholar]
- 124.Chandarana H, Do RK, Mussi TC, et al. The effect of liver iron deposition on hepatic apparent diffusion coefficient values in cirrhosis. AJR Am J Roentgenol. 2012 Oct;199(4):803–808. doi: 10.2214/AJR.11.7541. [DOI] [PubMed] [Google Scholar]
- 125.Metwally MA, Zein CO, Zein NN. Clinical significance of hepatic iron deposition and serum iron values in patients with chronic hepatitis C infection. The American journal of gastroenterology. 2004 Feb;99(2):286–291. doi: 10.1111/j.1572-0241.2004.04049.x. [DOI] [PubMed] [Google Scholar]
- 126.Chandarana H, Taouli B. Diffusion and perfusion imaging of the liver. European journal of radiology. 2010 Dec;76(3):348–358. doi: 10.1016/j.ejrad.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 127.Alonzi R, Hoskin P. Functional imaging in clinical oncology: magnetic resonance imaging- and computerised tomography-based techniques. Clin Oncol (R Coll Radiol) 2006 Sep;18(7):555–570. doi: 10.1016/j.clon.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 128.Kamel IR, Liapi E, Reyes DK, Zahurak M, Bluemke DA, Geschwind JF. Unresectable hepatocellular carcinoma: serial early vascular and cellular changes after transarterial chemoembolization as detected with MR imaging. Radiology. 2009 Feb;250(2):466–473. doi: 10.1148/radiol.2502072222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cui Y, Zhang XP, Sun YS, Tang L, Shen L. Apparent diffusion coefficient: potential imaging biomarker for prediction and early detection of response to chemotherapy in hepatic metastases. Radiology. 2008 Sep;248(3):894–900. doi: 10.1148/radiol.2483071407. [DOI] [PubMed] [Google Scholar]
- 130.Anzidei M, Napoli A, Zaccagna F, et al. Liver metastases from colorectal cancer treated with conventional and antiangiogenetic chemotherapy: evaluation with liver computed tomography perfusion and magnetic resonance diffusion-weighted imaging. Journal of computer assisted tomography. 2011 Nov-Dec;35(6):690–696. doi: 10.1097/RCT.0b013e318230d905. [DOI] [PubMed] [Google Scholar]
- 131.Marugami N, Tanaka T, Kitano S, et al. Early detection of therapeutic response to hepatic arterial infusion chemotherapy of liver metastases from colorectal cancer using diffusion-weighted MR imaging. Cardiovasc Intervent Radiol. 2009 Jul;32(4):638–646. doi: 10.1007/s00270-009-9532-8. [DOI] [PubMed] [Google Scholar]
- 132.Koh DM, Scurr E, Collins D, et al. Predicting response of colorectal hepatic metastasis: value of pretreatment apparent diffusion coefficients. AJR Am J Roentgenol. 2007 Apr;188(4):1001–1008. doi: 10.2214/AJR.06.0601. [DOI] [PubMed] [Google Scholar]
- 133.Kierans AS, Elazzazi M, Braga L, et al. Thermoablative treatments for malignant liver lesions: 10-year experience of MRI appearances of treatment response. AJR Am J Roentgenol. 2010 Feb;194(2):523–529. doi: 10.2214/AJR.09.2621. [DOI] [PubMed] [Google Scholar]
- 134.Kamel IR, Bluemke DA, Ramsey D, et al. Role of diffusion-weighted imaging in estimating tumor necrosis after chemoembolization of hepatocellular carcinoma. AJR Am J Roentgenol. 2003 Sep;181(3):708–710. doi: 10.2214/ajr.181.3.1810708. [DOI] [PubMed] [Google Scholar]
- 135.Chen CY, Li CW, Kuo YT, et al. Early response of hepatocellular carcinoma to transcatheter arterial chemoembolization: choline levels and MR diffusion constants--initial experience. Radiology. 2006 May;239(2):448–456. doi: 10.1148/radiol.2392042202. [DOI] [PubMed] [Google Scholar]
- 136.Rhee TK, Omary RA, Gates V, et al. The effect of catheter-directed CT angiography on yttrium-90 radioembolization treatment of hepatocellular carcinoma. J Vasc Interv Radiol. 2005 Aug;16(8):1085–1091. doi: 10.1097/01.RVI.0000177063.92678.21. [DOI] [PubMed] [Google Scholar]
- 137.Kamel IR, Reyes DK, Liapi E, Bluemke DA, Geschwind JF. Functional MR imaging assessment of tumor response after 90Y microsphere treatment in patients with unresectable hepatocellular carcinoma. J Vasc Interv Radiol. 2007 Jan;18(1 Pt 1):49–56. doi: 10.1016/j.jvir.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 138.Mannelli L, Kim S, Hajdu CH, Babb JS, Taouli B. Serial diffusion-weighted MRI in patients with hepatocellular carcinoma: Prediction and assessment of response to transarterial chemoembolization. Preliminary experience. European journal of radiology. 2013 Apr;82(4):577–582. doi: 10.1016/j.ejrad.2012.11.026. [DOI] [PubMed] [Google Scholar]
- 139.Deng J, Miller FH, Rhee TK, et al. Diffusion-weighted MR imaging for determination of hepatocellular carcinoma response to yttrium-90 radioembolization. J Vasc Interv Radiol. 2006 Jul;17(7):1195–1200. doi: 10.1097/01.RVI.0000227234.81718.EB. [DOI] [PubMed] [Google Scholar]
- 140.Rhee TK, Naik NK, Deng J, et al. Tumor response after yttrium-90 radioembolization for hepatocellular carcinoma: comparison of diffusion-weighted functional MR imaging with anatomic MR imaging. J Vasc Interv Radiol. 2008 Aug;19(8):1180–1186. doi: 10.1016/j.jvir.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 141.Mannelli L, Kim S, Hajdu CH, Babb JS, Clark TW, Taouli B. Assessment of tumor necrosis of hepatocellular carcinoma after chemoembolization: diffusion-weighted and contrast-enhanced MRI with histopathologic correlation of the explanted liver. AJR Am J Roentgenol. 2009 Oct;193(4):1044–1052. doi: 10.2214/AJR.08.1461. [DOI] [PubMed] [Google Scholar]
- 142.Lim KS. Diffusion-weighted MRI of hepatocellular carcinoma in cirrhosis. Clinical radiology. 2013 Sep 10; doi: 10.1016/j.crad.2013.07.022. [DOI] [PubMed] [Google Scholar]
- 143.Yuan Z, Ye XD, Dong S, et al. Role of magnetic resonance diffusion-weighted imaging in evaluating response after chemoembolization of hepatocellular carcinoma. European journal of radiology. 2010 Jul;75(1):e9–14. doi: 10.1016/j.ejrad.2009.05.040. [DOI] [PubMed] [Google Scholar]
- 144.Yaghmai V, Besa C, Kim E, Gatlin JL, Siddiqui NA, Taouli B. Imaging assessment of hepatocellular carcinoma response to locoregional and systemic therapy. AJR Am J Roentgenol. 2013 Jul;201(1):80–96. doi: 10.2214/AJR.13.10706. [DOI] [PubMed] [Google Scholar]
- 145.Lewin M, Poujol-Robert A, Boelle PY, et al. Diffusion-weighted magnetic resonance imaging for the assessment of fibrosis in chronic hepatitis C. Hepatology. 2007 Sep;46(3):658–665. doi: 10.1002/hep.21747. [DOI] [PubMed] [Google Scholar]
- 146.Wang Y, Ganger DR, Levitsky J, et al. Assessment of chronic hepatitis and fibrosis: comparison of MR elastography and diffusion-weighted imaging. AJR Am J Roentgenol. 2011 Mar;196(3):553–561. doi: 10.2214/AJR.10.4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Goyal A, Sharma R, Bhalla AS, Gamanagatti S, Seth A. Diffusion-weighted MRI in inflammatory renal lesions: all that glitters is not RCC! European radiology. 2013 Jan;23(1):272–279. doi: 10.1007/s00330-012-2577-0. [DOI] [PubMed] [Google Scholar]
- 148.Kiryu S, Dodanuki K, Takao H, et al. Free-breathing diffusion-weighted imaging for the assessment of inflammatory activity in Crohn’s disease. Journal of magnetic resonance imaging: JMRI. 2009 Apr;29(4):880–886. doi: 10.1002/jmri.21725. [DOI] [PubMed] [Google Scholar]
- 149.Poyraz AK, Onur MR, Kocakoc E, Ogur E. Diffusion-weighted MRI of fatty liver. Journal of magnetic resonance imaging: JMRI. 2012 May;35(5):1108–1111. doi: 10.1002/jmri.23519. [DOI] [PubMed] [Google Scholar]
- 150.Kim SY, Lee SS, Park B, et al. Reproducibility of measurement of apparent diffusion coefficients of malignant hepatic tumors: effect of DWI techniques and calculation methods. Journal of magnetic resonance imaging: JMRI. 2012 Nov;36(5):1131–1138. doi: 10.1002/jmri.23744. [DOI] [PubMed] [Google Scholar]
- 151.Le Bihan DBE, Lallemand D, Aubin ML, Vignaud J, Laval-Jeantet M. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology. 1988;168(2):497–505. doi: 10.1148/radiology.168.2.3393671. [DOI] [PubMed] [Google Scholar]
- 152.Hayashi T, Miyati T, Takahashi J, et al. Diffusion analysis with triexponential function in liver cirrhosis. Journal of magnetic resonance imaging: JMRI. 2013 Jul;38(1):148–153. doi: 10.1002/jmri.23966. [DOI] [PubMed] [Google Scholar]
- 153.Dyvorne HA, Galea N, Nevers T, et al. Diffusion-weighted imaging of the liver with multiple b values: effect of diffusion gradient polarity and breathing acquisition on image quality and intravoxel incoherent motion parameters--a pilot study. Radiology. 2013 Mar;266(3):920–929. doi: 10.1148/radiol.12120686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yoon JH, Lee JM, Yu MH, Kiefer B, Han JK, Choi BI. Evaluation of hepatic focal lesions using diffusion-weighted MR imaging: Comparison of apparent diffusion coefficient and intravoxel incoherent motion-derived parameters. Journal of magnetic resonance imaging: JMRI. 2013 Apr 30; doi: 10.1002/jmri.24158. [DOI] [PubMed] [Google Scholar]
- 155.Andreou A, Koh DM, Collins DJ, et al. Measurement reproducibility of perfusion fraction and pseudodiffusion coefficient derived by intravoxel incoherent motion diffusion-weighted MR imaging in normal liver and metastases. European radiology. 2013 Feb;23(2):428–434. doi: 10.1007/s00330-012-2604-1. [DOI] [PubMed] [Google Scholar]
- 156.Folpe AL, Lyles RH, Sprouse JT, Conrad EU, 3rd, Eary JF. (F-18) fluorodeoxyglucose positron emission tomography as a predictor of pathologic grade and other prognostic variables in bone and soft tissue sarcoma. Clinical cancer research: an official journal of the American Association for Cancer Research. 2000 Apr;6(4):1279–1287. [PubMed] [Google Scholar]
- 157.Rakheja R, Makis W, Skamene S, et al. Correlating metabolic activity on 18F-FDG PET/CT with histopathologic characteristics of osseous and soft-tissue sarcomas: a retrospective review of 136 patients. AJR Am J Roentgenol. 2012 Jun;198(6):1409–1416. doi: 10.2214/AJR.11.7560. [DOI] [PubMed] [Google Scholar]
- 158.Kwee TC, Takahara T, Ochiai R, et al. Complementary roles of whole-body diffusion-weighted MRI and 18F-FDG PET: the state of the art and potential applications. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2010 Oct;51(10):1549–1558. doi: 10.2967/jnumed.109.073908. [DOI] [PubMed] [Google Scholar]
- 159.Tsushima Y, Takano A, Taketomi-Takahashi A, Endo K. Body diffusion-weighted MR imaging using high b-value for malignant tumor screening: usefulness and necessity of referring to T2-weighted images and creating fusion images. Acad Radiol. 2007 Jun;14(6):643–650. doi: 10.1016/j.acra.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 160.Rakheja R, Demello L, Chandarana H, et al. Comparison of the Accuracy of PET/CT and PET/MRI Spatial Registration of Multiple Metastatic Lesions. AJR Am J Roentgenol. 2013 Nov;201(5):1120–1123. doi: 10.2214/AJR.13.11305. [DOI] [PubMed] [Google Scholar]
- 161.Ho KC, Lin G, Wang JJ, Lai CH, Chang CJ, Yen TC. Correlation of apparent diffusion coefficients measured by 3T diffusion-weighted MRI and SUV from FDG PET/CT in primary cervical cancer. European journal of nuclear medicine and molecular imaging. 2009 Feb;36(2):200–208. doi: 10.1007/s00259-008-0936-5. [DOI] [PubMed] [Google Scholar]
- 162.Wu X, Korkola P, Pertovaara H, Eskola H, Jarvenpaa R, Kellokumpu-Lehtinen PL. No correlation between glucose metabolism and apparent diffusion coefficient in diffuse large B-cell lymphoma: a PET/CT and DW-MRI study. European journal of radiology. 2011 Aug;79(2):e117–121. doi: 10.1016/j.ejrad.2011.04.062. [DOI] [PubMed] [Google Scholar]
- 163.Gu J, Khong PL, Wang S, Chan Q, Law W, Zhang J. Quantitative assessment of diffusion-weighted MR imaging in patients with primary rectal cancer: correlation with FDG-PET/CT. Molecular imaging and biology: MIB: the official publication of the Academy of Molecular Imaging. 2011 Oct;13(5):1020–1028. doi: 10.1007/s11307-010-0433-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Rakheja R, Chandarana H, Demello L, et al. Correlation Between Standardized Uptake Value and Apparent Diffusion Coefficient of Neoplastic Lesions Evaluated With Whole-Body Simultaneous Hybrid PET/MRI. AJR Am J Roentgenol. 2013 Nov;201(5):1115–1119. doi: 10.2214/AJR.13.11304. [DOI] [PubMed] [Google Scholar]
- 165.Turner R, Le Bihan D, Maier J, Vavrek R, Hedges LK, Pekar J. Echo-planar imaging of intravoxel incoherent motion. Radiology. 1990 Nov;177(2):407–414. doi: 10.1148/radiology.177.2.2217777. [DOI] [PubMed] [Google Scholar]
- 166.Dietrich O, Biffar A, Baur-Melnyk A, Reiser MF. Technical aspects of MR diffusion imaging of the body. European journal of radiology. 2010 Dec;76(3):314–322. doi: 10.1016/j.ejrad.2010.02.018. [DOI] [PubMed] [Google Scholar]