Abstract
The PhoP/PhoQ two-component system controls the extracellular magnesium depletion response in Salmonella enterica. Previous studies have shown that PhoP is unable to up-regulate its target genes in the absence of PhoQ function. In this work, we demonstrate that PhoP overexpression can substitute for PhoQ- and phosphorylation-dependent activation. Either a high concentration of PhoP or activation via phosphorylation stimulates PhoP self-association.
Salmonella enterica serovar Typhimurium responds to environmental magnesium deprivation by inducing the transcription of the PhoP-PhoQ regulon. This regulon is controlled by the activity of the PhoP/PhoQ two-component system (15, 27). PhoQ is the bifunctional sensor protein that detects extracellular changes in magnesium concentration and, by modulating its phosphatase activity, determines the phosphorylation state of PhoP, the transcriptional regulator (6).
In several two-component systems (1, 2, 5, 9, 16, 22, 23) phosphorylation of the response regulator either is essential or greatly increases its binding affinity for the promoter regions of its target genes. For some response regulators, this activation is achieved by promoting cooperative oligomerization (3, 8, 10, 11, 13, 20, 22). On the other hand, for effectors such as PhoB (4, 12), Fix J (11), NarL (28), and SpoOA (18) phosphorylation induces a conformational change in the protein relieving an autoinhibitory effect. Here we report that, when overexpressed, PhoP is able to activate its target genes independently from its phosphorylation status, in a concentration-dependent manner. We demonstrate that protein-protein interaction is enhanced by phosphorylation, although it can also occur with the unphosphorylated form of PhoP.
PhoP overexpression induces pcgF in the absence of PhoQ function.
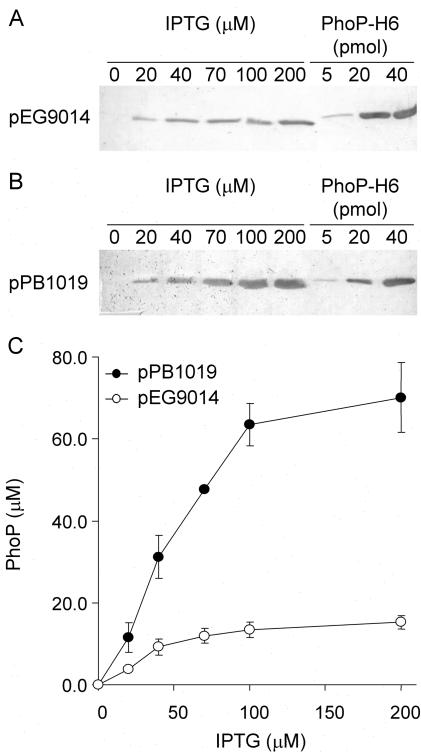
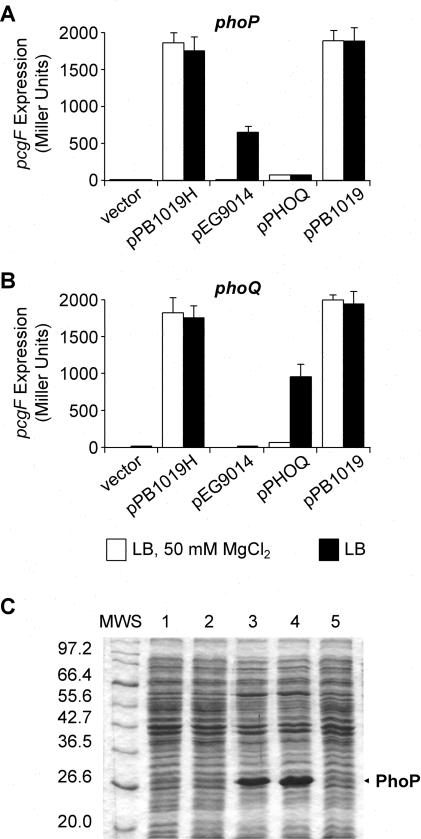
We expressed PhoP as a His-tagged fusion protein from plasmid pPB1019H, constructed by cloning the PCR product obtained using primers 5′-phoP-F (3′-GAGGATCCATATGATGCGCGTACTGG-5′) and 3′-phoP-R (3′-TCCAAGCTTAGTGGTGGTGGTGGTGGTGGCGCAATTCAAAAAGATATC-5′), between the BamHI and the HindIII sites of pUHE21-2lacIq (26). Surprisingly, pPB1019H was able to induce the expression of pcgF::MudJ (a representative PhoP-activated gene [pag]) (27) in both a phoP and a phoQ strain, under activating (Luria-Bertani medium [LB]) or repressing (LB plus 50 mM MgCl2) conditions for the PhoP/PhoQ system. This was in sharp contrast to the result obtained when using pEG9014 (a low-level PhoP expression plasmid) (26), where pcgF activation was dependent upon its cognate kinase PhoQ and repressed by extracellular Mg2+. As expected, expression of PhoQ from pEG9050 (26) restored pcgF activation in the phoQ but not in the phoP strain. In order to eliminate the His tag as a possible cause of this effect, plasmid pPB1019 was constructed by replacing the NsiI-HindIII fragment of pPB1019H with the corresponding fragment excised and purified from pEG9014 (26). As a result, pPB1019 differed from pEG9014 in only three extra nucleotides introduced into the spacing region between the putative ribosomal binding site and the start codon of phoP. pPB1019 showed the same PhoQ- and Mg2+-independent complementation behavior as did pPB1019H, indicating that the His tag was not responsible for this phenotype. Identical results were obtained with several pags tested (data not shown). Interestingly, a strong band corresponding to PhoP was detectable only by Coomassie blue-stained sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) when the cell extract from the pPB1019-complemented strain was analyzed. On the other hand, expression of the PhoP protein from the pEG9014-complemented strain was detectable only by Western blot analysis using polyclonal anti-PhoP antiserum (see Fig. 4).
FIG. 4.
Determination of plasmid-dependent PhoP intracellular concentration. (A and B) Whole-cell extracts obtained from cultures of Salmonella strain EG9558 (phoP7953::Tn10 pcgF9281::MudJ) harboring pEG9014 (A) or pPB1019 (B), grown in LB, and induced with IPTG as indicated were analyzed by SDS-PAGE (12% polyacrylamide gels), followed by transfer to nitrocellulose and development using rabbit polyclonal anti-PhoP antibodies. PhoP-H6 was also loaded and used as a standard to calculate the PhoP concentration. The immunoblots are representative of five independent experiments. (C) The intracellular PhoP concentration was calculated for Salmonella strain EG9558 (phoP7953::Tn10 pcgF9281::MudJ) harboring either pPB1019 or pEG9014, grown in LB, and induced with different IPTG concentrations as indicated.
Induction of pags depends on the intracellular concentration of PhoP.
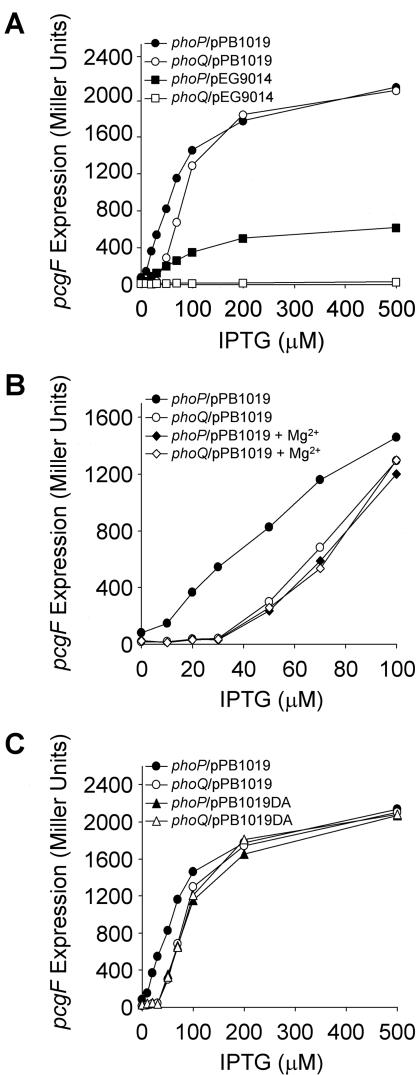
To determine whether mere overexpression of PhoP could create PhoQ independence, we varied the concentration of IPTG (isopropyl-β-d-thiogalactopyranoside) in the bacterial growth medium while expressing PhoP from either pEG9014 or pPB1019 (see Fig. 2A). Differing from pEG9014, when expression of PhoP was driven from pPB1019, pcgF expression reached the same maximal level in the presence and in the absence of phoQ.
FIG. 2.
PhoP overexpression can compensate for PhoQ- and Mg2+-dependent induction of pcgF. β-Galactosidase activity was determined from Salmonella strain EG9558 (phoP7953::Tn10 pcgF9281::MudJ) or PB1209 (phoQ5996::Tn10 pcgF9281::MudJ), harboring either pEG9014 or pPB1019 and grown in LB (A), harboring pPB1019 and grown in LB or LB plus 50 mM MgCl2 (B), and harboring either pPB1019 or pPB1019DA and grown in LB (C) and induced with different IPTG concentrations as indicated. The results are the averages of three independent assays performed in duplicate.
Previous studies have demonstrated that PhoQ responds to the extracellular magnesium concentration and controls the phosphorylation status of PhoP (6, 14). Maximal pcgF expression achieved with pPB1019 was identical in otherwise isogenic phoP and phoQ strains (Fig. 2A). However, with the use of IPTG concentrations below 100 μM, a different induction profile was evident, depending on the presence of the sensor kinase PhoQ. Besides, IPTG-controlled expression of pcgF was monitored in cells grown under either inducing (LB) or repressing (LB plus 50 mM MgCl2) conditions as shown in Fig. 2B. In the presence of magnesium at low IPTG concentrations, pcgF expression in the phoQ wild-type strain was down-regulated to the levels observed under either inducing or repressing conditions in the phoQ background. Thus, to attain induction of pags, PhoQ-dependent activation of PhoP can be effectively bypassed, provided that an intracellular threshold concentration of PhoP is reached.
PhoPD52A is able to promote gene transcription.
We next examined pcgF induction when overexpressing a PhoPD52A mutant (6) that is not phosphorylated (Fig. 2C). phoP was replaced by phoPD52A in pPB1019, resulting in pPB1019DA (which achieves high levels of PhoP expression similar to those obtained from pPB1019 [Fig. 1C, lane 4]). pPB1019DA was introduced by electroporation into pcgF::MudJ and pcgF::MudJ phoQ::Tn10 strains. pcgF expression was identical in the two genetic backgrounds and indistinguishable from that obtained when using pPB1019 under repressing conditions (Fig. 2C). As expected, expression of PhoPD52A from plasmid pEG9014DA did not complement a phoP strain (data not shown). Our results indicate that, even when PhoP phosphorylation is eliminated, overexpression of PhoP can activate pcgF.
FIG. 1.
PhoP overexpression induces pcgF in a PhoQ-independent manner. (A and B) β-Galactosidase activity from Salmonella strain EG9558 (phoP7953::Tn10 pcgF9281::MudJ) (A) or EG9531 (phoQ5996::Tn10 pcgF9281::MudJ) (B), harboring plasmid pUHE-21-2 (vector), pPB1019H, pEG9014, pEG9050 (pPHOQ), or pPB1019, grown in LB or in LB plus 50 mM MgCl2, and induced with 0.5 mM IPTG, was measured as described previously (24). The results are the averages of three independent assays performed in duplicate; error bars correspond to the standard deviations. (C) Protein profile of whole-cell extracts (30 μg of total protein/well) analyzed by SDS-PAGE with gels stained with Coomassie brilliant blue. Salmonella strain EG9558 (phoP7953::Tn10 pcgF9281::MudJ) harbored the vector (lane 1), pEG9014 (lane 2), pPB1019 (lane 3), pPB1019DA (lane 4), or pEG9014DA (lane 5), respectively, where DA represents a substitution of alanine for aspartic acid at position 52 in PhoP. MWS, protein broad-range molecular mass standards (New England Biolabs). Numbers at left are molecular masses in kilodaltons.
Quantification of the intracellular concentration of PhoP.
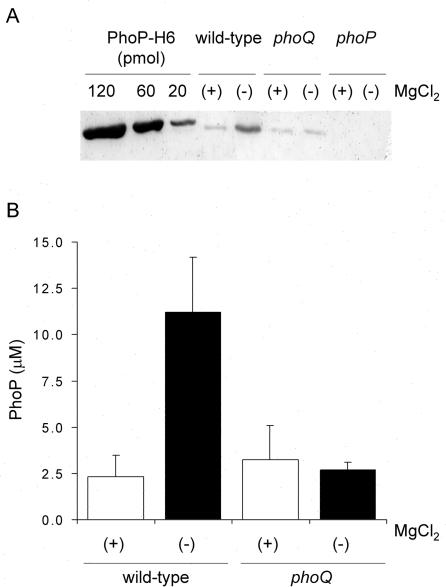
In order to quantify the PhoP protein at each induction point, we performed densitometry on immunodetection assays. A 3-ml culture was grown to exponential phase (optical density of 0.6), pelleted by gentle centrifugation (6,000 × g), and resuspended in 300 μl of phosphate-buffered saline. An aliquot of this cell suspension was withdrawn to determine CFU per milliliter by serial dilutions and LB agar plating. In parallel, a 20-μl aliquot was mixed with 5× SDS-PAGE sample buffer (2.5% β-mercaptoethanol, 9% glycerol, 10% SDS, 600 mM Tris-HCl [pH 6.8], 0.006% bromophenol blue) and loaded into denaturing polyacrylamide gels. Purified PhoP-H6 (molecular mass, 26.5 kDa) was used as a standard in the Western blot assays. Calculations were based on the assumption of a bacterial cellular volume of 1.9 fl (7) and a molecular mass of PhoP of 25.6 kDa. The results plotted in Fig. 3B show the modulation of PhoP concentration as a function of the extracellular Mg2+ in the wild-type Salmonella compared to the isogenic phoQ strain. The estimated basal intracellular concentration of PhoP protein was 2.7 ± 1.1 μM, either in the phoQ strain or in the wild-type strain when down-regulated by high Mg2+ concentration conditions (i.e., phoP transcription from the PhoP-independent promoter) (26). In LB with no added Mg2+, PhoP reached a maximum concentration of 11.2 ± 2.9 μM. The results shown in Fig. 3 set a range of intracellular PhoP concentrations between 2 and 3 μM when uninduced and an increase of about four- to fivefold upon induction.
FIG. 3.
Physiological modulation of PhoP intracellular concentration. (A) Whole-cell extracts (30 μg of total protein/well) from Salmonella wild-type or phoQ or phoP strains grown in LB (−) or in LB plus 50 mM MgCl2 (+) were analyzed by SDS-PAGE (12% polyacrylamide gels), followed by transfer to nitrocellulose and development using rabbit polyclonal anti-PhoP antibodies. Different quantities of purified PhoP-H6 protein were also loaded and used as references to calculate the PhoP concentration. The immunoblot shown is a representative result from five independent experiments. (B) PhoP intracellular concentration was calculated for Salmonella wild-type or phoQ strains grown in LB (−) or in LB plus 50 mM MgCl2 (+). The results are the averages of five independent assays, and the error bars correspond to the standard deviations.
The maximal induction obtained from pEG9014 corresponds to an estimated concentration of 15.2 ± 1.6 μM PhoP (Fig. 4C). This concentration of PhoP still enables PhoQ to down-regulate pag expression in the presence of Mg2+ (Fig. 1 and 2). In contrast, maximal intracellular concentrations of PhoP of approximately 70 μM were achieved from pPB1019 (Fig. 4C). At an intracellular concentration of 30 μM PhoP(obtained from pPB1019 when induction was carried out over 50 μM IPTG), induction of the pags becomes independent of PhoQ regulation (Fig. 4 and 2B). This result was identical for PhoPD52A (Fig. 2C).
PhoP phosphorylation enhances protein-protein interaction.
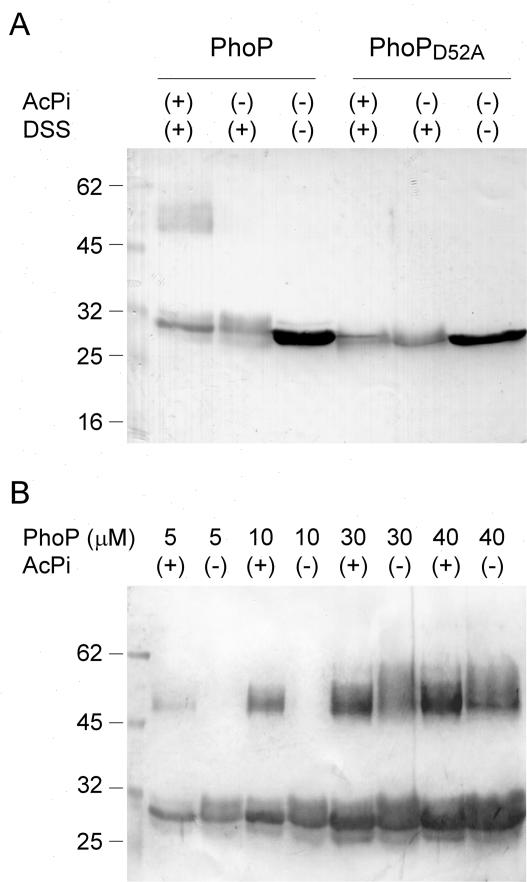
We investigated whether in vitro phosphorylation of purified PhoP influenced its self-association as previously reported for FixJ, NtrC, PhoB, NarL, and ArcA (11, 13, 19, 28). PhoP or PhoPD52A was incubated at a final concentration of 5 μM in 50 mM KCl-20 mM MgCl2-20% glycerol-0.05% Tween 20-50 mM HEPES (pH 8.0) at 25°C for 3 h with or without 25 mM acetyl phosphate (Fig. 5A). A 1 mM concentration of disuccinimidyl suberate (DSS; Sigma Chemical Co., St. Louis, Mo.) was subsequently added for 30 min at 25°C. In this experiment approximately 80% of PhoP was phosphorylated, as determined by reversed-phase high-pressure liquid chromatography (16). The presence of an immunoreactive band that migrated as a dimer of PhoP was observed only when phosphorylated PhoP was present in the assay mixture (Fig. 5A, lane 1). Besides, Fig. 5B indicates that self-association also occurs in the absence of PhoP phosphorylation, although dimerization is favored by phosphorylation. Unphosphorylated PhoP required concentrations of ≥30 μM for dimer formation, and less dimer was produced. This level of expression of PhoP could be reached only in pPB1019-overexpressing cells and not in the wild-type 14028s strain under our experimental conditions. Purified PhoPD52A was soluble only at concentrations below 7 μM, and so we were unable to include it in this analysis.
FIG. 5.
PhoP self-association depends on concentration or phosphorylation. (A) Affinity-purified PhoP or PhoPD52A (5 μM) previously incubated with or without acetyl phosphate (AcPi) was subjected to cross-linking with DSS. (B) Increasing concentrations of purified PhoP previously incubated with (+) or without (−) acetyl phosphate (AcPi) were subjected to cross-linking with DSS. Samples were analyzed by SDS-PAGE (12% polyacrylamide gels), followed by transfer to nitrocellulose and development using rabbit polyclonal anti-PhoP antibodies. Numbers at left are molecular masses in kilodaltons.
Based on the results presented, we can estimate that the autoregulation feedback triggered by limiting Mg2+ conditions increases the basal intracellular concentration of PhoP, generated from the Mg2+-independent promoter phoPP2 (26), approximately fivefold (Fig. 4A). Furthermore, we have shown that a nonphysiological 12-fold increase in the concentration of PhoP from the basal level is needed to circumvent the effect naturally induced by phosphorylation to promote pag expression. In addition, our in vitro cross-linking results demonstrate that PhoP is able to self-associate independently of its phosphorylation status, although protein-protein interaction is clearly enhanced upon phosphorylation (Fig. 5). This protein-protein interaction is favored as PhoP concentration increases. In fact, our in vitro results correlate well with our in vivo observations in that they show that a PhoP protein concentration above 30 μM favors protein-protein interaction and PhoQ-independent induction of the pags. The PhoQ-independent pag activation was achieved when PhoP levels were over three times the maximal level in a haploid cell. Nevertheless, it is surprising that there is so much capacity for pag expression that is not used at least under the growth conditions tested. Thus, it is tempting to speculate that there might be some inducing condition that has not been identified yet which would allow the normal cell to reach the levels of expression of the overproducing PhoP strain.
In summary, our results clearly show that phosphorylation is not an absolute requirement for PhoP to induce expression of its target genes, although under physiological conditions it is required for PhoP activation (21, 27). Therefore, it is reasonable to postulate that overexpression can substitute for phosphorylation simply by mass action that favors an interaction among monomers at high PhoP concentrations. It is this form, which appears to be a dimer, that exhibits enhanced affinity for DNA. In this context, it was previously shown that UhpA could activate transcription in the absence of the phosphodonor UhpB if present at a sufficiently high concentration. It was postulated that this effect correlated with the occupancy of multiple sites with different affinities in the target DNA sequences (9). In the case of OmpR, binding of the unphosphorylated protein to adjacent sites may be cooperative and cooperative interactions might be further stimulated upon phosphorylation (17). Chen et al. proposed that, for PhoP in Bacillus subtilis, both phosphorylation and dimerization are essential for the regulator to be transcriptionally active (8). This appears not to be the case for Salmonella PhoP, as present analysis of the PhoP-binding motif reveals a consensus single direct repeat in the promoter region of the pags that are directly regulated by PhoP (21, 25).
Lastly, our results highlight that, although response regulators are classified into subfamilies on the basis of structural similarities, their detailed reaction mechanisms even within the subfamily can be quite distinct.
Acknowledgments
We thank the anonymous referees for valuable suggestions.
This work was supported by grants from Agencia Nacional de Promoción Científica y Tecnológica (Argentina), Fundación Antorchas, the Third World Academy of Sciences (Trieste, Italy), CONICET, and Ministerio de Salud de la República Argentina. L.J.K. is supported by GM-58746 from the National Institutes of Health and MCB-0243085 from the National Science Foundation. E.G.V. is a career investigator of the National Research Council (CONICET, Argentina), and S.L. and M.E.C. are fellows of the same institution. M.E.C. was awarded an ASM International Fellowship to perform some of these studies in the laboratory of L.J.K. at Oregon Health Sciences University, Portland. F.C.S. is a member of the Rosario National University Research Council (CIUNR) and CONICET; he is also an International Research Scholar of the Howard Hughes Medical Institute.
REFERENCES
- 1.Aguirre, A., S. Lejona, E. García Véscovi, and F. C. Soncini. 2000. Phosphorylated PmrA interacts with the promoter region of ugd in Salmonella enterica serovar Typhimurium. J. Bacteriol. 182:3874-3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aiba, H., F. Nakasai, S. Mizushima, and T. Mizuno. 1989. Phosphorylation of a bacterial activator, OmpR, by a protein kinase, EnvZ, results in stimulation of its DNA binding ability. J. Biochem. 106:5-7. [DOI] [PubMed] [Google Scholar]
- 3.Asayama, M., A. Yamamoto, and Y. Kobayashi. 1995. Dimer form of phosphorylated Spo0A, a transcriptional regulator, stimulates the spo0F transcription at the initiation of sporulation in Bacillus subtilis. J. Mol. Biol. 250:11-23. [DOI] [PubMed] [Google Scholar]
- 4.Blanco, A. G., M. Sola, F. X. Gomis-Rüth, and M. Coll. 2002. Tandem DNA recognition by PhoB, a two-component signal transduction transcriptional activator. Structure 10:701-713. [DOI] [PubMed] [Google Scholar]
- 5.Boucher, P. E., K. Murakami, A. Ishihama, and S. Stibitz. 1997. Nature of DNA binding and RNA polymerase interaction of the Bordetella pertussis BvgA transcriptional activator at the fha promoter. J. Bacteriol. 179:1755-1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castelli, M. E., E. García Véscovi, and F. C. Soncini. 2000. The phosphatase activity is the target for Mg2+ regulation of the sensor protein PhoQ in Salmonella. J. Biol. Chem. 275:22948-22954. [DOI] [PubMed] [Google Scholar]
- 7.Cayley, D. S., H. J. Guttman, and M. T. Record, Jr. 2000. Biophysical characterization of changes in amounts and activity of Escherichia coli cell and compartment water and turgor pressure in response to osmotic stress. Biophys. J. 78:1748-1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen, Y., C. Birck, J. P. Samama, and F. M. Hulett. 2003. Residue R113 is essential for PhoP dimerization and function: a residue buried in the asymmetric PhoP dimer interface determined in the PhoPN three-dimensional crystal structure. J. Bacteriol. 185:262-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dahl, J. L., B. Y. Wei, and R. J. Kadner. 1997. Protein phosphorylation affects binding of the Escherichia coli transcription activator UhpA to the uhpT promoter. J. Biol. Chem. 272:1910-1919. [DOI] [PubMed] [Google Scholar]
- 10.Da Re, S., S. Bertagnoli, J. Fourment, J. M. Reyrat, and D. Kahn. 1994. Intramolecular signal transduction within the FixJ transcriptional activator: in vitro evidence for the inhibitory effect of the phosphorylatable regulatory domain. Nucleic Acids Res. 22:1555-1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Da Re, S., J. Schumacher, P. Rousseau, J. Fourment, C. Ebel, and D. Kahn. 1999. Phosphorylation-induced dimerization of the FixJ receiver domain. Mol. Microbiol. 34:504-511. [DOI] [PubMed] [Google Scholar]
- 12.Ellison, D. W., and W. R. McCleary. 2000. The unphosphorylated receiver domain of PhoB silences the activity of its output domain. J. Bacteriol. 182:6592-6597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fiedler, U., and V. Weiss. 1995. A common switch in activation of the response regulators NtrC and PhoB: phosphorylation induces dimerization of the receiver modules. EMBO J. 14:3696-3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.García Véscovi, E., F. C. Soncini, and E. A. Groisman. 1996. Mg2+ as an extracellular signal: environmental regulation of Salmonella virulence. Cell 84:165-174. [DOI] [PubMed] [Google Scholar]
- 15.Groisman, E. A. 2001. The pleiotropic two-component regulatory system PhoP-PhoQ. J. Bacteriol. 183:1835-1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Head, C. G., A. Tardy, and L. J. Kenney. 1998. Relative binding affinities of OmpR and OmpR-phosphate at the ompF and ompC regulatory sites. J. Mol. Biol. 281:857-870. [DOI] [PubMed] [Google Scholar]
- 17.Huang, K. J., C. Y. Lan, and M. M. Igo. 1997. Phosphorylation stimulates the cooperative DNA-binding properties of the transcription factor OmpR. Proc. Natl. Acad. Sci. USA 94:2828-2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ireton, K., D. Z. Rudner, K. J. Siranosian, and A. D. Grossman. 1993. Integration of multiple developmental signals in Bacillus subtilis through the Spo0A transcription factor. Genes Dev. 7:283-294. [DOI] [PubMed] [Google Scholar]
- 19.Jeon, Y., Y. S. Lee, J. S. Han, J. B. Kim, and D. S. Hwang. 2001. Multimerization of phosphorylated and non-phosphorylated ArcA is necessary for the response regulator function of the Arc two-component signal transduction system. J. Biol. Chem. 276:40873-40879. [DOI] [PubMed] [Google Scholar]
- 20.Lee, J., J. Owens, I. Hwang, C. Meares, and S. Kustu. 2000. Phosphorylation-induced signal propagation in the response regulator NtrC. J. Bacteriol. 182:5188-5195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lejona, S., A. Aguirre, M. L. Cabeza, E. Garcíia Véscovi, and F. C. Soncini. 2003. Molecular characterization of the Mg2+-responsive PhoP-PhoQ regulon in Salmonella enterica. J. Bacteriol. 185:6287-6294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu, W., and F. M. Hulett. 1997. Bacillus subtilis PhoP binds to the phoB tandem promoter exclusively within the phosphate starvation-inducible promoter. J. Bacteriol. 179:6302-6310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Makino, K., M. Shinagawa, M. Amemura, T. Kawamoto, M. Yamada, and A. Nakata. 1989. Signal transduction in phosphate regulon of Escherichia coli involves phosphotransfer between PhoR and PhoB proteins. J. Mol. Biol. 210:551-559. [DOI] [PubMed] [Google Scholar]
- 24.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 25.Minagawa, S., H. Ogasawara, A. Kato, K. Yamamoto, Y. Eguchi, T. Oshima, H. Mori, A. Ishihama, and R. Utsumi. 2003. Identification and molecular characterization of the Mg2+ stimulon of Escherichia coli. J. Bacteriol. 185:3696-3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soncini, F. C., E. García Véscovi, and E. A. Groisman. 1995. Transcriptional autoregulation of the Salmonella typhimurium phoPQ operon. J. Bacteriol. 177:4364-4371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soncini, F. C., E. García Véscovi, F. Solomon, and E. A. Groisman. 1996. Molecular basis of the magnesium deprivation response in Salmonella typhimurium: identification of PhoP-regulated genes. J. Bacteriol. 178:5092-5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang, J. H., G. Xiao, R. P. Gunsalus, and W. L. Hubbell. 2003. Phosphorylation triggers domain separation in the DNA binding response regulator NarL. Biochemistry 42:2552-2559. [DOI] [PubMed] [Google Scholar]