Abstract
We have previously shown in sheep that 10 days of modest chronic increase in maternal cortisol resulting from maternal infusion of cortisol (1 mg/kg/day) caused fetal heart enlargement and Purkinje cell apoptosis. In subsequent studies we extended the cortisol infusion to term, finding a dramatic incidence of stillbirth in the pregnancies with chronically increased cortisol. To investigate effects of maternal cortisol on the heart, we performed transcriptomic analyses on the septa using ovine microarrays and Webgestalt and Cytoscape programs for pathway inference. Analyses of the transcriptomic effects of maternal cortisol infusion for 10 days (130 day cortisol vs 130 day control), or ∼25 days (140 day cortisol vs 140 day control) and of normal maturation (140 day control vs 130 day control) were performed. Gene ontology terms related to immune function and cytokine actions were significantly overrepresented as genes altered by both cortisol and maturation in the septa. After 10 days of cortisol, growth factor and muscle cell apoptosis pathways were significantly overrepresented, consistent with our previous histologic findings. In the term fetuses (∼25 days of cortisol) nutrient pathways were significantly overrepresented, consistent with altered metabolism and reduced mitochondria. Analysis of mitochondrial number by mitochondrial DNA expression confirmed a significant decrease in mitochondria. The metabolic pathways modeled as altered by cortisol treatment to term were different from those modeled during maturation of the heart to term, and thus changes in gene expression in these metabolic pathways may be indicative of the fetal heart pathophysiologies seen in pregnancies complicated by stillbirth, including gestational diabetes, Cushing's disease and chronic stress.
Keywords: cortisol, fetal heart, late gestation, mitochondria, metabolism
during normal human and ovine pregnancy, fetal corticosteroid production is low until very late in gestation. In most, if not all, species, the marked increase in fetal secretion of corticosteroids as term approaches is critical for final organ maturation and survival at birth (36), including glucocorticoid receptor-mediated effects on cardiac maturation (53). In contrast to these positive, maturational effects of the normal pattern of corticosteroids, chronic and/or premature increases in cortisol are thought to produce adverse effects on the developing fetus, including effects on the developing cardiovascular system and heart. Antenatal administration of glucocorticoids may produce enlargement of the heart in humans (4, 68), and acute administration of cortisol into the ovine fetus produces hypertension and cardiac hypertrophy (37). Direct administration of cortisol into the coronary artery also produces cardiac enlargement with myocyte proliferation in the ovine fetus (21, 54).
We have found that chronic systemic maternal cortisol administration producing only a modest (1.5-fold) increase in maternal plasma cortisol concentrations also produces enlargement of the late gestation fetal heart (24, 47). In this model, which was designed to mimic maternal cortisol responses to chronic stress, cortisol was administered over a 10- to 14-day period starting at about 110- to 120-days gestation (0.75–0.90 gestation) in the sheep. Maternal cortisol administration increased the heart weight-to-body weight ratio, thickness of both ventricular free walls and the septum, and increased myocyte proliferation through the mineralocorticoid receptor, but increased Purkinje fiber apoptosis through the glucocorticoid receptor (GR) (14, 47). The proliferation in this model, as in the intracardiac cortisol infusion by Giraud and colleagues (21), occurs in the absence of an increase in fetal arterial pressure. The period of maternal cortisol infusion in this study is a period of rapid fetal growth, but also one in which the fetal heart is normally transitioning from proliferation to terminal differentiation (6, 26). Our results suggested that exposure to excess cortisol during late gestation might alter the trajectory of myocyte maturation, cardiac size, and cardiac function. However, other studies have suggested that the effects of antenatal cortisol or glucocorticoid exposure may be transitory, with recovery of fetal heart size (41, 45).
To assess the changes in the fetal heart occurring with chronic administration of cortisol over this period of terminal differentiation and remodeling of the fetal heart, we extended the duration of the maternal infusion of cortisol from 115 days gestation to birth. We found that when the duration of cortisol exposure is extended, there is a high incidence of stillbirth or fetal death at the time of labor and delivery, resulting in stillbirth in three of four ewes allowed to deliver spontaneously (27). In further study with this model in which the pregnancy was terminated to allow collection of fetal tissues, we were able to collect tissues from seven cortisol-infused fetal lambs and eight control fetal lambs at 139–144 days, although there were five additional stillbirths in the cortisol-infused group. Overall there was no effect of the more prolonged maternal cortisol infusion on heart weight or dimensions. The maternal physiology in these pregnancies appeared normal in terms of uterine blood flow and estrone and progesterone concentrations, although the ewes are both hypercortisolemic and hyperglycemic (27). Fetal demise appeared to occur late in pregnancy and appeared to be associated with the onset of labor, suggesting that the poor fetal outcome may be related to survival of the stress of labor and delivery and might be related to a functional deficit in the hearts not revealed by measurement of cardiac dimensions. To test the hypothesis that longer-term infusion of cortisol results in changes in gene expression in the heart that would reveal altered biologic processes, we undertook a transcriptomic analysis of the hearts from the four groups of fetuses: 130-day fetuses of control ewes, 130-day fetuses of cortisol-infused ewes, term fetuses of control ewes, and term fetuses of cortisol-infused ewes. We hypothesized that cortisol would produce differential gene expression at both 130 days and term, but that the pattern of induced genes would differ between the two gestational ages, with a predominance of proliferation-related genes upregulated at 130 days, but not at term, and with greater effects related to cardiac metabolism at term. We also hypothesized that some genes that are differentially regulated (DR) with maternal cortisol infusion at term would be in pathways that are activated or suppressed as an adaptive response to the earlier insult.
MATERIALS AND METHODS
Pregnant ewes and their fetuses were studied. All animal use was approved by the University of Florida Institutional Animal Care and Use Committee. Ewes were infused with cortisol (1 mg/kg/day cortisol sodium succinate; Pfizer, New York, NY) by continuous infusion; control ewes were not infused with cortisol but were otherwise similarly surgically prepared and sampled. Details of the animal use have been previously reported (14, 47). In the first study, hearts collected from singleton fetuses after 10 days of intravenous cortisol infusion into the ewe (130-day CORT, n = 6; 130 ± 1 days gestation) and from control ewes (130-day CON, n = 6; 130 ± 1 days gestation). In the subsequent study, cortisol was infused beginning on day 115–116 of gestation, and ewes were studied until signs of early labor were evident, or by 144 days, so that euthanasia was performed at days 139–144 of gestation. In this cohort there were seven cortisol-infused ewes with singleton fetuses (140-day CORT, n = 7; 142 ± 1 days gestation) and seven control ewes, one with twin fetuses (140-day CON, n = 8; 143 ± 1 days gestation); in each group one lamb was delivered live. Fetal hearts were excised and weighed, and samples of both ventricular free walls and septum were dissected and rapidly frozen in liquid nitrogen. The tissue samples were stored at −80 until the time of RNA extraction.
In both studies there was a significant increase in maternal cortisol produced by the infusion of cortisol. In the 130-day study fetal cortisol was increased (CORT 3.4 ± 0.6 ng/ml vs CON 1.5 ± 0.6 ng/ml) (47); fetuses in the study to term were not catheterized, so the values at 140 days are not available. As previously reported, maternal infusion of cortisol to 130 days increased fetal heart weight and wall thicknesses (47); however, in the studies to term neither fetal heart weight or fetal ventricular wall thicknesses were significantly increased (LV wall thickness CON, 5.2 ± 0.3 mm; CORT, 5.4 ± 0.3 mm; RV wall thickness CON, 4.5 ± 0.3 mm; CORT, 4.6 ± 0.3 mm; septal wall thickness CON, 6.7 ± 0.3; CORT, 6.8 ± 0.2 mm for the live lambs at term, n = 7–8).
Transcriptomic analysis.
We performed a transcriptomic analysis of gene expression in septal tissue by methodology previously used to identify transcriptomic responses to hypoxia in the fetal hypothalamus (64). Septum contains two functionally significant types of myocytes, cardiomyocytes, and the specialized cardiomyocytes, Purkinje fibers, of the conductive pathway in the heart. These are both potentially critical in the resulting pathophysiology, since septal enlargement and increased caspase-3 staining in septal Purkinje cells were evident at 130 days (14, 47), and because bradycardia at birth is observed in some clinical cases of adverse pregnancy outcome, including maternal diabetes (55, 65, 66). Messenger RNA was extracted from septa and purified with Trizol followed by on-column DNase digestion (Qiagen RNeasy Plus kits; Qiagen Sciences, Germantown, MD). RNA integrity numbers (RIN) for the RNA samples were ≥ 8.8. RNA (500 ng) was labeled with Cy5 using the Agilent QuickAmp Labeling kit to generate cRNA with specific activities of 11.45 ± 0.11 pmol/μg with yields of 8.67 ± 0.15 μg; array hybridization was performed as previously described (64). One sample had an RIN of 7 but was not an outlier by either microarray quality control tests or probe hybridization results compared with the other microarrays, so it was included in the gene expression analyses. We used the Agilent-019921 Sheep Gene Expression Microarray 8x15k, G4813A (GPL14112) platform. The annotation of this array platform was described in a previous publication from our laboratory group (46). The raw normalized array data for this experiment have been deposited in the National Center for Biotechnology Information's Gene Expression Omnibus (GEO) (12) and are accessible through GEO Series accession number GSE54237 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE54237).
Two-way analysis of variance with treatment group and gestational age as the factors was performed with JMP Genomics 6.0 (SAS Institute, Cary, NC). Prior to analysis, features flagged as nonuniform outliers were omitted, probes with <6 expression values per group were excluded (73 probes), and transcript levels were normalized to the median expression value on each array. Webgestalt (61, 69) was used to identify overrepresented biological or molecular processes and overrepresented cellular components associated with the DR genes using P < 0.05 as the criterion for statistical significance after Benjamini-Hochberg multiple test correction of the P value, and accepting at least two genes per category (3). Network inference of the DR genes was also performed using GeneMANIA (63), a plugin of Cytoscape (9). BiNGO (38), also a plugin of Cytoscape, was used for gene ontology analysis.
Genes differentially expressed as a result of cortisol treatment at each gestational age (i.e., at either 130 days or term) were determined as differentially expressed genes in 130-day CON vs 130-day CORT or 140-day CON vs 140-day CORT. Differentially expressed genes as a function of gestational age were determined by comparison of 140-day CON compared with 130-day CON. To test the hypothesis that changes in the heart produced by cortisol at 130 days reflect early maturation of normal cortisol-induced effects, we compared genes and their pathways differentially expressed in 130-day CORT vs 130-day CON septa to those differentially expressed at 140-day CON compared with 130-day CON septa. We also tested the hypothesis that genes altered by cortisol treatment to 140 days would be the same as those changed during the maturation from 130 days to 140 days gestation, i.e., that the 140-day CORT effect is simply a magnification of the normal effect of CORT near term, by comparison of DR genes in 140-day CON vs 130-day CON compared with 140-day CON vs 140-day CORT.
Array expression data for selected genes were validated by real-time quantitative PCR using TaqMan probes or Sybr Green (Applied Biosystems, Life Technologies) (Table 1). All primer sets were tested for PCR efficiency and for a single melting point product if SyBr Green chemistry was used.
Table 1.
| Gene Name | Human Gene MIM # | Reference Sequence | Forward Primer | Reverse Primer | Probe Sequence |
|---|---|---|---|---|---|
| CYP26B1 | 605207 | XM_004006067.1 | CACCCTGAAGCCATCAACGT | GGATGGCCATACGGAAGGT | N/A |
| PDK4 | 602527 | XM_004007738.1 | TTACACGTACTCCACTGCACCAA | AGCCAGCGGAGCATTCC | N/A |
| SOCS3 | 604176 | GU168729.1 | CAACGTGGCCACTCTCCAA | AGTCCAGGTGGCCGTTGA | TCTCTGTCGGAAGACC |
| TXNIP | 606599 | XM_004002420.1 | CCCCAAAGCTGCCATTGT | CACCTTGGTCTGGCCATTG | N/A |
| MT-ND1 | N/A | NC_001941 | CCATCGCCGACGCAAT | AGGATGTGGCGGGTCGTA | N/A |
Mitochondrial DNA.
To test for changes in mitochondrial DNA (mtDNA) content, we isolated total DNA from cardiac tissue using the QIAamp DSP DNA minikit (Qiagen), according to the manufacturer's directions. mtDNA was quantified with qRT-PCR with the ovine mitochondrial gene MT-ND1 normalized against the ovine nuclear, single copy gene, AT2R (corrected for sex differences in copy number). A TaqMan probe was used for AT2R (49) and SyBr Green for the MT-ND1, both genes amplified with close to 100% efficiency, and there was a single melting point product for MT-ND1.
Markers of oxidative stress.
To test for lipid peroxidation and glycosylation secondary to tissue hypoxia in the term fetal hearts, we performed Western blots. Antibodies against modified protein products of lipid peroxidation [anti-4-hydroxynonenal (HNE) Abcam 46545] and glycosylation [O-linked N-acetylglucosamine (NAG) ThermoScientific MAI-072] were used. Crude mitochondrial and cytosol preparations were prepared from left ventricle from 140-day CON and 140-day CORT by a modification of the method of Hu (22). Heart tissue was minced in 10 volumes of buffer H (5 mM HEPES pH 7.5, 250 mM mannitol, 0.1 mM EDTA, and 0.1% BSA), spun at 300 g for 10 min, and the supernatant kept. The pellet was resuspended in the same volume of buffer H, spun again, and the supernatants pooled. After a 15 min spin at 8,000 g, the supernatant was collected as the cytosolic fraction and the pellet as the mitochondrial fraction. Protein content was determined in each fraction with the Pierce 660 nm protein assay using BSA as the standard, and then 25 μg of cytosolic and 50 μg of mitochondrial fractions were run on a 4–20% Bis-Tris gel, transferred to nitrocellulose, and stained with Ponceau S. The blots were blocked with 5% nonfat dried milk, incubated with primary antibody at 1 μg/ml for HNE and 1:1,000 dilution for NAG, followed by washing and the appropriate secondary antibody. Chemiluminescence was generated with Bio-Rad Immunstar, imaged with the Chemidoc XRS system and Quantity One software (Bio-Rad, Hercules, CA), and the bands' intensities normalized against total protein content of the lane. Differences in optical density were analyzed by Student's t-test.
Immunohistochemical analysis of collagen deposition and nuclear density.
Tissues and sections were prepared as previously reported (4). To assess collagen deposition in the heart, sections of hearts collected from 140-day CON and 140-day CORT fetuses (n = 5/group, randomly selected) were stained with picrosirius red and imaged using Image J [National Institutes of Health (NIH), Bethesda, MD] as previously described for sections from the 130-day fetuses (47). The average area stained per section was compared by Student's t-test.
To assess nuclear density, we stained sections of the heart with 1 μg/ml Hoechst 33342 (Invitrogen, Carlsbad, CA); slides were deidentified to assure that the analysis was done blind as to the experimental group of each section. Five images were captured at ×10 magnification from the left ventricular free wall of each heart through both bright-field and fluorescent filters. The mean of the number of nuclei per tissue area was calculated for each animal using Image J (NIH). Tissue area was calculated from bright-field images at ×10 after color inversion and using the Huang thresholding method to set color threshold. The DAPI-stained nuclei were counted with the particle analysis plug-in for Image J (10, 56) after conversion of the image to a 16-bit image with thresholding by the Huang method. The particle size limits were set to include the smallest nuclei observed as well as nuclei in the process of mitosis. The same threshold and size limits were applied to all images. At least 25,475 nuclei were counted within a tissue area of at least 1 mm2 for each animal. Two cortisol-infused animals were excluded from this analysis, one for lack of tissue, and the other because of loss of tissue adhesion to the slides, perhaps due to poor fixation, resulting in group sizes of n = 5 for 140-day CORT and n = 8 for 140-day CON. The mean nuclei per tissue area values were compared by Student's t-test.
RESULTS
Septal genes altered by maternal cortisol treatment to 130-days gestation.
We found 447 probes to be DR in septa of 130-day CORT fetuses of cortisol-infused ewes compared with 130-day CON fetuses with no exogenous maternal cortisol infusion (Fig. 1, top). We could assign 430 probes unique Entrez gene IDs, and these were analyzed by Webgestalt analysis (69). The major biological processes identified were immune system process (110 genes, P = 1.92E-10), cell signaling (162 genes, P = 2.58E-5), response to stimulus (235 genes, P = 1.25E-7), and within these, the analysis identified overrepresented processes related to leukocyte and T-cell activation, adaptive immune response, production of cytokines and cytokine-mediated signaling, response to interferon-γ, and JAK-STAT signaling (Table 2). Major molecular function categories identified were DNA binding, especially of transcriptional regulatory sites; growth factor activity; cytokine receptor binding; and protease binding. The modeling of the pathways of the genes using Cytoscape and its plug-ins Genemania, ClusterOne, and BiNGO produced similar results, albeit with some additional, predicted functions such as cellular calcium homeostasis (3 of 184 genes in the pathway, P = 0.038) and apoptosis (P = 0.001, 8 of 567 genes) and specifically muscle cell apoptosis (P = 0.039, 1 of 4 genes). Since by 130 days, cortisol resulted in increased activated caspase-3 staining in Purkinje fibers, indicative of apoptosis (14), and the septa were enlarged by hyperplasia (47), the array analyses agree with the cardiac phenotype we had previously observed in these fetuses following maternal cortisol treatment for 10 days to 130-day gestation.
Fig. 1.

Volcano plots showing probes upregulated (red), downregulated (green), and unchanged (black) in fetal septa of ewes treated with cortisol from ∼120 to 130 days of gestation (top) or from ∼115 of gestation to term (bottom). The x-axis represents the difference in the expression between cortisol and control, changes in the positive direction being greater in cortisol than control. The y-axis is the negative log of the P value, the solid line represents P = 0.05, the level of significance accepted in the analyses; probes above this line were significantly differently regulated by cortisol in these septa. CON, control; CORT, cortisol; d, day.
Table 2.
Biological processes, molecular functions, and cellular components modeled as differentially regulated by increased maternal cortisol from ∼120 to 130 days gestation
| ID | Genes in Gene Set and Category/Total Genes in Category, n | Adj. P Value | |
|---|---|---|---|
| Biological Process | |||
| Cytokine-mediated signaling pathway | GO:0019221 | 34/339 | 1.69E-08 |
| Regulation of immune response | GO:0050776 | 41/555 | 5.14E-07 |
| T cell activation | GO:0042110 | 29/336 | 3.15E-06 |
| Positive regulation of cell activation | GO:0050867 | 24/241 | 3.33E-06 |
| Cytokine production | GO:0001816 | 33/425 | 3.58E-06 |
| Positive regulation of immune system process | GO:0002684 | 38/551 | 6.46E-06 |
| Positive regulation of JAK-STAT cascade | GO:0046427 | 11 of 51 | 7.23E-06 |
| Adaptive immune response | GO:0002250 | 221/209 | 1.58E-05 |
| Regulation of tyrosine Phosphorylation of STAT protein | GO:0042509 | 10 of 50 | 4.66E-05 |
| Response to drug | GO:0042493 | 28/371 | 4.66E-05 |
| Cellular response to interferon-γ | GO:0071346 | 13/90 | 4.78E-05 |
| Intracellular protein kinase cascade | GO:0007243 | 48/867 | 5.61E-05 |
| Regulation of immune effector process | GO:0002697 | 20/213 | 6.02E-05 |
| Positive regulation of leukocyte activation | GO:0002696 | 21/233 | 6.05E-05 |
| Cellular response to chemical stimulus | GO:0070887 | 72/1,547 | 6.05E-05 |
| Response to interferon-γ | GO:0034341 | 14/109 | 6.17E-05 |
| Cytokine receptor binding | GO:0005126 | 19/214 | 0.0004 |
| Molecular Function | |||
| Chromatin binding | GO:0003682 | 24/315 | 0.0004 |
| Chemokine receptor activity | GO:0004950 | 6 of 25 | 0.0023 |
| Protein homodimerization activity | GO:0042803 | 30/571 | 0.0054 |
| DNA-(apurinic or apyrimidinic site) lyase activity | GO:0003906 | 4 of 12 | 0.0054 |
| Cytokine binding | GO:0019955 | 8 of 60 | 0.0054 |
| Core promoter proximal Region sequence-specific DNA binding | GO:0000987 | 6 of 35 | 0.0054 |
| RNA polymerase II transcription factor binding | GO:0001085 | 7 of 56 | 0.0107 |
| Damaged DNA binding | GO:0003684 | 6 of 49 | 0.0223 |
| Peptide hormone binding | GO:0017046 | 5 of 34 | 0.0231 |
| Oxidoreductase activity, acting on paired donors, with incorporation or reduction of molecular oxygen, another compound as 1 donor, and incorporation of 1 atom of oxygen | GO:0016716 | 2 of 3 | 0.0241 |
| Leukotriene-C4 synthase activity | GO:0004464 | 2 of 3 | 0.0241 |
| Double-stranded DNA binding | GO:0003690 | 11/154 | 0.0241 |
| RNA polymerase II regulatory region sequence-specific DNA binding | GO:0000977 | 7 of 73 | 0.027 |
| Co-SMAD binding | GO:0070410 | 3 of 11 | 0.027 |
| Protein-hormone receptor activity | GO:0016500 | 3 of 11 | 0.027 |
| Protease binding | GO:0002020 | 6 of 56 | 0.0294 |
| Growth factor activity | GO:0008083 | 11/164 | 0.0297 |
| Protein heterodimerization activity | GO:0046982 | 19/379 | 0.0327 |
| Cellular Component | |||
| External side of plasma membrane | GO:0009897 | 18/203 | 0.0006 |
| Integral to plasma membrane | GO:0005887 | 53/1,220 | 0.0016 |
| Cell surface | GO:0009986 | 28/495 | 0.0016 |
| Immunological synapse | GO:0001772 | 5 of 22 | 0.0035 |
| Cytoplasm | GO:0005737 | 258/9,130 | 0.0035 |
| Extracellular space | GO:0005615 | 39/856 | 0.0035 |
| Receptor complex | GO:0043235 | 13/168 | 0.0062 |
| Lysosome | GO:0005764 | 18/345 | 0.0373 |
| Nuclear chromosome | GO:0000228 | 16/285 | 0.0373 |
| Endosome lumen | GO:0031904 | 3 of 11 | 0.0373 |
| Endosome | GO:0005768 | 25/565 | 0.0473 |
Septal genes altered by maternal cortisol treatment to term.
Following a more extended increase in maternal cortisol, from 115 days to term, 513 probes were DR between the 140-day CON and 140-day CORT groups (Fig. 1, bottom), 499 of which could be identified and were used to model pathways using Webgestalt. Immune function was again significantly overrepresented in biological process (81 genes, P = 3.7E-3), molecular function, and cellular component analyses, but responses to stimuli (234 genes, P = 3.7E-3), which included responses to hypoxia and to nutrients, cell death (77 genes, P = 5.2E-3), and organ development, in association with mitochondrial matrix, lipid metabolism, and endoplasmic reticulum membrane, were also identified (Table 3). Additionally, thin muscle filaments were identified as a cellular component differentially effected by CORT (Table 3). Modeling in Cytoscape with either Genemania alone or with Genemania plus BiNGO identified response to hypoxia as the major biological function modeled (P = 6.7E-6, 149 of 14,291 genes) with genes in the response to chemical stimulus, and immune system process pathways also significantly overrepresented in the 140-day CORT compared with CON groups.
Table 3.
Biological processes, molecular functions, and cellular components modeled as differentially regulated by increased maternal cortisol from ∼115 to 140 days gestation
| ID | Genes in Gene Set and Category/Total Genes in Category, n | Adj. P Value | |
|---|---|---|---|
| Biological Process | |||
| Response to hypoxia | GO:0001666 | 27/248 | 5.67E-06 |
| Response to abiotic stimulus | GO:0009628 | 52/807 | 3.11E-05 |
| Cellular lipid metabolic process | GO:0044255 | 50/813 | 0.0001 |
| Response to organic substance | GO:0010033 | 89/1,818 | 0.0001 |
| Cellular response to organic substance | GO:0071310 | 61/1,129 | 0.0005 |
| Cell surface receptor signaling pathway | GO:0007166 | 101/2,325 | 0.002 |
| Response to drug | GO:0042493 | 27/371 | 0.002 |
| Response to hormone stimulus | GO:0009725 | 42/723 | 0.0022 |
| Response to nutrient | GO:0007584 | 20/244 | 0.0037 |
| Regulation of immune system process | GO:0002682 | 46/865 | 0.0052 |
| cell death | GO:0008219 | 77/1,704 | 0.0052 |
| Lipid biosynthetic process | GO:0008610 | 33/544 | 0.0052 |
| Response to metal ion | GO:0010038 | 20/259 | 0.0065 |
| Organ development | GO:0048513 | 104/2,552 | 0.007 |
| Response to vitamin | GO:0033273 | 15/167 | 0.007 |
| Defense response | GO:0006952 | 54/1,107 | 0.007 |
| Cellular response to oxygen levels | GO:0071453 | 10 of 79 | 0.007 |
| Activation of immune response | GO:0002253 | 22/305 | 0.007 |
| Small molecule metabolic process | GO:0044281 | 102/2,515 | 0.0114 |
| Intracellular protein transmembrane import | GO:0044743 | 18/239 | 0.0114 |
| Immune response-activating signal transduction | GO:0002757 | 18/235 | 0.0114 |
| Response to zinc ion | GO:0010043 | 7 of 42 | 0.0114 |
| Molecular Function | |||
| Binding | GO:0005488 | 374/11,955 | 0.0055 |
| Protein binding | GO:0005515 | 247/7,337 | 0.0213 |
| Protein homodimerization activity | GO:0042803 | 31/571 | 0.0429 |
| Demethylase activity | GO:0032451 | 5 of 22 | 0.0429 |
| Cellular Component | |||
| Cytoplasm | GO:0005737 | 331/9,130 | 1.77E-12 |
| Mitochondrion | GO:0005739 | 66/1,525 | 0.0026 |
| Extracellular space | GO:0005615 | 42/856 | 0.0043 |
| I-κB/NF-κB complex | GO:0033256 | 3 of 5 | 0.0043 |
| Endoplasmic reticulum | GO:0005783 | 56/1,282 | 0.006 |
| Nuclear outer membrane-endoplasmic reticulum membrane network | GO:0042175 | 38/789 | 0.0078 |
| Complement component C1 complex | GO:0005602 | 2 of 2 | 0.0082 |
| BclIII/NF-κB2 complex | GO:0033257 | 2 of 2 | 0.0082 |
| Receptor complex | GO:0043235 | 13/168 | 0.0082 |
| Angiogenin-PRI complex | GO:0032311 | 2 of 2 | 0.0082 |
| Organelle membrane | GO:0031090 | 89/2373 | 0.0124 |
| CD40 receptor complex | GO:0035631 | 3 of 11 | 0.0303 |
| B cell receptor complex | GO:0019815 | 2 of 4 | 0.0388 |
| Muscle thin filament tropomyosin | GO:0005862 | 2 of 4 | 0.0388 |
| Membrane-enclosed lumen | GO:0031974 | 116/3,385 | 0.0388 |
| Cell leading edge | GO:0031252 | 15/257 | 0.0415 |
We confirmed the increase in expression of several genes that are critical regulators in pathways identified by the analysis as differentially expressed at term and that were greatly increased in expression (Fig. 2). For the response to nutrient and/or response to hypoxia pathways we confirmed that suppressor of cytokine signaling 3 (SOCS3, 604176) and cytochrome P450, family 26, subfamily B, polypeptide 1, (CYP26B1, 605207) were upregulated. In the mitochondrial matrix cellular component we confirmed that pyruvate dehydrogenase kinase 4 (PDK4, 602527) and thioredoxin interacting protein (TXNIP, 606599) were upregulated (Fig. 2).
Fig. 2.

Validation of the microarray and pathway analyses with quantitative real-time PCR. Microarray and pathway analyses suggested that TXNIP (top right), PDK4 (bottom right), and CYP26B1 (bottom left) gene expression were significantly increased in 140-day CORT (red) compared with 140-day CON (black) in the response to nutrient and to hypoxia pathways. Pathway analyses suggested that responses to nutrient were altered and PCR confirmed an increase in SOCS3, part of this pathway, but not found on the microarray. All of these genes were significantly differently expressed at P < 0.05 (shown by *); note the difference in y-axis scale between plots.
As approximately one-fifth of the genes DR in 140-day CORT were associated with mitochondria, the mitochondrial matrix was identified as a cellular component DR by 140-day CORT in Webgestalt analyses, and as mitochondria are also susceptible to hypoxic damage, we examined the mitochondrial DNA content of the hearts by RT-PCR as an index of relative mitochondrial number and found that 140-day CORT caused a significant decrease in mitochondrial DNA content in the left ventricle (Table 4). There was no change in the number of nuclei per tissue area of the hearts with cortisol treatment to 140 days (Table 4); therefore, the decrease in mitochondrial DNA content is unlikely to be due to an increase in nuclear density that would increase the denominator of the ratio used to determine mitochondrial DNA content (mitochondrial genome #/nuclear genome #) to artificially cause the illusion of reduced mitochondrial number.
Table 4.
Comparison of 140-day CORT to 140-day CON septa (collagen content, ANP, BNP, and CNP gene expression) or left ventricle (mitochondrial DNA content and HNE and NAG protein modifications)
| Control | Cortisol | |
|---|---|---|
| Collagen content | 16.15 ± 2.12 | 14.0 ± 1.93 |
| HNE-cytosolic | 0.18 ± 0.01 | 0.16 ± 0.01 |
| NAG-cytosolic | 4.64 ± 0.36 | 4.37 ± 0.59 |
| HNE-mitochondrial | 0.38 ± 0.02 | 0.37 ± 0.02 |
| NAG-mitochondrial | 2.15 ± 0.28 | 2.49 ± 0.45 |
| Nuclei/μm2 tissue | 0.024 ± 0.001 | 0.025 ± 0.004 |
| Mitochondrial DNA content | 1.27 ± 0.05 | 0.40 ± 0.03* |
| ANP expression | 1.08 ± 0.20 | 0.98 ± 0.18 |
| BNP expression | 1.03 ± 0.29 | 1.06 ± 0.19 |
| CNP expression | 1.32 ± 0.33 | 1.91 ± 0.29* |
CORT, cortisol; CON, control.
Significantly different from control, P < 0.05.
Maturational effects.
The number of genes DR by gestational age in the comparison of the 130-day CON and 140-day CON septa was far greater than those regulated by CORT at either age; however, the common genes were not always regulated in the same direction (Figs. 3 and 4). Similarly, the magnitudes of changes in gene expression were much greater during the maturational changes (Fig. 3). Modeling the 87 genes that were both DR by cortisol at 130 days (130-day CORT vs 130-day CON; of 157 genes) and by gestational age (130-day CON vs 140-day CON, of 1,027 genes) showed that the cortisol-suppressed genes also downregulated with maturation were predominantly involved in regulating the immune response (P = 6.47E-13), especially of responses to stress (P = 2.99E-9), to cytokine-mediated signaling pathways (P = 4.33E-9) and to interferon-γ (P = 6.67E-7) (Table 5). Modeling of the 218 commonly upregulated genes in 130-day CORT (of 282) and increasing gestational age from 130–140 days (of 2,576) suggests the genes induced by cortisol at 130 days reflect genes also normally induced later in gestation. These genes are involved in organ development, including the cardiovascular system (P = 0.012), in regulation of increased cytosolic calcium concentration (P = 0.007), and in positive regulation of muscle contraction (P = 0.018), as well as in cytokine-mediated signaling (P = 0.007), cell activation (P = 0.007) and JAK-STAT signaling (P = 0.002) (Table 6).
Fig. 3.
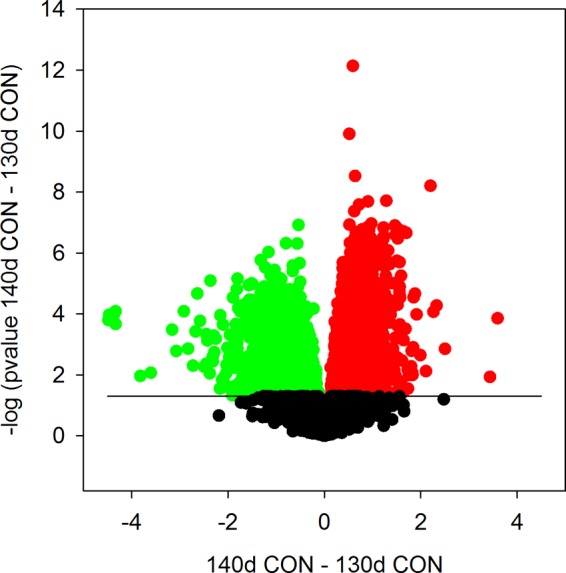
Volcano plot showing probes upregulated (red), downregulated (green), and unchanged (black) in fetal septa of 140 days gestation compared with 130 days gestation. The x-axis represents the difference between the expression at 140 and 130 days gestation, changes in the positive direction being greater at 140 days than at 130 days. Probes above the line indicating P = 0.05 were significantly differently regulated by maturation of these septa. Maturation significantly altered more genes than did cortisol at either age; the magnitude of change was also greater in many cases (Difference in scale of y-axes in Figs. 1 and 3 indicates a greater effect of gestational age than of cortisol on gene expression).
Fig. 4.
Volcano plots of the expression distribution of the probes on the array in the fetal septa, illustrating the maturational effects (difference between 140-day CON and 130-day CON, ○) with the probes altered in 130-day CORT (difference of 130-day CORT and 130-day CON) (top) or 140-day CORT (difference of 140-day CORT and 140-day CON) (bottom) shown in colors. Probes significantly differentially expressed with maturation are drawn above the solid line (P < 0.05). The red symbols are those probes whose expression was significantly increased by CORT (P < 0.05, at 130 day, top, or at term, 140 days, bottom) (i.e., 140 days vs 130 days in CON fetuses). The green symbols are the probes significantly decreased by CORT (P < 0.05; 130-day CORT, top, or 140-day CORT, bottom). Note that some genes are changed by CORT, but not by maturation (colored symbols below the significance line), and some were changed in the opposite direction by CORT compared with the effect of gestational age (e.g., red symbols on the negative x-axis side of the volcano plot, or green symbols on the positive x-axis side of the plot). In all cases y-axis values reflect the P values for gene expression due to the maturational effect.
Table 5.
Biological processes, molecular functions and cellular components modeled by commonly downregulated genes by both CORT to 130 days and maturation to term
| ID | Genes in Gene Set and Category/Total Genes in Category, n | Adj. P Value | |
|---|---|---|---|
| Biological Process | |||
| Regulation of immune response | GO:0050776 | 17/555 | 5.35E-07 |
| Defense response to virus | GO:0051607 | 10/182 | 3.04E-06 |
| Adaptive immune response based on somatic recombination of immune receptors built from immunoglobulin superfamily domains | GO:0002460 | 10/192 | 4.51E-06 |
| Type I interferon-mediated signaling pathway | GO:0060337 | 7/72 | 5.85E-06 |
| Interferon-γ-mediated signaling pathway | GO:0060333 | 7/75 | 6.84E-06 |
| Lymphocyte-mediated immunity | GO:0002449 | 9/177 | 1.82E-05 |
| Regulation of cytokine production | GO:0001817 | 12/378 | 3.11E-05 |
| Leukocyte migration | GO:0050900 | 10/248 | 3.16E-05 |
| Positive regulation of immune system process | GO:0002684 | 14/551 | 4.45E-05 |
| Regulation of T cell proliferation | GO:0042129 | 7/107 | 5.22E-05 |
| Hemopoiesis | GO:0030097 | 13/498 | 6.63E-05 |
| Molecular Function | |||
| Telomerase activity | GO:0003720 | 2/4 | 0.0069 |
| Protein homodimerization activity | GO:0042803 | 10/571 | 0.0137 |
| Lyase activity | GO:0016829 | 5/159 | 0.0247 |
| Double-stranded RNA binding | GO:0003725 | 3/44 | 0.0251 |
| Telomeric DNA binding | GO:0042162 | 2/16 | 0.0365 |
| Cytokine activity | GO:0005125 | 5/207 | 0.0365 |
| Cytokine binding | GO:0019955 | 3/60 | 0.0365 |
| Cytokine receptor binding | GO:0005126 | 5/214 | 0.0365 |
| Cellular Component | |||
| Phagocytic vesicle membrane | GO:0030670 | 3/32 | 0.0122 |
| Telomerase holoenzyme complex | GO:0005697 | 2/6 | 0.0122 |
| External side of plasma membrane | GO:0009897 | 6/203 | 0.0122 |
| Cytosol | GO:0005829 | 24/2,372 | 0.0122 |
| Extracellular space | GO:0005615 | 12/856 | 0.0134 |
| MHC protein complex | GO:0042611 | 3/38 | 0.0134 |
| ER to Golgi transport vesicle membrane | GO:0012507 | 3/37 | 0.0134 |
| Nuclear telomere cap complex | GO:0000783 | 2/12 | 0.0152 |
| Endomembrane system | GO:0012505 | 17/1,771 | 0.0307 |
| Immunological synapse | GO:0001772 | 2/22 | 0.0307 |
| Cytoplasmic stress granule | GO:0010494 | 2/23 | 0.0307 |
| Integral to lumenal side of endoplasmic reticulum membrane | GO:0071556 | 2/28 | 0.0349 |
Table 6.
Biological processes and molecular functions modeled by commonly upregulated genes by both CORT to 130 days and maturation to term
| ID | Genes in Gene Set and Category/Total Genes in Category, n | Adj. P Value | |
|---|---|---|---|
| Biological Process | |||
| Positive regulation of JAK-STAT cascade | GO:0046427 | 7/51 | 0.0021 |
| Regulation of digestive system process | GO:0044058 | 4/20 | 0.0077 |
| Cytokine-mediated signaling pathway | GO:0019221 | 15/339 | 0.0077 |
| Positive regulation of cell activation | GO:0050867 | 12/241 | 0.0077 |
| Elevation of cytosolic calcium ion concentration | GO:0007204 | 10/172 | 0.0077 |
| Gland development | GO:0048732 | 13/277 | 0.0077 |
| Cardiovascular system development | GO:0072358 | 23/754 | 0.0124 |
| Cell communication | GO:0007154 | 88/4,770 | 0.0124 |
| Inositol trisphosphate biosynthetic process | GO:0032959 | 3/9 | 0.0124 |
| Negative regulation of transcription from RNA polymerase II promoter | GO:0000122 | 17/485 | 0.0167 |
| Polyol biosynthetic process | GO:0046173 | 4/24 | 0.0167 |
| Positive regulation of muscle contraction | GO:0045933 | 4/26 | 0.0189 |
| Paraxial mesoderm morphogenesis | GO:0048340 | 3/11 | 0.0189 |
| Response to drug | GO:0042493 | 14/371 | 0.0206 |
| Response to anoxia | GO:0034059 | 2/3 | 0.0206 |
| Endocrine system development | GO:0035270 | 8/136 | 0.0206 |
| Regulation of hormone levels | GO:0010817 | 14/378 | 0.0242 |
| Molecular Function | |||
| Chromatin binding | GO:0003682 | 17/315 | 0.0002 |
| Cytokine receptor binding | GO:0005126 | 11/214 | 0.0042 |
| G protein-coupled peptide receptor activity | GO:0008528 | 8/117 | 0.0042 |
| Transcription regulatory region sequence-specific DNA binding | GO:0000976 | 9/143 | 0.0042 |
| Core promoter proximal region sequence-specific DNA binding | GO:0000987 | 5/35 | 0.0042 |
| Protein-hormone receptor activity | GO:0016500 | 3/11 | 0.008 |
| Co-SMAD binding | GO:0070410 | 3/11 | 0.008 |
| chemokine receptor activity | GO:0004950 | 4/25 | 0.008 |
| Oxidoreductase activity, acting on paired donors, with incorporation or reduction of molecular oxygen, another compound as 1 donor, and incorporation of 1 atom of oxygen | GO:0016716 | 2/3 | 0.0098 |
| Transforming growth factor-β receptor, pathway-specific cytoplasmic mediator activity | GO:0030618 | 2/5 | 0.0212 |
| tRNA (guanine) methyltransferase activity | GO:0016423 | 2/5 | 0.0212 |
| R-SMAD binding | GO:0070412 | 3/18 | 0.0212 |
| Phosphodiesterase I activity | GO:0004528 | 2/5 | 0.0212 |
| Protein dimerization activity | GO:0046983 | 24/972 | 0.0212 |
| RNA polymerase II regulatory region sequence-specific DNA binding | GO:0000977 | 5/73 | 0.0274 |
| Type I transforming growth factor-β receptor binding | GO:0034713 | 2/7 | 0.0334 |
| RNA polymerase II activating transcription factor binding | GO:0001102 | 3/25 | 0.0356 |
| Structure-specific DNA binding | GO:0043566 | 8/195 | 0.0356 |
| E-box binding | GO:0070888 | 3/28 | 0.0439 |
| RNA polymerase II transcription factor binding transcription factor activity | GO:0001076 | 5/87 | 0.0439 |
| RNA polymerase II core promoter proximal region sequence-specific DNA binding transcription factor activity involved in positive regulation of transcription | GO:0001077 | 4/57 | 0.0465 |
| signal transducer activity | GO:0004871 | 33/1,628 | 0.0465 |
| Cellular Component | |||
| None identified in analysis | |||
Neither the commonly up- nor downregulated genes were indicative of responses to hypoxia or nutrients, the major pathways suggested by modeling the genes DR in the 140-day CORT group, nor of muscle cell apoptosis, which was modeled with increased cortisol in the 130-day CORT group. The 60 genes changed in 130-day CORT, but not by gestational age, suggest that the cellular components affected are lysosome (10 genes P = 8.4E-3), cell surface, plasma membrane, and growth cone (2 genes, P = 0.04), but no biological processes or molecular functions were modeled at a significance level of P < 0.05.
Although the transcriptomic analysis suggests pathways related to the response to hypoxia are activated by excess cortisol to term, Western blot analyses with antibodies against oxidative stress markers commonly observed in hypoxic adult tissues did not show increased evidence of CORT-induced oxidative stress in 140-day CORT vs 140-day CON in cytosolic or mitochondrial fractions of left ventricle (Table 4). Likewise the expression of genes such as ANP, BNP, and collagens was not altered by cortisol treatment at either 130 days or term, nor was there evidence of collagen deposition in the hearts (Table 4). The lack of decrease in nuclear number per tissue area is consistent with the lack of collagen deposition in the hearts as hypertrophy secondary to collagen deposition, as is commonly seen in hypoxic adult hearts, would reduce this ratio.
DISCUSSION
The transcriptomic analyses of the fetal hearts suggest that chronic increases in maternal cortisol concentrations, such as may occur with maternal stress, have important consequences for the fetal heart. The chronic exposure to higher-than-normal maternal cortisol concentrations leads to changes in expression of genes, with an overrepresentation of genes related to immune function, cell signaling, and growth factor activity at 130 days, but an overrepresentation of genes related to metabolism by term.
As birth approaches, there is a significant shift in myocyte size and morphology and remodeling of the ventricles, with relative thinning of the right ventricular wall and thickening of the left ventricular free wall and with the formation of a collagenous sheath around the Purkinje fibers (7). Major cellular level changes begin in the heart before 130 days of gestation and proceed throughout birth and early extrauterine life (reviewed in Ref. 52). Driven by changes in concentrations of cortisol (53), as well as thyroid hormone (8), these ready the heart for the change to extrauterine life. These changes include maturation of the cardiomyocytes from a mononucleated, proliferative phenotype to a binucleated, nonproliferative, terminally differentiated phenotype, and changes in cellular collagen deposition, calcium signaling, and cellular energetics, with an increase in the number and distribution pattern of mitochondria in the cardiomyocytes (5, 39), so that the mitochondria are exquisitely positioned to efficiently provide the ATP required for muscle contraction and impulse propagation. To accommodate this, there are also shifts in metabolism. The fetal heart relies on lactate and glucose as major energy sources in contrast to the adult heart, which relies on fatty acids; that transition occurs in the first few weeks of extrauterine life in the sheep (2, 15). Although the fetal heart can metabolize fatty acids (2), fetal plasma levels of fatty acids are too low to be a useful source of energy. It is also probable that fetal oxygen levels are insufficient to effectively metabolize fats, because although the amount of ATP generated by fatty acid beta oxidation is greater than that from metabolism of lactate or glucose, the efficiency per mole of oxygen is less. Overall there are extensive cellular changes in late fetal heart development that are known to be controlled, at least indirectly, by the prepartum cortisol surge (53). The present results demonstrate that a modest, but chronic, increase in cortisol such as generated in our studies might influence normal cardiac maturation and function though effects on fetal cardiac metabolism.
One hypothesis for the effects of cortisol on the fetal heart was that increasing cortisol 1.5-fold between 120 and 130 days gestation (47) produces cardiac enlargement primarily by accelerating the normal late-gestational maturation of the heart; however, the transcriptomic analysis suggests that although some genes altered by cortisol at 130 days are those altered at 140 days in the control fetuses, this hypothesis is an oversimplification of the effect of cortisol on the fetal heart. In our earlier studies the effects of maternal cortisol did not correspond to the changes found with maturation when a subset of genes related to IGF and angiotensin were considered (48, 49). In this more extensive comparison using gene array, we found that some of the genes up- or downregulated in both 130-day CORT and in the normal maturation of the heart are changed in the same direction, the upregulated genes apparently involved in the maturation of the heart (suggested by gene ontology terms such as organ and cardiovascular system development) and the downregulated in modulation of the immune system in the heart (cytokine signaling and interferon-γ responses). The common upregulated genes have diverse actions, including calcium channel signaling and chemokine receptor expression, and suggest cortisol might contribute to maturation by mechanisms previously identified as GR-related in the mouse (53). The downregulated genes, while consistent with the known ability of cortisol to dampen immune responses in the adult (42), suggest that there may be a requirement for modulation of immune processes for the proper development of the heart during late gestation. However, there are many genes that are either unchanged in one group compared with the other or changed in the opposing direction by cortisol compared with the changes occurring during normal maturation. The analyses of the genes whose expression was affected in 130-day CORT, but not during maturation, indicate potential effects on the plasma membrane and specifically the growth cone. While it is tempting to hypothesize that this might underlie the Purkinje cell apoptosis we had previously observed in 130-day CORT, we did not examine the growth cones of the cardiac nerves, nor were many genes involved in these pathways, making the significance of the results unclear. Nonetheless, after only 10 days of infusion, a modest increase in fetal cortisol appears to induce genes with possible deleterious effects, as well a number that would later be induced in the course of normal maturation.
Previous studies from our laboratory (49) had also suggested that the effects seen in 130-day CORT were not through induction of genes associated with known inducers of cardiomyocyte proliferation such as IGFs or angiotensin, nor was the pattern similar to that seen for other genes near term (58, 59). The effects of cortisol also were dissimilar to those commonly observed in the enlarged mature heart such as collagen deposition or hypertrophy in response to increased afterload from hypertension. This difference is consistent with the fact that these cortisol concentrations are not high enough to increase fetal arterial pressure, and there was no ischemic event in these hearts. The gene array also did not identify changes in pathways associated with hypertrophic growth or ischemia. Expression of ANP or BNP and collagen content, indicators of heart failure in adults, were also not significantly changed. Indeed, microarray analyses followed by gene pathway modeling were employed to try to understand the mechanism of action of cortisol, as the previously hypothesized mechanisms of cortisol in maturation did not seem to be involved in the observed effects of cortisol.
Transcriptomic analysis also did not support the hypothesis that longer periods of cortisol exposure to term would exacerbate the cortisol effects occurring at 130 days. The pathways activated by cortisol, as modeled by comparison of 140-day CORT vs 140-day CON, suggested responses to hypoxia and to nutrients were altered and that these could involve mitochondria, lipid metabolism, and the endoplasmic reticulum. These responses were not modeled as effects of cortisol at 130-day CORT. However, we saw no evidence of fetal hypoxemia produced in the earlier studies with maternal infusion of cortisol to 130 days (24, 47), suggesting that chronic hypoxemia does not occur in this model with rather modest hyperglycemia. Although it is possible that the enlargement of the heart muscle, if not in concert with development of blood vessels to supply adequate oxygen and nutrients to the heart, could lead to tissue hypoxia and nutrient deficits, the hypoxia-induced genes HIF1α and ARNT aryl hydrocarbon receptor nuclear were not altered on the array. Of the 27 “hypoxia”-related genes, six of these were also in the nutrient pathway, and many others, including placental growth factor, corticotropin-releasing hormone receptor 1, and caveolin 1, are not specifically hypoxia related. In the 140-day hearts we did not find evidence of proteins modified by either lipid peroxidation or O-linked N-glycosylation end products as would be expected following production of reactive oxygen species, which could result after acute hypoxia. Thus it is perhaps more likely that the 140-day CORT effects are responses to changes in nutrient supply, i.e., adaptations to the chronic hyperglycemia secondary to cortisol-induced hyperglycemia in the ewe (24). Supporting this hypothesis is the increased expression of four genes, PDK4, TXNIP, SOCS3, and CYP26B1, known to alter cellular metabolism, increase insulin resistance, or decrease glucose utilization in the 140-day CORT septa. PDK4 is a gene regulated by both insulin and cortisol; PDK4 decreases pyruvate entry into the TCA cycle by phosphorylating and thereby inactivating the pyruvate dehydrogenase complex (25). TXNIP, which may function as a metabolic control protein in the heart (67), has recently been shown to increase insulin resistance and decrease insulin sensitivity in bone tissue from Cushing's disease patients (34). Increased expression of CYP26B1, which metabolizes all trans retinoic acid, may decrease glucose uptake (33). In cardiomyocytes subjected to hyperglycemia, retinoic acid/retinoid X receptor (RAR/RXR) signaling is impaired, leading to apoptosis through oxidative stress and NF-κB signaling (44, 57); however, in these fetal hearts increased breakdown of retinoic acid by CYP26B1 may reduce RAR/RXR activation, leading to apoptosis. Finally, SOCS3 limits glucose uptake by interfering with phosphorylation events downstream of the insulin receptor (13). Therefore, the gene expression pattern suggests that the 140-day CORT septa have impaired glucose uptake and metabolism.
The results of the transcriptomic analysis suggest that in the face of increased glucose levels, downregulation of carbohydrate metabolizing pathways protect the heart from damage due to oxidative stress, allowing adaptation until labor. The gene expression patterns at 140 days suggest that the stillbirth of these lambs may result from inadequate metabolic capacity of the heart as labor and delivery progress. The fetal heart withstands even rather severe chronic hypoxia without failing (50, 51), so it is unlikely that chronic hypoxia directly contributes to the fetal demise or to the transcriptomic patterns found in these hearts. However, acute hypoxia occurring secondarily to labor, coupled with the decrease in mitochondrial number in the 140-day CORT hearts, could decrease the efficient functioning of the fetal heart, potentially to the point of failure. Fetal sheep have been shown to have more pronounced bradycardic responses to hypoxia at term than at 125–130 days gestation (17). Studies in which ewes were infused or injected with dexamethasone have found that glucocorticoid exposure prolongs the bradycardia and vasoconstriction in response to acute hypoxia and increases the severity of acidemia (16, 23); in those studies it was suggested that the effect of glucocorticoid was to exaggerate both parasympathetic and sympathetic responses. Our array results suggest an effect of cortisol to alter energy availability in the heart at term, which may contribute to, or exaggerate, the bradycardia at term after chronic exposure to cortisol. Interestingly, the array results indicate that there was no increase in expression of the mitochondrial biogenesis markers PPARGC1A (604517), SIRT1(604479), SIRT3 (604481), NOS3 (163729), NRF1 (600879), TFAM (600438), the subunits of AMPK (602739, 602740, 600497, 602743, 602742, 602741), or MFN2 (608507) (20) as a result of the maternal cortisol infusion in the term hearts, suggesting that there is no drive to correct the apparent deficit in mitochondrial number. The transcriptomic response suggests that cardiac insulin resistance may also occur as an adaptation to chronic hypercortisolemia. Cardiac insulin resistance is observed in the rat prior to the heart failure associated with pressure overload hypertrophy and manifests as decreased insulin-stimulated glucose oxidation and glycolysis and decreased mitochondrial oxidative metabolism (70). If the 140-day CORT hearts are insulin resistant, they could be prone to fail at term as left ventricular afterload increases at birth. Indeed this is a potential explanation for the perinatal outcome, as eight of 15 fetuses in the cortisol-infused ewes died after the start of labor or in the immediate postpartum period.
These results in the hypercortisolemic, and presumably hyperglycemic fetus, appear to be in contrast to those in fetuses with growth restriction secondary to placental restriction. In placental restriction the overall fetal growth is compromised, but heart weight-to-body weight ratio is maintained, albeit with relatively more mononucleated cardiomyocytes and relatively larger cardiomyocytes (43), suggesting delayed transition to the terminally differentiated phenotype. In these hearts IGF-2 and IGF receptor expression is more abundant (62). These gene changes appear to be growth-sparing adaptations to sparsity of nutrients secondary to placental restriction, whereas in the fetuses of hypercortisolemic ewes changes in gene expression occur in the midst of plentiful glucose and may be growth-limiting adaptations.
Some developmental effects of cortisol are thought to be mediated indirectly by other factors, including thyroid hormone. Both the endogenous increase in cortisol and exogenous glucocorticoid treatment increase thyroid hormones in the fetus (18, 19). We therefore considered whether the effects of increased maternal cortisol found in fetuses at either 130 days or at term could be mediated through a direct effect of cortisol at the classical glucocorticoid response element (GRE) or indirectly through the thyroid hormone transcription factor. Neither GRE nor thyroid hormone response element was suggested as the predominant transcription factor by transcription factor analysis with Webgestalt. Of the genes differentially expressed as a result of maternal cortisol treatment to 130 days, there were no genes with GRE, and the T3 receptor (T3R) was a transcription factor targeting only seven genes (of 248 known T3R-regulated genes in the ontology databases, adjusted P value = 0.03). At term, the classical GRE was recognized as a transcription factor for 10 genes DR by cortisol (of 276 possible genes with GRE, adjusted P value = 0.0045) as was the T3R (10 genes of 248, adjusted P value= 0.0027). These findings suggest that cortisol-activated receptors may act primarily via protein-protein interactions with other transcription factors or that cortisol is acting indirectly for example through cortisol-mediated maternal, and the resultant fetal, hyperglycemia. Interestingly, the estrogen-related receptor 1 (ESRRA) was implicated in control of genes DR by cortisol at both 130 days and term. ESRRA is a member of the ESRR family of orphan receptors that has been shown in the mouse and in human hearts (30) to have profound effects coordinating cardiac metabolism, structure, and function (1, 11) and regulates thyroid hormone receptor synthesis (60). ESRRA was a suggested transcription factor for 26 DR genes of a possible 1,023 regulated by ESRRA, adjusted P value = 0.0002 at 130 days, and of 38 genes (of 1,023) at 140 days with an adjusted P value of 1.35E-8, suggesting that ESSRA has more importance than the GRE or T3R. The ESRR family member ESRRγ, which interacts with, or possibly is redundant to, ESRRA (11), regulates the expression of PDK4 (32), one of the genes upregulated by cortisol at 140 days, although in the transcription factor analysis PDK4 was not shown in the ESRRA-regulated genes. ESRRA has an important role, in concert with PPARGC1A, in regulating mitochondrial function (1, 40), including fission and fusion (35) and mitochondrial phospholipid synthesis (31), suggesting that ESSRA may play an important role in the mechanism by which increased maternal cortisol leads to decreased septal mitochondrial DNA content. But, as there is no published evidence to show that cortisol regulates the expression of the ESRRs, it is probable that this is an indirect effect mediated through the maternal and fetal hyperglycemia secondary to hypocortisolemia.
Thyroid hormone has also been shown to have effects on the fetal or neonatal heart that are not mediated by the classical TRE. Thyroid hormone mediates the switch from the fetal to adult form of TTN (titin), an important protein in the contractile apparatus of the heart, via increased phosphorylation of Akt (28, 29). Thyroid hormone also suppresses mitotic activity of growth factor-stimulated cardiomyocytes by increasing the activity of CDKN1A (p21) and decreasing the activity of CCND1 (cyclin D1) (8). Transcription factor modeling would miss this type of thyroid hormone action. But while our transcriptomics analyses did not indicate enrichment of Akt pathways following maternal cortisol treatment, at 140 days, CCND1 expression was significantly increased by cortisol and was part of the response to nutrient pathway. This suggests that cortisol would delay the terminal differentiation of the cardiomyocytes, an opposite action to that of T3. Incidentally, the expression of TTN was not affected by maternal cortisol treatments but was increased during the maturation from 130- to 140-days gestation. Nonetheless, it would be interesting to examine the subtypes of TTN in the fetuses of cortisol-infused ewes, as the stiffness of the heart is regulated in part by the proportions and phosphorylation status of the TTN subtypes present in the heart.
Our studies have indicated that chronic increases in cortisol during pregnancy, outside of the normal late-gestational window that is so important for proper fetal development, have potentially disastrous effects on fetal survival at birth (27). In those hearts transcriptomic analysis indicates effects of chronic exposure to increased cortisol that overlap with, but are largely distinct from, the effects of normal cardiac maturation. More importantly, the results indicate that chronically increased maternal cortisol stimulates a shift in cardiac metabolism that mirrors some aspects of the failing adult heart and that could prove maladaptive during labor and the immediate neonatal period. We conclude that these molecular responses might explain the increased incidence of stillbirth in pregnancies complicated by Cushing's disease or diabetes and suggest that metabolic support to the heart during this period could be beneficial.
GRANTS
This work was supported by National Institutes of Health Grants DK-60820 and HD-57871.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: E.M.R., C.E.W., M.B.R., and M.K.-W. conception and design of research; E.M.R., A.A., and M.K.-W. performed experiments; E.M.R., M.B.R., A.A., and M.K.-W. analyzed data; E.M.R., C.E.W., M.B.R., A.A., and M.K.-W. interpreted results of experiments; E.M.R. and M.K.-W. prepared figures; E.M.R. and M.K.-W. drafted manuscript; E.M.R., C.E.W., M.B.R., A.A., and M.K.-W. edited and revised manuscript; E.M.R., C.E.W., M.B.R., A.A., and M.K.-W. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Keith Walker and Xiaodi Feng for assistance in the quantification of nuclei and collagen in the hearts and Dr. Christiaan Leeuwenburg for advice on quantification of mitochondria.
Present address for M. B. Rabaglino: Departamento de Reproducción Animal, Facultad de Agronomía y Veterinaria, Universidad Nacional de Rio Cuarto, Rio Cuarto, Argentina.
REFERENCES
- 1.Alaynick WA, Kondo RP, Xie W, He W, Dufour CR, Downes M, Jonker JW, Giles W, Naviaux RK, Giguere V, Evans RM. ERRgamma directs and maintains the transition to oxidative metabolism in the postnatal heart. Cell Metab 6: 13–24, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Bartelds B, Knoester H, Smid GB, Takens J, Visser GH, Penninga L, van der Leij FR, Beaufort-Krol GC, Zijlstra WG, Heymans HS, Kuipers JR. Perinatal changes in myocardial metabolism in lambs. Circulation 102: 926–931, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Stat Soc Ser B 57: 289–300, 1995 [Google Scholar]
- 4.Boeuf B, Maragnes P, Belzic I, Lacotte J, Bonte JB, Guillois B. [Glucocorticoid-induced hypertrophic cardiomyopathy in premature infants: apropos of 4 cases]. Arch Pediatr 4: 152–157, 1997 [DOI] [PubMed] [Google Scholar]
- 5.Brook WH, Connell S, Cannata J, Maloney JE, Walker AM. Ultrastructure of the myocardium during development from early fetal life to adult life in sheep. J Anat 137: 729–741, 1983 [PMC free article] [PubMed] [Google Scholar]
- 6.Burrell JH, Boyn AM, Kumarasamy V, Hsieh A, Head SI, Lumbers ER. Growth and maturation of cardiac myocytes in fetal sheep in the second half of gestation. Anat Rec A Discov Mol Cell Evol Biol 274: 952–961, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Canale E, Smolich JJ, Campbell GR. Differentiation and innervation of the atrioventricular bundle and ventricular Purkinje system in sheep heart. Development 100: 641–651, 1987 [DOI] [PubMed] [Google Scholar]
- 8.Chattergoon NN, Giraud GD, Louey S, Stork P, Fowden AL, Thornburg KL. Thyroid hormone drives fetal cardiomyocyte maturation. FASEB J 26: 397–408, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cline MS, Smoot M, Cerami E, Kuchinsky A, Landys N, Workman C, Christmas R, Avila-Campilo I, Creech M, Gross B, Hanspers K, Isserlin R, Kelley R, Killcoyne S, Lotia S, Maere S, Morris J, Ono K, Pavlovic V, Pico AR, Vailaya A, Wang PL, Adler A, Conklin BR, Hood L, Kuiper M, Sander C, Schmulevich I, Schwikowski B, Warner GJ, Ideker T, Bader GD. Integration of biological networks and gene expression data using Cytoscape. Nat Protoc 2: 2366–2382, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collins TJ. ImageJ for microscopy. Biotechniques 43: 25–30, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Dufour CR, Wilson BJ, Huss JM, Kelly DP, Alaynick WA, Downes M, Evans RM, Blanchette M, Giguere V. Genome-wide orchestration of cardiac functions by the orphan nuclear receptors ERRalpha and gamma. Cell Metab 5: 345–356, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Edgar R, Domrachev M, Lash AE. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res 30: 207–210, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Emanuelli B, Peraldi P, Filloux C, Sawka-Verhelle D, Hilton D, Van Obberghen E. SOCS-3 is an insulin-induced negative regulator of insulin signaling. J Biol Chem 275: 15985–15991, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Feng X, Reini S, Richards E, Wood CE, Keller-Wood M. Cortisol stimulates proliferation and apoptosis in the late gestation fetal heart: differential effects of mineralocorticoid and glucocorticoid receptors. Am J Physiol Regul Integr Comp Physiol 305: R343–R350, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fisher DJ, Heymann MA, Rudolph AM. Myocardial oxygen and carbohydrate consumption in fetal lambs in utero and in adult sheep. Am J Physiol Heart Circ Physiol 238: H399–H405, 1980 [DOI] [PubMed] [Google Scholar]
- 16.Fletcher AJ, Gardner DS, Edwards CM, Fowden AL, Giussani DA. Cardiovascular and endocrine responses to acute hypoxaemia during and following dexamethasone infusion in the ovine fetus. J Physiol 549: 271–287, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fletcher AJ, Gardner DS, Edwards CM, Fowden AL, Giussani DA. Development of the ovine fetal cardiovascular defense to hypoxemia towards full term. Am J Physiol Heart Circ Physiol 291: H3023–H3034, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Forhead AJ, Curtis K, Kaptein E, Visser TJ, Fowden AL. Developmental control of iodothyronine deiodinases by cortisol in the ovine fetus and placenta near term. Endocrinology 147: 5988–5994, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Forhead AJ, Jellyman JK, Gardner DS, Giussani DA, Kaptein E, Visser TJ, Fowden AL. Differential effects of maternal dexamethasone treatment on circulating thyroid hormone concentrations and tissue deiodinase activity in the pregnant ewe and fetus. Endocrinology 148: 800–805, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Gesing A, Masternak MM, Wang F, Joseph AM, Leeuwenburgh C, Westbrook R, Lewinski A, Karbownik-Lewinska M, Bartke A. Expression of key regulators of mitochondrial biogenesis in growth hormone receptor knockout (GHRKO) mice is enhanced but is not further improved by other potential life-extending interventions. J Gerontol A Biol Sci Med Sci 66: 1062–1076, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giraud GD, Louey S, Jonker S, Schultz J, Thornburg KL. Cortisol stimulates cell cycle activity in the cardiomyocyte of the sheep fetus. Endocrinology 147: 3643–3649, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Hu Y, Suarez J, Fricovsky E, Wang H, Scott BT, Trauger SA, Han W, Hu Y, Oyeleye MO, Dillmann WH. Increased enzymatic O-GlcNAcylation of mitochondrial proteins impairs mitochondrial function in cardiac myocytes exposed to high glucose. J Biol Chem 284: 547–555, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jellyman JK, Gardner DS, Edwards CM, Fowden AL, Giussani DA. Fetal cardiovascular, metabolic and endocrine responses to acute hypoxaemia during and following maternal treatment with dexamethasone in sheep. J Physiol 567: 673–688, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jensen E, Wood CE, Keller-Wood M. Chronic alterations in ovine maternal corticosteroid levels influence uterine blood flow and placental and fetal growth. Am J Physiol Regul Integr Comp Physiol 288: R54–R61, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Jeoung NH, Harris RA. Role of pyruvate dehydrogenase kinase 4 in regulation of blood glucose levels. Korean Diabetes J 34: 274–283, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jonker SS, Zhang L, Louey S, Giraud GD, Thornburg KL, Faber JJ. Myocyte enlargement, differentiation, and proliferation kinetics in the fetal sheep heart. J Appl Physiol 102: 1130–1142, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Keller-Wood M, Feng X, Wood CE, Richards EM, Anthony RV, Dahl GE, Tao S. Elevated maternal cortisol leads to relative maternal hyperglycemia and increased stillbirth in ovine pregnancy. Am J Physiol Regul Integr Comp Physiol [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kruger M, Babicz K, von Frieling-Salewsky M, Linke WA. Insulin signaling regulates cardiac titin properties in heart development and diabetic cardiomyopathy. J Mol Cell Cardiol 48: 910–916, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Kruger M, Sachse C, Zimmermann WH, Eschenhagen T, Klede S, Linke WA. Thyroid hormone regulates developmental titin isoform transitions via the phosphatidylinositol-3-kinase/AKT pathway. Circ Res 102: 439–447, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Kwon DH, Eom GH, Kee HJ, Nam YS, Cho YK, Kim DK, Koo JY, Kim HS, Nam KI, Kim KK, Lee IK, Park SB, Choi HS, Kook H. Estrogen-related receptor gamma induces cardiac hypertrophy by activating GATA4. J Mol Cell Cardiol 65: 88–97, 2013 [DOI] [PubMed] [Google Scholar]
- 31.Lai L, Wang M, Martin OJ, Leone TC, Vega RB, Han X, Kelly DP. A role for peroxisome proliferator-activated receptor gamma coactivator 1 (PGC-1) in the regulation of cardiac mitochondrial phospholipid biosynthesis. J Biol Chem 289: 2250–2259, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee JH, Kim EJ, Kim DK, Lee JM, Park SB, Lee IK, Harris RA, Lee MO, Choi HS. Hypoxia induces PDK4 gene expression through induction of the orphan nuclear receptor ERRgamma. PLoS One 7: e46324, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee YM, Lee JO, Jung JH, Kim JH, Park SH, Park JM, Kim EK, Suh PG, Kim HS. Retinoic acid leads to cytoskeletal rearrangement through AMPK-Rac1 and stimulates glucose uptake through AMPK-p38 MAPK in skeletal muscle cells. J Biol Chem 283: 33969–33974, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lekva T, Bollerslev J, Sahraoui A, Scholz H, Boyum H, Evang JA, Godang K, Aukrust P, Ueland T. Thioredoxin interacting protein is a potential regulator of glucose and energy homeostasis in endogenous Cushing's syndrome. PLoS One 8: e64247, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liesa M, Borda-d'Agua B, Medina-Gomez G, Lelliott CJ, Paz JC, Rojo M, Palacin M, Vidal-Puig A, Zorzano A. Mitochondrial fusion is increased by the nuclear coactivator PGC-1beta. PLoS One 3: e3613, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liggins GC. The role of cortisol in preparing the fetus for birth. Reprod Fertil Dev 6: 141–150, 1994 [DOI] [PubMed] [Google Scholar]
- 37.Lumbers ER, Boyce AC, Joulianos G, Kumarasamy V, Barner E, Segar JL, Burrell JH. Effects of cortisol on cardiac myocytes and on expression of cardiac genes in fetal sheep. Am J Physiol Regul Integr Comp Physiol 288: R567–R574, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Maere S, Heymans K, Kuiper M. BiNGO: a Cytoscape plugin to assess overrepresentation of gene ontology categories in biological networks. Bioinformatics 21: 3448–3449, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Marin-Garcia J, Ananthakrishnan R, Goldenthal MJ. Heart mitochondrial DNA and enzyme changes during early human development. Mol Cell Biochem 210: 47–52, 2000 [DOI] [PubMed] [Google Scholar]
- 40.McDermott-Roe C, Ye J, Ahmed R, Sun XM, Serafin A, Ware J, Bottolo L, Muckett P, Canas X, Zhang J, Rowe GC, Buchan R, Lu H, Braithwaite A, Mancini M, Hauton D, Marti R, Garcia-Arumi E, Hubner N, Jacob H, Serikawa T, Zidek V, Papousek F, Kolar F, Cardona M, Ruiz-Meana M, Garcia-Dorado D, Comella JX, Felkin LE, Barton PJ, Arany Z, Pravenec M, Petretto E, Sanchis D, Cook SA. Endonuclease G is a novel determinant of cardiac hypertrophy and mitochondrial function. Nature 478: 114–118, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mildenhall L, Battin M, Bevan C, Kuschel C, Harding JE. Repeat prenatal corticosteroid doses do not alter neonatal blood pressure or myocardial thickness: randomized, controlled trial. Pediatrics 123: e646–e652, 2009 [DOI] [PubMed] [Google Scholar]
- 42.Miller AH, Spencer RL, Pearce BD, Pisell TL, Azrieli Y, Tanapat P, Moday H, Rhee R, McEwen BS. Glucocorticoid receptors are differentially expressed in the cells and tissues of the immune system. Cell Immunol 186: 45–54, 1998 [DOI] [PubMed] [Google Scholar]
- 43.Morrison JL, Botting KJ, Dyer JL, Williams SJ, Thornburg KL, McMillen IC. Restriction of placental function alters heart development in the sheep fetus. Am J Physiol Regul Integr Comp Physiol 293: R306–R313, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Nizamutdinova IT, Guleria RS, Singh AB, Kendall JA, Jr, Baker KM, Pan J. Retinoic acid protects cardiomyocytes from high glucose-induced apoptosis through inhibition of NF-kappaB signaling pathway. J Cell Physiol 228: 380–392, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O'Sullivan L, Cuffe JS, Paravicini TM, Campbell S, Dickinson H, Singh RR, Gezmish O, Black MJ, Moritz KM. Prenatal exposure to dexamethasone in the mouse alters cardiac growth patterns and increases pulse pressure in aged male offspring. PLoS One 8: e69149, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rabaglino MB, Richards E, Denslow N, Keller-Wood M, Wood CE. Genomics of estradiol-3-sulfate action in the ovine fetal hypothalamus. Physiol Genomics 44: 669–677, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reini SA, Dutta G, Wood CE, Keller-Wood M. Cardiac corticosteroid receptors mediate the enlargement of the ovine fetal heart induced by chronic increases in maternal cortisol. J Endocrinol 198: 419–427, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reini SA, Wood CE, Jensen E, Keller-Wood M. Increased maternal cortisol in late gestation ewes decreases fetal cardiac expression of 11β-HSD2 mRNA and the ratio of AT1 to AT2 receptor mRNA. Am J Physiol Regul Integr Comp Physiol 291: R1708–R1716, 2006 [DOI] [PubMed] [Google Scholar]
- 49.Reini SA, Wood CE, Keller-Wood M. The ontogeny of genes related to ovine fetal cardiac growth. Gene Expr Patt 9: 122–128, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reller MD, Morton MJ, Giraud GD, Reid DL, Thornburg KL. The effect of acute hypoxaemia on ventricular function during beta-adrenergic and cholinergic blockade in the fetal sheep. J Dev Physiol 11: 263–269, 1989 [PubMed] [Google Scholar]
- 51.Reller MD, Morton MJ, Thornburg KL. Right ventricular function in the hypoxaemic fetal sheep. J Dev Physiol 8: 159–166, 1986 [PubMed] [Google Scholar]
- 52.Rog-Zielinska EA, Richardson RV, Denvir M, Chapman K. Glucocorticoids and foetal heart maturation; implications for prematurity and foetal programming. J Mol Endocrinol 52: R125–R135 2014 [DOI] [PubMed] [Google Scholar]
- 53.Rog-Zielinska EA, Thomson A, Kenyon CJ, Brownstein DG, Moran CM, Szumska D, Michailidou Z, Richardson J, Owen E, Watt A, Morrison H, Forrester LM, Bhattacharya S, Holmes MC, Chapman KE. Glucocorticoid receptor is required for foetal heart maturation. Hum Mol Genet 22: 3269–3282, 2013 [DOI] [PubMed] [Google Scholar]
- 54.Rudolph AM, Roman C, Gournay V. Perinatal myocardial DNA and protein changes in the lamb: effect of cortisol in the fetus. Pediatr Res 46: 141–146, 1999 [DOI] [PubMed] [Google Scholar]
- 55.Schafer-Graf UM, Wockel A. [Severe diabetic fetopathy due to undiagnosed gestational diabetes mellitus]. Dtsch Med Wochenschr 131: 1151–1154, 2006 [DOI] [PubMed] [Google Scholar]
- 56.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Meth 9: 671–675, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Singh AB, Guleria RS, Nizamutdinova IT, Baker KM, Pan J. High glucose-induced repression of RAR/RXR in cardiomyocytes is mediated through oxidative stress/JNK signaling. J Cell Physiol 227: 2632–2644, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sundgren NC, Giraud GD, Schultz JM, Lasarev MR, Stork PJ, Thornburg KL. Extracellular signal-regulated kinase and phosphoinositol-3 kinase mediate IGF-1 induced proliferation of fetal sheep cardiomyocytes. Am J Physiol Regul Integr Comp Physiol 285: R1481–R1489, 2003 [DOI] [PubMed] [Google Scholar]
- 59.Sundgren NC, Giraud GD, Stork PJ, Maylie JG, Thornburg KL. Angiotensin II stimulates hyperplasia but not hypertrophy in immature ovine cardiomyocytes. J Physiol 548: 881–891, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vanacker JM, Bonnelye E, Delmarre C, Laudet V. Activation of the thyroid hormone receptor alpha gene promoter by the orphan nuclear receptor ERR alpha. Oncogene 17: 2429–2435, 1998 [DOI] [PubMed] [Google Scholar]
- 61.Wang J, Duncan D, Shi Z, Zhang B. WEB-based GEne SeT AnaLysis Toolkit (WebGestalt): update 2013. Nucleic Acids Res 41: W77–W83, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang KC, Zhang L, McMillen IC, Botting KJ, Duffield JA, Zhang S, Suter CM, Brooks DA, Morrison JL. Fetal growth restriction and the programming of heart growth and cardiac insulin-like growth factor 2 expression in the lamb. J Physiol 589: 4709–4722, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Warde-Farley D, Donaldson SL, Comes O, Zuberi K, Badrawi R, Chao P, Franz M, Grouios C, Kazi F, Lopes CT, Maitland A, Mostafavi S, Montojo J, Shao Q, Wright G, Bader GD, Morris Q. The GeneMANIA prediction server: biological network integration for gene prioritization and predicting gene function. Nucleic Acids Res 38: W214–W220, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wood CE, Rabaglino MB, Chang EI, Denslow N, Keller-Wood M, Richards E. Genomics of the fetal hypothalamic cellular response to transient hypoxia: endocrine, immune, and metabolic responses. Physiol Genomics 45: 521–527, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yli BM, Kallen K, Khoury J, Stray-Pedersen B, Amer-Wahlin I. Intrapartum cardiotocography (CTG) and ST-analysis of labor in diabetic patients. J Perinat Med 39: 457–465, 2011 [DOI] [PubMed] [Google Scholar]
- 66.Yli BM, Kallen K, Stray-Pedersen B, Amer-Wahlin I. Intrapartum fetal ECG and diabetes. J Matern Fetal Neonatal Med 21: 231–238, 2008 [DOI] [PubMed] [Google Scholar]
- 67.Yoshioka J, Imahashi K, Gabel SA, Chutkow WA, Burds AA, Gannon J, Schulze PC, MacGillivray C, London RE, Murphy E, Lee RT. Targeted deletion of thioredoxin-interacting protein regulates cardiac dysfunction in response to pressure overload. Circ Res 101: 1328–1338, 2007 [DOI] [PubMed] [Google Scholar]
- 68.Yunis KA, Bitar FF, Hayek P, Mroueh SM, Mikati M. Transient hypertrophic cardiomyopathy in the newborn following multiple doses of antenatal corticosteroids. Am J Perinatol 16: 17–21, 1999 [DOI] [PubMed] [Google Scholar]
- 69.Zhang B, Kirov S, Snoddy J. WebGestalt: an integrated system for exploring gene sets in various biological contexts. Nucleic Acids Res 33: W741–W748, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang L, Jaswal JS, Ussher JR, Sankaralingam S, Wagg C, Zaugg M, Lopaschuk GD. Cardiac insulin-resistance and decreased mitochondrial energy production precede the development of systolic heart failure after pressure-overload hypertrophy. Circ Heart Fail 6: 1039–1048, 2013 [DOI] [PubMed] [Google Scholar]



