Abstract
The exercise pressor reflex is greater in rats with ligated femoral arteries than it is in rats with freely perfused femoral arteries. The exaggerated reflex in rats with ligated arteries is attenuated by stimulation of μ-opioid and δ-opioid receptors on the peripheral endings of thin-fiber muscle afferents. The effect of stimulation of κ-opioid receptors on the exercise pressor reflex is unknown. We tested the hypothesis that stimulation of κ-opioid receptors attenuates the exercise pressor reflex in rats with ligated, but not freely perfused, femoral arteries. The pressor responses to static contraction were compared before and after femoral arterial or intrathecal injection of the κ-opioid receptor agonist U62066 (1, 10, and 100 μg). Femoral arterial injection of U62066 did not attenuate the pressor responses to contraction in either group of rats. Likewise, intrathecal injection of U62066 did not attenuate the pressor response to contraction in rats with freely perfused femoral arteries. In contrast, intrathecal injection of 10 and 100 μg of U62066 attenuated the pressor response to contraction in rats with ligated femoral arteries, an effect that was blocked by prior intrathecal injection of the κ-opioid receptor antagonist nor-binaltorphimine. In rats with ligated femoral arteries, the pressor response to stimulation of peripheral chemoreceptors by sodium cyanide was not changed by intrathecal U62066 injections, indicating that these injections had no direct effect on the sympathetic outflow. We conclude that stimulation of spinal, but not peripheral, κ-opioid receptors attenuates the exaggerated exercise pressor reflex in rats with ligated femoral arteries.
Keywords: skeletal muscle afferents, exercise, peripheral artery disease, spiradoline
activation of the exercise pressor reflex is responsible, at least in part, for the cardiovascular and ventilatory adjustments to exercise (13). The afferent arm of the reflex comprises group III and IV muscle afferents, which respond predominantly to mechanical and metabolic stimuli, respectively (14, 19). Group III and IV afferents, which together are termed thin-fiber afferents, synapse onto interneurons in the dorsal horn of the spinal cord (10). These interneurons, in turn, project initially to neurons in the ventrolateral medulla and the nucleus of the solitary tract (9, 29), and eventually to autonomic preganglionic neurons. The functional consequences of activating the exercise pressor reflex during exercise include increases in arterial blood pressure, heart rate (HR), and ventilation, all of which contribute to the increases in exercising skeletal muscle blood flow and O2 delivery (1, 2, 27).
In cats, dogs, and rats, acute or chronic muscle ischemia exaggerates the exercise pressor reflex (8, 19, 27, 32, 36). Our laboratory recently reported, for example, that the reflex is larger in rats whose femoral arteries were ligated 72 h before the start of the experiment than it was in rats whose femoral arteries were freely perfused (36). When the femoral artery of rats is ligated, the collateral circulation provides sufficient blood flow to the hindlimb muscles when they are at rest, but does not provide sufficient flow when they are exercising (30, 40). This blood flow pattern to the hindlimb muscles during both rest and exercise closely approximates the blood flow patterns to resting and exercising leg muscles of patients with peripheral arterial disease (4). In addition, the exercise pressor reflex evoked by either static or dynamic exercise is exaggerated in patients with peripheral arterial disease compared with the reflex evoked in healthy control subjects (4–6, 22).
Stimulation of opioid receptors inhibits afferent nociceptive transmission. Specifically, stimulation of both spinal and peripheral μ-, δ-, and κ-opioid receptors has been shown to exert potent antinociceptive effects in a variety of pain models (15, 23, 24, 26). Our laboratory has demonstrated recently that stimulation of μ-opioid and δ-opioid receptors located on the peripheral endings of hindlimb muscle afferents attenuated the exaggerated exercise pressor reflex in rats with ligated femoral arteries (17, 37). The effects of κ-opioid receptor stimulation, however, on the exercise pressor reflex in this preparation are unknown.
On the basis of the findings described above, the present investigation was conducted to determine whether peripheral and spinal κ-opioid receptor stimulation would attenuate the exaggerated exercise pressor reflex seen in rats whose femoral arteries were ligated. Specifically, we tested the hypothesis that femoral arterial injection, as well as intrathecal injection of U62066, a selective κ-opioid receptor agonist, would attenuate the pressor responses to static contraction in decerebrate, unanesthetized rats, whose femoral arteries were ligated 72 h prior to the experiment. We also tested the hypothesis that femoral arterial or intrathecal injection of U62066 would not attenuate the pressor responses to static contraction in decerebrate, unanesthetized rats whose femoral arteries were freely perfused.
METHODS
All procedures and protocols described in the present investigation were reviewed and approved by the Institutional Animal Care and Use Committee of the Penn State College of Medicine. Adult male Sprague-Dawley rats (n = 73, body weight range 343–500 g) were used in this study. Rats were housed in a temperature-controlled environment (24 ± 1°C) on a 12:12-h light-dark cycle with food (standard rat chow) and tap water available ad libitum. In 39 rats, the left femoral artery was ligated 72 h prior to the beginning of the experiment. Specifically, rats were anesthetized with 4% isoflurane (balance O2), after which the left femoral artery was surgically exposed and ligated tightly (5–0 silk suture) just distal to the inguinal ligament. Three rats were subjected to a sham surgery, which consisted of exposing the femoral artery, and then passing a suture under the artery without tying it. Experiments described below were completed in rats whose left femoral artery was ligated 72 h before the experiment (“ligated”; n = 39), rats subjected to the sham surgery (“sham”; n = 3), or in rats who were not subjected to any surgery and thus had patent femoral arteries (“freely perfused”; n = 31).
Surgical Procedures
On the day of the experiment, all rats were anesthetized with 3–4% isoflurane (balance O2). The trachea was cannulated, and the lungs were mechanically ventilated (Harvard Apparatus) with the gaseous anesthetic until the decerebration procedure was completed. The right jugular vein and right carotid artery were cannulated with polyethylene (PE)-50 catheters to inject fluids and to measure arterial blood pressure (P23 XL, Statham), respectively. Heart rate was calculated beat to beat from the arterial pressure pulse with a Gould Biotach. The left carotid artery was cannulated with either: 1) a PE-10 catheter with the tip advanced to ∼3–4 mm above the bifurcation of the abdominal aorta (n = 26; see Femoral arterial injection), 2) a PE-50 catheter inserted retrogradely for measurement for arterial blood pressure (n = 39; see Intrathecal injection and Control experiments), or 3) a PE-50 catheter inserted antegradely with the tip advanced until ∼2 mm distal to the bifurcation of the common carotid artery (n = 8; see Control experiments). In the 26 rats whose left carotid artery was cannulated with PE-10, reversible snares (2–0 silk suture) were placed around the right iliac artery and vein and the abdominal aorta and vena cava (above the tip of the PE-10 catheter). For all rats, arterial blood gases and pH were measured periodically throughout the experiment with a blood gas analyzer (ABL 80 FLEX, Radiometer) and were maintained within normal limits (PaCO2: 35–45 mmHg, PaO2: ∼100 mmHg, pH: 7.35–7.45) by adjusting ventilation and/or administration of intravenous sodium bicarbonate (8.5%). Core temperature was measured by a rectal probe and maintained at ∼37–38°C by a heating lamp.
For all of the rats in the femoral arterial injection and intrathecal injection treatment groups (n = 57), a laminectomy was performed to expose the lower lumbar spinal cord from L2 to L5. For rats in the femoral arterial injection treatment group, a pool was formed using the skin on the back, which was filled with warmed mineral oil (37.5°C). The dura was cut from L2-L5 and reflected so that the L4 and L5 ventral spinal roots (which innervate the muscles of the hindlimb) could be isolated and then cut close to their exit from the spinal cord. For rats in the intrathecal injection treatment group, the dura was cut at L3-L4, and a saline-filled PE-10 catheter was inserted intrathecally with the tip pointing rostrally and secured at the L2 level with Kwik-Sil (World Precision Instruments). The left tibial nerve was then surgically exposed and isolated. For all rats in the femoral arterial injection and intrathecal injection treatment groups, the left calcaneal bone was severed and the triceps surae (gastrocnemius, soleus, and plantaris complex) muscles were exposed and isolated. The severed end of the calcaneal tendon was then linked by string to a force transducer (Grass Instruments, FT10) which, in turn, was attached to a rack-and-pinion.
All rats were placed in a Kopf customized stereotaxic frame and spinal unit with clamps placed around the pelvis and rostral lumbar vertebrae. Dexamethasone (0.2 mg iv) was injected to minimize brain stem edema. A precollicular decerebration procedure was performed, and all neural tissue rostral to the section was aspirated. Bleeding was controlled with small pieces of oxidized regenerated cellulose (Ethicon, Johnson and Johnson), and the cranial cavity was packed with cotton. Anesthesia was terminated, and the rats were ventilated with room air and given a minimum of 60 min to recover and stabilize prior to the initiation of any experimental protocol. All experiments were performed in decerebrated instead of anesthetized rats, given the evidence indicating that anesthesia prevents the exercise pressor reflex in this species (31).
Experimental Procedures
Femoral arterial injection.
The cut peripheral ends of the L4 and L5 ventral roots were placed on a shielded stimulating electrode. Baseline muscle tension was set at ∼100 g by manually turning the rack-and-pinion. The hindlimb muscles were statically contracted for 30 s by electrically stimulating the cut L4 and L5 ventral roots (40 Hz, 0.1-ms pulse duration, ∼2× motor threshold). Following recovery (∼5–10 min), we stimulated κ-opioid receptors by injecting 1, 10, or 100 μg of the selective κ-opioid receptor agonist U62066 {(±)-[5α, 7α, 8β]-3,4-Dichloro-N-methyl-N-[7-(1-pyrrolidinyl)-1-oxaspiro[4.5]dec-8-yl]benzeneacetamide mesylate salt; spiradoline; Sigma-Aldrich} into the arterial supply of the left hindlimb via the left carotid artery catheter (see above). Before injecting U62066, we tightened the reversible snares around the right iliac artery and vein and the abdominal aorta and vena cava, a maneuver that both directed the injectate toward and partially trapped the injectate within the left hindlimb circulation. The snares were released 5 min following the injection, and the hindlimb was allowed to reperfuse for 10 min. The static contraction protocol was then repeated as described above. Initially, rats were injected with 1 μg U62066. If the preparation were stable (e.g., if bleeding were controlled and arterial blood gasses and pH were within normal limits), serial injection of 10- and 100-μg doses of U62066 were used. In later experiments, some rats received 10 and/or 100 μg of U62066 only. The volume and vehicle for injection for all U62066 doses were 100 μl of saline. In five freely perfused and five ligated rats that were injected with 100 μg U62066, we subsequently injected 1 μg (in 100 μl of saline) of the selective δ-opioid agonist DPDPE ([D-Pen2,5]enkephalin, [D-Pen2, D-Pen5]enkephalin; Tocris). Our laboratory has reported recently that this DPDPE dose attenuated the exercise pressor reflex in both freely perfused and ligated rats (17). Thus, injection of DPDPE served as a positive control confirming that U62066 injected into the femoral artery had access to the endings of the thin-fiber afferents innervating the hindlimb muscles. We also injected Evans blue dye (100 μl) in the same manner as that used for the drug injections. In all rats, the left triceps surae muscles were stained blue, a finding that indicated that U62066 injected into the femoral artery had access to the left hindlimb circulation in both groups of rats. Ventral root stimulation was used to initiate contraction in the femoral arterial injection experiments to replicate the experimental protocols used in the previous studies from our laboratory, which investigated the effects of peripheral μ- and δ-opioid receptor stimulation on the exercise pressor reflex (17, 37).
In five additional ligated rats, we investigated the effects of 100 μg of U62066 (in 100 μl of saline) injected into the jugular vein on the pressor and cardioaccelerator responses to the static contraction. Blood flow to and from the left hindlimb circulation was likely substantially reduced, but not abolished, when the abdominal and right leg snares were tightened. These experiments were performed, therefore, to determine whether systemic circulation of U62066 could explain the slightly, but not significantly, lower peak pressor responses to static contraction that we observed in ligated rats following femoral arterial injection of 100 μg U62066 (see results).
Intrathecal injection.
The need to maintain an intact dura prevented us from evoking contraction by ventral root stimulation. In the following experiments, therefore, we used tibial nerve stimulation to evoke contraction. Rats were tilted head-up at a 15° angle, which was maintained throughout the experiment to prevent the rostral migration of U62066 injected intrathecally. The left tibial nerve was placed on a shielded stimulating electrode. Baseline muscle tension was set at ∼100 g. After a minimum 30-s baseline period, we statically contracted the triceps surae muscle for 30 s by electrically stimulating the tibial nerve (40 Hz, 0.01-ms pulse duration, ∼2 × motor threshold). Following recovery (∼5–10 min), we stimulated κ-opioid receptors by injecting 1, 10, or 100 μg of U62066 intrathecally. After 10 min, we repeated the static contraction protocol. Initially, rats were injected with 1 μg of U62066. If no attenuation of the pressor response was observed and the preparation was stable, serial drug injection and contraction protocols were performed with 10 and/or 100 μg of U62066. In later experiments, some rats were injected only with the 10 and/or 100 μg of U62066. All intrathecal U62066 doses were dissolved in 10 μl of saline and flushed with 60 μl of saline, the latter being the dead space of the catheter. Subsequent to drug injections and contractions, all rats were paralyzed with pancuronium bromide (0.5 mg/kg iv), and the tibial nerve was stimulated to ensure that the pressor responses observed during contraction were not the result of electrical activation of the axons of thin-fiber afferents in the tibial nerve. In each of the rats used in this protocol, 10 μl of blue dye (followed by a 60-μl flush) was injected intrathecally. Blue dye never reached the medulla in any of the rats examined.
Control Experiments
To investigate the possibility that pain or discomfort from the surgical procedure, rather than femoral artery ligation per se, could account for either the exaggerated exercise pressor reflex or the fact that 10 and 100 μg of U62066 injected intrathecally attenuated the reflex in ligated rats, we measured the pressor and cardioaccelerator responses to contraction in sham rats (n = 3). In the sham experiments, static contraction and intrathecal injections of 10 and 100 μg of U62066 were performed exactly as described above for the intrathecal injection protocol.
In five ligated rats, we attempted to block the attenuating effects of intrathecal injection of 10 and 100 μg of U62066 that was observed in the intrathecal injection protocol (see results). Blockade was achieved by prior intrathecal injection of the selective κ-opioid receptor antagonist nor-binaltorphimine [nor-BNI; Sigma-Aldrich (28)]. Nor-BNI's peak antagonistic effect on κ-opioid receptors occurs ∼2 h following administration. This prompted us to wait about 75 min before the first contraction was initiated. We injected 1 μg of nor-BNI intrathecally (in 10 μl of saline followed by 60-μl saline flush) immediately following placement of the intrathecal catheter, which was prior to the decerebration procedure and the 60-min postanesthesia recovery period. Static contraction of the hindlimb muscles was evoked as described in the intrathecal injection protocol, and the pressor and cardioaccelerator responses were compared among the nor-BNI, 10 μg, and 100 μg U62066 conditions. In preliminary experiments (n = 2), intrathecal injection of 1 μg nor-BNI only 10 min prior to intrathecal U62066 injections and subsequent contractions did not block the attenuating effects of either 10 or 100 μg of U62066.
To directly investigate the possibility that intrathecal injections of either 10 or 100 μg of U62066 acted within the brain stem, we activated the peripheral chemoreceptors by intracarotid artery injection of sodium cyanide (25 μg/kg). Specifically, in eight ligated rats whose left carotid artery was cannulated (PE-50) antegradely, we compared the pressor responses to sodium cyanide before and after intrathecal injections of l0 and 100 μg of U62066.
Data Analysis
In all experiments, mean arterial blood pressure (MAP; in mmHg), HR (in bpm), and muscle tension were displayed continuously in real-time with a Spike 2 data acquisition system (Cambridge Electronic Design). Data were recorded and stored on a computer hard drive (Dell) for future off-line analysis. Baseline MAP and HR values were determined from the 30-s baseline period that preceded contraction. The pressor (increases in MAP) and cardioaccelerator (increases in HR) responses to static contraction and sodium cyanide injection were calculated as the difference between the peak MAP and HR values obtained during contraction and the baseline values. The tension-time index (TTI; in kg/s) for static contraction was calculated by subtracting the area under the tension trace for the 30-s baseline period from the area under the tension trace for the 30-s contraction period (contraction-baseline).
All data are expressed as means ± SE. Statistical comparisons were performed with either repeated-measures or nonrepeated-measures ANOVAs as appropriate. When indicated by the ANOVA result, individual means were compared with Holm-Sidak post hoc tests. Significance was accepted at P < 0.05.
RESULTS
Femoral Arterial Injection
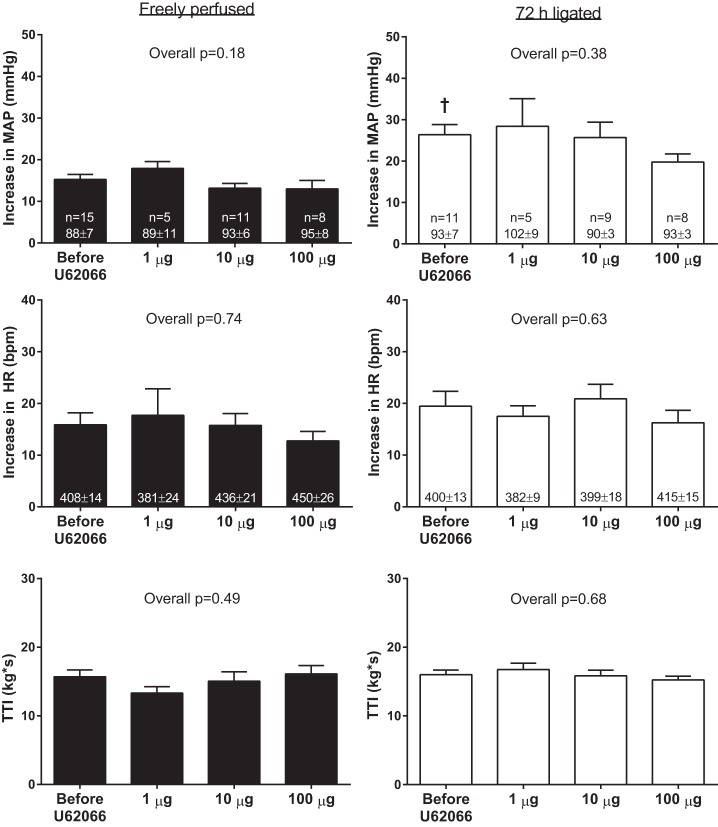
Consistent with our previous report (37), the peak pressor response to static contraction evoked by L4 and L5 ventral root stimulation was greater in ligated rats (26 ± 2 mmHg) than it was in freely perfused rats (16 ± 1 mmHg; P < 0.01). Femoral arterial injection of 1, 10, and 100 μg of U62066 had no effect on baseline MAP or HR in either freely perfused or ligated rats. Most importantly, there were no effects of any dose of U62066, injected into the femoral artery, on the pressor or cardioaccelerator responses to static contraction in either freely perfused or ligated rats (Fig. 1). There were no differences between the TTIs among conditions (Fig. 1). Average peak tension development was ∼600–700 g, and it did not differ among conditions in either freely perfused (overall P = 0.87) or ligated (overall P = 0.87) rats.
Fig. 1.
Effects of femoral arterial injection of 1, 10, and 100 μg of the κ-opioid receptor agonist U62066 on the pressor and cardioaccelerator responses to static contraction in freely perfused and ligated rats. Sample sizes and baseline values are indicated within mean bars for their corresponding conditions. MAP, mean arterial pressure; HR, heart rate; TTI, tension-time index. Data are expressed as means ± SE. †P < 0.05 vs. before U62066 in freely perfused rats.
In subsets of freely perfused and ligated rats, which received femoral arterial injections of 100 μg of U62066, 1 μg of the δ-opioid agonist DPDPE injected in the same manner as U62066 significantly attenuated the pressor responses to static contraction (Fig. 2). Femoral arterial injection of 1 μg DPDPE also attenuated the cardioaccelerator responses to static contraction in ligated rats but not in freely perfused rats. The TTIs were not different between the 100 μg U62066 and 1 μg DPDPE condition (Fig. 2). These findings are similar to a recent investigation from our laboratory (17) and demonstrate that U62066 injected into the femoral artery had access to the entire hindlimb circulation in both groups of rats.
Fig. 2.
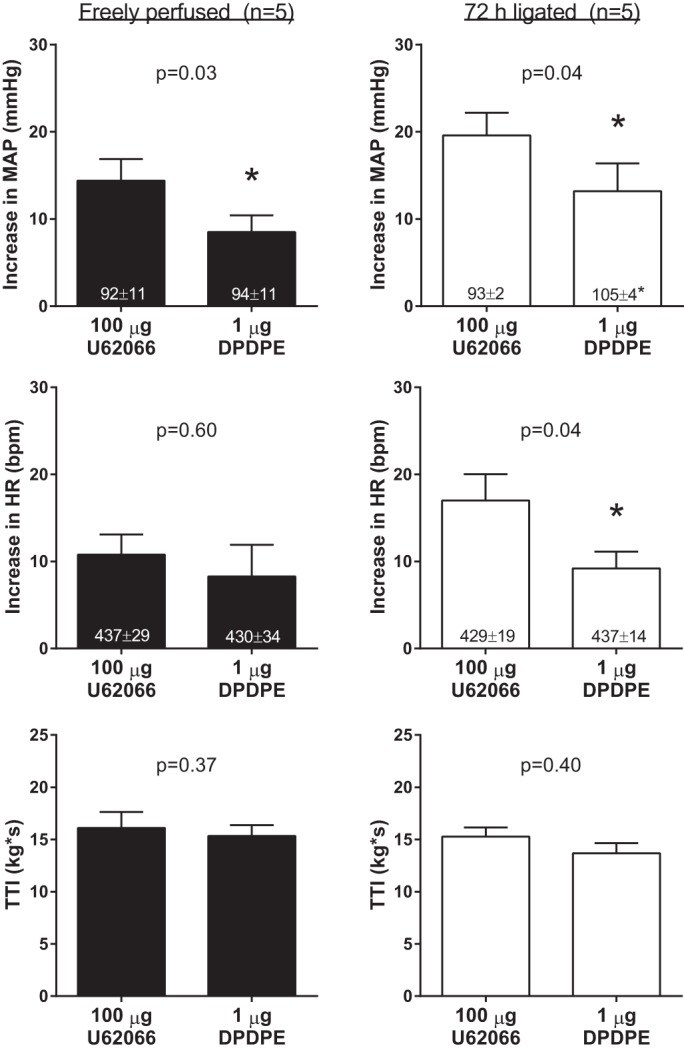
Effects of femoral arterial injection of 1 μg of the δ-opioid receptor agonist [D-Pen2,5]enkephalin, [D-Pen2, D-Pen5]enkephalin (DPDPE) on the pressor and cardioaccelerator responses to static contraction in five freely perfused and five ligated rats. Experiments were performed in subsets of freely perfused and ligated rats that received femoral arterial injection of 100 μg of the κ-opioid receptor agonist U62066. Baseline values are indicated within mean bars for their corresponding conditions. Data are expressed as means ± SE. *P < 0.05 vs. 100 μg U62066.
To further investigate the slight attenuation of the pressor response to static contraction caused by femoral arterial injection of 100 μg of U62066 found in our experiments (see Fig. 1), we injected 100 μg of the agonist into the jugular vein in five additional ligated rats. We found that 100 μg of U62066 injected into the jugular vein markedly and significantly attenuated the pressor and cardioaccelerator responses to static contraction (Fig. 3). The TTIs were not different before and after 100 μg of U62066 (Fig. 3). This suggested to us that the slightly lower pressor response observed following femoral arterial injection of 100 μg U62066 in ligated rats was likely explained by the κ-opioid agonist circulating to the spinal cord.
Fig. 3.
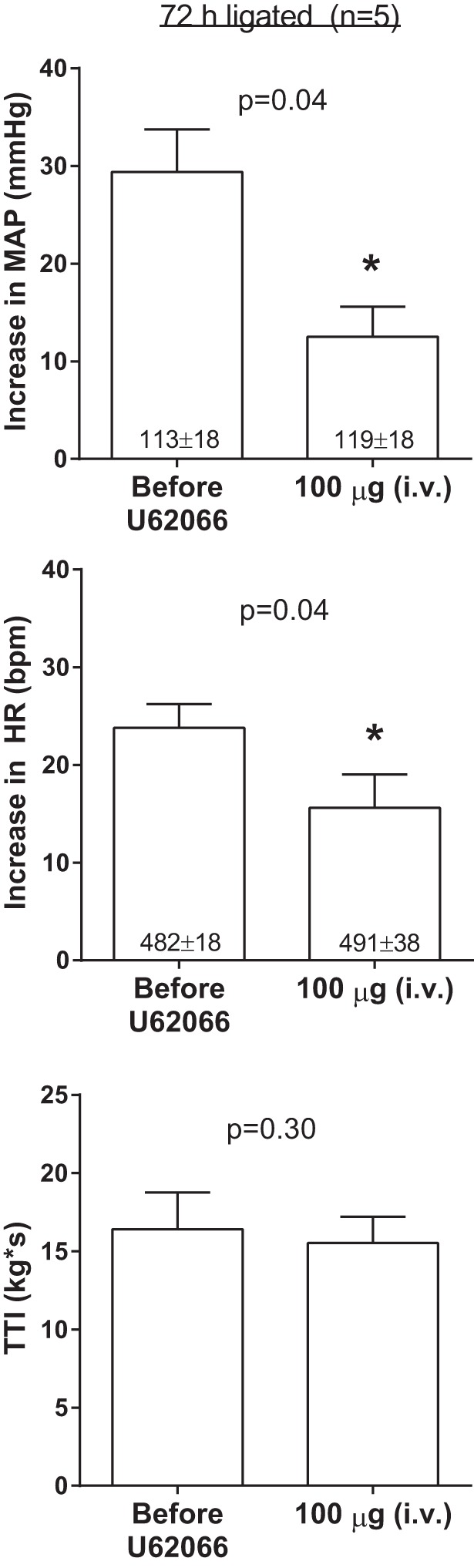
Effects of intravenous (jugular vein) injections of 100 μg of the κ-opioid receptor agonist U62066 on the pressor and cardioaccelerator responses to static contraction in five ligated rats. Baseline values are indicated within mean bars for their corresponding conditions. Data are expressed as means ± SE. *P < 0.05 vs. before U62066.
Intrathecal Injection
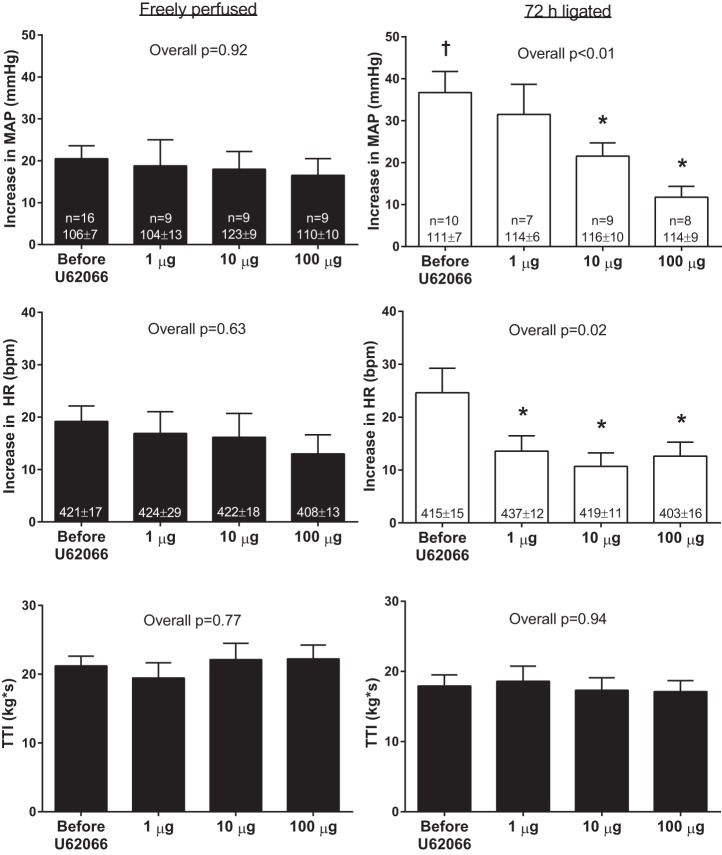
Similar to our finding in the femoral arterial injection protocol, the pressor response to static contraction was greater in ligated rats (37 ± 5 mmHg) than it was in freely perfused rats (20 ± 3 mmHg, P < 0.01). Intrathecal injection of 1, 10, and 100 μg of U62066 had no effect on baseline MAP or HR in either freely perfused or ligated rats. Likewise, intrathecal injection of 1, 10, and 100 μg of U62066 had no effect on the pressor and cardioaccelerator responses to static contraction in freely perfused rats (Fig. 4, left). Intrathecal injection of 10 and 100 μg, but not 1 μg, of U62066 significantly attenuated the pressor response to contraction in ligated rats (Fig. 4, right; see original tracings in Fig. 5). The difference in the pressor responses between the 10 and 100 μg U62066 conditions did not reach statistical significance (P = 0.27). The cardioaccelerator responses to contraction were significantly attenuated by each dose of U62066 in the ligated rats (Fig. 4, right). There were no differences in TTIs among conditions (Fig. 4). Average peak tension development was ∼700–1,000 g, and it did not differ among conditions in either freely perfused (overall P = 0.61) or ligated (overall P = 0.96) rats. Neuromuscular blockade via pancuronium bromide injection abolished the pressor responses to tibial nerve stimulation in both freely perfused (1 ± 1 mmHg, P = 0.18) and ligated (1 ± 1 mmHg, P = 0.07) rats, findings that indicated that responses evoked by contraction were not caused by electrical activation of the axons of thin-fiber afferents.
Fig. 4.
Effects of intrathecal injection of 1, 10, and 100 μg of the κ-opioid receptor agonist U62066 on the pressor and cardioaccelerator responses to static contraction in freely perfused and ligated rats. Sample sizes and baseline values are indicated within means for their corresponding conditions. Data are expressed as means ± SE. †P < 0.05 vs. before U62066 in freely perfused rats, *P < 0.05 vs. before U62066 within ligated rats.
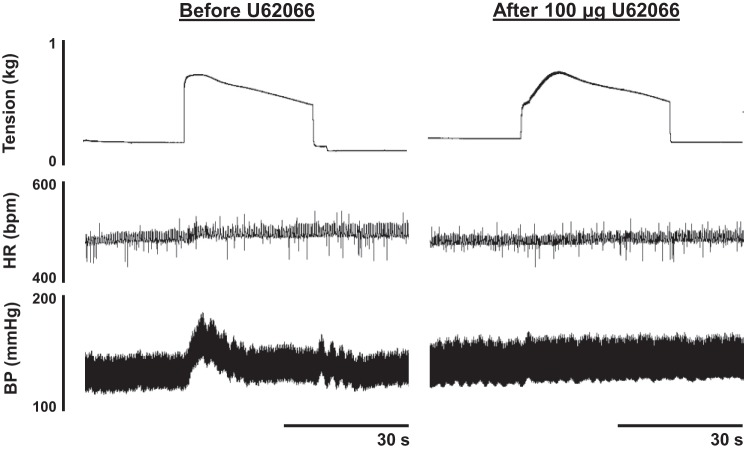
Fig. 5.
An example of the pressor and cardioaccelerator responses to static contraction from a ligated rat before U62066 and after intrathecal injection of 100 μg of U62066. Note the marked attenuation of the pressor and cardioaccelerator responses to contraction following intrathecal U62066 injection. BP; blood pressure.
Control Experiments
The pressor and cardioaccelerator responses to static contraction in three sham rats before U62066 injection were 20 ± 3 mmHg and 9 ± 2 bpm, respectively. These responses were not different from those found in the freely perfused rats (see Fig. 4; pressor response: P = 0.93, cardioaccelerator response: P = 0.17). Likewise, the pressor and cardioaccelerator responses to contraction in sham rats were not attenuated following intrathecal injection of 10 (pressor: 20 ± 3 mmHg, cardioaccelerator: 9 ± 3) or 100 (pressor: 20 ± 3 mmHg, cardioaccelerator: 7 ± 3) μg of U62066. Pancuronium bromide injection abolished the pressor (1 ± 1 mmHg; P = 0.37) and cardioaccelerator (1 ± 1 bpm; P = 0.42) responses to tibial nerve stimulation in the sham rats.
When compared with the responses found for ligated rats before U62066 from the intrathecal injection protocol (see Fig. 4), intrathecal injection of 1 μg of the κ-opioid receptor antagonist nor-BNI had no effect on the pressor (before U62066: 37 ± 5 mmHg; nor-BNI: 32 ± 8 mmHg, P = 0.59) or cardioaccelerator (before U62066: 25 ± 5 bpm, nor-BNI: 14 ± 3 bpm, P = 0.59) responses to static contraction. This indicates that endogenous spinal κ-opioid receptor stimulation did not modulate the exercise pressor reflex in ligated rats. Nevertheless, nor-BNI, injected intrathecally ∼75 min before contraction, prevented the attenuating effects of intrathecal injection of 10 and 100 μg of U62066 on the pressor and cardioaccelerator responses to contraction (Fig. 6). This finding confirms that the attenuating effects of intrathecal U62066 injections were mediated via κ-opioid receptors. There were no differences in the peak tensions or TTIs among conditions. Pancuronium bromide injection abolished the pressor (1 ± 1 mmHg; P = 0.98) and cardioaccelerator (1 ± 1 bpm; P = 0.98) responses to tibial nerve stimulation in the rats injected with nor-BNI.
Fig. 6.
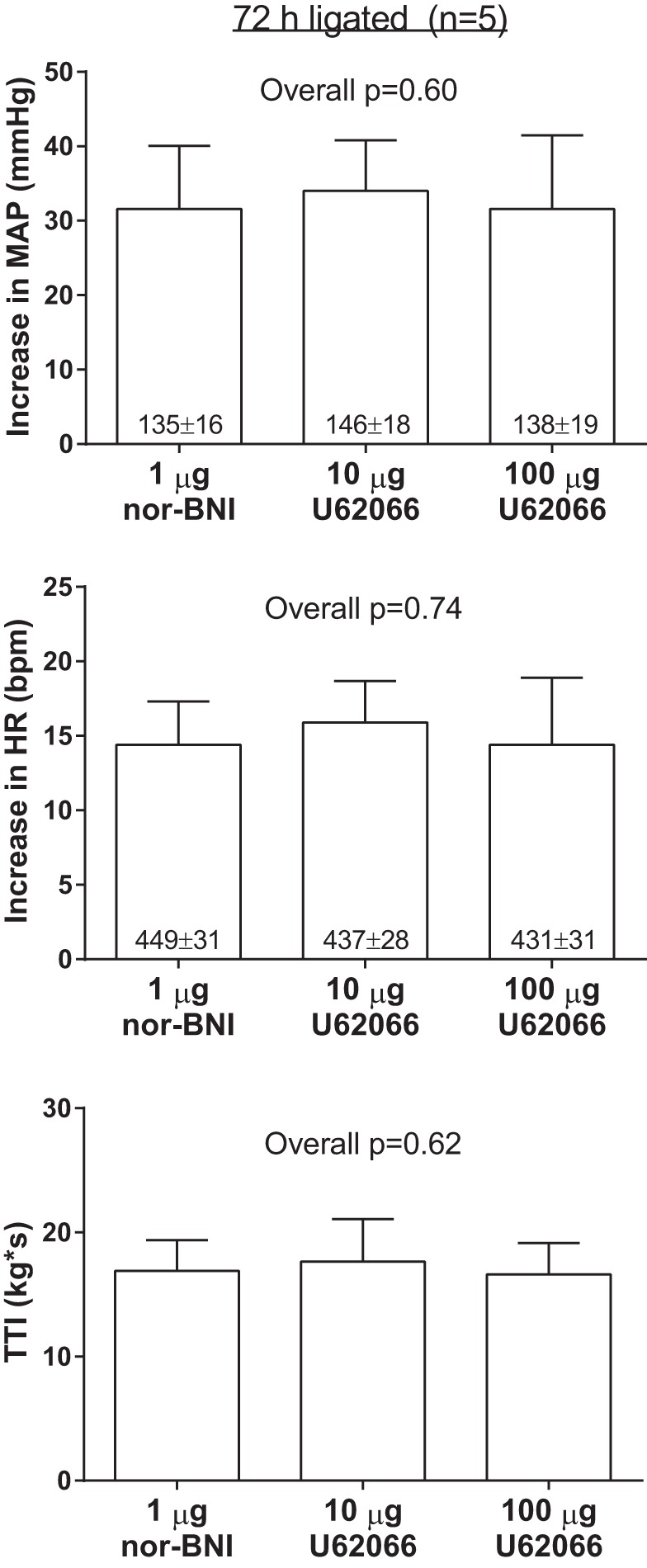
Effects of intrathecal injection of 10 and 100 μg of the κ-opioid receptor agonist U62066 on the pressor and cardioaccelerator responses to static contraction in ligated rats in the presence of prior intrathecal injection of 1 μg of the κ-opioid receptor antagonist nor-binaltorphimine (nor-BNI). Baseline values are indicated within mean bars for their corresponding conditions. Data are expressed as means ± SE.
Intrathecal injection of either 10 or 100 μg of U62066 had no effect on the pressor and cardiodecelerator responses to intracarotid artery injection of sodium cyanide (25 μg/kg) in ligated rats (Fig. 7). This finding indicates that the attenuations of the pressor responses to contraction observed following intrathecal injections of 10 and 100 μg of U62066 in ligated rats were mediated via local spinal κ-opioid receptor stimulation and were not the result of the rostral migration of the drug to have direct inhibitory effects on either thoracico-lumbar or medullary neurons controlling the sympathetic outflow.
Fig. 7.
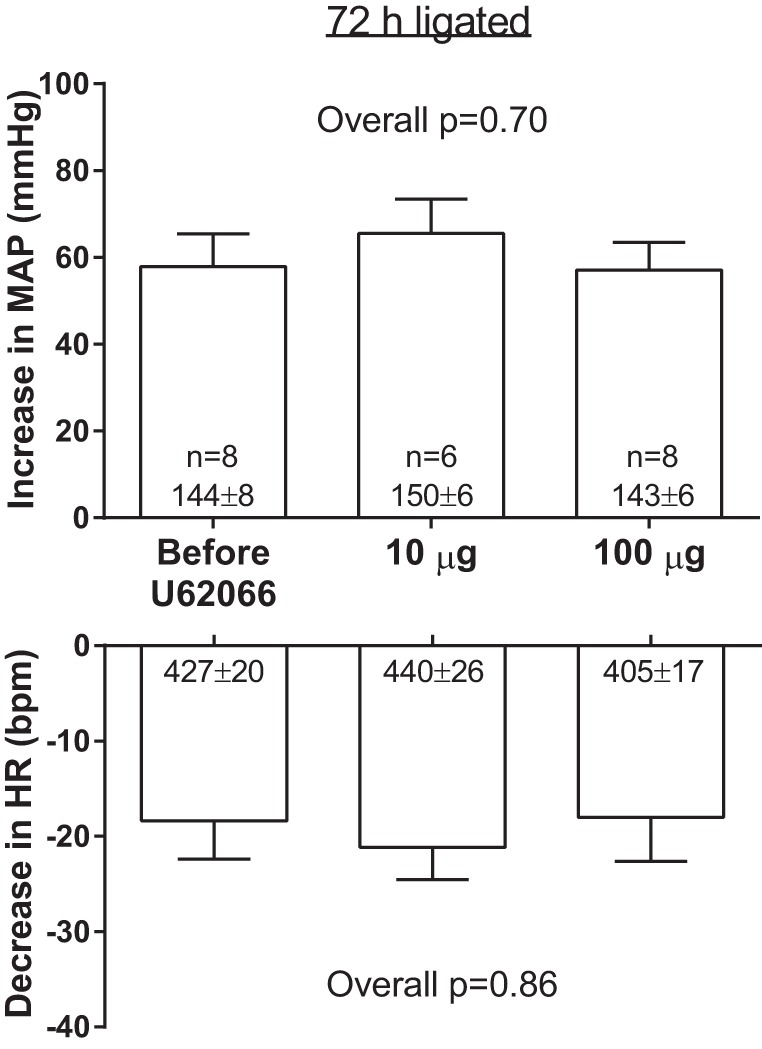
Effects of intrathecal injection of 10 and 100 μg of the κ-opioid receptor agonist U62066 on the peak pressor responses and reductions in HR following sodium cyanide injection. Sample sizes and baseline values are indicated within mean bars for their corresponding conditions. Data are expressed as means ± SE.
DISCUSSION
We found that intrathecal, but not femoral arterial, injection of the selective κ-opioid receptor agonist U62066 attenuated the exaggerated exercise pressor reflex in rats whose femoral arteries were ligated 72 h before the start of the experiment. In contrast, neither intrathecal nor femoral arterial injection of U62066 had any effect on the exercise pressor reflex in rats whose femoral arteries were freely perfused. We also found that intrathecal injections of U62066 had no effect on the exercise pressor reflex in sham rats. Intrathecal injection of the selective κ-opioid receptor antagonist nor-BNI had no effect on the exaggerated exercise pressor reflex in rats with ligated femoral arteries. Nor-BNI did, however, block the attenuating effects of intrathecal injection of U62066 in ligated rats. Intrathecal injections of U62066 did not attenuate the pressor responses to the cyanide-induced stimulation of arterial chemoreceptors, a finding that supports the notion that the attenuation of the exercise pressor reflex by intrathecal U62066 was not attributable to its migration to either the spinal sympathetic outflow or to the medulla. Considered together, our findings demonstrate that stimulation of spinal, but not peripheral, κ-opioid receptors, attenuated the exaggerated exercise pressor reflex in rats whose femoral arteries were ligated 72 h before the start of our experiments.
Our laboratory has paid particular attention to the role played by opioid receptors on the peripheral endings of thin-fiber afferents in attenuating the exercise pressor reflex. Previously, we have shown that stimulation of μ-opioid receptors on the endings of group IV afferents attenuated the reflex in rats with ligated femoral arteries, but had no effect on the reflex in rats with freely perfused arteries (37). We have also shown that stimulation of δ-opioid receptors on the endings of group III afferents attenuated the reflex in both rats with ligated femoral arteries and those with freely perfused femoral arteries (17). In contrast, we now show that stimulation of κ-opioid receptors on the peripheral endings of group III and IV afferents had no effect on the exercise pressor reflex in both rats with ligated femoral arteries, as well as in rats with freely perfused arteries. The stimulus applied to the κ-opioid receptors in our experiments was 100-fold greater than that used in our previous experiments, in which we stimulated μ-opioid and δ-opioid receptors in the periphery. Consequently, we think it is unlikely that 100 μg of U62066, injected into the femoral artery, was below threshold when we were attempting to stimulate κ-opioid receptors on the peripheral endings of group III and IV afferents.
Our present finding that intra-arterial hindlimb injection of a κ-opioid receptor agonist did not attenuate the exercise pressor reflex in freely perfused or ligated rats raises the possibility that κ-opioid receptors are not found on the group III and IV muscle afferents. We are not aware of any studies investigating the presence of κ-opioid receptors on the group III and IV afferents involved in the exercise pressor reflex specifically. Our original hypothesis that intra-arterial U62066 injection would attenuate the exercise pressor reflex in ligated rats was based on studies that have reported antinociceptive effects of peripherally administered κ-opioid receptor agonists (3, 21, 39). We should point out, however, that the antinociceptive effects of peripherally administered κ-opioid receptor agonists are controversial. For example, some studies reported that peripherally administered κ-opioid receptor agonists were antinociceptive, an effect that was prevented by prior administration of a κ-opioid receptor antagonist (3, 39). In contrast, another study reported that peripheral administration of κ-opioid receptor agonists had no effect on nociception (38). Still another study reported that peripherally administered κ-opioid receptor agonists were antinociceptive, but that the effect was not blocked by κ-opioid receptor antagonists (35). The resolution to these different findings may lie in the modality, location, and intensity of the noxious stimulus applied to the periphery (16, 21, 25).
Our finding that spinal κ-opioid receptor stimulation attenuated the exercise pressor reflex in rats with ligated femoral arteries raises the issue of whether the κ-opioid receptors stimulated by U62066 were located on the central endings of group III and IV afferents or were located postsynaptically on interneurons in the dorsal horn of the spinal cord. We can only speculate on this issue, but the fact that we could not attenuate the exercise pressor reflex with high doses of U62066 injected into the femoral artery suggests to us that the attenuation of the reflex by intrathecal injection of this κ-opioid agonist was attributable to the inhibition of interneurons in the dorsal horn receiving input from group III and IV afferents. It seems reasonable to suggest that if femoral artery ligation had increased the number of κ-opioid receptors on the central endings of group III and IV afferents, then this increase would have also been expressed on the peripheral endings of these afferents as well. We would be surprised to find that the protein comprising the κ-opioid receptor would be transported from the cell body in only one direction, namely toward the spinal cord.
The effects of κ-opioid receptor agonists on the discharge of dorsal horn neurons have been investigated. For the most part, the findings point to an inhibitory effect of κ-opioid receptor agonists on the responses of neurons located in the deep dorsal horn to a mechanical stimulus applied to an inflamed ankle. Specifically, iontophoretic application of a κ-opioid receptor agonist decreased the responses to pressure applied to an inflamed ankle in 7 of 15 of the dorsal horn neurons tested, increased the responses to pressure in 4 of 15 of the neurons tested, and had no effect on the responses in the remaining four neurons (34). In contrast, superfusion of the spinal cord with κ-opioid receptor agonists has been reported to increase the responses of neurons in the superficial dorsal horn of rats to mechanical stimuli, as well as to increase the size of their receptive fields, effects were thought to be hyperalgesic (12). These somewhat contradictory findings may be explained by differences between the location of the neurons tested, as well as by differences between the methods of applying the κ-opioid agonists. Nevertheless, further clarification and investigation are needed.
Dynorphin A and B are the endogenous ligands for κ-opioid receptors. Although dynorphin can be found in the rat dorsal root ganglia, the amount as assessed by radioimmunoassay per gram of tissue is much less than that found in the dorsal horn (7). In addition, the number of dynorphin cell bodies in laminas I, II, and V of the rat dorsal horn has been reported to be increased by inflammatory stimuli (11, 33). The effect of femoral artery ligation on the number of dynorphin-containing cells in the rat dorsal horn remains to be determined. Nevertheless, the location of dynorphin-containing interneurons in the dorsal horn, namely, laminae I, II, and V, is the same as that of the central terminals of group III and IV muscle afferents (10, 18, 20). This correspondence, in turn, raises the possibility that these dynorphin-containing interneurons could have an inhibitory effect on input arising from the group III and IV afferents stimulated by exercise. Our data, however, do not support this possibility because nor-BNI, a selective κ-opioid receptor antagonist injected intrathecally, did not increase the exercise pressor reflex, a finding that would have been expected if endogenous κ-opioid receptors were stimulated when the hindlimb muscles of ligated rats were contracted.
In conclusion, we found that stimulation of spinal, but not peripheral, κ-opioid receptors attenuated the exaggerated exercise pressor reflex to static contraction in decerebrate, unanesthetized rats whose femoral arteries were ligated 72 h before the experiment. Given that femoral artery ligation in the rat does not impact blood flow at rest but does reduce blood flow capacity during exercise to 10–20% of normal (30, 40), our preparation offers useful parallels to the blood flow patterns to skeletal muscle in patients with peripheral arterial disease. In addition, our findings add to previous reports from our laboratory demonstrating that peripheral μ-opioid and δ-opioid receptor stimulation in rats with ligated femoral arteries (17, 37) attenuated the exercise pressor reflex. Specifically, stimulation of peripheral μ-opioid and δ-opioid receptors, but not peripheral κ-opioid receptors, may prove useful in the treatment of both claudication and the exaggerated pressor responses to exercise in patients with peripheral arterial disease. Although the present investigation suggests that spinal κ-opioid receptor stimulation may also relieve both claudication and the exaggerated pressor responses to exercise in these patients, our finding that U62066 has only a central action may preclude its usefulness.
GRANTS
This work was supported by National Institutes of Health Grants HL-096570 and AR-059397.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: S.W.C. and M.P.K. conception and design of research; S.W.C. performed experiments; S.W.C. analyzed data; S.W.C., A.J.S., K.Y., and M.P.K. interpreted results of experiments; S.W.C. prepared figures; S.W.C. and M.P.K. drafted manuscript; S.W.C., A.J.S., K.Y., and M.P.K. edited and revised manuscript; S.W.C., A.J.S., K.Y., and M.P.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We would like to thank Ms. Joyce Kim for excellent technical assistance.
REFERENCES
- 1.Amann M, Blain GM, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. Group III and IV muscle afferents contribute to ventilatory and cardiovascular response to rhythmic exercise in humans. J Appl Physiol 109: 966–976, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amann M, Runnels S, Morgan DE, Trinity JD, Fjeldstad AS, Wray DW, Reese VR, Richardson RS. On the contribution of group III and IV muscle afferents to the circulatory response to rhythmic exercise in humans. J Physiol 589: 3855–3866, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Auh QS, Ro JY. Effects of peripheral κ-opioid receptor activation on inflammatory mechanical hyperalgesia in male and female rats. Neurosci Lett 524: 111–115, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baccelli G, Reggiani P, Mattioli A, Corbellini E, Garducci S, Catalano M. The exercise pressor reflex and changes in radial arterial pressure and heart rate during walking in patients with arteriosclerosis obliterans. Angiology 50: 361–374, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Bakke EF, Hisdal J, Jorgensen JJ, Kroese A, Stranden E. Blood pressure in patients with intermittent claudication increases continuously during walking. Eur J Vasc Endovasc Surg 33: 20–25, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Bakke EF, Hisdal J, Kroese AJ, Jorgensen JJ, Stranden E. Blood pressure response to isometric exercise in patients with peripheral atherosclerotic disease. Clin Physiol Funct Imag 27: 109–115, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Botticelli LJ, Cox BM, Goldstein A. Immunoreactive dynorphin in mammalian spinal cord and dorsal root ganglia. Proc Natl Acad Sci USA 78: 7783–7786, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coote JH, Hilton SM, Perez-Gonzalez JF. The reflex nature of the pressor response to muscular exercise. J Physiol 215: 789–804, 1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Craig AD. Distribution of brainstem projections from spinal lamina I neurons in the cat and the monkey. J Comp Neurol 361: 225–248, 1995 [DOI] [PubMed] [Google Scholar]
- 10.Craig AD, Mense S. The distribution of afferent fibers from the gastrocnemius-soleus muscle in the dorsal horn of the cat as revealed by the transport of horseradish peroxidase. Neurosci Lett 41: 233–238, 1983 [DOI] [PubMed] [Google Scholar]
- 11.Hollt V, Haarmann I, Millan MJ, Herz A. Prodynorphin gene expression is enhanced in the spinal cord of chronic arthritic rats. Neurosci Lett 73: 90–94, 1987 [DOI] [PubMed] [Google Scholar]
- 12.Hylden JL, Nahin RL, Traub RJ, Dubner R. Effects of spinal κ-opioid receptor agonists on the responsiveness of nociceptive superficial dorsal horn neurons. Pain 44: 187–193, 1991 [DOI] [PubMed] [Google Scholar]
- 13.Kaufman MP, Forster HV. Reflexes controlling circulatory, ventilatory and airway responses to exercise. In: Handbook of Physiology. Exercise: Regulation and Integration of Multiple Systems II Control of Respiratory and Cardiovascular Systems, edited by Rowell LB, Shepherd JT. Bethesda, MD: Am. Physiol. Soc., 1996, sect 12, chapt. 10, p. 381–447 [Google Scholar]
- 14.Kaufman MP, Longhurst JC, Rybicki KJ, Wallach JH, Mitchell JH. Effects of static muscular contraction on impulse activity of groups III and IV afferents in cats. J Appl Physiol 55: 105–112, 1983 [DOI] [PubMed] [Google Scholar]
- 15.Kim HJ, Seol TK, Lee HJ, Yaksh TL, Jun JH. The effect of intrathecal μ, δ, κ, and α-2 agonists on thermal hyperalgesia induced by mild burn on hind paw in rats. J Anesth 25: 884–891, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Kivell B, Prisinzano TE. Kappa-opioids and the modulation of pain. Psychopharmacology (Berl) 210: 109–119, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Leal AK, Yamauchi K, Kim J, Ruiz-Velasco V, Kaufman MP. Peripheral δ-opioid receptors attenuate the exercise pressor reflex. Am J Physiol Heart Circ Physiol 305: H1246–H1255, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Light AR, Perl ER. Spinal termination of functionally identified primary afferent neurons with slowly conducting myelinated fibers. J Comp Neurol 186: 133–150, 1979 [DOI] [PubMed] [Google Scholar]
- 19.McCloskey DI, Mitchell JH. Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol 224: 173–186, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mense S, Craig ADIII. Spinal and supraspinal terminations of primary afferent fibers from the gastrocnemius-soleus muscle in the cat. Neuroscience 26: 1023–1035, 1988 [DOI] [PubMed] [Google Scholar]
- 21.Millan MJ. Kappa-opioid receptors and analgesia. Trends Pharmacol Sci 11: 70–76, 1990 [DOI] [PubMed] [Google Scholar]
- 22.Muller MD, Drew RC, Blaha CA, Mast JL, Cui J, Reed AB, Sinoway LI. Oxidative stress contributes to the augmented exercise pressor reflex in peripheral arterial disease patients. J Physiol 590: 6237–6246, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagasaka H, Awad H, Yaksh TL. Peripheral and spinal actions of opioids in the blockade of the autonomic response evoked by compression of the inflamed knee joint. Anesthesiology 85: 808–816, 1996 [DOI] [PubMed] [Google Scholar]
- 24.Nagasaka H, Yaksh TL. Effects of intrathecal μ, δ, and κ agonists on thermally evoked cardiovascular and nociceptive reflexes in halothane-anesthetized rats. Anesth Analg 80: 437–443, 1995 [DOI] [PubMed] [Google Scholar]
- 25.Negus SS, O'Connell R, Morrissey E, Cheng K, Rice KC. Effects of peripherally restricted κ opioid receptor agonists on pain-related stimulation and depression of behavior in rats. J Pharmacol Exp Ther 340: 501–509, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Normandin A, Luccarini P, Molat JL, Gendron L, Dallel R. Spinal μ and δ opioids inhibit both thermal and mechanical pain in rats. J Neurosci 33: 11703–11714, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O'Leary DS, Augustyniak RA, Ansorge EJ, Collins HL. Muscle metaboreflex improves O2 delivery to ischemic active skeletal muscle. Am J Physiol Heart Circ Physiol 276: H1399–H1403, 1999 [DOI] [PubMed] [Google Scholar]
- 28.Portoghese PS, Lipkowski AW, Takemori AE. Binaltorphimine and nor-binaltorphimine, potent and selective κ-opioid receptor antagonists. Life Sci 40: 1287–1292, 1987 [DOI] [PubMed] [Google Scholar]
- 29.Potts JT, Lee SM, Anguelov PI. Tracing of projection neurons from the cervical dorsal horn to the medulla with the anterograde tracer biotinylated dextran amine. Auton Neurosci 98: 64–69, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Prior BM, Lloyd PG, Ren J, Li H, Yang HT, Laughlin MH, Terjung RL. Time course of changes in collateral blood flow and isolated vessel size and gene expression after femoral artery occlusion in rats. Am J Physiol Heart Circ Physiol 287: H2434–H2447, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Smith SA, Mitchell JH, Garry MG. Electrically induced static exercise elicits a pressor response in the decerebrate rat. J Physiol 537: 961–970, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stebbins CL, Longhurst JC. Potentiation of the exercise pressor reflex by muscle ischemia. J Appl Physiol 66: 1046–1053, 1989 [DOI] [PubMed] [Google Scholar]
- 33.Stevens CW, Kajander KC, Bennett GJ, Seybold VS. Bilateral and differential changes in spinal μ, δ and κ opioid binding in rats with a painful, unilateral neuropathy. Pain 46: 315–326, 1991 [DOI] [PubMed] [Google Scholar]
- 34.Stiller RU, Grubb BD, Schaible HG. Neurophysiological evidence for increased kappa opioidergic control of spinal cord neurons in rats with unilateral inflammation at the ankle. Eur J Neurosci 5: 1520–1527, 1993 [DOI] [PubMed] [Google Scholar]
- 35.Su X, Sengupta JN, Gebhart GF. Effects of κ-opioid receptor-selective agonists on responses of pelvic nerve afferents to noxious colorectal distension. J Neurophysiol 78: 1003–1012, 1997 [DOI] [PubMed] [Google Scholar]
- 36.Tsuchimochi H, McCord JL, Hayes SG, Koba S, Kaufman MP. Chronic femoral artery occlusion augments exercise pressor reflex in decerebrated rats. Am J Physiol Heart Circ Physiol 299: H106–H113, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsuchimochi H, McCord JL, Kaufman MP. Peripheral μ-opioid receptors attenuate the augmented exercise pressor reflex in rats with chronic femoral artery occlusion. Am J Physiol Heart Circ Physiol 299: H557–H565, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tyers MB. A classification of opiate receptors that mediate antinociception in animals. Br J Pharmacol 69: 503–512, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vanderah TW, Schteingart CD, Trojnar J, Junien JL, Lai J, Riviere PJ. FE200041 (D-Phe-D-Phe-D-Nle-D-Arg-NH2): A peripheral efficacious κ-opioid agonist with unprecedented selectivity. J Pharmacol Exp Ther 310: 326–333, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Yang HT, Feng Y, Allen LA, Protter A, Terjung RL. Efficacy and specificity of bFGF increased collateral flow in experimental peripheral arterial insufficiency. Am J Physiol Heart Circ Physiol 278: H1966–H1973, 2000 [DOI] [PubMed] [Google Scholar]