Abstract
The diarrheagenic pathogen enteropathogenic Escherichia coli (EPEC) dynamically modulates the survival of infected host intestinal epithelial cells. In the initial stages of infection, several prosurvival signaling events are activated in host cells. These include the phosphorylation of epidermal growth factor receptor (EGFR) and the consequent activation of the phosphatidylinositol-3 kinase/Akt pathway. While studying this pathway in infected epithelial cells, we observed EGFR depletion at later stages of infection, followed subsequently by a decrease in phospho-EGFR. EGFR loss was not dependent on receptor phosphorylation, or on canonical proteasome- and lysosome-dependent processes. Although a type III secretion mutant (ΔescN) stimulated EGFR phosphorylation, it failed to induce receptor degradation. To identify the specific EPEC effector molecule(s) that influenced EGFR stability, epithelial cells infected with isogenic mutant EPEC strains were examined. An EPEC ΔespF strain failed to induce EGFR degradation, whereas EPEC ΔespZ accentuated receptor loss in infected cells. Given the known and contrasting effects of EspF and EspZ on caspase activation, and the known role of proteases in cleaving EGFR, we explored the effect of caspase inhibitors on infection-dependent EGFR loss. The pan-caspase inhibitor Q-VD-OPh blocked EPEC-induced EGFR cleavage in a dose-dependent manner. Taken together, our data suggest that EPEC EspF stimulates caspase-dependent EGFR cleavage and loss, whereas EspZ inhibits this process. Whereas EGFR phosphorylation contributes to the survival of host cells early in infection, EspF-driven caspase activation and consequent EGFR loss likely induce a precipitous increase in host cell death later in the infectious process.
Keywords: EPEC, EGFR degradation, EGFR phosphorylation, EspF, EspZ, caspase
intestinal pathogens, particularly those that are predominantly extracellular, have to contend with niche-specific parameters, including the relatively steady turnover of intestinal epithelial cells. The diarrheagenic pathogen enteropathogenic Escherichia coli (EPEC) subverts intestinal epithelial function by injecting specific proteins into host cells via a type III secretion system (T3SS). It is now increasingly evident that the pathogen dynamically controls and alters host cell physiology via these “effector” proteins (41). Among other effects, EPEC virulence factors modulate the survival of intestinal epithelial cells. It has been appreciated for some time that intestinal epithelial cells infected by EPEC, and related pathogens, show minimal cell death early in infection (7, 8, 14, 29) and a dramatic decrease in viability during the later stages of the infectious process.
Crane et al. (7) were the first to describe death in EPEC-infected cells as having features of both apoptosis and necrosis and first to make the suggestion that the pathogen regulates host cell survival via multiple pathways. Thus the EPEC secreted molecule EspF localizes to the mitochondria, causes membrane depolarization, and induces caspase-dependent cell death pathways (8, 17, 22–24). Some EPEC strains also translocate the inhibitory calmodulin Cif, which induces host cell cycle arrest at both G1/S and G2/M and triggers delayed apoptosis (30, 31, 36). Prolonged infection of epithelial cells results in caspase-independent death; however, the responsible EPEC molecules and corresponding host cell pathways have not been well defined.
On the other hand, EPEC also elaborates several effector molecules that inhibit host cell death. For instance, EspZ-dependent prosurvival signaling in host cells contributes to direct, and indirect, inhibition of caspase activation (4, 29, 32, 33). In addition, the secreted effectors NleH and NleD also contribute to cytoprotection. NleH interacts with Bax inhibitor-1 to prevent elevated cytoplasmic Ca2+ levels, nuclear condensation, caspase-3 activation, and membrane blebbing (15). NleD has a zinc metalloprotease activity that cleaves JNK and p38, thereby blocking JNK-mediated proapoptotic signaling (2).
We previously demonstrated the activation of the EGFR/phosphatidylinositol-3 kinase/Akt pathway in EPEC-infected intestinal epithelial cells and showed that it promotes the survival of these host cells (28). Secreted factor/s in EPEC culture supernatant are sufficient to induce EGFR phosphorylation (28). However, we also observed the loss of total EGFR at later time points postinfection. In the present study, we define the mechanisms regulating EGFR stability in infected cells and demonstrate that the secreted effector proteins EspZ and EspF modulate EGFR levels. Functional implications of EGFR regulation on host cell survival and EPEC colonization include disease progression and bacterial dissemination, and these are also discussed.
METHODOLOGY
Cell lines.
The human intestinal epithelial cell lines Caco-2BBE (C2BBE), a brush border-expressing subclone of the Caco-2 cell line (25), and T84 were used in this study. C2BBE cells were cultured in Dulbecco's modified Eagle's medium (DMEM) with 25 mM glucose, 10% fetal bovine serum, and 20 mM HEPES (Invitrogen, Grand Island, NY) at 37°C in the presence of 5% CO2. Cells between passages 25 and 45 were used for experiments at days 7–10 postseeding. T84 cells were cultured in a 1:1 mixture of Ham's F12 medium and DMEM with 2.5 mM l-glutamine supplemented with 5% fetal bovine serum (Invitrogen) at 37°C in the presence of 5% CO2.
Growth of bacteria and infection of cell lines.
Bacterial strains used in this study include the enteropathogenic Escherichia coli O127:H6 strain E2348/69 (EPEC) and its isogenic derivatives (Table 1). C2BBE or T84 culture medium was changed to serum-free DMEM 14 h prior to bacterial infection. For infections, overnight bacterial cultures (in Luria-Bertani broth) were subcultured at 1:20 dilution in serum-free DMEM and grown to midlog growth phase (OD600 nm = 0.4) at 37°C. Bacteria were added to the apical surface of C2BBE or T84 cells corresponding to an initial multiplicity of infection (MOI) of 20 or 100 (C2BBE), or 200 (T84; consistent with most previous studies from diverse laboratories, higher EPEC MOIs are required to observe comparable changes in this cell line) as required. Following incubation at 37°C in a 5% CO2 water-jacketed incubator for 1 h, unattached bacteria were removed by washing with DMEM, fresh medium was added, and cell culture plates were incubated for additional time periods. Times indicated in the figures are from the point of initial addition of bacteria. For inhibitor studies, cells were pretreated with appropriate dilutions of the tyrosine kinase inhibitor tyrphostin AG1478 (EMD Millipore, Billerica, MA) or the pan-caspase inhibitor Q-VD-OPh (Sigma-Aldrich, St. Louis, MO), the proteasomal inhibitor MG-132 or the lysosomal inhibitor Bafilomycin A1 (Sigma-Aldrich), for 2 h prior to infection. Inhibitor levels were maintained throughout the course of subsequent infection.
Table 1.
List of strains
| Strain Name Used in This Paper | Description/Relevant Genotype | Reference |
|---|---|---|
| EPEC | EPEC E2348/69 strain (serotype O127:H6); isolated from an infant during an outbreak in Taunton, United Kingdom in 1969; E2348/69 Nalr | 37 |
| ΔespZ | EPEC derivative MK41 with a nonpolar espZ deletion; E2348/69 Nalr Kmr::ΔespZ::aphA-3 | 16 |
| ΔespF | EPEC derivative UMD874 with the nonpolar espF deletion; E2348/69 Nalr Kmr::ΔespF::aphA-3 | 16, 19 |
| ΔescN | EPEC derivative CVD4524 with the nonpolar escN deletion; E2348/69 Nalr Kmr::ΔescN::aphA-3 | 6, 20 |
| DH5α/vector | DH5α harboring the cosmid vector pCVD551 | 18 |
| DH5α/pLEE | DH5α harboring pCVD462 (cloned LEE) | 18 |
Immunoblot analysis.
Infected monolayers, and uninfected controls, were washed with sterile PBS, scraped, and total protein extracted in cell lysis buffer [20 mM Tris (pH 7.5), 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM β-glycerol phosphate, 1 mM Na3VO4, 1 μg/ml leupeptin, and 1 mM PMSF] (Sigma-Aldrich). Extracts were sonicated on ice three times to facilitate cell lysis. Protein concentrations were determined by use of the Pierce Coomassie (Bradford) Protein Assay Kit (Thermo Fisher Scientific, Rockford, IL). For immunoblot analyses, extracts (300 to 500 μg) were separated on 8% SDS-PAGE by use of the Protean II xi apparatus (Bio-Rad, Richmond, CA). Separated proteins were transferred to 0.2-μm nitrocellulose membranes (Transblot Cell Apparatus, Bio-Rad), blocked with 5% nonfat milk in Tris-buffered saline containing Tween 20 (TBST) and incubated with antibodies to pEGFR Tyr1068 (Epitomics, Burlingame, CA), EGFR (Santa Cruz Biotechnology, Dallas, TX), and actin (Sigma-Aldrich) at appropriate dilutions in blocking solution overnight at 4°C. Blots were incubated in horseradish peroxidase-conjugated goat anti-rabbit antibody (Sigma-Aldrich) for 1 h at room temperature. Membranes were washed three times for 5 min in TBST between each incubation step, and developed with SuperSignal West Femto Chemiluminescent Substrate (Thermo Fisher Scientific, Rockford, IL).
RESULTS
EPEC induces EGFR loss in infected intestinal epithelial cells.
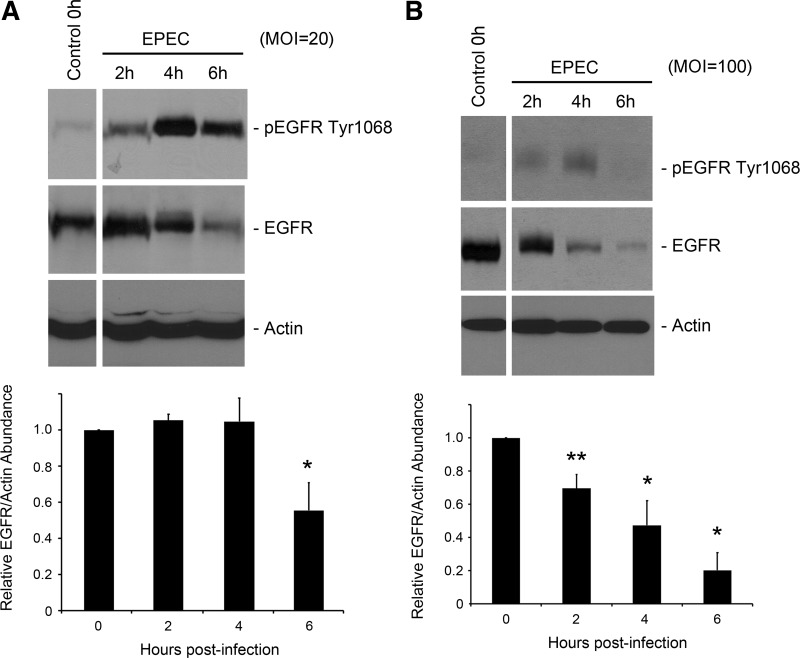
In our previous study on EGFR phosphorylation in EPEC-infected intestinal epithelial cells (28), we observed the depletion of pEGFR at late time points postinfection. Similarly, in this work, and in EPEC-infected C2BBE cells (MOI = 20), peak EGFR phosphorylation was evident at 4 h postinfection, with a subsequent reduction in pEGFR at 6 h postinfection (Fig. 1A). Additionally, there was a reduction in total EGFR levels at 6 h postinfection. EGFR depletion was EPEC dose dependent, with a more rapid decline in receptor levels in cells infected at a higher MOI (100; Fig. 1B).
Fig. 1.
Epidermal growth factor receptor (EGFR) degradation in infected cells is enteropathogenic Escherichia coli (EPEC) dose dependent. C2BBE cells were treated with medium alone or infected with EPEC at multiplicity of infection (MOI) = 20 (A) or MOI = 100 (B). Total protein extracts were prepared, separated, and analyzed for pEGFR Tyr1068, EGFR, and actin abundance by Western hybridization. Blots shown are representative of 3 similar experiments. Densitometric analysis was performed to determine EGFR abundance relative to actin and normalized to levels in control uninfected cells. Significant changes were assessed by ANOVA with Bonferroni post hoc test. *P < 0.01 and **P < 0.01 for specific conditions compared with uninfected control. Error bars represent standard error.
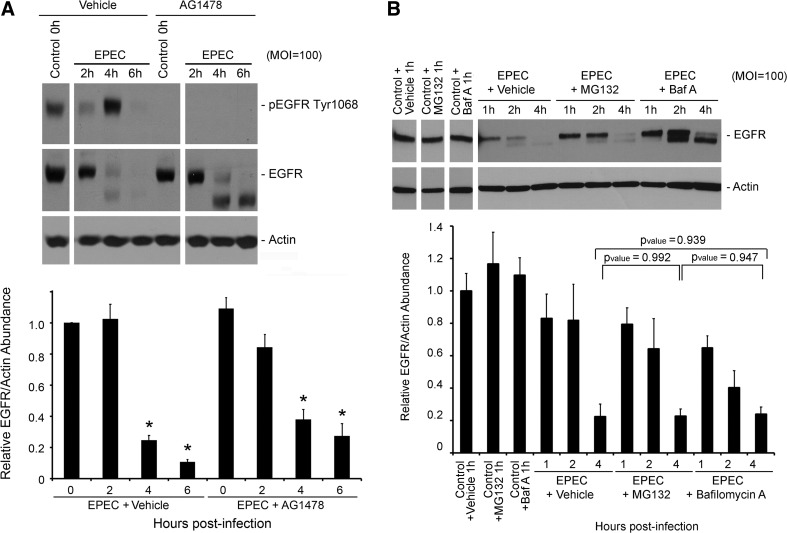
EPEC-induced EGFR degradation is phosphorylation independent.
In the canonical pathway, downregulation of EGFR signaling involves internalization via clathrin-mediated endocytosis, ubiquitination of the phosphorylated receptor, and subsequent proteasomal degradation (1, 10, 21). Therefore, the EGFR kinase inhibitor tyrphostin AG1478 was used to assess the contribution of EGFR phosphorylation and activation to degradation in infected cells. Although AG1478 effectively abrogated EGFR phosphorylation in EPEC-infected cells, it did not significantly alter the kinetics of EGFR loss (Fig. 2A). Following activation and endocytosis, EGFR is typically degraded via lysosome- or proteasome-dependent processes (35). However, infection-induced EGFR loss was evident even in the presence of proteasomal (MG132) and lysosomal (bafilomycin A) inhibitors, respectively, suggesting alternate mechanisms for this process (Fig. 2B).
Fig. 2.
A: EPEC induces phosphorylation-independent degradation of EGFR. C2BBE cells were pretreated with the tyrosine kinase inhibitor AG1478 (1 μM) or vehicle control (DMSO) for 2 h prior to infection with EPEC at MOI = 100. Inhibitor was maintained throughout the course of infection. Total protein extracts isolated at specified time points were immunoblotted for pEGFR Tyr1068, EGFR, and actin. Blots shown are representative of 3 similar experiments. Densitometric analysis was performed to determine EGFR abundance relative to actin and normalized to levels in control uninfected cells. Significant changes were assessed by ANOVA with Bonferroni post hoc test. *P < 0.01 for specific conditions compared with uninfected control. Error bars represent standard error. B: EPEC-induced EGFR degradation is not mediated by proteosomal and lysosomal processes. C2BBE cells were pretreated with the proteosomal inhibitor MG132 (10 μM), lysosomal inhibitor bafilomycin A (100 nM; Baf A), or vehicle control (DMSO) for 2 h prior to infection with EPEC at MOI = 100. Inhibitor was maintained throughout the course of infection. Total protein extracts isolated at specified time points were immunoblotted for EGFR and actin. Blots shown are representative of 3 similar experiments. Densitometric analysis was performed to determine EGFR abundance relative to actin and normalized to levels in control uninfected cells. P values for specific conditions compared were assessed by ANOVA with Bonferroni post hoc test. EGFR loss at 4 h postinfection was not significantly affected by MG132 or bafilomycin A. Error bars represent standard error.
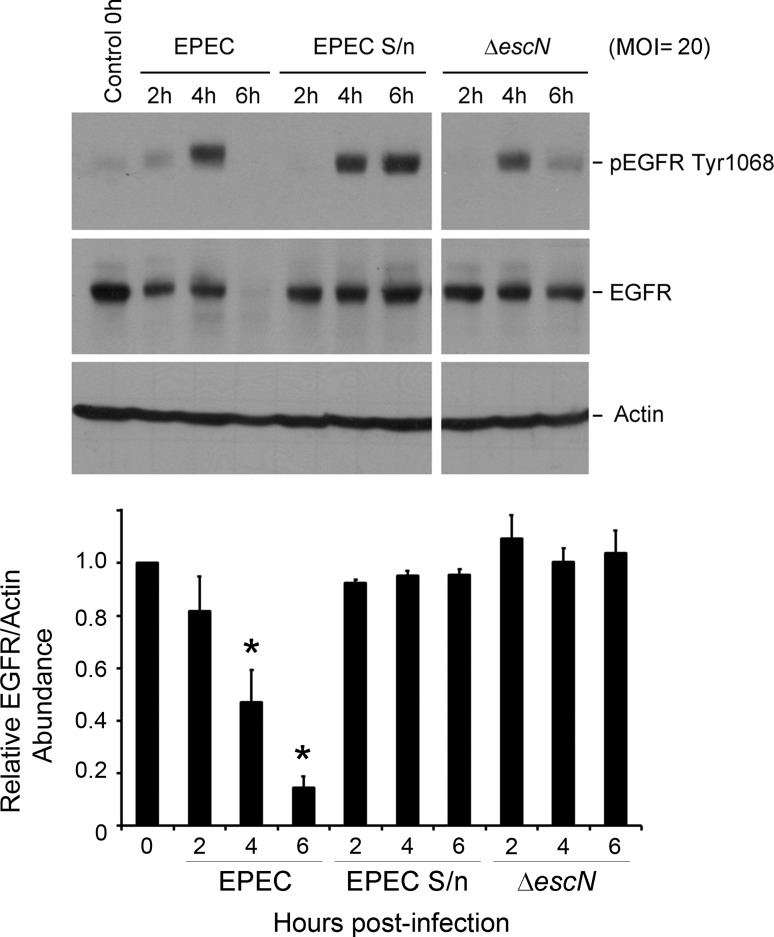
EGFR depletion is mediated by EPEC translocated effector(s).
To define the mechanism of EPEC-induced EGFR loss, C2BBE cells were treated with wild-type (WT) EPEC, filtered EPEC culture supernatant, or a type III secretion-defective mutant (ΔescN). Whereas EGFR phosphorylation was evident with all three treatments (Fig. 3), receptor loss was observed only in WT EPEC-infected cells. This further supports the contention that receptor loss is likely not a consequence of phosphorylation-dependent events, and that it is dependent on one or more type III-secreted effector proteins.
Fig. 3.
A secreted EPEC component promotes EGFR phosphorylation, but not its degradation. C2BBE cells were treated with medium alone or filter-sterilized EPEC supernatants (EPEC S/n), or infected with wild-type EPEC or an isogenic secretion-defective strain (ΔescN) at MOI = 20. Total protein extracts collected at specific time points were immunoblotted for pEGFR Tyr1068, EGFR, and actin. Blots shown are representative of 3 similar experiments; for each blot, the spacing after lane 7 denotes the cropping of irrelevant lanes. Densitometric analysis was performed to determine EGFR abundance relative to actin and normalized to levels in control uninfected cells. Significant changes were assessed by ANOVA with Bonferroni post hoc test. *P < 0.01 for specific conditions compared with uninfected control. Error bars represent standard error.
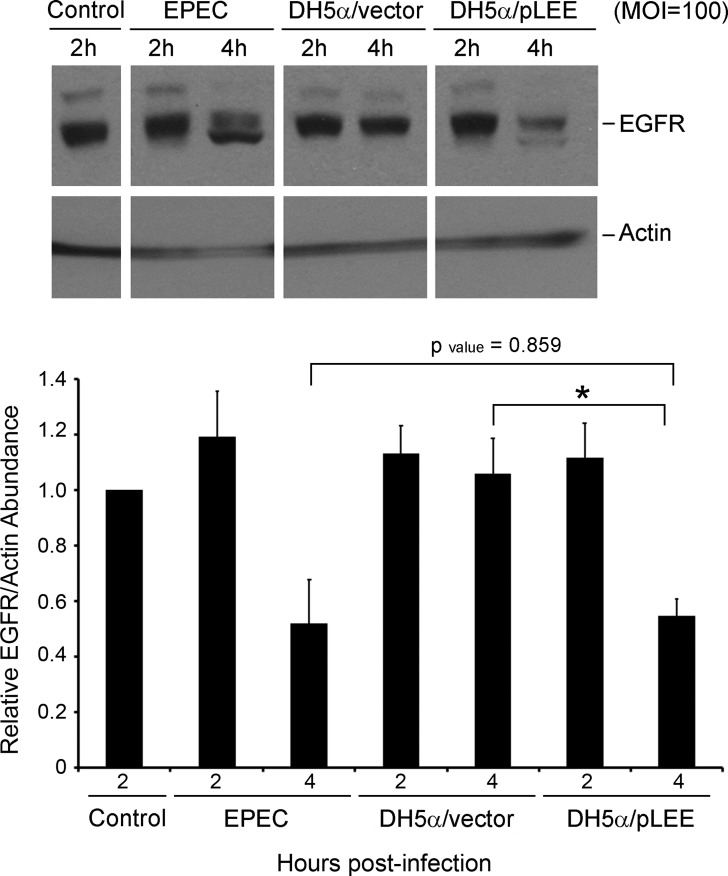
To determine whether the effector/s responsible for EGFR degradation are encoded within the 35-kb EPEC pathogenicity island (locus of enterocyte effacement, LEE), C2BBE cells were infected with a nonpathogenic E. coli strain (DH5α) harboring plasmid-encoded LEE (DH5α/pLEE). This strain was previously demonstrated to support type III secretion-dependent translocation of LEE-encoded effectors into host cells (18). EGFR loss was observed in DH5α/pLEE-infected C2BBE cells, but not in DH5α/vector-treated cells, implicating one or more LEE-encoded effectors in this process (Fig. 4). Analyses were restricted to 2- and 4-h infected cells since the focus was solely on determining whether the relevant effectors were LEE-encoded, and since the strain background (DH5α) precluded direct comparison to EPEC-infected host cells.
Fig. 4.
The EPEC locus of enterocyte effacement (LEE) is necessary and sufficient to promote EGFR degradation. C2BBE cells were treated with medium alone or infected with EPEC, DH5α harboring the cosmid vector pCVD551 (DH5α/vector) or DH5α harboring the LEE-encoding pCVD462 (DH5α/pLEE) at MOI = 100. Total protein extracts isolated at specific time points were immunoblotted for EGFR and actin. Blots shown are representative of 3 similar experiments. Densitometric analysis was performed to determine EGFR abundance relative to actin and normalized to levels in control uninfected cells. P values for specific conditions compared were assessed by ANOVA with Bonferroni post hoc test; *P < 0.01. Error bars represent standard error.
EPEC EspF stimulates the EGFR loss in infected intestinal epithelial cells.
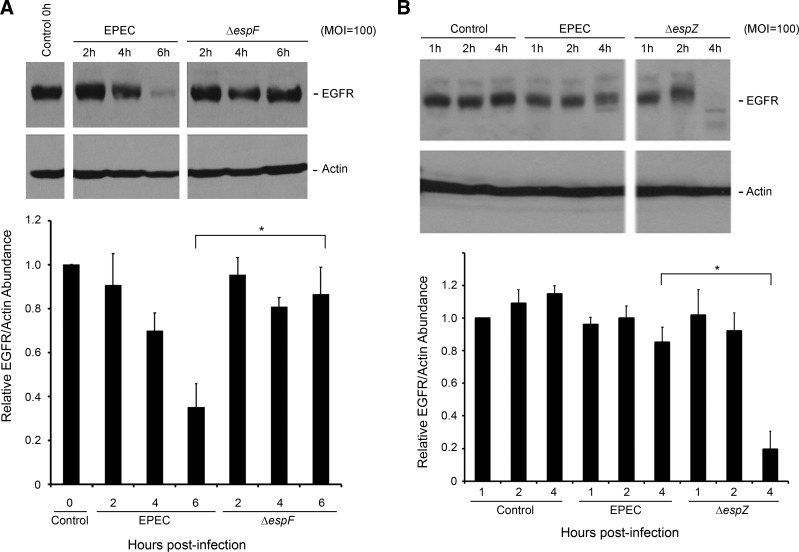
To identify effector(s) influencing EGFR loss, we evaluated phospho- and total EGFR levels in C2BBE cells infected with isogenic mutants lacking specific virulence proteins. As with WT EPEC infection, EGFR depletion was observed in C2BBE cells infected with EPEC Δtir, Δmap, ΔespG, ΔespH, ΔnleA, ΔnleH1/2, ΔespG2, and ΔfliC (data not shown), ruling out a role for the corresponding proteins in this process. In the case of ΔespF- and ΔespZ-infected cells, however, the kinetics of EGFR loss was clearly different from that of WT EPEC-infected cells (Fig. 5). In ΔespF-infected cells, EGFR persisted at 6 h postinfection, in contrast to WT-infected C2BBE cells (Fig. 5A), suggesting that EspF contributes to EGFR loss. To verify whether EspF- and EspZ-dependent EGFR alterations were cell line specific, we performed similar studies in T84 cells. Consistent with the observations in C2BBE cells, EPEC induced EGFR loss in T84 cells, and this phenotype was dependent on EspF (Fig. 6). Compared with C2BBE cells, however, EGFR loss in infected T84 cells was more modest at 6 h postinfection.
Fig. 5.
EspF promotes EGFR degradation, whereas EspZ protects infected cells from EGFR loss. C2BBE cells were treated with medium alone or infected with the wild-type EPEC, ΔespF mutant (A), or ΔespZ mutant (B) at MOI = 100. Total protein extracts collected were analyzed for the abundance of EGFR and actin by Western hybridization. Blots shown are representative of 4 similar experiments. Densitometric analysis was performed to determine EGFR abundance relative to actin and normalized to levels in control uninfected cells. Significant changes were assessed by ANOVA with Bonferroni post hoc test. *P < 0.01 for specific conditions compared. Error bars represent standard error.
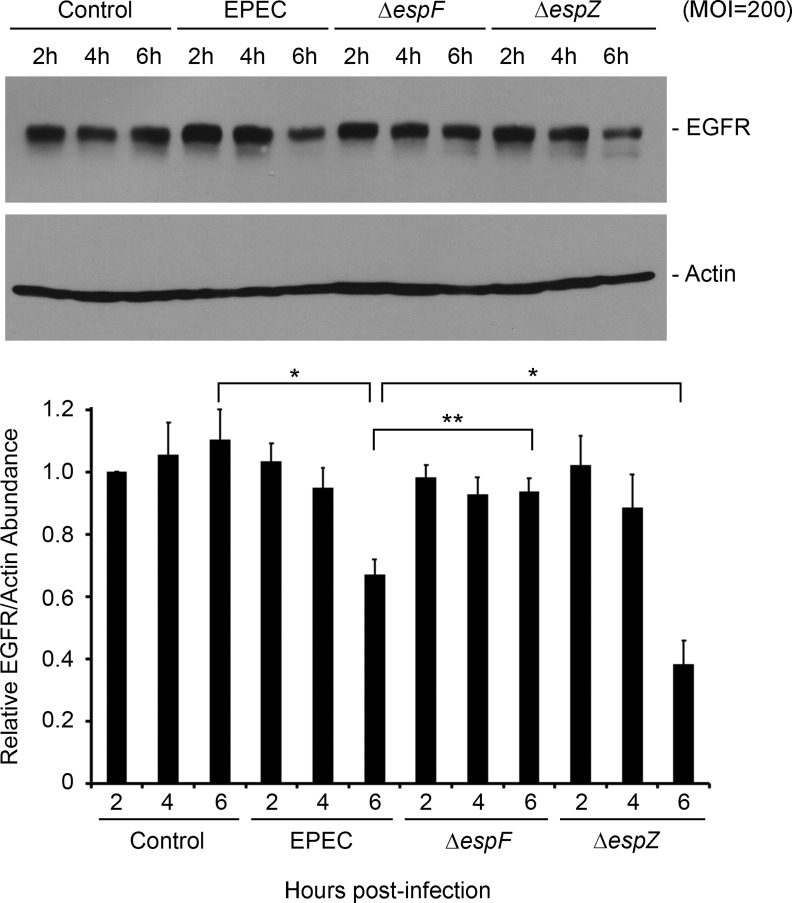
Fig. 6.
Validation of EPEC-mediated EGFR degradation in T84 cells. T84 cells were treated with medium alone or infected with EPEC, ΔespF mutant, or ΔespZ strain at MOI = 200. Total protein extracts isolated at specific time points were analyzed for the abundance of EGFR and actin by Western hybridization. Blots shown are representative of 3 similar experiments. Densitometric analysis was performed to determine EGFR abundance relative to actin and normalized to levels in control uninfected cells. Significant changes were assessed by ANOVA with Bonferroni post hoc test. *P < 0.01 and **P < 0.05 for specific conditions compared. Error bars represent standard error.
EPEC EspZ protects against EGFR loss from infected intestinal epithelial cells.
In contrast to ΔespF, ΔespZ infection of both C2BBE and T84 cells resulted in increased loss of EGFR relative to WT EPEC-infected cells (Figs. 5B and 6). Thus, although EGFR is substantially preserved at 4 h postinfection in WT EPEC-infected C2BBE cells, it is almost completely depleted in ΔespZ-infected cells at this time point. The time course of EGFR depletion paralleled the caspase activation in ΔespZ-infected C2BBE cells that we previously reported (29). Since ΔespZ-infected host cells undergo extensive rounding, detachment, and death by 6 h postinfection (29), EGFR levels were not assessed for this time point.
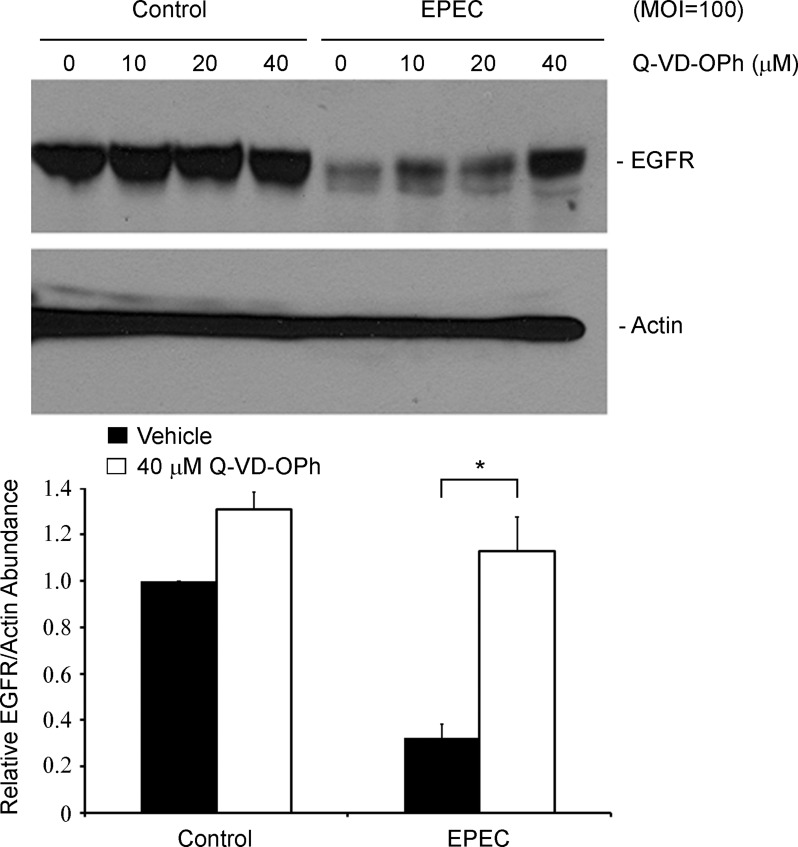
EGFR persistence in infected epithelial cells is dictated by EspF- and EspZ-dependent regulation of caspase activity.
Both EspF and EspZ have been implicated in the regulation of the intrinsic apoptotic pathway caspases (3, 7 and 9) and the survival of EPEC-infected intestinal epithelial cells. EspF localizes to the mitochondria and induces a loss in mitochondrial potential, and consequent activation of caspases 9 and 3 (8, 22–24), whereas EspZ inhibits caspase 3/7/9 activation (29). Caspases, or cysteine-dependent aspartate-directed proteases, have previously been implicated in site-specific EGFR cleavage (DVVD1012 and DNPD1171) (13). Therefore, the pan-caspase inhibitor Q-VD-OPh was used to probe caspase dependence of EGFR loss in EPEC-infected C2BBE cells (Fig. 7). Consistent with our hypothesis, a Q-VD-OPh dose-dependent restoration of total EGFR was observed in EPEC-infected epithelial cells. Taken together, our results suggest that activated caspases degrade EGFR in EPEC-infected cells. EspF stimulates EGFR cleavage by activating caspases, whereas EspZ delays EGFR loss by inhibiting caspase activation.
Fig. 7.
EGFR loss during EPEC infection is caspase dependent. C2BBE cells were pretreated with increasing concentrations of the pan-caspase inhibitor Q-VD-OPh (0, 10, 20, or 40 μM) for 2 h. Cells were then treated with medium alone or infected with EPEC at MOI = 100. At 5 h postinfection, total protein extracts were isolated and immunoblotted for EGFR and actin. Densitometric analysis was performed to determine EGFR abundance relative to actin and normalized to levels in control uninfected cells (0 μM Q-VD-OPh). Significant changes were assessed by ANOVA with Bonferroni post hoc test; *P < 0.01, n = 3. Error bars represent standard error.
DISCUSSION
Multiple studies have revealed that EPEC manipulates the survival of host intestinal epithelial cells by eliciting both cytoprotective and cytolethal responses, likely in a temporally regulated manner (7, 8, 15, 28, 29, 33). Although we previously reported on the protective effects of EPEC-induced EGFR phosphorylation, this study demonstrates a type III secretion-dependent downregulation of EGFR signaling later in infection. The effector protein EspF stimulates caspase-dependent EGFR cleavage, whereas the early secreted EPEC molecule EspZ curtails these effects. It is interesting to note that phospho-EGFR levels initially increase even as total EGFR levels begin to decrease in infected cells, possibly suggesting that the phosphorylated receptor is relatively refractory to caspase-dependent cleavage. Formally, however, we cannot rule out the possibility of an alternate role for EGFR loss in infected cells, in addition to, or independent of, the canonical phosphorylation-related signaling pathways.
Caspase-mediated EGFR cleavage has been reported in apoptotic human keratinocyte HaCaT and human cervical epithelial HeLa cell lines (20), as well as during UVA damage of HaCaT cells (26). Curiously, the ubiquitin ligase c-Cbl was also degraded in EPEC-infected cells in a type III secretion-dependent manner (data not shown); it is unclear whether EGFR cleavage precipitates c-Cbl loss or whether c-Cbl is independently cleaved by caspases (11, 40). Although caspase-mediated cleavage of c-Cbl has not been reported, in silico analysis of the protein using the caspase cleavage prediction server CASVM version 1.0 (39) revealed the presence of four putative DxxD caspase cleavage sites (DPFD-435, DDDD-459, DDDD-460, and DGYD-775).
In an earlier study, we demonstrated EGFR-dependent phosphorylation of Akt Ser473 (pAkt) in infected epithelial cells (28). In macrophages, WT EPEC induced a transient increase in pAkt, but, interestingly, Akt phosphorylation was sustained in cells infected with a T3SS-deficient strain (5, 26). EPEC-induced dephosphorylation of host proteins, including pAKT Ser473, contributes to bacterial evasion of phagocytosis by macrophages (12, 26). Although EGFR signaling in EPEC-infected macrophages has not been studied, caspase-dependent downregulation of EGFR could be one mechanism contributing to the eventual loss of pAkt.
EGFR signaling has been implicated in the pathogenesis of several bacterial infections. EGFR activation by Neisseria gonorrhoeae and Salmonella typhimurium enhances bacterial invasion of host epithelial cells (9, 34). EGFR activation in enterohemorrhagic Escherichia coli (O157:H7)- and Helicobacter pylori-infected epithelial cells, respectively, leads to enhanced interleukin-8 production (38). Importantly, pathogenic bacteria have mechanisms to influence the duration of EGFR signaling, typically to sustain it for long periods. Mechanisms include the manipulation of endocytic and/or proteasomal/lysosomal degradation steps in the ligand-induced EGFR downregulation pathway. Helicobacter pylori CagA, for instance, prevents receptor endocytosis, and the prolonged surface exposure of EGFR likely contributes to sustained increase in EGFR signaling and to the neoplastic transformation of host cells (3). Similarly, the Shigella flexneri type III-secreted effector protein IpgD prolongs EGFR signaling by preventing endocytosis and impairing lysosomal degradation (27).
Our observations are consistent with a precipitous loss of viability of infected cells during late stages of EPEC infection. Although host cell survival during the initial phase of infection, promoted by EPEC effector molecules such as EspZ, may be critical for colonization and pathogenesis, it is likely that accelerated cell death during later stages may promote symptomatic disease and facilitate bacterial dispersion.
GRANTS
This research project was supported by the National Institutes of Health (V. K. Viswanathan; NIAID1R01AI081742). Work in the Viswanathan laboratory is also supported by the USDA CSREES Hatch Program (ARZT-5704100-A02-140).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
J.L.R., G.V., and V.V. conception and design of research; J.L.R. and K.A.R. performed experiments; J.L.R., G.V., and V.V. analyzed data; J.L.R., G.V., and V.V. interpreted results of experiments; J.L.R. and K.A.R. prepared figures; J.L.R. drafted manuscript; J.L.R., K.A.R., G.V., and V.V. edited and revised manuscript; G.V. and V.V. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Ross Calvin Monasky for assistance with the experiments.
REFERENCES
- 1.Alwan HA, van Zoelen EJ, van Leeuwen JE. Ligand-induced lysosomal epidermal growth factor receptor (EGFR) degradation is preceded by proteasome-dependent EGFR de-ubiquitination. J Biol Chem 278: 35781–35790, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Baruch K, Gur-Arie L, Nadler C, Koby S, Yerushalmi G, Ben-Neriah Y, Yogev O, Shaulian E, Guttman C, Zarivach R, Rosenshine I. Metalloprotease type III effectors that specifically cleave JNK and NF-kappaB. EMBO J 30: 221–231, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bauer B, Bartfeld S, Meyer TF. H. pylori selectively blocks EGFR endocytosis via the non-receptor kinase c-Abl and CagA. Cell Microbiol 11: 156–169, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Berger CN, Crepin VF, Baruch K, Mousnier A, Rosenshine I, Frankel G. EspZ of enteropathogenic and enterohemorrhagic Escherichia coli regulates type III secretion system protein translocation. MBio 3: e00317–12, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Celli J, Olivier M, Finlay BB. Enteropathogenic Escherichia coli mediates antiphagocytosis through the inhibition of PI 3-kinase-dependent pathways. EMBO J 20: 1245–1258, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collington GK, Booth IW, Donnenberg MS, Kaper JB, Knutton S. Enteropathogenic Escherichia coli virulence genes encoding secreted signalling proteins are essential for modulation of Caco-2 cell electrolyte transport. Infect Immun 66: 6049–6053, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crane JK, Majumdar S, Pickhardt DF., 3rd. Host cell death due to enteropathogenic Escherichia coli has features of apoptosis. Infect Immun 67: 2575–2584, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crane JK, McNamara BP, Donnenberg MS. Role of EspF in host cell death induced by enteropathogenic Escherichia coli. Cell Microbiol 3: 197–211, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Galan JE, Pace J, Hayman MJ. Involvement of the epidermal growth factor receptor in the invasion of cultured mammalian cells by Salmonella typhimurium. Nature 357: 588–589, 1992 [DOI] [PubMed] [Google Scholar]
- 10.Galcheva-Gargova Z, Theroux SJ, Davis RJ. The epidermal growth factor receptor is covalently linked to ubiquitin. Oncogene 11: 2649–2655, 1995 [PubMed] [Google Scholar]
- 11.Gibson S, Tu S, Oyer R, Anderson SM, Johnson GL. Epidermal growth factor protects epithelial cells against Fas-induced apoptosis requirement for Akt activation. J Biol Chem 274: 17612–17618, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Goosney DL, Celli J, Kenny B, Finlay BB. Enteropathogenic Escherichia coli inhibits phagocytosis. Infect Immun 67: 490–495, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He YY, Huang JL, Chignell CF. Cleavage of epidermal growth factor receptor by caspase during apoptosis is independent of its internalization. Oncogene 25: 1521–1531, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Heczko U, Carthy CM, O'Brien BA, Finlay BB. Decreased apoptosis in the ileum and ileal Peyer's patches: a feature after infection with rabbit enteropathogenic Escherichia coli O103. Infect Immun 69: 4580–4589, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hemrajani C, Berger CN, Robinson KS, Marches O, Mousnier A, Frankel G. NleH effectors interact with Bax inhibitor-1 to block apoptosis during enteropathogenic Escherichia coli infection. Proc Natl Acad Sci USA 107: 3129–3134, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanack KJ, Crawford JA, Tatsuno I, Karmali MA, Kaper JB. SepZ/EspZ is secreted and translocated into HeLa cells by the enteropathogenic Escherichia coli type III secretion system. Infect Immun 73: 4327–4337, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kenny B, Jepson M. Targeting of an enteropathogenic Escherichia coli (EPEC) effector protein to host mitochondria. Cell Microbiol 2: 579–590, 2000 [DOI] [PubMed] [Google Scholar]
- 18.McDaniel TK, Kaper JB. A cloned pathogenicity island from enteropathogenic Escherichia coli confers the attaching and effacing phenotype on E. coli K-12. Mol Microbiol 23: 399–407, 1997 [DOI] [PubMed] [Google Scholar]
- 19.McNamara BP, Donnenberg MS. A novel proline-rich protein, EspF, is secreted from enteropathogenic Escherichia coli via the type III export pathway. FEMS Microbiol Lett 166: 71–78, 1998 [DOI] [PubMed] [Google Scholar]
- 20.McNamara BP, Koutsouris A, O'Connell CB, Nougayrede JP, Donnenberg MS, Hecht G. Translocated EspF protein from enteropathogenic Escherichia coli disrupts host intestinal barrier function. J Clin Invest 107: 621–629, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Melikova MS, Kondratov KA, Kornilova ES. Two different stages of epidermal growth factor (EGF) receptor endocytosis are sensitive to free ubiquitin depletion produced by proteasome inhibitor MG132. Cell Biol Int 30: 31–43, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Nagai T, Abe A, Sasakawa C. Targeting of enteropathogenic Escherichia coli EspF to host mitochondria is essential for the bacterial pathogenesis: critical role of the 16th leucine residue in EspF. J Biol Chem 289: 2998–3011, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Nougayrede JP, Donnenberg MS. Enteropathogenic Escherichia coli EspF is targeted to mitochondria and is required to initiate the mitochondrial death pathway. Cell Microbiol 6: 1097–1111, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Nougayrede JP, Foster GH, Donnenberg MS. Enteropathogenic Escherichia coli effector EspF interacts with host protein Abcf2. Cell Microbiol 9: 680–693, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Peterson MD, Mooseker MS. Characterization of the enterocyte-like brush border cytoskeleton of the C2BBe clones of the human intestinal cell line, Caco-2. J Cell Sci 102: 581–600, 1992 [DOI] [PubMed] [Google Scholar]
- 26.Quitard S, Dean P, Maresca M, Kenny B. The enteropathogenic Escherichia coli EspF effector molecule inhibits PI-3 kinase-mediated uptake independently of mitochondrial targeting. Cell Microbiol 8: 972–981, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Ramel D, Lagarrigue F, Pons V, Mounier J, Dupuis-Coronas S, Chicanne G, Sansonetti PJ, Gaits-Iacovoni F, Tronchere H, Payrastre B. Shigella flexneri infection generates the lipid PI5P to alter endocytosis and prevent termination of EGFR signaling. Sci Signal 4: ra61, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Roxas JL, Koutsouris A, Viswanathan VK. Enteropathogenic Escherichia coli-induced epidermal growth factor receptor activation contributes to physiological alterations in intestinal epithelial cells. Infect Immun 75: 2316–2324, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roxas JL, Wilbur JS, Zhang X, Martinez G, Vedantam G, Viswanathan VK. The enteropathogenic Escherichia coli-secreted protein EspZ inhibits host cell apoptosis. Infect Immun 80: 3850–3857, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Samba-Louaka A, Nougayrede JP, Watrin C, Jubelin G, Oswald E, Taieb F. Bacterial cyclomodulin Cif blocks the host cell cycle by stabilizing the cyclin-dependent kinase inhibitors p21 and p27. Cell Microbiol 10: 2496–2508, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Samba-Louaka A, Nougayrede JP, Watrin C, Oswald E, Taieb F. The enteropathogenic Escherichia coli effector Cif induces delayed apoptosis in epithelial cells. Infect Immun 77: 5471–5477, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shames SR, Croxen MA, Deng W, Finlay BB. The type III system-secreted effector EspZ localizes to host mitochondria and interacts with the translocase of inner mitochondrial membrane 17b. Infect Immun 79: 4784–4790, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shames SR, Deng W, Guttman JA, de Hoog CL, Li Y, Hardwidge PR, Sham HP, Vallance BA, Foster LJ, Finlay BB. The pathogenic E. coli type III effector EspZ interacts with host CD98 and facilitates host cell prosurvival signalling. Cell Microbiol 12: 1322–1339, 2010 [DOI] [PubMed] [Google Scholar]
- 34.Swanson KV, Griffiss JM, Edwards VL, Stein DC, Song W. Neisseria gonorrhoeae-induced transactivation of EGFR enhances gonococcal invasion. Cell Microbiol 13: 1078–1090, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sweeney C, Carraway KL., 3rd. Negative regulation of ErbB family receptor tyrosine kinases. Br J Cancer 90: 289–293, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taieb F, Nougayrede JP, Watrin C, Samba-Louaka A, Oswald E. Escherichia coli cyclomodulin Cif induces G2 arrest of the host cell cycle without activation of the DNA-damage checkpoint-signalling pathway. Cell Microbiol 8: 1910–1921, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Taylor J. Infectious infantile enteritis, yesterday and today. Proc R Soc Med 63: 1297–1301, 1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wallasch C, Crabtree JE, Bevec D, Robinson PA, Wagner H, Ullrich A. Helicobacter pylori-stimulated EGF receptor transactivation requires metalloprotease cleavage of HB-EGF. Biochem Biophys Res Commun 295: 695–701, 2002 [DOI] [PubMed] [Google Scholar]
- 39.Wee LJ, Tan TW, Ranganathan S. SVM-based prediction of caspase substrate cleavage sites. BMC Bioinformatics 7, Suppl 5: S14, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Widmann C, Gibson S, Johnson GL. Caspase-dependent cleavage of signaling proteins during apoptosis. A turn-off mechanism for anti-apoptotic signals. J Biol Chem 273: 7141–7147, 1998 [DOI] [PubMed] [Google Scholar]
- 41.Wong AR, Pearson JS, Bright MD, Munera D, Robinson KS, Lee SF, Frankel G, Hartland EL. Enteropathogenic and enterohaemorrhagic Escherichia coli: even more subversive elements. Mol Microbiol 80: 1420–1438, 2011 [DOI] [PubMed] [Google Scholar]