Abstract
Adenosine is a purine metabolite that can mediate anti-inflammatory responses in the digestive tract through the A2A adenosine receptor (A2AAR). We examined the role of this receptor in the control of inflammation in the adoptive transfer model of colitis. Infection of A2AAR−/− mice with Helicobacter hepaticus increased colonic inflammation scores compared with uninfected A2AAR controls. Comparison of T cell subsets in wild-type and A2AAR−/− mice revealed differences in markers associated with activated helper T (Th) cells and regulatory T (Treg) cells. Previous studies showed that expression of A2AAR on CD45RBHI and CD45RBLO Th cells is essential for the proper regulation of colonic inflammation. Adoptive transfer of CD45RBHI with CD45RBLO from wild-type mice into RAG1−/−/A2AAR−/− mice induced severe disease within 3 wk, although transfer of the same subsets into RAG1−/− mice does not induce colitis. This suggests that the presence of A2AAR on recipient cells is also important for controlling colitis. To investigate the role of A2AAR in myeloid cells, chimeric recipients were generated by injection of bone marrow from RAG1−/− or RAG1−/−/A2AAR−/− mice into irradiated RAG1−/− mice. After adoptive transfer, these recipients did not develop colitis, regardless of A2AAR expression by the donor. Together, our results suggest that the control of inflammation in vivo is dependent on A2AAR signaling through multiple cell types that collaborate in the regulation of colitis by responding to extracellular adenosine.
Keywords: colitis, adenosine, A2A adenosine receptor, adoptive transfer, regulatory T cells
the nucleoside adenosine accumulates in areas of hypoxia or inflammation and can stimulate responses in a number of cell types through ligation of one of four G protein-coupled receptors: A1 adenosine receptor (A1AR), A2AAR, A2BAR, and A3AR. The downstream effects of these receptors vary on the basis of the cell type and the different G protein subunits involved (12, 17).
Adenosine has been shown to be a significant immune modulator, and adenosine receptors are expressed on a number of cells involved in inflammation, including lymphocytes, myeloid cells, and endothelial cells (25). One of the primary effects of adenosine on these cells is the generation of an anti-inflammatory response. A major receptor that mediates these responses is A2AAR. Recent studies have focused on the role of A2AAR in controlling or suppressing inflammation in models of disease. A2AAR is the predominant receptor on CD4+ helper T (Th) cells and has been examined for its roles in controlling cytokine production, particularly IFN-γ, IL-2, and TNF-α (2, 13, 24, 26, 28). Stimulation of A2AAR also suppresses proinflammatory responses, including adhesion, extravasation, and activation of neutrophils and other granulocytes, in myeloid cells (18). While A2AAR−/− mice appear healthy, they manifest a more inflamed phenotype in response to provocative stimuli (2, 28).
The traditional CD45RB transfer model of colitis focuses on the role of Th cells in the development of colitis. It is becoming evident, however, that other cell lineages [i.e., neutrophils, dendritic cells, and innate lymphoid cells (ILCs)] play an important role in the development of this multifactorial disease (8, 9). When immunodeficient recipient mice received CD45RBHI Th cells along with oral A2AAR agonist treatment, the agonist appeared to attenuate inflammation and proinflammatory cytokine production compared with vehicle controls (26). A2AAR agonists have also been shown to ameliorate ileitis in a mouse model of spontaneous ileitis (27). The decrease in disease severity induced by A2AAR agonists in both models could be due to stimulation of A2AAR on T cells, myeloid cells, or cells of multiple nonlymphoid and stromal tissues.
To further examine the role of A2AAR in the regulation of T cell-mediated colitis, we assessed disease in A2AAR−/− mice that were or were not exposed to a microbial stress. Furthermore, to dissect which cell types were targets of adenosine, we compared the role of A2AAR in the control of T cell-mediated colitis in recombination-activating gene-deficient (RAG1−/−) mice, as well as in RAG1−/−/A2AAR−/− (double-knockout) mice. The results suggest that control of colitis requires signaling through A2AAR expressed by lymphoid and nonlymphoid cells.
MATERIALS AND METHODS
Mice.
C57BL/6 and RAG1−/− mice were purchased from Jackson Laboratory. Adora2a-deficient (A2AAR−/−) mice were inbred onto the C57BL/6 background (by J. Linden). RAG1−/−/A2AAR−/− mice inbred onto a C57BL/6 background were generated in the authors' laboratory [forward primer, common for wild-type (WT) or A2AAR−/−: GGGCTCCTCGGTGTACAT; reverse primer for A2AAR−/−: CCCACAGATCTAGCCTTA; reverse primer for WT: TGTCACGTCCTGCACGAC]. All mice were maintained in conventional housing at the University of Virginia or the University of California, San Diego, using procedures approved by the respective Institutional Animal Care and Use Committee.
Inoculation with Helicobacter hepaticus.
H. hepaticus was cultured in Brucella broth layered over blood agar (5% sheep blood) in a microaerophilic (90% N2-5% CO2-5% O2) chamber. Female A2AAR−/− mice at 5–10 wk of age were fasted overnight before being inoculated with 1 × 108 colony-forming units of H. hepaticus and monitored for signs of disease. Once signs of disease were noticed, mice were euthanized, and colons were removed and fixed in Bouin's fixative overnight, transferred to 70% ethanol, and processed for histology. Paraffin-embedded sections were deparaffinized, rehydrated, and stained with hematoxylin and eosin. Sections were evaluated for histological damage following a scoring protocol wherein tissue thickness, polymorphonuclear (PMN) and mononuclear cell infiltration, epithelial damage, and infiltration of the submucosa and muscularis were examined.
CD4+ T cell isolation and fluorescein-activated cell sorting.
Mice were euthanized and spleens were extracted, disrupted into a single-cell suspension using frosted glass slides, and filtered through a 70-μm cell strainer. The resulting suspension was enriched for CD4+ cells using microbeads (L3T4, Miltenyi Biotec). Enriched cells were incubated with anti-CD16/32 (Fc Block) for 10 min prior to incubation with fluorescently conjugated anti-mouse monoclonal antibodies for 30 min. After two washes, cells were fixed and permeabilized using the FoxP3 staining buffer set (eBioscience, San Diego, CA) and incubated with anti-FoxP3 for 30 min. Cells were washed and assayed on a Cyan ADP 9 color analyzer (Beckman Coulter, Fullerton, CA). Antibodies used in this study were anti-CD4-peridinin chlorophyll protein complex (PerCP) or anti-CD4-FITC (RM4-5), anti-CD45RB-allophycocyanin (APC)/Cy7 or anti-CD45RB-phycoerythrin (PE) (C363-16A), anti-CD25-PE/Cy7 (PC61.5), anti-FoxP3-AF647 (FJK-16s), anti-FR4-FITC (eBio12A5), anti-GITR-FITC (DTA-1), anti-CD39-PE (24DMS1), and anti-CD73-eFluor450 (TY2.3). Results were analyzed using FloJo software (TreeStar, Ashland, OR).
Adoptive transfers.
To evaluate which cells expressing A2AAR regulate inflammation in the gastrointestinal tract, we employed the CD45RB transfer model of colitis (29, 36). Splenocytes from C57BL/6 mice were enriched using CD4+ microbeads (L3T4, Miltenyi Biotec) and sorted into subsets on the basis of expression of CD4+ and CD45RB. CD45RBHI (5 × 105 cells) and CD45RBLO (1 × 105 cells) Th cells from C57BL/6 mice were injected intraperitoneally into RAG1−/− or RAG1−/−/A2AAR−/− recipients. Mice were weighed and monitored weekly for signs of disease. After mice showed evidence of colitis (e.g., wasting and soft stools), they were euthanized, colons were removed, and a 50- to 75-mg piece of the midcolon was collected for cytokine analysis by ELISA. The remainder of the colon was fixed in Bouin's fixative, and hematoxylin-eosin-stained sections were evaluated for histological damage following a scoring protocol wherein tissue thickness, PMN and mononuclear cell infiltration, epithelial damage, and infiltration of the submucosa and muscularis were examined.
Immunohistochemistry.
Similar 3-μm colonic sections were stained with a polyclonal anti-myeloperoxidase (MPO) antibody (Novus Biologicals, Littleton, CO), and tissue-bound peroxidase activity was visualized with 3,3′-diaminobenzidine. Hematoxylin was used for nuclear counterstaining. The number of MPO+ cells was counted in 10 different fields at an original magnification of ×400 and expressed per field.
ELISA.
A 50- to 75-mg piece of the midcolon was removed and homogenized in PBS containing protease inhibitor. The resulting suspension was centrifuged for 20 min at 10,000 g at 4°C. Supernatants were collected and assayed by ELISA for IFN-γ, IL-10, TNF-α (BD Biosciences), and IL-17A (eBioscience). Cytokine levels were calculated on the basis of a standard curve and normalized to protein concentration.
Bone marrow chimeras.
Female RAG1−/− mice were irradiated with 600 rad (6 Gy) twice at an interval of 4 h. Immediately following the second dose of radiation, 7 × 106 bone marrow cells obtained from the femurs of RAG1−/− or RAG1−/−/A2AAR−/− female mice were injected intravenously into the tail vein of the irradiated mice. Mice were allowed to recover until at least two consecutive blood samples [analyzed using a Hemavet analyzer (Drew Scientific, Waterbury, CT)] revealed reconstitution of myeloid cells and body weight returned to 100% of preirradiation values (9–10 wk). At that time, reconstituted mice received an adoptive transfer of WT CD45RBHI and CD45RBLO Th cells intraperitoneally and were monitored as described above. All reconstituted mice were kept on water containing 0.24 mg/ml trimethoprim and 1.2 mg/ml sulfamethoxazole beginning 5 days prior to irradiation and continuing until 5 days prior to adoptive transfer.
Statistical analysis.
Values are means ± SE. Data were compared by ANOVA followed by Tukey's post hoc comparison (if ANOVA was significant) or Student's t-test (unpaired) unless normality tests demonstrated a need for nonparametric tests, in which case a Kruskal-Wallis test was performed. Results were considered significant if P ≤ 0.05.
RESULTS
H. hepaticus induces colitis in A2AAR−/− mice.
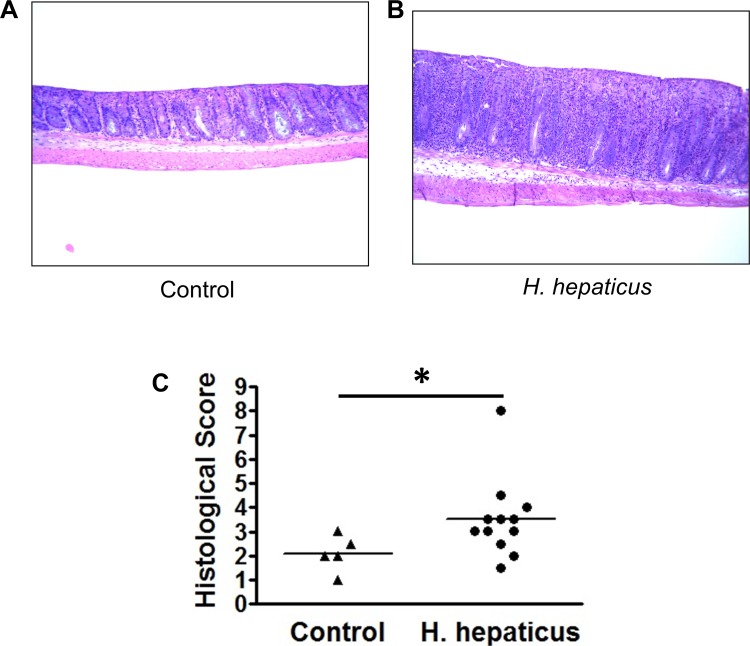
Female A2AAR−/− mice were inoculated with H. hepaticus and monitored weekly for signs of colonic inflammation (soft stools, blood in stools, and rectal prolapse). After 33 wk, mice were euthanized and colons were removed and processed for histology. All A2AAR−/− mice given a control gavage of PBS had normal colonic histology with healthy epithelium and no signs of pathological immune infiltration (Fig. 1A). Of the 12 A2AAR−/− mice inoculated with H. hepaticus, 3 developed rectal prolapse and moderate-to-severe colitis (Fig. 1B), suggesting an increase in the sensitivity of these mice to luminal provocation. Histological scores indicated a significant increase in the inflammation in the H. hepaticus-inoculated mice that included infiltration with PMN cells (Fig. 1C). The importance of the bacterial stimulus is appreciated in view of the fact that rectal prolapse and colitis have never been observed in A2AAR−/− mice maintained in our facilities over the last 8 years.
Fig. 1.
Induction of colitis in A2A adenosine receptor (A2AAR)-deficient (A2AAR−/−) mice after inoculation with H. hepaticus. A and B: A2AAR−/− mice were inoculated with PBS (control) or 1 × 108 colony-forming units of H. hepaticus and monitored for signs of disease. C: at 33 wk postinoculation, mice were euthanized, and colon tissues were fixed, stained, and scored for histological damage. Dot plot represents combined data from 2 separate experiments; horizontal bars represent means [n = 5 (control) and 12 (H. hepaticus)]. *P < 0.05.
Changes in T cell subsets in A2AAR−/− mice.
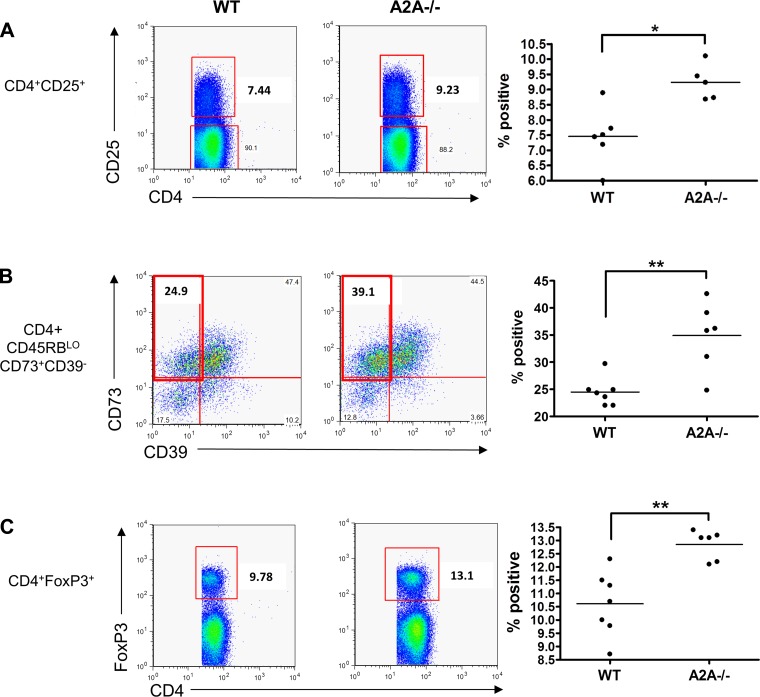
In view of the development of colitis after H. hepaticus administration in the A2AAR−/− mice, we hypothesized that A2AAR−/− mice have fewer regulatory T (Treg) cells and, thus, less control over inflammation, than their WT counterparts. Therefore, we used flow cytometry to look for differences in the expression of Treg markers on noninfected T cells from WT and A2AAR−/− mice. Splenic CD4+ Th cells were analyzed for seven surface markers (CD4, CD45RB, CD25, CD73, CD39, FR4, and GITR) and intracellular FoxP3 using simultaneous, 10-parameter flow cytometry. A2AAR−/− mice had a higher percentage of CD4+CD25+ splenocytes than WT mice, which could be indicative of an increase in activated Th and/or Treg cells (Fig. 2A). Within the CD45RBLO compartment of CD4+ cells, a greater percentage of CD73+CD39− cells was found in the A2AAR−/− mice, suggesting an increase in primed uncommitted Th cells (Fig. 2B). Moreover, these mice also had an increased percentage of CD4+ FoxP3+ cells (Fig. 2C), suggesting a higher percentage of Treg cells in the A2AAR−/− mice. All other Th cell subsets investigated were not significantly different. Thus the increased susceptibility of the A2AAR−/− mice to infection is not due to any detectable shortage of Th cells that express markers associated with Treg cells.
Fig. 2.
A2AAR−/− helper T (Th) cells express more activation and regulatory T (Treg) cell markers than wild-type (WT) cells. CD4+ splenocytes from WT and A2AAR−/− (A2A−/−) mice were isolated and stained for fluorescein-activated cell sorting. A2AAR−/− Th cells expressed more CD25 (A) and FoxP3 (C) than WT cells. Within the CD45RBLO population, which contains a subset of Treg cells, more A2AAR−/− cells expressed CD73 in the absence of CD39 (B), suggesting an activated, and not strictly Treg, phenotype (n = 5–7 per group). *P < 0.01, **P < 0.005.
RAG1−/−/A2AAR−/− mice develop severe colitis rapidly after adoptive transfer.
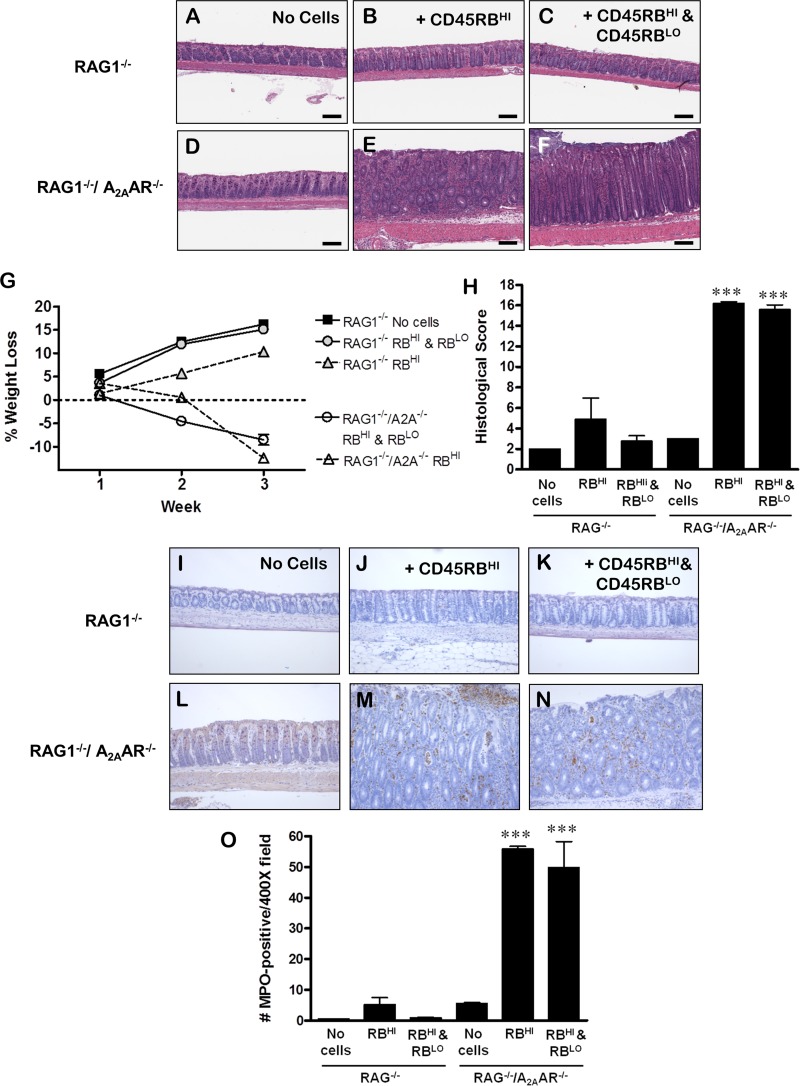
A previous study from our laboratory demonstrated the importance of A2AAR expression on T cells in the development of colitis in the CD45RB model (26). To determine whether A2AAR expression is essential only on T cells or whether adenosine signaling in other (recipient) cell types is important in the control of colitis in this model, we created a RAG1−/−/A2AAR−/− mouse. WT CD45RBHI cells, alone or in combination with WT CD45RBLO cells, were adoptively transferred into RAG1−/− or RAG1−/−/A2AAR−/− mice. After 2 wk, RAG1−/−/A2AAR−/− recipients suffered significant weight loss (Fig. 3G) and developed perianal inflammation and soft stools (unpublished observations). Interestingly, the RAG1−/−/A2AAR−/− mice that received CD45RBHI cells and those that received CD45RBHI with CD45RBLO cells showed this rapid onset of disease. Neither group of RAG1−/− recipients developed disease by 21–27 days posttransfer (Fig. 3G), although those receiving CD45RBHI cells alone developed colitis if the experiment was continued to 8 wk posttransfer (data not shown, similar to Ref. 26). The rapid onset of wasting was associated with the severity of colonic inflammation in RAG1−/−/A2AAR−/− mice, including significant thickening, large infiltrates of PMN and mononuclear cells, significant damage to the epithelium, edema, and immune infiltration of the submucosa and muscularis (Fig. 3, E, F, and H). Neutrophil infiltration was significant, as evidenced by the dramatic increase in MPO+ cells in the tissue (Fig. 3, M–O). RAG1−/− mice did not develop significant colitis at this early time point and, therefore, had low histological damage scores and little infiltration of MPO+ cells, although the RAG1−/− group that received CD45RBHI cells alone was beginning to show signs of immune infiltration (Fig. 3, B, H, J, and O). RAG1−/− and RAG1−/−/A2AAR−/− mice that did not receive an adoptive transfer of Th cells did not develop significant intestinal inflammation (Fig. 3, A, D, H, I, L, and O).
Fig. 3.
A2AAR on recipient cells is essential for proper control of colitis in the adoptive transfer model. A–H: CD45RBHI cells alone or with CD45RBLO cells were adoptively transferred into RAG1−/− or RAG1−/−/A2AAR−/− (double-knockout) mice. RAG1−/−/A2AAR−/− mice developed severe colitis 24–27 days after transfer of WT CD45RBHI (E) or WT CD45RBHI with WT CD45RBLO (F) Th cells, as shown by wasting (G) and severe histological inflammation and damage scores (H). At the same time point, RAG1−/− mice that received WT CD45RBHI (B) or WT CD45RBHI with WT CD45RBLO (C) Th cells had not developed wasting (G) or colitis, although the WT CD45RBHI group was beginning to show signs of inflammation (H). Control mice from the RAG1−/− (A) and RAG1−/−/A2AAR−/− (D) recipient groups that did not receive an adoptive transfer did not develop colitis (H). I–O: sections of colon from RAG1−/− or RAG1−/−/A2AAR−/− mice 24–27 days after adoptive transfer of WT CD45RBHI cells alone or with WT CD45RBLO cells were stained with antibodies to myeloperoxidase (MPO). RAG1−/− (I) or RAG1−/−/A2AAR−/− (L) control mice that received no cells had few MPO+ cells. RAG1−/− mice that received CD45RBHI cells (J) had slightly more MPO+ cells than RAG1−/− mice that received CD45RBHI and CD45RBLO cells (K), but the difference was not significant. RAG1−/−/A2AAR−/− mice that received WT CD45RBHI cells alone (M) or with WT CD45RBLO cells (N) had the greatest numbers of MPO+ cells, significantly more than all other groups (O). Scale bars, 150 μm. P < 0.05, by ANOVA (G). P < 0.0001 by ANOVA; ***P < 0.001 by pair-wise comparison vs. all other groups (H and O). Data in G were pooled from 2 separate experiments (n = 3 per group). Data in H and O were pooled from 3 separate experiments (n = 2–8 per group, n = 2 for untreated controls).
Proinflammatory cytokines are expressed in association with the rapid onset of disease in RAG1−/−/A2AAR−/− mice.
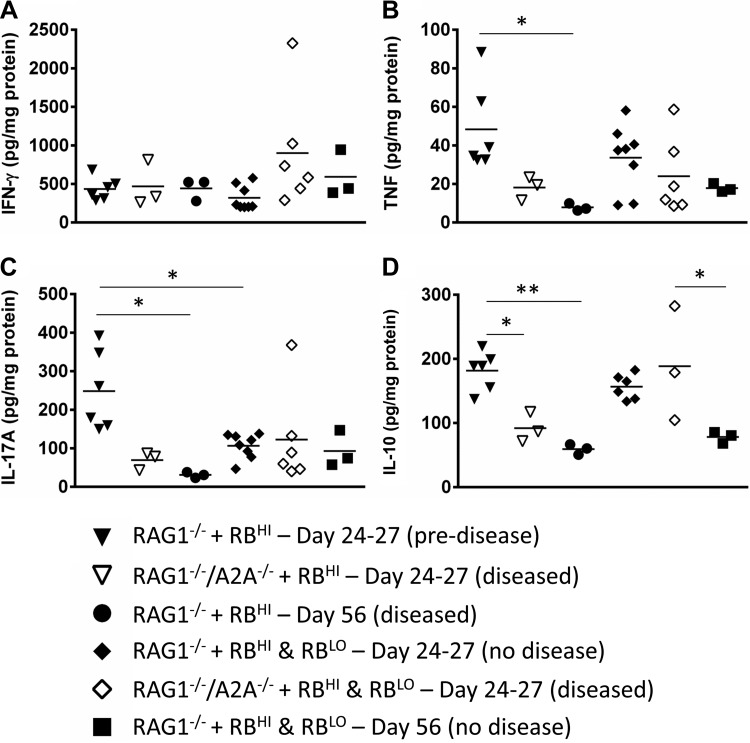
The expression of key proinflammatory (IFN-γ, IL-17A, and TNF-α) and anti-inflammatory (IL-10) cytokines was measured in the colonic tissue. IFN-γ levels did not differ significantly between the groups (Fig. 4A). TNF and IL-17A were increased before the onset of disease in RAG1−/− mice that received CD45RBHI cells and decreased when disease was severe (Fig. 4, B and C). Among the groups that received CD45RBHI cells, IL-10 levels were highest in RAG1−/− mice before the onset of colitis, suggesting an attempt to control the development of disease (Fig. 4D). As with the proinflammatory cytokines, these levels dropped before the onset of severe colitis. The only difference between RAG1−/−/A2AAR−/− recipient mice and their RAG1−/− counterparts receiving the same cell types was an increase in the production of IL-10 in RAG1−/−/A2AAR−/− mice at the onset of disease after transfer of both CD45RBHI and CD45RBLO cells (Fig. 4D). This is interesting, as it suggests that at least some immunosuppressive function is maintained in these mice after cotransfer with the Treg-containing CD45RBLO cells, even after the development of severe colitis. To validate the assessment of the cytokines in the colon, mesenteric lymph node cells from each cohort were pooled and assayed for intracellular cytokine expression by flow cytometry. Th cells from RAG1−/− mice receiving CD45RBHI cells alone had a higher percentage of cells expressing IFN-γ or IL-17A than the cohort receiving both CD45RBHI and CD45RBLO cells. The percentage of Th cells expressing these cytokines was much higher in RAG1−/−/A2AAR−/− mice after receiving CD45RBHI alone or CD45RBHI and CD45RBLO cells (data not shown). Together, these data confirm the disruption of cytokine regulation associated with disease.
Fig. 4.
Cytokine expression in the colon of RAG1−/−/A2AAR−/− mice after adoptive transfer is similar to that in the colon of RAG1−/− recipients. At the time the animals were euthanized, a portion of the colon was collected, processed, and assayed by ELISA for IFN-γ (A), TNF (B), IL-17A (C), and IL-10 (D). RAG1−/−/A2AAR−/− mice were euthanized at the onset of disease (24–27 days posttransfer). RAG1−/− mice were euthanized at 24–27 days posttransfer (before disease onset in these mice) or at the time of disease onset (56 days posttransfer). Tissue IFN-γ levels were not significantly different between the 4 groups at 24–27 days posttransfer or at the onset of disease (A). TNF-α levels (B) were highest in RAG1−/− mice 24–27 days after adoptive transfer of CD45RBHI cells (▼) but decreased by the time of disease onset in these mice (○). IL-17A (C) was also higher in RAG1−/− mice 24–27 days after transfer of CD45RBHI cells (▼) than in those that received cotransfer with CD45RBLO at the same time point (⧫), but levels dropped by disease onset (●). IL-10 (D) was higher in RAG1−/− mice 24–27 days after transfer of CD45RBHI alone (▼) or cotransfer with CD45RBLO (⧫) but dropped by the onset of colitis (●). At disease onset, RAG1−/−/A2AAR−/− mice that received CD45RBHI and CD45RBLO cells (◇) had significantly greater amounts of tissue IL-10 than RAG1−/− mice that received CD45RBHI and CD45RBLO cells (D, ■). *P ≤ 0.05; ** P ≤ 0.005. Dot plots of cytokine levels for RAG1−/− mice before the onset of disease and RAG1−/−/A2AAR−/− mice at the onset of disease (24–27 days posttransfer) represent pooled data from 3 separate experiments (n = 3–8 per group). Dot plots of cytokine levels at the onset of disease in RAG1−/− mice represent data from 1 experiment (n = 3 per group).
Rapid onset of severe colitis in RAG1−/−/A2AAR−/− mice is due to the lack of A2AAR on stromal or radiation-resistant myeloid cells.
To determine whether the accelerated onset of colitis in RAG1−/−/A2AAR−/− recipient mice was due to A2AAR-deficient myeloid cells, we created chimeric mice to be used as recipients for the adoptive transfer experiment. The chimeric mice will have myeloid cells reflecting the phenotype of the different donor strains of mice and stromal cells of the recipient. To determine if the absence of A2AAR on bone marrow-derived myeloid cells was sufficient to accelerate the colitis in the RAG1−/− recipients, we transferred the bone marrow of RAG1−/− (RAG1−/− → RAG1−/−) or RAG1−/−/A2AAR−/− (RAG1−/−/A2AAR−/− → RAG1−/−) mice into irradiated RAG1−/− recipients. Thus, in the RAG1−/−/A2AAR−/− → RAG1−/− mice, the stromal cells expressed A2AAR, but the myeloid cells from the bone marrow did not. RAG1−/− → RAG1−/− chimeric mice expressed A2AAR on both stromal and myeloid cells. After confirmation that the bone marrow cells had engrafted, the chimeric mice received an adoptive transfer of CD45RBHI and CD45RBLO Th cells (an adoptive transfer that induces disease in RAG1−/−/A2AAR−/−, but not RAG1−/−, mice). Control, nonchimeric mice also received an adoptive transfer. As expected, control RAG1−/− mice did not develop colitis after adoptive transfer of CD45RBHI and CD45RBLO Th cells, but RAG1−/−/A2AAR−/− mice developed severe colitis within 2 wk posttransfer and had to be euthanized by 5 wk posttransfer (Fig. 5). RAG1−/−/A2AAR−/− → RAG1−/− chimeric mice did not develop colitis as quickly as the control RAG1−/−/A2AAR−/− mice following adoptive transfer of CD45RBHI and CD45RBLO Th cells. At 9 wk posttransfer, these mice had weight loss and clinical scores similar to RAG1−/− → RAG1−/− chimeric mice that did not receive adoptive transfer, demonstrating that the lack of A2AAR on bone marrow-derived myeloid cells is not sufficient for the rapid onset of severe colitis induced by adoptive transfer of CD45RBHI and CD45RBLO Th cells into RAG1−/−/A2AAR−/− mice. This suggests that stromal or radiation-resistant myeloid cells also play an important role.
Fig. 5.
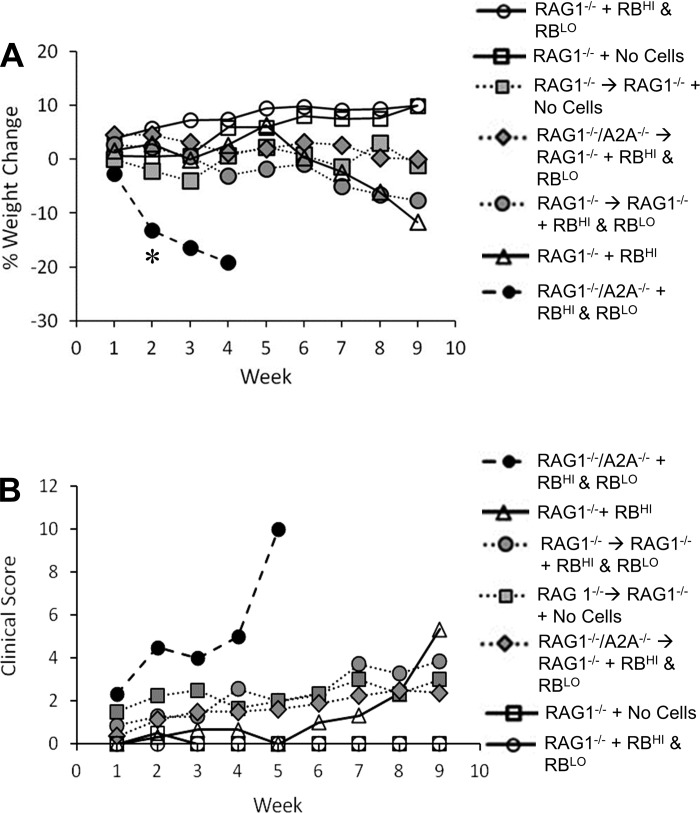
Colitis in RAG1−/−/A2AAR −/− hosts is not solely dependent on bone marrow-derived cells. RAG1−/− mice were irradiated and reconstituted with RAG1−/− (RAG1−/− → RAG1−/−) or RAG1−/−/A2AAR−/− (RAG1−/−/A2AAR−/− → RAG1−/−) bone marrow. After recovery, mice received an adoptive transfer of CD45RBHI Th cells and CD45RBLO Th cells from WT mice. Body weight (A) and clinical disease (B) were monitored weekly. Scoring system for clinical disease (B) incorporated weight loss, hair coat grooming, eye alertness, nasal discharge, activity level, and posture. Colitis induced by CD45RBHI cells was still controlled by CD45RBLO cells in RAG1−/−/A2AAR−/− → RAG1−/− chimeric mice, suggesting that A2AAR-deficient myeloid cells are not solely responsible for the severe disease caused by this transfer in RAG1−/−/A2AAR−/− mice. Data were pooled from 2 separate experiments; n = 2–8 survivors per group (all groups started with 7–8 animals, but after 2 wk mortality decreased the size of the “RAG1−/−/A2AAR−/− + RBHI & RBLO” group to 2 animals). *P < 0.05.
DISCUSSION
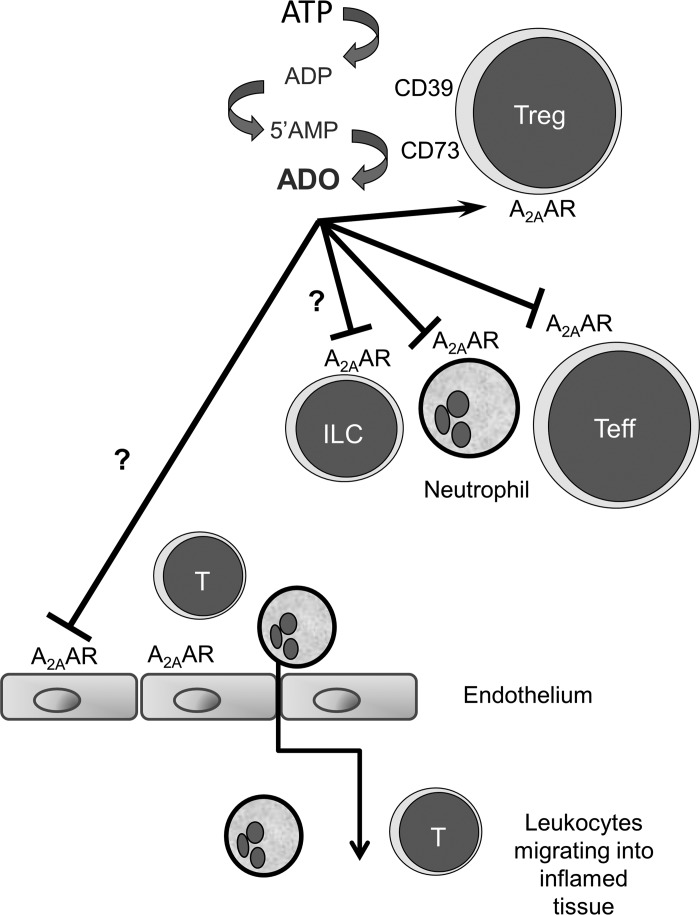
Inflammatory bowel disease is believed to reflect an excessive host response to luminal bacteria. The abnormalities in immune regulation have been modeled using the CD45RB adoptive transfer model of colitis to probe which cells that express A2AAR contribute to the control of colitis (26). The expression of genes in transferred T cells and the inflamed tissue in this model have been reported to more closely resemble changes in human disease than other approaches such as the dextran sodium sulfate or trinitrobenzene sulfonic acid model (36). While the microbial triggers of human disease are not known, H. hepaticus has been used as a microbial provocation that enhances colitis in several animal models in which immune regulation is disrupted (23, 37). In the current study we have applied these approaches to evaluate the role of adenosine in maintaining immunological homeostasis to H. hepaticus and the role of A2AAR expressed by nonlymphoid cells in the control of disease. The current study shows that extracellular adenosine has an essential role in the control of colitis by targeting A2AAR on lymphoid and nonlymphoid cells (Fig. 6).
Fig. 6.
Role of A2AAR on different cell types in the CD45RB model of colitis. In inflammation, ATP accumulates due to cell death and, in some cases, is produced by bacteria. It is clear from past studies that Treg cells express the enzymes CD39 and CD73, which metabolize ATP to ADP, 5′-AMP, and then adenosine (ADO). Adenosine plays an important role in enhancing the function of Treg cells and the suppression of inflammation mediated by activated Th (Teff) cells, neutrophils, and possibly other inflammatory cells such as innate lymphoid cells (ILCs). While expression of A2AAR on T cells is essential for the optimal control of colitis in this model (26), the current study highlights an important and previously unknown role for expression of A2AAR on recipient cells as well. Data from chimeric mice suggest that radiation-sensitive myeloid cells are not the only lineage affected by adenosine, as stromal or radiation-resistant myeloid cells such as ILCs may also respond to adenosine and play an essential role in the control of colitis. A2AAR expression on stromal cells (particularly vascular endothelium) may regulate recruitment of immune/inflammatory cells to the gut, such that the absence of A2AAR favors accumulation of proinflammatory cells. For example, engagement of A2AAR may impair expression of chemokines or adhesion molecules and, thereby, restrict egress of leukocytes from the circulation. These possibilities require additional investigation.
Colitis caused by H. hepaticus is an interesting model to study the control of immunological homeostasis, as it is known that disease is prevented by Treg cells (23). Treg cells inhibit host responses through several mechanisms (32, 33), including the production of IL-10 (23), transforming growth factor-β1 (4), or IL-35 (10) and cytokine deprivation (30). In addition, Treg cells express CD39 and CD73, two enzymes that convert ATP to adenosine (1, 7, 11, 20). Production of adenosine by Treg cells contributes to immune regulation in vitro (7, 11, 20) and controls gastrointestinal inflammation in vivo (1, 2, 16, 26). Typically, adenosine can act in a paracrine or autocrine fashion to activate A2AAR and increase intracellular cAMP, possibly leading to the direct transfer of cAMP from Treg to target cells (5, 6) or to cAMP-mediated inhibition of proinflammatory cytokine production (3, 13, 17, 24, 26). The critical role of A2AAR was supported by our observation that A2AAR−/− mice developed colitis after inoculation with H. hepaticus, a phenomenon not observed in WT mice (23). A2AAR−/− mice do not develop inflammatory disease under normal conditions but rapidly develop disease upon provocation. For example, A2AAR−/− mice inoculated with Helicobacter felis developed more severe gastritis than WT mice (2), and concanavalin A-induced liver injury was significantly greater in A2AAR−/− than WT mice (28). However, since multiple cell lineages express A2AAR, the cellular targets for adenosine have not been completely defined.
Colitis caused by H. hepaticus is controlled by IL-10 and Treg cells (23); thus it is possible that the absence of A2AAR−/− decreased the number or activity of Treg cells. However, our results showed that A2AAR−/− mice had a greater percentage of Treg (FoxP3+) cells than WT mice. Treg cells from A2AAR−/− mice appear to be functional, as they are able to suppress in vitro (unpublished data). Furthermore, Th cells from A2AAR−/− mice secrete IL-10 in amounts equivalent to WT Th cells in vitro (26), and IL-10 production was evident in tissues or Th cells from diseased mice. IL-10 production is greater in A2AAR−/− CD45RBLO cells in vitro than WT cells (unpublished data), a finding that does not explain their defect in the CD45RB transfer model. As we have been unable to associate any deficiency in adenosine production or function with an effect on transforming growth factor-β1 or IL-35 (unpublished observations), the data presented here suggest that a “pool” of extracellular adenosine is directly responsible for controlling target cells of multiple lineages to maintain immunological homeostasis.
While Treg cells from A2AAR−/− mice can mediate regulatory function in vitro, a previous study from our laboratory showed that Treg cells (as represented by the heterogenous group of CD45RBLO cells) lacking this receptor were unable to suppress WT or A2AAR−/− effectors in the CD45RB transfer model of colitis (26). These observations are verified by a three- to fourfold increase in histological damage scores compared with cotransfer of WT CD45RBHI and CD45RBLO. In addition, A2AAR−/− CD45RBHI cells were not suppressed by WT CD45RBLO cells (26). A2BAR has also been studied for its role in the development of colitis. A2BAR is the predominant adenosine receptor on colonic epithelium and is expressed on some immune cell types, including myeloid cells and possibly T cells (14). The role of A2BAR in the development of colitis is controversial, however, as some groups demonstrate detrimental effects (19, 21, 22), while others suggest protective effects (15), of A2BAR stimulation. The role of A2AAR in controlling inflammation is better understood, and because we did not see a change in A2BAR expression in Th cell subsets of A2AAR−/− mice or colonic tissue of RAG1−/−/A2AAR−/− mice (unpublished observations), we focused solely on the role of A2AAR in colitis. The difference we observed in the A2AAR dependency of Treg responses in vitro and in vivo suggests that suppression in vivo is more dependent on adenosine signaling through A2AAR in T cells. Another interpretation suggests an important role for non-T (host) cells in the control of T cell-mediated colitis.
A2AAR are expressed by myeloid cells, natural killer cells, and endothelial cells (14, 18). Previous research has shown that A2AAR agonists suppress inflammatory disease in animal models (2, 26, 27). The specific target of these agonists could be myeloid or nonimmune cells, as well as lymphocytes. A2AAR signaling can have significant suppressive effects on neutrophils by inhibiting adhesion, extravasation, activation, cytokine production, and oxidative burst (18). Furthermore, the function of macrophages and dendritic cells is regulated by A2AAR (25), and these cells are prominent in the colitis model. Therefore, we speculated that myeloid cells may also be targets for adenosine, and we created a RAG1−/−/A2AAR−/− mouse for use as a recipient in adoptive transfer experiments. Severe colitis developed rapidly in these animals after transfer of CD45RBHI Th cells alone or with CD45RBLO cells. This is contrary to findings in adoptive transfer experiments using RAG1−/−/A2BAR−/− mice (19), further supporting the divergent roles of these receptors in the development of colitis. The rapid onset of disease in the RAG1−/−/A2AAR−/− mice suggests a role for adenosine signaling in myeloid cells in this model. To test whether the lack of adenosine stimulation on myeloid cells accounts for the early onset of disease in RAG1−/−/A2AAR−/− recipients, we produced chimeric mice. However, colitis did not develop after the cotransfer of CD45RBHI and CD45RBLO cells in any of the chimeric recipients in which myeloid cells were derived from RAG1−/−/A2AAR−/− mice. These results suggest two alternative explanations. First, nonlymphoid cells may mediate the suppressive effects of A2AAR signaling. Endothelial cells, for example, express A2AAR, and stimulation of the receptor leads to decreased inflammatory cytokine secretion and adhesion molecule expression (18). This may have dramatic effects on the activation, adhesion, and extravasation of immune cells into the colon. Another possibility is the role of A2AAR signaling on a radiation-resistant cell type derived from the bone marrow that persisted and was able to control inflammation in these chimeras. ILCs are radiation-resistant (31), and we have found that both ILC1 and ILC3 express A2AAR (unpublished data). ILCs play a role in the development of inflammatory disease (35), although it is challenging to enrich them sufficiently to investigate their role directly. No ILC-specific transcription factor has been identified, limiting the potential use of a conditional knockout. Still, the expression of A2AAR on ILC1 subsets from the spleen and ILC3 subsets from Peyer's patches of the gut suggests an interesting future research direction to elucidate their role in the development of colitis. It is difficult to pinpoint the specific contributions of multiple lineages that express A2AAR, as the effects of manipulating A2AAR function may be masked by redundant processes.
In summary, this study provides new insights into the importance of adenosine in the control of inflammation through its effects on myeloid or nonlymphoid cells. The generation of a RAG1−/−/A2AAR−/− mouse as a recipient in adoptive transfer suggests a prominent role for adenosine signaling in these cells in the regulation of colitis. While adenosine is produced by several sources, including Treg cells, this mediator targets a more complex regulatory axis than was previously envisaged. Future studies are needed to evaluate the relative contribution of multiple cell lineages that respond to adenosine in the complex control of immunological homeostasis.
GRANTS
This work was supported by a Research Fellowship Award from the Crohn's and Colitis Foundation of America (to C. C. Kurtz) and National Institute on Allergy and Infectious Diseases Grants AI-070491 and AI-079145 (to P. B. Ernst).
DISCLOSURES
P. B. Ernst collaborates with Lewis and Clark Pharmaceuticals on studies of adenosine function. No other conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.C.K., I.D., M.N., S.H.F., J.L., C.F.W., and P.B.E. are responsible for conception and design of the research; C.C.K., I.D., M.N., and V.B. performed the experiments; C.C.K., I.D., M.N., and V.B. analyzed the data; C.C.K., I.D., S.H.F., V.B., J.L., C.F.W., and P.B.E. interpreted the results of the experiments; C.C.K. and I.D. prepared the figures; C.C.K. drafted the manuscript; C.C.K., I.D., M.N., S.H.F., V.B., J.L., C.F.W., and P.B.E. edited and revised the manuscript; C.C.K., I.D., M.N., S.H.F., V.B., J.L., C.F.W., and P.B.E. approved the final version of the manuscript.
ACKNOWLEDGMENTS
The authors thank Mary Harp, Elizabeth Wiznerowicz, Steven Black, Mohammad S. Alam, William G. Ross, and Jeffrey Wilson for technical assistance.
Present address of C. C. Kurtz: Department of Biology, University of Wisconsin Oshkosh, Oshkosh, WI 54901.
REFERENCES
- 1.Alam MS, Kurtz CC, Rowlett RM, Reuter BK, Wiznerowicz E, Das S, Linden J, Crowe SE, Ernst PB. CD73 is expressed by human regulatory T helper cells and suppresses proinflammatory cytokine production and Helicobacter felis-induced gastritis in mice. J Infect Dis 199: 494–504, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alam MS, Kurtz CC, Wilson JM, Burnette BR, Wiznerowicz EB, Ross WG, Rieger JM, Figler RA, Linden J, Crowe SE, Ernst PB. A2A adenosine receptor (AR) activation inhibits pro-inflammatory cytokine production by human CD4+ helper T cells and regulates Helicobacter-induced gastritis and bacterial persistence. Mucosal Immunol 2: 232–242, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armstrong JM, Chen JF, Schwarzschild MA, Apasov S, Smith PT, Caldwell C, Chen P, Figler H, Sullivan G, Fink S, Linden J, Sitkovsky MV. Gene dose effect reveals no Gs-coupled A2A adenosine receptor reserve in murine T-lymphocytes: studies of cells from A2A-receptor-gene-deficient mice. Biochem J 354: 123–130, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asseman C, Fowler S, Powrie F. Control of experimental inflammatory bowel disease by regulatory T cells. Am J Respir Crit Care Med 162: S185–S189, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Bopp T, Becker C, Klein M, Klein-Hessling S, Palmetshofer A, Serfling E, Heib V, Becker M, Kubach J, Schmitt S, Stoll S, Schild H, Staege MS, Stassen M, Jonuleit H, Schmitt E. Cyclic adenosine monophosphate is a key component of regulatory T cell-mediated suppression. J Exp Med 204: 1303–1310, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bopp T, Dehzad N, Reuter S, Klein M, Ullrich N, Stassen M, Schild H, Buhl R, Schmitt E, Taube C. Inhibition of cAMP degradation improves regulatory T cell-mediated suppression. J Immunol 182: 4017–4024, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Borsellino G, Kleinewietfeld M, Di MD, Sternjak A, Diamantini A, Giometto R, Hopner S, Centonze D, Bernardi G, Dell'Acqua ML, Rossini PM, Battistini L, Rotzschke O, Falk K. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood 110: 1225–1232, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Buonocore S, Ahern PP, Uhlig HH, Ivanov II, Littman DR, Maloy KJ, Powrie F. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature 464: 1371–1375, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chin AC, Parkos CA. Neutrophil transepithelial migration and epithelial barrier function in IBD: potential targets for inhibiting neutrophil trafficking. Ann NY Acad Sci 1072: 276–287, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Collison LW, Chaturvedi V, Henderson AL, Giacomin PR, Guy C, Bankoti J, Finkelstein D, Forbes K, Workman CJ, Brown SA, Rehg JE, Jones ML, Ni HT, Artis D, Turk MJ, Vignali DA. IL-35-mediated induction of a potent regulatory T cell population. Nat Immunol 11: 1093–1101, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, Chen JF, Enjyoji K, Linden J, Oukka M, Kuchroo VK, Strom TB, Robson SC. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med 204: 1257–1265, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eltzschig HK, Sitkovsky MV, Robson SC. Purinergic signaling during inflammation. N Engl J Med 367: 2322–2333, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Erdmann AA, Gao ZG, Jung U, Foley J, Borenstein T, Jacobson KA, Fowler DH. Activation of Th1 and Tc1 cell adenosine A2A receptors directly inhibits IL-2 secretion in vitro and IL-2-driven expansion in vivo. Blood 105: 4707–4714, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ernst PB, Thompson LF. Much ado about adenosine. Adenosine synthesis and function in regulatory T cell biology. J Immunol 185: 1993–1998, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frick JS, MacManus CF, Scully M, Glover LE, Eltzschig HK, Colgan SP. Contribution of adenosine A2B receptors to inflammatory parameters of experimental colitis. J Immunol 182: 4957–4964, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Friedman DJ, Kunzli BM, Rahim YI, Sevigny J, Berberat PO, Enjyoji K, Csizmadia E, Friess H, Robson SC. CD39 deletion exacerbates experimental murine colitis and human polymorphisms increase susceptibility to inflammatory bowel disease. Proc Natl Acad Sci USA 106: 16788–16793, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hasko G, Cronstein B. Regulation of inflammation by adenosine. Front Immunol 4: 85, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hasko G, Cronstein BN. Adenosine: an endogenous regulator of innate immunity. Trends Immunol 25: 33–39, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Ingersoll SA, Laroui H, Kolachala VL, Wang L, Garg P, Denning TL, Gewirtz AT, Merlin D, Sitaraman SV. A2BAR expression in non-immune cells plays an important role in the development of murine colitis. Dig Liver Dis 44: 819–826, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kobie JJ, Shah PR, Yang L, Rebhahn JA, Fowell DJ, Mosmann TR. T regulatory and primed uncommitted CD4 T cells express CD73, which suppresses effector CD4 T cells by converting 5′-adenosine monophosphate to adenosine. J Immunol 177: 6780–6786, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Kolachala V, Ruble B, Vijay-Kumar M, Wang L, Mwangi S, Figler H, Figler R, Srinivasan S, Gewirtz A, Linden J, Merlin D, Sitaraman S. Blockade of adenosine A2B receptors ameliorates murine colitis. Br J Pharmacol 155: 127–137, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kolachala VL, Vijay-Kumar M, Dalmasso G, Yang D, Linden J, Wang L, Gewirtz A, Ravid K, Merlin D, Sitaraman SV. A2B adenosine receptor gene deletion attenuates murine colitis. Gastroenterology 135: 861–870, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kullberg MC, Jankovic D, Gorelick PL, Caspar P, Letterio JJ, Cheever AW, Sher A. Bacteria-triggered CD4+ T regulatory cells suppress Helicobacter hepaticus-induced colitis. J Exp Med 196: 505–515, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lappas CM, Rieger JM, Linden J. A2A adenosine receptor induction inhibits IFN-γ production in murine CD4+ T cells. J Immunol 174: 1073–1080, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Linden J. Regulation of leukocyte function by adenosine receptors. Adv Pharmacol 61: 95–114, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naganuma M, Wiznerowicz EB, Lappas CM, Linden J, Worthington MT, Ernst PB. Critical role for adenosine A2A receptors in the T cell mediated regulation of colitis. J Immunol 177: 2765–2769, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Odashima M, Bamias G, Rivera-Nieves J, Linden J, Nast CC, Moskaluk CA, Marini M, Sugawara K, Kozaiwa K, Otaka M, Watanabe S, Cominelli F. Activation of A2A adenosine receptor attenuates intestinal inflammation in animal models of inflammatory bowel disease. Gastroenterology 129: 26–33, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Ohta A, Sitkovsky M. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature 414: 916–920, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Ostanin DV, Bao J, Koboziev I, Gray L, Robinson-Jackson SA, Kosloski-Davidson M, Price VH, Grisham MB. T cell transfer model of chronic colitis: concepts, considerations, and tricks of the trade. Am J Physiol Gastrointest Liver Physiol 296: G135–G146, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pandiyan P, Zheng L, Ishihara S, Reed J, Lenardo MJ. CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+ T cells. Nat Immunol 8: 1353–1362, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Romera-Hernandez M, Aparicio-Domingo P, Cupedo T. Damage control: Rorγt+ innate lymphoid cells in tissue regeneration. Curr Opin Immunol 25: 156–160, 2013 [DOI] [PubMed] [Google Scholar]
- 32.Sakaguchi S, Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T. Regulatory T cells: how do they suppress immune responses? Int Immunol 21: 1105–1111, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity 30: 636–645, 2009 [DOI] [PubMed] [Google Scholar]
- 35.Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, Koyasu S, Locksley RM, McKenzie AN, Mebius RE, Powrie F, Vivier E. Innate lymphoid cells—a proposal for uniform nomenclature. Nat Rev Immunol 13: 145–149, 2013 [DOI] [PubMed] [Google Scholar]
- 36.te Velde AA, de KF, Sterrenburg E, Pronk I, ten Kate FJ, Hommes DW, van Deventer SJ. Comparative analysis of colonic gene expression of three experimental colitis models mimicking inflammatory bowel disease. Inflamm Bowel Dis 13: 325–330, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Ward JM, Anver MR, Haines DC, Melhorn JM, Gorelick P, Yan L, Fox JG. Inflammatory large bowel disease in immunodeficient mice naturally infected with Helicobacter hepaticus. Lab Anim Sci 46: 15–20, 1996 [PubMed] [Google Scholar]