Abstract
Colorectal cancer is a heterogeneous disease that is one of the major causes of cancer death in the U.S. There is evidence that lifestyle factors like diet can modulate the course of this disease. Demonstrating the benefit and mechanism of action of dietary interventions against colon cancer will require studies in preclinical models. Many mouse models have been developed to study colon cancer but no single model can reflect all types of colon cancer in terms of molecular etiology. In addition, many models develop only low-grade cancers and are confounded by development of the disease outside of the colon. This review will discuss how mice can be used to model human colon cancer and it will describe a variety of new mouse models that develop colon-restricted cancer as well as more advanced phenotypes for studies of late-state disease.
Keywords: animal, preclinical, chemoprevention, mutation, sporadic
sporadic colorectal cancer is the third most common malignancy in the U.S. with a 1:5 lifetime risk of developing the disease (3). Although both men and women are affected, there is some evidence that men have a higher incidence and earlier onset than women (1, 84). Five-year survival rates are high for people with early stages of colorectal cancer but fall dramatically at the later stages. Thus research to improve the diagnosis, prevention, and treatment of colorectal cancer is crucial to reducing colorectal cancer mortality.
Chemoprevention is the term given to efforts that block cancer initiation, slow tumor growth and metastasis, or inhibit cancer recurrence after treatment. The basic premise of chemoprevention is that by extending the natural history of colorectal cancer, we can reduce the number of people who need treatment, expand the window during which highly treatable low-grade cancers are detected and diagnosed, and reduce the chance of cancer recurrence after treatment (113). Many population-based studies suggest that dietary factors are important chemopreventative agents against colorectal cancer (59, 69). However, studies identifying associations between dietary factors and cancer risk cannot prove causation, and this makes the links between diet and colorectal cancer tenuous. Clinical trials carefully designed to test the impact of dietary agents on colorectal cancer are practical for some (e.g., biomarker studies, cancer recurrence) but not other (e.g., lifelong changes, studies of tumor promotion, progression, or metastasis) chemoprevention goals. For example, human tumors develop over a lifetime and are usually diagnosed in middle-aged adults. This makes early intervention studies aimed at regulating cancer initiation and preventing tumor development impractical from a cost and sample size standpoint.
As a result, alternative strategies to test the biological validity of various chemoprevention hypotheses are needed. Preclinical animal models provide the best hope for the careful evaluation of dietary chemoprevention strategies, to study the molecular mechanisms of colorectal carcinogenesis, and to translate mechanistic hypotheses derived from cell models into whole organisms. Many animal models for colorectal cancer have been developed and used to understand colon carcinogenesis or to test dietary prevention strategies (Table 1). Interested readers should refer to earlier reviews for detailed descriptions of long-standing models like the ApcMin mouse or those whose cancer results from chemical induction (48, 91, 108). In this review, I will provide a perspective for how mouse models can be developed to best reflect human colon cancer. In addition, I will discuss how a number of recent models have improved our ability to study colorectal cancer and dietary chemoprevention.
Table 1.
A summary of models used to study dietary prevention
| Model | Examples | Reference |
|---|---|---|
| Spontaneous cancer | “Western” diet (high fat, low Ca, vitamin D) | 48 |
| Chemically induced | AOM, NMU, DMH, MNNG | 91 |
| APCmin mouse | 48 | |
| Genetic modification | ||
| Global | 11 APC lines including APCΔ15, APCΔ14; Msh2; Mlh1; PTEN; Smad3 | 48, 108, 131 |
| Floxed alleles | LSL-KrasG12D, Msh2 (exon 2), APC (exon 14, exon 15) | 48 |
| Intestine-specific promoter | Villin, Car1, CDX2P9.5, Fabl | See text |
| Inducible | Cre-ERT2, rtTA, AhRc/CYP1A1 | 48 |
| Xenograft | Cell lines: SW480, SW620, HCT116, KM12SM, LIM1215 | See text |
| Patient-derived xenografts (PDX) | ||
| Allograft | CT26 cells | See text |
What Makes an Animal Model Useful for Chemoprevention Research?
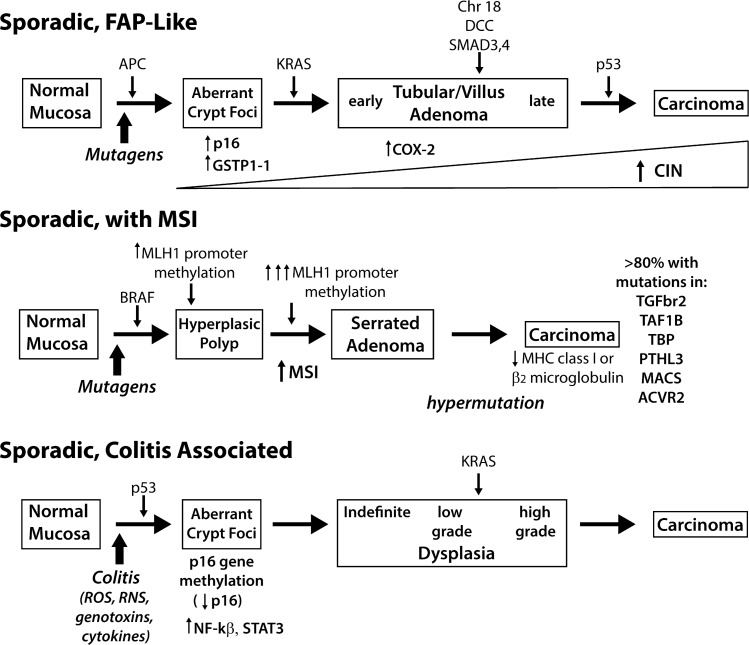
Although it may seem obvious, the goal for using mouse models to study dietary chemoprevention is to gain insight into the value of specific agents for use against human colon cancer. Because of this, the starting point for choosing an animal model has to be how it relates to the human disease. Historically, colon cancer has been viewed in three broad categories: tumors characterized by chromosomal instability (CIN) and progressing from adenoma to carcinoma, those arising from microsatellite instability (MSI), and those associated with inflammation-induced dysplasia. These cancers are formed by sporadic mutations in specific genes that serve as drivers for the initiation and progression of cancer. However, recent advances in cancer genomics are showing that these categorizations, although useful, are overly simplistic. In this section I will provide a brief overview of the molecular etiology and pathological features of colorectal cancer in humans. This information is summarized in Fig. 1.
Fig. 1.
Molecular changes associated with colorectal cancer development. Colorectal cancer is a heterogeneous disease. Traditional pathways for the molecular etiology of sporadic colon cancer are 1) tumors that develop through an adenoma-to-carcinoma progression that is similar to the heritable cancer familial adenomatous polyposis (FAP), 2) tumors that are characterized by high levels of microsatellite instability (MSI) due to inactivation of mismatch repair enzymes, similar to Lynch Syndrome, and 3) tumors that develop from dysplastic lesions resulting from recurrent bouts of colonic inflammation. CIN, chromosomal instability; MHC, major histocompatibility complex; ROS, reactive oxygen species; RNS, reactive nitrogen species. Pathological features leading to the development of each tumor are shown. Common gene mutations or epigenetic changes leading to initiation and progression are shown on top of arrows leading from one phenotype to the next. Additional biochemical features of specific phenotypes are presented below the phenotype.
Molecular and pathological features of human colorectal cancer.
Most sporadic colorectal cancers arise from benign adenomatous polyps over a course of several decades. These tumors are typically found in the descending colon and rectum and they are initiated through the mechanism of CIN (37). Our understanding of this type of cancer started with studies on the rare human colorectal cancer susceptibility syndrome, familial adenomatous polyposis (FAP), a disease caused by germline mutations in the tumor suppressor gene APC. APC is a 2,843-amino acid protein that is part of the canonical WNT signaling pathway. In the cytoplasm it helps form a destruction complex that reduces the cytoplasmic level of β-catenin (93), whereas in the nucleus it sequesters (82) and inhibits β-catenin binding to the TCF/LEF transcriptional complex (100) and mediates β-catenin export to cytoplasm (40, 82, 92). As a part of a complex formed with the TCF/LEF family of transcription factors, β-catenin regulates genes whose protein products stimulate cell proliferation and delay differentiation. The loss of APC function in FAP patients or in animal models leads to the development of pedunculated adenomatous polyps (119). Progression of these polyps to invasive and metastatic cancer (i.e., adenoma-to-carcinoma progression) is driven by the sequential accumulation of mutations in at least three additional pathways: the MAPK pathway (i.e., KRAS-activating mutations), TGF-β-induced differentiation pathway (e.g., deletions that disrupt SMAD genes), and DNA repair pathways (i.e., inactivation of TP53); this is known as the Vogelstein model (29).
Cancers found in the ascending colon account for 20% of all colon cancers and they are distinct from those that develop through the adenoma-to-carcinoma progression. Our initial understanding of these cancers comes from the study of Lynch Syndrome (a.k.a. hereditary nonpolyposis colorectal cancer, or HNPCC). HNPCC is cause by germline mutations in mismatch repair genes (90% by MSH2 and MLH1 gene mutations; 10% by MSH6, PMS2, or MLH3 gene mutations) (35). Inactivation of these DNA mismatch repair (MMR) mechanisms is associated with MSI (51) and dysregulation of MMR increases the potential for mutations in other oncogenes, e.g., a high percentage of HNPCC colon cancers exhibit increased WNT signaling (45) and β-catenin gene (CTNNB1) mutations (75). Sporadic colon cancers with MSI commonly result from hypermethylation of MMR gene promoters, particularly MLH1 (23), and these tumors have a high frequency of mutations in the BRAF gene and in genes controlling the canonical WNT pathway (109), as well as inactivating mutations of the type II TGF-β receptor (67) and the proapoptotic gene BAX (86). Histologically, MSI-associated tumors exhibit an exophytic or sessile gross phenotype and are characterized microscopically by excessive mucin production, poor epithelial differentiation, and lymphocytic infiltration (51). A unique morphological variant of hyperplastic polyp with a serrated glandular morphology and BRAF mutations is a precursor lesion to MSI cancers.
Recent research demonstrates that a form of MSI exists that is independent of mutations in or hypermethylation of genes for MMR enzymes: elevated microsatellite alterations at selected tetranucleotide repeats (EMAST) or MSI-low (41, 120). Moderately and poorly differentiated colorectal tumors have higher frequencies of EMAST (56.9 and 40%, respectively) than either well-differentiated colorectal tumors (12.5%) or adenomas (33%) (56). Although the importance of EMAST in tumors is not yet known with certainty, patients with stage II or III colorectal cancer and EMAST tumors were found to have a worse prognosis, with a shorter time to tumor recurrence, and were more likely to develop distal metastasis than patients that has microsatellite stable or MSI-high tumors (33). This is consistent with the observation that EMAST may increase the frequency of loss of heterozygosity in humans (125). EMAST is characterized by a change in the cellular distribution of MSH3 from the nucleus to the cytosol (41), and this redistribution is enhanced by oxidative stress in a number of colorectal cancer cell lines (111).
The risk of colon cancer can be increased by inflammation, especially as seen in conditions like Crohn's disease or ulcerative colitis in humans or in rodents cycled through multiple rounds of colitis with agents like dextran sodium sulfate (DSS) (49, 90). Like distal colon cancer, inflammation-associated colon cancer is strongly associated with CIN but in contrast to distal colon cancer, the disease follows a dysplasia-to-carcinoma progression that includes accumulation of mutations in some, but not all, of the genes from the Vogelstein model (112) (Fig. 1). For example, p53 gene mutations are a late event in sporadic cancer of the distal colon whereas p53 mutations are commonly seen in dysplastic lesions that precede colitis-associated colon cancer (12). In contrast, it is unclear whether APC mutations are important during colitis-associated cancer. Although several small studies report a 30–50% incidence of APC mutations in colitis-associated colon tumors (39, 50, 87), others found few, if any, APC gene mutations in such tumors (5a, 57, 114). Inflammation can also accelerate the development of colon cancer that has been initiated by deletion of a single APC gene allele or with a chemical carcinogen (49). Proposed mechanisms for how inflammation initiates or promotes the early stages of colon cancer include: genotoxin-induced mutations resulting from microorganisms that breech the epithelial barrier, DNA damage resulting from reactive oxygen species (ROS), cytokine-induced regulation of microRNAs, and aberrant DNA methylation resulting from mislocalization of DNA methyltransferases (4, 17, 121). Although inflammation is a critical modifier of colon cancer, and mouse models for inflammation-induced colon cancer exist, these have been described extensively elsewhere and will not be discussed further here (107).
There is significant mutational diversity in colorectal cancer.
After reading the brief description of colorectal cancer development above, one might get the impression that all colon cancers follow predictable molecular paths. However, significant heterogeneity exists in the mutational profile among (83) and within (36) tumors. The scope of colon tumor diversity was first described by Sjoblom et al. (102), who sequenced the exomes of 13,023 genes in 11 human colon tumors and found that each tumor had an average of 93 mutated genes. More recently, The Cancer Genome Atlas research network conducted exome sequencing on 224 colon tumors and found that distal colon tumors have a median of 58 nonsilent mutations and that 24 genes were mutated in a significant number of cases (14a). Although the genes from the Vogelstein model were commonly mutated (e.g., 81% of tumors contain APC mutations, 60% p53, 43% KRAS, 9% SMAD4), it is clear that they don't define all cases of distal colon cancer. This is consistent with the “unique tumor principle” suggested by Ogino and Goel (83). This principle states that the intertumor histological heterogeneity observed in clinical samples is due to the fact that each tumor, in each patient, arises through a unique pathway of molecular disruption that is unlikely to be exactly recapitulated in any other tumor (83). More recently, Gerlinger et al. (36) extended this concept by showing extensive intratumoral heterogeneity across multiple tumor biopsies from four patients with metastatic renal cell carcinoma, e.g., >63% of all somatic mutations were not detectable across every tumor region sampled. This suggests the existence of branched evolutionary tumor growth, with some ubiquitous common mutations in all tumor cells but heterogeneity developing at later stages of tumor growth (128). It is likely that similar evolutionary diversity exists during development of tumors in animal models but this has not yet been experimentally demonstrated.
Mutations in tumors are frequently categorized as “drivers” responsible for the primary development of the tumors or “passengers” that occur as a consequence of the driver mutations and have no functional significance in carcinogenesis. Of the somatic mutations Sjoblom et al. (102) observed in a typical tumor, 5–12 were classified as driver mutations that modulate 12–20 core pathways (55). The implication of the driver/passenger mutation model is that preventative and therapeutic approaches could be targeted to driver mutations or pathways influenced by driver mutations. However, the distinction between driver and passenger mutations may be overstated. Recent evidence shows that passenger mutations occur in genes may have a modest individual impact on cells but their cumulative impact alters cancer progression (72). Regardless, both the diversity of driver mutations and the potential importance of passenger mutations complicate the challenge of making mouse models that perfectly recapitulate human colorectal cancer.
Defining the Factors that Make a Good Mouse Model for Dietary Prevention and Colon Cancer Research
The diversity of human colorectal cancer makes it impossible for a single animal model to adequately represent all forms of the disease. Notwithstanding this challenge, I believe that certain fundamental characteristics are important for maximizing the translational potential of mouse models for dietary chemoprevention.
First, the metabolism of the nutrient or bioactive agent has to be similar between mice and humans. Without this, results from mice are unlikely to apply to humans. For example, our understanding of the role of β-carotene in lung carcinogenesis was delayed because, in contrast to humans, most laboratory animals break down β-carotene in their intestine and thus do not absorb it intact (118). Related to this, the mechanism of action for a dietary agent should be present in the cancer stage represented by the mouse. High serum levels of the vitamin D metabolite 25 hydroxyvitamin D have been proposed to reduce colorectal cancer risk by serving as a substrate for local conversion to the active hormone 1,25-dihydroxyvitamin D, a ligand that activates gene transcription through the vitamin D receptor (VDR). However, Xu et al. (123) showed that VDR mRNA and protein expression were lower in the small intestine and colon tissue of ApcMin mice compared with wild-type mice, thus making it more difficult to study the role of vitamin D status on colon cancer prevention in this model.
Second, since the research goal is to study sporadic colorectal cancer, the cancer that develops in the mouse model should be limited to the large intestine. Primary tumors that develop in other sites have the potential to disrupt the health of the animal in ways that confound interpretation of the study results. ApcMin mice classically used for dietary chemoprevention studies develop many more small intestinal tumors than colon tumors (79). The small intestinal tumors frequently hemorrhage, causing anemia that negatively affects the general health of the mouse. In addition, the oxidized heme in the intestine after hemorrhage can promote tumorigenesis but this mechanism reflects only a subgroup of human sporadic colorectal cancers (38). Mlh1−/− mice used to model colon cancer originating from defects in DNA mismatch repair and microsatellite instability develop cancer in both lymphoid tissue and in the intestine (27). Similarly, whereas 70% of MutS homologue 2 (Msh2) knockout mice developed intestinal tumors after 6 mo of age, these mice succumb primarily to lymphomas (89). Because of the extracolonic effects, global gene deletion or transgenic mouse models more closely reflect rare heritable cancers like FAP and HNPCC in which the heritable mutations are found in all cells and where extracolonic manifestations are present in two-thirds of affected individuals.
Third, the pathological and molecular features of colorectal tumors in the mouse model, as well as the timing and location of those features, should be similar to those observed in a subtype of human colorectal cancer. This will increase the potential of a mouse study to translate into the complexity of human cancer. Because modifying the mouse genome is now commonplace, deleting or overexpressing any gene or microRNA is achievable. Many such models are available and have been reviewed elsewhere (48, 108). In addition, large scale gene knockout mouse projects are making libraries of embryonic stem (ES) cells with single gene deletions; as of 2011 almost 17,000 genes had been deleted in ES cells by various teams around the world (25). This effort is dramatically reducing the barriers to study the role of specific genes in colorectal cancer. The pathological features of rodent models of intestinal cancer was recently published; interested readers should also refer to this report (119). In particular, this report indicates that newer mouse models with multiple genetic alterations develop intestinal tumors that closely resemble the morphology of human tumors. The report also identifies a website for sharing information about the histological features of published models (mmgint.org). However, there is a significant limitation to the use of global gene knockouts and transgenic mice for dietary chemoprevention studies; the defect is present in embryos so dietary interventions can't be implemented before the genetic modification has had an influence on cell biology. This can be overcome with inducible transgenes or gene knockouts so that dietary interventions can be introduced prior to the mutation event.
Finally, the models should capture the microenvironmental features relevant to human colon cancer. For colitis-associated colon cancer this will include cellular interactions and factors produced by infiltrating immune cells that induce DNA damage (e.g., oxidative stress), regulate cancer-relevant cell growth, or promote angiogenesis (17). In addition, there is a growing appreciation that genotoxins or metabolites from the gut microbiome can influence carcinogenesis or tumor growth (5, 21). Unfortunately these issues are often ignored by researchers.
New Mouse Models for Colon Cancer Research
The great advantage of the mouse as a research model is the relative ease with which it can be genetically modified. Genetically modified mice offer the potential to precisely recapitulate specific molecular changes relevant to human colorectal cancer. As I noted earlier, there are many mouse models for colon cancer research (Table 1; Refs. 48, 108). This section will discuss new mouse models that have been developed over the last 5 years.
Tissue-specific and inducible models.
Controlled induction of genetic mutations is possible in mice and the most common system for doing this is by genomic recombination using Cre recombinase (Cre), a bacteriophage enzyme that deletes sequences between two LoxP DNA sites (11, 30). This approach requires a transgenic mouse expressing Cre and a mouse with LoxP sites flanking a biologically important DNA sequence within a gene. In addition, a modified version of the Cre protein has been designed that allows tamoxifen-inducible control of Cre activity (31). Another system for tissue-specific inducible transgene activation is the reverse tetracycline activator system (32, 52). This approach requires development of mice that express the reverse tetracycline-regulated transactivator (rtTA), mice that have a transgene whose promoter is controlled by the rtTA, and, if the transgene encodes for Cre, mice with a gene containing LoxP sites. The complications of this approach make it less common.
Most genetically modified mouse models were generated to have a single gene defect in all cells in the body so they are often confounded by the existence of precancerous or cancerous lesions in other tissues. This can be limited with transgenes driven by tissue-specific promoters such as 1) the Villin promoter, which limits intestinal transgene expression to the epithelial cells along the crypt-villus axis and from the duodenum to the colon (10, 28, 64); 2) the intestinal fatty acid binding protein promoter (Fabpl4xat-132), which limits intestinal transgene expression to the proximal small intestine, cecum, and colon (95); and 3) a 6.3-kb fragment of the cytokeratin 19 (CK19) promoter that is expressed throughout the intestinal epithelium (74). Unfortunately, each of these promoter systems has expression that extends beyond the colon (i.e., villin in small intestine and kidney; Fabpl in ileum, bladder, and testes; CK19 in small intestine, stomach, pancreatic ducts, and hepatic ducts) (48). Because of this, over the last several years researchers have developed mouse models that use promoters from genes with a more restricted cell or tissue distribution. However, even with these advances we do not yet have mouse models where transgene expression is limited to the epithelium of the just proximal colon, just the distal colon, or just the rectum.
Lgr5 has been identified as a marker of one type of intestinal stem cell (9). Barker et al. (9) used homologous recombination of the Lgr5 gene locus to create a gene that marks cells expressing Lgr5 with EGFP and that permits tamoxifen-inducible Cre-mediated deletion of floxed alleles from intestinal stem cells (Lgr5-EGFP-IRES-Cre-ERT2 mice). Inducing deletion of both APC alleles with this model led to formation of colon adenomas while inducing APC allele deletion in more mature intestinal epithelial cells did not (8). This demonstrates that stem cells can be the cells of origin for colon cancer. However, there are two weaknesses with this model. First, Lgr5 is expressed in the stem cells throughout the gastrointestinal tract, as well as in a number of other tissues (e.g., hair follicle) (7). Second, the expression of the Lgr5-EGFP-IRES-Cre-ERT2 transgene is not uniform across the intestine; based on EGFP expression only 10–15% of crypts express the transgene. As a result, although this mouse may be useful to study early carcinogenic transformation in vivo, it may also lead to confounding phenotypes in other tissues and more mice may be needed if tumor incidence is low.
Hinoi et al. (42) developed a mouse model using a 9.5-kb fragment of the CDX2 gene promoter to drive Cre expression (CDX2P9.5-NLS-Cre). After birth, this transgene has expression that is limited to the ileum, cecum, and colon. However, the CDX2P9.5-NLS promoter is transiently expressed in the posterior of the mouse within multiple tissues during embryonic development. As a result, when the promoter is used to drive Cre for the recombination of floxed alleles, the gene is knocked out in the proximal intestine as well as in the muscle, skin, and bones of the rear legs, and in essential organs like the kidney and spleen. This explains why CDX2P9.5-NLS;Cre;Apc580D/580D embryos were not viable. Depending on the gene being studied, it is also possible that CDX2P9.5-NLS;Cre mice may develop additional cancers outside the colon. Although the adverse effects of fetal and extraintestinal expression could be overcome by coupling the CDX2P9.5-NLS promoter to Cre-ERT2, allowing recombination of floxed alleles after birth, this model has not yet been reported.
The CDX2P9.5-NLS promoter construct has also been used to construct the CDX2P9.5-G22-Cre mouse (2). The “G22” refers to the addition of 22 guanine repeats in the transgene coding region before the normal translation start site for Cre. The repeats are out of phase with the Cre start site; this means that Cre can be transcribed and translated only after a somatic frameshift mutation occurs, thus making embryonic expression unlikely. When spontaneous, single-base deletions in the G22 repeat occur, it puts the Cre coding sequence in phase, which allows translation of a functional Cre enzyme and deletion of floxed alleles. β-Galactosidase expression from a CDX2P9.5-G22-LacZ transgene was limited to the ileum and proximal colon. This makes the CDX2P9.5-G22-LacZ mouse an interesting model to study the effects of dietary agents on spontaneous mutations in the colon.
A unique approach to restrict the temporal and spatial activity of Cre was reported by Hung et al. (43). To get recombination of floxed alleles limited to the colon of adult mice, they introduced an adenovirus vector expressing Cre through the rectum to infect colonic epithelial cells. This approach provides precise control over when and where Cre will be expressed. However, the technique requires anesthetizing the mice, applying surgical clamps to the distal colon, and creating abrasive injury to the colonic mucosa before a 30-min adenoviral infection period. Without the injury to stimulate cell proliferation, floxed alleles in the self-renewing stem cells of the colonic crypt are not infected by the adenovirus and deletion of floxed alleles does not occur (99).
A final recent model is one developed in my laboratory. We used a bioinformatics approach to identify Carbonic anhydrase-1 (CA) as a gene that is highly expressed in the colon of mice. We modified the CA promoter/enhancer to drive Cre expression (CAC) (124). CAC transgene expression affects ∼10% of large intestinal epithelial cells. In the distal colon transgene expression occurs from the crypt base to the luminal surface in groups of three to six crypts whereas in the proximal colon expression is mainly in the surface epithelium. When the CAC transgene is used to remove a lox-STOP-lox signal in ROSA26R mice, β-galactosidase expression is seen at 14.5 days postcoitum and a small amount of staining is also seen in the liver. When we used the CAC mouse to drive recombination of the APC580D locus and delete one APC allele, recombination caused 15% of the mice to develop at least one distal colon tumor by 20 wk of age. The penetrance of colon cancer can be increased several ways in CAC mice. First, deletion of two APC alleles results in 100% penetrance of colon cancers, with florid tumor growth in the distal colon by 6 wk of age (124). We also used the CAC mouse to delete both a single APC580D allele and a lox-stop-lox allele controlling expression of a constitutively active form of KRAS (G12D) (13). By 15 wk of age 100% of the double-mutant mice develop an average of 4.6 tumors in the distal colon. In none of these cases (single or double APC deletion, APC/KRAS double mutant) did tumors develop in the small intestine or liver; this large-intestine-specific expression makes the CAC model unique among genetically mouse models. In addition, we have recently learned that a 5-day course of DSS leads to activation of the CAC transgene in the epithelium of both the proximal and distal colon (unpublished data). This likely explains our published observation that tumor incidence and multiplicity are dramatically increased in CAC mice with one floxed APC allele following DSS treatment (124). Thus using the CAC model to delete a floxed APC allele may be particularly useful for studies on the role of dietary agents in inflammation-induced colon cancer.
Diversity in the colon cancer phenotypes of mice with APC gene mutations.
The most commonly modified gene in mice used for dietary chemoprevention research is APC. Although APC mutant models have been available for a long time, there are many such models and it is confusing which of them should be used for dietary prevention studies. This section will give an overview of the mutant APC models.
APC truncation mutations occur in >90% of FAP patients and in >75% of sporadic colon cancers. Because of the critical role Wnt signaling plays in development, homozygosity for most germline APC truncation mutations are embryonically lethal. As a result, FAP patients and genetically modified mice with APC gene modifications are heterozygotes and loss of heterozygosity of the remaining wild-type Apc allele is required for adenoma formation (63). In terms of dietary prevention, the protection from acquisition of a “second hit” that leads mice heterozygous for APC mutations to form aberrant crypt foci or adenomas is a critical end point.
Over 90% of germline APC mutations in FAP patients (76) and somatic APC mutations in colorectal tumors (77) occur within exon 15 in a cluster between codons 1286 and 1513 and this results in truncation of the APC protein to between 426 and 504 amino acids long (vs. 2,843 for the wild-type protein). The most common mutant APC mouse model is the ApcMin mouse that was generated in an N-ethyl-N-nitrosourea-chemical mutagenesis screen (79). The ApcMin mouse expresses a truncated APC protein 850 amino acids long, resulting from a mutation at codon 2549 (105). In addition to the ApcMin mouse, there are 11 genetically engineered APC mutant mice with germline modifications and 2 mutant lines with LoxP sites flanking either exon 14 or exon 15 (48, 131). There is significant phenotypic heterogeneity in these APC mutant mice in terms of tumor number and location. All global APC mutants form more tumors in the small intestine compared with the colon and several form tumors at extraintestinal sites (131). Several of the APC mouse models have phenotypes similar to ApcMin mice (i.e., APCΔ716, APCΔ474, APC1309), in several the phenotype is more severe (APC1322T, APCΔSAMP, APCΔ14, APC580S or Δ580), and a very high intestinal tumor burden is seen in APCΔ15 mice. Recently, the APC1572T mouse has been shown to have mammary cancer but not intestinal cancer (34). This suggests that the last SAMP repeat in APC accounts for the mammary phenotype of APC gene truncation. This phenotypic variability reflects the importance of structural features necessary for protein-protein interactions affecting APC function. Important motifs within APC confer the ability to bind to β-catenin [three 15-amino-acid repeats between residues 1014 and 1210 (106), seven 20-amino-acid repeats between amino acids 1034 and 2130 (93)] or to the destruction complex through Axin (104) (three serine-alanine-methionine-proline repeats between amino acids 1034 and 2130).
In addition to the phenotypic variation that exists among APC mutants, classical genetic mapping strategies have revealed 13 loci that modify the ApcMin phenotype when it is present on different genetic backgrounds (71); similar genetic modifiers of cancer phenotypes are likely to exist in humans as well as to affect cancers with other molecular etiologies. Thus it is critical that chemoprevention researchers control the genetic background of their mouse models to limit this diversity (especially when crossing lines).
Variation in the gut microbiome can influence the development of colon cancer.
It has long been recognized that the microbes within the colon can ferment dietary starch and fiber and that the resultant fermentation products can influence colon carcinogenesis (22, 97). In addition, fewer adenomas develop in APC mutant mice raised in germ-free (GF) conditions (19, 26, 58) and in Smad3 knockout mice grown in specific pathogen-free housing (65) whereas colonic levels of specific microbes are associated with increased colon cancer in animals and humans [e.g., F. nucleatum (15, 53)]. These examples reveal a role for the gut microbiota in the etiology of colon cancer, and recent evidence suggests that this is due to specific microbial metabolites or genotoxins that directly influence the integrity of DNA (44). Consistent with this hypothesis, Conlon et al. (20) found that feeding resistant starches to rats increased gut short-chain fatty acid (SCFA) and reduced gut ammonia levels and that these changes were inversely (SCFA) and positively (ammonia) associated with DNA strand breaks in colonocytes. Also, Arthur et al. (4) used IL-10 knockout mice with monocolonization to show that only mice whose colons had been colonized with microbes expressing the bacterial genotoxin colibactin developed colon tumors in response to azoxymethane (AOM).
The impact of gut microbes on colon cancer is clearly seen in mice lacking the mucin 2 (Muc2) gene. Mucin 2 is a large gel-forming protein that makes up the bulk of the mucus layer coating colonic epithelial cells. Mucins are secreted by goblet cells and provide a barrier between the gut microbiome and the epithelial barrier (47). In contrast to normal mice, gut bacteria are in direct contact with the intestinal epithelium of Muc2 knockout mice and they extend deep into the crypt where the normal intestinal stem cell resides. Muc2 knockout mice also have more intestinal epithelial cell proliferation than normal mice and 68% of Muc2 knockout mice develop intestinal cancer within 1 year (115). In addition, deletion of Muc2 from Apc1638N/+ and ApcMin/+ mice increased the incidence of tumors, increased the number of tumors per mouse, and shifted tumor burden toward the large intestine, where bacterial loads are greater (127). Thus the Muc2 knockout mouse is an excellent model for researchers who want to learn whether a dietary agent can either reduce the presence of genotoxins from the gut microbiome or stimulate epithelial cell systems for that prevent or repair genotoxin-mediated DNA damage.
For people conducting dietary prevention studies that are not explicitly examining the gut microbiome, there is a cautionary note that must be sounded. It is clear that dietary changes can modify the gut microbiome. For example, David et al. (24) found that, just days after feeding humans subjects a low-fiber, animal protein-based diet, the diversity of the gut microbiome significantly increased to reflect species that were more tolerant of bile. In mice, even the simple act of switching between two commercial chow diets increased microbial diversity in the gut and this increased the sensitivity of mice to DSS-induced colitis (80). In terms of cancer, Mai et al. (66) found that a cancer-protective diet high in olive oil and supplemented with a dried fruit and vegetable extract increased levels of a bacteria from family Lachnospiraceae (order Clostridiales). This suggests that targeting specific bacterial groups by diet could be an effective colon cancer prevention strategy. Unfortunately, we have not identified all of the protective or harmful gut microbes yet nor have we reached the point where we can reliably and predictably alter the gut microbial population by diet. As a result, it is critical for cancer researchers to control their diets and limit unintended variability in diet composition. This would argue strongly against the use of chow-type diets formulated on a least-cost ingredient basis where there is significant batch-to-batch variability in the ingredients used.
Models of late stage colorectal cancer.
Mice with single gene modifications generally have mild phenotypes that do not develop past adenoma. This feature makes them useful for early carcinogenesis studies or for examining the function of specific pathways. Although many dietary cancer prevention studies focus on modifying the early stages of cancer, dietary factors many also have an impact at later stages that could make them valuable adjuvant therapies to complement traditional treatment regimes. The following section will focus on mouse models of advanced colon cancer.
COMBINING GENE MUTANTS TO MAKE MICE WITH MORE ADVANCED CANCER PHENOTYPES.
Breeding mice to combine genetic modifications into a single mouse can cause more advanced phenotypes that allow researchers to examine the role that dietary factors can play in later stages of cancer. First, a number of studies have used mice with K-RAS activating mutations to modify tumor development in other genetically modified mouse models. This approach uses mice where the endogenous Kras allele has been replaced with an allele containing a floxed translational stop site (Lox-STOP-Lox, LSL) in front of a gene encoding either a constitutively active Kras [i.e., glycine to aspartate at codon 12, LSL-KrasG12D mice (46) or glycine to valine, LSL-KrasG12V (60)]. When Cre is used to recombine the LSL locus in LSL-KrasG12D/+ mice, the colons have a phenotype of epithelial hyperplasia (14). However, when the LSL-KrasG12D allele is combined with the ApcΔ14 allele (floxed exon 14) to make LSL-KrasG12D/+;ApcΔ14/+ mice, Cre-mediated recombination of both alleles induces development of tumors that are more advanced than those from ApcΔ14/+ mouse, i.e., uniform, high-grade hyperplasia, a complete lack of terminally differentiated cells, loss of cell polarity, and fused glands with serrated borders. In addition, combining the LSL-KrasG12D allele with either the ApcMin genotype or AOM chemical induction increased the number of colonic lesions (61). Only minor effects were observed in the intestine of the LSL-KrasG12V mice after Cre-mediated recombination (61), but this mutant accelerated tumorigenesis in ApcMin mice (61) as well as the cancer caused by DMH treatment (62). Similarly, although Cre-mediated recombination of floxed TGF-β receptor 2 (TGFb2Rloxp/loxp) alleles caused formation of intestinal neoplasms, in LSL-KrasG12D/+;TGFb2Rloxp/loxp transgenic mice recombination results in the formation of tumors that metastasize through a β-catenin-independent mechanism (110).
Disruption of enzymes controlling DNA mismatch repair enhances accumulation of mutations in other oncogenes, e.g., tumors from Msh2−/− mice acquire Apc gene mutations (88). When either Mlh1 or Msh2 knockout mice are crossed to mice heterozygous for a mutated Apc allele, intestinal tumorigenesis is markedly increased (27). Similarly, crossing Msh3 knockout mice to Apc1638N/+ mice increased the number of frameshift mutations in the wild-type Apc allele and this caused higher incidence and more tumors per mouse compared with the Apc1638N/+ mice (16). Finally, combining Msh2-null mice with LSL-KrasG12V mice increased the number of adenomas per mouse fivefold after Cre-mediated recombination (60).
Pten−/− mice do not develop intestinal neoplasia, but, when they are crossed with ApcMin mice, larger, more invasive tumors develop than the adenomas and adenocarcinomas normally seen in the ApcMin mouse (98). Marsh et al. (68) later confirmed this observation by demonstrating that mice with conditional deletion of one Apc580S and one floxed Pten allele in intestinal epithelial cells had more advanced adenocarcinomas than mice with just one deleted Apc allele. Also, although deletion of the TGFbr2 gene alone has a minimal impact on intestinal tumorigenesis, when TGFbr2 deletion is combined with PTEN knockout in the intestinal epithelium, 86% of the mice develop malignant tumors in both the small intestine and colon and 8% of the tumor-bearing mice have metastases (129).
The protein mitochondrial transcription factor A (TFAM) is required for expression and maintenance of mitochondrial DNA (mtDNA) (54). Woo et al. (122) found that mice heterozygous for the TFAM knockout (Tfam+/−) have mild mtDNA depletion but no other overt phenotypes. However, they also showed that when these mice are crossed with ApcMin mice, their offspring have increased tumor number and faster tumor growth. This was proposed to be due to increased generation of ROS from mitochondria, suggesting these double-mutant mice might be particularly useful to study the impact of dietary agents on antioxidant systems or on DNA repair mechanisms.
A final model worth considering could be useful to evaluate the impact of dietary agents on CIN, a central feature of tumors developing in the distal colon. Bub1 is a serine/threonine kinase critical for the mitotic checkpoint, and Bub1 hypomorphic mice are prone to aneuploidy and cancer (96). When Bub1 hypomorphic mice are crossed with ApcMin mice, colon tumor incidence increased from 25 to 90% owing to loss of heterozygosity of the remaining wild-type APC allele and/or duplication of a copy of chromosome 18 with the ApcMin allele (6).
XENOGRAFTS, ALLOGRAFTS, AND MODELS OF METASTASIS.
After tumors have developed, the goal of dietary chemoprevention is to limit tumor cell growth, block neovascularization and angiogenesis, and enhance antitumor immunity. When researchers want to study the impact of a treatment on established tumors they use xenografts of human primary tumor cells and cell lines [e.g., HCT116, KM12SM, LIM1215, SW480 (126), SW620 (73)] implanted in immunodeficient mice, or allografts of mouse tumor cells implanted into genetically matched hosts. Some evidence suggests that human cell xenografts have more clinical predictive value than mouse cell allografts (116). However, human xenografts in immunodeficient mice eliminate the ability to study antitumor immunity that influences tumor growth and angiogenesis whereas mouse cell allografts can be used to study this biology (94, 117). Unfortunately, both mouse and human cell lines suffer from mutational drift with extended culture (70) and no single cell line models the diversity of human cancer (14a). As a result, patient-derived xenografts (PDX) are being utilized to capture the mutational diversity of human colorectal cancer and to develop personalized treatment regimens (101). Although the challenge of acquiring tumors suggests use of PDX will be limited to researchers in hospital environments, The Jackson Laboratories now has a PDX resource available with many colorectal cancer tumors (http://jaxservices.jax.org/invivo/pdx.html). Given the promise of this approach, similar repositories are likely to be developed elsewhere in the future. In addition, the available tumor samples will likely be fully sequenced so that researchers can choose samples to test based on classification of their mutational spectrum.
Colon cancer generally metastasizes to two tissues: liver and lung. A general weakness of animal models of colorectal cancer is that they rarely develop advanced tumors that metastasize to other tissues. Because of this, researchers interested in the impact of dietary agents on metastasis have been limited to transplantation of cancer cell lines into mice. Morikawa et al. (78) first reported that intrasplenic injection of primary human colon tumor cells into nude mice (Balb/c) leads to lung and liver metastases. Later it was shown that both intravenous and intrasplenic injection of the mouse colon tumor cell line CT26 cells (from Balb/c mice) cause lung and liver metastases in genetically matched hosts (130). Zhang et al. (132) recently reported a modification of this method that significantly improved the efficiency of metastasis. They passed CT26 cells through three rounds of in vivo selection to identify the CT26-FL3 cell line. Injecting these cells directly into the cecum resulted in 90% of the mice developing multiple liver tumors whereas just 8% of mice with cecal injection of standard CT26 cells developed single metastatic liver tumors. Recently, Chowdhury et al. (18) developed a metastasis model whereby HCT116 cells are first grown as xenografts in nude mice then removed and reimplanted orthotopically into the colon where 68% of mice develop either lung or liver metastases. Finally, PDXs also metastasize to lung and liver when implanted into the cecum of nude mice (85).
Several genetically modified mouse models develop metastasis. Smad3 knockout mice on the 129/Sv background developed aggressive colorectal adenocarcinomas within 4–6 mo and this was accompanied by frequent metastasis to regional lymph nodes (133). Hung et al. (43) used surgical introduction of a Cre-expressing adenovirus to induce recombination of floxed alleles in the distal colon of ApcΔ580/+;LSL-KrasG12D/+ mice. Carcinomas appeared in these mice within 20 wk of the adenovirus injection and spontaneous gross liver metastases were observed by 24 wk after the injection. Finally, although neither Villin-Cre;LSL-KrasG12D/+ nor Villin-Cre;TGFb2Rloxp/loxp double transgenic mice form intestinal neoplasms, 15% of Villin-Cre;LSL-KrasG12D/+;TGFb2Rloxp/loxp triple transgenic mice developed lymph node and lung metastases by 22 wk of age through a β-catenin-independent mechanism (110).
Conclusion
The field of dietary cancer prevention holds great promise for reducing cancer incidence, slowing cancer progression, improving cancer outcomes, and limiting cancer recurrence. However, the challenge facing the field is to conduct studies that demonstrate a direct role for a dietary pattern or agent on the course of cancer, i.e., to move past the associations identified in population studies. This is extremely challenging to do with clinical trials, especially for lifelong prevention strategies. As a result, animal models will be a critical component in the process of validating hypothesis regarding dietary chemoprevention. However, to realize this promise of dietary cancer prevention, researchers need to carefully choose the preclinical models they use in their studies. This selection should be based on a clear understanding of human colorectal cancer, recognition of how animal models relate to the human disease, an appreciation for the strengths and weakness of the model they choose, and a clear hypothesis for how the dietary intervention might influence carcinogenesis or cancer cells in the model. Our emerging understanding of the molecular diversity of human colorectal cancer is going to make this challenge greater, and it is clear that neither common models like the ApcMin mouse nor any other single model is sufficient for testing all dietary cancer prevention strategies for all subtypes of colorectal cancer. Fortunately, there are now a variety of excellent models that should vastly improve the quality of research resulting from preclinical studies on dietary prevention of colorectal cancer.
GRANTS
This research was supported by NIH award CA165240 to J. C. Fleet.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
J.C.F. prepared figures, drafted manuscript, edited and revised manuscript, approved final version of manuscript.
REFERENCES
- 1.Abotchie PN, Vernon SW, Du XL. Gender differences in colorectal cancer incidence in the United States, 1975–2006. J Womens Health 21: 393–400, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akyol A, Hinoi T, Feng Y, Bommer GT, Glaser TM, Fearon ER. Generating somatic mosaicism with a Cre recombinase-microsatellite sequence transgene. Nat Methods 5: 231–233, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Cancer Society. Colorectal Cancer Facts & Figures 2011–2013. Atlanta, GA: American Cancer Society, 2011 [Google Scholar]
- 4.Arthur JC, Perez-Chanona E, Muhlbauer M, Tomkovich S, Uronis JM, Fan TJ, Campbell BJ, Abujamel T, Dogan B, Rogers AB, Rhodes JM, Stintzi A, Simpson KW, Hansen JJ, Keku TO, Fodor AA, Jobin C. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science 338: 120–123, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Attene-Ramos MS, Wagner ED, Plewa MJ, Gaskins HR. Evidence that hydrogen sulfide is a genotoxic agent. Mol Cancer Res 4: 9–14, 2006 [DOI] [PubMed] [Google Scholar]
- 5a.Aust DE, Terdiman JP, Willenbucher RF, Chang CG, Molinaro-Clark A, Baretton GB, Loehrs U, Waldman FM. The APC/beta-catenin pathway in ulcerative colitis-related colorectal carcinomas: a mutational analysis. Cancer 94: 1421–1427, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Baker DJ, Jin F, Jeganathan KB, van Deursen JM. Whole chromosome instability caused by Bub1 insufficiency drives tumorigenesis through tumor suppressor gene loss of heterozygosity. Cancer Cell 16: 475–486, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barker N, Clevers H. Leucine-rich repeat-containing G-protein-coupled receptors as markers of adult stem cells. Gastroenterology 138: 1681–1696, 2010 [DOI] [PubMed] [Google Scholar]
- 8.Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, van den Born M, Danenberg E, Clarke AR, Sansom OJ, Clevers H. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature 457: 608–611, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449: 1003–1007, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Beuling E, Baffour-Awuah NY, Stapleton KA, Aronson BE, Noah TK, Shroyer NF, Duncan SA, Fleet JC, Krasinski SD. GATA factors regulate proliferation, differentiation, and gene expression in small intestine of mature mice. Gastroenterology 140: 1219–1229, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Birling MC, Gofflot F, Warot X. Site-specific recombinases for manipulation of the mouse genome. Methods Mol Biol 561: 245–263, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Burmer GC, Crispin DA, Kolli VR, Haggitt RC, Kulander BG, Rubin CE, Rabinovitch PS. Frequent loss of a p53 allele in carcinomas and their precursors in ulcerative colitis. Cancer Commun 3: 167–172, 1991 [DOI] [PubMed] [Google Scholar]
- 13.Byun AJ, Hung KE, Fleet JC, Bronson RT, Mason JB, Garcia PE, Crott JW. Colon-specific tumorigenesis in mice driven by Cre-mediated inactivation of Apc and activation of mutant Kras. Cancer Lett 347: 191–195, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calcagno SR, Li S, Colon M, Kreinest PA, Thompson EA, Fields AP, Murray NR. Oncogenic K-ras promotes early carcinogenesis in the mouse proximal colon. Int J Cancer 122: 2462–2470, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14a.Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature 487: 330–337, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castellarin M, Warren RL, Freeman JD, Dreolini L, Krzywinski M, Strauss J, Barnes R, Watson P, Allen-Vercoe E, Moore RA, Holt RA. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res 22: 299–306, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen PC, Kuraguchi M, Velasquez J, Wang Y, Yang K, Edwards R, Gillen D, Edelmann W, Kucherlapati R, Lipkin SM. Novel roles for MLH3 deficiency and TLE6-like amplification in DNA mismatch repair-deficient gastrointestinal tumorigenesis and progression. PLoS Genet 4: e1000092, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiba T, Marusawa H, Ushijima T. Inflammation-associated cancer development in digestive organs: mechanisms and roles for genetic and epigenetic modulation. Gastroenterology 143: 550–563, 2012 [DOI] [PubMed] [Google Scholar]
- 18.Chowdhury S, Ongchin M, Sharratt E, Dominguez I, Wang J, Brattain MG, Rajput A. Intra-tumoral heterogeneity in metastatic potential and survival signaling between iso-clonal HCT116 and HCT116b human colon carcinoma cell lines. PLoS One 8: e60299, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colnot S, Niwa-Kawakita M, Hamard G, Godard C, Le Plenier S, Houbron C, Romagnolo N, Berrebi D, Giovannini M, Perret C. Colorectal cancers in a new mouse model of familial adenomatous polyposis: influence of genetic and environmental modifiers. Lab Invest 84: 1619–1630, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Conlon MA, Kerr CA, McSweeney CS, Dunne RA, Shaw JM, Kang S, Bird AR, Morell MK, Lockett TJ, Molloy PL, Regina A, Toden S, Clarke JM, Topping DL. Resistant starches protect against colonic DNA damage and alter microbiota and gene expression in rats fed a Western diet. J Nutr 142: 832–840, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cuevas-Ramos G, Petit CR, Marcq I, Boury M, Oswald E, Nougayrede JP. Escherichia coli induces DNA damage in vivo and triggers genomic instability in mammalian cells. Proc Natl Acad Sci USA 107: 11537–11542, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cummings JH, Bingham SA. Dietary fibre, fermentation and large bowel cancer. Cancer Surv 6: 601–621, 1987 [PubMed] [Google Scholar]
- 23.Cunningham JM, Christensen ER, Tester DJ, Kim CY, Roche PC, Burgart LJ, Thibodeau SN. Hypermethylation of the hMLH1 promoter in colon cancer with microsatellite instability. Cancer Res 58: 3455–3460, 1998 [PubMed] [Google Scholar]
- 24.David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, Biddinger SB, Dutton RJ, Turnbaugh PJ. Diet rapidly and reproducibly alters the human gut microbiome. Nature 505: 559–563, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dolgin E. Mouse library set to be knockout. Nature 474: 262–263, 2011 [DOI] [PubMed] [Google Scholar]
- 26.Dove WF, Clipson L, Gould KA, Luongo C, Marshall DJ, Moser AR, Newton MA, Jacoby RF. Intestinal neoplasia in the ApcMin mouse: independence from the microbial and natural killer (beige locus) status. Cancer Res 57: 812–814, 1997 [PubMed] [Google Scholar]
- 27.Edelmann W, Yang F, Kuraguchi M, Heyer J, Lia M, Kneitz B, Fan KH, Brown AMC, Lipkin M, Kucherlapati R. Tumorigenesis in Mlh1 and Mlh1/Apc1638N mutant mice. Cancer Res 59: 1301–1307, 1999 [PubMed] [Google Scholar]
- 28.El Marjou F, Janssen KP, Chang BH, Li M, Hindie V, Chan L, Louvard D, Chambon P, Metzger D, Robine S. Tissue-specific and inducible Cre-mediated recombination in the gut epithelium. Genesis 39: 186–193, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell 61: 759–767, 1990 [DOI] [PubMed] [Google Scholar]
- 30.Feil R. Conditional somatic mutagenesis in the mouse using site-specific recombinases. Hand Exp Pharmacol 178: 3–28, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Feil R, Wagner J, Metzger D, Chambon P. Regulation of Cre recombinase activity by mutated estrogen receptor ligand-binding domains. Biochem Biophys Res Commun 237: 752–757, 1997 [DOI] [PubMed] [Google Scholar]
- 32.Furth PA, St OL, Boger H, Gruss P, Gossen M, Kistner A, Bujard H, Hennighausen L. Temporal control of gene expression in transgenic mice by a tetracycline-responsive promoter. Proc Natl Acad Sci USA 91: 9302–9306, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garcia M, Choi C, Kim HR, Daoud Y, Toiyama Y, Takahashi M, Goel A, Boland CR, Koi M. Association between recurrent metastasis from stage II and III primary colorectal tumors and moderate microsatellite instability. Gastroenterology 143: 48–50, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gaspar C, Franken P, Molenaar L, Breukel C, van der Valk M, Smits R, Fodde R. A targeted constitutive mutation in the APC tumor suppressor gene underlies mammary but not intestinal tumorigenesis. PLoS Genet 5: e1000547, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Geiersbach KB, Samowitz WS. Microsatellite instability and colorectal cancer. Arch Pathol Lab Med 135: 1269–1277, 2011 [DOI] [PubMed] [Google Scholar]
- 36.Gerlinger M, Rowan AJ, Horswell S, Larkin J, Endesfelder D, Gronroos E, Martinez P, Matthews N, Stewart A, Tarpey P, Varela I, Phillimore B, Begum S, McDonald NQ, Butler A, Jones D, Raine K, Latimer C, Santos CR, Nohadani M, Eklund AC, Spencer-Dene B, Clark G, Pickering L, Stamp G, Gore M, Szallasi Z, Downward J, Futreal PA, Swanton C. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med 366: 883–892, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gervaz P, Bucher P, Morel P. Two colons-two cancers: paradigm shift and clinical implications. J Surg Oncol 88: 261–266, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Gilsing AM, Fransen F, de Kok TM, Goldbohm AR, Schouten LJ, de Bruine AP, van Engeland M, van den Brandt PA, de Goeij AF, Weijenberg MP. Dietary heme iron and the risk of colorectal cancer with specific mutations in KRAS and APC. Carcinogenesis 34: 2757–2766, 2013 [DOI] [PubMed] [Google Scholar]
- 39.Greenwald BD, Harpaz N, Yin J, Huang Y, Tong Y, Brown VL, McDaniel T, Newkirk C, Resau JH, Meltzer SJ. Loss of heterozygosity affecting the p53, Rb, and mcc/apc tumor suppressor gene loci in dysplastic and cancerous ulcerative colitis. Cancer Res 52: 741–745, 1992 [PubMed] [Google Scholar]
- 40.Henderson BR. Nuclear-cytoplasmic shuttling of APC regulates beta-catenin subcellular localization and turnover. Nat Cell Biol 2: 653–660, 2000 [DOI] [PubMed] [Google Scholar]
- 41.Hile SE, Shabashev S, Eckert KA. Tumor-specific microsatellite instability: do distinct mechanisms underlie the MSI-L and EMAST phenotypes? Mutat Res 743–744: 67–77, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hinoi T, Akyol A, Theisen BK, Ferguson DO, Greenson JK, Williams BO, Cho KR, Fearon ER. Mouse model of colonic adenoma-carcinoma progression based on somatic Apc inactivation. Cancer Res 67: 9721–9730, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Hung KE, Maricevich MA, Richard LG, Chen WY, Richardson MP, Kunin A, Bronson RT, Mahmood U, Kucherlapati R. Development of a mouse model for sporadic and metastatic colon tumors and its use in assessing drug treatment. Proc Natl Acad Sci USA 107: 1565–1570, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Irrazabal T, Belcheva A, Girardin SE, Martin A, Philpott DJ. The multifaceted role of the intestinal microbiota in colon cancer. Mol Cell 54: 309–320, 2014 [DOI] [PubMed] [Google Scholar]
- 45.Isinger-Ekstrand A, Therkildsen C, Bernstein I, Nilbert M. Deranged Wnt signaling is frequent in hereditary nonpolyposis colorectal cancer. Fam Cancer 10: 239–243, 2011 [DOI] [PubMed] [Google Scholar]
- 46.Jackson EL, Willis N, Mercer K, Bronson RT, Crowley D, Montoya R, Jacks T, Tuveson DA. Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras. Genes Dev 15: 3243–3248, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Johansson ME, Phillipson M, Petersson J, Velcich A, Holm L, Hansson GC. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci USA 105: 15064–15069, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Johnson RL, Fleet JC. Animal models of colorectal cancer. Cancer Metastasis Rev 32: 39–61, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kanneganti M, Mino-Kenudson M, Mizoguchi E. Animal models of colitis-associated carcinogenesis. J Biomed Biotechnol 2011: 342637, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kern SE, Redston M, Seymour AB, Caldas C, Powell SM, Kornacki S, Kinzler KW. Molecular genetic profiles of colitis-associated neoplasms. Gastroenterology 107: 420–428, 1994 [DOI] [PubMed] [Google Scholar]
- 51.Kim H, Jen J, Vogelstein B, Hamilton SR. Clinical and pathological characteristics of sporadic colorectal carcinomas with DNA replication errors in microsatellite sequences. Am J Pathol 145: 148–156, 1994 [PMC free article] [PubMed] [Google Scholar]
- 52.Kistner A, Gossen M, Zimmermann F, Jerecic J, Ullmer C, Lubbert H, Bujard H. Doxycycline-mediated quantitative and tissue-specific control of gene expression in transgenic mice. Proc Natl Acad Sci USA 93: 10933–10938, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kostic AD, Chun E, Robertson L, Glickman JN, Gallini CA, Michaud M, Clancy TE, Chung DC, Lochhead P, Hold GL, El-Omar EM, Brenner D, Fuchs CS, Meyerson M, Garrett WS. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe 14: 207–215, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Larsson NG, Wang J, Wilhelmsson H, Oldfors A, Rustin P, Lewandoski M, Barsh GS, Clayton DA. Mitochondrial transcription factor A is necessary for mtDNA maintenance and embryogenesis in mice. Nat Genet 18: 231–236, 1998 [DOI] [PubMed] [Google Scholar]
- 55.Leary RJ, Lin JC, Cummins J, Boca S, Wood LD, Parsons DW, Jones S, Sjöblom T, Park BH, Parsons R, Willis J, Dawson D, Willson JKV, Nikolskaya T, Nikolsky Y, Kopelovich L, Papadopoulos N, Pennacchio LA, Wang TL, Markowitz SD, Parmigiani G, Kinzler KW, Vogelstein B, Velculescu VE. Integrated analysis of homozygous deletions, focal amplifications, and sequence alterations in breast and colorectal cancers. Proc Natl Acad Sci USA 105: 16224–16229, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee SY, Chung H, Devaraj B, Iwaizumi M, Han HS, Hwang DY, Seong MK, Jung BH, Carethers JM. Microsatellite alterations at selected tetranucleotide repeats are associated with morphologies of colorectal neoplasias. Gastroenterology 139: 1519–1525, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Leedham SJ, Graham TA, Oukrif D, McDonald SA, Rodriguez-Justo M, Harrison RF, Shepherd NA, Novelli MR, Jankowski JA, Wright NA. Clonality, founder mutations, and field cancerization in human ulcerative colitis-associated neoplasia. Gastroenterology 136: 542–550.e6, 2009 [DOI] [PubMed] [Google Scholar]
- 58.Li Y, Kundu P, Seow SW, de Matos CT, Aronsson L, Chin KC, Karre K, Pettersson S, Greicius G. Gut microbiota accelerate tumor growth via c-jun and STAT3 phosphorylation in APCMin/+ mice. Carcinogenesis 33: 1231–1238, 2012 [DOI] [PubMed] [Google Scholar]
- 59.Lofano K, Principi M, Scavo MP, Pricci M, Ierardi E, Di Leo A. Dietary lifestyle and colorectal cancer onset, recurrence, and survival: myth or reality? J Gastrointest Cancer 44: 1–11, 2013 [DOI] [PubMed] [Google Scholar]
- 60.Luo F, Brooks DG, Ye H, Hamoudi R, Poulogiannis G, Patek CE, Winton DJ, Arends MJ. Conditional expression of mutated K-ras accelerates intestinal tumorigenesis in Msh2-deficient mice. Oncogene 26: 4415–4427, 2007 [DOI] [PubMed] [Google Scholar]
- 61.Luo F, Brooks DG, Ye H, Hamoudi R, Poulogiannis G, Patek CE, Winton DJ, Arends MJ. Mutated K-rasAsp12 promotes tumourigenesis in ApcMin mice more in the large than the small intestines, with synergistic effects between K-ras and Wnt pathways. Int J Exp Pathol 90: 558–574, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Luo F, Poulogiannis G, Ye H, Hamoudi R, Zhang W, Dong G, Arends MJ. Mutant K-ras promotes carcinogen-induced murine colorectal tumourigenesis, but does not alter tumour chromosome stability. J Pathol 223: 390–399, 2011 [DOI] [PubMed] [Google Scholar]
- 63.Luongo C, Moser AR, Gledhill S, Dove WF. Loss of Apc+ in intestinal adenomas from Min mice. Cancer Res 54: 5947–5952, 1994 [PubMed] [Google Scholar]
- 64.Madison BB, Dunbar L, Qiao XT, Braunstein K, Braunstein E, Gumucio DL. Cis elements of the villin gene control expression in restricted domains of the vertical (crypt) and horizontal (duodenum, cecum) axes of the intestine. J Biol Chem 277: 33275–33283, 2002 [DOI] [PubMed] [Google Scholar]
- 65.Maggio-Price L, Treuting P, Zeng W, Tsang M, Bielefeldt-Ohmann H, Iritani BM. Helicobacter infection is required for inflammation and colon cancer in SMAD3-deficient mice. Cancer Res 66: 828–838, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mai V, Colbert LH, Perkins SN, Schatzkin A, Hursting SD. Intestinal microbiota: a potential diet-responsive prevention target in ApcMin mice. Mol Carcinog 46: 42–48, 2007 [DOI] [PubMed] [Google Scholar]
- 67.Markowitz S, Wang J, Myeroff L, Parsons R, Sun L, Lutterbaugh J, Fan RS, Zborowska E, Kinzler KW, Vogelstein B, Brattain M, Willson JKV. Inactivation of the type II TGF-beta receptor in colon cancer cells with microsatellite instability. Science 268: 1336–1338, 1995 [DOI] [PubMed] [Google Scholar]
- 68.Marsh V, Winton DJ, Williams GT, Dubois N, Trumpp A, Sansom OJ, Clarke AR. Epithelial Pten is dispensable for intestinal homeostasis but suppresses adenoma development and progression after Apc mutation. Nat Genet 40: 1436–1444, 2008 [DOI] [PubMed] [Google Scholar]
- 69.Marshall JR. Prevention of colorectal cancer: diet, chemoprevention, and lifestyle. Gastroenterol Clin North Am 37: 73–82, vi, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Masramon L, Vendrell E, Tarafa G, Capella G, Miro R, Ribas M, Peinado MA. Genetic instability and divergence of clonal populations in colon cancer cells in vitro. J Cell Sci 119: 1477–1482, 2006 [DOI] [PubMed] [Google Scholar]
- 71.McCart AE, Vickaryous NK, Silver A. Apc mice: models, modifiers and mutants. Pathol Res Pract 204: 479–490, 2008 [DOI] [PubMed] [Google Scholar]
- 72.McFarland CD, Korolev KS, Kryukov GV, Sunyaev SR, Mirny LA. Impact of deleterious passenger mutations on cancer progression. Proc Natl Acad Sci USA 110: 2910–2915, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McNutt NS, Mak LL, Kim YS. Comparison of cell peripheries in the human colonic adenocarcinoma cell lines SW480 and SW620 grown in floating chamber culture, cover slip culture, athymic (nude) mice, and BALB/c mice. Lab Invest 44: 309–323, 1981 [PubMed] [Google Scholar]
- 74.Means AL, Xu Y, Zhao A, Ray KC, Gu G. A CK19(CreERT) knockin mouse line allows for conditional DNA recombination in epithelial cells in multiple endodermal organs. Genesis 46: 318–323, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Miyaki M, Iijima T, Kimura J, Yasuno M, Mori T, Hayashi Y, Koike M, Shitara N, Iwama T, Kuroki T. Frequent mutation of β-catenin and APC genes in primary colorectal tumors from patients with hereditary nonpolyposis colorectal cancer. Cancer Res 59: 4506–4509, 1999 [PubMed] [Google Scholar]
- 76.Miyoshi Y, Ando H, Nagase H, Nishisho I, Horii A, Miki Y, Mori T, Utsunomiya J, Baba S, Petersen G. Germ-line mutations of the APC gene in 53 familial adenomatous polyposis patients. Proc Natl Acad Sci USA 89: 4452–4456, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Miyoshi Y, Nagase H, Ando H, Horii A, Ichii S, Nakatsuru S, Aoki T, Miki Y, Mori T, Nakamura Y. Somatic mutations of the APC gene in colorectal tumors: mutation cluster region in the APC gene. Hum Mol Genet 1: 229–233, 1992 [DOI] [PubMed] [Google Scholar]
- 78.Morikawa K, Walker SM, Jessup JM, Fidler IJ. In vivo selection of highly metastatic cells from surgical specimens of different primary human colon carcinomas implanted into nude mice. Cancer Res 48: 1943–1948, 1988 [PubMed] [Google Scholar]
- 79.Moser AR, Pitot HC, Dove WF. A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science 247: 322–324, 1990 [DOI] [PubMed] [Google Scholar]
- 80.Nagy-Szakal D, Mir SA, Ross MC, Tatevian N, Petrosino JF, Kellermayer R. Monotonous diets protect against acute colitis in mice: epidemiologic and therapeutic implications. J Pediatr Gastroenterol Nutr 56: 544–550, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Neufeld KL, Zhang F, Cullen BR, White RL. APC-mediated downregulation of beta-catenin activity involves nuclear sequestration and nuclear export. EMBO Rep 1: 519–523, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ogino S, Goel A. Molecular classification and correlates in colorectal cancer. J Mol Diagn 10: 13–27, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Potter JD, Slattery ML, Bostick RM, Gapstur SM. Colon cancer: a review of the epidemiology. Epidemiol Rev 15: 499–545, 1993 [DOI] [PubMed] [Google Scholar]
- 85.Puig I, Chicote I, Tenbaum SP, Arques O, Herance JR, Gispert JD, Jimenez J, Landolfi S, Caci K, Allende H, Mendizabal L, Moreno D, Charco R, Espin E, Prat A, Elez ME, Argiles G, Vivancos A, Tabernero J, Rojas S, Palmer HG. A personalized preclinical model to evaluate the metastatic potential of patient-derived colon cancer initiating cells. Clin Cancer Res 19: 6787–6801, 2013 [DOI] [PubMed] [Google Scholar]
- 86.Rampino N, Yamamoto H, Ionov Y, Li Y, Sawai H, Reed JC, Perucho M. Somatic frameshift mutations in the BAX gene in colon cancers of the microsatellite mutator phenotype. Science 275: 967–969, 1997 [DOI] [PubMed] [Google Scholar]
- 87.Redston MS, Papadopoulos N, Caldas C, Kinzler KW, Kern SE. Common occurrence of APC and K-ras gene mutations in the spectrum of colitis-associated neoplasias. Gastroenterology 108: 383–392, 1995 [DOI] [PubMed] [Google Scholar]
- 88.Reitmair AH, Cai JC, Bjerknes M, Redston M, Cheng H, Pind MT, Hay K, Mitri A, Bapat BV, Mak TW, Gallinger S. MSH2 deficiency contributes to accelerated APC-mediated intestinal tumorigenesis. Cancer Res 56: 2922–2926, 1996 [PubMed] [Google Scholar]
- 89.Reitmair AH, Redston M, Cai JC, Chuang TC, Bjerknes M, Cheng H, Hay K, Gallinger S, Bapat B, Mak TW. Spontaneous intestinal carcinomas and skin neoplasms in Msh2-deficient mice. Cancer Res 56: 3842–3849, 1996 [PubMed] [Google Scholar]
- 90.Rizzo A, Pallone F, Monteleone G, Fantini MC. Intestinal inflammation and colorectal cancer: a double-edged sword? World J Gastroenterol 17: 3092–3100, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rosenberg DW, Giardina C, Tanaka T. Mouse models for the study of colon carcinogenesis. Carcinogenesis 30: 183–196, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rosin-Arbesfeld R, Townsley F, Bienz M. The APC tumour suppressor has a nuclear export function. Nature 406: 1009–1012, 2000 [DOI] [PubMed] [Google Scholar]
- 93.Rubinfeld B, Souza B, Albert I, Muller O, Chamberlain SH, Masiarz FR, Munemitsu S, Polakis P. Association of the APC gene product with beta-catenin. Science 262: 1731–1734, 1993 [DOI] [PubMed] [Google Scholar]
- 94.Rutkowski MR, Stephen TL, Conejo-Garcia JR. Anti-tumor immunity: myeloid leukocytes control the immune landscape. Cell Immunol 278: 21–26, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Saam JR, Gordon JI. Inducible gene knockouts in the small intestinal and colonic epithelium. J Biol Chem 274: 38071–38082, 1999 [DOI] [PubMed] [Google Scholar]
- 96.Schliekelman M, Cowley DO, O'Quinn R, Oliver TG, Lu L, Salmon ED, Van Dyke T. Impaired Bub1 function in vivo compromises tension-dependent checkpoint function leading to aneuploidy and tumorigenesis. Cancer Res 69: 45–54, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Scott KP, Gratz SW, Sheridan PO, Flint HJ, Duncan SH. The influence of diet on the gut microbiota. Pharmacol Res 69: 52–60, 2013 [DOI] [PubMed] [Google Scholar]
- 98.Shao J, Washington MK, Saxena R, Sheng H. Heterozygous disruption of the PTEN promotes intestinal neoplasia in APCmin/+ mouse: roles of osteopontin. Carcinogenesis 28: 2476–2483, 2007 [DOI] [PubMed] [Google Scholar]
- 99.Shibata H, Toyama K, Shioya H, Ito M, Hirota M, Hasegawa S, Matsumoto H, Takano H, Akiyama T, Toyoshima K, Kanamaru R, Kanegae Y, Saito I, Nakamura Y, Shiba K, Noda T. Rapid colorectal adenoma formation initiated by conditional targeting of the Apc gene. Science 278: 120–123, 1997 [DOI] [PubMed] [Google Scholar]
- 100.Sierra J, Yoshida T, Joazeiro CA, Jones KA. The APC tumor suppressor counteracts beta-catenin activation and H3K4 methylation at Wnt target genes. Genes Dev 20: 586–600, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Siolas D, Hannon GJ. Patient-derived tumor xenografts: transforming clinical samples into mouse models. Cancer Res 73: 5315–5319, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sjoblom T, Jones S, Wood LD, Parsons DW, Lin J, Barber TD, Mandelker D, Leary RJ, Ptak J, Silliman N, Szabo S, Buckhaults P, Farrell C, Meeh P, Markowitz SD, Willis J, Dawson D, Willson JK, Gazdar AF, Hartigan J, Wu L, Liu C, Parmigiani G, Park BH, Bachman KE, Papadopoulos N, Vogelstein B, Kinzler KW, Velculescu VE. The consensus coding sequences of human breast and colorectal cancers. Science 314: 268–274, 2006 [DOI] [PubMed] [Google Scholar]
- 104.Spink KE, Polakis P, Weis WI. Structural basis of the Axin-adenomatous polyposis coli interaction. EMBO J 19: 2270–2279, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Su LK, Kinzler KW, Vogelstein B, Preisinger AC, Moser AR, Luongo C, Gould KA, Dove WF. Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC gene. Science 256: 668–670, 1992 [DOI] [PubMed] [Google Scholar]
- 106.Su LK, Vogelstein B, Kinzler KW. Association of the APC tumor suppressor protein with catenins. Science 262: 1734–1737, 1993 [DOI] [PubMed] [Google Scholar]
- 107.Sussman DA, Santaolalla R, Strobel S, Dheer R, Abreu MT. Cancer in inflammatory bowel disease: lessons from animal models. Curr Opin Gastroenterol 28: 327–333, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Taketo MM, Edelmann W. Mouse models of colon cancer. Gastroenterology 136: 780–798, 2009 [DOI] [PubMed] [Google Scholar]
- 109.Thorstensen L, Lind GE, Lovig T, Diep CB, Meling GI, Rognum TO, Lothe RA. Genetic and epigenetic changes of components affecting the WNT pathway in colorectal carcinomas stratified by microsatellite instability. Neoplasia 7: 98–108, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Trobridge P, Knoblaugh S, Washington MK, Munoz NM, Tsuchiya KD, Rojas A, Song X, Ulrich CM, Sasazuki T, Shirasawa S, Grady WM. TGF-beta receptor inactivation and mutant Kras induce intestinal neoplasms in mice via a beta-catenin-independent pathway. Gastroenterology 136: 1680–1688, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tseng-Rogenski SS, Chung H, Wilk MB, Zhang S, Iwaizumi M, Carethers JM. Oxidative stress induces nuclear-to-cytosol shift of hMSH3, a potential mechanism for EMAST in colorectal cancer cells. PLoS One 7: e50616, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ullman TA, Itzkowitz SH. Intestinal inflammation and cancer. Gastroenterology 140: 1807–1816, 2011 [DOI] [PubMed] [Google Scholar]
- 113.Umar A, Dunn BK, Greenwald P. Future directions in cancer prevention. Nat Rev Cancer 12: 835–848, 2012 [DOI] [PubMed] [Google Scholar]
- 114.Umetani N, Sasaki S, Watanabe T, Shinozaki M, Matsuda K, Ishigami H, Ueda E, Muto T. Genetic alterations in ulcerative colitis-associated neoplasia focusing on APC, K-ras gene and microsatellite instability. Jpn J Cancer Res 90: 1081–1087, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Velcich A, Yang W, Heyer J, Fragale A, Nicholas C, Viani S, Kucherlapati R, Lipkin M, Yang K, Augenlicht L. Colorectal cancer in mice genetically deficient in the mucin Muc2. Science 295: 1726–1729, 2002 [DOI] [PubMed] [Google Scholar]
- 116.Voskoglou-Nomikos T, Pater JL, Seymour L. Clinical predictive value of the in vitro cell line, human xenograft, and mouse allograft preclinical cancer models. Clin Cancer Res 9: 4227–4239, 2003 [PubMed] [Google Scholar]
- 117.Waldner M, Schimanski CC, Neurath MF. Colon cancer and the immune system: the role of tumor invading T cells. World J Gastroenterol 12: 7233–7238, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wang XD, Liu C, Bronson RT, Smith DE, Krinsky NI, Russell M. Retinoid signaling and activator protein-1 expression in ferrets given beta-carotene supplements and exposed to tobacco smoke [see comments]. J Natl Cancer Inst 91: 60–66, 1999 [DOI] [PubMed] [Google Scholar]
- 119.Washington MK, Powell AE, Sullivan R, Sundberg JP, Wright N, Coffey RJ, Dove WF. Pathology of rodent models of intestinal cancer: progress report and recommendations. Gastroenterology 144: 705–717, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Watson MM, Berg M, Soreide K. Prevalence and implications of elevated microsatellite alterations at selected tetranucleotides in cancer. Br J Cancer. 2014. Apr 1. 10.1038/bjc.2014.167 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Westbrook AM, Wei B, Braun J, Schiestl RH. Intestinal inflammation induces genotoxicity to extraintestinal tissues and cell types in mice. Int J Cancer 129: 1815–1825, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Woo DK, Green PD, Santos JH, D'Souza AD, Walther Z, Martin WD, Christian BE, Chandel NS, Shadel GS. Mitochondrial genome instability and ROS enhance intestinal tumorigenesis in APCMin/+ mice. Am J Pathol 180: 24–31, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Xu H, Posner GH, Stevenson M, Campbell FC. Apc(MIN) modulation of vitamin D secosteroid growth control. Carcinogenesis 31: 1434–1441, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Xue Y, Johnson R, DeSmet M, Snyder PW, Fleet JC. Generation of a transgenic mouse for colorectal cancer research with intestinal cre expression limited to the large intestine. Mol Cancer Res 8: 1095–1104, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yamada K, Kanazawa S, Koike J, Sugiyama H, Xu C, Funahashi K, Boland CR, Koi M, Hemmi H. Microsatellite instability at tetranucleotide repeats in sporadic colorectal cancer in Japan. Oncol Rep 23: 551–561, 2010 [PMC free article] [PubMed] [Google Scholar]
- 126.Yang JL, Hanley JR, Yu Y, Berney CR, Russell PJ, Crowe PJ. In vivo overexpression of c-erbB-2 oncoprotein in xenografts of mice implanted with human colon cancer lines. Anticancer Res 17: 3463–3468, 1997 [PubMed] [Google Scholar]
- 127.Yang K, Popova NV, Yang WC, Lozonschi I, Tadesse S, Kent S, Bancroft L, Matise I, Cormier RT, Scherer SJ, Edelmann W, Lipkin M, Augenlicht L, Velcich A. Interaction of Muc2 and Apc on Wnt signaling and in intestinal tumorigenesis: potential role of chronic inflammation. Cancer Res 68: 7313–7322, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yap TA, Gerlinger M, Futreal PA, Pusztai L, Swanton C. Intratumor heterogeneity: seeing the wood for the trees. Sci Transl Med 4: 127ps110, 2012 [DOI] [PubMed] [Google Scholar]
- 129.Yu M, Trobridge P, Wang Y, Kanngurn S, Morris SM, Knoblaugh S, Grady WM. Inactivation of TGF-β signaling and loss of PTEN cooperate to induce colon cancer in vivo. Oncogene 33: 1538–1547, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zeelenberg IS, Ruuls-Van Stalle L, Roos E. The chemokine receptor CXCR4 is required for outgrowth of colon carcinoma micrometastases. Cancer Res 63: 3833–3839, 2003 [PubMed] [Google Scholar]
- 131.Zeineldin M, Neufeld KL. Understanding phenotypic variation in rodent models with germline Apc mutations. Cancer Res 73: 2389–2399, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhang Y, Davis C, Ryan J, Janney C, Pena MM. Development and characterization of a reliable mouse model of colorectal cancer metastasis to the liver. Clin Exp Metastasis 30: 903–918, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhu Y, Richardson JA, Parada LF, Graff JM. Smad3 mutant mice develop metastatic colorectal cancer. Cell 94: 703–714, 1998 [DOI] [PubMed] [Google Scholar]