Abstract
Biotin is essential for the normal function of pancreatic beta cells. These cells obtain biotin from their surroundings via transport across their cell membrane. Little is known about the uptake mechanism involved, how it is regulated, and how it is affected by internal and external factors. We addressed these issues using the mouse-derived pancreatic beta-TC-6 cells and freshly isolated mouse and human primary pancreatic beta cells as models. The results showed biotin uptake by pancreatic beta-TC-6 cells occurs via a Na+-dependent, carrier-mediated process, that is sensitive to desthiobiotin, as well as to pantothenic acid and lipoate; the process is also saturable as a function of concentration (apparent Km = 22.24 ± 5.5 μM). These cells express the sodium-dependent multivitamin transporter (SMVT), whose knockdown (with doxycycline-inducible shRNA) led to a sever inhibition in biotin uptake. Similarly, uptake of biotin by mouse and human primary pancreatic islets is Na+-dependent and carrier-mediated, and both cell types express SMVT. Biotin uptake by pancreatic beta-TC-6 cells is also adaptively regulated (via transcriptional mechanism) by extracellular substrate level. Chronic treatment of pancreatic beta-TC-6 cells with bacterial lipopolysaccharides (LPS) leads to inhibition in biotin uptake. This inhibition is mediated via a Toll-Like receptor 4-mediated process and involves a decrease in membrane expression of SMVT. These findings show, for the first time, that pancreatic beta cells/islets take up biotin via a specific and regulated carrier-mediated process, and that the process is sensitive to the effect of LPS.
Keywords: biotin, pancreatic beta cells, biotin uptake mechanism, transport regulation
the water-soluble vitamin biotin, also known as vitamin B7 or vitamin H, is required for normal cellular functions, growth, and development. Biotin acts as a cofactor (as a carboxyl carrier) for five carboxylases that catalyze indispensable steps in intermediate metabolism including gluconeogenesis and fatty acid metabolism (reviewed in Refs. 34, 35). Emerging evidences also point to a role for biotin in normal immune function and in the regulation of gene expression (reviewed in Refs. 24, 29). In reference to the latter, biotin status has been shown to influence the expression of more than 2,000 human genes, at both the transcriptional and post-transcriptional levels (3, 30, 44). The affected genes include those that are critical for maintaining the differentiated phenotype of pancreatic beta cells, preservation of their mass (i.e., their proportion), and for insulin secretion (3, 30, 38, 44). In reference to the latter, the vitamin has been shown to increases the expression of pancreatic and duodenal homeobox 1 (Pdx-1) (44), a critical transcription factor for the expression of insulin, and several genes involved in its synthesis and secretion by pancreatic beta cells (2). In pancreatic beta cells, biotin appears to increase the expression of insulin receptor (7) and glucokinase (3, 30, 39), a rate-limiting enzyme in glucose-stimulated insulin secretion (20). It also decreases the expression of several gluconeogenic genes (6, 36). Finally, biotin supplementation appears to have the ability to reduce hyperglycemia and improve glucose tolerance in humans (5, 15, 20), as well as in animal models of diabetes (26, 46).
Despite the importance of biotin for the health and function of pancreatic beta cells, and its apparent influence on insulin and glucose physiology, little is known about the mechanism involved in biotin uptake by these cells, how the process is regulated, and how factors that affect the health/function of pancreatic cells (like bacterial lipopolysaccharides, LPS) affect biotin uptake. Like all other mammalian cells, pancreatic beta cells cannot synthesize biotin endogenously, and thus must obtain it from their surroundings (circulation) via transport across cell membrane. The aims of this study were to delineate the mechanism and regulation of biotin uptake by pancreatic beta cells and to examine the effect of chronic exposure to LPS on the uptake process. We used the mouse-derived culture pancreatic beta-TC-6 cells as well as freshly isolated primary mouse and human pancreatic islets. The results showed biotin uptake by pancreatic beta cells/islets is via a specific Na+-dependent carrier-mediated process that involves the sodium-dependent multivitamin transporter (SMVT) system. This process is adaptively regulated by the substrate level in the extracellular compartment and is sensitive to LPS.
MATERIALS AND METHODS
Materials.
Radioactive 3H-biotin and 3H-pantothenic acid (specific activity: 60 Ci/mmol and 50 Ci/mmol, respectively; radiochemical purity for both >97%) were purchased from American Radiolabeled Chemicals (ARC) (St. Louis, MO). Other reagents/chemicals used in these studies were purchased from commercial vendors, and all were of either analytical or molecular biology grades. Specific primers used for PCR amplifications were from Sigma Genosys (Woodlands, TX).
Cell culture and uptake studies.
The mouse-derived beta-TC-6 cells (ATCC, Rockville, MD) were maintained under standard condition (5% CO2 and 37°C) in DMEM growth medium supplemented with 15% FBS and antibiotics. To make a biotin-deficient medium, we used a custom-made vitamin deficient medium (DMEM from GIBCO-BRL, Grand Island, NY) supplemented with 2.5% of dialyzed FBS (treated with avidin-agarose) (28). For knocking down SMVT using the inducible shRNA approach, cells were maintained in DMEM supplemented with 10% of Clontech's Tet system approved FBS (Clontech Laboratories, Mountain View, CA). TLR4 gene inactivation was done by commercially available dominant negative mutant (pZERO-mTLR4; Invivogen, CA). Uptake of 3H-biotin was measured in cells incubated in Krebs-Ringer buffer (pH 7.4 unless otherwise stated) at 37°C as described before (23); uptake was liner as a function of time for 8 min (data not shown). Uptake was expressed in term of milligram cellular protein per unit time (i.e., femtomoles per milligram protein per minute). Protein concentrations were determined using a Bio-Rad Dc protein assay kit (Hercules, CA).
Preparation of mouse and human primary pancreatic islets for uptake studies.
Mouse primary pancreatic islets were isolated by enzymatic digestion followed by Ficoll gradient centrifugation method as reported by us before (23). Briefly, mice (8–10 wk old) were euthanized using ketamine and xylazine (Intraperitoneal injection of 100 and 10 mg/kg, respectively). The pancreas was isolated, minced, and digested with collagenase IV (1 mg/ml) containing DNase I (0.1 mg/ml) for 30 min with occasional shaking at 37°C. After digestion, pancreatic tissues were filtered through nylon mesh and the filtrate was subjected to discontinuous Ficoll gradient (25, 23, 20, and 11%, respectively) for isolation of islets. Islets were collected from the interface of 20 and 11% of Ficoll, subsequently washed three times, and used for uptake studies as described before (23). Cell viability was assessed using Trypan blue, with the result showing 85–90% viability.
Primary human pancreatic islets were obtained from healthy adult organ donors (National Disease Research Interchange, Philadelphia, PA). After receiving the islets, samples were centrifuged (1,500 rpm for 8 min), washed, and resuspended in KR buffer, then used for uptake/molecular investigations. Islets viability was checked by Trypan blue and found to be around 70%.
Quantitative real-time PCR.
Total RNA samples were isolated using TRIzol (Invitrogen, Carlsbad, CA) method as described in the manufacture's protocol. The isolated RNA samples were subjected to DNaseI treatment to remove DNA contamination, and first strand c-DNAs were synthesized using iScript reverse transcriptase kit (Bio-Rad). Relative gene expression was measured by quantitative real-time PCR using gene-specific primers, and levels were normalized relative to mouse or human β-actin (for mSMVT, forward 5′-GGATCTGTGGGACTGTGA-3′ and reverse 5′-CACATCTGTCCAGATGACA-3′; for β-actin, forward 5′-ATCCTCTTCCTCCCTGGA-3′ and reverse 5′-TTCATGGATGCCACAGGA-3′; for hSMVT, forward 5′-TGTCTACCTTCTCCATCATGGA-3′ and reverse 5′-TAGAGCCCAATGGCAAGAGA-3′; for; for β-actin, forward 5′-GTCAGGTCATCACTATCGGC-3′ and reverse 5′-CATGGATGCCACAGGATTCC-3′) following 2−ΔΔct method (19).
SMVT knockdown by inducible shRNA in beta-TC-6 cells.
Three SMVT gene-specific shRNA target sequences (5′-AATGGGCTGTCTTCCTGTG-3′ and 5′-CACAGGAAGACAGCCCATT-3′) were designed for cloning into the pSingle-tTS-shRNA vector following manufacturer's protocol (Clontech Laboratories, Mountain View, CA). Briefly, the complementary strands were annealed to each other, and the double stranded DNA was ligated to the pSingle-tTS-shRNA vector (which was linearized with XhoI and HindIII). The construct was transformed into XL1 blue Escherichia coli, and the isolated plasmid was sequenced to verify the insert.
The recombinant pSingle-tTS-shRNA plasmids were then transfected into cultured pancreatic beta-TC-6 cells; 48 h after transfection, cells were maintained in G418 (500 μg/ml) for selection. Gene knockdown was induced by treating the cells with doxycycline (800 ng/ml) for 72 h. Cell populations treated in identical manner, but without doxycycline, were used as controls.
Western blot analysis.
Total protein was isolated using RIPA buffer (Sigma) in presence of a protease inhibitors cocktail (Roche, Nutley, NJ). Total isolated protein was loaded onto NuPAGE 4–12% Bis-Tris gradient minigels (Invitrogen) and subjected to Western blot analysis. The blot was coincubated with anti-mSMVT antibody (raised in rabbit) and monoclonal anti-β actin antibody followed by incubation with anti-rabbit IR 800 dye and anti-mouse IR 680 dye (LI-COR, Lincoln, Nebraska) secondary antibodies (1:25,000) at room temperature for 1 h. The expressions of respective proteins were quantified by using Odyssey application software (version 3.0) in Odyssey Infrared imaging system (LI-COR).
Cell surface biotinylation assay.
The effect of LPS on the expression of the SMVT protein at the cell membrane of pancreatic beta-TC-6 was examined by a biotinylation assay using a cell surface biotinylation kit (Pierce Biotechnology, Rockford, IL). Briefly, cells were maintained under serum starved condition overnight and treated with LPS (1 μg/ml) for 24 h (42). Cell surface biotinylation was performed by treating cells with sulfo-NHS-SS-biotin followed by isolation of surface proteins by incubating the cell lysate with streptavidine-agarose beads following manufacturer's protocol. Relative surface expression of SMVT was determined with respect to total cellular SMVT by mean of Western blotting.
Statistical analysis.
Data shown here are means ± SE of at least 3 separate experiments and were analyzed for significance using the Student's t-test. Uptake data were expressed as femtomoles per milligram protein per unit time or as percentage relative to simultaneously performed controls. To determine transport kinetic parameters of the carrier-mediated process, uptake by the latter component was determined by subtracting uptake by diffusion from total uptake at each concentration examined. The apparent Km and Vmax of the carrier-mediated process were determined using Michaelis-Menten equation in Graph Pad Prism software (Version 5.03).
RESULTS
Physiological Characterization of the Biotin Uptake Process of Pancreatic Beta Cells/Islets
General characteristics.
Isosmotic replacement of Na+ in the incubation buffer with another monovalent cation (K+) led to a significant (P < 0.01) inhibition in the initial rate of biotin (5 nM) uptake by pancreatic beta-TC-6 cells (155 ± 13 and 20 ± 6 fmol·mg protein−1·min−1 in the presence and absence of Na+, respectively). The initial rate of biotin uptake by pancreatic beta-TC-6 cells was also pH dependent with a significantly (P < 0.01) higher uptake at pH 7.4 than at pH 6.5 and 5.5 (as percentage to uptake at pH 7.4: 100 ± 0.88, 65.16 ± 3.19, 48.32 ± 4.46, respectively). We examined the effect of unlabeled biotin, its structural analog desthiobiotin, and that of lipoate and pantothenic acid (all at 500 μM) on the initial rate of 3H-biotin (5 nM) uptake, and observed a significant (P < 0.01 for all) inhibition in 3H-biotin uptake in the presence of all these compounds (as percentage: 100 ± 3.84, 13.57 ± 2.44, 13.72 ± 4.61, 12.67 ± 3.71, and 11.57 ± 3.13 for control and in the presence of unlabeled biotin, desthiobiotin, lipoate, and pantothenic acid, respectively).
Similar findings were obtained with freshly isolated primary mouse pancreatic islets in that the initial rate of biotin uptake was significantly (P < 0.05) higher at physiological pH 7.4 compared with pH 6.5 and 5.5 (11.1 ± 0.42, 8.73 ± 0.46, and 3.61 ± 0.65 fmol·mg protein−1·min−1, respectively; P < 0.05), and that Na+ replacement with K+ led to a significant (P < 0.05) inhibition in uptake (as percentage: 100 ± 19.12 and 13.3 ± 5.8 in the presence and absence of Na+, respectively). Moreover, unlabeled biotin (1 mM) caused a significant (P < 0.01) inhibition in the initial rate of uptake of 3H-biotin (5 nM) (as percentage: 100 ± 10 and 35.7 ± 7 in the absence and presence of unlabeled biotin, respectively). In other studies, we extended the investigations to the human situation and examined the effect of Na+ removal (replacement with Li+) and that of unlabeled biotin (1 mM) in the incubation medium on the initial rate of 3H-biotin (5 nM) uptake by primary human pancreatic islets. The results showed a significant inhibition in biotin uptake upon Na+ replacement [uptake of 2.46 ± 0.15 and 0.43 ± 0.9 fmol·mg protein−1·min−1 (P < 0.05) in the presence and absence of Na+, respectively], and in the presence of unlabeled biotin [in percentage: 100 ± 5.3 and 14.51 ± 5.04 (P < 0.01) in the absence and presence of unlabeled biotin, respectively].
Collectively, the above described results suggest that biotin uptake by mouse and human pancreatic beta cells/islets is mediated via a Na+-dependent carrier-mediated mechanism.
Kinetic parameter of biotin uptake by pancreatic beta cells.
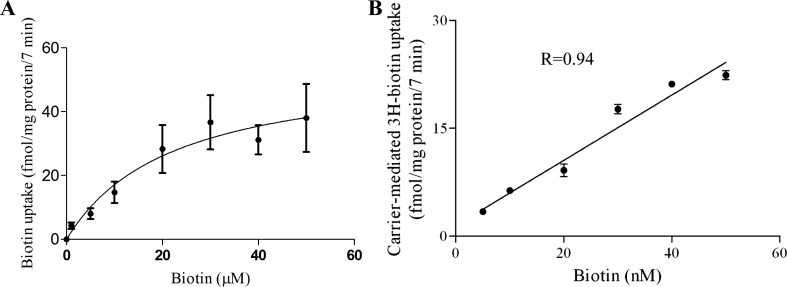
Kinetic parameters of the biotin uptake process of pancreatic beta-TC-6 cells were determined by examining the initial rate of biotin uptake as a function of substrate concentration. The results showed that uptake includes a saturable component over the micromolar range (Fig. 1A) with a Vmax and an apparent Km of 55.1 ± 5.9 fmol·mg protein−1·7 min−1 and 22.24 ± 5.5 μM, respectively (Fig. 1A). Since studies by others (13, 45) have suggested the possible existence of another biotin uptake system that functions at the nanomolar range, we tested the possible existence of such a system by examining uptake as a function of that concentration range. No evidence for the existence of such a system was observed, as biotin uptake was linear over this range (Fig. 1B).
Fig. 1.
Uptake of biotin by pancreatic beta-TC-6 cells as a function of substrate concentration. Confluent monolayers were incubated at 37°C in Krebs-Ringer buffer, pH 7.4, in the presence of micromolar (1–50 μM) (A) and nanomolar (5–50 nM) (B) concentrations of biotin. Uptake by the carrier-mediated system was calculated as described in materials and methods. Values are means ± SE of at least 3 separate uptake determinations. When not visible, error bars are smaller than the displayed symbol.
Molecular identity of the biotin uptake system of pancreatic beta cells/islets.
The SMVT system is expressed in a variety of tissues (41). Here we examined whether mouse and human pancreatic beta cells/islets express the SMVT system at the mRNA levels. The results showed that SMVT is indeed expressed, albeit at different levels, in pancreatic beta-TC-6 cells and in mouse and human primary pancreatic islets. Human pancreatic islets showed more expression of SMVT than cultured and primary mouse pancreatic beta-TC-6 cells, respectively.
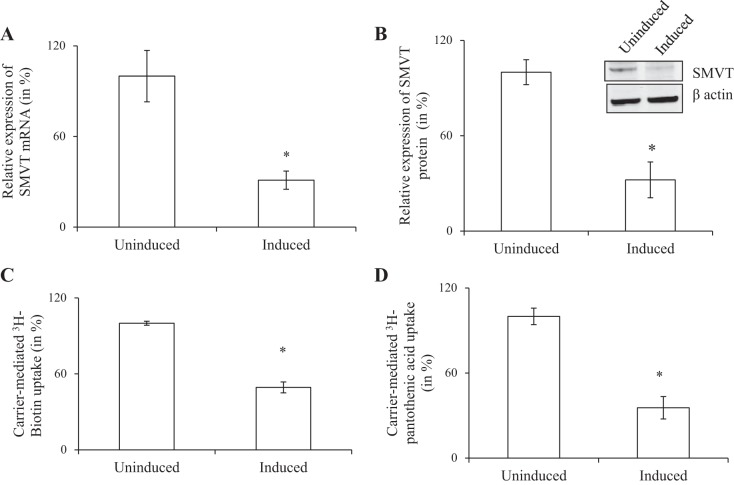
To examine the relative contribution of the SMVT system toward carrier-mediated biotin uptake by pancreatic beta-TC-6 cells, we used the approach of gene silencing with shRNA. We stably transfected the cells with doxycycline-inducible shRNA targeted to mouse SMVT. Gene knockdown was induced by incubating the transfected cells with doxycycline. The effectiveness of the gene silencing was verified by demonstrating a significant reduction (around 70%; P < 0.01 for both) in expression of SMVT at the mRNA and protein levels in the shRNA transfected and induced cells compared with noninduced cells (Fig. 2, A and B). The consequence of knocking down the SMVT system on carrier-mediated biotin uptake was determined with the results showing ∼60% (P < 0.01) inhibition in carrier-mediated biotin uptake in induced cells expressing shRNA compared with noninduced cells (Fig. 2C). These findings suggest that the SMVT system is the major biotin uptake carrier by pancreatic beta-TC-6 cells. Since SMVT also transports pantothenic acid, we tested if knocking down SMVT (with the same shRNA approach) also inhibits uptake of this substrate. The results indeed showed a significant decrease in 3H-pantothenic acid uptake (P < 0.05) in induced cells expressing shRNA compared with noninduced cells (Fig. 2D).
Fig. 2.
Effect of knocking down the sodium-dependent multivitamin transporter (SMVT) system of pancreatic beta-TC-6 cells on carrier-mediated biotin and pantothenic acid uptake. Following induction of the knockdown with doxycycline (DOX) (see materials and methods), the following were examined: level of expression of the SMVT mRNA (quantitative real-time PCR; expression was relative to beta-actin) (A), level of expression of the SMVT protein (Western blotting; expression was relative to respective beta-actin) (B), initial rate of carrier-mediated biotin uptake (C), and the effect of knocking down the SMVT system on carrier-mediated pantothenic acid uptake (another substrate of the SMVT system) (D). Inset in B shows a representative gel image. All values are means ± SE of at least 3 independent experiments. *P < 0.01.
The human SLC5A6 (the gene that encodes SMVT) has two promoters (promoter 1 and 2; P1 and P2) with activity of P1 being higher than that of P2 in a number of tissues, as we reported before (8, 27). Thus we determined the relative activity of these two promoters in pancreatic beta-TC-6 cells [the human SLC5A6 5′-promoters are active in mice in vivo (27)]. The results showed a significantly (P < 0.01) higher P1 activity than P2 (Fig. 3), suggesting these cells utilize the former promoter to a greater extent than the latter in driving the transcription of this gene in pancreatic beta cells.
Fig. 3.
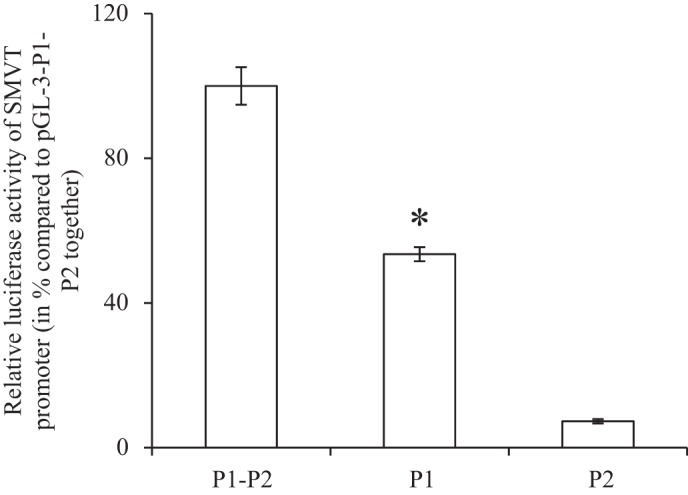
Relative activities of the SLC5A6 promoters 1 and 2 in pancreatic beta-TC-6 cells promoters. Promoter constructs pGL3-P1P2, pGL3-P1, and pGL3-P2 were transiently expressed in pancreatic beta-TC-6 cells followed (48 h) by determination of firefly luciferase activity. Activity of the latter was normalized relative to activity of simultaneously expressed Renilla luciferase and expressed as %. All values are means ± SE of at least 3 independent experiments. *P < 0.01 in comparison of P1 to P2.
Regulation of the Biotin Uptake by Pancreatic Beta Cells
Adaptive regulation by extracellular substrate level.
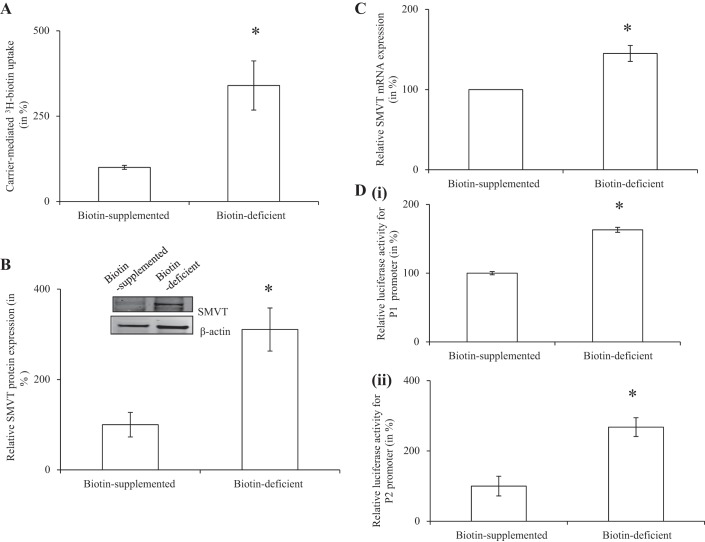
We examined whether the biotin uptake process of pancreatic beta cells is adaptively regulated by the prevailing vitamin level. We examined the initial rate of biotin uptake by pancreatic beta-TC-6 cells maintained in biotin-deficient medium and in biotin oversupplemented (100 μM) medium (see materials and methods). The results showed a significantly (P < 0.01) higher 3H-biotin (5 nM) uptake in cells maintained in biotin-deficient medium compared with those maintained in biotin oversupplemented medium (Fig. 4A). Expression of the SMVT protein and mRNA were both found to be significantly (P < 0.01 for both) higher in the cells that were maintained in the biotin-deficient medium compared with those in the oversupplemented medium (Fig. 4, B and C). We further examined if the adaptive regulation in biotin uptake is mediated by induction of the SMVT promoters. For this we used the full-length human SMVT promoters P1 and P2 fused to the firefly luciferase reporter gene (27, 28). Promoter constructs were transfected into pancreatic beta-TC-6 cells and maintained for 96 h in biotin-deficient and oversupplemented medium. The result showed a significantly (P < 0.01 for both) higher promoter activity in cells maintained in the biotin-deficient medium compared with those in the oversupplemented medium (Fig. 4D, i and ii).
Fig. 4.
Adaptive regulation of SMVT as a function of extracellular biotin level. Pancreatic beta-TC-6 cells were maintained in biotin-deficient or biotin oversupplemented (100 μM) medium for 96 h followed by determination of initial rate of carrier-mediated uptake (A), level of SMVT protein (Western blotting) (B), level of SMVT mRNA (quantitative real-time PCR (C), and activity of the SLC5A6 promoter 1 (i) and promoter 2 (ii) (D). Results are means ± SE of at least 3 independent experiments. *P < 0.05.
Possible regulation of biotin uptake in pancreatic beta cells by intracellular signaling pathways and by high glucose levels.
We examined whether the process of biotin uptake by mouse pancreatic beta cells is regulated by intracellular signaling pathways, focusing on those pathways that regulate the uptake of other nutrients in a variety of other cellular systems [e.g., protein kinase A (PKA), Ca2+/calmodulin, protein tyrosine kinase (PTK), and protein kinase C (PKC)] (11; reviewed in Ref. 32). Using specific modulators of these regulatory pathways, the results showed that although modulators of PKA (dibutyryl cAMP and forskolin), Ca2+/calmodulin (calmidazolium chloride), and PTK (genistein)-mediated pathways have no effect on biotin uptake by pancreatic beta-TC-6 cells (data not shown), the classic PKC activator phorbol 12-myristate 13-acetate (PMA), but not its negative control (4α-PMA), caused a significant (P < 0.05) inhibition (as percentage: 100 ± 6, 75.7 ± 3.5, and 106.1 ± 6.7 for control, PMA-treated, and 4α-PMA-treated cells, respectively).
Since biotin plays a role in normal pancreatic beta cells homeostasis and function (38), and high glucose levels affect pancreatic physiology (9, 17, 18, 37), we also investigated the effect of prolonged exposure (96 h) of pancreatic beta-TC6 cells to a high glucose (26 mM) level on biotin uptake. The results, however, showed no effect of such a treatment on biotin uptake (as percentage: 100 ± 11.3 and 114 ± 8.8 in the presence of 5.5 and 26 mM glucose, respectively), suggesting that biotin uptake is not affected by hyperglycemia.
Effect of bacterial LPS on biotin uptake by pancreatic beta cells.
Gut microbiota has recently been proposed as an environmental factor that increases the risk of metabolic disorders such as diabetes mellitus. Patients with Type 2 diabetes have altered gut microbial diversity and an increase in the level of circulating bacterial LPS, which is associated with a low-grade endotoximia (4, 16, 43). LPS has been shown to exert deleterious effects on the health/function of pancreatic beta cells, and it does so via Toll-Like receptor 4 (TLR4)–mediated mechanism (1). The effect of LPS on biotin uptake by pancreatic beta cells is not known and, therefore, was examined using pancreatic beta-TC-6 cells. The results showed that exposure (24 h) of the cells to clinically relevant LPS concentrations (1 μg/ml) (1, 42) lead to a significant and concentration-dependent inhibition in the initial rate of biotin uptake (Fig. 5A) (LPS did not affect cell viability as indicated by Trypan blue analysis where >95% of the treated cells were found to be viable). Since LPS exerts its effect via TLR4, we examined the effect of inhibiting the expression of this receptor by expressing a dominant negative mutant on the ability of LPS to inhibit biotin uptake by pancreatic beta-TC-6 cells (see materials and methods). The results showed that such a treatment leads to significant (P < 0.01) protection against the inhibitory effect of LPS on biotin uptake by these cells (Fig. 5B).
Fig. 5.
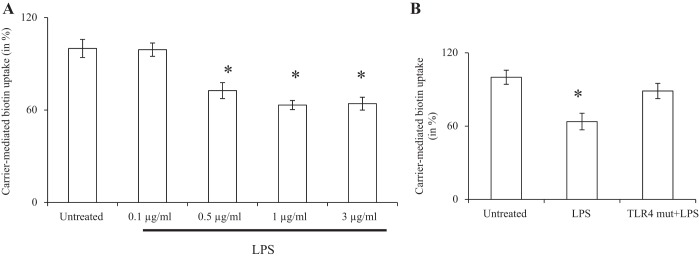
Effect of LPS on carrier-mediated biotin uptake by pancreatic beta cells: role of the TLR receptor. A: pancreatic beta-TC-6 cells were pretreated (for 24 h) with increased concentrations of LPS, and carrier-mediated biotin uptake was then determined. B: cells were transfected with TLR4 dominant negative plasmid and subjected to LPS pretreatment (24 h). Data are means ± SE of 3 independent experiments and expressed as % relative to simultaneously performed untreated control. *P < 0.01.
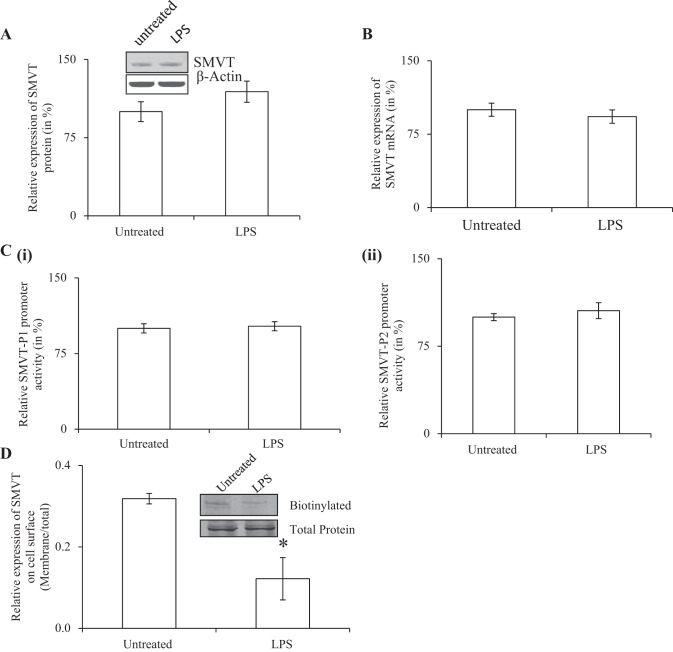
To determine if the inhibitory effect of LPS is mediated via inhibition in the expression of SMVT protein and mRNA, we examined the effect of LPS treatment of pancreatic beta-TC6 cells by Western blotting and quantitative PCR, respectively. The results showed LPS treatment to have no effect on either of these parameters (Fig. 6, A and B). We also examined the effect of LPS on activity of the SLC5A6 promoters transfected into pancreatic beta-TC-6 cells and again observed no effect (Fig. 6C, i and ii). These findings suggest that the inhibitory effect of LPS on biotin uptake by pancreatic beta cell is not mediated via changes in protein and RNA levels, nor in the transcription rate of the SLC5A6 gene. This led us to speculate that the effect is probably mediated by alteration in the expression of the SMVT protein at the cell membrane. To test this possibility, we performed biotinylation assays (see methods) and indeed found a significant (P < 0.05) reduction in the level of expression of the SMVT protein at the cell membrane with no change in level of expression of total cellular SMVT protein in LPS-treated cells compared with control cells (Fig. 6D).
Fig. 6.
Effect of exposure of pancreatic beta cells to LPS on molecular parameters of SMVT. Pancreatic beta-TC-6 cells were pre-exposed to LPS (1 μg/ml for 24 h) followed by determination of level of expression of SMVT protein (A) and mRNA (B) levels and activity of SLC5A6 promoter promoters 1 and 2 (C, i and ii). D shows the result of the biotinylation assay. Level of expression of biotinylated membranous SMVT was normalized relative to total SMVT in the cellular homogenate. Inset: a representative Western blot showing level of expression of SMVT at the cell membrane and in the total cellular homogenate. All methodologies are described in materials and methods. Data are means ± SE of 3 independent experiments and expressed as % relative to simultaneously performed untreated controls. *P < 0.05.
DISCUSSION
Previous in vitro and in vivo studies have shown that biotin is important for normal health/function of pancreatic beta cells. The vitamin plays a role in the expression of genes that are critical for maintaining the differentiated phenotype of pancreatic beta cells and in preserving their mass; it also plays a role in insulin secretion (3, 30, 39, 44) and in expression of glucokinase (30, 39) and Pdx1 (44), proteins that favor function and preservation of pancreatic beta cells (2, 21). Despite the importance of biotin for the normal homeostasis and function of the endocrine pancreas, little is known about how cells of this important organ take up biotin, how the process is regulated, and how internal and external factors affect the uptake process. Our objectives in the current investigations were to address these issues, and we used cultured mouse-derived pancreatic beta-TC-6 cells and complemented the findings with studies using freshly isolated mouse and human primary (native) pancreatic islets. The results showed the uptake of 3H-biotin by pancreatic beta-TC-6 cells to be Na+-dependent and carrier-mediated as indicated by the inhibition caused by unlabeled biotin and its structural analog desthiobiotin, and by the saturation in biotin uptake as a function of concentration. Similarly, uptake of biotin by native freshly isolated primary mouse and human pancreatic islets was Na+-dependent and carrier-mediated in nature. As seen with other cell types of the digestive system, biotin uptake by pancreatic beta cells was also inhibited by pantothenic acid and lipoate, thus pointing to possible involvement of the SMVT in the uptake process. Indeed, mouse and human pancreatic beta cells/islets were found, by Western blotting and qualitative PCR, to express the SMVT system. Studies on biotin uptake in certain other cellular systems have suggested possible involvement of another (non-SMVT) biotin uptake carrier that operates in the nanomolar range (13, 45), but no such evidence were found in the current studies. The latter was shown by the lack of saturation in the initial rate of biotin uptake over the nanomolar range, and by the SMVT shRNA knockdown approach which established a major role for the SMVT system in biotin uptake by pancreatic beta cells.
Following delineation of the mechanism of biotin uptake by pancreatic beta cells/islets, we examined potential regulation of the uptake process by extracellular and intracellular factors. Biotin uptake by pancreatic beta-TC-6 cells was shown to be adaptively regulated by the prevailing biotin level in the extracellular environment, and was higher in cells maintained under a biotin-deficient condition compared with those maintained in the presence of a high level of biotin. This adaptive regulation in biotin uptake was associated with parallel changes in the level of expression of the SMVT protein and mRNA, as well as in the activity of the SLC5A6 promoters. These findings suggest possible involvement of a transcriptional mechanism(s) in mediating the observed adaptive response in pancreatic beta cells biotin uptake process. We also examined possible regulation of the pancreatic beta cells biotin uptake process by specific intracellular regulatory pathways and found that specific modulators of the PKA, PTK, and Ca2+/calmodulin-mediated pathways had no effect on biotin uptake. On the other hand, a role for the PKC-mediated pathway was suggested by the effect of the PKC modulator PMA (but not by its negative control 4α-PMA) on biotin uptake by pancreatic beta cells. It is interesting to mention here that biotin uptake by intestinal epithelial cells is also under the regulation of this intracellular regulatory pathway, suggesting that different cells may use the same regulatory mechanism to regulate their biotin uptake (32).
The intestinal microbiota appears to be an important environmental factor that influences the risk of metabolic disorders like diabetes mellitus (4). Studies have also shown that subjects with Type 2 diabetes have altered microbiota diversity and an enrichment in gram-negative bacteria (which express LPS) (16, 43). Indeed there is an increase in the level of LPS in the circulation of those subjects and a low-grade endotoxemia, which are believed to play a role in the onset of the metabolic disorders (1). Pancreatic beta cells express significant levels of TLR4 which make them sensitive to the effect of LPS (10, 14, 40). Circulating LPS binding to TLR4 leads to activation of the nuclear factor kappa-light-chain-enhancer of activated B-cells (NF-κB), p38 mitogen-activated protein kinases (p38 MAPK), activator protein 1 (AP-1), and interferon-inducible inflammatory gene expression (1, 14). We observed that chronic exposure of pancreatic beta-TC-6 cells to LPS lead to a significant inhibition in biotin uptake. This inhibition was not associated with changes in the level of expression of the total cellular SMVT protein, nor changes in level of its mRNA or activity of its gene promoters, but rather due to a decrease in the level of expression of the SMVT protein at the cell membrane as demonstrated by biotinylation assay. This is similar to what others have seen in studies on the inhibitory effects of LPS on the serotonin transporter in intestinal epithelial cells (22).
In summary, our studies show for the first time that biotin uptake by mouse and human pancreatic beta cells/islets is mediated via a regulated carrier-mediated mechanism that involves the SMVT system. This study also shows that the bacterial LPS inhibits biotin uptake by reducing the amount of the SMVT protein that is expressed at the cell membrane.
GRANTS
This work was supported by grants from the Department of Veterans Affairs, the National Institute of Diabetes and Digestive and Kidney Diseases Grant DK58057, and the National Institute on Alcohol Abuse and Alcoholism Grant AA018071.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
A.G., T.V.S., and H.M.S. conception and design of research; A.G. and T.V.S. performed experiments; A.G., T.V.S., and H.M.S. analyzed data; A.G., T.V.S., and H.M.S. interpreted results of experiments; A.G. and T.V.S. prepared figures; A.G. and H.M.S. drafted manuscript; A.G., T.V.S., and H.M.S. edited and revised manuscript; A.G., T.V.S., and H.M.S. approved final version of manuscript.
REFERENCES
- 1.Amyot J, Semache M, Ferdaoussi M, Fontés G, Poitout V. Lipopolysaccharides impair insulin gene expression in isolated islets of langerhans via toll-like receptor-4 and NF-kB signalling. PLoS One 7: e36200, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Babu DA, Deering TG, Mirmira RG. A feat of metabolic proportions: Pdx1orchestrates islet development and function in the maintenance of glucose homeostasis. Mol Genet Metab 92: 43–55, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borboni P, Magnaterra R, Rabini RA, Staffolani R, Porzio O, Sesti G, et al. Effect of biotin on glucokinase activity, mRNA expression and insulin release in cultured beta-cells. Acta Diabetol 33: 154–8, 1996 [DOI] [PubMed] [Google Scholar]
- 4.Cani PD, Delzenne NM. Gut microflora as a target for energy and metabolic homeostasis. Curr Opin Clin Nutr Metab Care 10: 729–734, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Coggeshall JC, Heggers JP, Robson MC, Baker H. Biotin status and plasma glucose in diabetics. Ann NY Acad Sci 447: 389–392, 1985 [Google Scholar]
- 6.Dakshinamurti K, Li W. Transcriptional regulation of liver phosphoenolpyruvate carboxykinase by biotin in diabetic rats. Mol Cell Biochem 132: 127–132, 1994 [DOI] [PubMed] [Google Scholar]
- 7.De La Vega LA, Stockert RJ. Regulation of the insulin and asialoglycoprotein receptors via cGMP-dependent protein kinase. Am J Physiol Cell Physiol 279: C2037–C2042, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Dey S, Subramanian VS, Chatterjee NS, Rubin SA, Said HM. Characterization of the 5′ regulatory region of the human sodium-dependent multivitamin transporter, hSMVT. Biochim Biophys Acta, 1574: 187–192, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Garcia-Ocaña A, Alonso LC. Glucose mediated regulation in Beta cell proliferation. Open Endocrinol J 4: 55–65, 2010 [Google Scholar]
- 10.Garay-Malpartida HM, Mourao RF, Mantovani M, Santos IA, Sogayar MC, Goldberg AC. Toll-like receptor 4 (TLR4) expression in human and murine pancreatic beta-cells affects cell viability and insulin homeostasis. BMC Immunol 12: 18, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghosal A, Subramanian VS, Said HM. Role of the putative N-glycosylation and PKC-phosphorylation sites of the human sodium-dependent multivitamin transporter (hSMVT) in function and regulation. Biochim Biophys Acta 1808: 2073–2080, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghosal A, SaidHM Mechanism and regulation of vitamin B2 (riboflavin) uptake by mouse and human pancreatic B-cells/islets: physiological and molecular aspects. Am J Physiol Gastrointest Liver Physiol 303: G1052–G1058, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grafe F, Wohlrab W, Neubert RH, Brandsch M. Transport of biotin in human keratinocytes. J Investig Dermatol 120: 428–433, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Kiely A, Robinson A, McClenaghan NH, Flatt PR, Newsholme P. Toll like receptor agonist induced changes in clonal rat BRIN-BD11 beta-cell insulin secretion and signal transduction. J Endocrinol 202: 365–373, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Koutsikos D, Fourtounas C, Kapetanaki A, Agroyannis B, Tzanatos H, Rammos G, Kopelias I, Bosiolis B, Bovoleti O, Darema M, Sallum G. Oral glucose tolerance test after high-dose i.v. biotin administration in normoglucemic hemodialysis patients. Ren Fail 18: 131–137, 1996 [DOI] [PubMed] [Google Scholar]
- 16.Larsen N, Vogensen FK, van den Berg FW, Nielsen DS, Andreasen AS, Pedersen BK, Al-Soud W, Sørensen SJ, Hansen LH, Jakobsen M. Gut microbiota in human adults with type 2 diabetes differs from nondiabetic adults. PLoS One 5: e9085, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ling Z, Kiekens R, Mahler T, Schuit FC, Pipeleers-Marichal M, Sener A, Kloppel G, Malaisse WJ, Pipeleers DG. Effects of chronically elevated glucose levels on the functional properties of rat pancreatic beta-cells. Diabetes 45: 1774–1782, 1996 [DOI] [PubMed] [Google Scholar]
- 18.Ling Z, Pipeleers DG. Prolonged exposure of human beta cells to elevated glucose levels results in sustained cellular activation leading to a loss of glucose regulation. J Clin Invest 98: 2805–2812, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Livak KJ, Schmittgen TD. Analysis of the relative gene expression data using realtime quantative PCR and the 2(−delta delta C(T)) method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Maebashi M, Makino Y, Furukawa Y, Ohinata K, Kimura S, Sato T. Therapeutic evaluation of the effect of biotin on hyperglycemia in patients with non-insulin dependent diabetes mellitus. J Clin Biochem Nutr 14: 211–218, 1993 [Google Scholar]
- 21.Matschinsky Banting Lecture FM. A lesson in metabolic regulation inspired by the glucokinase glucose sensor paradigm. Diabetes 45: 223–241, 1996 [DOI] [PubMed] [Google Scholar]
- 22.Mendoza C, Matheus N, Iceta R, Mesonero JE, Alcalde AI. Lipopolysaccharide induces alteration of serotonin transporter in human intestinal epithelial cells. Innate Immun 15: 243–250, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Mee L, Nabokina SM, Sekar VT, Subramanian VS, Maedler K, Said HM. Pancreatic beta cells and islets take up thiamin by a regulated carrier-mediated process: studies using mice and human pancreatic preparations. Am J Physiol Gastrointest Liver Physiol 297: G197–G206, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mock DM. Biotin. In: Handbook of Vitamins, edited by Zempleni J, McCormick DB, Suttie JW. New York: CRC, 2006, p. 361–377 [Google Scholar]
- 25.Padgett LE, Broniowska KA, Hansen PA, Corbett JA, Tse HM. The role of reactive oxygen species and proinflammatory cytokines in type 1 diabetes pathogenesis. Ann NY Acad Sci 1281: 16–35, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reddi A, DeAngelis B, Frank O, Lasker N, Baker H. Biotin supplementation improves glucose and insulin tolerances in genetically diabetic KK mice. Life Sci 42: 1,323–1,330, 1988 [DOI] [PubMed] [Google Scholar]
- 27.Reidling JC, Said HM. Regulation of the human biotin transporter hSMVT promoter by KLF-4 and AP-2: confirmation of promoter activity in vivo. Am J Physiol Cell Physiol 292: C1305–C1312, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Reidling J, Nabokina SM, Said HM. Molecular mechanisms involved in the adaptive regulation of human intestinal biotin uptake: A study of the hSMVT system. Am J Physiol Gastrointest Liver Physiol 292: G275–G281, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Rodriguez-Melendez R, Zempleni J. Regulation of gene expression by biotin (review). J Nutr Biochem 14: 680–690, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Romero-Navarro G, Cabrera-Valladares G, German MS, Matschinsky FM, Velazquez A, Wang J, Fernandez-Mejia C. Biotin regulation of pancreatic glucokinase and insulin in primary cultured rat islets and in biotin-deficient rats. Endocrinology 140: 4,595–4,600, 1999 [DOI] [PubMed] [Google Scholar]
- 31.Said HM, Ma TY, Grant K. Regulation of riboflavin intestinal uptake by protein kinase A: 387 studies with Caco-2 cells. Am J Physiol Gastrointest Liver Physiol 267: G955–G959, 1994 [DOI] [PubMed] [Google Scholar]
- 32.Said HM. Cellular uptake of biotin: mechanisms and regulation. J Nutr 129: 490s–493s, 1999 [DOI] [PubMed] [Google Scholar]
- 33.Said HM. Recent advances in carrier-mediated absorption of water-soluble vitamins. Ann Review Physiol 66: 419–446, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Said HM. Cell and molecular aspects of human intestinal biotin absorption. J Nutr 139: 158–162, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Said HM. Recent advances in transport of water-soluble vitamins in organs of the digestive system: a focus on the colon and the pancreas. Am J Physiol Gastrointest Liver Physiol 305: G601–G610, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sugita Y, Shirakawa H, Sugimoto R, Furukawa Y, Komai M. Effect of biotin treatment on hepatic gene expression in streptozotocin-induced diabetic rats. Biosci Biotechnol Biochem 72: 1,290–1,298, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Tang C, Han P, Oprescu AI, Simon Lee C, Gyulkhandanyan AV, Chan GNY, Wheeler MB, Giacca A. Evidence for a role of superoxide generation in glucose-induced β-cell dysfunction in vivo. Diabetes 56: 2,722–2,731, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Vega-Monroy LD, Larrieta E, German MS, Baez-Saldana A, Fernandez-Mejia C. Effects of biotin supplementation in the diet on insulin secretion, islet gene expression, glucose homeostasis and beta-cell proportion. J Nutr Biochem 24: 169–177, 2013 [DOI] [PubMed] [Google Scholar]
- 39.Vilches-Flores A, Tovar AR, Marin-Hernandez A, Rojas-Ochoa A, Fernandez-Mejia C. Biotin increases glucokinase expression via soluble guanylate cyclase/protein kinase G, adenosine triphosphate production and autocrine action of insulin in pancreatic rat islets. J Nutr Biochem 21: 606–612, 2009 [DOI] [PubMed] [Google Scholar]
- 40.Vives-Pi M, Somoza N, Fernandez-Alvarez J, Vargas F, Caro P, Alba A, Gomis R, Labeta MO, Pujol-Borrell R. Evidence of expression of endotoxin receptors CD14, toll-like receptors TLR4 and TLR2 and associated molecule MD-2 and of sensitivity to endotoxin (LPS) in islet beta cells. Clin Exp Immunol 133: 208–218, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang H, Huang W, Fei YJ, Xia H, Yang-Feng TL, Leibach FH, Devoe LD, Ganapathy V, Prasad PD. Human placental Na-dependent multivitamin transporter. Cloning, functional expression, gene structure, and chromosomal localization. J Biol Chem 274: 14875–14883, 1999 [DOI] [PubMed] [Google Scholar]
- 42.Wen L, Peng J, Zhenjun Li Wong FS. The effect of innate immunity on autoimmune diabetes and the expression of toll-like receptors on pancreatic islets. J Immunol 172: 3173–3180, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Wu X, Ma C, Han L, Nawaz M, Gao F, Zhang X, Yu P, Zhao C, Li L, Zhou A, Wang J, Moore JE, Millar BC, Xu J. Molecular characterization of the faecal microbiota in patients with type II diabetes. Curr Microbiol 61: 69–78, 2010 [DOI] [PubMed] [Google Scholar]
- 44.Yoshikawa H, Tajiri Y, Sako Y, Hashimoto T, Umeda F, Nawata H. Effects of biotin on glucotoxicity of lipotoxicity in rat pancreatic islets. Metabolism 51: 163–168, 2002 [DOI] [PubMed] [Google Scholar]
- 45.Zempleni J, Mock DM. Uptake and metabolism of biotin by human peripheral blood mononuclear cells. Am J Physiol Cell Physiol 275: C382–C388, 1998 [DOI] [PubMed] [Google Scholar]
- 46.Zhang H, Osada K, Maebashi M, Ito M, Komai M, Furukawa Y. A high biotin diet improves the impaired glucose tolerance of long-term spontaneously hyperglycemic rats with non-insulin-dependent diabetes mellitus. J Nutr Sci Vitaminol (Tokyo) 42: 517–526, 2009 [DOI] [PubMed] [Google Scholar]