Abstract
Pseudomonas aeruginosa and members of the Burkholderia cepacia complex often coexist in both the soil and the lungs of cystic fibrosis patients. To gain an understanding of how these different species affect each other's physiology when coexisting, we performed a screen to identify P. aeruginosa genes that are induced in the presence of Burkholderia. A random gene fusion library was constructed in P. aeruginosa PA14 by using a transposon containing a promoterless lacZ gene. Fusion strains were screened for their ability to be induced in the presence of Burkholderia strains in a cross-streak assay. Three fusion strains were induced specifically by Burkholderia species; all three had transposon insertions in genes known to be iron regulated. One of these fusion strains, containing a transposon insertion in gene PA4467, was used to characterize the inducing activity from Burkholderia. Biochemical and genetic evidence demonstrate that ornibactin, a siderophore produced by nearly all B. cepacia strains, can induce P. aeruginosa PA4467. Significantly, PA4467 is induced early in coculture with an ornibactin-producing but not an ornibactin-deficient B. cepacia strain, indicating that ornibactin can be produced by B. cepacia and detected by P. aeruginosa when the two species coexist.
Microbial populations in most natural environments consist of a multitude of species, yet laboratory studies of bacterial physiology have focused predominately on pure cultures. Relatively little is known at the molecular level about how different bacteria interact with each other when present in the same environment. Specifically, there is a need to define how the gene expression patterns of a given bacterium are affected by the presence of other microbial species.
To gain insight into ways in which different bacterial species may affect each other, we investigated the interaction between two ubiquitous soil microorganisms, Pseudomonas aeruginosa and members of the Burkholderia cepacia complex. These bacterial species are phylogenetically distinct yet often coexist in diverse environments. P. aeruginosa, a member of the gamma proteobacteria, and Burkholderia species, members of the beta proteobacteria, are both catabolically versatile and highly resistant to many antibiotics (1, 23, 38, 47). Burkholderia strains are characterized by a high level of genomic diversity, and the B. cepacia complex is now comprised of at least nine species (10, 11, 22, 25). These species include B. cepacia, initially isolated as an onion pathogen, and Burkholderia multivorans and Burkholderia vietnamiensis, which have been investigated for use as biocontrol and bioremediation agents (5, 9, 39, 40). P. aeruginosa has been found ubiquitously in soil environments (2, 38, 47). Since both genera are common soil isolates and have such similar phenotypic properties, it is likely that they occupy similar niches in many natural environments.
Although they are primarily soil inhabitants, both P. aeruginosa and many Burkholderia species are also opportunistic human pathogens. P. aeruginosa is a major source of nosocomial infections, causing disease in a wide range of immunocompromised patients (24). In addition, P. aeruginosa colonizes the lungs of almost all cystic fibrosis (CF) patients at an early age. While antibiotic treatment can temporarily decrease bacterial levels in the lungs of CF patients, Pseudomonas infections are usually chronic (14).
Subsequent to P. aeruginosa infection, CF patients frequently develop secondary infections by a number of other microbial species. Prominent among these are members of the Burkholderia genus, which have emerged as particularly problematic pathogens for CF patients (6, 14, 15, 17). Patients with Burkholderia infections often succumb to what is termed the “cepacia syndrome,” or necrotizing pneumonia, which is usually fatal (7, 49). Currently, it is unclear how the presence of Burkholderia leads to such a rapid decline in health.
Because P. aeruginosa and B. cepacia are found in the same natural environments and can coinfect human hosts, we wanted to investigate how Burkholderia affects P. aeruginosa gene expression. In order to examine interactions between P. aeruginosa and Burkholderia in an unbiased manner, we prepared a random gene fusion library in P. aeruginosa and performed a screen using a cross-streak assay on solid medium. This screen allowed us to identify Pseudomonas genes that are induced in the presence of secreted or cell-associated factors from Burkholderia. The gene fusions were used in an assay to identify the inducing activity from Burkholderia. We were able to determine that ornibactin, a siderophore (iron-chelating molecule) produced by Burkholderia, can strongly affect P. aeruginosa gene expression under the conditions tested. This demonstrates that competition for iron can be a factor in Pseudomonas-Burkholderia interactions.
MATERIALS AND METHODS
Media, reagents, and bacterial strains.
Bacterial strains were grown in Luria-Bertani broth (LB) or succinate minimal medium (27). Tetracycline (TET) was used at concentrations of 150 or 75 μg/ml, and carbenicillin was used at a concentration of 750 μg/ml. 5-Bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) was used at a concentration of 100 μg/ml. Strains used for conditioned medium experiments are indicated in Table 1.
TABLE 1.
Induction of PA4467::Tn5lacZ with medium conditioned by Burkholderia or non-Burkholderia species
| Lab collection no. | Strain | Miller units ± SD | Reference or source |
|---|---|---|---|
| ZK 2878 | B. cepacia complex, CF isolate 5 | 295 ± 11 | J. Burns, University of Washington |
| ZK 2715 | B. vietnamiensis G4 | 284 ± 28 | 41 |
| ZK 2875 | B. cepacia complex, CF isolate 2 | 255 ± 24 | J. Burns |
| ZK 2714 | B. cepacia 5424 | 235 ± 76 | 27 |
| ZK 2716 | B. multivorans 17616 | 230 ± 11 | 27 |
| ZK 2877 | B. cepacia complex, CF isolate 3-2 | 225 ± 18 | J. Burns |
| ZK 3331 | B. cepacia K56-2 | 208 ± 19 | 27 |
| ZK 2876 | B. cepacia complex, CF isolate 3-1 | 121 ± 2 | J. Burns |
| ZK 2718 | B. cepacia 25416 | 92 ± 12 | 27 |
| ZK 2610 | Staphylococcus aureus strain Newman | 45 ± 1 | Lab stock |
| ZK 2757 | Bacillus subtilis 3610 | 38 ± 11 | Lab stock |
| ZK 2745 | Candida albicans SC5314 | 36 ± 4 | Lab stock |
| ZK 126 | Escherichia coli K-12 | 32 ± 1 | Lab stock |
| ZK 2511 | Enterococcus sp. clinical isolate | 20 ± 7 | Lab stock |
| ZK 2466 | P. aeruginosa PA14 | 20 ± 3 | Lab stock |
Transposon library construction and insertion mapping.
A random library of Tn5-B21 transposon insertion mutants (lacZ fusion strains) was generated in P. aeruginosa PA14 as described previously (36, 43). The transposon library consists of mutants derived from 20 independent conjugations. Transposon insertion sites were identified by arbitrary PCR amplification as described previously (8, 37). PCR products were sequenced, and results were compared to the P. aeruginosa genome database (http://www.pseudomonas.com).
Cross-streak assay.
Cultures of Burkholderia strains were grown in LB at 37°C overnight, and 100 μl was streaked down the center of square petri dishes containing LB agar with X-Gal. These plates were incubated for 24 h at 30°C. Fusion strains were taken from LB-TET (75 μg/ml) plates, individually resuspended in 100 μl of LB, and allowed to incubate at 37°C for 4 h. A toothpick was used to streak each fusion strain suspension from the edge of the plate towards the Burkholderia streak, with a small area of overlap. Plates were incubated at 30°C for 42 h and observed for the appearance of blue pigment (indicative of β-galactosidase activity) in the P. aeruginosa fusion strains.
Liquid induction assay.
Conditioned medium was obtained from cultures grown in LB or succinate minimal medium with or without 10 mM ornithine at 30° for 40 h. Cells were pelleted by centrifugation, and the supernatant was filtered through a 0.2 μm-pore-size filter. Conditioned medium was added 1:1 with fresh medium (LB or succinate minimal) to the assay.
PA4467::Tn5lacZ (fusion I) cells were prepared for the β-galactosidase assay as follows: liquid overnight cultures were grown in LB-TET (75 μg/ml) at 37°C, diluted 1:200 in LB with TET, allowed to grow to an optical density at 600 nm (OD600) of 0.3, and diluted again 1:200 in LB with TET. The cultures were centrifuged when they reached an OD600 of 0.1, and the pellets were resuspended in 1/20 of the original volume of LB. Concentrated cells were added to the assay medium such that the final cell density was an OD600 of 0.1. Fusion cells were incubated in the indicated medium for 45 min at 37°C with shaking. After incubation, cells were placed immediately on ice, β-galactosidase assays were performed, and Miller units were calculated as described previously (31). PAO1 lacZ fusion strains (pvdJ, pvdA, regA, and pvdS) were grown with carbenicillin and assayed for induction as described above.
RNA isolation and microarray analysis.
Total RNA was isolated from P. aeruginosa PAO1 cells that were treated as follows. PAO1 cultures were harvested at an OD600 of 0.3, centrifuged, and resuspended in LB, an equal volume of Burkholderia Bc2-conditioned medium and LB, or an equal volume of PAO1-conditioned medium and LB. For conditioned media, Burkholderia Bc2 and PAO1 were grown in LB at 37°C for 24 h and filter sterilized. P. aeruginosa was incubated in these conditions at 37°C for 30 min with shaking. RNA was isolated with RNeasy columns (QIAGEN) after treating the cells with 3 mg of lysozyme/ml. The GeneChip probes were prepared according to the protocol supplied by Affymetrix, and data were analyzed by using MICROARRAY SUITE software (Affymetrix).
Biochemical techniques.
Ferric chloride was added to various media to test for the presence of iron-binding compounds. One microliter of a FeCl3 · 6H2O 25% (wt/vol) solution was added to 500 μl of conditioned medium. A change in color relative to fresh medium indicated the presence of an iron-chelating compound (29). The chrome azurol S (CAS) assay with or without shuttle solution was used as described previously (42). Absorbance at 630 nm was measured relative to water alone. The ratio of absorbance with the medium of interest mixed with the CAS solution to that of water or fresh medium with the CAS solution was designated the relative CAS activity and is a measure of the iron-chelating activity.
Ornibactin purification and quantitation.
Ornibactin was purified as described previously (13, 30, 48) with the following modifications. Burkholderia strain K56-2 was grown in 150 ml of succinate medium with 10 mM ornithine at 30°C for 40 h. Cells were pelleted by centrifugation, and the supernatant was removed and filtered through a 0.2 μm-pore-size filter. Conditioned medium was applied to a C18 column (15 by 1.5 cm), rinsed with water, and eluted with 100% methanol. This eluant was concentrated by evaporation, added to a Sephadex LH-20 column (30 by 1.5 cm), and eluted with 100% methanol. After testing for CAS activity, every fifth CAS-active fraction was pooled, and the solution was concentrated by evaporation. Fifty microliters was applied to a silica gel G thin-layer chromatography (TLC) plate. The TLC plate was developed in butanol-acetic acid-water (3:1:1). After drying, the plate was sprayed with 0.1 M FeCl3 in 0.1 N HCl.
CAS-active fractions were dried to completeness through evaporation, resuspended in water, and pooled. This was used as the ornibactin stock solution because the TLC plate indicated that no other iron-binding compounds were present and this is the preparation used in other studies involving ornibactin (30). The concentration of ornibactin in the stock solution was quantitated by using the molar extinction coefficient of ornibactin in water (1,267 M−1 cm−1) as described by Meyer et al. (30). The CAS assay was used to correlate purified ornibactin and ornibactin present in K56-2-conditioned medium.
Mixed cultures.
P. aeruginosa and Burkholderia strains were grown overnight in succinate medium with 20 μM ferrous sulfate. Cultures were diluted into fresh succinate medium at 1:100 of the desired final OD600 and incubated at 30°C until cultures reached the desired optical densities. When fusion I cultures were at an OD600 of 0.1 and Burkholderia cultures were at an OD600 of either 0.1 or 1.0, aliquots were centrifuged, rinsed, and resuspended in warm succinate medium. Cells were added to 5 ml of warm succinate medium at the following densities: fusion I to an OD600 of 0.1 and Burkholderia K56-2 or K56pvdA::tp to an OD600 of either 0.1 or 1.0. Cultures were incubated with shaking at 30°C. Aliquots were taken at the indicated time points, and 10−5 and 10−6 dilutions were plated in duplicate on LB plates. Plates were incubated for 48 h at 37°C. The remaining samples were placed on ice and used for β-galactosidase assays. Cell counts and β-galactosidase activity normalized to cell number were obtained by counting P. aeruginosa and Burkholderia colonies. The colonies were readily distinguishable on the basis of morphology. It was previously determined that, at the dilutions used, the presence of one species did not affect the ability of the other species to grow. To determine the β-galactosidase activity normalized to fusion I cell number, calculations were performed as described for Miller units (see above), with the OD600 value replaced with CFU/ml and multiplied by a factor of 108. This value was designated β-galactosidase activity/CFU.
RESULTS
Isolation of Burkholderia-responsive P. aeruginosa gene fusions.
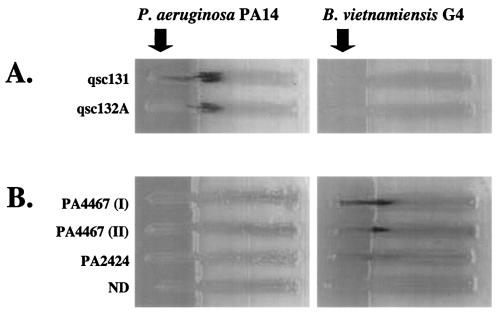
A screen was designed to identify P. aeruginosa gene fusions induced by either secreted or cell-associated factors from Burkholderia species. The screen was based on an assay in which random P. aeruginosa lacZ gene fusions are cross-streaked against a Burkholderia strain. For fusions that are induced by Burkholderia, lacZ expression should be high proximal and low distal to the cross-streak. To validate the screen, we tested gene fusions that are induced by secreted P. aeruginosa quorum-sensing molecules, the homoserine lactones PAI-1 and PAI-2 (41). These gene fusions were in a strain background unable to synthesize either PAI-1 or PAI-2 (lasI rhlI) (52). When cross-streaked with wild-type P. aeruginosa, lacZ expression was evident proximal to the cross-streak (Fig. 1A).
FIG. 1.
P. aeruginosa PA14 and B. vietnamiensis G4 were tested for their ability to induce either P. aeruginosa PAO1 (lasI rhlI) quorum-sensing-controlled fusions (qsc131 and qsc132A) (A) or P. aeruginosa PA14 Tn5lacZ fusions (B). ND indicates that the transposon insertion site of the fusion was not determined. B. vietnamiensis and P. aeruginosa PA14 strains were incubated on LB-X-Gal plates for 24 h at 30°C prior to inoculation of the fusion strains. Plates were photographed after 40 h of coincubation at 30°C.
A library of approximately 6,000 transposon insertion strains was screened for fusions that are specifically induced by Burkholderia. A Tn5lacZ insertion library was constructed in P. aeruginosa strain PA14 as described previously (35, 36). The transposon contains a promoterless lacZ gene, creating a transcriptional fusion upon insertion. Isolated fusion strains were tested in the cross-streak assay for their ability to become induced in the presence of Burkholderia strains. Separate LB agar plates containing the chromogenic galactoside X-Gal were inoculated with vertical streaks of two different Burkholderia strains, B. vietnamiensis G4 (an environmental isolate) and B. cepacia 5424 (a CF clinical isolate). The Burkholderia strains were allowed to grow for 24 h before the P. aeruginosa fusion strains were cross-streaked with them. The P. aeruginosa fusions were observed over a period of 42 h for the appearance of blue pigment proximal to the Burkholderia streak. Of the fusion strains screened, 36 initial candidates were apparently inducible by Burkholderia. These fusions were rescreened against a number of other microbial species to rule out fusions that were not induced specifically by Burkholderia. Candidate fusions were also screened against wild-type P. aeruginosa to rule out fusions that were induced by general nutrient depletion. Of the 36 initial candidates, three fusions were identified as being induced specifically by Burkholderia (Fig. 1B).
Induction of P. aeruginosa gene fusions by Burkholderia-conditioned medium.
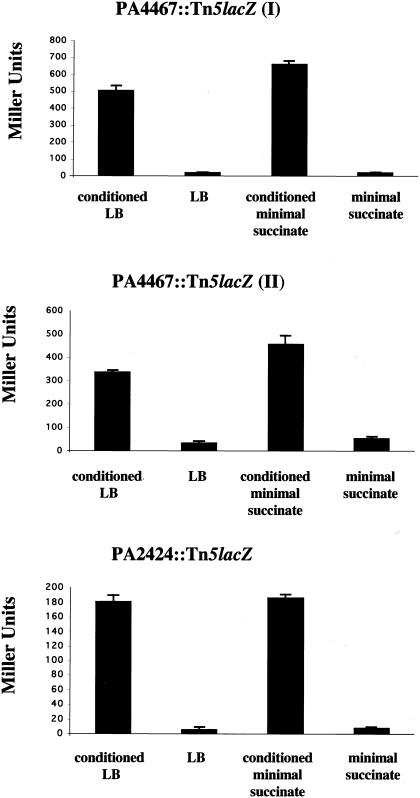
To quantitate the level of induction of the fusions by Burkholderia, we utilized a liquid β-galactosidase assay. Burkholderia strains were grown in either rich or succinate minimal medium to stationary phase, and cell-free supernatants of these cultures were collected and referred to as conditioned medium. Early-exponential-phase cultures (OD600 of 0.1) of the P. aeruginosa fusion strains were resuspended in a 1:1 mixture of Burkholderia-conditioned medium and fresh medium. Fusion cells were then incubated for 45 min before the β-galactosidase assays were performed (Fig. 2). All three P. aeruginosa fusion strains were induced in the presence of Burkholderia-conditioned medium relative to medium alone, regardless of whether rich or minimal medium was used. In both types of conditioned media, the fusions were induced between 10- and 20-fold. For all of our subsequent experiments, we focused on a single fusion strain, PA4467::Tn5lacZ (I), or fusion I for short.
FIG. 2.
Fusions were tested for their ability to be induced by Burkholderia-conditioned medium in a liquid β-galactosidase assay. The assay was performed and Miller units were calculated as described in Materials and Methods. All three fusion strains were induced by both rich (LB) and minimal (succinate) media conditioned by B. vietnamiensis G4. The results shown are the averages of data from three separate experiments (error bars indicate standard deviations [SD]).
In addition to the initial Burkholderia strains tested in the cross-streak assay, we found that numerous strains, representing multiple species in the B. cepacia complex, strongly induced fusion I. Microbial strains were inoculated into LB and allowed to grow for 40 h at 30°C, and the resulting conditioned media were tested for their ability to induce the fusion. All nine of the Burkholderia strains tested strongly induced the fusion, while conditioned media from other microbial species, including P. aeruginosa itself, did not induce the fusion (Table 1).
Transposon insertions into iron-regulated genes.
The transposon insertion sites of the three Burkholderia-responsive fusions were identified by using arbitrary PCR and DNA sequence analysis. Two of the fusion strains contain transposon insertions in or directly following the same open reading frame (ORF), PA4467. We called these fusion I (insertion site 4,997,281) and fusion II (insertion site 4,996,465), respectively. The ORF of unknown function is located at the end of an operon containing the manganese-cofactored superoxide dismutase and a fumarate hydratase. The operon has been shown to be induced under low-iron conditions, and biochemical studies show that Fur, the ferric uptake regulator, binds in the promoter region (18, 19). While this operon has also been shown to be regulated by the PAI-1 quorum-sensing system, regulation by iron has been shown to be dominant (3, 20). The third fusion strain, fusion III (insertion site 2,717,251), contains a transposon insertion in PA2424, a gene recently named pvdL for its role in pyoverdine biosynthesis. The pvdL gene encodes a nonribosomal peptide synthetase and is thought to be in a bicistronic operon with a gene, PA2425, encoding a putative thioesterase. The operon is upregulated in low-iron conditions and is under the control of the alternative sigma factor, PvdS (33).
Microarray analysis of P. aeruginosa genes induced by Burkholderia.
Microarray experiments were performed to complement the screen to identify P. aeruginosa genes induced in the presence of Burkholderia-conditioned medium. A mid-exponential-phase culture of P. aeruginosa (strain PAO1) was incubated for 30 min in (i) LB, (ii) an equal volume of Burkholderia Bc2-conditioned medium and LB, and (iii) an equal volume of P. aeruginosa PAO1-conditioned medium and LB. RNA was isolated, and Affymetrix GeneChips were used to identify P. aeruginosa genes induced in the presence of Burkholderia-conditioned medium. Table 2 shows all of the genes whose levels of expression increased 20-fold or greater in the presence of Burkholderia-conditioned medium relative to LB alone. Among the most highly induced genes in the microarray experiment were those identified in the genetic screen, PA2424 and the operon containing PA4467 (PA4467-PA4471). In addition, other genes known to be iron regulated, such as those involved in pyoverdine and pyochelin biosynthesis, were highly induced. While PA2424 was also induced in P. aeruginosa-conditioned medium relative to LB, the level of induction was much lower than that with Burkholderia-conditioned medium. In addition, other known iron-regulated genes were not induced in P. aeruginosa-conditioned medium relative to LB. This indicates that the pattern of gene induction in the presence of Burkholderia is not a generic response to conditioned media from stationary-phase cultures. The results further indicate that there is an overlap between genes induced in low-iron conditions and those induced by Burkholderia-conditioned medium.
TABLE 2.
P. aeruginosa PAO1 genes with increased expression in Burkholderia-conditioned medium relative to LBa
| ORF | Gene | Induction (fold) in Burkholderia-conditioned LB versus LBb | Induction (fold) in Pseudomonas-conditioned LB versus LBb,c |
|---|---|---|---|
| PA2411 | 158 ± 5 | 7 ± 2 | |
| PA4471 | 152 ± 2 | NC | |
| PA2424 | pvdL | 124 ± 2 | 6 ± 1 |
| PA2452 | 124 ± 3 | NC | |
| PA3407 | hasA | 115 ± 2 | NC |
| PA4470 | fumC | 111 ± 2 | NC |
| PA2413 | 91 ± 2 | NC | |
| PA4469 | 82 ± 1 | NC | |
| PA2412 | 71 ± 2 | NC | |
| PA4468 | sodM | 66 ± 1 | NC |
| PA2400 | 60 ± 2 | 4 ± 1 | |
| PA2399 | pvdD | 58 ± 4 | NC |
| PA2393 | 52 ± 2 | NC | |
| PA2034 | 52 ± 2 | NC | |
| PA4710 | 45 ± 1 | NC | |
| PA2386 | pvdA | 42 ± 2 | NC |
| PA2402 | 38 ± 1 | NC | |
| PA4467 | 38 ± 1 | NC | |
| PA2396 | pvdF | 36 ± 2 | NC |
| PA2426 | pvdS | 36 ± 3 | NC |
| PA2392 | 32 ± 3 | NC | |
| PA4228 | pchD | 32 ± 2 | NC |
| PA4896 | 28 ± 1 | NC | |
| PA2394 | 28 ± 2 | NC | |
| PA2033 | 26 ± 2 | NC | |
| PA2444 | glyA | 24 ± 5 | 13 ± 4 |
| PA3530 | bfd | 24 ± 3 | NC |
| PA4168 | 23 ± 4 | NC | |
| PA2397 | pvdE | 22 ± 1 | NC |
| PA1999 | 20 ± 3 | 15 ± 3 | |
| PA2401 | 20 ± 1 | NC | |
| PA0672 | 20 ± 3 | NC |
Results are shown for genes induced more than 20-fold.
Data shown are the averages of two experiments ± SD.
NC, no change above background.
Iron regulation of fusion I expression.
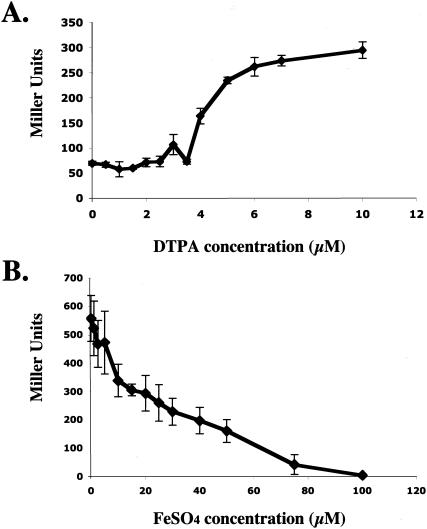
Because both PA4467 and pvdL are known to be iron regulated, we confirmed that fusion I could be induced under iron-limiting conditions. We monitored fusion I β-galactosidase activity in medium with increasing concentrations of diethylenetriaminepentaacetic acid (DTPA), an iron-chelating agent (21). As predicted, iron limitation was sufficient to induce fusion I (Fig. 3A), as the fusion was induced in the presence of DTPA at concentrations greater than 4 μM. To test whether excess iron could suppress the inducing capability of Burkholderia-conditioned medium, we added increasing concentrations of ferrous sulfate to B. vietnamiensis G4-conditioned medium. As shown in Fig. 3B, the addition of iron incrementally decreased the induction of fusion I by conditioned medium, suggesting that iron limitation was required for the ability of Burkholderia-conditioned medium to induce the fusion.
FIG. 3.
(A) DTPA, a synthetic iron chelator, was used to test whether iron depletion of fresh medium was able to induce fusion I. DTPA was added to succinate minimal medium at the concentrations shown. Values are the averages of data from three separate experiments (error bars indicate SD). (B) The ability of ferrous sulfate to suppress induction by B. vietnamiensis G4-conditioned medium was tested. The indicated concentrations of ferrous sulfate were added to conditioned succinate minimal medium. The induction level was determined as described in Materials and Methods. Values are the averages of data from three separate experiments (error bars indicate SD).
Iron-chelating activity in Burkholderia-conditioned medium.
From the above results, we hypothesized that an iron-chelating activity from Burkholderia was sequestering iron away from P. aeruginosa in these experiments. To test this hypothesis, we used several assays to detect the presence of iron chelators in Burkholderia-conditioned medium. Both the ferric chloride and CAS assays (see Materials and Methods) can detect the presence of iron-chelating compounds (29, 42). With both assays, Burkholderia-conditioned medium tested positive for iron-chelating activity (data not shown). We wanted to investigate whether this iron-chelating activity corresponded to the activity that induced fusion I.
Previous studies had identified four siderophores made in various combinations by Burkholderia strains: pyochelin, salicylic acid, cepabactin, and ornibactin (28, 30, 44, 46, 48). Darling et al. (13) showed that roughly 90% of all B. cepacia complex strains tested produced ornibactin and salicylic acid, whereas pyochelin and cepabactin were produced by far fewer strains. In addition, Meyer et al. (30) found that one well-characterized strain, ATCC 25416, produced ornibactin at a level of 109 mg/liter, while cepabactin and pyochelin were produced at levels of 46 mg/liter and 11 mg/liter, respectively.
Several studies have examined the ability of P. aeruginosa to utilize Burkholderia siderophores. Both pyochelin and salicylic acid are known to be made and used by P. aeruginosa to scavenge iron (12, 26, 51). In a study examining the use of heterologous siderophores, Meyer (26) demonstrated that cepabactin could be taken up and used for iron acquisition by P. aeruginosa. However, studies with radiolabeled ornibactin showed that P. aeruginosa could not take it up over a 15-min assay period, suggesting that ornibactin cannot be used as an iron source (30).
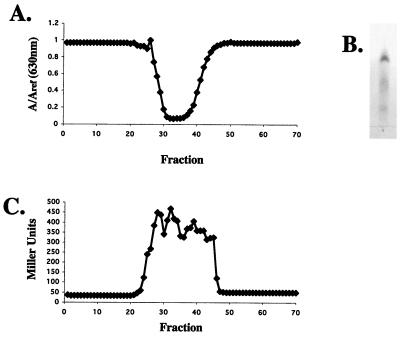
We hypothesized that ornibactin may make iron unavailable to P. aeruginosa, causing iron-regulated operons to be induced. To test this hypothesis, we purified ornibactin using the published protocol (13, 30, 48). In the final step of the purification process, we tested fractions obtained from a Sephadex LH-20 column for iron-chelating activity using the CAS assay (Fig. 4A). CAS-active fractions were pooled, run on a TLC plate, and sprayed with ferric chloride. Three bands with Rf values of 0.25, 0.18, and 0.09 were detected, indicating the presence of ornibactin (Fig. 4B). The column fractions were then tested for their ability to induce fusion I. As seen in Fig. 4C, fractions containing purified ornibactin were able to induce fusion I.
FIG. 4.
(A) Fractions from a Sephadex LH-20 column were tested for iron-chelating activity. Two hundred microliters of each 1-ml fraction was concentrated, resuspended in water, and tested in the CAS assay. (B) The CAS-active fractions were pooled and run on a silica gel G TLC plate, which was then sprayed with ferric chloride. The only iron-reactive spots were the three bands indicative of the three forms of ornibactin (containing acyl side chains of different lengths; Rf values, 0.25, 0.18, and 0.09). (C) One hundred microliters of each 1-ml fraction was dried by lyophilization, resuspended in buffered medium, mixed 1:1 with fresh succinate medium, and tested for its ability to induce fusion I.
Ornibactin requirement for fusion I induction by Burkholderia-conditioned medium.
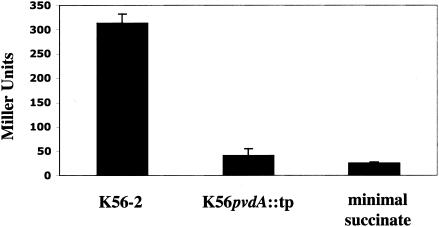
To test whether ornibactin production was necessary for the inducing capability of Burkholderia-conditioned medium, we obtained a strain defective in ornibactin biosynthesis from P. Sokol (University of Calgary, Calgary, Alberta, Canada). The parental strain, B. cepacia K56-2 is an isolate from a CF patient and is a member of the genomovar III group of the B. cepacia complex. It was shown to produce ornibactin, salicylic acid, and negligible amounts of pyochelin. A nonpolar replacement mutant in pvdA, a gene encoding an l-ornithine N5-oxygenase, is defective in ornibactin production (45). We tested the ability of conditioned medium from the wild-type strain and from the mutant strain to induce fusion I. While the wild-type strain induced the fusion, the mutant strain did not (Fig. 5). These data indicate that ornibactin is necessary for the ability of Burkholderia K56-2-conditioned medium to induce fusion I.
FIG. 5.
Conditioned succinate minimal media from ornibactin-producing strain B. cepacia K56-2 and the ornibactin-deficient mutant K56pvdA::tp were tested for their ability to induce fusion I. Burkholderia strains were grown in succinate minimal medium with 10 mM ornithine for 40 h at 30°C. Values are the averages of data from four separate experiments (error bars indicate SD).
Conditioned media from K56-2 and K56pvdA::tp were also used to test whether ornibactin biosynthesis is necessary for the ability of Burkholderia to induce other Pseudomonas iron-regulated genes. As shown in Table 3, conditioned medium from the wild-type strain but not the ornibactin-deficient mutant is able to induce the iron-regulated pyoverdine biosynthesis genes pvdJ and pvdA, as well as iron-regulated transcriptional regulators pvdS and regA (34). This finding indicates that the induction of numerous iron-regulated genes in the presence of Burkholderia is due to ornibactin production.
TABLE 3.
Induction of P. aeruginosa PAO1 iron-regulated genes by conditioned media from wild-type and ornibactin-deficient Burkholderia strains
| lacZ fusion | Conditioned media (Miller units ± SD)b
|
||
|---|---|---|---|
| SMMa | SMM + K56-2 | SMM + K56pvdA::tp | |
| pvdA | 18 ± 6 | 81 ± 29 | 11 ± 4 |
| regA | 31 ± 26 | 172 ± 69 | 23 ± 7 |
| pvdJ | 17 ± 7 | 289 ± 160 | 20 ± 5 |
| pvdS | 31 ± 10 | 412 ± 266 | 29 ± 1 |
SMM, succinate minimal medium.
Values are the averages of results from three separate experiments.
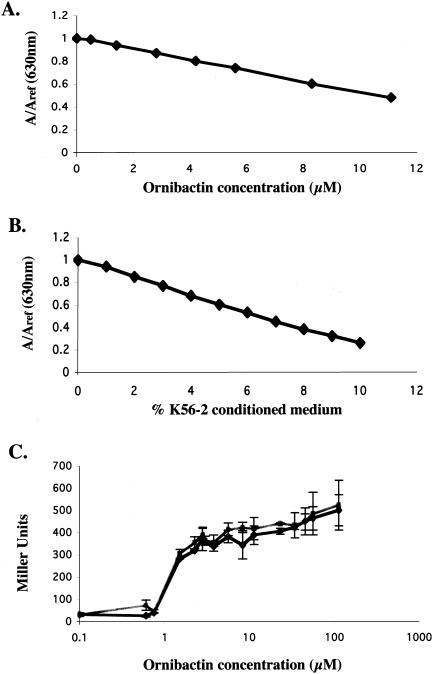
To test whether ornibactin accounted for all of the inducing activity present in Burkholderia-conditioned medium, we determined the concentration of ornibactin in B. cepacia K56-2-conditioned medium. Using a published molar extinction coefficient, we determined the concentration of ornibactin in our purfied preparation. This allowed us to correlate ornibactin concentration with CAS activity (Fig. 6A). Using dilutions of K56-2-conditioned medium in the CAS assay, we made a second curve correlating conditioned medium with CAS activity (Fig. 6B). Equating CAS activities from both curves, we could assign values of ornibactin concentration to the Burkholderia-conditioned medium. After growth of Burkholderia K56-2 in succinate minimal medium supplemented with 10 mM ornithine, the conditioned medium contained 166 μM ornibactin. A dose-response experiment was performed in which known amounts of ornibactin were added to cultures of the fusion I strain. The induction levels were compared to those of fusion cells incubated with conditioned medium corresponding to equivalent amounts of ornibactin. As shown in Fig. 6C, purified ornibactin and K56-2-conditioned medium with equivalent ornibactin concentrations induced fusion I to the same level. This result indicates that ornibactin is the major activity in Burkholderia K56-2 conditioned medium responsible for the induction of this fusion.
FIG. 6.
The concentration of ornibactin in K56-2-conditioned succinate medium was determined. (A) Dilutions of the ornibactin stock solution were assigned activity in the CAS assay. (B) Dilutions of K56-2-conditioned medium, shown as the percentage of conditioned medium in water added to the CAS assay, were assigned CAS activity. (C) Known ornibactin concentrations (squares) and the volumes of conditioned medium that corresponded to the known ornibactin concentrations (diamonds) were tested for induction of fusion I. Values are the averages of data from three separate experiments (error bars indicate SD). The volume differences were made up with buffered medium (minimal medium lacking succinate) and were mixed 1:1 with fresh succinate medium.
Induction of fusion I in mixed cultures of P. aeruginosa and Burkholderia.
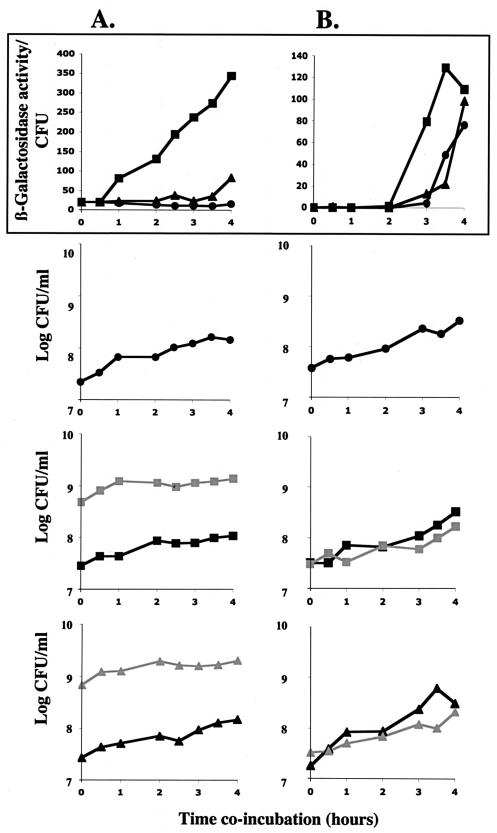
To address the role of ornibactin in P. aeruginosa-Burkholderia interactions, we examined the effect of ornibactin production on P. aeruginosa in mixed cultures. Specifically, we compared the expression level of fusion I in the presence of an ornibactin-producing B. cepacia strain relative to an ornibactin-deficient B. cepacia strain. B. cepacia cultures at an OD600 of 1.0 were pelleted by centrifugation and rinsed to remove any residual ornibactin. These cells were mixed with fusion I cells at an OD600 of 0.1, such that the majority of the cells were Burkholderia cells. The cultures were incubated at 30°C, and aliquots were taken for CFU counts and β-galactosidase assays at various time points. As shown in Fig. 7A, fusion I cells were induced immediately in the presence of an ornibactin-producing but not an ornibactin-deficient B. cepacia strain even though the growth rates of the two B. cepacia strains were roughly equivalent.
FIG. 7.
The ability of B. cepacia K56-2 or K56pvdA::tp to induce fusion I in mixed cultures was determined under different conditions. (A) Burkholderia cells were in the majority and in stationary phase (added at an OD600 of roughly 1.0 to fusion I cells at an OD600 of 0.1). (B) There were equal numbers of Burkholderia cells and fusion I cells and Burkholderia cells were in early logarithmic growth (added at an OD600 of 0.1 to fusion I cells at an OD600 of 0.1). The top panels indicate the expression levels of fusion I cells incubated alone (black circles), with B. cepacia K56-2 (black squares), or with K56pvdA::tp (black triangles). The lower three sets of panels show growth (log CFU/ml) of the strains in the different mixed cultures; fusion I cells (black circles, black squares, and black triangles) are in culture alone, with B. cepacia K56-2 (gray squares), or with B. cepacia K56pvdA::tp (gray triangles). The experiments shown are representative of the results obtained; the experiments were repeated four times.
To examine whether ornibactin could induce fusion I when the two species are mixed at comparable numbers and physiological states, we added both species in the early exponential phase at an OD600 of 0.1. As shown in Fig. 7B, ornibactin production by Burkholderia species is able to induce fusion I even when both strains are inoculated at a low density. While PA4467 is known to become induced in stationary phase, it is reproducibly induced earlier in the presence of an ornibactin-producing B. cepacia strain. Importantly, this occurs even though the cultures are not initially iron limited and overall CFU counts and growth rates in the different cultures are equivalent. This demonstrates that in conditions where P. aeruginosa and Burkholderia are found in similar physiological states and numbers, ornibactin can play a role in the response of Pseudomonas to the presence of Burkholderia.
DISCUSSION
We describe here a genetic approach to examine the interaction of two microbial species. We show that the production of the siderophore ornibactin by members of the B. cepacia complex can significantly alter the expression of P. aeruginosa gene PA4467. It has been shown previously that the operon containing PA4467 is controlled by Fur, a regulatory factor that derepresses the operon under iron-limiting conditions (18). Since ornibactin was shown to not be taken up by P. aeruginosa (30), it is likely that the mechanism by which ornibactin causes PA4467 induction is by limiting the amount of iron available to Pseudomonas. Iron availability is a global regulatory signal for P. aeruginosa; numerous genes, including many virulence factors, are induced under low-iron conditions (50). Therefore, ornibactin, as a nonutilizable siderophore, likely causes a global response, inducing a large number of P. aeruginosa genes. The induction of many iron-regulated genes by Burkholderia-conditioned medium in our microarray experiment supports this idea. Furthermore, we show that a range of iron-regulated genes are induced by conditioned medium from ornibactin-producing but not ornibactin-deficient Burkholderia strains.
We wanted to determine whether the observed effect of ornibactin on P. aeruginosa occurred in Burkholderia-Pseudomonas mixed cultures. To address this, we tested whether ornibactin could induce PA4467 when the two species were mixed at comparable numbers and in comparable physiological states. We show that even when the two species are mixed during early exponential phase, an ornibactin-producing Burkholderia strain, but not an ornibactin-deficient strain, induces P. aeruginosa PA4467. This occurs despite equivalent growth rates and cell numbers in the different cultures. This suggests that, in some environments where the two species are in similar physiological states, ornibactin may be produced by Burkholderia and detected by P. aeruginosa.
It is well established that both soil and the lungs of CF patients are environments with low concentrations of bioavailable iron (50). Siderophores are important for microbial growth in the soil and have been isolated directly from soil in several studies (4, 32). In addition, P. aeruginosa siderophores have been isolated from the sputa of CF patients (16). Similar studies have not yet been done with ornibactin, but it is likely that ornibactin, produced in vitro by the vast majority of clinical Burkholderia strains, is also produced in the lungs of CF patients. Supporting this, studies by Sokol et al. have shown that a strain deficient in ornibactin production is less virulent than its ornibactin-producing parent in a mouse model (45). Thus, it appears likely that, like P. aeruginosa siderophores, ornibactin is synthesized by Burkholderia in both the soil and the lungs of CF patients. Further studies are required to address the significance of ornibactin production in Burkholderia-Pseudomonas interactions in both the CF lung and soil environments.
Acknowledgments
We thank Pamela Sokol, Tom Lessie, Mike Vasil, and Jane Burns for strains, Andrea Donnelly for her assistance in the genetic screen, and members of the Kolter laboratory for critical reading of the manuscript.
We also thank the Cystic Fibrosis Foundation for subsidizing the Affymetrix GeneChip. This work was supported by grants from the NIH (GM58213) and CFF (LORY00V0).
REFERENCES
- 1.Ballard, R. W., N. J. Palleroni, M. Doudoroff, R. Y. Stanier, and M. Mandel. 1970. Taxonomy of the aerobic pseudomonads: Pseudomonas cepacia, P. marginata, P. alliicola and P. caryophylli. J. Gen. Microbiol. 60:199-214. [DOI] [PubMed] [Google Scholar]
- 2.Bano, N., and J. Musarrat. 2003. Characterization of a new Pseudomonas aeruginosa strain NJ-15 as a potential biocontrol agent. Curr. Microbiol. 46:324-328. [DOI] [PubMed] [Google Scholar]
- 3.Bollinger, N., D. J. Hassett, B. H. Iglewski, J. W. Costerton, and T. R. McDermott. 2001. Gene expression in Pseudomonas aeruginosa: evidence of iron override effects on quorum sensing and biofilm-specific gene regulation. J. Bacteriol. 183:1990-1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bossier, P., M. Höfte, and W. Verstraete. 1988. Ecological significance of siderophores in soil. Adv. Microb. Ecol. 10:385-414. [Google Scholar]
- 5.Burkholder, W. 1950. Sour skin, a bacterial rot of onion bulbs. Phytopathology 40:115-118. [Google Scholar]
- 6.Burns, J. L. 2001. Burkholderia cepacia—a transmissible cystic fibrosis pathogen. J. Pediatr. 139:618-619. [DOI] [PubMed] [Google Scholar]
- 7.Burns, J. L., and L. Saiman. 1999. Burkholderia cepacia infections in cystic fibrosis. Pediatr. Infect. Dis. J. 18:155-156. [DOI] [PubMed] [Google Scholar]
- 8.Caetano-Anolles, G. 1993. Amplifying DNA with arbitrary oligonucleotide primers. PCR Methods Appl. 3:85-94. [DOI] [PubMed] [Google Scholar]
- 9.Chang, H.-K., and G. J. Zylstra. 1999. Role of quinolinate phosphoribosyl transferase in degradation of phthalate by Burkholderia cepacia DBO1. J. Bacteriol. 181:3069-3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coenye, T., and P. Vandamme. 2003. Diversity and significance of Burkholderia species occupying diverse ecological niches. Environ. Microbiol. 5:719-729. [DOI] [PubMed] [Google Scholar]
- 11.Coenye, T., P. Vandamme, J. J. LiPuma, J. R. Govan, and E. Mahenthiralingam. 2003. Updated version of the Burkholderia cepacia complex experimental strain panel. J. Clin. Microbiol. 41:2797-2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cox, C. D., K. L. Rinehart, Jr., M. L. Moore, and J. C. Cook, Jr. 1981. Pyochelin: novel structure of an iron-chelating growth promoter for Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 78:4256-4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Darling, P., M. Chan, A. D. Cox, and P. A. Sokol. 1998. Siderophore production by cystic fibrosis isolates of Burkholderia cepacia. Infect. Immun. 66:874-877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Govan, J. R., and V. Deretic. 1996. Microbial pathogenesis in cystic fibrosis: mucoid Pseudomonas aeruginosa and Burkholderia cepacia. Microbiol. Rev. 60:539-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Govan, J. R., J. E. Hughes, and P. Vandamme. 1996. Burkholderia cepacia: medical, taxonomic and ecological issues. J. Med. Microbiol. 45:395-407. [DOI] [PubMed] [Google Scholar]
- 16.Haas, B., J. Kraut, J. Marks, S. C. Zanker, and D. Castignetti. 1991. Siderophore presence in sputa of cystic fibrosis patients. Infect. Immun. 59:3997-4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hart, C. A., and C. Winstanley. 2002. Persistent and aggressive bacteria in the lungs of cystic fibrosis children. Br. Med. Bull. 61:81-96. [DOI] [PubMed] [Google Scholar]
- 18.Hassett, D. J., M. L. Howell, U. A. Ochsner, M. L. Vasil, Z. Johnson, and G. E. Dean. 1997. An operon containing fumC and sodA encoding fumarase C and manganese superoxide dismutase is controlled by the ferric uptake regulator in Pseudomonas aeruginosa: fur mutants produce elevated alginate levels. J. Bacteriol. 179:1452-1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hassett, D. J., M. L. Howell, P. A. Sokol, M. L. Vasil, and G. E. Dean. 1997. Fumarase C activity is elevated in response to iron deprivation and in mucoid, alginate-producing Pseudomonas aeruginosa: cloning and characterization of fumC and purification of native FumC. J. Bacteriol. 179:1442-1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hassett, D. J., J. F. Ma, J. G. Elkins, T. R. McDermott, U. A. Ochsner, S. E. West, C. T. Huang, J. Fredericks, S. Burnett, P. S. Stewart, G. McFeters, L. Passador, and B. H. Iglewski. 1999. Quorum sensing in Pseudomonas aeruginosa controls expression of catalase and superoxide dismutase genes and mediates biofilm susceptibility to hydrogen peroxide. Mol. Microbiol. 34:1082-1093. [DOI] [PubMed] [Google Scholar]
- 21.Kazmierski, W. M., G. Wolberg, J. G. Wilson, S. R. Smith, D. S. Williams, H. H. Thorp, and L. Molina. 1996. Iron chelates bind nitric oxide and decrease mortality in an experimental model of septic shock. Proc. Natl. Acad. Sci. USA 93:9138-9141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lessie, T. G., W. Hendrickson, and B. D. Manning, and R. Devereux. 1996. Genomic complexity and plasticity of Burkholderia cepacia. FEMS Microbiol. Lett. 144:117-128. [DOI] [PubMed] [Google Scholar]
- 23.Lessie, T. G., and T. Gaffney. 1986. Catabolic potential of Pseudomonas cepacia, p. 439-481. In J. R. Sokatch and L. N. Ornston (ed.), The bacteria: a treatise on structure and function. Academic Press, New York, N.Y.
- 24.Lyczak, J. B., C. L. Cannon, and G. B. Pier. 2000. Establishment of Pseudomonas aeruginosa infection: lessons from a versatile opportunist. Microbes Infect. 2:1051-1060. [DOI] [PubMed] [Google Scholar]
- 25.Mahenthiralingam, E., T. Coenye, J. W. Chung, D. P. Speert, J. R. Govan, P. Taylor, and P. Vandamme. 2000. Diagnostically and experimentally useful panel of strains from the Burkholderia cepacia complex. J. Clin. Microbiol. 38:910-913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meyer, J. M. 1992. Exogenous siderophore-mediated iron uptake in Pseudomonas aeruginosa: possible involvement of porin OprF in iron translocation. J. Gen. Microbiol. 138:951-958. [DOI] [PubMed] [Google Scholar]
- 27.Meyer, J. M., and M. A. Abdallah. 1978. The fluorescent pigment of Pseudomonas fluorescens: biosynthesis, purification and physiochemical properties. J. Gen. Microbiol. 107:319-328. [Google Scholar]
- 28.Meyer, J. M., D. Hohnadel, and F. Halle. 1989. Cepabactin from Pseudomonas cepacia, a new type of siderophore. J. Gen. Microbiol. 135:1479-1487. [DOI] [PubMed] [Google Scholar]
- 29.Meyer, J. M., and A. Stintzi. 1998. Iron metabolism and siderophores in Pseudomonas and related species, p. 201-243. In T. C. Montie (ed.), Pseudomonas. Plenum Press, New York, N.Y.
- 30.Meyer, J. M., V. T. Van, A. Stintzi, O. Berge, and G. Winkelmann. 1995. Ornibactin production and transport properties in strains of Burkholderia vietnamiensis and Burkholderia cepacia (formerly Pseudomonas cepacia). Biometals 8:309-317. [DOI] [PubMed] [Google Scholar]
- 31.Miller, J. H. 1992. A short course in bacterial genetics. Cold Spring Harbor Press, Cold Spring Harbor, N.Y.
- 32.Mirleau, P., S. Delorme, L. Philippot, J. Meyer, S. Mazurier, and P. Lemanceau. 2000. Fitness in soil and rhizosphere of Pseudomonas fluorescens C7R12 compared with a C7R12 mutant affected in pyoverdine synthesis and uptake. FEMS Microbiol. Ecol. 34:35-44. [DOI] [PubMed] [Google Scholar]
- 33.Mossialos, D., U. Ochsner, C. Baysse, P. Chablain, J. P. Pirnay, N. Koedam, H. Budzikiewicz, D. U. Fernandez, M. Schafer, J. Ravel, and P. Cornelis. 2002. Identification of new, conserved, non-ribosomal peptide synthetases from fluorescent pseudomonads involved in the biosynthesis of the siderophore pyoverdine. Mol. Microbiol. 45:1673-1685. [DOI] [PubMed] [Google Scholar]
- 34.Ochsner, U. A., P. J. Wilderman, A. I. Vasil, and M. L. Vasil. 2002. GeneChip expression analysis of the iron starvation response in Pseudomonas aeruginosa: identification of novel pyoverdine biosynthesis genes. Mol. Microbiol. 45:1277-1287. [DOI] [PubMed] [Google Scholar]
- 35.O'Toole, G. A., and R. Kolter. 1998. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol. Microbiol. 30:295-304. [DOI] [PubMed] [Google Scholar]
- 36.O'Toole, G. A., and R. Kolter. 1998. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: a genetic analysis. Mol. Microbiol. 28:449-461. [DOI] [PubMed] [Google Scholar]
- 37.O'Toole, G. A., L. A. Pratt, P. I. Watnick, D. K. Newman, V. B. Weaver, and R. Kolter. 1999. Genetic approaches to study of biofilms. Methods Enzymol. 310:91-109. [DOI] [PubMed] [Google Scholar]
- 38.Palleroni, N. J. 1984. Genus I. Pseudomonas Migula 1894, 237AL, p. 141-199. In N. R. Krieg and J. G. Holt (ed.), Bergey's manual of systematic bacteriology, vol. 1. Williams & Wilkins, Baltimore, Md. [Google Scholar]
- 39.Parales, R. E., J. L. Ditty, and C. S. Harwood. 2000. Toluene-degrading bacteria are chemotactic towards the environmental pollutants benzene, toluene, and trichloroethylene. Appl. Environ. Microbiol. 66:4098-4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parke, J. L., and D. Gurian-Sherman. 2001. Diversity of the Burkholderia cepacia complex and implications for risk assessment of biological control strains. Annu. Rev. Phytopathol. 39:225-258. [DOI] [PubMed] [Google Scholar]
- 41.Pesci, E. C., and B. H. Iglewski. 1997. The chain of command in Pseudomonas quorum sensing. Trends Microbiol. 5:132-135. [DOI] [PubMed] [Google Scholar]
- 42.Schwyn, B., and J. B. Neilands. 1987. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 160:47-56. [DOI] [PubMed] [Google Scholar]
- 43.Simon, R., J. Quandt, and W. Klipp. 1989. New derivatives of transposon Tn5 suitable for mobilization of replicons, generation of operon fusions and induction of genes in gram-negative bacteria. Gene 80:161-169. [DOI] [PubMed] [Google Scholar]
- 44.Sokol, P. A. 1986. Production and utilization of pyochelin by clinical isolates of Pseudomonas cepacia. J. Clin. Microbiol. 23:560-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sokol, P. A., P. Darling, D. E. Woods, E. Mahenthiralingam, and C. Kooi. 1999. Role of ornibactin biosynthesis in the virulence of Burkholderia cepacia: characterization of pvdA, the gene encoding l-ornithine N5-oxygenase. Infect. Immun. 67:4443-4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sokol, P. A., C. J. Lewis, and J. J. Dennis. 1992. Isolation of a novel siderophore from Pseudomonas cepacia. J. Med. Microbiol. 36:184-189. [DOI] [PubMed] [Google Scholar]
- 47.Stanier, R. Y., N. J. Palleroni, and M. Doudoroff. 1966. The aerobic pseudomonads: a taxonomic study. J. Gen. Microbiol. 43:159-271. [DOI] [PubMed] [Google Scholar]
- 48.Stephan, H., S. Freund, W. Beck, G. Jung, J. M. Meyer, and G. Winkelmann. 1993. Ornibactins—a new family of siderophores from Pseudomonas. Biometals 6:93-100. [DOI] [PubMed] [Google Scholar]
- 49.Thomassen, M. J., C. A. Demko, J. D. Klinger, and R. C. Stern. 1985. Pseudomonas cepacia colonization among patients with cystic fibrosis. A new opportunist. Am. Rev. Respir. Dis. 131:791-796. [DOI] [PubMed] [Google Scholar]
- 50.Vasil, M. L., and U. A. Ochsner. 1999. The response of Pseudomonas aeruginosa to iron: genetics, biochemistry and virulence. Mol. Microbiol. 34:399-413. [DOI] [PubMed] [Google Scholar]
- 51.Visca, P., A. Ciervo, V. Sanfilippo, and N. Orsi. 1993. Iron-regulated salicylate synthesis by Pseudomonas spp. J. Gen. Microbiol. 139:1995-2001. [DOI] [PubMed] [Google Scholar]
- 52.Whiteley, M., K. M. Lee, and E. P. Greenberg. 1999. Identification of genes controlled by quorum sensing in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 96:13904-13909. [DOI] [PMC free article] [PubMed] [Google Scholar]