Abstract
Patients with idiopathic pulmonary fibrosis (IPF) often do worse following infection, but the cause of the decline is not fully understood. We previously demonstrated that infection with a murine gamma herpes virus (γHV-68) could exacerbate established lung fibrosis following administration of fluorescein isothiocyanate (McMillan et al. Am J Respir Crit Care Med 177: 771–780, 2008). In the present study, we anesthetized mice and injected saline or bleomycin intratracheally on day 0. On day 14, mice were anesthetized again and infected with either a Gram-negative bacteria (Pseudomonas aeruginosa), or with H1N1 or γHV-68 viruses. Measurements were then made on days 15, 21, or 35. We demonstrate that infection with P. aeruginosa does not exacerbate extracellular matrix deposition post-bleomycin. Furthermore, fibrotic mice are effectively able to clear P. aeruginosa infection. In contrast, bleomycin-treated mice develop worse lung fibrosis when infected with γHV-68, but not when infected with H1N1. The differential ability of γHV-68 to cause increased collagen deposition could not be explained by differences in inflammatory cell recruitment or whole lung chemokine and cytokine responses. Alveolar epithelial cells from γHV-68-infected mice displayed increased expression of TGFβ receptor 1, increased SMAD3 phosphorylation, and evidence of apoptosis measured by cleaved poly-ADP ribose polymerase (PARP). The ability of γHV-68 to augment fibrosis required the ability of the virus to reactivate from latency. This property appears unique to γHV-68, as the β-herpes virus, cytomegalovirus, did not have the same effect.
Keywords: lung, fibrosis, collagen, virus, bacteria
fibrosis is a condition characterized by the deposition of extracellular matrix (ECM) proteins such as collagen and fibronectin causing stiffening of interstitial tissue or airways when it occurs in the lung. Fibrosis can be triggered by known agents such as allergens, toxic chemicals, and radiation or can occur for unknown reasons such as in the case of idiopathic pulmonary fibrosis (IPF) (50). IPF is a progressive disease eventually causing death from respiratory insufficiency usually within 2–5 years of diagnosis. Lung transplantation is the only proven therapy in the United States and this procedure has a median patient survival rate of 3 years (1). The pathogenesis of IPF remains unknown but key events likely involve continuous cycles of injury and abnormal repair with evidence suggesting that the variation in fibrotic lesions is due to repeated lung injury over the course of the disease (11). Chronic viral infection, mainly herpes viruses, have been implicated as one cause of ongoing epithelial injury and therefore have been implicated as a cofactor in either the initiation or exacerbation of the disease (reviewed in 28).
Previous studies have shown the presence of Epstein-Barr virus (EBV), cytomegalovirus, herpes simplex virus 1, as well as human herpes viruses (HHV)-7 and -8 in lung tissue of human IPF patients (15, 17, 35, 38). However, this is somewhat controversial as other studies found no association between herpes viral infection and IPF (47, 51). There is strong evidence in animal models linking γ-herpes virus infection with development of fibrosis in T helper type 2 (Th2) biased mice (6, 8, 19, 27). In these cases, fibrosis was associated with persistent reactivation of the virus and development of alternatively activated macrophages (8, 25–27). Additionally, infection of aged mice with murine γ herpes virus-68 (γHV-68) results in development of lung fibrosis (29, 39) and the pathogenic mechanisms have been shown to include epithelial cell stress and apoptosis and enhanced susceptibility of fibroblasts to viral-induced TGFβ secretion.
We have previously shown that γHV-68 infection given prior to stimulation with bleomycin or fluorescein isothiocyanate (FITC) can augment development of lung fibrosis (44). Possible mechanisms involve alterations in alveolar epithelial cells such as increased synthesis of cysteinyl leukotrienes, induction of transforming growth factor (TGF)-β, and recruitment of circulating fibrocytes (44). Similarly, infection of mice with γHV-68 after the establishment of lung fibrosis can also worsen deposition of collagen within the lung, and this increased fibrosis correlated with enhanced production of IL-13, IFNγ, and TNFα (23). Exacerbation of fibrosis required virus capable of replication as UV-inactivated virus did not exacerbate disease (23).
This study aimed to investigate whether the ability to exacerbate established pulmonary fibrosis in mice was unique to γHV-68 or whether other inflammatory/infectious insults could augment fibrosis. Thus we sought to determine whether an acute bacterial infection with Pseudomonas aeruginosa (P. aeruginosa) or an acute viral infection that does not establish latency, influenza A (H1N1), could augment fibrotic outcomes.
P. aeruginosa is a Gram-negative opportunistic human pathogen that rarely causes disease in healthy individuals. However, P. aeruginosa is responsible for life-threatening infections in immunocompromised patients, the elderly, and following prolonged hospitalization (42). Clearance of P. aeruginosa from the lungs requires a functional innate immune system with the involvement of macrophages and polymorphonuclear leukocytes (PMNs) (20). Influenza A is a RNA virus that replicates in the respiratory epithelium leading to the infiltration of inflammatory cells, mainly mononuclear leukocytes and small numbers of PMNs. Innate defense against influenza A infection involves the production of high levels of type I interferons by infected epithelial cells, alveolar macrophages (AMs), recruited conventional dendritic cells (cDCs), PMNs, and NK cells (9, 16, 32). DCs lining the airways play key roles in activating effector CD8 T cells mediating viral clearance and protection (3, 22). In contrast, γHV-68 can infect a variety of cells within the lung including epithelial cells, fibroblasts, macrophages, and B cells (36, 37). There is low level induction of type I interferon, and plasmacytoid DCs are necessary to activate cDCs in vitro (49). Production of both IFNγ and perforin are important for viral control (34, 40). In this study we used bleomycin to establish fibrosis in mice, and then compared the ability of P. aeruginosa, H1N1, and γHV-68 to exacerbate the fibrotic response.
MATERIALS AND METHODS
Animals.
Male wild-type C57BL/6 (B6) mice were obtained from The Jackson Laboratory (Bar Harbor, ME). Mice were housed under pathogen-free conditions and provided food and water ad libitum. All animal experiments complied with university and federal guidelines for humane use and care. The University of Michigan Committee on Use and Care of Animals approved these experiments.
P. aeruginosa PA01 infection.
As previously described, P. aeruginosa PA01 inoculum was prepared and mice were injected intratracheally with a sublethal dose of 5 × 105 colony forming units (CFU) (2, 21). For exacerbation studies, mice were infected with P. aeruginosa on day 14 post-bleomycin.
Quantification of bacterial burden in the lung and blood.
Mice were euthanized 24 h following intratracheal infection with P. aeruginosa. Blood and whole lung samples were collected. Bacterial burden in whole lung and blood samples were analyzed by CFU assay as previously described (2).
Total lung leukocyte preparation.
Whole lung samples were harvested from mice and collagenase-digested as previously described (14). For each sample total viable cells were counted on a hemocytometer by trypan blue exclusion. Differential analysis was done using the total lung cells isolated from the collagenase-digested whole lung samples to determine the percentage of neutrophils, eosinophils, and monocytes/macrophages as previously described (14).
Flow cytometry.
Whole lungs were enzymatically digested using collagenase and DNase (24) and leukocytes were isolated. Leukocytes were incubated with Fc block (1:100) clone 24G2 (BD Pharmingen, San Diego, CA) for 15 min, then stained with combinations of anti-mouse CD45, CD4, CD8, T cell receptor β, NK1.1, and CD19 directly conjugated antibodies available from BD Pharmingen. Cells were analyzed on a FACScan (BD Biosciences, Mountain View, CA).
Bleomycin model of pulmonary fibrosis.
Mice were given bleomycin intratracheally as described previously (24).
Viral infection.
Mice were anesthetized and infected intranasally with 50 plaque-forming units (PFU) of influenza A virus (A/PR/8/34) (H1N1) or 5 × 104 PFU of γHV-68 clone WUMS (American Type Culture Collection) in 20 μl saline. The dose of 50 PFU for H1N1 corresponds to a nonlethal dose in wild-type mice. γHV-68 is not lethal in wild-type mice even at higher doses (0.5–1 × 106). Viral infection occurred on day 14 post-bleomycin challenge. In some experiments, mice were infected with 5 × 104 PFU γHV-68 containing a mutation in the v-cyclin gene (ΔORF72) or the marker rescue (essentially wild-type) viral control (43) or were infected with 5 × 104 PFU murine cytomegalovirus (CMV).
Lung collagen measurements.
Collagen deposition in the lungs of all mice from each treatment group was measured using a hydroxyproline assay post-bleomycin challenge as described previously (24).
Enzyme-linked immunoassay/ELISA.
Whole lung homogenates were prepared for analysis of cytokines and chemokines as described previously (14). Each ELISA assay was done using a Duoset ELISA kit (R&D Systems, Minneapolis, MN) according to manufacturer's instructions.
Alveolar epithelial cell (AEC) isolation.
AECs were isolated from mice that had been treated with bleomycin on day 0 and infected with H1N1 or γHV-68 on day 14. AECs were prepared on day 21 using a previously described method (37).
Semiquantitative real-time RT-PCR.
RNA was extracted from the AECs or left lung using TRIzol reagent (Invitrogen, Carlsbad, CA), then analyzed by real-time RT-PCR on an Applied Biosytems StepOne Plus thermocycler (Applied Biosystems, Foster City, CA). Gene specific primers and probes were purchased from Sigma-Aldrich (St Louis, MO). Relative expression was calculated using the comparative CT method with beta actin (β-actin) as an internal standard gene control. Fold change in mRNA was quantified using the ΔΔCT method.
Western blot analysis.
AECs were lysed in radioimmunoprecipitation assay buffer with protease inhibitor cocktail (Sigma) for 15 min at 4°C and centrifuged. Total protein concentrations in the supernatants were determined by the Bicinchoninic acid assay (Pierce). Equal amounts of protein from each sample were separated on a 4–20% gradient SDS-polyacrylamide gel and transferred to a PVDF membrane (Amersham/GE Healthcare, Pittsburgh, PA). PVDF membrane was then probed with rabbit polyclonal PARP, SMAD3 (Cell Signaling, Beverly, MA) and β-actin (Sigma).
Statistical analysis.
Statistical analysis was measured by analysis of variance (3 or more comparisons) with a Bonferroni post hoc test or Student t-test (2 comparisons) using GraphPad Prism 6 software (San Diego, CA). Data shown represent means ± SE. P < 0.05 was considered significant.
RESULTS
P. aeruginosa infection has no effect on bleomycin-induced pulmonary fibrosis.
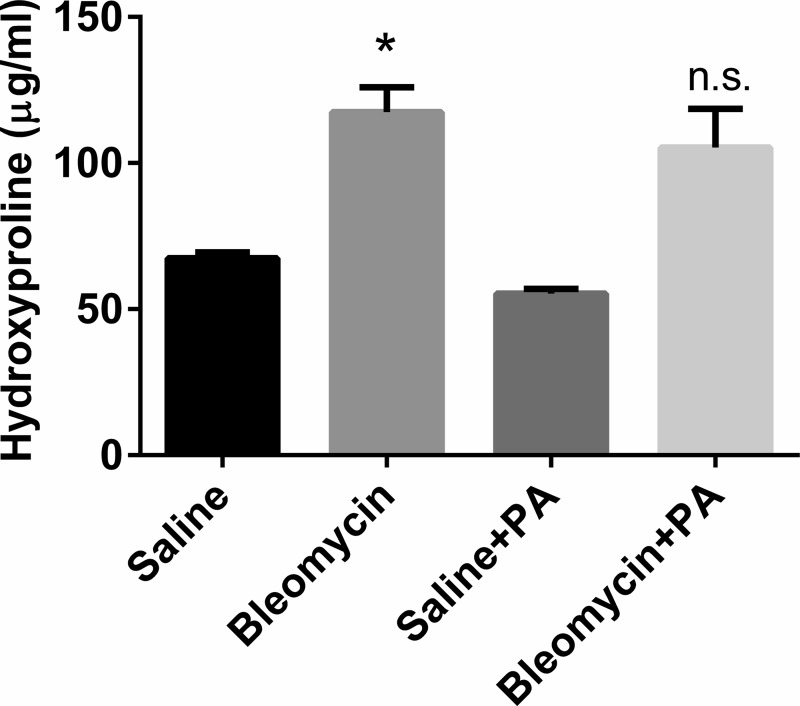
To determine the effects of P. aeruginosa infection on established pulmonary fibrosis, mice were given intratracheal saline or bleomycin on day 0. On day 14, mice were then given 5 × 105 CFU P. aeruginosa intratracheally or were mock infected. All lungs were harvested on day 21 and lung collagen content was measured by hydroxyproline assay. As expected, bleomycin-treated mice showed significant increases in collagen content compared with saline controls. However, subsequent infection of bleomycin-treated mice with P. aeruginosa showed no significant increase in collagen content compared with bleomycin-treated mice that were mock infected (Fig. 1). Thus P. aeruginosa infection in wild-type mice does not exacerbate bleomycin-induced fibrotic response in the lungs. Furthermore, at this dose of infection, there was no difference in the survival of bleomycin-treated mice that were mock infected or infected with P. aeruginosa (data not shown).
Fig. 1.
Pseudomonas aeruginosa infection does not exacerbate bleomycin-induced fibrosis. Wild-type mice were given bleomycin or saline intratracheally on day 0. On day 14, half of the mice in each group were given P. aeruginosa (PA) intratracheally or saline as a vehicle control. Lungs were harvested on day 21 for hydroxyproline assay. Data shown represent n = 6–10 mice per group pooled from 3 independent experiments. *P < 0.05. ns, not significant.
Fibrotic mice do not show increased susceptibility to P. aeruginosa.
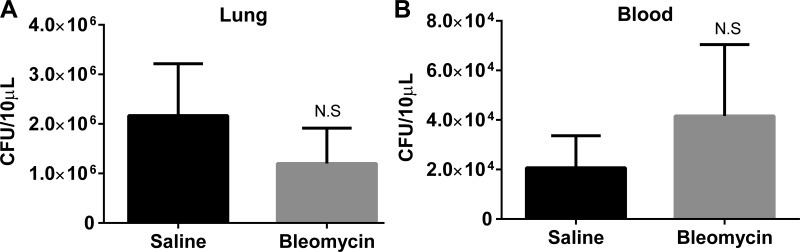
To determine whether the bleomycin-treated mice were more susceptible to infection with P. aeruginosa, mice were treated with saline or bleomycin as previously described. On day 14, both groups of mice were infected with 5 × 105 CFU P. aeruginosa. Blood and lungs were collected on day 15 (24 h postinfection, a time point noted for maximal bacterial growth postinfection) (2) and plated for CFU assay. Mice treated with bleomycin prior to infection with P. aeruginosa showed no difference in bacterial load in the lung (Fig. 2A) or in the blood (Fig. 2B) compared with mice first treated with saline.
Fig. 2.
Bleomycin-treated mice show no defect in the clearance of P. aeruginosa infection. Mice were first treated with saline or bleomycin on day 0 followed by infection with P. aeruginosa on day 14. On day 15 lungs (A) and blood (B) were collected for colony-forming units (CFU) analysis. Data represent n = 5–8 mice per group from 2 independent experiments. NS, not significant.
H1N1 influenza A infection does not exacerbate bleomycin-induced fibrosis.
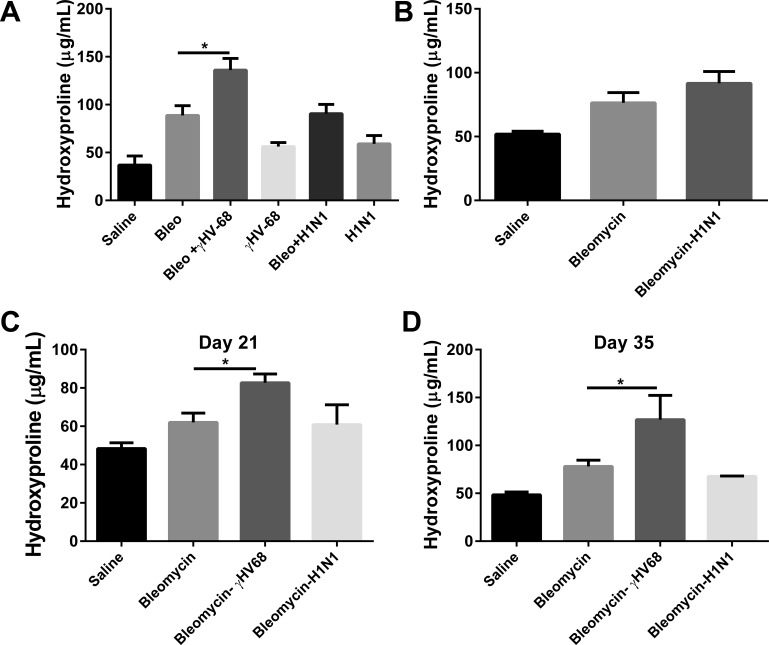
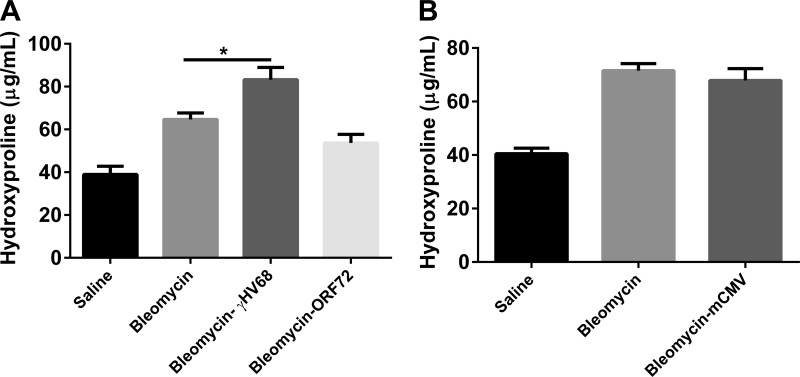
We next wanted to determine whether the exacerbation of established pulmonary fibrosis could occur with an acute viral infection that does not establish latency. Wild-type mice were treated with bleomycin or saline on day 0. On day 14, a time of established pulmonary fibrosis, bleomycin-treated mice received 5 × 104 PFU γHV-68 or 50 PFU H1N1 intranasally or were mock infected. These doses were chosen to be nonlethal in control mice. Lungs were harvested on day 21 to measure lung collagen content by hydroxyproline assay. Figure 3A demonstrates that subsequent γHV-68 infection resulted in significantly more collagen deposition in the lungs than did bleomycin treatment followed by mock infection or bleomycin followed by H1N1 infection (P < 0.05). These data replicate early findings that γHV-68 can exacerbate established lung fibrosis (23). However, H1N1 was not able to exacerbate fibrosis at day 21. To see if H1N1 might be able to exacerbate bleomycin-induced fibrosis at an earlier time point that corresponded to expected peak viral replication, mice injected with bleomycin on day 0 were infected with H1N1 on day 18, and lungs were harvested for hydroxyproline content on day 21 (Fig. 3B); however, no elevations in collagen deposition were noted at this time point either. To determine whether H1N1 or γHV-68 infection altered fibrosis at a later time point, another experiment was set up to harvest lungs at days 21 and 35 following the same initial treatments (Fig. 3, C and D). Levels of fibrosis in γHV-68-infected mice were still the highest of all groups, and the only ones to show significant increases over bleomycin and mock infection. Figure 4 shows representative histology of lungs from all three groups of mice harvested at day 21.
Fig. 3.
H1N1 infection does not exacerbate bleomycin-induced pulmonary fibrosis. A: mice were given bleomycin (Bleo) or saline intratracheally on day 0. On day 14, bleomycin- or saline-treated mice received γ-herpes virus-68 (γHV-68), H1N1, or saline intranasally. Lungs were harvested for collagen determination on day 21. B: on day 18, bleomycin-treated mice received H1N1 or saline intranasally. Lungs were harvested 3 days postinfection to measure collagen content and were compared with mice treated with saline alone. Data represents n = 5–8 mice per group collected in 2 independent experiments. C and D: in a separate experiment, mice were treated with saline or bleomycin on day 0, viral or mock infections occurred at day 14, and lungs were harvested at day 21 or day 35; n = 3–6 mice per group. *P < 0.05.
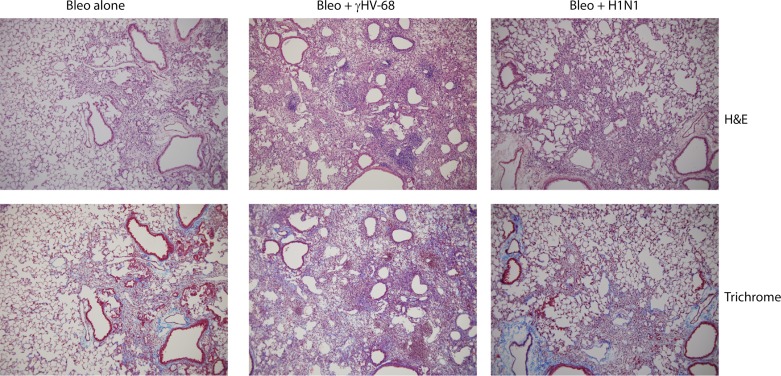
Fig. 4.
Histological analyses. Immunohistochemistry showing representative lungs of mice treated with bleomycin + vehicle control (saline), bleomycin + γHV-68 infection, or bleomycin + H1N1 infection. Shown are hematoxylin and eosin (H&E) or Masson's trichrome staining. Magnification is 200×. Left panels: mice treated with bleomycin were harvested on day 21 postinjection and show diffuse mononuclear infiltrates and collagen deposition noted as blue coloration in the bottom panel. Middle panels: mice were treated with bleomycin on day 0 and infected with γHV-68 on day 14. Lungs were harvested on day 21 and show both focal areas of dense mononuclear inflammatory cells as well as diffuse inflammation. Collagen deposition is noted within the interstitium. Right panels: mice were injected with bleomycin on day 0 and H1N1 on day 14. Lungs were harvested on day 21 and show diffuse mononuclear infiltration. While collagen deposition is seen within the interstitium, it is similar to that noted in mice treated with bleomycin alone. Overall, the mice treated with bleomycin + γHV-68 show the greatest degree of lung involvement. Representative of n = 4 lungs in each group.
γHV-68 replication is enhanced post-bleomycin and the ability to reactivate from latency is required for exacerbation of fibrotic response.
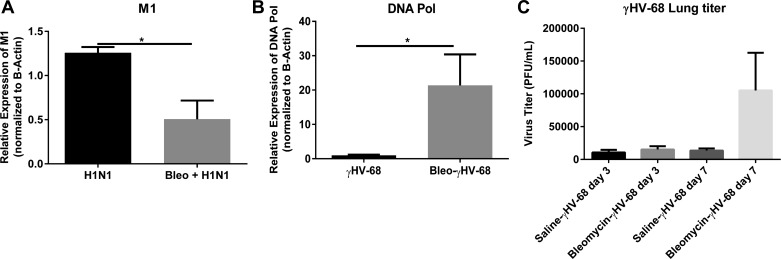
To determine the levels of viral replication which were present in the lungs on day 21, total lung RNA was subjected to RT-PCR analysis for expression of the influenza M1 gene (Fig. 5A) or for the lytic γHV-68 DNA polymerase (DNA pol) (Fig. 5B). These levels were compared with animals that had been given saline prior to viral infection. Regarding H1N1 infection, expression of M1 was significantly decreased 7 days postinfection (dpi) between mice that were pretreated with saline or pretreated with bleomycin. This suggests that bleomycin administration did not make the mice more susceptible to H1N1 replication measured at 7 dpi. Because of the low level inoculum of H1N1 (50 PFU) or perhaps due to viral clearance, we could not detect virus by plaque assay in the lungs at either day 3 or day 7 postinfection in saline or bleomycin-pretreated mice (data not shown). In contrast, the γHV-68 DNA pol gene was significantly elevated 7 dpi in bleomycin-treated compared with saline-treated mice confirming earlier observations (23). In addition, we were able to demonstrate virus by plaque assay following γ-68 infection (Fig. 5C).
Fig. 5.
γHV-68 replicates to a greater extent than does H1N1 post-bleomycin. Mice were injected on day 0 with saline or bleomycin. On day 14 mice were infected with γHV-68 or H1N1. On day 21, lungs were collected and levels of H1N1 viral M1 (A) or γHV-68 DNA polymerase (DNA pol) (B) gene expression were measured by real-time RT-PCR. C: similarly, lungs were collected from mice infected with γHV-68 on days 17 and 21, and virus titers in the lungs were measured by plaque assay. Data shown are from n = 3–5 lungs/group representative of 2 experiments.
Exacerbation of lung fibrosis by γHV-68 requires the ability to reactivate from latency and is not a property shared by cytomegalovirus (CMV).
A v-cyclin mutant γHV-68 (ΔORF72) is 100-fold decreased in its ability to reactivate from latency (43). In our hands, mice infected with ΔORF72 display decreased replication within the lung compared with wild-type marker rescue virus by day 3 postinfection (data not shown). To see if on-going viral replication was required to promote fibrosis, we infected bleomycin-treated mice with mock control, marker-rescue virus, or ΔORF72 mutant virus and analyzed collagen deposition at day 21 (Fig. 6A). Wild-type (marker rescue) virus significantly enhanced fibrosis whereas ΔORF72 did not. Surprisingly, infection with a β herpes virus, CMV, did not exacerbate bleomycin-induced fibrosis either (Fig. 6B). We were unable to plaque infectious CMV from the lungs of these C57Bl/6 mice, but we did confirm that expression of viral 1E1 and envelope gB proteins were detectable by real-time RT-PCR (data not shown).
Fig. 6.
Exacerbation of bleomycin-induced fibrosis is specific to γHV-68 and dependent on viral replication. Mice were first treated with saline or bleomycin on day 0. On day 14 mice were infected with 5 × 104 PFU of γHV-68 containing a mutation in the v-cyclin gene (ΔORF72) or marker rescue (essentially wild-type) virus (A) or 5 × 104 murine cytomegalovirus (mCMV) (B) or were mock infected. On day 21, lungs were harvested for collagen determination. Data shown represents n = 5–8 mice per group collected in two independent experiments. *P < 0.05.
The profibrotic effects of γHV-68 compared with H1N1 and P. aeruginosa infection are not explained by inflammatory cell recruitment.
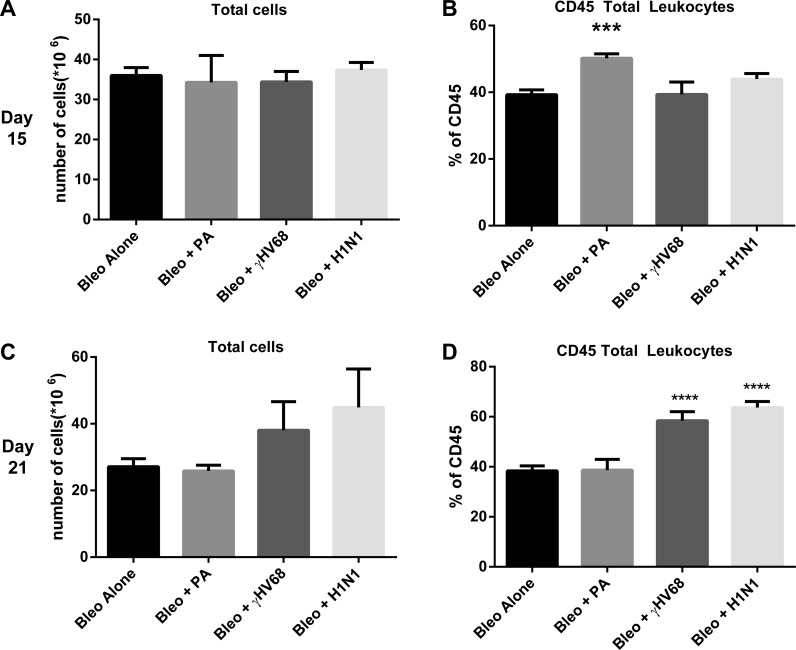
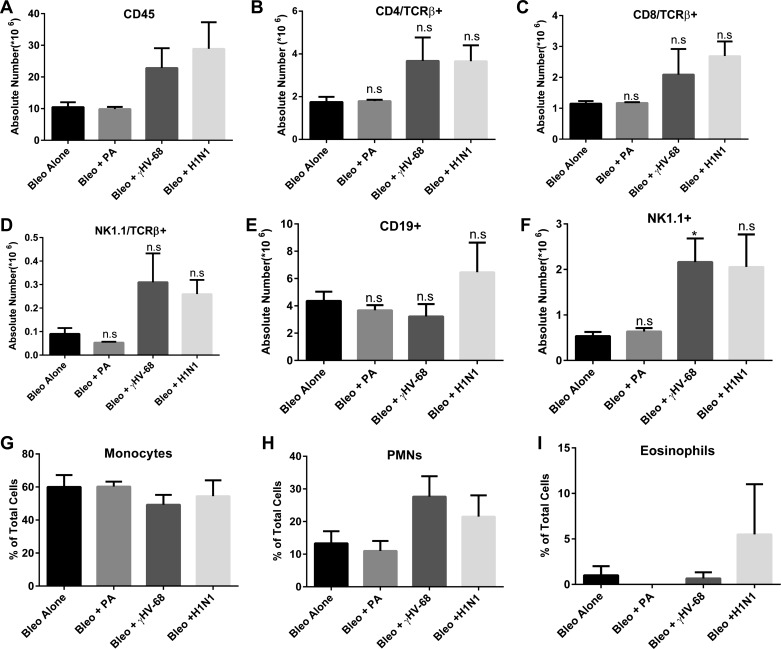
To determine whether the inflammatory cell composition was different following the various infections, mice were treated with bleomycin on day 0. Next, bleomycin-treated mice were infected with P. aeruginosa, H1N1, or γHV-68 on day 14. Lungs were harvested on days 15 or 21, which represent 1 and 7 dpi, respectively. Single-cell suspensions were isolated, counted, and analyzed by flow cytometry to assess leukocyte populations. As demonstrated in Fig. 7A, total cells were not different between groups at day 15, but there was a noticeable increase in the percentage of CD45+ leukocytes in the P. aeruginosa infected mice at this time point (Fig. 7B). This increase was attributable to an influx of PMNs in these mice, as would be expected for this bacterial infection (data not shown). This increased percentage of leukocytes in the P. aeruginosa group was not maintained at day 21, consistent with rapid clearance of the organism from the lungs (Fig. 7C). In contrast, the viral infected mice showed increased percentages of CD45+ leukocytes on day 21, consistent with the recruitment of leukocytes in response to viral infection. Both H1N1 and γHV-68 infected mice showed similar increases in leukocyte accumulation in the lung (Fig. 7D), although histologic evidence in Fig. 4 suggests that γHV-68-infected mice show both diffuse and focal inflammatory infiltrates. Because H1N1 infected mice did not exacerbate fibrosis whereas γHV-68 infected mice did, we analyzed the various leukocyte subsets between these two groups. There were no discernable differences in the percentages of CD4, CD8, NK, NK-T, CD19, monocyte, PMN, or eosinophil cell types between these groups (Fig. 8). Thus differential accumulation of leukocyte subsets could not explain why γHV-68 infection augments fibrotic responses in the lung whereas H1N1 infection did not.
Fig. 7.
Leukocyte recruitment is enhanced following infection. Mice were injected with bleomycin on day 0. On day 14, mice were infected with saline, P. aeruginosa, H1N1, or γHV-68. Lungs were harvested from n = 4 mice each on day 15 or day 21. Total lung leukocytes were enumerated (A and C) and percentage of CD45+ leukocytes were assessed (B and D) by flow cytometry. Data are from 1 experiment representative of 2. ***P < 0.001, ****P < 0.0001.
Fig. 8.
Leukocyte recruitment is enhanced following infection. Mice were injected with bleomycin on day 0. On day 14, mice were infected with saline, P. aeruginosa, H1N1, or γHV-68. Lungs were harvested from n = 4 mice each on day 15 or day 21. Total lung leukocytes were enumerated, and absolute numbers of CD45+ (A), CD4 TcRβ (B), CD8 TcRβ (C), Nk1.1 TcRβ (D), CD19 (E), and NK1 (F) positive cells were determined based on flow cytometry. Differential analysis was done to determine the percentage of monocytes/macrophages (G), neutrophils (H), and eosinophils (I). Data shown are from one experiment representative of 2. *P < 0.05.
Differences in profibrotic mediators do not explain the ability of γHV-68, but not H1N1, to exacerbate fibrosis.
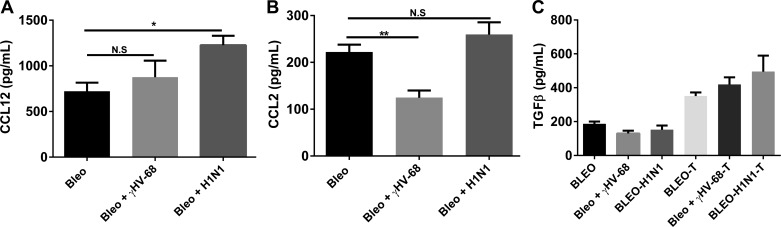
We next examined production of several pro- and antifibrotic mediators by ELISA. Expression of CCL12 (Fig. 9A) was elevated in both viral infections, but only reached significance in the H1N1-infected mice. Expression of CCL2 (Fig. 9B) is decreased post-γHV-68 infection, but is unchanged post-H1N1. Expression of active and total TGFβ (Fig. 9C) is similar in all groups.
Fig. 9.
Differences in CCL12, CCL2, and TGFβ cannot explain the differential ability of γHV-68, but not H1N1, to exacerbate ECM deposition post-bleomycin. Mice were treated with bleomycin on day 0 and infected with saline, γHV-68, or H1N1 on day 14. On day 21, lungs were harvested and whole lung homogenates were analyzed by ELISA for CCL12 (A), CCL2 (B), levels of active (C, left side) or acid-activated total TGFβ (C, right side). n = 3–5 mice/group representative of 2 experiments. *P < 0.05. **P < 0.01.
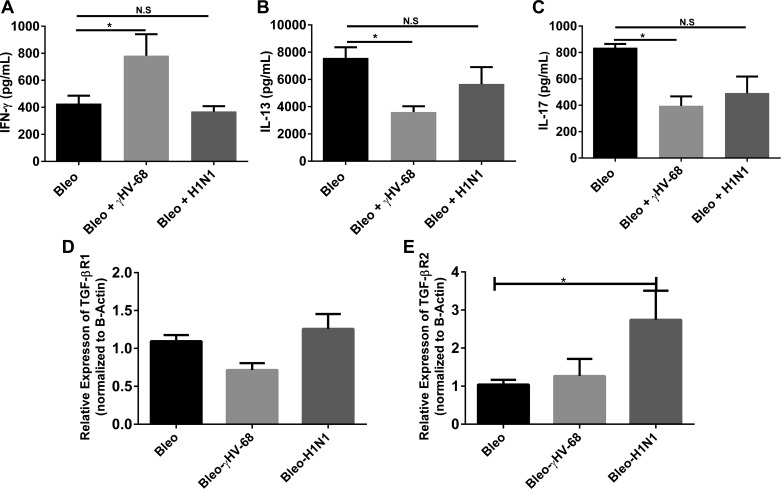
When evaluating Th1, Th2, and Th17 cytokines, IFNγ was elevated in γHV-68-infected mice compared with bleomycin plus mock infection (Fig. 10A), confirming earlier results in FITC and γHV-68-infected mice (23). Interestingly, levels of IFNγ were reduced in H1N1-infected mice, perhaps consistent with the observation that H1N1 replication is resolving at this time point. Levels of IL-13 (Fig. 10B) and IL-17 (Fig. 10C) were reduced in both groups of virally infected mice compared with the bleomycin control group, but only reached significance in γHV-68-infected animals. This is also consistent with the earlier observation that γHV-68 can augment fibrosis in the absence of Th2 cytokines (23). Previous studies have shown that aged mice, which are susceptible to γHV-68-induced fibrosis, have elevated levels of TGF-β receptors on lung fibroblasts (29). However, in the present studies, levels of TGFβR1 (Fig. 10D) were not different, and levels of TGFβRII (Fig. 10E) were elevated only in H1N1-infected mice when measured in the whole lung.
Fig. 10.
Differences in Th1, Th2, and Th17 and TGFβ receptors in whole lung do not explain the differential ability of γHV-68, but not H1N1, to exacerbate ECM deposition post-bleomycin. Mice were treated with bleomycin on day 0 and infected with saline, γHV-68, or H1N1 on day 14. On day 21, lungs were harvested and whole lung homogenates were analyzed by ELISA for IFNγ (A), IL-13 (B), and IL-17 (C); n = 5 for each group. In D and E, whole lung RNA was prepared and analyzed for expression of TGFβRI and II by real-time RT-PCR, n = 3–5 for each group; all representative of 2 experiments. *P < 0.05.
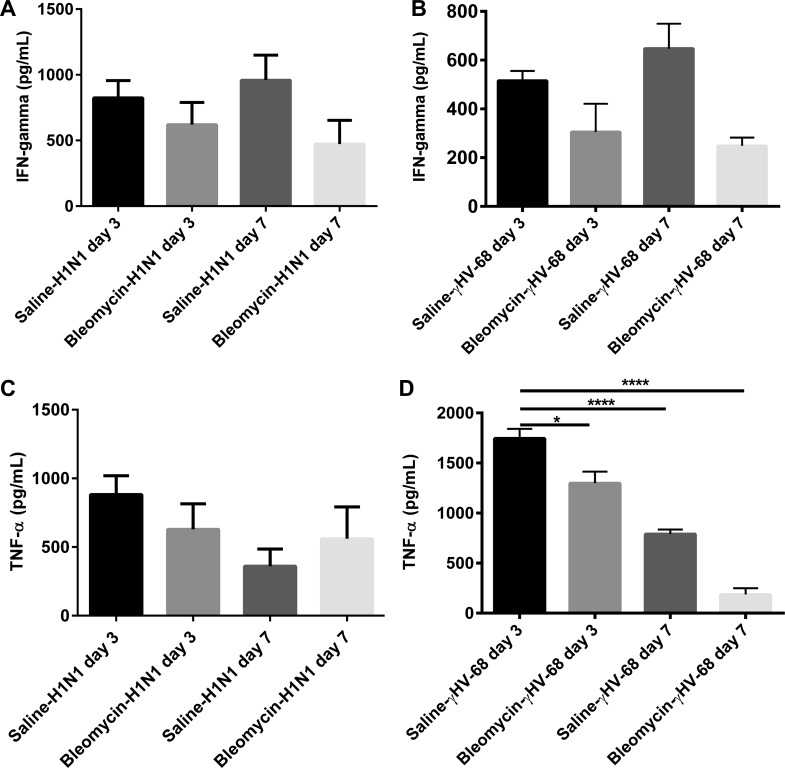
When we compared the induction of IFNγ or TNFα in mice treated with either virus alone compared with the amount made in response to viral infection post-bleomycin, we noted that pretreatment with bleomycin did not alter levels of either cytokine in response to H1N1 infection significantly. However, the ability of bleomycin-treated mice to produce TNFα was significantly inhibited post-γHV68 infection, and production of IFNγ tended to be lower (Fig. 11). These results may indicate a suboptimal antiviral response to γHV-68 in bleomycin-pretreated mice.
Fig. 11.
Bleomycin-treated mice show a defect in the production of TNFα to γHV-68 infection but not H1N1. Mice were treated with saline or bleomycin on day 0. On day 14 mice from each treatment group were infected with 50 PFU H1N1 or 5 × 104 PFU of γHV-68. On days 17 and 21 (days 3 and 7 postinfection), lungs were harvested and whole lung homogenates were analyzed by ELISA for IFNγ (A and B) and TNFα (C and D). Data shown are representative from one experiment; n = 3–4/mice per group. *P < 0.05, ****P < 0.0001.
Alveolar epithelial cells are more sensitive to TGFβ signaling and show evidence of apoptosis post-γHV-68 infection.
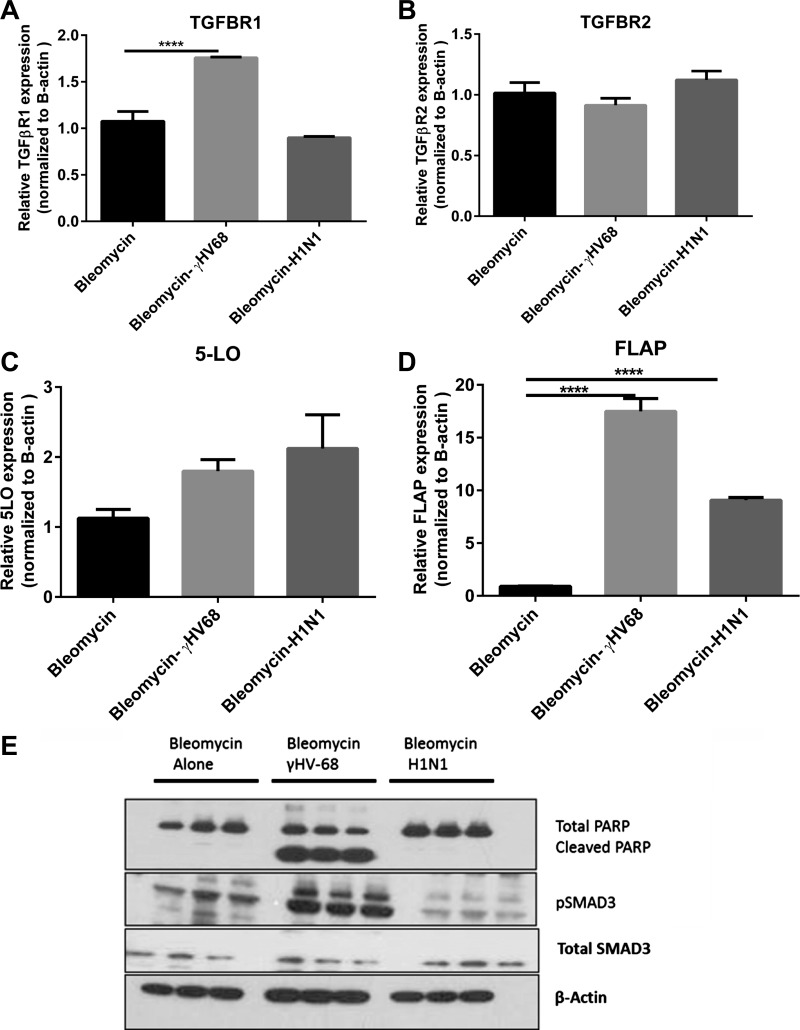
Human studies that have associated exacerbation of lung fibrosis with herpes virus have shown presence of virus in alveolar epithelial cells (AECs), and because latent infection of AECs with γHV-68 have demonstrated elevated production of cysteinyl leukotrienes (44), we isolated AECs from bleomycin + mock infection, bleomycin + H1N1, or bleomycin + γHV-68-infected mice on day 21 and analyzed them for expression of leukotriene synthetic enzymes and expression of TGFβ receptors and analyzed their protein lysates for evidence of SMAD3 phosphorylation and apoptosis via cleaved PARP (Fig. 12). Expression of leukotriene synthetic enzymes [5-lipoxygenase (5-LO) and 5-LO activating protein (FLAP)] were increased in response to both infections. However, only infection with γHV-68 resulted in increased expression of TGFβR1, increased evidence of SMAD3 phosphorylation, and increased evidence of apoptosis as noted by cleaved PARP.
Fig. 12.
Alveolar epithelial cells (AECs) from bleomycin and γHV-68-treated mice show increased sensitivity to TGFβ signaling, and increased evidence of leukotriene synthesis and apoptosis post-γHV-68 infection. Mice were treated with bleomycin on day 0. On day 14, some mice were infected with saline, H1N1, or γHV-68. Primary AECs were isolated from the lungs of all groups of mice on day 21. Total RNA was isolated and by real time RT-PCR we measured the expression of TGFβ receptor I/II (A, B), 5-lipoxygenase (5-LO; C), and 5-LO activating protein (FLAP; D). Phosphorylated SMAD3, total SMAD3, cleaved PARP, total PARP, and beta actin were detected by Western blotting (E). Data in all panels represent n = 3/group. ****P < 0.0001.
DISCUSSION
The cause of IPF is unknown, yet several lines of evidence have suggested that viral infections may play a role either as initiating or exacerbating agents. Mounting clinical evidence suggests that patients with IPF have T cells with low expression of CD28 (10, 13). This suggests chronic activation of T cells in IPF patients, potentially due to underlying and undiagnosed infections. Thus the goal of this study was to determine whether both bacterial and viral infections could exacerbate bleomycin-induced fibrosis.
Even though P. aeruginosa has the capability of infecting epithelial cells (33) and IPF patients tend to do poorly when they develop bacterial pneumonia (31), P. aeruginosa infection was effectively cleared from the lungs and did not exacerbate fibrosis. While our results suggest that this bacteria would not worsen fibrosis due to enhanced ECM deposition, there is caution in extrapolating these results to humans. The progressive nature of IPF is not modeled by bleomycin; thus, it is likely that in humans with chronic disease and more diminished lung capacity, a bacterial infection could be far more devastating, and certainly an influx of inflammatory cells might worsen dyspnea even if it does not alter ECM deposition.
Our results using bleomycin verified earlier results using FITC as a fibrotic stimulus (23) and demonstrated that γHV-68 could exacerbate ECM deposition post-bleomycin stimulus. The ability of γHV-68 to do this likely involves ability of the virus to reactivate from latency as the ΔORF72 mutant was not able to do this. Our unpublished observations suggest that the first 3 days of replication by ΔORF72 and γHV-68 are similar, but viral gene expression is significantly diminished by 7 days postinfection in ΔORF72-infected mice. Thus exacerbation of fibrosis likely requires on-going viral replication or spread within the AECs. These data are consistent with earlier studies showing that ongoing viral replication is necessary for fibrosis in Th2-biased mice as well (26). It may also be a unique feature of γHV-68 or perhaps of γ-herpes viruses in general since CMV (a β-herpes virus) did not enhance fibrosis. It should be noted that our experiments used the same dose of γHV-68 and CMV; however, C57Bl/6 mice are relatively resistant to CMV infection and we could not plaque infectious CMV from the lungs on day 7 postinfection (data not shown) whereas we could demonstrate infectious virus in γHV-68-infected mice (Fig. 5). We could demonstrate by RT-PCR that CMV viral RNA was expressed (data not shown), albeit at low levels. However, our earlier finding that murine adenovirus type 1 (MAV1) was also unable to exacerbate fibrosis following FITC challenge (23) and our current results with H1N1 suggest that this is not a feature of all viral infections that can infect AECs. Caution should be used when interpreting these data, however, since it is clear that the rates of infection are different for all these viruses.
When examining a variety of pro-and antifibrotic mediators and the composition of the inflammatory cell influx that followed both infections, no notable differences could explain the discrepancy between the ability of γHV-68, but not H1N1 to exacerbate fibrosis. This prompted us to look at changes that might be specific to AECs, the initial site of infection, and viral replication within the lung. We have observed that γHV-68 can replicate in AECs in culture without lytic destruction of all the cells. In contrast, H1N1 may be more likely to ultimately destroy all infected epithelial cells. Our results in vivo suggest that γHV-68-infected mice have AECs that are more responsive to TGFβ signaling and show signs of apoptosis. This is consistent with earlier work showing that γHV-68 infection in aged mice is associated with AEC apoptosis and ER stress (39). One caveat was that our analyses of AECs were done at day 7 postinfection, a time point when H1N1 replication was diminished, but γHV-68 replication was on-going. It is possible that the prolonged replication of γHV-68 at 7 dpi may cause more epithelial stress. We have previously demonstrated that AECs isolated from mice with latent γHV-68 infection overproduce profibrotic factors such as TGFβ and cysteinyl leukotrienes (37, 44). Our current data confirm that infection with γHV-68 and H1N1 both significantly upregulate FLAP in AECs, enzymatic machinery necessary for cysteinyl leukotriene synthesis; however, induction of FLAP was highest with γHV-68. Because cysteinyl leukotrienes can promote fibrocyte proliferation (45) and activation of resident lung fibroblasts (5, 30), this could promote ECM deposition post-viral infection. When we looked for evidence of TGFβ signaling in AECs, we observed elevated TGFβR1 in AECs from γHV-68-infected mice. This resulted in increased evidence of TGFβ activation of these cells at this time point as demonstrated by increased phospho-SMAD3 expression. Ultimately, we believe these AECs may be undergoing apoptosis in vivo as there was evidence of cleaved PARP, a marker of apoptosis. Thus we speculate that the ability of γHV-68 to undergo persistent rounds of reactivation and an enhanced sensitivity of the infected AECs to respond to TGFβ leads to ongoing apoptosis in AECs along with induction of profibrotic factors such as cysteinyl leukotrienes, which ultimately promote ECM deposition in these mice. Because H1N1 has also been shown to induce apoptotic machinery in AECs as a way to promote viral replication (46), it is not clear why our results differ with these two viral infections. These results may merely reflect the doses of virus used, the extent of ultimate damage to the AECs, alterations in the ability to repair damaged epithelium following each infection or additional signaling cascades induced by the distinct viruses that we have not yet identified.
While our results in AECs highlight cell-specific increases in susceptibility to TGFβ signaling post-γHV-68 infection, we did not observe differences in total or active TGFβ in the lungs of bleomycin-treated mice infected with γHV-68 or H1N1. As this cytokine is often activated locally on the cell surface, it is likely that measurements in lung homogenates do not accurately reflect levels available during cell-cell communication. However, we were surprised that levels of TGFβRII were actually elevated in H1N1-infected mice within the whole lung. One caveat of these interpretations however is that receptor expression levels were measured in whole lungs, not in isolated fibroblasts. As TGFβ receptors can be expressed on numerous cell types, it is not clear what cells may be overexpressing TGFβRII in H1N1-infected mice. Because TGFβRII can interact with various other cellular proteins such as cyclin B2 (18) endoglin (CD105) (4) or TGFβRIII (12), this may result in differential cell activation of some cell types in the H1N1-infected mice that may further explain the discrepancies between outcomes with H1N1 vs. γHV-68.
Finally, the differences in cell types that are readily infected by each virus may play an additional role. We have demonstrated that γHV-68 is readily found as both lytic and latent infection in lung AECs, fibroblasts, macrophages, and B cells (37). However, H1N1 tends to restrict replication predominantly to the epithelial cells within the lung (48) and in our hands does not replicate well in macrophages. It is interesting that one study has suggested that H1N1 can replicate more effectively in type II AECs from IPF patients (7). Additionally, a recent case report noted acute exacerbation of IPF following H1N1 vaccination (41). Thus, as mentioned before, bleomycin may not be effectively modeling all the epithelial changes noted in patients with IPF. It should also be noted that while H1N1 infection at the doses used in this study do not appear to worsen ECM deposition, that is not to say that H1N1 infection is not detrimental to fibrotic lungs. At higher doses of H1N1 (500 PFU), bleomycin-treated mice were highly susceptible to rapid death, most likely from acute lung injury (data not shown). However, this dose was also lethal in some control mice.
In summary, γHV-68 is able to exacerbate bleomycin-induced fibrosis or FITC-induced fibrosis (23) and stimulate collagen deposition. Infection with P. aeruginosa, H1N1, and CMV did not exacerbate bleomycin-induced fibrosis at the doses tested in our studies. The difference in the ability of γHV-68, but not the other pathogens tested, to exacerbate collagen deposition requires the ability of γHV-68 to undergo reactivation from latency as demonstrated by our experiments with the ΔORF72 mutant virus. Additionally, we have demonstrated evidence of enhanced sensitivity to TGFβ signaling in AECs from γHV-68-infected mice, likely leading to enhanced profibrotic release of cysteinyl leukotrienes, AEC stress, and apoptosis.
GRANTS
This work was supported by National Institutes of Health Grants HL-115618 (B. B. Moore) and T32-AI-007413 (S. L. Ashley).
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.L.A., Y.J., and T.A.M. performed experiments; S.L.A., Y.J., T.A.M., and B.B.M. analyzed data; S.L.A., Y.J., and B.B.M. interpreted results of experiments; S.L.A. prepared figures; S.L.A. drafted manuscript; S.L.A. and B.B.M. edited and revised manuscript; S.L.A., Y.J., T.A.M., L.F.v.D., Y.L., and B.B.M. approved final version of manuscript; L.F.v.D., Y.L., and B.B.M. conception and design of research.
ACKNOWLEDGMENTS
We thank Carol Wilke for technical help and advice in many experiments.
Work performed by Y. Jegal occurred at the University of Michigan during a sabbatical year. We thank Carol Wilke for technical help and advice in many experiments.
REFERENCES
- 1.Adkins JM, Collard HR. Idiopathic pulmonary fibrosis. Semin Respir Crit Care Med 33: 433–439, 2012 [DOI] [PubMed] [Google Scholar]
- 2.Ballinger MN, Aronoff DM, McMillan TR, Cooke KR, Olkiewicz K, Toews GB, Peters-Golden M, Moore BB. Critical role of prostaglandin E2 overproduction in impaired pulmonary host response following bone marrow transplantation. J Immunol 177: 5499–5508, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature 392: 245–252, 1998 [DOI] [PubMed] [Google Scholar]
- 4.Barbara NP, Wrana JL, Letarte M. Endoglin is an accessory protein that interacts with the signaling receptor complex of multiple members of the transforming growth factor-beta superfamily. J Biol Chem 274: 584–594, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Baud L, Perez J, Denis M, Ardaillou R. Modulation of fibroblast proliferation by sulfidopeptide leukotrienes: effect of indomethacin. J Immunol 138: 1190–1195, 1987 [PubMed] [Google Scholar]
- 6.Efstathiou S, Ho YM, Hall S, Styles CJ, Scott SD, Gompels UA. Murine herpesvirus 68 is genetically related to the gammaherpesviruses Epstein-Barr virus and herpesvirus saimiri. J Gen Virol 71: 1365–1372, 1990 [DOI] [PubMed] [Google Scholar]
- 7.Fujino N, Kubo H, Ota C, Suzuki T, Takahashi T, Yamada M, Suzuki S, Kondo T, Nagatomi R, Tando Y, Yamaya M. Increased severity of 2009 pandemic influenza A virus subtype H1N1 infection in alveolar type II cells from patients with pulmonary fibrosis. J Infect Dis 207: 692–693, 2013 [DOI] [PubMed] [Google Scholar]
- 8.Gangadharan B, Hoeve MA, Allen JE, Ebrahimi B, Rhind SM, Dutia BM, Nash AA. Murine gammaherpesvirus-induced fibrosis is associated with the development of alternatively activated macrophages. J Leukoc Biol 84: 50–58, 2008 [DOI] [PubMed] [Google Scholar]
- 9.GeurtsvanKessel CH, Willart MA, van Rijt LS, Muskens F, Kool M, Baas C, Thielemans K, Bennett C, Clausen BE, Hoogsteden HC, Osterhaus AD, Rimmelzwaan GF, Lambrecht BN. Clearance of influenza virus from the lung depends on migratory langerin+CD11b- but not plasmacytoid dendritic cells. J Exp Med 205: 1621–1634, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilani SR, Vuga LJ, Lindell KO, Gibson KF, Xue J, Kaminski N, Valentine VG, Lindsay EK, George MP, Steele C, Duncan SR. CD28 down-regulation on circulating CD4 T-cells is associated with poor prognoses of patients with idiopathic pulmonary fibrosis. PLos One 5: e8959, 2010. 10.1371/journal.pone.0008959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harari S, Caminati A. IPF: new insight on pathogenesis and treatment. Allergy 65: 537–553, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Henis YI, Moustakas A, Lin HY, Lodish HF. The types II and III transforming growth factor-beta receptors form homo-oligomers. J Cell Biol 126: 139–154, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herazo-Maya JD, Noth I, Duncan SR, Kim S, Ma SF, Tseng GC, Feingold E, Juan-Guardela BM, Richards TJ, Lussier Y, Huang Y, Vij R, Lindell KO, Xue J, Gibson KF, Shapiro SD, Garcia JG, Kaminski N. Peripheral blood mononuclear cell gene expression profiles predict poor outcome in idiopathic pulmonary fibrosis. Sci Transl Med 5: 205ra136, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hubbard LL, Ballinger MN, Thomas PE, Wilke CA, Standiford TJ, Kobayashi KS, Flavell RA, Moore BB. A role for IL-1 receptor-associated kinase-M in prostaglandin E2-induced immunosuppression post-bone marrow transplantation. J Immunol 184: 6299–6308, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kelly BG, Lok SS, Hasleton PS, Egan JJ, Stewart JP. A rearranged form of Epstein-Barr virus DNA is associated with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 166: 510–513, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Kumagai Y, Takeuchi O, Kato H, Kumar H, Matsui K, Morii E, Aozasa K, Kawai T, Akira S. Alveolar macrophages are the primary interferon-alpha producer in pulmonary infection with RNA viruses. Immunity 27: 240–252, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Lasithiotaki I, Antoniou KM, Vlahava VM, Karagiannis K, Spandidos DA, Siafakas NM, Sourvinos G. Detection of herpes simplex virus type-1 in patients with fibrotic lung diseases. PLos One 6: e27800, 2011. 10.21371/journal.pone.0027800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu JH, Wei S, Burnette PK, Gamero AM, Hutton M, Djeu JY. Functional association of TGF-beta receptor II with cyclin B. Oncogene 18: 269–275, 1999 [DOI] [PubMed] [Google Scholar]
- 19.Lok SS, Haider Y, Howell D, Stewart JP, Hasleton PS, Egan JJ. Murine gammaherpes virus as a cofactor in the development of pulmonary fibrosis in bleomycin resistant mice. Eur Respir J 20: 1228–1232, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Lovewell RR, Patankar YR, Berwin B. Mechanisms of phagocytosis and host clearance of Pseudomonas aeruginosa. Am J Physiol Lung Cell Mol Physiol 306: L591–L603, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mancuso P, Standiford TJ, Marshall T, Peters-Golden M. 5-Lipoxygenase reaction products modulate alveolar macrophage phagocytosis of Klebsiella pneumoniae. Infect Immun 66: 5140–5146, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McGill J, Heusel JW, Legge KL. Innate immune control and regulation of influenza virus infections. J Leukoc Biol 86: 803–812, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McMillan TR, Moore BB, Weinberg JB, Vannella KM, Fields WB, Christensen PJ, van Dyk LF, Toews GB. Exacerbation of established pulmonary fibrosis in a murine model by gammaherpesvirus. Am J Respir Crit Care Med 177: 771–780, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moore BB, Paine R, 3rd, Christensen PJ, Moore TA, Sitterding S, Ngan R, Wilke CA, Kuziel WA, Toews GB. Protection from pulmonary fibrosis in the absence of CCR2 signaling. J Immunol 167: 4368–4377, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Mora AL, Torres-Gonzalez E, Rojas M, Corredor C, Ritzenthaler J, Xu J, Roman J, Brigham K, Stecenko A. Activation of alveolar macrophages via the alternative pathway in herpesvirus-induced lung fibrosis. Am J Respir Cell Mol Biol 35: 466–473, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mora AL, Torres-Gonzalez E, Rojas M, Xu J, Ritzenthaler J, Speck SH, Roman J, Brigham K, Stecenko A. Control of virus reactivation arrests pulmonary herpesvirus-induced fibrosis in IFN-gamma receptor-deficient mice. Am J Respir Crit Care Med 175: 1139–1150, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mora AL, Woods CR, Garcia A, Xu J, Rojas M, Speck SH, Roman J, Brigham KL, Stecenko AA. Lung infection with gamma-herpesvirus induces progressive pulmonary fibrosis in Th2-biased mice. Am J Physiol Lung Cell Mol Physiol 289: L711–L721, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Naik PK, Moore BB. Viral infection and aging as cofactors for the development of pulmonary fibrosis. Expert Rev Respir Med 4: 759–771, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naik PN, Horowitz JC, Moore TA, Wilke CA, Toews GB, Moore BB. Pulmonary fibrosis induced by gamma-herpesvirus in aged mice is associated with increased fibroblast responsiveness to transforming growth factor-beta. J Gerontol A Biol Sci Med Sci 67: 714–725, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Phan SH, McGarry BM, Loeffler KM, Kunkel SL. Binding of leukotriene C4 to rat lung fibroblasts and stimulation of collagen synthesis in vitro. Biochemistry 27: 2846–2853, 1988 [DOI] [PubMed] [Google Scholar]
- 31.Rangappa P, Moran JL. Outcomes of patients admitted to the intensive care unit with idiopathic pulmonary fibrosis. Crit Care Resusc 11: 102–109, 2009 [PubMed] [Google Scholar]
- 32.Sanders CJ, Doherty PC, Thomas PG. Respiratory epithelial cells in innate immunity to influenza virus infection. Cell Tissue Res 343: 13–21, 2011 [DOI] [PubMed] [Google Scholar]
- 33.Schmiedl A, Kerber-Momot T, Munder A, Pabst R, Tschernig T. Bacterial distribution in lung parenchyma early after pulmonary infection with Pseudomonas aeruginosa. Cell Tissue Res 342: 67–73, 2010. 10.1007/s00441-00010-01036-y [DOI] [PubMed] [Google Scholar]
- 34.Sparks-Thissen RL, Braaten DC, Hildner K, Murphy TL, Murphy KM, Virgin HWt. CD4 T cell control of acute and latent murine gammaherpesvirus infection requires IFNgamma. Virology 338: 201–208, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Stewart JP, Egan JJ, Ross AJ, Kelly BG, Lok SS, Hasleton PS, Woodcock AA. The detection of Epstein-Barr virus DNA in lung tissue from patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 159: 1336–1341, 1999 [DOI] [PubMed] [Google Scholar]
- 36.Stewart JP, Usherwood EJ, Ross A, Dyson H, Nash T. Lung epithelial cells are a major site of murine gammaherpesvirus persistence. J Exp Med 187: 1941–1951, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stoolman JS, Vannella KM, Coomes SM, Wilke CA, Sisson TH, Toews GB, Moore BB. Latent infection by gammaherpesvirus stimulates profibrotic mediator release from multiple cell types. Am J Physiol Lung Cell Mol Physiol 300: L274–L285, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tang YW, Johnson JE, Browning PJ, Cruz-Gervis RA, Davis A, Graham BS, Brigham KL, Oates JA, Jr, Loyd JE, Stecenko AA. Herpesvirus DNA is consistently detected in lungs of patients with idiopathic pulmonary fibrosis. J Clin Microbiol 41: 2633–2640, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Torres-Gonzalez E, Bueno M, Tanaka A, Krug LT, Cheng DS, Polosukhin VV, Sorescu D, Lawson WE, Blackwell TS, Rojas M, Mora AL. Role of endoplasmic reticulum stress in age-related susceptibility to lung fibrosis. Am J Respir Cell Mol Biol 46: 748–756, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsai CY, Hu Z, Zhang W, Usherwood EJ. Strain-dependent requirement for IFN-gamma for respiratory control and immunotherapy in murine gammaherpesvirus infection. Viral Immunol 24: 273–280, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Umeda Y, Morikawa M, Anzai M, Sumida Y, Kadowaki M, Ameshima S, Ishizaki T. Acute exacerbation of idiopathic pulmonary fibrosis after pandemic influenza A (H1N1) vaccination. Intern Med 49: 2333–2336, 2010 [DOI] [PubMed] [Google Scholar]
- 42.Van Delden C, Iglewski BH. Cell-to-cell signaling and Pseudomonas aeruginosa infections. Emerg Infect Dis 4: 551–560, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Dyk LF, Virgin HWt, Speck SH. The murine gammaherpesvirus 68 v-cyclin is a critical regulator of reactivation from latency. J Virol 74: 7451–7461, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vannella KM, Luckhardt TR, Wilke CA, van Dyk LF, Toews GB, Moore BB. Latent herpesvirus infection augments experimental pulmonary fibrosis. Am J Respir Crit Care Med 181: 465–477, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vannella KM, McMillan TR, Charbeneau RP, Wilke CA, Thomas PE, Toews GB, Peters-Golden M, Moore BB. Cysteinyl leukotrienes are autocrine and paracrine regulators of fibrocyte function. J Immunol 179: 7883–7890, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang X, Tan J, Zoueva O, Zhao J, Ye Z, Hewlett I. Novel pandemic influenza A (H1N1) virus infection modulates apoptotic pathways that impact its replication in A549 cells. Microbes Infect 16: 178–186, 2014 [DOI] [PubMed] [Google Scholar]
- 47.Wangoo A, Shaw RJ, Diss TC, Farrell PJ, du Bois RM, Nicholson AG. Cryptogenic fibrosing alveolitis: lack of association with Epstein-Barr virus infection. Thorax 52: 888–891, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weinheimer VK, Becher A, Tonnies M, Holland G, Knepper J, Bauer TT, Schneider P, Neudecker J, Ruckert JC, Szymanski K, Temmesfeld-Wollbrueck B, Gruber AD, Bannert N, Suttorp N, Hippenstiel S, Wolff T, Hocke AC. Influenza A viruses target type II pneumocytes in the human lung. J Infect Dis 206: 1685–1694, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weslow-Schmidt JL, Jewell NA, Mertz SE, Simas JP, Durbin JE, Flano E. Type I interferon inhibition and dendritic cell activation during gammaherpesvirus respiratory infection. J Virol 81: 9778–9789, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wilson MS, Wynn TA. Pulmonary fibrosis: pathogenesis, etiology and regulation. Mucosal Immunol 2: 103–121, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zamo A, Poletti V, Reghellin D, Montagna L, Pedron S, Piccoli P, Chilosi M. HHV-8 and EBV are not commonly found in idiopathic pulmonary fibrosis. Sarcoidosis Vasc Diffuse Lung Dis 22: 123–128, 2005 [PubMed] [Google Scholar]