Abstract
Severe bronchospasm refractory to β-agonists continues to cause significant morbidity and mortality in asthmatic patients. We questioned whether chloride channels/transporters are novel targets for the relaxation of airway smooth muscle (ASM). We have screened a library of compounds, derivatives of anthranilic and indanyloxyacetic acid, that were originally developed to antagonize chloride channels in the kidney. We hypothesized that members of this library would be novel calcium-activated chloride channel blockers for the airway. The initial screen of this compound library identified 4 of 20 compounds that relaxed a tetraethylammonium chloride-induced contraction in guinea pig tracheal rings. The two most effective compounds, compounds 1 and 13, were further studied for their potential to either prevent the initiation of or relax the maintenance phase of an acetylcholine (ACh)-induced contraction or to potentiate β-agonist-mediated relaxation. Both relaxed an established ACh-induced contraction in human and guinea pig ex vivo ASM. In contrast, the prevention of an ACh-induced contraction required copretreatment with the sodium-potassium-chloride cotransporter blocker bumetanide. The combination of compound 13 and bumetanide also potentiated relaxation by the β-agonist isoproterenol in guinea pig tracheal rings. Compounds 1 and 13 hyperpolarized the plasma cell membrane of human ASM cells and blocked spontaneous transient inward currents, a measure of chloride currents in these cells. These functional and electrophysiological data suggest that modulating ASM chloride flux is a novel therapeutic target in asthma and other bronchoconstrictive diseases.
Keywords: organ bath, niflumic acid, electrophysiology, NKCC, bumetanide
the worldwide prevalence of asthma is between 7 and 10% and it is implicated in 1 of 250 deaths (21). The pathogenesis of asthma involves chronic airway inflammation, increased mucus secretion, irritability of airway parasympathetic nerves, and hypertrophy and hyperresponsiveness of airway smooth muscle (ASM). Relaxation of ASM remains an important goal in the acute treatment of bronchospasm, although no new classes of drugs have been developed in the past few decades. There is a need for novel therapeutics to treat acute bronchospasm and control severe asthma because many patients are resistant to current therapies.
Severe asthma was defined by a task force supported by the European Respiratory Society and American Thoracic Society as “asthma which requires treatment with high dose inhaled corticosteroids plus a second controller and/or systemic corticosteroids to prevent it from becoming uncontrolled or which remains uncontrolled despite this therapy” (4). Severe asthma accounts for only 5–10% of asthmatic patients but accounts for a large portion of health care costs and morbidity associated with asthma (23). Uncontrolled asthma is defined by the Global Initiative for Asthma on the basis of six characteristics including daytime symptoms, limitations of activities, nocturnal symptoms, limitations of activities, need for rescue treatment, lung function, and exacerbations in the past month. By these guidelines, 43.7% of patients were found to have uncontrolled asthma, despite treatment of 78.5% with an inhaled corticosteroid and β-agonist (22). β-Agonists remain first-line therapy in the treatment of an acute asthma exacerbation, but chronic overuse leads to tachyphylaxis. New drugs that relieve ASM contraction acutely and also work synergistically with β-agonists would be an excellent addition to currently available therapies.
Chloride channels are a potential novel target on ASM. Chloride currents can contribute to ASM contraction, as activation leads to chloride efflux from the cell, which leads to depolarization of the membrane that further triggers a voltage-dependent calcium influx and further depolarization, which is a component of contraction (15). In addition to their potential role on the plasma membrane, chloride channels may also modulate calcium release from internal stores [i.e., sarcoplasmic reticulum (SR)] as necessary ions to maintain a neutral charge across the SR membrane with calcium flux (10). Chloride channel modulators have shown promise as a potential novel class of therapeutics to relax ASM (29). Modulation of the ligand-gated GABAA (6) and glycine channel (31), calcium-activated chloride channels (CaCCs) (11, 32), and the sodium-potassium-chloride cotransporter (NKCC) (2, 12, 28) have all been shown to be important in ASM relaxation. Thus CaCCs and NKCC are two potential targets on ASM that control cellular chloride flux.
Chloride channels are ubiquitous, and blockers are currently being investigated as possible therapeutic targets in vascular smooth muscle for hypertension, kidneys for diuretics, and gastrointestinal smooth muscle for diarrhea and as possible antitumor agents in cancer therapy (30). We have screened a library of 20 compounds, derivatives of anthranilic and indanyloxyacetic acid, that were previously developed as chloride channel antagonists in the kidney and trachea (20). We hypothesized that members of this library would be novel chloride channel blockers that facilitate ASM relaxation and that further analysis of the structural and mechanistic differences could aid in novel bronchodilator drug development.
METHODS
Materials.
All materials were obtained from Sigma-Aldrich (St. Louis, MO) unless otherwise specified. Tetrodotoxin was purchased from Calbiochem, EMD Biosciences (La Jolla, CA). MK571 was purchased from Tocris (Minneapolis, MN). Membrane potential dye [fluorescent imaging plate reader (FLIPR) blue reagent] was obtained from Molecular Devices (Sunnyvale, CA).
Guinea pig tracheal rings.
Animal protocols were approved by the Columbia University Animal Care and Use Committee. Male Hartley guinea pigs weighing ∼400 g were anesthetized with intraperitoneal injection of pentobarbital sodium (100 mg/kg). Trachea were removed and transferred to ice-cold Krebs-Henseleit (KH) buffer of the following composition (in mM): NaCl 118, KCl 5.6, CaCl2 0.5, MgSO4 0.24, NaH2PO4 1.3, NaHCO3 25, glucose 5.6, pH 7.4. Epithelial-denuded tracheal rings were prepared with use of a dissecting microscope. Epithelium was removed by gently passing cotton fibers through the lumen of the tracheal rings. The rings were suspended in 4-ml water jacketed organ baths (37°C: Radnoti Glass Technology, Monrovia, CA) and connected to a Grass FT03 force transducer (Grass Telefactor, West Warwick, RI) with silk sutures. The transducers were coupled to the computer with BioPac hardware and data were collected by using Acqknowledge 7.3.3 software (BioPac Systems, Goleta, CA). KH buffer was bubbled with a gas mixture of 95% oxygen and 5% carbon dioxide, and buffer was exchanged every 15 min for 1 h during equilibration of tracheal rings at 1 g resting tension. Unless specified otherwise in the results, all baths received 10−5 M indomethacin to block the effects of endogenous prostanoids, 10−5 M N-vanillylnonanamide (capsaicin analog) to activate and subsequently block the effects of nonadrenergic, noncholinergic nerves, 10−6 M tetrodotoxin to block neuronal effects, and 10−5 M of pyrilamine to block effects of endogenous histamine release. Preliminary contractile challenges of each ring consisted of two cycles of acetylcholine (ACh) dose responses (100 nM–100 μM) to define the EC50 of acetylcholine for each tracheal ring.
Initial studies sought to determine whether an established contraction could be relaxed by these novel chloride channel blockers. Niflumic acid (NFA) and 5-nitro-2-(3-phenylpropylamino) benzoic acid (NPPB) are known chloride channel blockers and were used as positive controls. Tissue was contracted with either 10 mM tetraethylammonium chloride (TEA), 80 mM potassium gluconate, or an EC50 dose of ACh. When a plateau in contractile force was achieved, 100 μM of 5-chloro-2-((4-chlorobenzyl) amino) benzoic acid (compound 1), 5-chloro-2-(1-naphthyl methylamino) benzoic acid (compound 13), NFA, NPPB, or vehicles (0.1% DMSO or 0.1% ethanol) were added to separate organ baths as indicated in the figures. We chose 100 μM of compound 1 or compound 13 since this concentration of the classic chloride channel blockers NFA and NPPB have previously been shown to be effective at this concentration in an organ bath model (10). In cellular assays, the IC50 of NFA and NPPB has been reported to be up to 140 and 150 μM, respectively (11). For contractions induced by ACh, the ability of chloride channel blockers alone or in combination with bumetanide (Na+-K+-Cl− cotransporter inhibitor) to relax an established contraction was evaluated. The remaining amount of contractile force present at 30 min was expressed as the percent of initial force immediately before compound addition.
Separate studies were performed to determine whether pretreatment with these chloride channel blockers alone or in combination with blockade of the Na+-K+-Cl− cotransporter with bumetanide could attenuate the magnitude of an induced ACh contraction. Rings were pretreated for 15 min with 100 μM of compound 1, compound 13, or NPPB alone or in combination with 10 μM bumetanide before challenge with an EC50 concentration of ACh. The magnitude of the contraction was compared with an identical EC50 contraction with ACh that was performed before the pretreatment in each tracheal ring.
Separate studies were performed to determine whether the combined treatment with compound 13 and bumetanide could potentiate a β-agonist-induced relaxation of an ACh-induced contraction. Rings were contracted with an EC50 concentration of ACh and relaxation was induced with ½ log increments of isoproterenol (0.1 nM–10 μM) in the presence of vehicle (0.1% DMSO-0.1% ethanol) or 50 μM compound 13/10 μM bumetanide added simultaneously with the 0.5 nM concentration of isoproterenol.
Human tracheal strips.
Studies using deidentified human tissue were reviewed by the Columbia University Institutional Review Board and deemed not human subjects research. Tissues were obtained from healthy, normal organ donor discarded surgical waste subsequent to lung transplant surgery. Tissues were collected after transplant and placed in DMEM media (GIBCO, Grand Island, NY) and bubbled overnight in 5% carbon dioxide and 95% oxygen at 4°C. The following morning, the smooth muscle tissue was carefully dissected under a dissecting microscope and the epithelium was removed. The strips were anchored in the organ bath as described above by using KH buffer of the same composition. The strips were allowed to equilibrate for 1 h with buffer exchanges every 15 min and were treated with 10−6 M tetrodotoxin, 10−5 M pyrilamine, and 10−5 M MK571. Preliminary contractile challenges of each human ASM strip consisted of two cycles of ACh dose responses (100 nM–1 mM) to define the EC50 of ACh for each strip. Studies in human ASM sought to determine whether an established contraction could be relaxed by these novel chloride channel blockers alone or in combination with Na+-K+-Cl− cotransporter inhibition. Tissue was contracted with an EC50 dose of ACh. When a plateau in contractile force was achieved, 100 μM of compound 1, 100 μM compound 13, or 10 μM bumetanide, alone or in combination, or appropriate vehicles (0.1% DMSO or 0.1% ethanol) were added to separate organ baths as indicated in the figures. The remaining amount of contractile force present at 30 min was expressed as the percent of initial force immediately before compound addition.
Cultured human airway smooth muscle cells.
Immortalized human ASM cell lines modified to stably express human telomerase reverse transcriptase were a kind gift from Dr. William Gerthoffer (University of South Alabama, Mobile, AL) prepared as described previously (7) and grown in Dulbecco's modified Eagle's medium/F12 media (GIBCO), with 10% FBS and antibiotics.
FLIPR MEMBRANE POTENTIOMETRIC DYE ASSAY.
Immortalized human ASM cells were cultured on black-walled 96-well plates to 100% confluence and were washed four times with warmed (37°C) assay buffer of the following composition (in mM): NaCl 140, KCl 4.7, CaCl2 2.5, MgCl2 1.2, HEPES 11, d-glucose 10. A stock solution (100% dye) of FLIPR blue dye was prepared by reconstitution of 1 vial (125 mg) with 100 ml of assay buffer. A 50% working stock was prepared by further diluting the reconstituted blue dye 1:1 with assay buffer and was used to load cells (90 μl/well) over 45 min at 37°C in a humidified cell culture incubator (95% air-5% CO2). All reagents were dissolved in assay buffer. Fluorescence was measured with a FlexStation 3 (Molecular Devices) with an excitation wavelength of 530 nm, emission wavelength of 565 nm, and a cutoff filter of 550 nm. Baseline fluorescence was measured every 2 s for 1 min, then for 2 min after injection of the vehicle or compound of interest as indicated (by using the Flex-Station 3's integrated injection capabilities), and the maximal change in fluorescence was recorded.
PATCH-CLAMP ANALYSIS.
Cells were plated on poly-l-lysine 12-mm coverslips (BD) coated with 0.5 mg/ml collagen type I. The coverslips were transferred to 0.5-ml chambers on the stage of an inverted microscope (Nikon). Membrane currents were recorded with a tight-seal conventional whole-cell configuration. The extracellular solution contained (in mM) 130 NaCl, 5.5 TEA-Cl, 2.2 CaCl2, 1 MgCl2, 10 HEPES, 10 glucose, pH adjusted to 7.4 with NaOH. The pipette solution contained (in mM) 75 CsCl, 64 Cs-gluconate, 1 MgCl2, 10 HEPES, 3 Na2ATP, pH adjusted to pH 7.3 with CsOH (1). Cesium and TEA were used in intracellular and extracellular solutions, respectively, to block K+ channels to allow for the study of chloride channel activity. Whole-cell currents were recorded via Axopatch 200B and digitized via 1322A. Patch pipettes had resistances of 3–6 MΩ. All recordings were performed at room temperature. Events were counted as spontaneous transient inward currents (STICs) if their amplitude exceeded twofold of the baseline as detected with pCLAMP10 and analysis by Origin 8 software.
STATISTICS.
Data were analyzed by one-way ANOVA with repeated measures. The Bonferroni correction was applied for multiple comparisons. Statistical significance was established at P < 0.05, and all values are expressed as means ± SE. For organ bath experiments, n refers to the number of guinea pig tracheal rings or human ASM strips. For cellular assays, n refers to a well of a 96-well plate for FLIPR and a single cell for patch-clamp analysis.
RESULTS
Chloride channel blockers relax an established TEA-induced contraction.
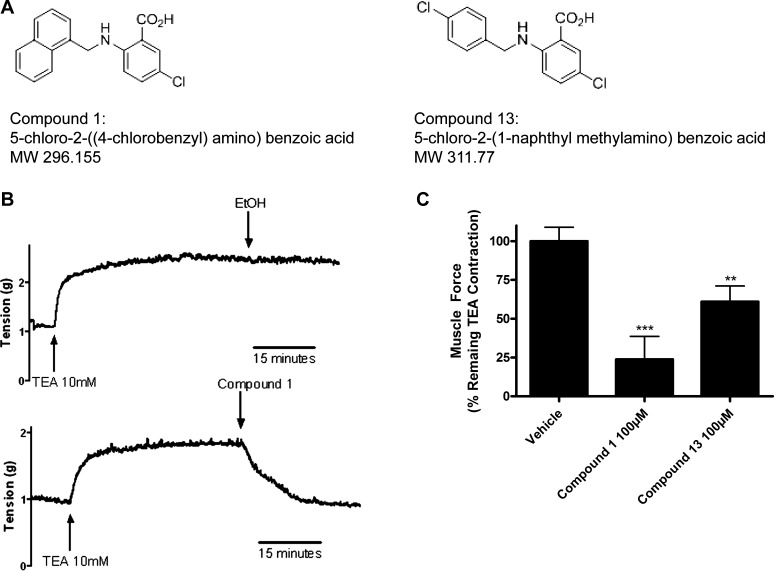
Chloride channel blockers were initially tested for their ability to reverse a depolarized induced contraction in guinea pig ASM, since chloride flux has been shown to be important in depolarization (16). All 20 compounds of the chloride channel blocker library were screened against a contraction induced by 10 mM TEA, a potassium channel blocker that causes a depolarization of ASM by blocking potassium efflux. The anthranilic acid derivatives compound 1 and compound 13 (Fig. 1A) relaxed a TEA-induced contraction in guinea pig tracheal rings (Fig. 1B), whereas derivatives of indanyloxyacetic acid failed to relax TEA-contracted guinea pig ASM (data not shown). Of the 20 chloride channel blockers screened, compounds 1 and 13 most potently relaxed a TEA-induced contraction and were therefore chosen for further study. Compound 1 (100 μM) relaxed 76.2% and compound 13 (100 μM) relaxed 39.0% of a TEA-induced contraction at 15 min after drug addition (P < 0.001 and P < 0.01; n = 9, respectively) (Fig. 1C).
Fig. 1.
Chloride channel blockers relax a tetraethylammonium chloride (TEA) contraction in guinea pig tracheal rings. A: structures of compounds 1 and 13. B: representative tracing of muscle force recordings in organ bath with guinea pig tracheal rings contracted with 10 mM TEA. Rings were treated with vehicle or compounds. Compound 1 completely relaxed TEA contraction within 15 min. C: 100 μM compound 1 and compound 13 relaxed a TEA contraction (23.8 ± 14.9, 61.0 ± 10.1, respectively, calculated as percent of remaining TEA contraction at 15 min). **P < 0.01, ***P < 0.001 compared with vehicle; n = 6–9.
Chloride channel blockers relax an established K-gluconate contraction.
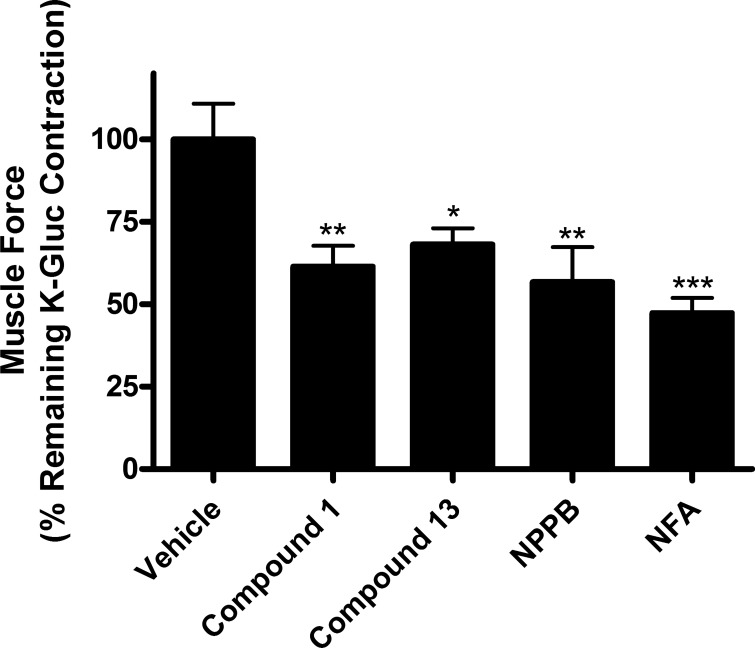
Many chloride channel blockers are known to also stimulate potassium channels (8). Thus we performed additional studies in the presence of a large extracellular concentration of potassium to ensure that our findings of compound 1 or 13 inhibiting a TEA-induced depolarizing contraction were not due to these compounds opening a potassium channel. Relaxation of ASM by either of the chloride channel blockers in the presence of a large potassium gradient (80 mM K-gluconate), which is unfavorable for K+ efflux, would suggest that neither compound 1 nor 13 was inducing relaxation of depolarization-induced contractions by opening of a potassium channel.
Both compounds 1 and 13 (100 μM) relaxed an 80 mM potassium gluconate-induced contraction [61.46% ± 6.3 (P < 0.01; n = 6), 68.17% ± 4.9 (P < 0.05; n = 6), respectively, percent of potassium gluconate-induced contraction at 30 min] (Fig. 2). Classic chloride channel blockers NPPB (100 μM) and NFA (100 μM) were used as positive controls and also showed significant relaxation [56.71% ± 10.6 (P < 0.01; n = 4), 47.36% ± 4.6 (P < 0.001; n = 4), respectively, percent of potassium gluconate-induced contraction at 30 min]. The ability of compounds 1 and 13 to functionally mimic classic chloride channel blockers is consistent with the conclusion that compounds 1 and 13 are blockers of chloride channels in ASM.
Fig. 2.
Chloride channel blockers relax potassium gluconate contraction in guinea pig tracheal rings. Chloride channel blockers relaxed 80 mM potassium gluconate contractions in guinea pig tracheal rings [compound 1: 61.5% ± 6.3; compound 13: 68.2% ± 4.9; 5-nitro-2-(3-phenylpropylamino) benzoic acid (NPPB): 56.7% ± 10.6; niflumic acid (NFA): 47.4% ± 4.6, calculated as percent of remaining potassium gluconate contraction at 30 min]. *P < 0.05, **P < 0.01, ***P < 0.001 compared with vehicle; n = 4–6.
Combination of chloride channel blockers and NKCC blockade attenuates the initiation of an ACh-induced contraction.
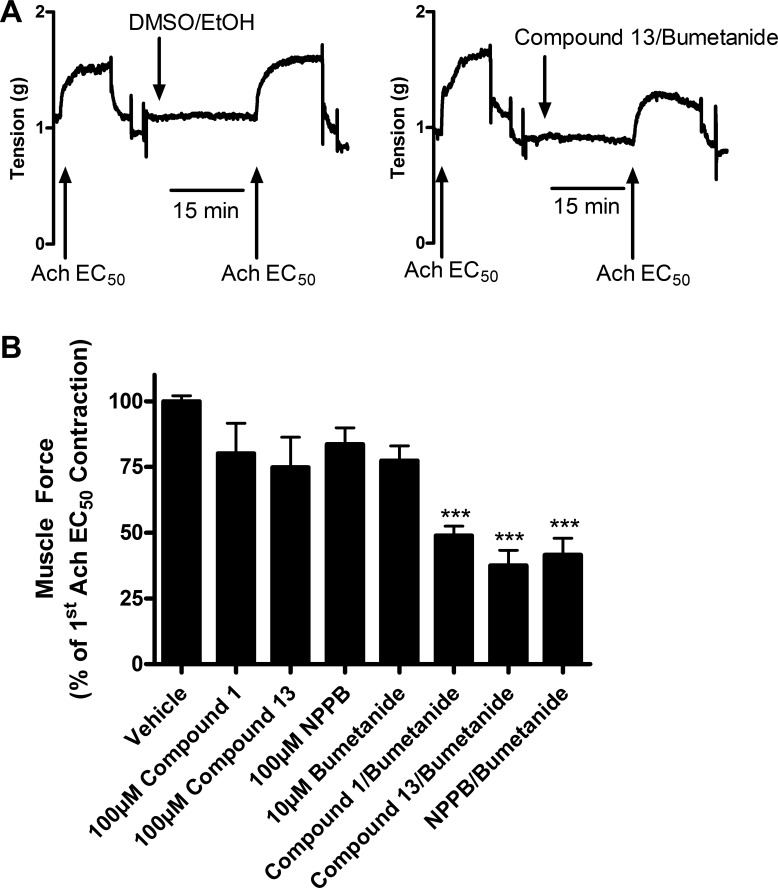
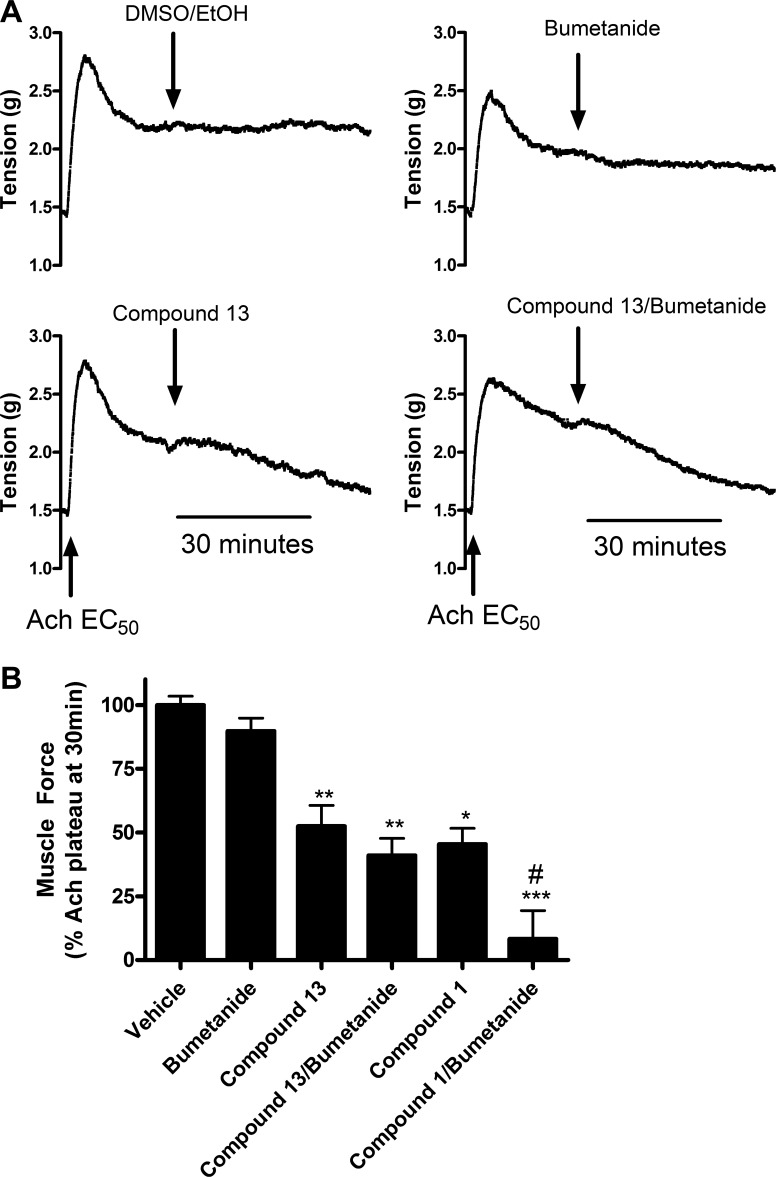
Compounds 1 and 13 were next tested for their abilities to attenuate the initiation phase of an ACh contraction, since ACh is a natural bronchoconstrictor and prevention of contraction would have clinical utility. Although separate pretreatment with chloride channel blockers or the NKCC blocker bumetanide failed to significantly attenuate the initiation of an ACh contraction, a combination of pretreatments with chloride channel blockers plus bumetanide significantly attenuated an ACh contraction compared with vehicle controls (Fig. 3A). With pretreatment with both compound 1 (100 μM) plus bumetanide (10 μM) for 15 min, an EC50 ACh contraction was only 48.9% ± 3.6 of vehicle (0.1% ethanol and 0.1% DMSO) control contractions (P < 0.001; n = 5), whereas the combination of compound 13 (100 μM) plus bumetanide (10 μM) or NPPB (100 μM) plus bumetanide (10 μM) showed similar levels of attenuation [37.5% ± 5.8 and 41.5% ± 6.3, respectively (P < 0.001; n = 5)]. The classical (NFA or NPPB) or novel (compounds 1 and 13) chloride channel blockers alone did not demonstrate a statistically significant attenuation of contraction (Fig. 3B). Therefore, to prevent or attenuate the initiation phase of an ACh contraction by blockade of chloride flux, a combination of compound 1 or 13 with an NKCC blocker was required.
Fig. 3.
Pretreatment with chloride channel blockers attenuates the initiation of an acetylcholine (ACh) contraction in guinea pig tracheal rings. A: representative tracing of tension recordings in organ bath with guinea pig tracheal rings contracted with ACh EC50 dose. Tracheal rings treated with vehicle show no attenuation of contraction, whereas pretreatment with compound 13 100 μM plus bumetanide 10 μM for 15 min shows attenuation of the initiation of contraction. B: pretreatment with a combination of chloride channel blockers plus bumetanide attenuated the initiation of an ACh contraction, whereas treatment with chloride channel blockers or bumetanide alone failed to attenuate the initiation of ACh contractions. Compound 1 100 μM/bumetanide 10 μM: 48.9% ± 3.6; compound 13 100 μM/bumetanide 10 μM: 37.5% ± 5.8; NPPB 100 μM/bumetanide 10 μM: 41.5% ± 6.3. ***P < 0.001 compared with vehicle; n = 5. Muscle force calculated as a percent of first ACh EC50 contraction.
Chloride channel blockers relax an established ACh contraction in guinea pig tracheal rings and human ASM.
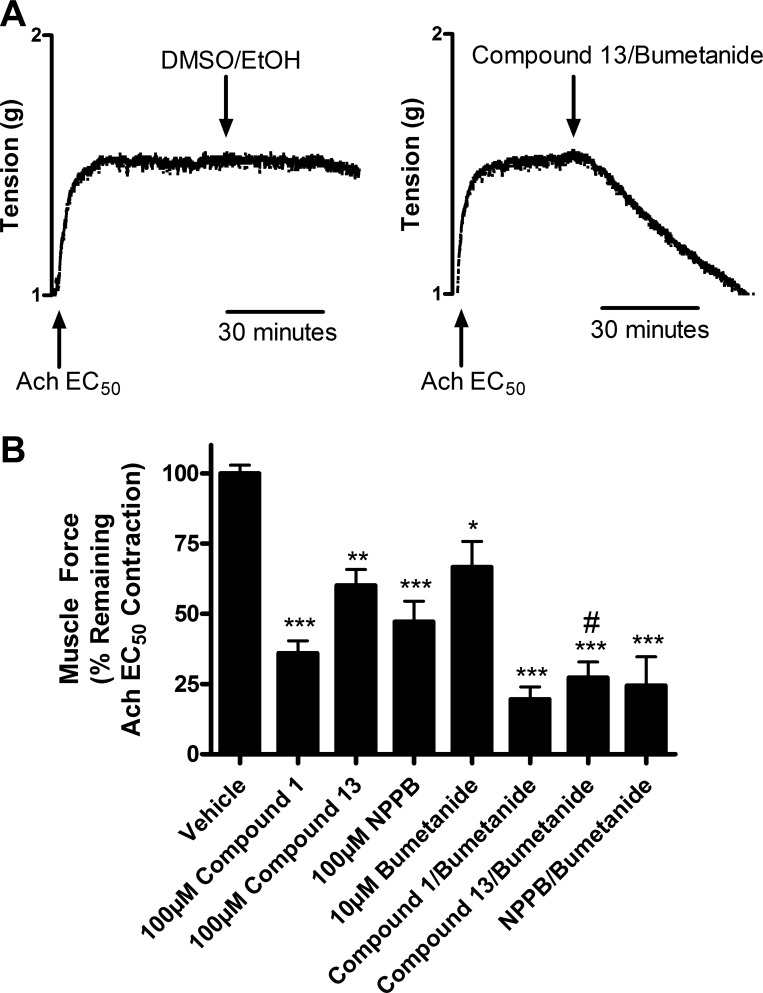
Chloride channel blockers were then tested against the plateau phase of an established ACh contraction. Guinea pig tracheal rings were precontracted with an EC50 dose of ACh and then chloride channel blockers were added to the organ bath to investigate whether the chloride channel blockers had an effect on the maintenance phase of contraction, in addition to the initiation of contraction as demonstrated above. Treatment with chloride channel blockers alone, bumetanide alone, or the combination all significantly relaxed an established ACh contraction (Fig. 4, A and B). Relaxation by compound 1, compound 13, or NPPB used alone was significant at 100 μM. Dose-response studies with compound 13 showed significant relaxation at 50 μM, but not at 10 μM (data not shown). After relaxation with compounds 1 and 13, guinea pig tracheal rings were extensively washed and recontracted with an EC50 concentration of ACh, which resulted in contractions of similar magnitude to those achieved in response to the initial ACh contractions. These results suggest that the effect of these chloride channel inhibitors was reversible and that these compounds were not having a nonspecific toxic effect on the tissue (data not shown). Relaxation induced by compound 13 (100 μM) (60.1% ± 5.7, P < 0.01; n = 6) but not compound 1 (100 μM) was further potentiated when combined with bumetanide (27.3% ± 5.6, P < 0.05 compared with compound 13 alone; n = 6) (Fig. 4B). Taken together these results demonstrate that chloride channel blockade or NKCC blockade alone was sufficient to relax an established ACh contraction. This is in contrast to the findings above that demonstrate that combined blockade of a chloride channel with the NKCC chloride cotransporter is required to attenuate the initiation of an ACh contraction.
Fig. 4.
Chloride channel blockers relax the maintenance phase of a preestablished ACh contraction in guinea pig tracheal rings. A: representative tracing of tension recordings of guinea pig tracheal rings contracted with an ACh EC50 dose. Treatment with vehicle (0.1% ethanol and 0.1% DMSO) showed no relaxation, whereas treatment with compound 13 100 μM and bumetanide 10 μM alone showed relaxation within 30 min. B: treatment with chloride channel blockers, bumetanide, or the combination relaxed an ACh contraction. Compound 13 (60.1% ± 5.7) relaxation was significantly increased when combined with bumetanide (27.3% ± 5.6) (calculated as percent of remaining ACh EC50 contraction at 30 min). *P < 0.05, **P < 0.01, ***P < 0.001 compared with vehicle; #P < 0.05 compared with compound 13 alone; n = 5–8.
We next determined whether separate blockade of the chloride channel or NKCC cotransporter was sufficient to relax an established ACh contraction in human ASM. In human ASM, vehicle (0.1% ethanol, or 0.1% DMSO) or bumetanide alone failed to relax an established ACh contraction, whereas compound 1 (100 μM) or 13 (100 μM) alone [45.4% ± 6.3 (P < 0.05; n = 9), 52.5% ± 8.1 (P < 0.01; n = 9), respectively] or in combination with bumetanide [8.2% ± 11.1 (P < 0.001; n = 13), 41.1% ± 6.7 (P < 0.001; n = 13), respectively] relaxed an established ACh contraction (Fig. 5, A and B). In human ex vivo ASM, only relaxation by compound 1 was significantly further increased when combined with bumetanide (P < 0.05 compared with compound 1 alone). Bumetanide alone did not demonstrate significant relaxation, and compound 1 relaxation instead of 13 (as seen in guinea pig organ bath data above) was significantly increased in combination with bumetanide, suggesting a potential species difference. However, in both species the novel chloride channel blockers alone significantly relaxed an established ACh contraction.
Fig. 5.
Chloride channel blockers relax an established ACh contraction in human airway smooth muscle. A: representative tracings of tension recordings of human airway smooth muscle (ASM) strips contracted with an ACh EC50 dose. Treatment with compound 13 100 μM or compound 13 in combination with bumetanide 10 μM showed significant relaxation within 30 min, whereas treatment with vehicle or bumetanide 10 μM alone showed no relaxation. B: compound 1 or 13 alone (45.4% ± 6.3, 52.5% ± 8.1) and in combination with bumetanide (8.2% ± 11.1, 41.1% ± 6.7) relaxed an ACh contraction, whereas vehicle or bumetanide treatment alone failed to relax an ACh contraction (calculated as percent of remaining ACh EC50 contraction at 30 min). *P < 0.05, **P < 0.001, ***P < 0.001 compared with vehicle; #P < 0.05 compared with compound 1 alone; n = 8–13.
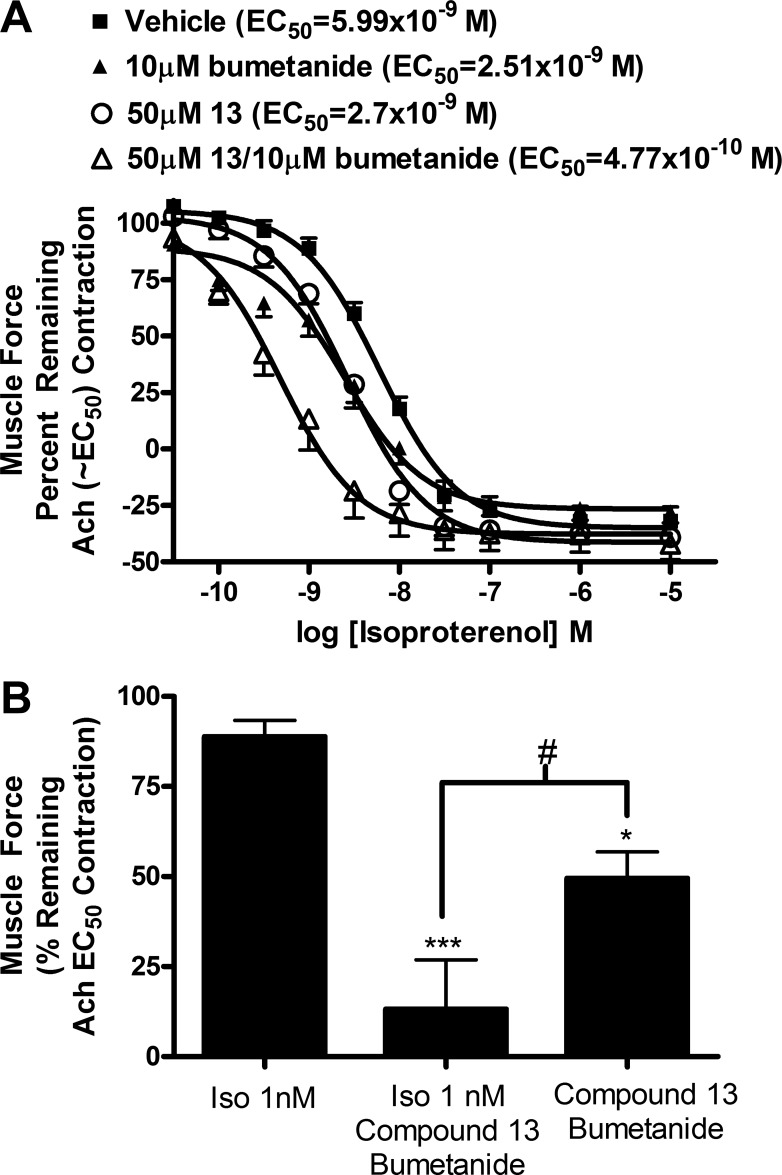
Combination of chloride channel blockers and bumetanide potentiate β-agonist relaxation by isoproterenol in guinea pig tracheal rings.
β-Agonists remain the first line of treatment for asthma or acute bronchospasm, although severe asthmatic patients have been observed to become refractory to these agents. Therefore, we tested whether the chloride channel blockers or modulation of chloride flux could be used in combination with β-agonists to potentiate their effects. The EC50 of isoproterenol-induced relaxation was shifted from 5.99 × 10−9 M to 4.77 × 10−10 M (P < 0.01; n = 6) when a combination of 50 μM compound 13 and 10 μM bumetanide was used with isoproterenol, which is a 12.6-fold or greater than one log shift of the EC50 of isoproterenol (Fig. 6A). Neither 10 μM bumetanide nor 50 μM compound 13 used separately with isoproterenol significantly shifted relaxation by isoproterenol [2.51 × 10−9, 2.7 × 10−9 M, respectively (P > 0.05)], with a 2.4-fold shift and 2.2-fold shift, respectively. Therefore, a combination of treatment with compound 13 and bumetanide potentiated the relaxation effect of isoproterenol. At 1 nM concentration of isoproterenol, ACh contraction was only relaxed 11.1 ± 4.5%, whereas in combination with 50 μM compound 13 and 10 μM bumetanide relaxation increased to 86.8 ± 13.7% (n = 6, P < 0.01), demonstrating a 7.8-fold difference in relaxation (Fig. 6B). Treatment with 50 μM compound 13 and 10 μM bumetanide without isoproterenol resulted in relaxation of 50.5 ± 7.4% (n = 10, P < 0.01 compared with 1 nM isoproterenol, P < 0.05 compared with 50 μM compound 13 + 10 μM bumetanide + 1 nM isoproterenol). These results suggest a synergistic rather than an additive effect, since the percent relaxation of combination treatment with isoproterenol, compound 13, and bumetanide (86.8%) is greater than the sum of isoproterenol relaxation + compound 13 + bumetanide relaxation (61.6%).
Fig. 6.
The combination of 50 μM of compound 13 plus 10 μM bumetanide potentiates relaxation of ACh contractions in guinea pig tracheal rings by isoproterenol. A: the EC50 of isoproterenol-mediated relaxation of an ACh contraction was shifted from 5.99 × 10−9 (vehicle) to 4.77 × 10−10 with a combination of 50 μM compound 13 and 10 μM bumetanide (P < 0.01). The 10 μM bumetanide or 50 μM compound 13 by themselves did not significantly shift relaxation by isoproterenol (2.51 × 10−9, 2.7 × 10−9 respectively, P > 0.05); n = 4–6. B: isoproterenol at 1 nM concentration relaxed ACh EC50 11.1% ± 4.5, whereas in combination with 10 μM bumetanide and 50 μM compound 13 the ACh EC50 contraction relaxed to 86.8% ± 13.7, demonstrating a 7.8-fold difference in relaxation. The 10 μM bumetanide plus 50 μM compound 13 relaxed an ACh EC50 contraction 50.5% ± 7.4 (calculated as percent of remaining ACh EC50 contraction at 7 min). *P < 0.05, ***P < 0.001 compared with iso 1 nM; #P < 0.05; n = 6–10.
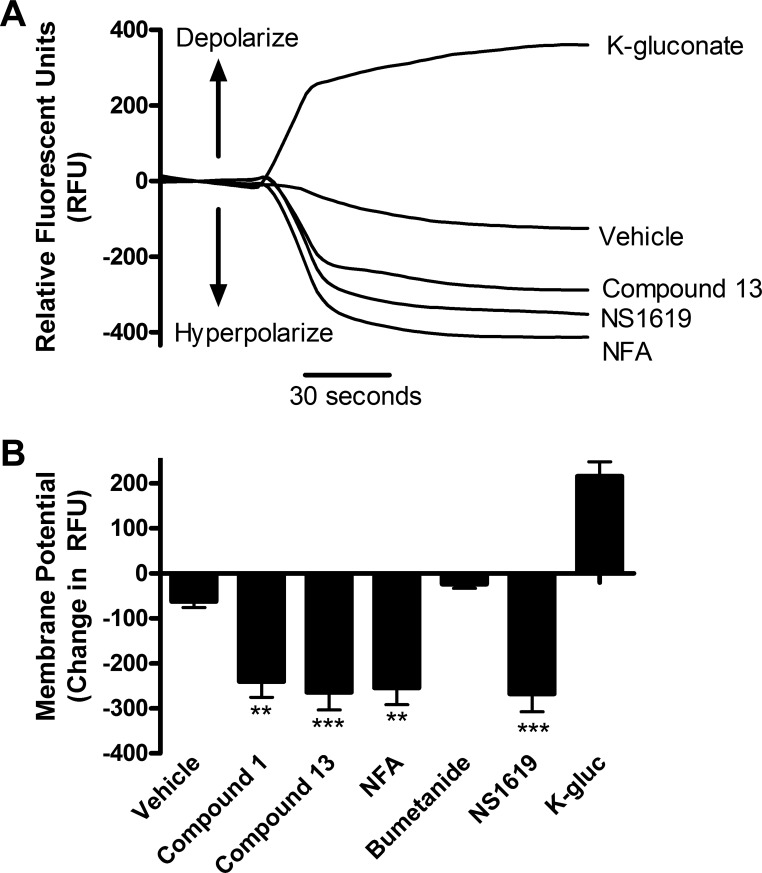
Chloride channel blockers hyperpolarize human ASM cells.
Human ASM cells were incubated with FLIPR membrane potentiometric dye to assess whether the chloride channel blockers changed membrane potential at the cellular level. FLIPR fluorescence increases with depolarization, and decreases with hyperpolarization (Fig. 7A). Potassium gluconate 40 mM was used as a positive control as a depolarizing agent, and NS1619 10 μM (K+ channel opener) was used as a positive control as a hyperpolarizing agent. The 100 μM compound 1 [−240.3 ± 35 relative fluorescent units (RFU), P < 0.01; n = 12], compound 13 (−264.2 ± 40 RFU, P < 0.001; n = 12), or NFA (−254.7 ± 37 RFU, P < 0.01; n = 12) all caused a hyperpolarization, whereas 10 μM bumetanide alone did not cause a significant change (Fig. 7B). Therefore, chloride channel blockade, but not NKCC blockade, hyperpolarized human ASM cells. Chloride channel blockers hyperpolarized membrane potential in human ASM cells, which is consistent with this being a cellular mechanism that contributed to relaxation demonstrated in organ bath studies.
Fig. 7.
Chloride channel blockers hyperpolarize human airway smooth muscle cells. A: representative tracing of continuous fluorescence recordings with fluorescent imaging plate reader (FLIPR) dye. Potassium gluconate (K-gluc) depolarizes, whereas NS1619 (K+-channel opener), compound 13, and NFA hyperpolarize human ASM cells. Potassium gluconate and NS1619 were used as controls as they are known to depolarize and hyperpolarize, respectively. B: change in relative fluorescent units (RFU) with chloride channel blockers. The 100 μM compound 1 (−240.3 ± 35), compound 13 (−264.2 ± 40), and NFA (−254.7 ± 37) all caused a decrease in membrane potential, whereas 10 μM bumetanide did not cause a significant change. **P < 0.01, ***P < 0.001 compared with vehicle; n = 12.
Chloride channel blockers inhibit STIC activity in human ASM cells.
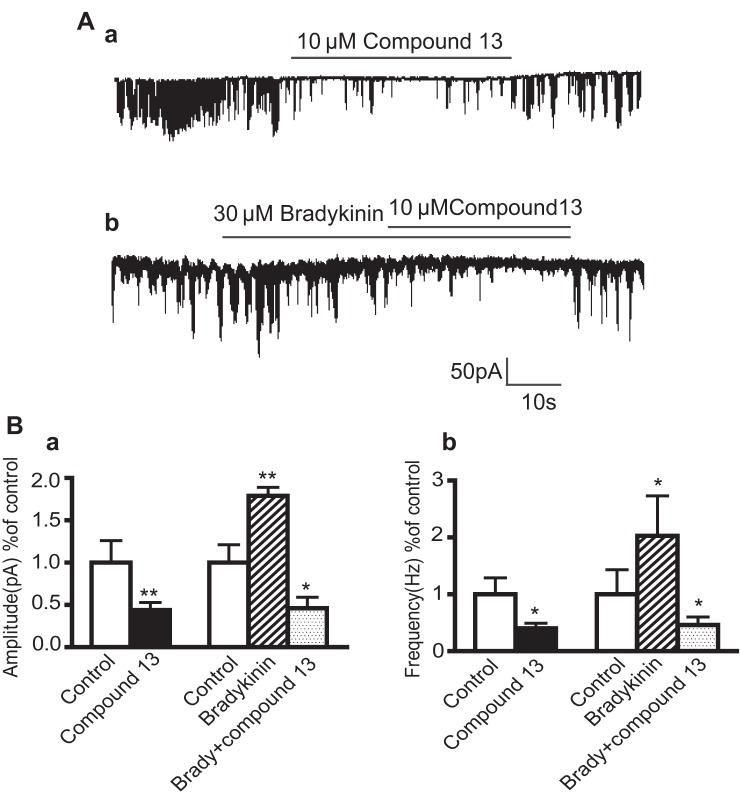
Many different types of chloride channels exist on human ASM cells, but one of the most important types of chloride channels involved in depolarization and airway hyperresponsiveness is the CaCC (5, 11, 32). Calcium sparks activate CaCCs, leading to chloride efflux and depolarization of the cell, which in turn leads to further contraction with the opening of voltage-dependent calcium channels (16). Therefore, compound 13 was tested for its specific ability to block this channel. The activity of this channel can be measured via electrophysiology by measuring STICs (5, 17). Under whole-cell voltage-clamp configuration, STICS were identified from human ASM cells by using a holding potential of −60 mV (Fig. 8Aa).
Fig. 8.
Effects of compound 13 and bradykinin on Ca2+- activated chloride channel activity [spontaneous transient inward current (STICs)] from cultured human airway smooth muscle cells. A, a: representative tracing of Ca2+-activated chloride channel current (STICs) in response to 10 μM compound 13 in human airway smooth muscle cells. b: Representative tracing of STICs in response to 10 μM compound 13 and 30 μM bradykinin in human airway smooth muscle cells. B, a: summary of effects of compound 13 on STICs under basal conditions. Compound 13 decreased the basal amplitude (control 1 ± 0.26, compound 13 0.44 ± 0.09) and frequency of STICs (control 1 ± 0.29, compound 13 0.4 ± 0.1) (n = 9). b: Bradykinin increased STICs and compound 13 decreased the amplitude (control 1 ± 0.21 bradykinin 1.79 ± 0.11; bradykinin plus compound 13 0.46 ± 0.13) and frequency (control 1 ± 0.43, bradykinin 2.03 ± 0.85, bradykinin plus compound 13 0.4 ± 0.14) of these bradykinin-induced STICs; n = 5. *P < 0.05, **P < 0.01 all compared with control groups. Values expressed as fold change with control normalized to 1.
The average peak amplitude and frequency of the current under control conditions was 124.2 ± 2 pA and 5.61 ± 2.7 Hz, respectively (calculated from 599 events in 14 cells). Compound 13 decreased both the amplitude (44% of control, P < 0.01) and frequency of STICs (40% of control, P < 0.05) under basal conditions (n = 9) (Fig. 8B). Elevations of intracellular calcium will increase STIC activity, and thus bradykinin was used in the present study because of its well-established ability to increase intracellular calcium in these cells. The bradykinin stimulation effect was inhibited by 10 μM compound 13 (Fig. 8Ab). Although bradykinin increased the amplitude (179%) and frequency (203%) compared with control, in the presence of 10 μM compound 13 and bradykinin the amplitude (46%) and frequency (40%) decreased to below control values (n = 5, P < 0.05) (Fig. 8Bb). This study suggests that compound 13 is a potential Ca2+-activated chloride channel blocker that may modulate plasma membrane potential and in turn human ASM contraction.
DISCUSSION
In the present study we have shown that chloride channels and transporters are important in both the initiation and maintenance of ASM contraction. A library of chloride channel blockers was first screened for their ability to relax a contraction induced by TEA depolarization. Two of these compounds that demonstrated potent relaxation were then evaluated for their ability to either prevent the initiation of a contraction, induced by an endogenous Gq-coupled bronchoconstrictor, ACh, or to relax an established ACh contraction. Furthermore, the effect of blockade of the chloride transporter, NKCC, either alone or in combination with chloride channel blockade, against the initiation or maintenance of an ACh contraction was measured. The chloride channel blockers were more effective in relaxing a preestablished ACh contraction (chloride channel blockers alone or NKCC blockade alone both effectively relaxed a contraction) than in inhibiting the initiation of a contraction (which required coblockade with NKCC inhibitors to significantly inhibit contraction).
One possible explanation for this difference is the subcellular localization of chloride channels in ASM cells and the effect of chloride channel blockade on calcium signaling at the plasma vs. SR membranes. In fact, the relative contribution of membrane potential to ASM function appears to be less important compared with other smooth muscle subtypes such as vascular smooth muscle. Thus it is possible that our functional effects of chloride channel blockade may be due to effects on SR calcium release due to a loss of charge balance (10). Janssen and colleagues (10) previously showed in bovine ASM that treatment with the chloride channel blocker NPPB reduced the magnitude of successive ACh contractions, whereas another chloride channel blocker, NFA, did not. They suggested that NPPB had a higher selectivity for chloride channels on the SR than NFA (24) and that NPPB may also be inhibiting chloride flux at the SR with resultant rundown into the SR calcium pools after repetitive contractile challenges. Although chloride efflux through calcium-activated chloride channels on the plasma membrane has been shown to be important in initial depolarization (16), which subsequently induces voltage-gated calcium entry into the cell and calcium-induced calcium release from the SR, Janssen and colleagues hypothesized that chloride flux on the SR membrane dictates SR calcium release and refilling by neutralizing the charge balance across the SR membrane. Thus, by blocking chloride channels on the SR, calcium may not be able to leave the SR to maintain a contraction. Our compounds were compared against NPPB and were found to have a similar potency and relaxation profile. Therefore, they may also play an important role in calcium signaling regulated at the SR in ASM. Chloride channel blockers may be more effective in blocking the maintenance of a contraction, since it requires constant refilling of calcium and release from the SR that may be inhibited by blocking chloride flux.
Several studies have shown the importance of calcium-activated chloride channel blockade in ASM. Huang et al. (11) showed that pretreatment with benzbromarone, a CaCC blocker, can partially attenuate a methacholine contraction in human ASM isolated from a single individual. Zhang et al. (32) have used an ovalbumin-sensitized mouse model to show that intravenous pretreatment with NFA or benzbromarone prevented airway hyperresponsiveness following inhaled methacholine. These studies differ from the present study in that pretreatment with our chloride channel blockers was insufficient to attenuate an ACh-induced contraction unless combined with an NKCC blocker (i.e., bumetanide). There are several variations in study design that may account for these differences. The studies conducted in isolated human bronchial rings were from a single individual and used 10 μM benzbromarone, a compound believed to act by inhibiting TMEM16 or anoctamin channels, the recently identified family of calcium-activated chloride channels. The specificity and potency of compounds 1 and 13 used in the present study for the TMEM16 family of channels is currently unknown. Zhang et al. used an in vivo sensitized mouse model whereas our studies were performed in nonsensitized ex vivo ASM from guinea pigs and humans. Thus our results may differ from these mouse studies due to species differences, the mode of delivery or differences in potency and specificity between the chloride channel blockers used, or the study of ex vivo ASM contraction vs. in vivo lung resistance. Importantly, we also investigated the ability of our compounds to relax an existing preestablished ACh contraction, which may more closely mimic a constricted airway during an asthmatic exacerbation. Thus our study is the first to show relaxation of a preestablished ACh contraction in ex vivo human and guinea pig ASM by chloride channel blockade.
NKCC was previously shown to be present in human (13) and guinea pig (27) ASM. As seen in our present study, NKCC inhibitors such as furosemide have been shown to play a role in ASM relaxation in guinea pig ASM (28). In these studies, more significant relaxation was seen in fetal and newborn airway tissue than in adult guinea pig tissue. Clinically furosemide has effectiveness in reducing airway resistance in premature human infants (18, 26) and in selective challenges in adults (2, 9, 25) but is not universally effective in all studies.
We have observed a significant relaxation of a preexisting contraction in the adult guinea pig with bumetanide but did not observe any significant relaxation with bumetanide treatment alone in adult human ASM. However, bumetanide did increase relaxation in combination with compound 1 compared with compound 1 alone in adult human ASM. Therefore, although NKCC blockade alone does not cause significant relaxation in adult human ASM, the combination of NKCC blockade with chloride channel blockade can cause relaxation. Interestingly, compound 13 relaxation was augmented in guinea pig with bumetanide, but compound 1 was augmented by bumetanide in human ASM, suggesting a potential species or age difference in compound 1's vs. compound 13's effects. Our studies are the first to use NKCC blockade in combination with CaCC blockade to prevent contraction, or to augment relaxation of a preexisting contraction.
Our studies demonstrate that pretreating guinea pig ASM with either chloride channel or NKCC blockers alone did not attenuate a subsequent ACh-induced contraction. In contrast, a combination of chloride channel and NKCC blockers as pretreatments inhibited the initial magnitude of an ACh contraction. The initial depolarization of plasma membrane is believed to involve chloride efflux (14, 16). Our results may be explained by a depletion of cytosolic chloride necessary for CaCC efflux by concomitant NKCC blockade. NKCC is a bidirectional cotransporter that moves chloride into and out of the cell on the basis of transmembrane gradients. ACh-induced contractions initiate a membrane depolarizing event by calcium-induced chloride efflux. We speculate that coblockade of CaCC and NKCC would have an additive effect by decreasing chloride efflux through the CaCC while concomitantly reducing available intracellular chloride to maintain this efflux. An alternative or complementary cellular mechanism that could account for these findings is additional effects at the SR. Blockade of CaCC on the SR could block calcium release, and refilling of the SR could be impaired by reduced cytosolic chloride that is necessary for maintaining charge balance across the SR membrane (10).
We have also shown that a combination of CaCC and NKCC blockade was able to potentiate relaxation of the β-agonist isoproterenol. This has clinical relevance and suggests that chloride channel blockers could be used in combination with β-agonists in patients with severe asthma where loss of potency of β-agonist occurs or to decrease the chronic dose of β-agonists. β-Agonists work through a PKA pathway with multiple downstream targets including modulation of intracellular calcium and hyperpolarization of the ASM cell through activation of calcium-activated potassium channels (3, 19). We have shown that compounds 1 and 13 hyperpolarize the ASM, and it is possible that potentiation of this hyperpolarization may be occurring with β-agonists since both hyperpolarize through different mechanisms. Another possible explanation for synergy is reduced SR calcium release due to chloride flux blockade as mentioned previously.
We have identified two cellular mechanisms that contribute to relaxation by compounds 1 and 13. In isolated human ASM cells our chloride channel blockers hyperpolarized the plasma cell membrane which would favor relaxation of ASM. Furthermore, we utilized STICs, an electrophysiological event resulting from calcium activation of CaCCs in ASM (17) to implicate this specific family of chloride channels in our compounds' effects. The reversible blockade of STICs by compound 13 would imply inhibition of plasma membrane CaCCs by our chloride channel blockers.
In conclusion, we have identified two chloride channel blockers that relax an ACh contraction and in combination with NKCC blockade prevent initiation of an ACh contraction and potentiate β-agonist relaxation. These two chloride channel blockers also hyperpolarize ASM cells, and compound 13 inhibits calcium-activated chloride channel activity. Modulation of chloride flux is a promising new treatment of asthma and bronchospasm.
GRANTS
This work was supported by National Institutes of Health grants (J. Danielsson) (GM008464) and (C. W. Emala) (GM065281).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
J.D., P.D.Y., A.R., D.W.L., and C.W.E. conception and design of research; J.D., X.W.F., and Y.Z. performed experiments; J.D., X.W.F., and Y.Z. analyzed data; J.D., P.D.Y., X.W.F., and C.W.E. interpreted results of experiments; J.D., A.R., and X.W.F. prepared figures; J.D. and C.W.E. drafted manuscript; J.D., A.R., X.W.F., D.W.L., and C.W.E. edited and revised manuscript; J.D., P.D.Y., A.R., X.W.F., Y.Z., D.W.L., and C.W.E. approved final version of manuscript.
REFERENCES
- 1.Bao R, Lifshitz LM, Tuft RA, Bellve K, Fogarty KE, ZhuGe R. A close association of RyRs with highly dense clusters of Ca2+-activated Cl− channels underlies the activation of STICs by Ca2+ sparks in mouse airway smooth muscle. J Gen Physiol 132: 145–160, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bianco S, Vaghi A, Robuschi M, Pasargiklian M. Prevention of exercise-induced bronchoconstriction by inhaled frusemide. Lancet 2: 252–255, 1988 [DOI] [PubMed] [Google Scholar]
- 3.Billington CK, Ojo OO, Penn RB, Ito S. cAMP regulation of airway smooth muscle function. Pulm Pharmacol Ther 26: 112–120, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, Adcock IM, Bateman ED, Bel EH, Bleecker ER, Boulet LP, Brightling C, Chanez P, Dahlen SE, Djukanovic R, Frey U, Gaga M, Gibson P, Hamid Q, Jajour NN, Mauad T, Sorkness RL, Teague WG. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J 43: 343–373, 2014 [DOI] [PubMed] [Google Scholar]
- 5.Gallos G, Remy KE, Danielsson J, Funayama H, Fu XW, Chang HY, Yim PD, Xu D, Emala CW., Sr Functional expression of the TMEM16 family of calcium activated chloride channels in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 305: L625–L634, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gallos G, Yim P, Chang S, Zhang Y, Xu D, Cook JM, Gerthoffer WT, Emala CW., Sr Targeting the restricted α-subunit repertoire of airway smooth muscle GABAA receptors augments airway smooth muscle relaxation. Am J Physiol Lung Cell Mol Physiol 302: L248–L256, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gosens R, Stelmack GL, Dueck G, McNeill KD, Yamasaki A, Gerthoffer WT, Unruh H, Gounni AS, Zaagsma J, Halayko AJ. Role of caveolin-1 in p42/p44 MAP kinase activation and proliferation of human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 291: L523–L534, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Greenwood IA, Leblanc N. Overlapping pharmacology of Ca2+-activated Cl− and K+ channels. Trends Pharmacol Sci 28: 1–5, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Grubbe RE, Hopp R, Dave NK, Brennan B, Bewtra A, Townley R. Effect of inhaled furosemide on the bronchial response to methacholine and cold-air hyperventilation challenges. J Allergy Clin Immunol 85: 881–884, 1990 [DOI] [PubMed] [Google Scholar]
- 10.Hirota S, Trimble N, Pertens E, Janssen LJ. Intracellular Cl− fluxes play a novel role in Ca2+ handling in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 290: L1146–L1153, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Huang F, Zhang H, Wu M, Yang H, Kudo M, Peters CJ, Woodruff PG, Solberg OD, Donne ML, Huang X, Sheppard D, Fahy JV, Wolters PJ, Hogan BL, Finkbeiner WE, Li M, Jan YN, Jan LY, Rock JR. Calcium-activated chloride channel TMEM16A modulates mucin secretion and airway smooth muscle contraction. Proc Natl Acad Sci USA 109: 16354–16359, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iwamoto LM, Gries DM, Nakamura KT. Loop diuretics and in vitro relaxation of human fetal and newborn mouse airways. Pediatr Res 50: 273–276, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Iwamoto LM, Nakamura KT, Wada RK. Immunolocalization of a Na-K-2Cl cotransporter in human tracheobronchial smooth muscle. J Appl Physiol 94: 1596–1601, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Janssen LJ. Acetylcholine and caffeine activate Cl− and suppress K+ conductances in human bronchial smooth muscle. Am J Physiol Lung Cell Mol Physiol 270: L772–L781, 1996 [DOI] [PubMed] [Google Scholar]
- 15.Janssen LJ, Killian K. Airway smooth muscle as a target of asthma therapy: history and new directions. Respir Res 7: 123, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Janssen LJ, Sims SM. Ca2+-dependent Cl− current in canine tracheal smooth muscle cells. Am J Physiol Cell Physiol 269: C163–C169, 1995 [DOI] [PubMed] [Google Scholar]
- 17.Janssen LJ, Sims SM. Spontaneous transient inward currents and rhythmicity in canine and guinea-pig tracheal smooth muscle cells. Pflügers Arch 427: 473–480, 1994 [DOI] [PubMed] [Google Scholar]
- 18.Kao LC, Warburton D, Sargent CW, Platzker AC, Keens TG. Furosemide acutely decreases airways resistance in chronic bronchopulmonary dysplasia. J Pediatr 103: 624–629, 1983 [DOI] [PubMed] [Google Scholar]
- 19.Kotlikoff MI, Kamm KE. Molecular mechanisms of beta-adrenergic relaxation of airway smooth muscle. Annu Rev Physiol 58: 115–141, 1996 [DOI] [PubMed] [Google Scholar]
- 20.Landry DW, Reitman M, Cragoe EJ, Jr, Al-Awqati Q. Epithelial chloride channel. Development of inhibitory ligands. J Gen Physiol 90: 779–798, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Masoli M, Fabian D, Holt S, Beasley R, Global Initiative for Asthma (GINA) Program. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy 59: 469–478, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Miedinger D, Neukomm E, Chhajed PN, Schnyder A, Naef M, Ackermann M, Leuppi JD. The use of the Asthma Control Test in general practice and its correlation with asthma control according to the GINA guidelines. Curr Med Res Opin 27: 2301–2308, 2011 [DOI] [PubMed] [Google Scholar]
- 23.Moore WC, Bleecker ER, Curran-Everett D, Erzurum SC, Ameredes BT, Bacharier L, Calhoun WJ, Castro M, Chung KF, Clark MP, Dweik RA, Fitzpatrick AM, Gaston B, Hew M, Hussain I, Jarjour NN, Israel E, Levy BD, Murphy JR, Peters SP, Teague WG, Meyers DA, Busse WW, Wenzel SE; National Heart, Lung, Blood Institute's Severe Asthma Research Program. Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute's Severe Asthma Research Program. J Allergy Clin Immunol 119: 405–413, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pollock NS, Kargacin ME, Kargacin GJ. Chloride channel blockers inhibit Ca2+ uptake by the smooth muscle sarcoplasmic reticulum. Biophys J 75: 1759–1766, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prandota J. Furosemide: progress in understanding its diuretic, anti-inflammatory, and bronchodilating mechanism of action, and use in the treatment of respiratory tract diseases. Am J Ther 9: 317–328, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Rastogi A, Luayon M, Ajayi OA, Pildes RS. Nebulized furosemide in infants with bronchopulmonary dysplasia. J Pediatr 125: 976–979, 1994 [DOI] [PubMed] [Google Scholar]
- 27.Rhoden KJ, Douglas JS. Evidence of Na-K-Cl cotransport in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 268: L551–L557, 1995 [DOI] [PubMed] [Google Scholar]
- 28.Stevens EL, Uyehara CF, Southgate WM, Nakamura KT. Furosemide differentially relaxes airway and vascular smooth muscle in fetal, newborn, and adult guinea pigs. Am Rev Respir Dis 146: 1192–1197, 1992 [DOI] [PubMed] [Google Scholar]
- 29.Townsend EA, Yim PD, Gallos G, Emala CW. Can we find better bronchodilators to relieve asthma symptoms? J Allergy (Cairo) 2012: 321949, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verkman AS, Galietta LJ. Chloride channels as drug targets. Nat Rev Drug Discov 8: 153–171, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yim PD, Gallos G, Xu D, Zhang Y, Emala CW. Novel expression of a functional glycine receptor chloride channel that attenuates contraction in airway smooth muscle. FASEB J 25: 1706–1717, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang CH, Li Y, Zhao W, Lifshitz LM, Li H, Harfe BD, Zhu MS, ZhuGe R. The transmembrane protein 16A Ca2+-activated Cl− channel in airway smooth muscle contributes to airway hyperresponsiveness. Am J Respir Crit Care Med 187: 374–381, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]