Abstract
Animal studies have shown that platelet-derived growth factor (PDGF) signaling is required for normal alveolarization. Changes in PDGF receptor (PDGFR) expression in infants with bronchopulmonary dysplasia (BPD), a disease of hypoalveolarization, have not been examined. We hypothesized that PDGFR expression is reduced in neonatal lung mesenchymal stromal cells (MSCs) from infants who develop BPD. MSCs from tracheal aspirates of premature infants requiring mechanical ventilation in the first week of life were studied. MSC migration was assessed in a Boyden chamber. Human lung tissue was obtained from the University of Rochester Neonatal Lung Biorepository. Neonatal mice were exposed to air or 75% oxygen for 14 days. PDGFR expression was quantified by qPCR, immunoblotting, and stereology. MSCs were isolated from 25 neonates (mean gestational age 27.7 wk); 13 developed BPD and 12 did not. MSCs from infants who develop BPD showed lower PDGFR-α and PDGFR-β mRNA and protein expression and decreased migration to PDGF isoforms. Lungs from infants dying with BPD show thickened alveolar walls and paucity of PDGFR-α-positive cells in the dysmorphic alveolar septa. Similarly, lungs from hyperoxia-exposed neonatal mice showed lower expression of PDGFR-α and PDGFR-β, with significant reductions in the volume of PDGFR-α-positive alveolar tips. In conclusion, MSCs from infants who develop BPD hold stable alterations in PDGFR gene expression that favor hypoalveolarization. These data demonstrate that defective PDGFR signaling is a primary feature of human BPD.
Keywords: prematurity, hyperoxia, lung development, PDGFR-α, PDGFR-β
over 25% of premature infants with birth weight less than 1,500 g develop bronchopulmonary dysplasia (BPD) (24). This translates to over 10,000 new cases of BPD annually (29). Survivors of BPD have abnormal lung structure and function even as adults (10, 15, 40), making BPD a leading cause of pediatric lung disease. The lungs of infants with BPD demonstrate fewer and larger alveoli, as well as poorly formed secondary crests (23), indicating interference with the normal ingrowth of secondary septa into larger precursor saccules. Furthermore, alveolar septa are thickened with collagen and elongated cells resembling fibroblasts (4). In infants with respiratory distress syndrome, α-smooth muscle actin-positive myofibroblasts appear in the alveolar septa as early as 4 days after birth (38). Within days to weeks, numerous myofibroblasts feature intense immunoreactivity for transforming growth factor (TGF)-β, a stimulus for myofibroblastic differentiation (38). Together, these results indicate that BPD may result in part from the abnormal migration and differentiation of mesenchymal progenitor cells within the interstitia of the terminal air spaces.
PDGF isoforms (PDGF-AA, PDGF-BB, PDGF-AB) stimulate migration and proliferation of lung fibroblasts in vitro (6, 31). PDGF binds to two high-affinity receptors: an α-receptor that binds both A- and B-chains and a β-receptor with high affinity for PDGF-B chains (18). In the saccular and alveolar stage of lung development, Pdgfra-expressing cells migrate to the tips of secondary alveolar septa and differentiate into α-smooth muscle actin- and elastin-producing myofibroblasts required for alveogenesis (7, 8, 26, 30, 32, 33, 41). PDGF-A-deficient mice surviving beyond birth fail to form alveoli owing to the failure of PDGFR-α mesenchymal progenitor cells to migrate distally to the site of secondary crest formation (8). PDGF-B- and PDGFR-β-deficient embryos show reduced recruitment and proliferation of vascular smooth muscle cells and pericytes during blood vessel formation (19).
We have isolated mesenchymal stromal cells (MSCs) from tracheal aspirates of premature infants with respiratory distress (20). Using array-based analysis, we have shown that unstimulated MSCs show a gene expression pattern associated with alveolar septal fibroblasts (36). Isolation and characterization of neonatal lung MSCs therefore provides the opportunity to examine gene expression patterns of alveolar mesenchymal cells ex vivo.
In this study, we hypothesized that there are stable differences in the expression of PDGFR-α and -β between neonatal lung MSCs from infants who develop BPD and cells from infants who do not develop this disease. We also hypothesized that similar differences would be found in the lungs from infants with BPD, as well as the lungs of hyperoxia-exposed neonatal mice, a model of BPD.
METHODS
Patients.
We examined tracheal aspirates from infants admitted to the Newborn Intensive Care Unit, as approved by the Institutional Review Board of the University of Michigan Medical School. Entry criteria included gestational age at birth ≤32 wk, mechanical ventilation for respiratory distress, and age ≤7 days.
Ethics statement.
The animal study was performed in strict accordance with the NIH Guide for the Care and Use of Laboratory Animals recommendations. The protocol was approved by the University of Michigan Committee on Use and Care of Animals.
Cell culture.
Neonatal lung MSCs were isolated as described previously (20, 36). Unstimulated passage two or three MSCs were plated for 24 h in 10% fetal bovine serum, then serum starved for 2 h prior to harvesting.
Animal model.
Two- to 3-day-old C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME) were exposed to air or 75% oxygen for 14 days by use of a polypropylene chamber coupled to an oxygen controller and sensor (BioSpherix, Lacona, NY) (35).
Quantitative real-time PCR.
MSC mRNA was extracted with RNeasy Plus Mini Kit (Qiagen, Valencia, CA). Mouse whole-lung RNA was prepared by use of TRIzol (Invitrogen, Carlsbad, CA). Gene expression of human and mouse PDGFRA, PDGFRB, GAPDH, and human Ki67 were quantified with SYBR Green technology. The primers used are listed in Table 1. Relative gene expression was analyzed with the 2−ΔCT algorithm.
Table 1.
Primers used for quantitative real-time PCR
| Gene Name | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| hPDGFRA | GGTCTTGGAAGTGAGCAGT | ACATCTGGGTCTGGCACATA |
| hPDGFRB | GTGCTCACCATCATCTCCCT | ACTCAATCACCTTCCATCGG |
| hKi-67 | AGCACGTCGTGTCTCAAGAT | GTTCCCTGAGCAACACTGTCT |
| hGAPDH | CGACCACTTTGTCAAGCTCA | AGGGGTCTACATGGCAACTG |
| mPDGFRA | TGCAGTTGCCTTACGACTCCAGAT | AGCCACCTTCATTACAGGTTGGGA |
| mPDGFRB | ACTACATCTCCAAAGGCAGCACCT | TGTAGAACTGGTCGTTCATGGGCA |
| mGAPDH | TCCACTCACGGCAAATTCAAC | CGCTCCTGGAAGATGGTGATG |
Immunoblotting.
Lysates were resolved by SDS-PAGE, and transferred to a nitrocellulose membrane. Membranes were blocked in 5% milk for 1 h in room temperature and probed with antibodies against PDGFR-α (Santa Cruz Biotechnology, Santa Cruz, CA) and PDGFR-β (Cell Signaling, Danvers, MA).
Immunoassay.
Protein levels of PDGF-AA and PDGF-BB in tracheal aspirate supernatant fluid were determined using a multiplex immune assay (EMD Millipore, Billerica, MA).
Immunohistochemistry of mouse lung tissue.
Lungs were perfused with 5 mM EDTA, inflated to 30 cmH2O pressure with 4% paraformaldehyde, and fixed in formalin overnight. Slides were probed with anti-PDGFR-α (Santa Cruz Biotechnology, Santa Cruz, CA) and stained with a biotinylated anti-rabbit IgG-avidin horseradish peroxidase and diaminobenzidine detection system (Vector Labs, Burlingame, CA). Immunoadsorption of the PDGFR-α antibody was carried out by coincubating the anti-PDGFR-α antibody at a 1:2,000 dilution with five times the concentration of PDGFR-α blocking peptide (Santa Cruz Biotechnology, sc-338 P) for 1 h with mixing at room temperature.
Mouse lung tissue preparation for fluorescence microscopy.
Lungs were harvested as described above and placed in cassettes in random orientation to allow the systematic uniform random sampling required for stereological morphometry (14). From a random start, paraffin-embedded lungs were sectioned every 250 μm, and 5-μm-thick sections were probed with Cy3-labeled mouse anti-α-smooth muscle actin antibody (clone 1A4, Sigma-Aldrich), AlexaFluor 488-conjugated rabbit anti-PDGFR-α antibody (Santa Cruz Biotechnology), or isotype control antibodies. Nuclei were visualized with Hoechst 33342 (Sigma-Aldrich).
Stereological morphometry of mouse alveolar tip volumes.
To quantify the volume of alveolar tips expressing PDGFR-α, we combined fluorescence microscopy and stereological methods. The tips of secondary alveolar crests were identified by expression of α-smooth muscle actin. For each experimental section, rabbit IgG- and mouse IgG-stained slides were used to set the background staining to 0. Stereological volume determinations were performed as described (3) by systematic-uniform random sampling (14, 34). Harvested lungs were inflated to 30 mm pressure with formalin, tied off at the trachea, trimmed of all other tissue, and placed in PBS for an initial measurement of lung volume by the water displacement assessment. Tissue was fixed for paraffin sectioning, with the first cut at a random starting point followed by 8–12 systematic-uniform slices at 500-μm intervals thereafter. To determine reference volume initially, we superimposed low-power (×5) images of each section at 500 μm onto a 0.645-cm2 grid (NIH ImageJ, Bethesda, MD) to determine total sectional area by the Cavalieri point-counting method (14). The Cavalieri lung volume, Vref, was calculated as Vref = ∑A × t × K, where ∑A is the sum of the reference area on each section, t is the tissue thickness, and K is the sampling interval. Randomly chosen fields of each systematically cut 5-μm-thick tissue section were photographed at ×200. A systematic randomly oriented 8 × 10-point crossgrid (NIH ImageJ) was superimposed on each image, and both the total number of points falling on alveolar tips and the number of points falling on PDGFR-α-containing alveolar tips were counted. The fractional object volume for the whole lung, Vobj, was calculated as Vobj = [∑(Po)/∑(Pref)]*Vref, where ∑(Po) is the sum of the points counted in a lung, ∑(Pref) is the sum of all reference points counted in a systematic sectioning of the lung, and Vref is the reference volume. Given the assumption that the proportion PDGFR-α (+) tips is uniform through the lung, and since Vv = Vobj/Vref, the volume density, Vv, was calculated as the sum of the points in the reference space, i.e., total points landing on PDGFR-α (+) tips, divided by the total number of points counted (3). To calculate the total volume of PDGFR-α-positive alveolar tips, we multiplied Vv by lung volume, as obtained by the water displacement method. Fifty-eight slides from six hyperoxia-exposed animals and 40 slides from five air-exposed animals were examined.
Immunohistochemistry of lung tissue from infants with BPD.
Human lung tissue sections were obtained from the University of Rochester Lung Biorepository under a protocol approved by the Institutional Review Board of Strong Memorial Hospital (Rochester, NY). Specimens were obtained from infants who died in the intensive care nursery. The diagnosis of BPD was based on premature delivery, need for chronic respiratory support, requirement for supplemental oxygen after 36 wk gestation, chest radiographs, and pathological tissue diagnosis at autopsy. Specimens were also obtained from infants succumbing to nonpulmonary disorders. Samples were processed within 6 h of death. These specimens were not suitable for stereological analysis and therefore were used for comparative histology and immunohistochemistry. Immunohistochemistry was performed as described above, using anti-PDGFR-α antibody.
Chemotaxis assay.
Migration of cells to PDGF-AA (PeproTech, Rocky Hill, NJ, cat. no. 100-13A) (30 ng/ml), PDGF-BB (PeproTech, cat. no. 100-14B) (10 ng/ml) or serum-free medium, as control, was assessed after 4 h incubation in a 12-well Boyden chamber (Neuroprobe, Gaithersburg, MD) by use of an 8-μm pore size membrane. For each experiment, MSCs from three patients who developed BPD and three who did not were used. Membranes were stained (toluidine blue stain) and cells on the distal side of the membrane were counted. Four random high-power fields (×200) for each sample and condition were examined. Chemotaxis index was calculated as the number of MSCs migrating toward the growth factor divided by the number of MSCs migrating toward control serum-free medium.
Statistical analysis.
All data were described as means ± SE or median and interquartile range. An unpaired t-test or Mann-Whitney test was used for comparison. P values were considered statistically significant if they were <0.05.
RESULTS
Patient data.
Lung MSCs from 25 neonates were used in this study. Thirteen of the 25 babies (52%) required supplemental oxygen at 36 wk, a clinical definition of BPD (16, 24). Characteristics of the patients are described in Table 2. Gestational age and birth weight were significantly lower, and the number of surfactant doses, day of sampling, days requiring mechanical ventilation, and days on oxygen were significantly higher in the infants who developed BPD.
Table 2.
Characteristics of patients with neonatal lung MSCs isolated from tracheal aspirates, used for the in vitro studies
| BPD | Non-BPD | P | |
|---|---|---|---|
| n | 13 | 12 | |
| Male gender, n (% of total) | 9 (69%) | 8 (67%) | 1.00 (Fisher's exact test) |
| Gestational age, mean ± SD, wk | 26.3 ± 2.1 | 29.3 ± 1.9 | <0.005 (t-test) |
| Birth weight, mean ± SD, g | 893.1 ± 23 | 1303 ± 26 | <0.001 (t-test) |
| Surfactant dose, mean ± SD | 2.4 ± 0.7 | 1.3 ± 0.5 | <0.0001 (t-test) |
| Suspected chorioamnionitis, n (% of total) | 3 (23%) | 2 (17%) | 1.00 (Fisher's exact test) |
| Vent days, mean ± SD, days | 35 ± 22 | 5 ± 4 | <0.0001 (t-test) |
| O2 days, median (IQR) | 188 (113) | 27 (33) | <0.0001 (Mann-Whitney test) |
| Died, n (% of total) | 1 (8%) | 0 | 1.00 (Fisher's exact test) |
| Day of sampling, median (IQR) | 4 ± 3 | 1 ± 1 | <0.01 (Mann-Whitney test) |
MSCs, mesenchymal stromal cells; BPD, bronchopulmonary dysplasia; IQR, interquartile range.
Neonatal lung MSCs from infants who develop BPD show lower mRNA and protein expression of PDGFR-α and PDGFR-β.
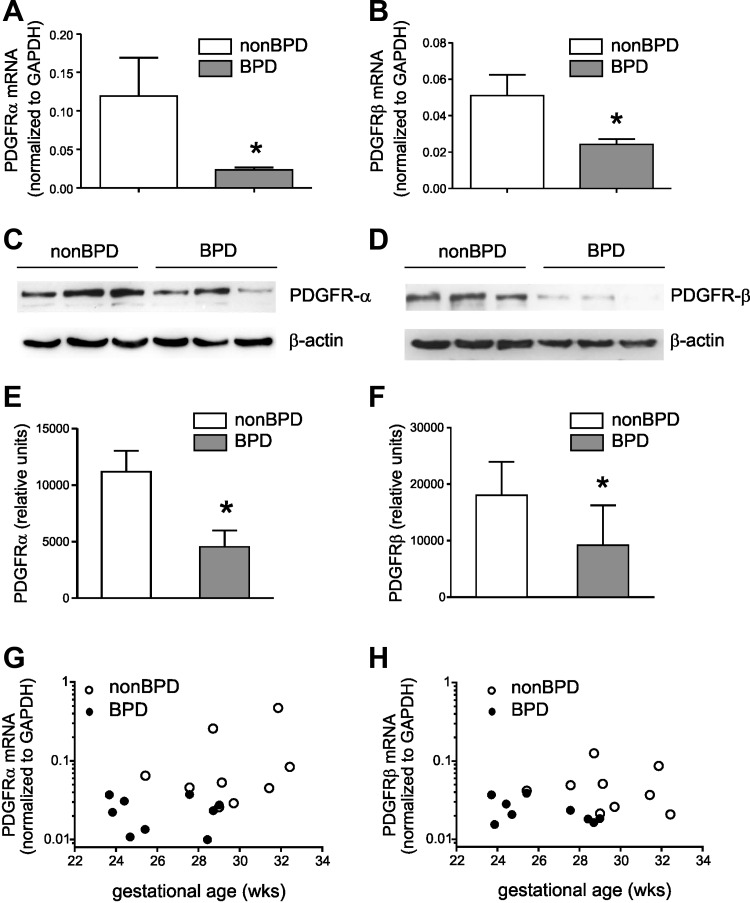
We examined MSCs from 13 infants who developed BPD and MSCs from 12 infants who did not develop this disease. RT-PCR analysis demonstrated that MSCs from infants who develop BPD showed lower mRNA expression of PDGFRA (Fig. 1A) and PDGFRB (Fig. 1B). Immunoblots confirmed reduced PDGFR-α (Fig. 1, C and E) and PDGFR-β (Fig. 1, D and F) protein expression in MSCs from infants who developed BPD. We also examined the relationship between PDGFRA and PDGFRB mRNA expression and gestational age at birth. For the same gestational age PDGFRA mRNA expression (Fig. 1G) was lower in MSCs from infants who develop BPD. A similar relationship was observed for PDGFRB mRNA expression (Fig. 1H). Finally, we measured PDGF-AA and PDGF-BB levels in tracheal aspirate fluid. There was no difference in PDGF concentration between aspirates from babies who developed BPD compared with aspirates from babies who did not develop BPD (PDGF-AA, 114 ± 73 vs. 85 ± 46 pg/ml, means ± SD; PDGF-BB, 142 ± 71 vs. 227 ± 278 pg/ml, means ± SD).
Fig. 1.
Neonatal lung mesenchymal stromal cells (MSCs) from infants who develop bronchopulmonary dysplasia (BPD) show lower mRNA and protein expression of PDGFR-α and PDGFR-β. mRNA expression was assessed by RT-PCR and protein expression was assessed by immunoblotting. Unstimulated MSCs from infants who developed BPD showed significantly lower mRNA expression of PDGFRA (A) and PDGFRB (B) (N = 12–13 for each group, *P < 0.05, Mann-Whitney test). Representative immunoblots confirm decreased protein expression of PDGFR-α (170 kDa, C) and PDGFR-β (190 kDa, D). MSCs from 3 infants who developed BPD and 3 infants who did not develop BPD are shown. E and F: densitometry analysis group mean data (N = 11–12 for each group, *P < 0.05, unpaired t-test). The relationships between PDGFRA (G) and PDGFRB mRNA expression (H) and gestational age demonstrate that, for the same gestational age, PDGFRA and PDGFRB mRNA expression is lower in MSCs from infants who develop BPD.
Lungs of infants with BPD show a paucity of PDGFR-α-positive mesenchymal cells in thickened dysmorphic alveolar walls.
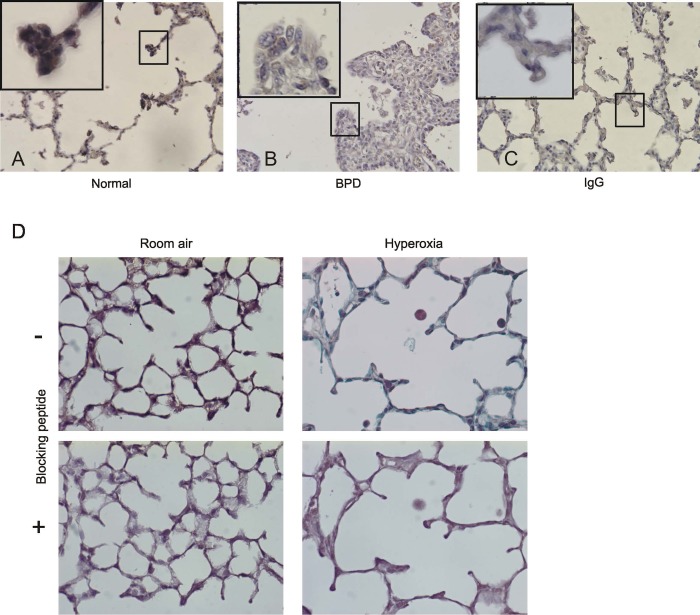
Lung sections from age matched full-term infants (Fig. 2A) were immunostained and compared with lungs from infants dying of BPD (Fig. 2B). Immunostaining of the full-term infant lung showed PDGFR-α-positive cells at the tips of the alveolar septa. Immunostaining of lungs from infants dying of BPD showed distorted lung architecture with thickened alveolar walls, fewer and poorly formed alveolar secondary crests, and reduced PDGFR-α content at the alveolar septal tips. To confirm the specificity of the PDGFR-α-positive staining, lung sections from mice exposed to room air or hyperoxia were immunostained after immunoadsorption of the primary antibody with an antigenic blocking peptide. Figure 2D demonstrates that PDGFR-α-positive staining at the tips of secondary crests is abolished following immunoadsorption with blocking peptide.
Fig. 2.
Lungs of infants dying with BPD show a paucity of PDGFR-α-positive mesenchymal cells in the thickened alveolar walls. Compared with the lung of an age matched full-term infant dying of a nonpulmonary cause (A and C), immunostained lung sections from infants dying with BPD (B) show widened alveolar spaces and thickened alveolar walls with fewer alveolar secondary crests and a paucity of brown-staining PDGFR-α-positive cells at the tips of the dysmorphic alveolar septa (original magnification, ×400; insets, ×1,000 magnification). C shows normal lung stained with a control antibody. In D, lungs from air-exposed mice show brown PDGFR-α staining at the tips of secondary crests. The lungs of hyperoxia-exposed mice show reduced number of PDGFR-α-positive tips. Staining is decreased when lung sections were probed in the presence of antigenic peptide. (Original magnification, ×400).
Neonatal lung MSCs from infants who develop BPD show decreased migration to PDGF.
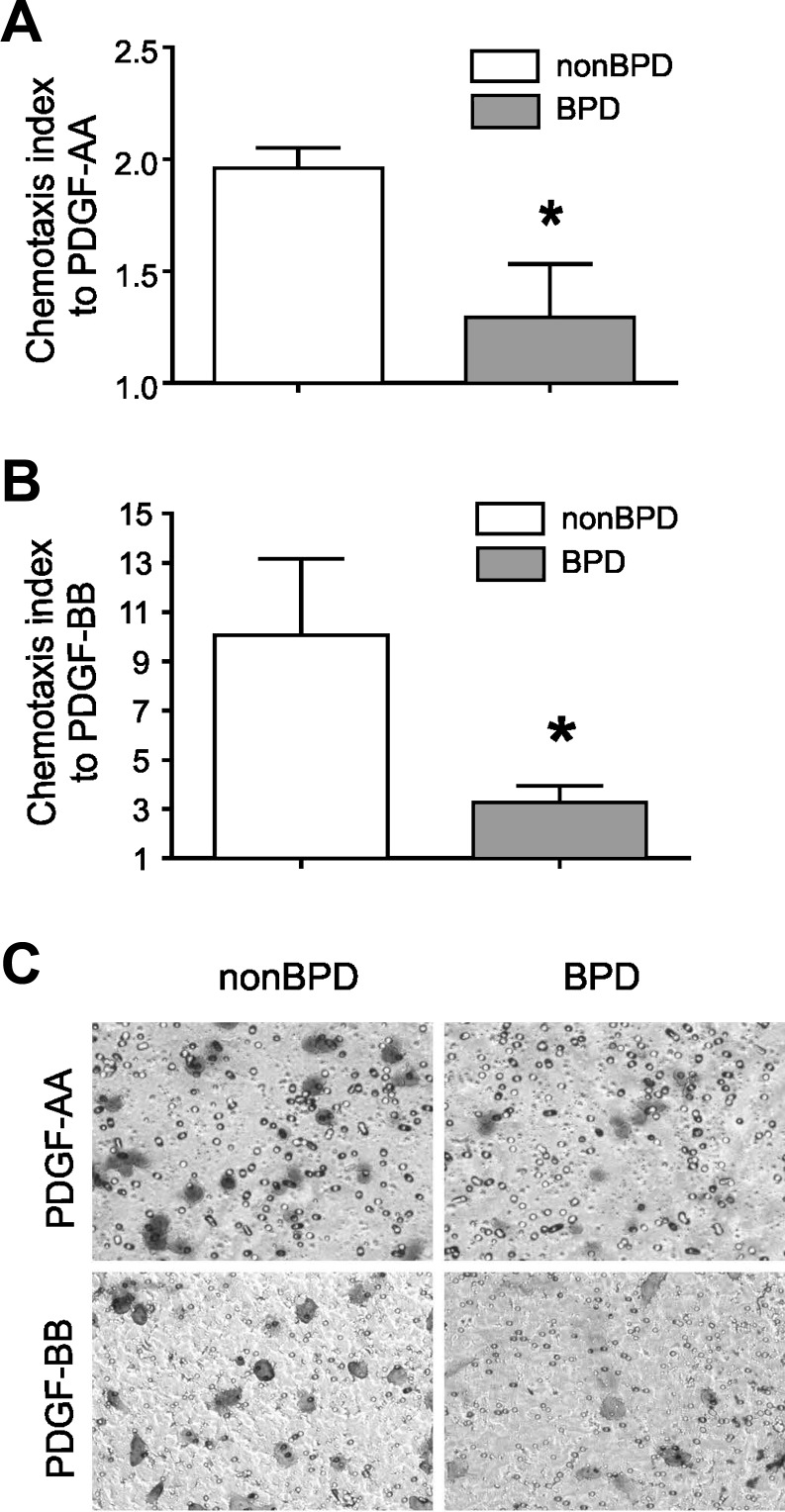
To further investigate the functional effect of decreased PDGFR expression, we assessed migration of the cells to PDGF-AA (30 ng/ml) and PDGF-BB (10 ng/ml) using a Boyden chamber. Compared with cells from infants not developing BPD, MSCs from infants developing BPD showed decreased migration to both PDGF-AA and PDGF-BB (Fig. 3, A–C).
Fig. 3.
Neonatal lung MSCs from infants who develop BPD show decreased migration to PDGF. Migration of neonatal lung MSCs in response to PDGF-AA (30 ng/ml) and PDGF-BB (10 ng/ml) was assessed after 4-h incubation in 12-well Boyden chamber. PDGF-AA (A) and PDGF-BB (B). Group mean chemotaxis indexes for PDGF-AA (A) and PDGF-BB (B) are shown (*P < 0.05, unpaired t-test) compared with those for neonatal lung MSCs from infants not developing disease. In C, a representative membrane, stained after 4 h incubation with PDGF-AA or PDGF-BB, shows that fewer MSCs from infants who developed BPD cross through the membrane (toluidine blue stain). Results shown are representative of 3 independent experiments.
Effect of TGF-β1 treatment on MSC PDGFR-α expression.
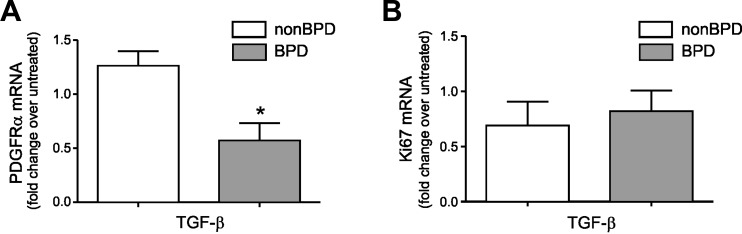
Overexpression of TGF-β is sufficient for development of a BPD phenotype in neonatal mice, including proliferation of α-actin-positive cells within the alveolar septal walls and hypoalveolarization (17, 39). To examine the effects of TGF-β1 on PDGFR-α expression in vitro, we treated neonatal lung MSCs with TGF-β1 (1 ng/ml) in serum-free medium for 48 h. In contrast to MSCs from infants who do not develop BPD, MSCs from infants who develop BPD showed PDGFR-α downregulation in response to TGF-β1 (Fig. 4A). In addition, we examined the effects of TGF-β1 on MSC expression of the cell proliferation marker, Ki67. Following treatment with TGF-β1 (1 ng/ml), Ki67 was equally expressed in MSCs from infants who develop BPD and MSCs from infants who do not develop disease (Fig. 4B). These data suggest that the differences in PDGFR-α expression following treatment with TGF-β1 are not related to differences in proliferation.
Fig. 4.
Neonatal lung MSCs from infants who develop BPD show reduced PDGFR-α mRNA expression in response to treatment with TGF-β1. A: PDGFR-α mRNA expression after treatment with low dose of TGF-β1 (1 ng/ml for 48 h) was assessed by qPCR (N = 6–9 for each group, *P < 0.05, unpaired t-test). B: no change in Ki67 mRNA expression was observed upon treatment with 1 ng/ml TGF-β1.
Lungs from hyperoxia-exposed neonatal mice show significantly lower expression of PDGFR.
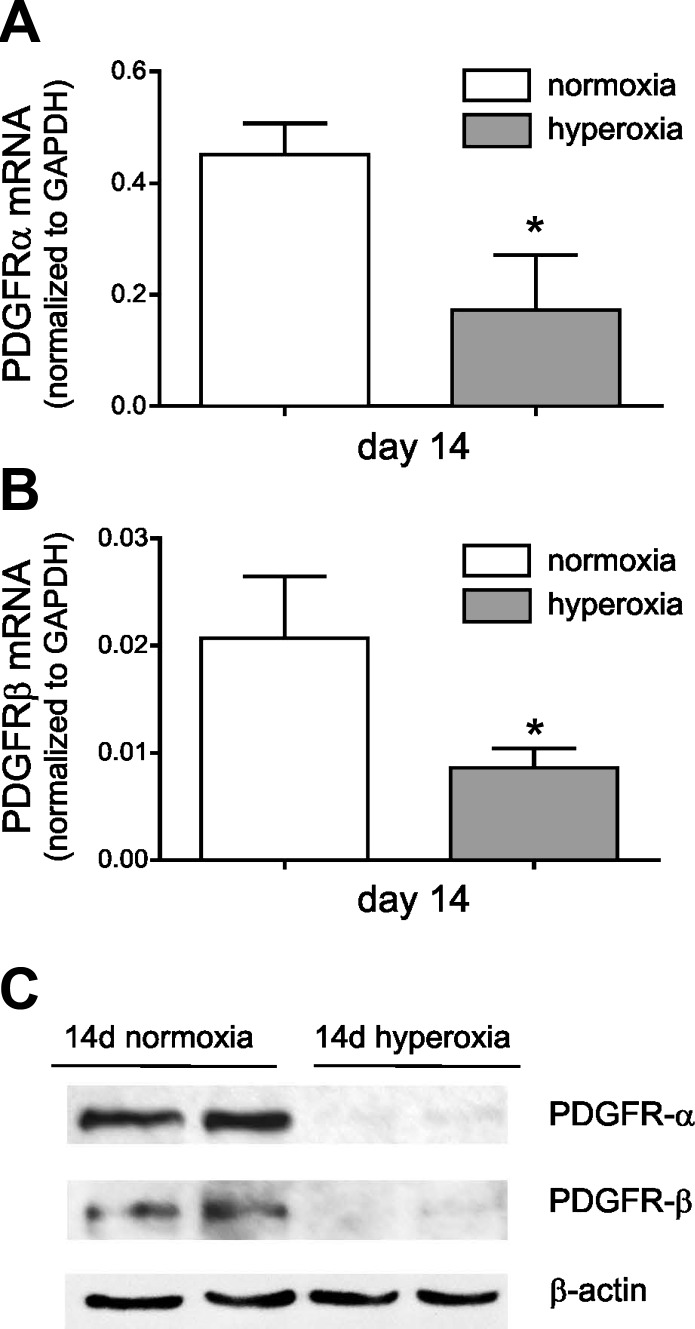
We examined PDGFR-α and PDGFR-β expression in hyperoxia-exposed neonatal mice, a model of BPD-like hypoalveolarization. Two- to 3-day-old C57BL/6J mice were exposed to air or 75% oxygen for 14 days. Total lung mRNA and protein lysates were harvested. Compared with lungs from air-exposed mice, hyperoxic exposure caused decreased mRNA (Fig. 5A) and protein (Fig. 5B) expression of PDGFR-α and PDGFR-β.
Fig. 5.
Lungs from hyperoxia-exposed neonatal mice show significantly lower expression of PDGFR-α and PDGFR-β. Two- to 3-day-old wild-type C57BL/6J mice were exposed to air or 75% oxygen for 14 days. Total lung mRNA and protein lysates were harvested. Lung mRNA encoding PDGFR-α (A) and PDGFR-β (B) was decreased after 14-day hyperoxic exposure (*different from same-day control, P < 0.05, unpaired t-test). C: immunoblotting analysis shows reduced protein expression of PDGFR-α (molecular weight, 170 kDa) and PDGFR-β (molecular weight, 190 kDa) in lungs from hyperoxia-exposed mice. Results are representative of 3 independent experiments.
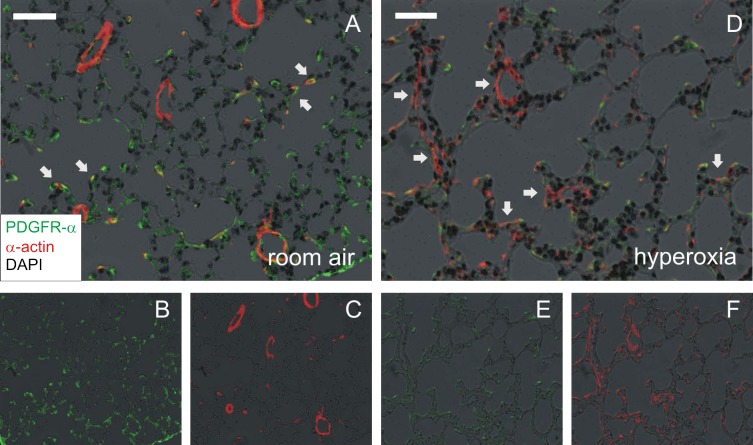
To further define the effects of hyperoxia on PDGFR-α expressing cells in the distal lung, we again exposed 2- to 3-day-old mice to 14 days of 75% oxygen or air. Mouse lungs were harvested for morphology as described above. Compared with air exposure (Fig. 6, A–C), lungs from hyperoxia-exposed mice showed fewer and larger air spaces and thickened alveolar walls (Fig. 6, D–F). Hyperoxic exposure also decreased PDGFR-α immunoreactivity at the tips of secondary crests in the alveolar septa, as identified by α-smooth muscle actin. Following hyperoxia exposure, there was a 2.7-fold decrease in the Vv of total alveolar tips and an 11-fold decrease in Vv of tips positive for PDGFR-α. (Table 3). Despite significant increases in lung volume, hyperoxia-exposed lungs showed a 9-fold decrease in the PDGFR-α(+) alveolar tips volume per lung and a 3.4-fold decrease in the total volume of PDGFR-α-positive alveolar tips normalized to the total volume of alveolar tips per lung.
Fig. 6.
In vivo hyperoxic exposure of neonatal mice decreases PDGFR-α immunofluorescence in alveolar septal tips. Lungs from 2- to 3-day-old wild-type C57BL/6J mice, exposed to air or 75% oxygen for 14 days, were perfused and harvested for morphology. Lung sections were immunostained with anti-PDGFR-α (green) and anti-α-smooth muscle actin Cy3 conjugate (red) or isotype control. Nuclei were stained with Hoechst 33342, shown as a negative grayscale image in black. Air-exposed lungs (A–C) showed PDGFR-α-positive, α-actin-positive alveolar myofibroblasts at the alveolar tips (A, arrows). A, merged image; B, PDGFR-α; C, α-actin. Lungs of hyperoxia-exposed mice show fewer and larger air spaces and thickened alveolar walls (D–F). Hyperoxic exposure also appeared to decrease the number of PDGFR-α-expressing alveolar myofibroblasts while increasing the number of PDGFR-a-negative, α-actin-positive myofibroblasts in the alveolar septa (D, arrows). D, merged image; E, PDGFR-α; F, α-actin. Magnification is ×200, and white bars represent 50 μm.
Table 3.
Hyperoxia exposure decreases the total volume of PDGFR-α positive cells at the alveolar tips
| Treatment | Room Air | Hyperoxia |
|---|---|---|
| Lung volume, ml | 0.25 ± 0.02 | 0.48 ± 0.03* |
| Alveolar tips Vv | 0.040 ± 0.0065 | 0.015 ± 0.0024† |
| Alveolar tips volume per lung, ml | 0.010 ± 0.0022 | 0.007 ± 0.0014 |
| PDGFR-α(+) alveolar tips Vv | 0.033 ± 0.0055 | 0.003 ± 0.0003* |
| PDGFR-α(+) alveolar tips volume per lung, ml | 0.009 ± 0.0019 | 0.001 ± 0.0001† |
| PDGFR-α(+) alveolar tips volume/alveolar tips volume per lung, ml | 0.819 ± 0.0390 | 0.238 ± 0.0672* |
Vv, volume density. Values are means ± SE; n = 5 for Room Air and n = 6 for Hyperoxia condition; different from Room Air group,
P < 0.001,
P < 0.01, unpaired t-test.
DISCUSSION
PDGF is a mesenchymal cell mitogen and chemoattractant (6, 7, 19, 26, 30, 31). During the pseudoglandular stage of lung development, PDGF-A is expressed by the branching lung epithelium, whereas PDGFR-α is expressed in the surrounding mesenchyme (8, 26). The requirement of PDGF for alveolarization has been established in animal studies (28). PDGF-A released from the growing bronchial epithelium promotes proliferation and disaggregation of PDGFR-α-positive progenitor cells, leading to their distal spread along the epithelial basement membrane to the tips of secondary alveolar septa. These cells then differentiate into α-smooth muscle actin- and elastin-producing myofibroblasts required for alveogenesis (8, 26, 30, 32, 33, 41). PDGF-A-deficient mice develop lung hypoalveolarization secondary to the loss of alveolar myofibroblasts and failure of alveolar septation (8, 26). Neonatal rats injected daily with the PDGF tyrosine kinase antagonist imatinib mesylate from day 1–7 of life show impaired alveologenesis as reflected by a decrease in secondary crests, increase in alveolar size, and decrease in alveolar number (25). Analogous to rodents undergoing PDGF-A blockade, mice undergoing hyperoxic exposure show lungs with arrested alveolar development that show a paucity of α-actin-positive myofibroblasts at the septal tips (21). Similarly, the lung pathology of surviving premature infants with BPD is characterized by fewer and larger alveoli, as well as poorly formed secondary crests, indicating interference with septation (23). However, changes in PDGFR expression in infants with BPD, a disease of hypoalveolarization, have not previously been examined.
In this study, we examined the PDGFR expression of MSCs isolated from the tracheal aspirates of 25 premature infants with respiratory distress. These cells demonstrate a gene expression pattern related to alveolar septal fibroblasts (36) and offer a window on examining processes occurring in mesenchymal cells of the distal lung. We found that lung MSCs from premature infants who develop BPD expressed lower levels of PDGFR-α. The lower levels of PDGFR-α were not related to gestational age. In addition, MSCs from infants who developed BPD showed reduced migration to PDGF in vitro compared with cells isolated from infants who did not develop this disease. Thus MSCs from infants developing BPD appeared to be functionally distinct from control cells. Finally, tissue from infants with BPD showed similar reductions in PDGFR-α staining. Together, these data show that MSCs from infants developing BPD hold stable alterations in PDGFR-α gene expression that favor reduced alveolarization. These data demonstrate for the first time that defective PDGFR-α signaling is a primary feature of human BPD.
PDGF-B and its receptor PDGFR-β play a role in recruitment of vascular smooth muscle cells and pericytes during blood vessel formation (19). Given the importance of angiogenesis in the development of mature alveoli (37), one might expect a correlation between alveolarization and PDGFR-β in the lungs of infants with BPD. Indeed, we found reduced PDGFR-β mRNA and protein expression in MSCs from infants developing BPD. During early postnatal lung growth in rats, neutralizing antibodies to either PDGF-B or a truncated soluble PDGFR-β each cause a significant reduction of DNA synthesis (9). Together, these results suggest that reduced PDGFR-β contributes to hypoalveolarization in BPD.
One important question is whether reduced PDGFR expression in MSCs from infants with BPD is related to the maturity of the infants studied. In the cohort studied, premature infants developing BPD tended to have a lower gestational age at birth and lower birthweight than premature infants who did not develop BPD. To test this, we examined the relationship between gestational age at birth and PDGFR mRNA expression, normalized to GAPDH. For the same gestational age, PDGFRA and PDGFRB mRNA expression tended to be lower in MSCs from infants who developed BPD, consistent with the notion that reduced expression was indeed defective in BPD. On the other hand, there appeared to be a positive relationship between gestational age and PDGFRA, but not PDGFRB, expression, suggesting that PDGFRA expression increases with fetal maturation. This being the case, the later day of sampling in BPD infants should not have contributed to the observed reduction in PDGFR expression. Also, we found no differences in tracheal aspirate PDGF-AA and PDGF-BB levels between aspirates from babies who develop BPD and aspirates from babies who do not develop BPD. Our results for PDGF-BB confirm a previous report showing no significant association between BPD and bronchoalveolar lavage fluid concentration of PDGF-BB in premature infants (2) and are consistent with the notion that differences in PDGFR expression appear to be the primary PDGF pathway defect associated with the development of BPD.
To further characterize this process in vivo, we examined the lungs of neonatal mice exposed to hyperoxia, which show arrested alveolar development and thickened alveolar walls analogous to BPD. Hyperoxic exposure was associated with decreased lung mRNA and protein expression of PDGFR-α. Previous studies examining the effects of hyperoxia on lung mRNA and protein expression have showed mixed results. Hyperoxia-exposed neonatal rats show increased PDGFR-α mRNA expression but decreased protein expression (9). Furthermore, in normoxic mice, PDGFR-α protein was localized primarily to the airway epithelium. In piglets, hyperoxia has no effect on the protein expression of PDGFR-α in the airway epithelium or alveolar septum. More recently, it was shown that mechanical ventilation of preterm lambs reduced lung PDGFR-α mRNA and protein expression in the distal lung (5). However, no studies have focused on PDGFR-α-expression at the tips of secondary alveolar septa, which is required for alveogenesis. We therefore performed immunofluorescent staining and stereological analysis on neonatal mice undergoing hyperoxic exposure. We found that hyperoxia decreased the volume fraction of PDGFR-α-expressing cells located at the tips of secondary alveolar crests, a condition that would favor reduced alveolarization.
Previously, we demonstrated that, compared with MSCs from infants not developing BPD, MSCs from infants developing BPD show higher phospho-glycogen synthase kinase-3β, β-catenin, and α-actin content (35), indicative of advanced myofibroblastic differentiation. We now show that MSCs from infants developing BPD express lower levels of PDGFR-α. One possible common factor in the development of this phenotype from a less differentiated mesenchymal progenitor cell may be exposure to TGF-β. Overexpression of TGF-β in newborn rodent lungs induces changes consistent with BPD, including hypoalveolarization (17, 39). We have previously shown that TGF-β1 induces neonatal lung MSC myofibroblastic differentiation (35). Finally, TGF-β has been shown to downregulate PDGFR expression in other lung mesenchymal cells including lung fibroblasts (27). In the present study, we found that low-dose TGF-β1 reduced PDGFR-α expression only in MSCs from infants who develop BPD. TGF-β1 did not affect the expression of the cell proliferation marker Ki67, suggesting that the reduced expression of PDGFR-α in MSCs from infants who developed BPD is not due to antimitogenic effects of TGF-β.
There are some limitations to our study. First, examining the components of tracheal aspirates may not provide an accurate picture of processes in the distal lung leading to the development of BPD. However, studies have shown neonatal tracheal aspirate fluid to have equal validity to bronchoalveolar lavage in the estimation of disaturated phosphatidylcholine (12) and IL-8 levels (11), and therefore these aspirates may reflect a practical alternative for obtaining lung fluid specimens. Second, it is possible that the lung MSCs isolated from infants who develop BPD represent a different population of cells that are recruited to the airway lumen, therefore explaining differences in PDGFR expression. Third, hyperoxic exposure of neonatal mice does not replicate the circumstances leading to human BPD. However, the lungs of neonatal mice and premature infants share similar developmental features. At birth, rodents exhibit a saccular stage of lung development and alveolarization proceeds in the first 2 postnatal weeks (1). In the human lung, saccules appear by 23 wk gestation (13, 22). The common reductions in PDGFR-α expression observed in MSCs from premature infants and lung tissue from hyperoxic newborn mice provide further evidence that the neonatal hyperoxia model constitutes a reasonably sound model of human BPD.
We conclude that neonatal lung MSCs from infants who develop BPD show reduced PDGFR-α expression, leading to impaired cell migration. PDGFR-α expression is also reduced in the alveoli of infants with BPD in situ. Given the requirement of PDGFR for septation and alveolarization, these data strongly suggest that defective PDGFR-α signaling is a primary feature of human BPD. Further understanding of MSC biology may provide further insight into the pathogenesis of BPD and lead to new therapeutic interventions.
GRANTS
This work was supported by NIH grants R01 HL79339 and K23 HL109149.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
A.P.P., J.K.B., and M.B.H. conception and design of research; A.P.P., J.K.B., T.X.C., M.N.R., M.J.L., J.L., Q.C., A.M.G., and G.S.P. performed experiments; A.P.P., J.K.B., M.N.R., and M.B.H. analyzed data; A.P.P. and M.B.H. interpreted results of experiments; A.P.P., J.K.B., and M.B.H. prepared figures; A.P.P., J.K.B., and M.B.H. drafted manuscript; A.P.P. and M.B.H. edited and revised manuscript; A.P.P., J.K.B., M.N.R., M.J.L., J.L., Q.C., A.M.G., G.S.P., and M.B.H. approved final version of manuscript.
REFERENCES
- 1.Amy RW, Bowes D, Burri PH, Haines J, Thurlbeck WM. Postnatal growth of the mouse lung. J Anat 124: 131–151, 1977 [PMC free article] [PubMed] [Google Scholar]
- 2.Been JV, Debeer A, van Iwaarden JF, Kloosterboer N, Passos VL, Naulaers G, Zimmermann LJ. Early alterations of growth factor patterns in bronchoalveolar lavage fluid from preterm infants developing bronchopulmonary dysplasia. Pediatr Res 67: 83–89, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Bentley JK, Deng H, Linn MJ, Lei J, Dokshin GA, Fingar DC, Bitar KN, Henderson WR, Jr, Hershenson MB. Airway smooth muscle hyperplasia and hypertrophy correlate with glycogen synthase kinase-3β phosphorylation in a mouse model of asthma. Am J Physiol Lung Cell Mol Physiol 296: L176–L184, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhatt AJ, Pryhuber GS, Huyck H, Watkins RH, Metlay LA, Maniscalco WM. Disrupted pulmonary vasculature and decreased vascular endothelial growth factor, Flt-1, and TIE-2 in human infants dying with bronchopulmonary dysplasia. Am J Respir Crit Care Med 164: 1971–1980, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Bland RD, Xu L, Ertsey R, Rabinovitch M, Albertine KH, Wynn KA, Kumar VH, Ryan RM, Swartz DD, Csiszar K, Fong KS. Dysregulation of pulmonary elastin synthesis and assembly in preterm lambs with chronic lung disease. Am J Physiol Lung Cell Mol Physiol 292: L1370–L1384, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Bonner JC, Osornio-Vargas AR, Badgett A, Brody AR. Differential proliferation of rat lung fibroblasts induced by the platelet-derived growth factor-AA, -AB, and -BB isoforms secreted by rat alveolar macrophages. Am J Respir Cell Mol Biol 5: 539–547, 1991 [DOI] [PubMed] [Google Scholar]
- 7.Boström H, Gritli-Linde A, Betsholtz C. PDGF-a/PDGF alpha-receptor signaling is required for lung growth and the formation of alveoli but not for early lung branching morphogenesis. Dev Dyn 223: 155–162, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Boström H, Willetts K, Pekny M, Levéen P, Lindahl P, Hedstrand H, Pekna M, Hellström M, Gebre-Medhin S, Schalling M, Nilsson M, Kurland S, Törnell J, Heath JK, Betsholtz C. PDGF-A signaling is a critical event in lung alveolar myofibroblast development and alveogenesis. Cell 85: 863–873, 1996 [DOI] [PubMed] [Google Scholar]
- 9.Buch S, Han RN, Cabacungan J, Wang J, Yuan S, Belcastro R, Deimling J, Jankov R, Luo X, Lye SJ, Post M, Tanswell AK. Changes in expression of platelet-derived growth factor and its receptors in the lungs of newborn rats exposed to air or 60% O2. Pediatr Res 48: 423–433, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Cutz E, Chiasson D. Chronic lung disease after premature birth. N Engl J Med 358: 743–745; author reply 745–746, 2008 [DOI] [PubMed] [Google Scholar]
- 11.D'Angio CT, Basavegowda K, Avissar NE, Finkelstein JN, Sinkin RA. Comparison of tracheal aspirate and bronchoalveolar lavage specimens from premature infants. Biol Neonate 82: 145–149, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Dargaville PA, South M, McDougall PN. Comparison of two methods of diagnostic lung lavage in ventilated infants with lung disease. Am J Respir Crit Care Med 160: 771–777, 1999 [DOI] [PubMed] [Google Scholar]
- 13.Dilly SA. Scanning electron microscope study of the development of the human respiratory acinus. Thorax 39: 733–742, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dorph-Petersen KA, Nyengaard JR, Gundersen HJ. Tissue shrinkage and unbiased stereological estimation of particle number and size. J Microsc 204: 232–246, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Eber E, Zach MS. Paediatric origins of adult lung disease {bullet} 8. Long term sequelae of bronchopulmonary dysplasia (chronic lung disease of infancy). Thorax 56: 317–323, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ehrenkranz RA, Walsh MC, Vohr BR, Jobe AH, Wright LL, Fanaroff AA, Wrage LA, Poole K; National Institutes of Child Health and Human Development Neonatal Research Network. Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics 116: 1353–1360, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Gauldie J, Galt T, Bonniaud P, Robbins C, Kelly M, Warburton D. Transfer of the active form of transforming growth factor-β1 gene to newborn rat lung induces changes consistent with bronchopulmonary dysplasia. Am J Pathol 163: 2575–2584, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hart CE, Forstrom JW, Kelly JD, Seifert RA, Smith RA, Ross R, Murray MJ, Bowen-Pope DF. Two classes of PDGF receptor recognize different isoforms of PDGF. Science 240: 1529–1531, 1988 [DOI] [PubMed] [Google Scholar]
- 19.Hellstrom M, Kalén M, Lindahl P, Abramsson A, Betsholtz C. Role of PDGF-B and PDGFR-β in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development 126: 3047–3055, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Hennrick KT, Keeton AG, Nanua S, Kijek TG, Goldsmith AM, Sajjan US, Bentley JK, Lama VN, Moore BB, Schumacher RE, Thannickal VJ, Hershenson MB. Lung cells from neonates show a mesenchymal stem cell phenotype. Am J Respir Crit Care Med 175: 1158–1164, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Hirakawa H, Pierce RA, Bingol-Karakoc G, Karaaslan C, Weng M, Shi GP, Saad A, Weber E, Mariani TJ, Starcher B, Shapiro SD, Cataltepe S. Cathepsin S deficiency confers protection from neonatal hyperoxia-induced lung injury. Am J Respir Crit Care Med 176: 778–785, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hislop A, Reid L. Development of the acinus in the human lung. Thorax 29: 90–94, 1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hussain NA, Siddiqui NH, Stocker JR. Pathology of arrested acinar development in postsurfactant bronchopulmonary dysplasia. Hum Pathol 29: 710–717, 1998 [DOI] [PubMed] [Google Scholar]
- 24.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med 163: 1723–1729, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Lau M, Masood A, Yi M, Belcastro R, Li J, Tanswell AK. Long-term failure of alveologenesis after an early short-term exposure to a PDGF-receptor antagonist. Am J Physiol Lung Cell Mol Physiol 300: L534–L547, 2011 [DOI] [PubMed] [Google Scholar]
- 26.Lindahl P, Karlsson L, Hellstrom M, Gebre-Medhin S, Willetts K, Heath J, Betsholtz C. Alveogenesis failure in PDGF-A-deficient mice is coupled to lack of distal spreading of alveolar smooth muscle cell progenitors during lung development. Development 124: 3943–3953, 1997 [DOI] [PubMed] [Google Scholar]
- 27.Lindroos PM, Coin PG, Badgett A, Morgan DL, Bonner JC. Alveolar macrophages stimulated with titanium dioxide, chrysotile asbestos, and residual oil fly ash upregulate the PDGF receptor-alpha on lung fibroblasts through an IL-1beta-dependent mechanism. Am J Respir Cell Mol Biol 16: 283–292, 1997 [DOI] [PubMed] [Google Scholar]
- 28.Madurga A, Mizikova I, Ruiz-Camp J, Morty RE. Recent advances in late lung development and the pathogenesis of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 305: L893–L905, 2013 [DOI] [PubMed] [Google Scholar]
- 29.Martin JA, Hamilton BE, Ventura SJ, Osterman MJ, Kirmeyer S, Mathews TJ, Wilson EC. Births: final data for 2009. Natl Vital Stat Rep 60: 1–70, 2011 [PubMed] [Google Scholar]
- 30.McGowan SE, Grossmann RE, Kimani PW, Holmes AJ. Platelet-derived growth factor receptor-alpha-expressing cells localize to the alveolar entry ring and have characteristics of myofibroblasts during pulmonary alveolar septal formation. Anat Rec (Hoboken) 291: 1649–1661, 2008 [DOI] [PubMed] [Google Scholar]
- 31.McGowan SE, McCoy DM. Platelet-derived growth factor-A and sonic hedgehog signaling direct lung fibroblast precursors during alveolar septal formation. Am J Physiol Lung Cell Mol Physiol 305: L229–L239, 2013 [DOI] [PubMed] [Google Scholar]
- 32.McGowan SE, Torday JS. The pulmonary lipofibroblast (lipid interstitial cell) and its contributions to alveolar development. Annu Rev Physiol 59: 43–62, 1997 [DOI] [PubMed] [Google Scholar]
- 33.Noguchi A, Reddy R, Kursar JD, Parks WC, Mecham RP. Smooth muscle isoactin and elastin in fetal bovine lung. Exp Lung Res 15: 537–552, 1989 [DOI] [PubMed] [Google Scholar]
- 34.Ochs M, Mühlfeld C. Quantitative microscopy of the lung: a problem-based approach. Part 1: basic principles of lung stereology. Am J Physiol Lung Cell Mol Physiol 305: L15–L22, 2013 [DOI] [PubMed] [Google Scholar]
- 35.Popova AP, Bentley JK, Anyanwu AC, Richardson MN, Linn MJ, Lei J, Wong EJ, Goldsmith AM, Pryhuber GS, Hershenson MB. Glycogen synthase kinase-3β/β-catenin signaling regulates neonatal lung mesenchymal stromal cell myofibroblastic differentiation. Am J Physiol Lung Cell Mol Physiol 303: L439–L448, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Popova AP, Bozyk PD, Goldsmith AM, Linn MJ, Lei J, Bentley JK, Hershenson MB. Autocrine production of TGF-β1 promotes myofibroblastic differentiation of neonatal lung mesenchymal stem cells. Am J Physiol Lung Cell Mol Physiol 298: L735–L743, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thebaud B, Ladha F, Michelakis ED, Sawicka M, Thurston G, Eaton F, Hashimoto K, Harry G, Haromy A, Korbutt G, Archer SL. Vascular endothelial growth factor gene therapy increases survival, promotes lung angiogenesis, and prevents alveolar damage in hyperoxia-induced lung injury: evidence that angiogenesis participates in alveolarization. Circulation 112: 2477–2486, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Toti P, Buonocore G, Tanganelli P, Catella AM, Palmeri ML, Vatti R, Seemayer TA. Bronchopulmonary dysplasia of the premature baby: an immunohistochemical study. Pediatr Pulmonol 24: 22–28, 1997 [DOI] [PubMed] [Google Scholar]
- 39.Vicencio AG, Lee CG, Cho SJ, Eickelberg O, Chuu Y, Haddad GG, Elias JA. Conditional overexpression of bioactive transforming growth factor-beta1 in neonatal mouse lung: a new model for bronchopulmonary dysplasia? Am J Respir Cell Mol Biol 31: 650–656, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Wong PM, Lees AN, Louw J, Lee FY, French N, Gain K, Murray CP, Wilson A, Chambers DC. Emphysema in young adult survivors of moderate-to-severe bronchopulmonary dysplasia. Eur Respir J 32: 321–328, 2008 [DOI] [PubMed] [Google Scholar]
- 41.Yamada M, Kurihara H, Kinoshita K, Sakai T. Temporal expression of alpha-smooth muscle actin and drebrin in septal interstitial cells during alveolar maturation. J Histochem Cytochem 53: 735–744, 2005 [DOI] [PubMed] [Google Scholar]